Abstract
Rodent studies demonstrate that supplementing the maternal diet with choline during pregnancy produces life-long cognitive benefits for the offspring. In contrast, the two experimental studies examining cognitive effects of maternal choline supplementation in humans produced inconsistent results, perhaps because of poor participant adherence and/or uncontrolled variation in intake of choline or other nutrients. We examined the effects of maternal choline supplementation during pregnancy on infant cognition, with intake of choline and other nutrients tightly controlled. Women entering their third trimester were randomized to consume, until delivery, either 480 mg choline/d (n = 13) or 930 mg choline/d (n = 13). Infant information processing speed and visuospatial memory were tested at 4, 7, 10, and 13 mo of age (n = 24). Mean reaction time averaged across the four ages was significantly faster for infants born to mothers in the 930 (vs. 480) mg choline/d group. This result indicates that maternal consumption of approximately twice the recommended amount of choline during the last trimester improves infant information processing speed. Furthermore, for the 480-mg choline/d group, there was a significant linear effect of exposure duration (infants exposed longer showed faster reaction times), suggesting that even modest increases in maternal choline intake during pregnancy may produce cognitive benefits for offspring.—Caudill, M. A., Strupp, B. J., Muscalu, L., Nevins, J. E. H., Canfield, R. L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study.
Keywords: saccade, reaction time, visuospatial memory, longitudinal
Demand for the essential nutrient choline is extremely high during prenatal development because of accelerations in fetal and placental tissue expansion, DNA methylation, and other physiologic processes (1–3). Choline supply is of particular importance for the developing brain because it is a precursor of acetylcholine, a key neurotransmitter for regulating neuronal proliferation, differentiation, migration, maturation, plasticity, and survival, as well as for synapse formation (4–8). Choline also provides substrate for the formation of phosphatidylcholine and sphingomyelin, principal components of neuronal and other cellular membranes required for signal transduction, brain development, and fetal growth (9–11). Moreover, choline is the primary dietary source of methyl groups, which, via epigenetic modifications, can exert lasting effects on gene expression (8, 12, 13).
More than 25 yr of research with rodents has consistently demonstrated that supplementing the maternal diet with additional choline produces life-long cognitive benefits for the offspring (14, 15). Furthermore, maternal choline supplementation not only improves offspring spatial memory and attentional function in young adult animals (16–20) but also can reduce or prevent normal age-related decline in those core cognitive functions (15). Maternal choline supplementation is also neuroprotective against a range of prenatal and early postnatal neural insults (21–25). The exact mechanisms responsible for those beneficial effects on cognitive performance are incompletely understood but are likely linked to the enduring neuroanatomic, neurochemical, molecular, and electrophysiologic changes that are caused by variations in prenatal choline supply (15, 24).
Despite numerous studies showing beneficial cognitive effects of maternal choline supplementation in animal models, only two experimental studies have been conducted, to our knowledge, to evaluate that putative effect in humans. Ross et al. (26) conducted a randomized, placebo-controlled trial of phosphatidylcholine supplementation (∼900 mg choline/d), beginning in the second trimester of pregnancy and continuing into the third postnatal month. At 5 wk of age, a greater percentage of the infants in the choline-supplemented group, relative to the control group, exhibited electrophysiologic evidence of cerebral inhibition in an auditory, evoked-response task—a characteristic associated with a reduced risk of both attentional dysfunction and schizophrenia as the child matures. In the other study, Cheatham et al. (27) randomized pregnant women to placebo or supplemental phosphatidylcholine (750 mg choline/d) from 18 wk gestation through 90 d postpartum. Infants were tested at both 10 and 12 mo of age using tasks of short-term, visuospatial memory and long-term, episodic memory but, contrary to predictions from the animal work, no effects of maternal choline supplementation were observed. It is not clear why this latter study did not observe a cognitive benefit of increased maternal choline intake. However, it could be due to poor participant adherence and/or uncontrolled variations in the intake of choline and other nutrients. In the current study, we sought to further examine that question in humans under conditions of highly controlled maternal intake of choline and other nutrients.
MATERIALS AND METHODS
Study design
The present study was a single-center, randomized, double-blind, parallel-group controlled feeding intervention conducted at Cornell University (3). Ethics approval was obtained from the Institutional Review Board for Human Study Participant Use at Cornell University. This trial was registered (NCT-1127022) at the U.S. National Library of Medicine (Bethesda, MD, USA; https://clinicaltrials.gov/); it was not overseen by a data monitoring committee and is now closed.
Participants
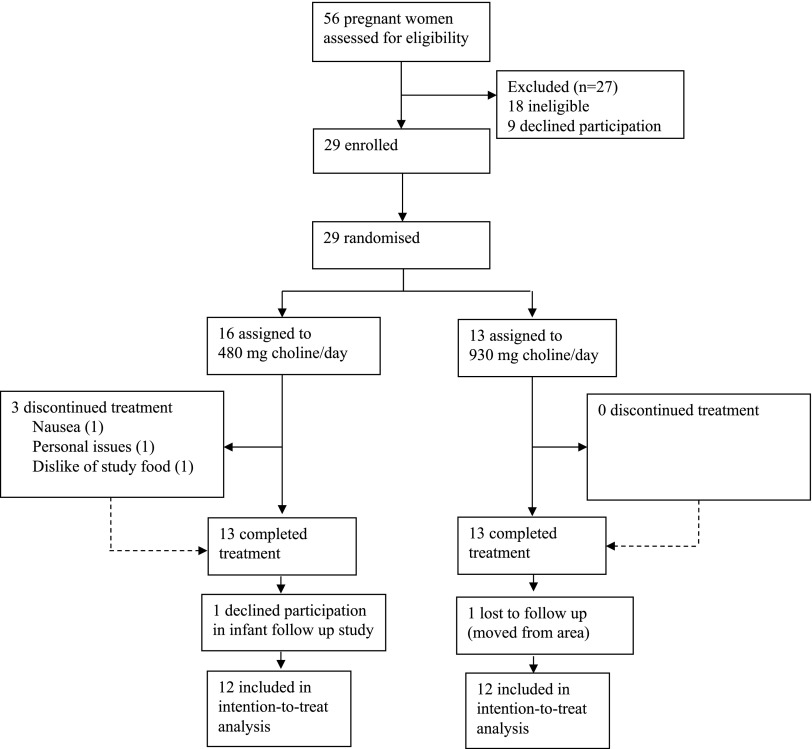
Pregnant women were recruited between January 2009 and October 2010 at maternity clinics throughout the Ithaca (NY, USA) region using flyers. Eligible participants were ≥21 yr old, entering their third trimester of pregnancy (self-reported), and were willing to comply with the study protocol, which required them to eat ≥5 meals/wk on-site, and consume only study-provided foods and beverages. Women were excluded if they were anemic (based on complete blood cell count), had blood markers of kidney and liver function outside of the reference range (based on blood chemistry profile), or reported having cardiovascular disease, cancer, type 1 or 2 diabetes mellitus, gastrointestinal disorders, or kidney or liver disease (based on health questionnaire). Additional exclusion criteria included use of prescription medications known to affect liver function; presence of >1 fetus; self-reported tobacco, drug, or alcohol use during gestation; or presence of pregnancy-associated complications (e.g., preeclampsia, gestational diabetes). Of the 29 women recruited, 26 (90%) completed the protocol, and 24 (83%) provided written, informed consent to have their infants participate in the cognitive-assessment component of the study (Fig. 1). One of the pregnant women in the 480 mg choline/d group inadvertently entered the study at gestational week 23 (rather than wk 27), which increased her exposure time to the study intervention.
Figure 1.
Trial profile. Flow of participants through the study screening, intervention, and infant follow-up study phases.
Randomization and masking
Upon study entry, pregnant women were randomized on a 1:1 ratio to receive either 480 or 930 mg choline/d. All women consumed the standard study diet (providing 380 mg choline/d) and a choline supplement of either 100 or 550 mg/d to achieve target choline doses of 480 or 930 mg/d, respectively. The supplement was mixed with cranberry–grape juice and served in 50-ml, color-coded, conical tubes (opaque blue or purple) to conceal the supplemental dose from the participant. Study personnel who performed the cognitive assessments and statistical analyses had no role in the intervention phase of the feeding study and were blinded to the choline dose assignment.
Procedures
Feeding study
Eligible pregnant women entered the feeding study on a rolling basis until target numbers of 13 in each choline group were achieved. The study diet (3) provided an average of 380 mg choline/d and 2100 calories/d across the 7-d rotational menu, which could be adjusted to meet caloric needs by adding or subtracting noncholine containing foods and beverages (e.g., white rice and sugar-sweetened beverages). Supplemental choline (choline chloride; Balchem, New Hampton, NY, USA) at intake levels of 100 or 550 mg was prepared by study personnel, as described in Yan et al. (3). On weekdays, participants consumed at least 1 meal/d and drank the choline supplement under the supervision of study personnel in the Human Metabolic Research Unit. All other weekday meals were provided as take-away food and were consumed off site. On weekends, participants consumed all meals off site and were instructed to consume the supplement with the take-away meal of their choice. Participant adherence to the designated two levels of choline intake was high, based on in-laboratory monitoring of the consumption of the choline supplement on 5 of 7 d/wk, completion of daily food checklists, and the return of the supplement and food containers. Further evidence for excellent adherence was provided by the finding that fasting plasma concentrations of choline and its metabolites were significantly greater in the women consuming 930 mg choline/d than in those consuming 480 mg choline/d (3).
To meet nutrient recommendations and consensus guidelines for pregnant women (11, 28) and to further ensure comparable nutrient intake, participants received the same daily, over-the-counter, prenatal multivitamin supplement (Pregnancy Plus; Fairhaven Health, Bellingham, WA, USA), a 200-mg docosahexaenoic acid supplement (Nature’s Way Neuromins; Schwabe North America, Green Bay, WI, USA), and a thrice weekly potassium and magnesium supplement (General Nutrition Corp., Pittsburgh, PA, USA). These supplements were consumed at the on-site meal during the week and with an off-site meal on weekends.
Most women completed the 12-wk feeding study before delivering their babies. Those who had not delivered after 12 wk continued the designated supplement regimen until delivery.
Data collection
Maternal and newborn information were obtained from medical charts at the time of delivery. Maternal information included due date, complications during the third trimester of pregnancy, and complications during labor or delivery. Newborn information included the date and method of delivery, gestational age, birth weight, and gender.
Cognitive testing
During 1-h laboratory visits at ∼4, 7, 10, and 13 mo of age, infants performed a visual attention task designed to measure the latency of saccadic eye movements to locations on a display screen, in which small, animated pictures appeared. Two types of saccades are distinguished in this task: 1) visually guided reactive saccades, which occur when the infant detects a peripheral stimulus and makes an abrupt shift in visual fixation to align the fovea with the visual target; and 2) memory-guided, anticipatory saccades, which occur when the infant predicts the appearance of a future picture based on detecting the spatiotemporal regularity of past picture onsets (29). Reactive saccades provide an early measure of information processing speed—widely acknowledged to be an important dimension of individual differences in human cognitive performance and intelligence (30). That is, infant saccade reaction time, assessed on 1 occasion as early as 3–4 mo of age, predicts information processing speed later in childhood (assessed in a manual reaction time paradigm) and childhood intelligent quotient (IQ) scores (31, 32). Moreover, longitudinal studies reveal that infant saccade reaction time shows lawful, age-related changes across the first year of life and robust stability of individual differences during infancy (33, 34). In contrast, the number of anticipatory saccades an infant makes depends greatly on the nature of the stimulus sequence presented and has not generally provided a reliable index of age-related changes or individual differences during infancy (33, 34). However, measures of visual anticipation in young infants have been shown to predict IQ scores in childhood (32) and some components of adult IQ (35).
For the present assessments, infants sat on their caregiver’s lap in a darkened room, 60 cm from a computer monitor, and viewed a sequence of animated images that appeared briefly at 10° to the left or right of visual center. Each image appeared for 700 ms and was followed by a 1.0-s interstimulus interval (blank screen). Infrared corneal reflection videography was used to record the eye region (at 30 Hz) as infants made visual fixation shifts during the 37-image sequence. The side of appearance of the first 7 images (baseline sequence) was unpredictable, whereas the subsequent 30 images appeared in a predictable left–right alternation (postbaseline sequence). For each video frame of eye movement data, eye position and stimulus position were scored off-line by human observers (blinded to the choline intake level of the mother) using computer-assisted software. Saccade latencies were computed as the difference between the time of stimulus onset and the time the infant’s eye began rotating to fixate at the location of the stimulus. A predictive saccade was defined as any fixation shift directed toward the next stimulus location that occurred before the actual onset of the stimulus (i.e., during the interstimulus interval) plus those saccades that occurred so quickly after stimulus onset that they could not have been triggered by the visual information itself (i.e., <133 ms) (34). Fixation shifts that followed the onset of the stimulus onset by ≥133 ms were defined as reactive (i.e., visually guided) saccades (34).
Outcomes
Our primary outcome was the mean saccade reaction time for the stimulus-guided fixation shifts. Our secondary outcome was the number of predictive saccades. Both outcomes were measured at ages 4, 7, 10, and 13 mo and were computed separately for fixation shifts to unpredictable stimuli during the baseline sequence and for fixation shifts during the postbaseline alternating sequence. As a result, each infant could contribute up to 2 data points for each outcome at each assessment age.
Statistical analysis
The sample size of the pregnant women who participated in the feeding study was based on a power calculation that predicted differences of 20% in biomarkers of choline metabolism (choline and betaine) with a power of 80% at an α of 0.05 between choline intake groups (i.e., 480 compared with 930 mg/d). For the mean saccade reaction time, analyses were powered at >80% to detect a difference of 10% between groups. All participants were included in all analyses by intention to treat. Statistical analyses were conducted using linear or logistic mixed-model methods in SAS 9.4 software (SAS Institute, Cary, NC, USA). Statistical significance was set at P < 0.05 for main effects and P < 0.10 for interactions. All tests were 2-tailed.
For mean saccade reaction time, we used linear, mixed-effects regression modeling for longitudinal data with random coefficients to estimate and test parameters of individual growth curves (36). Age was entered as the natural logarithm of postnatal age in days on each of the 4 testing dates to account for the nonlinearity of age-related change in saccade reaction time during that age period (34).
The effects of maternal choline intake level on mean saccade reaction time was first examined using an unadjusted model that included fixed effects for the independent variables choline intake (480 or 930 mg/d), infant age in days (∼4, 7, 10, and 13 mo), and image sequence type (unpredictable baseline vs. predictable postbaseline sequence). Random effects were estimated for the intercept and slope of age, for sequence type as a grouping variable, and for the individual child. We used restricted maximum-likelihood estimation and assumed an unstructured variance–covariance matrix. This model tested whether greater third-trimester, maternal choline intake resulted in faster infant mean saccade reaction time and whether the choline dose effect, if any, varied as a function of age, the type of image sequence, or the interaction of age and sequence type (i.e., all interactions included).
For adjusted analyses, we prespecified the inclusion of covariates known to be prognostic of infant cognitive outcomes. First, we included maternal age at conception and maternal education because recruitment for the feeding study did not include them as stratification variables. Second, we included gestational age, birth weight, and presence of labor or delivery complications because they were unknowable at the time of random assignment. Finally, ethnicity/race was included as a covariate because it affected the randomization (37) (via a minimization procedure). In this a priori model, covariates were entered only as fixed main effects (no interactions included). After the adjusted analyses, we conducted a secondary analysis to explore a refinement to our exposure estimation.
The same modeling approach was used for the secondary outcome, number of predictive saccades, with the following exceptions. First, because number of predictive saccades is a count variable, we used logistic, mixed-model regression methods with Poisson distribution and used the logarithm of the number of opportunities to anticipate a picture as an offset, using the SAS 9.4 Glimmix procedure. Second, because the number of predictive saccades has no confirmed pattern of age-related change, postnatal age was entered as an untransformed variable, and only a random intercept was included.
RESULTS
Data were obtained from 24 of the 26 eligible infants (92%). Table 1 shows baseline demographic and clinical characteristics of mothers and infants that were considered in the statistical models for the 480 and 930 mg choline/d groups. No adverse effects of the choline doses were reported.
TABLE 1.
Maternal health and demographics, pregnancy and delivery, and birth outcomes characteristics
| Characteristic | Third trimester choline intake | |
|---|---|---|
| 480 mg/d (n = 12) | 930 mg/d (n = 12) | |
| Health and demographicsa | ||
| Age (yr) | 28.8 (2.8) | 28.2 (4.0) |
| Body–mass index | 23.7 (3.2) | 23.4 (3.1) |
| Primiparous | 7 (58.3) | 4 (33.3) |
| Educationb | ||
| <4-yr college degree | 1 (8.3) | 4 (33.3) |
| ≥4-yr college degree | 11 (91.7) | 8 (66.7) |
| Ethnicityb | ||
| White | 8 (66.7) | 6 (50.0) |
| Black | 0 (0.0) | 1 (8.3) |
| Hispanic | 2 (16.7) | 2 (16.7) |
| Asian | 1 (8.3) | 3 (25.0) |
| Mixed | 1 (8.3) | 0 (0.0) |
| Pregnancy and delivery | ||
| Gestation length (wk)a | 40.2 (0.87) | 39.9 (0.42) |
| Labor/delivery complicationsb | 5 (41.7) | 4 (33.3) |
| Delivery method (vaginal)b | 10 (83.3) | 12 (100.0) |
| Birth outcomes | ||
| Gender (female)b | 2 (16.7) | 3 (25) |
| Birth weight (kg)a | 3.50 (0.43) | 3.47 (0.32) |
Data are means ± sd.
Data are numbers (%).
Saccade reaction time
For mean saccade reaction time, data were available for 139 of 192 possible participant visits (72%) and a total of 1477 stimulus presentation trials. Unadjusted regression analyses revealed that, when averaged across all ages and both sequence types (unpredictable, predictable), the estimated mean saccade reaction time for infants in the 930 mg choline/d group was 22.6 ms faster [confidence interval (CI): 1.3–43.8 ms; P = 0.03) than the mean saccade reaction time for infants in the 480 mg choline/d group (Supplemental Fig. 1). There was a highly significant effect of age at reaction time assessment (P < 0.0001), indicating that saccade reaction times became faster from 4 to 13 mo. However, that age-related increase in speed did not differ by choline intake group (interaction P = 0.3). There was also no effect of sequence type and no interactions involving any combination of intake group, sequence type, or age (all values P >0.2).
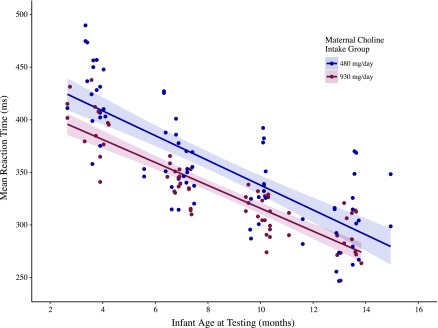
Adjusted analyses from the a priori model revealed a greater (33.8 ms; CI: 2.7–54.8 ms) beneficial effect of higher maternal choline intake, as compared with the unadjusted model estimate. The statistically significant difference (P = 0.03) in reaction time- between the two choline intake groups is illustrated in Fig. 2, which also shows the decline in mean saccade reaction time with increasing age for both groups (P < 0.0001) and that the effect of maternal choline intake level did not differ significantly as a function of age (age × choline intake group interaction P = 0.4). Gestational age was the only significant fixed-effect covariate (P = 0.04). The model estimated that each additional week of gestation was associated with an 18.3 ms decline (CI: 0.5–36.1) in mean saccade reaction time (when saccade reaction time was estimated at gestation age of 40 wk and infant age of 7 mo).
Figure 2.
Infant mean saccade reaction time as a function of age and maternal third-trimester choline intake group status. Data points are predicted values from the a priori mixed-effects regression model of maternal choline intake group on mean saccade reaction time, adjusted for the natural logarithm of infant age at testing, gestational age at birth, birth weight, presence of pregnancy or labor complications, maternal age, maternal ethnicity, and maternal education level. Each infant could contribute up to 2 data points for mean reaction time at each age, one for the baseline sequence and one for the postbaseline alternating sequence. The choline intake group effect is significant at P = 0.03. Lines represent least-squares linear-regression estimates and shadings represent 95% confidence limits.
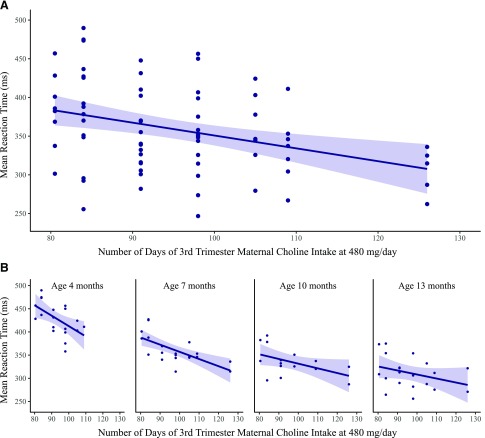
To further characterize dose–response in an intent-to-treat fashion, we explored the possibility that gestational age was serving as a proxy for differences in duration of choline exposure by including the number of days of exposure to the intervention in our a priori–adjusted model. In that model, there was a statistically significant effect of days of exposure (P = 0.02); the estimated effect of the choline dose was 51.3 ms (CI: 15.8–86.7 ms), and the effect of gestational age was no longer significant (P = 0.3). In a final model, we estimated the simple effects of days of exposure to the intervention, nested in dose, which revealed a significant effect of exposure days for the 480 mg choline/d group (b = −2.25; se = 0.64, P < 0.001), but no effect in the 930 mg choline/d group (b = −0.17; se = 1.27, P = 0.9). Figure 3A shows the dose–response for the predicted mean saccade reaction time by days of prenatal exposure to 480 mg choline/d intake for all ages combined, and Fig. 3B shows that relationship at each assessment age.
Figure 3.
Infant mean saccade reaction time as a function of the number of days of third-trimester maternal intake of 480 mg choline/d. A) Data points are predicted values of mean saccade reaction time for all ages combined (estimated at the mean age of 8.05 mo) from the a priori mixed-effects regression model, adjusted for days of maternal third-trimester intake of choline at 480 mg/d, the natural logarithm of infant age at testing, gestational age at birth, birth weight, presence of pregnancy or labor complications, maternal age, maternal ethnicity, and maternal education level. Each infant could contribute up to 2 data points for mean reaction time at each age, one for the baseline sequence and one for the postbaseline alternating sequence. The simple slope estimate for the effect of days of third-trimester maternal choline intake at 480 mg choline/d is −2.25 (se = 0.64, P < 0.001) and is represented by the least-squares fitted line. The shading represents the 95% confidence limit for the slope estimate. B) The same relationship estimated separately for each assessment age (4, 7, 10, and 13 mo), illustrating the consistency of the effect of days of choline intake within each assessment age. The reduced range for the abscissa in the plot of 4-mo data is due to missing data, at that age only, for the participant with the longest duration of prenatal exposure.
Predictive saccades
The unadjusted model revealed the expected finding that infants produced more predictive saccades during the predictable stimulus sequence than they did during the unpredictable baseline sequence (mean difference = 1.14; P < 0.0001). However, no significant effect of maternal choline intake during pregnancy was detected for the number of predictive saccades (mean difference = 0.08; P = 0.7). Adjustment for covariates in the a priori model did not alter that result (mean difference = 0.08; P = 0.6) but revealed that infants who experienced a medical complication during pregnancy or labor produced fewer predictive saccades than did infants without such complications (mean difference = 0.39; P = 0.01). The number of prenatal exposure days to choline was not related to the number of anticipatory saccades produced (P = 0.5).
DISCUSSION
The results of this controlled-feeding trial reveal significantly faster saccade reaction time, a measure of information processing speed (30, 34), among infants born to mothers consuming the higher level of choline intake throughout their third trimester. Infants of mothers randomly assigned to consume 930 mg choline/d were consistently movements than were the infants of mothers who consumed 480 mg choline/d. Notably, infants in the 930 mg choline/d group were consistently faster to react to pictures across the 4 assessment ages, indicating that the beneficial effect of a higher maternal choline intake on infant information processing speed endured for at least the first year of postnatal life. Moreover, those findings were not model dependent because they were observed in both unadjusted and a priori covariate-adjusted models. In light of prior evidence that infant saccade reaction time predicts IQ scores and information processing speed in early childhood (31, 32), our findings support the view that maternal choline supplementation has lasting beneficial effects on offspring cognitive function. Finally, our findings indicate that the last trimester of pregnancy constitutes a sensitive period for the functional effects of maternal choline supplementation on cognitive development, consistent with the animal data (19, 38). However, maternal choline supplementation during other stages of pregnancy and/or early postnatal development may also have lasting offspring effects, and the present study does not address their relative sensitivity.
Our findings also provide some support for a beneficial effect of consuming 480 mg choline/d, relative to women’s usual choline intake. Specifically, we found a significant linear dose–response showing that faster infant processing speed was associated with a greater number of days of fetal exposure to the 480 mg/d maternal choline dose, even after controlling for the gestational age at birth. That duration effect could have been found only if participants’ usual choline intake (i.e., prior to the start of the intervention) was substantially <480 mg/d; otherwise, if usual choline intake was approximately equal to 480 mg/d, then all participants would have had the same duration of exposure to that amount of choline throughout pregnancy, regardless of the number of days the intervention was administered. This inference is consistent with the evidence, discussed above, indicating that the average choline intake of pregnant women in the U.S. is ∼300–350 mg choline/d, with fewer than 25% of women consuming the adequate intake (AI) level (39–41). Considered in that light, the intake duration effect we report suggests that, even a modest increase in typical maternal choline intake during pregnancy would be beneficial for infant information processing speed, with possible long-term benefits for offspring cognitive function throughout life.
Although few studies have examined the effect of maternal choline intake on offspring cognition in humans, our finding of faster information processing speed among infants of mothers consuming more choline during pregnancy is broadly consistent with results from the randomized clinical trial by Ross et al. (26) showing improved auditory sensory gating among 5-wk-old infants of choline-supplemented mothers. The Ross finding and our present finding could both reflect enhanced gating of extrinsic stimuli by Ch6 cholinergic neurons in the brainstem reticular formation and/or Ch1 cholinergic neurons in the basal forebrain medial septal nucleus (42, 43). Another factor contributing to faster processing speed could be improved nerve conduction velocity because of greater availability of choline-derived cell membrane phospholipids (e.g., phosphatidylcholine and sphingomyelin) (44). Other mechanisms could relate to lasting changes in gene expression because of choline’s role as a primary dietary source of methyl groups (45, 46).
In contrast to the saccade reaction time findings, we found no evidence for an effect of maternal choline intake on the number of anticipatory saccades, a putative measure of spatial memory for sequential regularity. Cheatham et al. (27) also reported no effects of maternal choline supplementation on infant spatial memory assessed at 10 and 12 mo using a manual search task. These findings might indicate that prenatal choline does not affect the cognitive operations underlying spatial memory in humans (contrary to rodents) but such a conclusion would be premature given the paucity of experimental studies with human subjects. It is also possible that effects of prenatal choline on neural systems subserving spatial memory do not emerge until later in life, given that the neural systems underlying mature forms of spatial and episodic memory do not emerge until after 2 yr of age in humans (47). Consistent with that contention are the results of a large, prospective study by Boeke et al. (39), which reported a positive association between maternal self-reported, third-trimester choline intake and children’s performance on two visual memory tasks at age 7 yr.
The present study had several strengths which, in combination, likely increased our ability to detect an effect in a small sample of infants. First, during the intervention period, all participants consumed the same foods from a laboratory-prepared menu, with all nutrients controlled and consumed in recommended amounts. Second, participants’ consumption of the choline supplement occurred in the laboratory under the supervision of study personnel, thus ensuring that the two groups differed substantially in choline intake during the treatment period. In the 2 previous studies in this area, there was no control of dietary choline intake and adherence to the choline supplementation was inferred only from pill counts and other indirect measures of adherence (26, 27). A third factor that may have increased our ability to detect a cognitive benefit was our use of choline chloride as the supplemental form of the nutrient. Research with rodents has shown superior bioavailability of choline chloride to the brain, as compared to phosphatidylcholine, the supplement used in the two prior human studies (48). An additional strength of our study was the successful randomization, researcher and participant blinding, and the use of intention-to-treat analyses. Moreover, the longitudinal design of our study provided us with multiple assessments of infant information processing speed, enabling us to show consistency of the choline intake effect from 4 independent assessments that span the entire first year of postnatal life. A final strength is that we used infant reaction time as an outcome. Reaction time measures a central dimension of infant information processing and has been shown to predict cognitive performance later in life (31, 32).
There are several limitations of the present study. First, the small sample size, although sufficient for our stated hypotheses, limits generalizability of the findings. Second, the use of only 1 paradigm to assess effects of maternal choline intake on infant cognition limits conclusions about the specificity of the cognitive benefits. In addition, the lack of cognitive assessments extending beyond infancy precludes determination of the longevity of the benefits of maternal choline supplementation we observed during the first postnatal year. A final limitation is that the choline supplementation was implemented during only the final trimester of pregnancy, making it impossible to predict the effects of a longer period of supplementation.
In summary, choline supplementation of the diet among women in their third trimester of pregnancy, at a level exceeding the current AI, improved infant processing speed relative to maternal consumption of the AI. This finding suggests that the current AI level for choline during pregnancy may need to be increased for improved offspring cognitive functioning. In addition, our results provide evidence that maternal consumption of the AI of choline during this period also produces benefits relative to typical choline intake among pregnant women. If the benefits we report are lasting and are enabled through improved brain structure and function, as has been shown in the rodent studies, then increasing maternal choline intake could be a nutritional strategy for improving offspring cognition throughout life. Longitudinal studies that extend beyond infancy and incorporate multiple measures of cognition are needed to further explore this question in humans. Although the effects of increased maternal choline intake are expected to be small at an individual level, they are anticipated to be substantial at a societal level, including population-wide improvements in cognitive function (15) and reduced incidence of aging-related cognitive decline (49–51).
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the women participants for their extraordinary cooperation and compliance with the controlled feeding trial and for making their infants available for this neurobehavioral follow-up study. This work was funded, in part, by the Egg Nutrition Center, The Beef Checkoff, the U.S. Department of Agriculture (USDA) Cooperative State Research, Education, and Extension Service (special research Grant 00444528), The Institute for the Social Sciences Small Grants Program, a Bronfenbrenner Life Course Center Research Grant, and the U.S. National Institute of Food and Agriculture, and the USDA (Hatch Accession No. 1007195). None of the funding sources had any role in trial design, participant recruitment, data collection, data analysis, data interpretation, manuscript preparation, or any aspect pertinent to the study. The authors declare no conflicts of interest.
Glossary
- AI
adequate intake
- CI
confidence interval
- IQ
intelligent quotient
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. A. Caudill designed the controlled feeding trial, which was conducted by her research group; L. Muscalu and R. L. Canfield designed the infant assessment and collected the behavioral data; L. Muscalu scored the eye movement data and performed data management with assistance from R. L. Canfield; L. Muscalu, J. E. H. Nevins, and R. L. Canfield analyzed the data and created the figures; M. A. Caudill, B. J. Strupp, and R. L. Canfield interpreted the data; all authors wrote the report; and R. L. Canfield had full access to all the data in the study, and was responsible for submitting the final manuscript for publication.
REFERENCES
- 1.Zeisel S. H., Mar M. H., Zhou Z., da Costa K. A. (1995) Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J. Nutr. 125, 3049–3054 [DOI] [PubMed] [Google Scholar]
- 2.Caudill M. A. (2010) Pre- and postnatal health: evidence of increased choline needs. J. Am. Diet. Assoc. 110, 1198–1206 10.1016/j.jada.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 3.Yan J., Jiang X., West A. A., Perry C. A., Malysheva O. V., Devapatla S., Pressman E., Vermeylen F., Stabler S. P., Allen R. H., Caudill M. A. (2012) Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr. 95, 1060–1071 10.3945/ajcn.111.022772 [DOI] [PubMed] [Google Scholar]
- 4.Abreu-Villaça Y., Filgueiras C. C., Manhães A. C. (2011) Developmental aspects of the cholinergic system. Behav. Brain Res. 221, 367–378 10.1016/j.bbr.2009.12.049 [DOI] [PubMed] [Google Scholar]
- 5.Albright C. D., Friedrich C. B., Brown E. C., Mar M. H., Zeisel S. H. (1999) Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. Dev. Brain Res. 115, 123–129 10.1016/S0165-3806(99)00057-7 [DOI] [PubMed] [Google Scholar]
- 6.Cermak J. M., Blusztajn J. K., Meck W. H., Williams C. L., Fitzgerald C. M., Rosene D. L., Loy R. (1999) Prenatal availability of choline alters the development of acetylcholinesterase in the rat hippocampus. Dev. Neurosci. 21, 94–104 10.1159/000017371 [DOI] [PubMed] [Google Scholar]
- 7.Lauder J. M., Schambra U. B. (1999) Morphogenetic roles of acetylcholine. Environ. Health Perspect. 107 (Suppl 1), 65–69 10.1289/ehp.99107s165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisel S. H. (2011) The supply of choline is important for fetal progenitor cells. Semin. Cell Dev. Biol. 22, 624–628 10.1016/j.semcdb.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blusztajn J. K. (1998) Choline, a vital amine. Science 281, 794–795 10.1126/science.281.5378.794 [DOI] [PubMed] [Google Scholar]
- 10.Zeisel S. H. (2006) Choline: critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 26, 229–250 10.1146/annurev.nutr.26.061505.111156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine . (1998) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline, National Academies Press (US), Washington, DC: [PubMed] [Google Scholar]
- 12.Jiang X., Yan J., West A. A., Perry C. A., Malysheva O. V., Devapatla S., Pressman E., Vermeylen F., Caudill M. A. (2012) Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J. 26, 3563–3574 10.1096/fj.12-207894 [DOI] [PubMed] [Google Scholar]
- 13.Kovacheva V. P., Davison J. M., Mellott T. J., Rogers A. E., Yang S., O’Brien M. J., Blusztajn J. K. (2009) Raising gestational choline intake alters gene expression in DMBA-evoked mammary tumors and prolongs survival. FASEB J. 23, 1054–1063 10.1096/fj.08-122168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCann J. C., Hudes M., Ames B. N. (2006) An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci. Biobehav. Rev. 30, 696–712 10.1016/j.neubiorev.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 15.Meck W. H., Williams C. L. (2003) Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 27, 385–399 10.1016/S0149-7634(03)00069-1 [DOI] [PubMed] [Google Scholar]
- 16.Cheng R. K., MacDonald C. J., Williams C. L., Meck W. H. (2008) Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn. Mem. 15, 153–162 10.1101/lm.729408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loy R., Heyer D., Williams C. L., Meck W. H. (1991) Choline-induced spatial memory facilitation correlates with altered distribution and morphology of septal neurons. Adv. Exp. Med. Biol. 295, 373–382 10.1007/978-1-4757-0145-6_21 [DOI] [PubMed] [Google Scholar]
- 18.Meck W. H., Smith R. A., Williams C. L. (1988) Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev. Psychobiol. 21, 339–353 10.1002/dev.420210405 [DOI] [PubMed] [Google Scholar]
- 19.Meck W. H., Williams C. L. (1999) Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res. Dev. Brain Res. 118, 51–59 10.1016/S0165-3806(99)00105-4 [DOI] [PubMed] [Google Scholar]
- 20.Williams C. L., Meck W. H., Heyer D. D., Loy R. (1998) Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 794, 225–238 10.1016/S0006-8993(98)00229-7 [DOI] [PubMed] [Google Scholar]
- 21.Moon J., Chen M., Gandhy S. U., Strawderman M., Levitsky D. A., Maclean K. N., Strupp B. J. (2010) Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav. Neurosci. 124, 346–361 10.1037/a0019590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas J. D., Garrison M., O’Neill T. M. (2004) Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 26, 35–45 10.1016/j.ntt.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 23.Yang Y., Liu Z., Cermak J. M., Tandon P., Sarkisian M. R., Stafstrom C. E., Neill J. C., Blusztajn J. K., Holmes G. L. (2000) Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J. Neurosci. 20, RC109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blusztajn J. K., Mellott T. J. (2013) Neuroprotective actions of perinatal choline nutrition. Clin. Chem. Lab. Med. 51, 591–599 10.1515/cclm-2012-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strupp B. J., Powers B. E., Velazquez R., Ash J. A., Kelley C. M., Alldred M. J., Strawderman M., Caudill M. A., Mufson E. J., Ginsberg S. D. (2016) Maternal choline supplementation: a potential prenatal treatment for down syndrome and Alzheimer’s disease. Curr. Alzheimer Res. 13, 97–106 10.2174/1567205012666150921100311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross R. G., Hunter S. K., McCarthy L., Beuler J., Hutchison A. K., Wagner B. D., Leonard S., Stevens K. E., Freedman R. (2013) Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am. J. Psychiatry 170, 290–298 10.1176/appi.ajp.2012.12070940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheatham C. L., Goldman B. D., Fischer L. M., Costa K. A., da, Reznick J. S., Zeisel S. H. (2012) Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 96, 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koletzko B., Lien E., Agostoni C., Böhles H., Campoy C., Cetin I., Decsi T., Dudenhausen J. W., Dupont C., Forsyth S., Hoesli I., Holzgreve W., Lapillonne A., Putet G., Secher N. J., Symonds M., Szajewska H., Willatts P., Uauy R.; World Association of Perinatal Medicine Dietary Guidelines Working Group (2008) The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J. Perinat. Med. 36, 5–14 10.1515/JPM.2008.001 [DOI] [PubMed] [Google Scholar]
- 29.Canfield R. L., Haith M. M. (1991) Young infants’ visual expectations for symmetric and asymmetric stimulus sequences. Dev. Psychol. 27, 198–208 10.1037/0012-1649.27.2.198 [DOI] [Google Scholar]
- 30.Rose S. A., Feldman J. F. (1997) Memory and speed: their role in the relation of infant information processing to later IQ. Child Dev. 68, 630–641 10.2307/1132115 [DOI] [PubMed] [Google Scholar]
- 31.Dougherty T. M., Haith M. M. (1997) Infant expectations and reaction time as predictors of childhood speed of processing and IQ. Dev. Psychol. 33, 146–155 10.1037/0012-1649.33.1.146 [DOI] [PubMed] [Google Scholar]
- 32.Teubert M., Lohaus A., Fassbender I., Vöhringer I. A., Suhrke J., Poloczek S., Freitag C., Lamm B., Teiser J., Keller H., Knopf M., Schwarzer G. (2015) Moderation of stimulus material on the prediction of IQ with infants’ performance in the visual expectation paradigm: do greebles make the task more challenging? Infant Child Dev. 24, 522–537 10.1002/icd.1897 [DOI] [Google Scholar]
- 33.Canfield R. L., Wilken J., Schmerl L., Smith E. G. (1995) Age-related change and stability of individual differences in infant saccade reaction time. Infant Behav. Dev. 18, 351–358 10.1016/0163-6383(95)90023-3 [DOI] [Google Scholar]
- 34.Canfield R. L., Smith E. G., Brezsnyak M. P., Snow K. L. (1997) Information processing through the first year of life: a longitudinal study using the visual expectation paradigm. Monogr. Soc. Res. Child Dev. 62, 1–145 10.2307/1166196 [DOI] [PubMed] [Google Scholar]
- 35.Benson J. B., Cherny S. S., Haith M. M., Fulker D. W. (1993) Rapid assessment of infant predictors of adult IQ: midtwin–midparent analyses. Dev. Psychol. 29, 434–447 10.1037/0012-1649.29.3.434 [DOI] [Google Scholar]
- 36.Laird N. M., Ware J. H. (1982) Random-effects models for longitudinal data. Biometrics 38, 963–974 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- 37.Altman D. G. (1998) Adjustment for covariate imbalance. In Encyclopedia of Biostatistics (Armitage P., and Colton T., eds) Vol. 1, pp. 1000–1005, John Wiley & Sons, Chichester, UK [Google Scholar]
- 38.Mellott T. J., Williams C. L., Meck W. H., Blusztajn J. K. (2004) Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 18, 545–547 [DOI] [PubMed] [Google Scholar]
- 39.Boeke C. E., Gillman M. W., Hughes M. D., Rifas-Shiman S. L., Villamor E., Oken E. (2013) Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 177, 1338–1347 10.1093/aje/kws395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis E. D., Subhan F. B., Bell R. C., McCargar L. J., Curtis J. M., Jacobs R. L., Field C. J.; APrON Team (2014) Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br. J. Nutr. 112, 112–121 10.1017/S0007114514000555 [DOI] [PubMed] [Google Scholar]
- 41.Visentin C. E., Masih S., Plumptre L., Malysheva O., Nielsen D. E., Sohn K. J., Ly A., Lausman A. Y., Berger H., Croxford R., El-Sohemy A., Caudill M. A., O’Connor D. L., Kim Y. I. (2015) Maternal choline status, but not fetal genotype, influences cord plasma choline metabolite concentrations. J. Nutr. 145, 1491–1497 10.3945/jn.115.211136 [DOI] [PubMed] [Google Scholar]
- 42.Javitt D. C., Freedman R. (2015) Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am. J. Psychiatry 172, 17–31 10.1176/appi.ajp.2014.13121691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi Y., Isa T. (2002) Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Netw. 15, 731–741 10.1016/S0893-6080(02)00059-X [DOI] [PubMed] [Google Scholar]
- 44.Jana A., Pahan K. (2010) Sphingolipids in multiple sclerosis. Neuromolecular Med. 12, 351–361 10.1007/s12017-010-8128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang X., West A. A., Caudill M. A. (2014) Maternal choline supplementation: a nutritional approach for improving offspring health? Trends Endocrinol. Metab. 25, 263–273 10.1016/j.tem.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 46.Niculescu M. D., Zeisel S. H. (2002) Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J. Nutr. 132 (8, Suppl), 2333S–2335S [DOI] [PubMed] [Google Scholar]
- 47.Lavenex P., Banta Lavenex P. (2013) Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav. Brain Res. 254, 8–21 10.1016/j.bbr.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 48.Cheng W. L., Holmes-McNary M. Q., Mar M. H., Lien E. L., Zeisel S. H. (1996) Bioavailability of choline and choline esters from milk in rat pups. J. Nutr. Biochem. 7, 457–464 10.1016/0955-2863(96)00079-4 [DOI] [Google Scholar]
- 49.Meck W. H., Williams C. L., Cermak J. M., Blusztajn J. K. (2008) Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front. Integr. Neurosci. 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng R. K., Scott A. C., Penney T. B., Williams C. L., Meck W. H. (2008) Prenatal-choline supplementation differentially modulates timing of auditory and visual stimuli in aged rats. Brain Res. 1237, 167–175 10.1016/j.brainres.2008.08.062 [DOI] [PubMed] [Google Scholar]
- 51.Glenn M. J., Kirby E. D., Gibson E. M., Wong-Goodrich S. J., Mellott T. J., Blusztajn J. K., Williams C. L. (2008) Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 1237, 110–123 10.1016/j.brainres.2008.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds R. M. (2013) Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis—2012 Curt Richter Award winner. Psychoneuroendocrinology 38, 1–11 10.1016/j.psyneuen.2012.08.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.