Abstract
Cholesterol is an important component of plasma membranes (PMs) and the precursor of all steroid hormones. In steroidogenic tissues, upon hormone stimulation, there is a rapid transfer of cholesterol to the mitochondria, which is the site of the initial step in steroidogenesis. In the current study, we examined PM cholesterol trafficking for steroidogenesis. In a mitochondrial reconstitution assay, adrenal PMs supported steroidogenesis in the absence of additional transport proteins. Depletion of cholesterol in PMs by 50% eliminated the membranes’ ability to support steroidogenesis in vitro and reduced steroid production in intact Y1 adrenocortical cells. Syntaxin (STX)-5 and α-soluble N-ethylmaleimide-sensitive factor attachment protein (α-SNAP) are enriched in adrenal PMs, and adrenocorticotropic hormone treatment of rats recruited STX5 and α-SNAP to adrenal PMs and mitochondria. Immunodepletion of STX5 and α-SNAP from PMs decreased steroidogenesis supported by PMs in vitro. Protease digestion of PMs decreased, whereas recombinant STX5 or α-SNAP restored, the PMs’ ability to support steroidogenesis. Knockdown of either STX5 or α-SNAP in Y1 cells decreased stimulated steroidogenesis. These results indicate that STX5 and α-SNAP facilitate cholesterol trafficking from PMs to mitochondria for adrenal steroid synthesis and underscore the importance of vesicular trafficking of PM cholesterol for steroidogenesis.—Deng, B., Shen, W.-J., Dong, D., Azhar, S., Kraemer, F. B. Plasma membrane cholesterol trafficking in steroidogenesis.
Keywords: adrenal gland, mitochondria, SNARE proteins
Steroid hormones play important roles in almost every aspect of cellular metabolism, including carbohydrate regulation and lipid and protein metabolism, immune function (glucocorticoids), salt and water balance, and blood pressure regulation (mineralocorticoids) (1). They are also critically involved in the maintenance of secondary sex characteristics, reproductive functions, and muscle and bone growth (testosterone, progestin, and estrogens). Steroidogenesis is controlled by a complex interplay among several subcellular compartments, multiple enzymes, substrates, and products whereby cholesterol is converted to steroid hormones (2, 3).
The synthesis and uptake of cholesterol, which is an important component of cells, is tightly controlled by sterol regulatory element–binding protein-2 in animal cells, and ∼60–90% of total cellular cholesterol is concentrated in plasma membranes (PMs) (4). In contrast, cholesterol content in mitochondria and the endoplasmic reticulum (ER) is much lower than in PMs (5). Cholesterol is the precursor for the production of all steroid hormones (6, 7). In contrast to peptide hormones, steroids are not stored in appreciable amounts within cells and must be synthesized on demand. Upon hormonal stimulation of steroidogenesis, cholesterol must be trafficked to the inner mitochondrial membrane, where cytochrome P450 family 11, subfamily A, member 1, which converts cholesterol to pregnenolone, the first steroid product, is located (7, 8). Steroidogenic acute regulatory protein (StAR) and its partners are responsible for the rate-limiting movement of cholesterol from the outer to the inner mitochondria. Because StAR-mediated transfer of cholesterol to the inner mitochondrial membrane depletes cholesterol in the outer mitochondrial membrane, it is necessary for cholesterol content in the outer mitochondrial membrane to be replenished to maintain ongoing steroidogenesis. There are several different pathways that supply cholesterol for steroid production, including endogenous cholesterol biosynthesis in the ER, lipoprotein-derived cholesterol uptake via LDL receptor–mediated endocytosis or via scavenger receptor, class B1–mediated selective uptake, and hydrolysis of cholesteryl esters stored within lipid droplets (6). However, the mechanisms responsible for cholesterol trafficking to mitochondria for adrenal steroidogenesis from each of these sources are not fully understood.
Cholesterol is synthesized in the ER and is rapidly trafficked to the PM, primarily via nonvesicular mechanisms and subsequently can enter the endosomal recycling pool via vesicular transport (9). Likewise, LDL receptor–mediated cholesterol is delivered to the ER after lysosomal processing, mixing with the pool of newly synthesized cholesterol. Prior studies using Leydig and adrenal tumor cells have provided evidence that free cholesterol in the PM is primarily used for steroidogenesis (10, 11). Venugopal et al. (12) tracked cholesterol using mCherry-D4 (perfringolysin O) and found that PM provides cholesterol for steroidogenesis in Leydig cells. We recently showed that several soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins participate in trafficking lipid droplets to mitochondria, thereby delivering stored cholesterol to support steroidogenesis (7). The current experiments were undertaken to determine whether SNAREs are also involved in facilitating the movement of cholesterol from the PM to mitochondria for steroidogenesis.
MATERIALS AND METHODS
Chemicals and reagents
Pregnenolone and progesterone ELISA assay kits were purchased from Alpco (Salem, NH, USA). Cholesterol assay kits were purchased from Stanbio Laboratory (Boerne, TX, USA). Sphingomyelin (SM) assay kits and sphingomyelinase (SMase) were purchased from MilliporeSigma (Burlington, MA, USA). Phospholipid (phosphatidylcholine) assay kits were purchased from Cayman Chemical (Ann Arbor, MI, USA). Soybean l-1α-phosphatidylcholine, cholesterol, hydroxypropyl-β-cyclodextrin (HPCD), 22(R)-hydroxycholesterol, and trilostane were purchased from MilliporeSigma (Burlington, MA, USA) and abiraterone was obtained from Axon Biochemicals BV (Groningen, The Netherlands). Pierce RIPA buffer, Halt Protease Inhibitor Cocktail, and Halt Phosphatase Inhibitor Cocktail were from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies against syntaxin (STX)-5 (SC-365124), α-soluble N-ethylmaleimide-sensitive factor attachment protein (α-SNAP), STX-17, and voltage-dependent anion channel (VDAC)-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against SNAP25 (ab53723), SNAP23 (ab4114), STX-7 (ab68982), and GAPDH were obtained from Abcam Inc. (Cambridge, United Kingdom). Antibodies against VDAC2 and -3 and StarD3, -D4, and -D6 were purchased from Aviva Systems Biology Corp. (San Diego, CA, USA). Antibodies against NA+/K+-ATPase, calreticulin, β-actin, and assay kits for cytochrome c oxidase IV were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against α-SNAP, -23, and -25; STX-5 and-17; and StarD3 were authenticated and validated by immunoblots against their respective recombinant proteins and controls. Other antibodies were authenticated and validated by the companies from which they were purchased. Full-length recombinant STX-5 protein (long form) was purchased from Bio-Techne (Littleton, CO, USA), and short form recombinant STX 5 was purchased from OriGene Technologies (Rockville, MD, USA). Both protein preparations were more than 80% pure, as determined by SDS-PAGE and Coomassie blue staining.
Cholesterol–HPCD complexes were prepared with a cholesterol:HPCD ratio of 1:10 and 1:20 (mole ratio). In brief, 0.415 g of HPCD were dissolved in 0.83 ml of methanol. Separately, 11.7 mg cholesterol was dissolved in 58.5 µl chloroform. Then the cholesterol solution was slowly added to the HPCD solution to form a clear solution, which was then dried under a stream of nitrogen gas (13). The cholesterol–HPCD complex was dissolved in 30 ml cell culture medium (1:10, mole ratio) or 60 ml cell culture medium (1:20, mole ratio).
Animals
Adult male Sprague Dawley rats (about 200 g, 8 wk of age) were purchased from Harlan Laboratories (Indianapolis, IN, USA). All the rats were housed in the animal facility at the Veterans Affairs Palo Alto Health Care System for 1 wk on a standard chow diet to allow them to acclimate. All procedures were performed according to institution guidelines that had been approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Palo Alto Health Care System.
For the detection of SNARE protein movement in adrenals, rats were injected with adrenocorticotropic hormone (ACTH, 25 IU; Cortrosyn; Amphastar Pharmaceuticals, Rancho Cucamonga, CA, USA) 1 h before euthanasia. Control rats were injected with dexamethasone (40 μg per 100 g body weight; MilliporeSigma) 12 h before euthanasia to suppress any stress induced secretion of endogenous ACTH.
Subcellular fractionation
Adrenals were removed from age-matched male rats and used to isolate mitochondria, PMs, and ER. Adrenal glands from 4 rats were cleaned free of fat and finely minced with scissors in 35 mm dishes in cold 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)/sucrose buffer [10 mM HEPES and 250 mM sucrose (pH 7.4)]. The minced adrenals were then lightly homogenized in ice-cold HEPES/sucrose buffer 4 times. The homogenate was centrifuged at 700 g at 4°C for 10 min to sediment the nuclei and nonlysed cells. The supernatant was then centrifuged at 12,000 g at 4°C for 15 min to sediment the mitochondria. The pellet containing mitochondria was resuspended in 300 µl HEPES/sucrose buffer, and the supernatant was centrifuged at 100,000 g at 4°C for 1 h to sediment the PMs and ER. The resulting pellet was resuspended in 10 mM HEPES buffer containing 15% sucrose and then overlayed with a discontinuous sucrose gradient containing the following sucrose concentrations in 10 mM HEPES buffer: 1 ml 45% sucrose, 2 ml 30% sucrose, 1.5 ml 15% sucrose, and 0.5 ml 7.5% sucrose. The discontinuous sucrose gradient was then centrifuged at 100,000 g at 4°C for 1 h with a SW55Ti rotor (Beckman Coulter, Brea, CA, USA). After centrifugation, 2 clearly visible bands of membranes (a light membrane and a heavy membrane fraction) were collected with a syringe and needle (Supplemental Fig. S1).
Cloning, expression, and purification of recombinant proteins
Recombinant SNARE proteins, including α-SNAP, SNAP23, SNAP25, and STX17, as well as StAR [N-62, StAR lacking its N-terminal 62 aa, which retains its cholesterol transport activity and can support steroidogenesis (7, 14)], were expressed and purified as described by Lin et al. (7). In brief, α-SNAP, SNAP23, SNAP25, STX17, and StAR (N-62) cDNAs in a pET15b vector were transformed individually into BL21DE3 cells and induced by 1 mM isopropyl-1-thio-β-d-galactopyranoside for 3 h. The cells were collected by centrifugation, dissolved in lysis buffer, and sonicated 3 times with 10 bursts each. The cell lysates were removed after centrifugation at 12,000 g for 30 min. The expressed recombinant proteins contained in the supernatants were purified with Ni-nitrilotriacetic acid agarose (Qiagen, Germantown, MD, USA), according to the manufacturer’s protocol. The purified individual proteins were run on SDS-PAGE to document their purity (Supplemental Fig. S2) and stored at 20°C for further use.
Cell culture
Mouse adrenocortical cells (Y1) were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in F-12K medium supplemented with 2.5% fetal bovine serum, 15% horse serum, 100 U/ml penicillin, and 100 g/ml streptomycin. The cells (1 × 105) were seeded into 12 well plates for 1 d and then were treated with HPCD or HPCD+cholesterol for 1 h for manipulation of cellular cholesterol. They were then treated with 2.5 mM dibutyryl cAMP in F-12K medium for 3 h, and the medium was collected for progesterone detection with an ELISA assay kit. For manipulation of PM cholesterol, Y1 adrenocortical cells (2 × 107) were incubated with HPCD or HPCD+cholesterol (cholesterol:HPCD ratio 1:10 and 1:20) for 1 h and used to isolate PMs and ER according to the methods described above. Mouse Leydig tumor cells (MLTCs) were obtained from ATCC and were cultured in RPMI 1640 plus 10% fetal bovine serum, 100 U/ml penicillin, and 100 g/ml streptomycin.
Mitochondrial reconstitution assay
The reconstitution assay system was conducted according to our reported methods with slight modification (7). The current assay consisted of 0.01 µM succinic acid, 10 µg GTP, 5 µg (protein) PM, and 50 µg mitochondria in HEPES/sucrose buffer with a final volume of 100 µl. Where indicated, 1 μg of each of the recombinant proteins was added to the assay system (7). Before initiation, mitochondria were treated with 10 µM trilostane and 10 µM abiraterone for 2.5 min at room temperature and then added to the assay system. After 10 min incubation, the samples were centrifuged at 2500 g at 4°C for 10 min and the supernatants collected for pregnenolone detection using a pregnenolone ELISA assay kit. The sensitivity of the pregnenolone ELISA kit was 0.054 ng/ml. The specificity (% cross-reactivity) with pregnenolone reactivity set at 100% was as follows: progesterone 6.0%, dehydroisoandrosterone 5.2%, 5α-androstanediol 4.7%, epiandrosterone 1.0%, pregnenolone sulfate 0.4%, androstanedione 0.3%, 5α-androsterone, dehydroepiandrosterone (DHEA) SO4 0.2%, and etiocholanolone 0.1%. For each assay, controls were used that were prepared by spiking serum with defined quantities of pregnenolone. A standard curve was generated by using 0, 0.1, 0.4, 1.6, 6.4, and 25.6 ng/ml pregnenolone. All pregnenolone production assays were performed in the presence of the inhibitors trilostane and abiraterone, to prevent further metabolism of pregnenolone into other steroids. For SMase treatment, isolated PMs were digested with 100 mU/ml SMase for 30 min according to Das et al. (4) before addition to the reconstitution assay.
Transfections
STX-5 and α-SNAP silencer predesigned small interfering (si)RNAs were purchased from Thermo Fisher Scientific. Scrambled siRNA was used as a negative control. Y1 adrenocortical cells were transfected with 75 nM STX5 and α-SNAP siRNA, using PolyJet reagent (SignaGen Laboratories, Rockville, MD, USA) according to the manufacturer’s protocol in 12-well culture plates. After 2 d, the medium was replaced with fresh medium containing 2.5 mM dibutyryl cAMP and then was collected 3 h later for progesterone assay by ELISA. The sensitivity of the progesterone ELISA kit was 0.05 ng/ml. The specificity (percentage of cross-reactivity) with progesterone reactivity set at 100% was as follows: 20-α-dihydroprogesterone 0.03%, 20-β-dihydroprogesterone 3.27%, 5-α-pregnan-3,20 dione 3.46%, 17-α-hydroxyprogesterone 1.50%, pregnenolone 0.39%, cortisol 0.003%, 21-deoxycortisol 0.01%, 11-deoxycortisol 0.01%, corticosterone 0.30%, 11-deoxycorticosterone 0.83%, androstenedione 0.12%, testosterone 0.03%, estradiol <0.0012%, danazol <0.00012%, DHEA 0.02%, and DHEA-SO4 0.005%. For each assay, controls were used that were prepared by spiking serum with defined quantities of progesterone. A standard curve was generated by using 0, 0.12, 0.9, 3.0, 7.9, 15.0, and 36.0 ng/ml progesterone. For a positive control, 10 µM 22(R)-hydroxycholesterol was added 3 h before media collection. Cells were collected by RIPA for Western blot analysis.
Lipid measurements
Lipids were extracted by using the method of Folch et al. (15). Cholesterol concentration was measured with the Cholesterol LiquiColor assay kit (Stanbio Laboratory), which employs cholesterol esterase and cholesterol oxidase to convert total cholesterol ester and cholesterol to cholest-4-en-3-one and hydrogen peroxide. When combined with peroxidase/phenol-4-antipyrine reagent, the quinonimine chromogen with absorbance at 500 nm is produced. The intensity of the final red color is proportional to the amount of total cholesterol from 0 to 750 mg/dl. For each assay, a standard curve was generated using standards purchased from Stanbio Laboratory (no. 1012030). Phospholipids (phosphatidylcholine) were measured with the phosphatidylcholine assay kit from Cayman Chemical. The kit combines phosphatidylcholine-specific phospholipase D and choline oxidase to hydrolyze phosphatidylcholine and generate hydrogen peroxide, which then is catalyzed to generate a blue dye with an optimal absorption at 595 nm. A standard curve was performed in each assay, and the amount of phosphatidylcholine in each sample was calculated. The kit has a dynamic range of 20–150 mg/dl of phosphatidylcholine. SM was measured using the Sphingomyelin Quantification Assay Kit from MilliporeSigma. The assay system contains SMase and alkaline phosphatase, the combination of which allows the hydrolysis of SM to ceramide and phosphocholine, which is then converted to choline, producing a colorimetric signal for reading at 570 nm. The assay is linear from 1 µM to 1 mM SM. A standard curve was performed in each assay and the amount of SM in each sample calculated from the standard curve.
RESULTS
PMs can support adrenal mitochondrial steroidogenesis
Previously, we set up an in vitro assay system to measure mitochondrial steroidogenesis in which a lipid emulsion was used as a mimic for lipid droplets and to supply cholesterol to mitochondria. To determine whether PMs can directly supply cholesterol to support mitochondrial steroidogenesis, we first established a scheme to isolate mitochondria, PM, and ER, according to Lin et al. (7) and Radhakrishnan et al. (16) (Supplemental Fig. S1A). The PM and ER fractions were assessed by immunoblot analysis for Na+/K+-ATPase and calreticulin as markers for PM and ER, respectively. Results shown in Supplemental Fig. S1B, C indicate that the isolated PM fractions were ∼6-fold enriched in the PM marker Na+/K+-ATPase and contained minimal levels of the ER marker calreticulin. Total lipids from the PM preparations were extracted and the content of cholesterol, phospholipid and SM were assayed, with the following results: cholesterol 14.21 ± 1.65 ng/µg, phospholipid 136 ± 22.49 µM/µg, and SM 11.23 ± 1.28 ng/µg protein. Cytochrome c oxidase activity in the mitochondria preparations were assayed with and without n-dodecyl β-d-maltoside and used as an indicator of the integrity of the isolated mitochondria. Results indicated that 95% of mitochondria were intact (data not shown). To further evaluate the integrity of mitochondria, we conducted the mitochondrial reconstitution assay by using adrenal cytosol and lipid droplets, as described by Lin et al. (7). As expected, there was an ∼4.5-fold increase in the production of pregnenolone when a lipid emulsion was used as a source of cholesterol (Supplemental Fig. S1D).
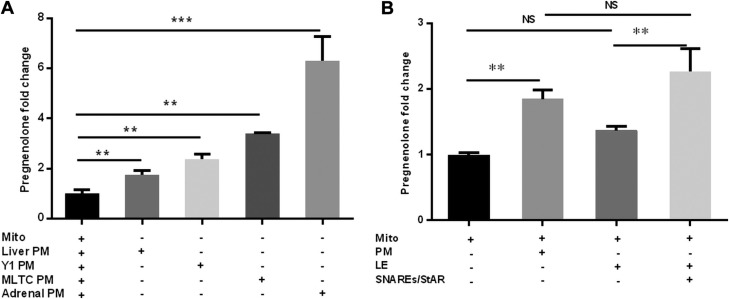
To test whether PMs can support adrenal mitochondrial steroidogenesis in the reconstitution assay, we added isolated rat adrenal PMs to mitochondria that had been isolated from rat adrenals in the absence of any other sources of cholesterol such as a lipid emulsion. The addition of PM isolated from rat adrenals to the mitochondrial preparation resulted in a 6-fold increase in pregnenolone production (Fig. 1A, P < 0.01). This finding is in contrast to that observed with addition of a lipid emulsion to the mitochondrial reconstitution assay, where there was no stimulation of pregnenolone production in the absence of SNARE proteins and StAR (7). When PMs that had been isolated from the MLTCs were added to rat adrenal mitochondria, there was a 2.4-fold increase in pregnenolone production, and a 3.4-fold increase when PMs isolated from Y1 adrenocortical cells were added in the mitochondrial reconstitution assay system, again in the absence of additional SNARE proteins and StAR. Although the liver is not a steroidogenic organ, when liver PMs were isolated and added to the reconstitution assay with isolated rat adrenal mitochondria, there was a small, yet significant (1.75-fold) increase in the production of pregnenolone. As a comparison to our previous publication where we showed that lipid emulsion mimicking lipid droplets and several SNARE proteins are involved in cholesterol transport to mitochondria for steroidogenesis, we compared the level of induction in steroidogenesis supported by a lipid emulsion and added SNAREs to that observed with addition of only PMs in the mitochondrial reconstitution system. PMs induced pregnenolone production to the same level as that supported by a lipid emulsion in the presence of recombinant SNAREs previously examined in the reconstitution system (Fig. 1B). These results revealed that PM alone can effectively support adrenal mitochondrial steroidogenesis.
Figure 1.
Plasma membranes can support steroidogenesis. PMs from rat adrenals, MLTCs, Y1 adrenocortical cells, and liver were purified and added to the in vitro mitochondrial reconstitution assay, in the absence of any other sources of cholesterol or accessory proteins. Pregnenolone production was measured by ELISA. A) The relative pregnenolone production was measured with rat adrenal PMs, MLTC, Y1 adrenocortical cell, and liver PMs as the sources of cholesterol. The basal pregnenolone production for mitochondria without PMs ranged from 0.5 to 3 ng/ml. B) Comparison of rat adrenal PMs and lipid emulsion as cholesterol sources for pregnenolone production in the absence or presence of accessory proteins. LE, lipid emulsion; Mito, mitochondria; NS, not significant; SNAREs/StAR, a cocktail containing recombinant SNAP23, SNAP25, α-SNAP, STX17, and N62-StAR. Results are representative of 3 independent experiments (n = 3). **P < 0.01, ***P < 0.001.
Role of PM cholesterol content in supporting steroidogenesis
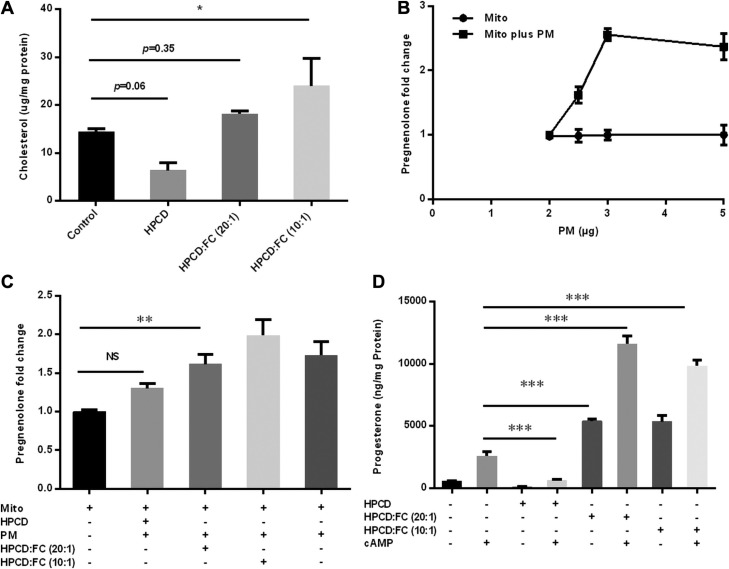
To determine whether manipulating PM cholesterol content could affect steroidogenesis in the mitochondrial reconstitution assay, we treated Y1 adrenocortical cells with HPCD to deplete PM cholesterol or treated cells with HPCD loaded with cholesterol to increase PM cholesterol. After incubation of the cells, PMs were isolated by sucrose gradient centrifugation. Analysis of the cholesterol concentration of the isolated PM indicated that incubation of cells with HPCD resulted in ∼50% depletion of PM cholesterol, whereas incubation with cholesterol-loaded HPCD (cholesterol:HPCD ratio1:10) approximately doubled PM cholesterol content, and incubation with HPCD containing half the amount of cholesterol (cholesterol/HPCD ratio 1:20) resulted in PM cholesterol content similar to that of control cells (Fig. 2A). Before examining the effects of PM with differing amounts of cholesterol in the reconstitution assay, a dose response curve of PM isolated from control Y1 adrenocortical cells was performed (Fig. 2B). Addition of PM isolated from control cells increased the mitochondrial production of pregnenolone at 2.5 µg (protein) of PM and saturated at 3 µg (protein) of PM within the assay. When PMs isolated from cells treated to manipulate their cholesterol content were examined in the mitochondrial reconstitution assay, cholesterol-depleted PMs did not significantly support steroid production, whereas steroid production was similar when normal and cholesterol-enriched PMs were used, perhaps reflecting the rapid saturation and narrow dose response of PM-supported steroid production in the reconstitution assay system (Fig. 2C). To determine whether this is reflected in intact cells, Y1 adrenocortical cells were treated with HPCD or with cholesterol-loaded HPCD complex for 1 h, before cAMP-stimulated steroid production was assessed. As expected, HPCD incubation depleted cellular cholesterol, whereas HPCD+cholesterol incubation increased cellular cholesterol (Supplemental Fig. S3). Cellular production of progesterone was reduced by cholesterol depletion and increased by cholesterol loading, consistent with cellular cholesterol stores influencing steroid production in the absence of lipoprotein-delivered cholesterol (Fig. 2D). It is noteworthy that progesterone production was markedly increased in cholesterol-loaded cells, even in the absence of cAMP stimulation, which suggests either that basal levels of StAR were sufficient to transport cholesterol into mitochondria or that enough oxidized cholesterol, which does not require StAR for transport to the inner mitochondrial membrane, was present to support steroid production.
Figure 2.
PM cholesterol affects steroidogenesis. Y1 adrenocortical cells were treated with HPCD to deplete PM cholesterol or cells treated with HPCD-loaded cholesterol to increase PM cholesterol. A) PMs were isolated from HPCD-treated Y1 adrenocortical cells, and cholesterol content was analyzed. B) Dose response of Y1 adrenocortical cell PMs in the mitochondrial reconstitution assay. C) Effect of cholesterol-manipulated PMs on steroid production in the mitochondrial reconstitution assay. D) Production of progesterone in Y1 adrenocortical cells pretreated with HPCD or with cholesterol-loaded HPCD complex for 1 h and then treated with cAMP. Mito, mitochondria. NS, not significant. Results are representative of 3 independent experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
SMase has no effect on cholesterol trafficking for steroidogenesis
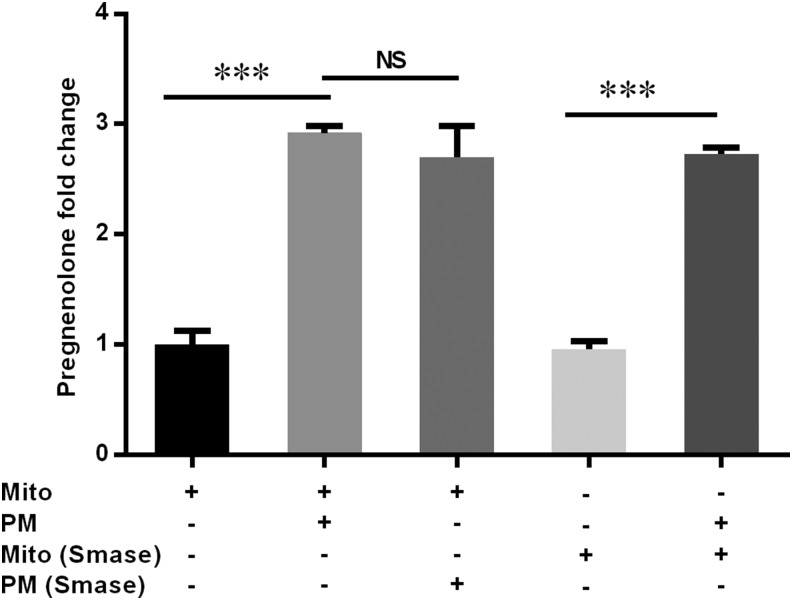
Cholesterol pools within the PMs are influenced by complex formation with SM. To determine whether the ability of PM to support steroid production is affected by SM binding of cholesterol, we digested isolated PMs with 100 mU/ml SMase for 30 min at 37°C. Incubation of mitochondria, with or without SMase, had no effect on pregnenolone production, demonstrating that SMase has no harmful effects on mitochondrial function (Fig. 3). When SMase-treated PMs were added to the reconstitution assay, pregnenolone production was supported to the same extent as with untreated PMs, even though there was complete depletion of SM and appearance of large amounts of ceramide (Supplemental Fig. S4).
Figure 3.
Effects of SM on steroid production. Rat PMs were digested with 100 mU/ml SMase for 30 min at 37°C and added to the mitochondrial reconstitution assay, and pregnenolone production was analyzed. The basal pregnenolone production for mitochondria without PM was 1.8 ± 0.2 ng/ml. Results are representative of 3 independent experiments (n = 3). ***P < 0.001.
Protein components from PMs facilitate mitochondrial steroidogenesis
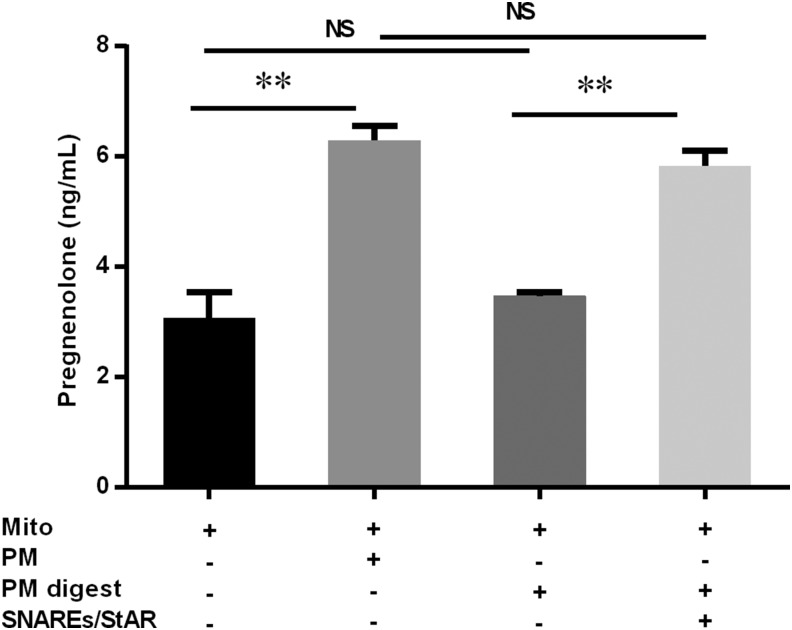
To determine whether there are protein components in PMs that are critical for facilitating mitochondrial steroidogenesis, PM preparations were treated with proteinase K before adding them to the reconstitution assay system. The addition of proteinase K-digested PM did not increase the production of pregnenolone in the mitochondrial reconstitution system, even though cholesterol content in the proteinase K-digested PMs was unaltered (Fig. 4). When purified SNARE proteins (SNAP23, SNAP25, α-SNAP, and STX17) and N62-StAR were added to the digested PM fraction, the pregnenolone production in the reconstitution system was restored to the level observed with undigested PMs. This result indicated that there are protein components in the PMs that are essential in the use of PM cholesterol for mitochondrial steroidogenesis, at least under the conditions of the in vitro system used.
Figure 4.
Protein components of PMs are necessary for steroidogenesis. Rat adrenal PM preparations were treated with proteinase K before they were added to the mitochondrial reconstitution assay system. Mito, mitochondria; PM digest, proteinase K-digested PM; SNAREs/StAR, a cocktail containing recombinant SNAP23, SNAP25, α-SNAP, STX17, and N62-StAR; NS, not significant. Results are representative of 3 independent experiments (n = 3). **P < 0.01.
Distribution of SNARE proteins in different cellular compartments
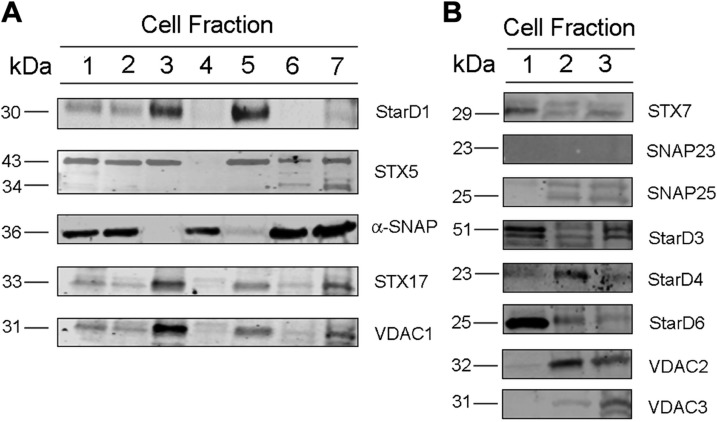
Because we had shown previously that SNARE proteins were involved in trafficking cholesterol in lipid droplets (7) and addition of SNAREs restored the ability of proteinase K-treated PMs to support steroidogenesis, we used immunoblot analysis to survey the distribution of several SNARE and lipid transport proteins, which could facilitate PM cholesterol utilization, in adrenal PM and other subcellular compartments. Subcellular fractions were prepared with differential centrifugations according to the scheme shown in Supplemental Fig. S1. Proteins were extracted and resolved on SDS-PAGE before blots were run using antibodies against several different SNARE proteins. Both STX5 and α-SNAP were present at relatively high levels in the isolated adrenal PM fraction (Fig. 5), whereas there was only very weak expression of StarD1, -3, and -6; STX7, -17, -23, and 25; and VDAC1 and -3 (Fig. 5B). VDAC2 and StarD4 were also relatively enriched in the isolated PM fraction.
Figure 5.
Distribution of proteins in cellular compartments. A) Subcellular fractions were isolated from rat adrenals and an immunoblot assay was conducted for StarD1, STX5, α-SNAP, STXx17, and VDAC1. Lane 1: total cell lysate; lane 2: total cell lysate minus nuclear fraction; lane 3: nuclear fraction and nonlysed cells; lane 4: cytosol; lane 5: mitochondria; lane 6: light membrane fraction (PM); lane 7: heavy membrane fraction (ER). B) Lane 1: whole-cell lysate; lane 2: PM; and lane 3: ER were assayed for STX7, SNAP23, SNAP25, StarD3, StarD4, StarD6, VDAC2 and VDAC3.
STX5 and α-SNAP move to the mitochondria and PM after ACTH stimulation
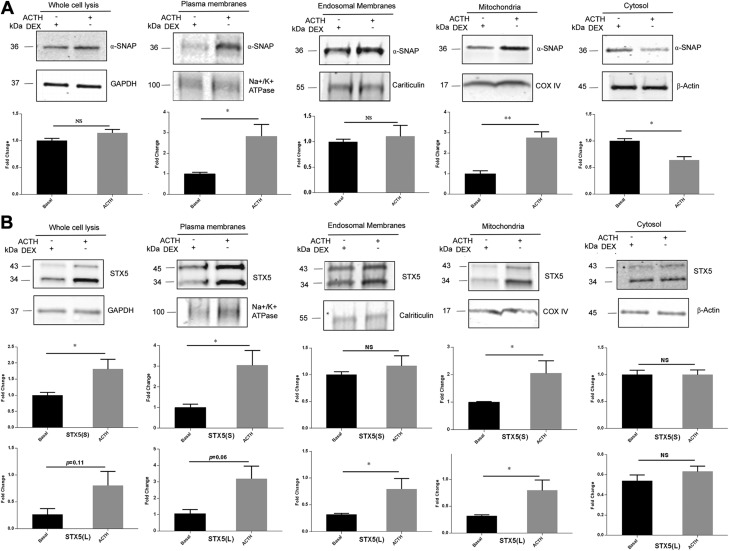
After stimulation with ACTH in vivo, there is a marked increase in steroidogenesis. We therefore analyzed whether there were any changes in protein subcellular distribution after in vivo ACTH stimulation. ACTH was injected 1 h before euthanasia in the experimental group, and basal control rats were injected with dexamethasone 12 h before euthanasia to prevent any stress-induced ACTH secretion. Adrenals were isolated for subcellular fractionation by differential sucrose gradient centrifugation. Protein preparations from whole-tissue extracts, PMs, ER, mitochondria, and cytosol fractions were resolved on SDS-PAGE followed by immunoblot analysis. NA+/K+- ATPase, calreticulin, cytochrome c oxidase, and β-actin were used as PM, ER, mitochondrial, and cytosol markers, respectively. GAPDH was used as control to normalize protein in total cell lysates. Our results indicated that the total amounts of α-SNAP in whole-tissue extracts were similar in basal conditions and after ACTH injection. However, after ACTH stimulation, α-SNAP was enriched in the PM and mitochondrial fractions, with significantly less α-SNAP in the cytosolic fraction, suggesting the translocation of α-SNAP from the cytosol to PM and mitochondria. The amount of α-SNAP in ER was not changed (Fig. 6A). There was a significant increase in the total cellular expression of the short form of STX5 after ACTH stimulation. The amount of short-form STX5 was enriched in the mitochondrial fraction after ACTH stimulation, with both the short and long form of STX5 enriched in the PM after ACTH. There were no changes in the amount of STX5 in ER and cytosol after ACTH treatment (Fig. 6B).
Figure 6.
STX5 and α-SNAP move to PMs and mitochondria after ACTH stimulation. Rats were injected with ACTH 1 h before euthanasia. Basal rats were injected with dexamethasone 12 h before euthanasia to prevent any stress-induced ACTH secretion. Rat adrenals were isolated for subcellular fractionation by differential sucrose gradient centrifugation, and 10 µg whole-cell lysate, PMs, endosomal membranes, mitochondria, and cytosol were subjected to immunoblot analysis for α-SNAP and STX5. GAPDH, NA+/K+-ATPase, calreticulin, cytochrome c oxidase IV, and β-actin were used as markers for whole-cell lysate, PMs, ER, mitochondria, and cytosol, respectively. A) Distribution of α-SNAP among subcellular fractions under basal conditions and after ACTH treatment. B) Distribution of STX5 among subcellular fractions under basal conditions and after ACTH treatment. NS, not significant. *P < 0.05, **P < 0.01, basal vs. ACTH treated.
Functional significance of STX5 and α-SNAP in PM cholesterol trafficking
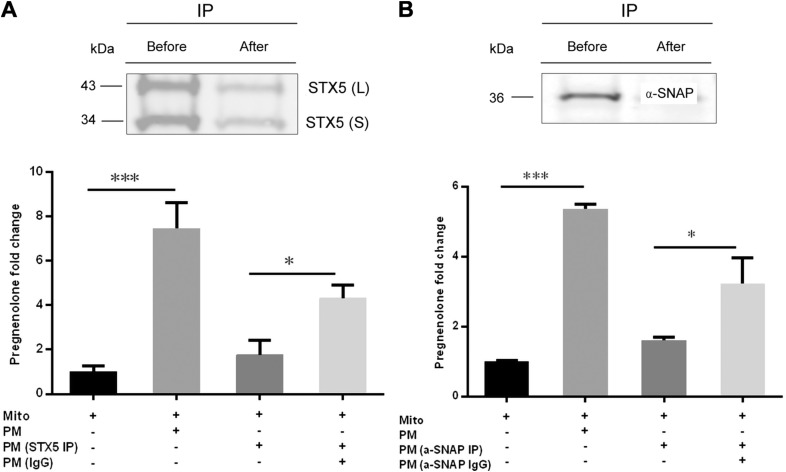
To explore potential roles of STX5 and α-SNAP in PM cholesterol trafficking, we used STX5 and α-SNAP antibodies to immunodeplete STX5 and α-SNAP from isolated adrenal PMs. Western blot analysis showed that immunoprecipitation (IP) using antibodies against STX5 (Fig. 7A) and α-SNAP (Fig. 7B) resulted in efficient depletion of the proteins from PMs. When the STX5 (Fig. 7C)- or α-SNAP (Fig. 7D)–depleted PMs were added to the mitochondrial reconstitution assay, the production of pregnenolone was significantly lower than observed in PMs immunoprecipitated with control IgG.
Figure 7.
STX5 and α-SNAP associated with PMs are essential for cholesterol trafficking. Rat adrenal PMs were immunodepleted using STX5 or α-SNAP antibodies and then added to the mitochondrial reconstitution assay. A) Rat adrenal PMs before and after STX5 IP were analyzed by immunoblot assay. B) Rat adrenal PMs before and after α-SNAP IP were analyzed by immunoblot assay. C) STX5 depleted PMs (STX5 IP) were added to the mitochondrial reconstitution assay. D) α-SNAP depleted PM (α-SNAP IP) were added to the mitochondrial reconstitution assay. IP, immunoprecipitated; L, long form of STX5; S, short form of STX5; Mito, mitochondria. Results are representative of 3 independent experiments (n = 3). *P < 0.05; ***P < 0.001.
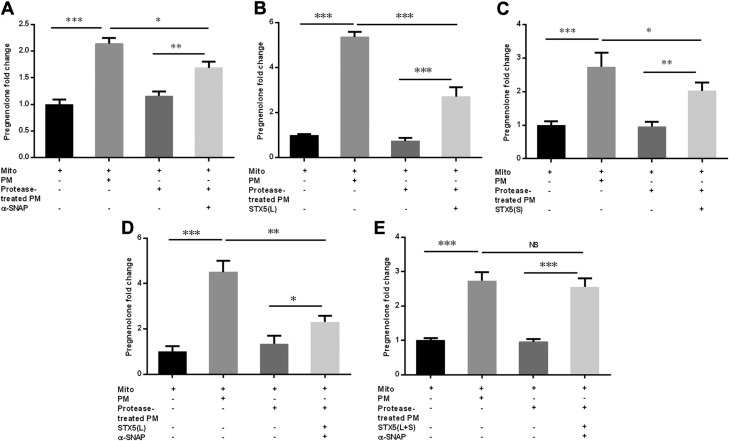
Next, we evaluated whether addition of STX5 and α-SNAP was sufficient to restore steroidogenesis in protease-treated PMs. PMs were treated with proteinase K and then incubated with purified recombinant STX5, or α-SNAP protein, or both before examining their ability to support steroidogenesis in the mitochondrial reconstitution assay. Results indicated that when α-SNAP (Fig. 8A), STX5 long form (Fig. 8B), or STX5 short form (Fig. 8C) was added to the protease-treated PMs, each of them stimulated steroidogenesis above that observed with PMs treated with protease alone; however, none of the individual proteins was able to achieve the same level of steroid production as that observed with intact PMs that had not been exposed to proteinase K. The incubation of proteinase K–treated PMs with both STX5 long form and α-SNAP (Fig. 8D) significantly stimulated steroidogenesis, yet still failed to achieve the same level of steroid production as that observed with intact PMs that had not been exposed to proteinase K. However, when proteinase K-treated PMs were incubated with α-SNAP and both the short and long forms of STX5 and examined in the mitochondrial reconstitution system, pregnenolone production was restored to the same level as that observed with intact PMs that had not been exposed to proteinase K (Fig. 8E). These results indicate that α-SNAP and STX5 (both short and long forms) associate with the PMs and are involved in facilitating PM cholesterol trafficking to mitochondria for steroidogenesis.
Figure 8.
Addition of STX5 and α-SNAP was sufficient to restore steroidogenesis in protease-treated PMs. Rat adrenal PMs were treated with proteinase K and then incubated with purified recombinant STX5, α-SNAP protein, or both, before their ability to support steroidogenesis was examined in a mitochondrial reconstitution assay. A) The effect of α-SNAP protein in restoring steroidogenesis in protease-treated PM. B) Effect of STX5 long-form protein in restoring steroidogenesis in protease-treated PM. C) Effect of STX5 short-form protein in restoring steroidogenesis in protease-treated PMs. D) Effect of STX5 long-form and α-SNAP protein together in restoring steroidogenesis in protease-treated PMs. E) Effect of STX5 long-form, STX5 short-form and α-SNAP protein together in restoring steroidogenesis in protease-treated PMs. The basal pregnenolone production for mitochondria without PM ranged from 0.5 to 1.9 ng/ml. L, long form of STX5; Mito, mitochondria; NS, not significant; S, short form of STX5. Results are representative of 3 independent experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
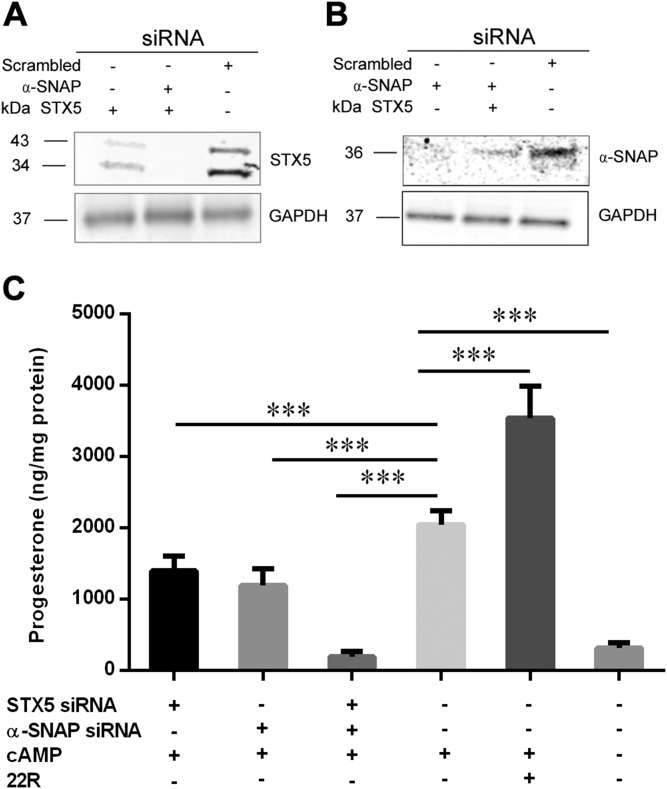
Knockdown of STX5 and α-SNAP decreases the production of progesterone
To further document the functional roles of STX5 and α-SNAP on trafficking PM cholesterol to mitochondria for steroid production, Y1 adrenocortical cells were transfected with siRNAs for STX5 and α-SNAP individually or in combination. Compared with the control (scrambled siRNA), STX5 and α-SNAP protein expression was significantly decreased in cells transfected with the respective siRNAs, without any apparent off target effects (Fig. 9A, B). Knockdown of either STX5 or α-SNAP individually resulted in a small, but significant, decrease in cAMP-stimulated steroid production; however, the combined knockdown of both STX5 and α-SNAP completely abrogated cAMP-stimulated steroid production, reducing levels to those observed in the basal state without cAMP incubation (Fig. 9C).
Figure 9.
Knockdown of STX5 and α-SNAP decreases production of progesterone in Y1 adrenocortical cells. Y1 adrenocortical cells were transfected with siRNAs for STX5 and α-SNAP individually or in combination for 48 h. Y1 adrenocortical cells were then treated with cAMP for 3 h, and media were analyzed for progesterone production. 22(R)-hydroxycholesterol was used as a positive control. A, B) Immunoblot analysis for STX5 (A) and α-SNAP (B). GAPDH was used as the loading control. C) Progesterone production in cells transfected with scrambled (control) or STX5 and α-SNAP siRNAs, individually or in combination. ***P < 0.001.
DISCUSSION
Cholesterol is the precursor of all vertebrate steroid hormones (3, 17). Cholesterol must be transported to mitochondria, which are cholesterol poor, to be converted to pregnenolone by cytochrome P450, family 11, subfamily A, member 1 after acute hormone stimulation of steroidogenesis (7). Previous research has indicated that several pathways, including endocytic lipoprotein-derived cholesterol uptake, scavenger receptor class B1–mediated selective cholesterol uptake, endogenous cholesterol synthesis, and the mobilization of cholesterol stored in lipid droplets, constitute sources of cholesterol for trafficking to mitochondria (6, 7, 18, 19). However, the relative contributions of each of these pathways for steroid production and whether similar mechanisms are used for cholesterol trafficking in each pathway have not been determined. Cholesterol is distributed heterogeneously among the membranes of the cellular compartments, with the PMs containing the highest concentrations of cholesterol: ∼60% of total cellular cholesterol (20).
In vitro reconstitution assays have been effective tools for studying the molecular mechanisms of intracellular trafficking (21, 22). In our previous work, we modified earlier systems to establish an in vitro mitochondrial reconstitution assay system for elucidating the transfer of cholesterol from lipid droplets to mitochondria by following pregnenolone production. In the current studies, we further modified our mitochondrial reconstitution system to study the trafficking of cholesterol from PMs to mitochondria for steroidogenesis. After isolating and purifying PMs by using sucrose gradient centrifugation and documenting their purity and slight ER contamination, we confirmed that PMs can supply cholesterol for steroidogenesis within this in vitro system. Venugopal et al. (12) used perfringolysin O fourth domain (D4) tagged with fluorescent proteins to track cholesterol and observed that PM cholesterol is imported into mitochondria for steroidogenesis in Leydig cells after dibutyryl cAMP–stimulated steroidogenesis.
In view of the substantial reservoir of cholesterol in PMs, we examined whether manipulating PM cholesterol content with cyclodextrin treatment (23, 24) influenced steroid production. Cyclodextrin incubation depleted PM cholesterol ∼50%, whereas incubation with cholesterol-loaded cyclodextrin approximately doubled PM cholesterol content. When examined in the mitochondrial reconstitution assay, cholesterol-depleted PMs did not significantly support steroid production, whereas steroid production was similar when normal and cholesterol-enriched PMs were used. These observations were partially mirrored in intact cells with cholesterol content that had been similarly manipulated. Thus, cellular production of progesterone in Y1 adrenocortical cells was reduced by cholesterol depletion, but, in contrast to the in vitro system, steroid production was increased by cholesterol loading, suggesting either a narrow dose response of the in vitro reconstitution system or that cholesterol enrichment with cyclodextrin results in the expansion of multiple pools of cholesterol in addition to the PM. This latter possibility is supported by several lines of evidence examining cholesterol overloading of cells (25–28). Das et al. (4) defined 3 pools of PM cholesterol: a pool of free cholesterol, an SM-sequestered pool of cholesterol, and an essential pool of cholesterol that is not influenced by SM. SMase-treated PMs supported pregnenolone production in the reconstitution assay to the same extent as untreated PMs, even though lipid composition was dramatically altered with complete depletion of SM and appearance of large amounts of ceramide. This observation contrasts with SMase treatment of cells where increased steroid production is observed and provides further evidence that SMase treatment of cells, similar to cellular cholesterol enrichment with cyclodextrin, results in the expansion of multiple cellular pools of cholesterol.
Because the addition of PMs isolated from rat adrenals to the in vitro mitochondrial reconstitution system resulted in a substantial increase in pregnenolone production in the absence of added SNARE proteins and StAR, which had been shown to be essential for utilization of cholesterol presented in lipid droplets (7), we explored whether there are protein components in the PMs that are critical for mitochondrial steroidogenesis. Consistent with this concept, proteinase K–treated PMs were unable to support production of pregnenolone in the mitochondrial reconstitution system even though PM cholesterol content was not altered. However, addition of purified SNARE proteins and StAR to the system restored the ability of proteinase K-treated PMs to support steroidogenesis.
SNARE proteins, which are characterized by an evolutionarily conserved central coiled–coil SNARE motif, are tail-anchored membrane proteins (29). Several SNAREs are capable of binding to cholesterol and are responsible for cholesterol distribution among intracellular membranes (30). In addition, several SNARE proteins, such as STX-17 (31), STX5 (7), SNAP23 (32), SNAP25 (33), and α-SNAP, are expressed in steroidogenic cells (7). When surveying SNARE expression in PMs, we found that STX5 and α-SNAP are highly enriched in PMs and that both STX-5 and α-SNAP are further enriched in adrenal PMs and mitochondria after ACTH stimulation. Research has shown that STX5 and α-SNAP form a protein complex to exert their effects (34). Immunodepletion of either STX5 or α-SNAP from PMs significantly reduced the ability of PMs to support pregnenolone production in the mitochondrial reconstitution assay. Moreover, the combination of α-SNAP and both short and long forms of STX5, but not individually, fully restored pregnenolone production supported by proteinase K–treated PMs to normal in the mitochondrial reconstitution system. Finally, knockdown of either STX5 or α-SNAP individually in Y1 adrenocortical cells resulted in a significant reduction in steroid production, but the combined knockdown of both STX5 and α-SNAP completely abrogated cAMP-stimulated steroid production, confirming the importance of STX5 and α-SNAP in steroid production.
It is noteworthy that there was a relatively small effect on steroid production observed with the addition of StAR to the mitochondrial reconstitution assay. This was likely because the bacterially produced StAR used in these studies was not phosphorylated, whereas StAR is normally phosphorylated during hormonal stimulation (35, 36), which modulates its activity (37). Moreover, it should not be surprising that the magnitude of the changes in steroid production generated in the mitochondrial reconstitution assay system is much lower than observed in intact cells or organisms, as the reconstitution assay system certainly lacks many components found in intact cells. Furthermore, the fact that SNARE proteins such as STX5 and α-SNAP stimulated significant increases in steroid production in the mitochondrial reconstitution assay does not negate the importance of StAR for the rate-limiting movement of cholesterol from the outer to the inner mitochondrial membrane (38), but highlights the importance of SNARE proteins in trafficking cholesterol to the outer mitochondrial membrane, which becomes apparent in an artificial in vitro mitochondrial reconstitution assay system optimized to examine cholesterol trafficking to mitochondria.
In summary, using an in vitro mitochondrial reconstitution assay system and cell knockdown experiments, we provide evidence that STX5 and α-SNAP are involved in facilitating the trafficking of cholesterol from PMs to mitochondria for adrenal steroid synthesis, underscoring the importance of vesicular trafficking in supporting the delivery of PM cholesterol for steroidogenesis.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by Merit Review Awards I01BX001923 (to S.A.) and I01BX000398 (to F.B.K.); and Senior Research Career Scientist Award IK6B004200 (to S.A.) from the U.S. Department of Veterans Affairs, Biomedical Laboratory Research Development Program. The authors declare no conflicts of interest.
Glossary
- α-SNAP
α-soluble N-ethylmaleimide-sensitive factor attachment protein
- ACTH
adrenocorticotropic hormone
- ER
endoplasmic reticulum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HPCD
hydroxypropyl-β-cyclodextrin
- IP
immunoprecipitation
- MLTC
mouse Leydig tumor cell
- PM
plasma membrane
- siRNA
small interfering RNA
- SM
sphingomyelin
- SMase
sphingomyelinase
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptor
- StAR
steroidogenic acute regulatory protein
- STX
syntaxin
- VDAC
voltage-dependent anion channel
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. Deng, W.-J. Shen, S. Azhar, and F. B. Kraemer designed the experiments and analyzed the data; B. Deng, W.-J. Shen, and D. Dong performed the research; and B. Deng, W.-J. Shen, S. Azhar, and F. B. Kraemer wrote the paper.
REFERENCES
- 1.Lindzey J., Korach K. S. (1997) Steroid hormones. In Endocrinology: Basic and Clinical Principles (Conn P. M., and Melmed S., eds.), pp. 47–62, Humana Press, Totowa, NJ, USA: 10.1007/978-1-59259-641-6_4 [DOI] [Google Scholar]
- 2.Miller W. L., Bose H. S. (2011) Early steps in steroidogenesis: intracellular cholesterol trafficking. J. Lipid Res. 52, 2111–2135 10.1194/jlr.R016675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne A. H., Hales D. B. (2004) Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25, 947–970 10.1210/er.2003-0030 [DOI] [PubMed] [Google Scholar]
- 4.Das A., Brown M. S., Anderson D. D., Goldstein J. L., Radhakrishnan A. (2014) Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife 3, 02882 10.7554/eLife.02882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvath S. E., Daum G. (2013) Lipids of mitochondria. Prog. Lipid Res. 52, 590–614 10.1016/j.plipres.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Kraemer F. B. (2007) Adrenal cholesterol utilization. Mol. Cell. Endocrinol. 265–266, 42–45 10.1016/j.mce.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Lin Y., Hou X., Shen W. J., Hanssen R., Khor V. K., Cortez Y., Roseman A. N., Azhar S., Kraemer F. B. (2016) SNARE-mediated cholesterol movement to mitochondria supports steroidogenesis in rodent cells. Mol. Endocrinol. 30, 234–247 10.1210/me.2015-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin L. A., Kennedy B. E., Karten B. (2016) Mitochondrial cholesterol: mechanisms of import and effects on mitochondrial function. J. Bioenerg. Biomembr. 48, 137–151 10.1007/s10863-014-9592-6 [DOI] [PubMed] [Google Scholar]
- 9.Ikonen E. (2008) Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138 10.1038/nrm2336 [DOI] [PubMed] [Google Scholar]
- 10.Freeman D. A. (1989) Plasma membrane cholesterol: removal and insertion into the membrane and utilization as substrate for steroidogenesis. Endocrinology 124, 2527–2534 10.1210/endo-124-5-2527 [DOI] [PubMed] [Google Scholar]
- 11.Gocze P. M., Freeman D. A. (1993) Plasma membrane cholesterol is utilized as steroidogenic substrate in Y-1 mouse adrenal tumor cells and normal sheep adrenal cells. Exp. Cell Res. 209, 21–25 10.1006/excr.1993.1279 [DOI] [PubMed] [Google Scholar]
- 12.Venugopal S., Martinez-Arguelles D. B., Chebbi S., Hullin-Matsuda F., Kobayashi T., Papadopoulos V. (2016) Plasma membrane origin of the steroidogenic pool of cholesterol used in hormone-induced acute steroid formation in Leydig cells. J. Biol. Chem. 291, 26109–26125 10.1074/jbc.M116.740928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purdy P. H., Graham J. K. (2004) Effect of adding cholesterol to bull sperm membranes on sperm capacitation, the acrosome reaction, and fertility. Biol. Reprod. 71, 522–527 10.1095/biolreprod.103.025577 [DOI] [PubMed] [Google Scholar]
- 14.Arakane F., Sugawara T., Nishino H., Liu Z., Holt J. A., Pain D., Stocco D. M., Miller W. L., Strauss J. F., III (1996) Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc. Natl. Acad. Sci. USA 93, 13731–13736 10.1073/pnas.93.24.13731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folch J., Lees M., Sloane Stanley G. H. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 16.Radhakrishnan A., Goldstein J. L., McDonald J. G., Brown M. S. (2008) Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 8, 512–521 10.1016/j.cmet.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller W. L., Auchus R. J. (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 10.1210/er.2010-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr B. R., Simpson E. R. (1981) Lipoprotein utilization and cholesterol synthesis by the human fetal adrenal gland. Endocr. Rev. 2, 306–326 10.1210/edrv-2-3-306 [DOI] [PubMed] [Google Scholar]
- 19.Shen W. J., Azhar S., Kraemer F. B. (2016) ACTH regulation of adrenal SR-B1. Front. Endocrinol. (Lausanne) 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesmin B., Maxfield F. R. (2009) Intracellular sterol dynamics. Biochim. Biophys. Acta 1791, 636–645 10.1016/j.bbalip.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. (1984) Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39, 405–416 10.1016/0092-8674(84)90019-9 [DOI] [PubMed] [Google Scholar]
- 22.Rothman J. E., Urbani L. J., Brands R. (1984) Transport of protein between cytoplasmic membranes of fused cells: correspondence to processes reconstituted in a cell-free system. J. Cell Biol. 99, 248–259 10.1083/jcb.99.1.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrom R. S., Liu X. (2007) Detergent and detergent-free methods to define lipid rafts and caveolae. Methods Mol. Biol. 400, 459–468 10.1007/978-1-59745-519-0_30 [DOI] [PubMed] [Google Scholar]
- 24.Stella V. J., He Q. (2008) Cyclodextrins. Toxicol. Pathol. 36, 30–42 10.1177/0192623307310945 [DOI] [PubMed] [Google Scholar]
- 25.Hao M., Lin S. X., Karylowski O. J., Wüstner D., McGraw T. E., Maxfield F. R. (2002) Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J. Biol. Chem. 277, 609–617 10.1074/jbc.M108861200 [DOI] [PubMed] [Google Scholar]
- 26.Lange Y., Ye J., Steck T. L. (2014) Essentially all excess fibroblast cholesterol moves from plasma membranes to intracellular compartments. PLoS One 9, e98482 10.1371/journal.pone.0098482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Ge M., Ciani L., Kuriakose G., Westover E. J., Dura M., Covey D. F., Freed J. H., Maxfield F. R., Lytton J., Tabas I. (2004) Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 279, 37030–37039 10.1074/jbc.M405195200 [DOI] [PubMed] [Google Scholar]
- 28.Wüstner D., Mondal M., Tabas I., Maxfield F. R. (2005) Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic 6, 396–412 10.1111/j.1600-0854.2005.00285.x [DOI] [PubMed] [Google Scholar]
- 29.Jung J. J., Inamdar S. M., Tiwari A., Choudhury A. (2012) Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6. Biosci. Rep. 32, 383–391 10.1042/BSR20120006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enrich C., Rentero C., Hierro A., Grewal T. (2015) Role of cholesterol in SNARE-mediated trafficking on intracellular membranes. J. Cell Sci. 128, 1071–1081 10.1242/jcs.164459 [DOI] [PubMed] [Google Scholar]
- 31.Steegmaier M., Oorschot V., Klumperman J., Scheller R. H. (2000) Syntaxin 17 is abundant in steroidogenic cells and implicated in smooth endoplasmic reticulum membrane dynamics. Mol. Biol. Cell 11, 2719–2731 10.1091/mbc.11.8.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant N. J., Hepp R., Krause W., Aunis D., Oehme P., Langley K. (1999) Differential expression of SNAP-25 isoforms and SNAP-23 in the adrenal gland. J. Neurochem. 72, 363–372 10.1046/j.1471-4159.1999.0720363.x [DOI] [PubMed] [Google Scholar]
- 33.Grosse J., Bulling A., Brucker C., Berg U., Amsterdam A., Mayerhofer A., Gratzl M. (2000) Synaptosome-associated protein of 25 kilodaltons in oocytes and steroid-producing cells of rat and human ovary: molecular analysis and regulation by gonadotropins. Biol. Reprod. 63, 643–650 10.1095/biolreprod63.2.643 [DOI] [PubMed] [Google Scholar]
- 34.Rabouille C., Kondo H., Newman R., Hui N., Freemont P., Warren G. (1998) Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell 92, 603–610 10.1016/S0092-8674(00)81128-9 [DOI] [PubMed] [Google Scholar]
- 35.Pon L. A., Hartigan J. A., Orme-Johnson N. R. (1986) Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J. Biol. Chem. 261, 13309–13316 [PubMed] [Google Scholar]
- 36.Pon L. A., Orme-Johnson N. R. (1988) Acute stimulation of corpus luteum cells by gonadotrophin or adenosine 3′,5′-monophosphate causes accumulation of a phosphoprotein concurrent with acceleration of steroid synthesis. Endocrinology 123, 1942–1948 10.1210/endo-123-4-1942 [DOI] [PubMed] [Google Scholar]
- 37.Arakane F., King S. R., Du Y., Kallen C. B., Walsh L. P., Watari H., Stocco D. M., Strauss J. F., III (1997) Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J. Biol. Chem. 272, 32656–32662 10.1074/jbc.272.51.32656 [DOI] [PubMed] [Google Scholar]
- 38.Stocco D. M. (2001) StAR protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol. 63, 193–213 10.1146/annurev.physiol.63.1.193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.