Abstract
Prostaglandin D2 and its cyclopentenone metabolites [cyclopentenone prostaglandins (CyPGs)], Δ12prostaglandin J2 and 15-deoxy-Δ12,14-prostaglandin J2, act through 2 GPCRs, d-type prostanoid 1 and the chemoattractant receptor homologous molecule expressed on type 2 T-helper cells (Crth2). In addition to its role in allergy and asthma, the role of Crth2 in the resolution of inflammation, to mediate the proresolving functions of endogenous CyPGs, is not well understood. We investigated the regulation of LPS or zymosan-induced inflammatory response by signals from the Crth2 receptor in macrophages that lack Crth2 expression [knockout (KO)]. Increased expression of proinflammatory genes, including Tnf-α, was observed in Crth2 KO cells. Targeting the endogenous biosynthetic pathway of CyPGs with indomethacin or HQL79, which inhibit cyclooxygenases or hematopoietic prostaglandin D synthase, respectively, or use of Crth2 antagonists recapitulated the proinflammatory phenotype as in Crth2 KO cells. Ligand-dependent activation of Crth2 by 13,14-dihydro-15-keto-prostaglandin D2 increased Ca2+ influx through store-operated Ca2+ entry (SOCE) accompanied by the up-regulation of stromal interaction molecule 1 and calcium release–activated calcium modulator 1 expression, suggesting that the proresolution effects of CyPG-dependent activation of SOCE could be mediated by Crth2 during inflammation. Interestingly, Crth2 signaling down-regulated the Ca2+-regulated heat stable protein 1 that stabilizes Tnf-α mRNA via the increased expression of microRNA 155 to dampen inflammatory responses triggered through the TNF-α–NF-κB axis. In summary, these studies present a novel regulatory role for Crth2 during inflammatory response in macrophages.—Diwakar, B. T., Yoast, R., Nettleford, S., Qian, F., Lee, T.-J., Berry, S., Huffnagle, I., Rossi, R. M., Trebak, M., Paulson, R. F., Prabhu, K. S. Crth2 receptor signaling down-regulates lipopolysaccharide-induced NF-κB activation in murine macrophages via changes in intracellular calcium.
Keywords: cyclopentenone prostaglandins, selenium, protein kinase A, cAMP, resolution of inflammation
The chemoattractant receptor homologous molecule expressed on type 2 T helper (Th2) cells [Crth2; G-protein coupled receptor 44 (Gpr44); CD294] receptor is one of the 2 d-type prostanoid (DP), 7 transmembrane GPCRs known to mediate actions of prostaglandin D2 (PGD2), derived from cyclooxygenase (COX) and prostaglandin D synthase (PGDS), and its cyclopentenone metabolites [cyclopentenone prostaglandins (CyPGs)], Δ12-prostaglandin J2 (Δ12-PGJ2) and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (1). Unlike PGD2, which predominantly acts through the DP1 receptor (PTGDR; DP), CyPGs exhibit high affinity and selectivity toward Crth2 in addition to acting through the cytoplasmic nuclear hormone receptor, peroxisome proliferator–activated receptor γ (PPAR-γ) (2, 3). Originally, Crth2 receptor was identified on Th2 cells, and subsequently it was shown to be expressed in a variety of immune cells, including type 2 innate lymphoid cells and macrophages (4–7). Crth2 receptor mediates a myriad of functions in immunocompetent cells such as allergic inflammation (8–10), proinflammatory functions (2, 4, 11), and antiinflammatory actions (6, 12, 13). These diverse actions of Crth2 signals depend on the cell type, concentration, affinity, and specificity of ligands. Although the role of Crth2 in allergy and asthma has been extensively studied, its role is less explored in macrophages expressing selenoproteins that generate sufficient levels of CyPGs during inflammation, as in the case of bacterial endotoxin LPS-induced inflammatory response (14, 15).
Activation of Crth2 by CyPGs decreases intracellular cAMP levels via downstream effector G-protein Gαi (4, 16). Mounting evidence suggests that higher levels of cAMP during inflammation leads to the activation of PKA to positively affect an increase in NF-κB–mediated transcription (17–19) in murine macrophages (20, 21), human monocytes (22), and other cell types (23–25) to promote inflammation. However, cellular compensatory circuits can effectively regulate such mechanisms via the modulation of second messengers, as seen in the case of the CyPG-Crth2 signaling axis. Agonist-dependent activation of Crth2 results in increased intracellular calcium ([Ca2+]i) concentration. In general, GPCRs activate store-operated Ca2+ entry (SOCE) to raise [Ca2+]i levels by mediating release of inositol 1,4,5-triphosphate (IP3) from membrane phospholipids, followed by IP3 binding to IP3 receptors (IP3Rs) on the surface of the endoplasmic reticulum (ER) to deplete the internal Ca2+ stores. Depletion of ER stores causes a conformational change in stromal interaction molecule 1 (STIM1) to promote its oligomerization and interaction with calcium release–activated calcium modulator 1 (ORAI1) channels at ER–plasma membrane junctions to activate influx of Ca2+ (26, 27). Surprisingly, there are no reports that demonstrate if Crth2 signals activate SOCE for Ca2+ mobilization in activated macrophages to negatively regulate inflammatory signaling pathways.
Activated NF-κB and expression of downstream target genes, such as Tnf-α, are a hallmark of inflammation, particularly in macrophages that play a pivotal and dual role in exacerbating inflammation as well as limiting inflammation by activating pathways of resolution. Interestingly, CyPGs are known to modulate levels of TNF-α and other cytokines by down-regulating the activation of NF-κB via Crth2-independent mechanisms through inhibition of IKK complexes as well as repression of NF-κB–dependent transcription via ligand-activated PPAR-γ and other mechanisms (28–30). In addition, the NF-κB pathway is regulated by miR-155, which suppresses IKK-β and IKK-ε as part of a negative feedback loop (31, 32). miR-155 also represses the expression of Ca2+-regulated heat stable protein 1 (Carhsp1), which stabilizes TNF-α through the 2 RNA binding motifs present within the cold shock domain to induce TNF-α mRNA decay in macrophages (33–35). Even though the literature is replete with data showing that specialized proresolving mediators such as lipoxins affect the proresolution program via signaling through cognate GPCRs mediated by miRs (36), it is not clear if CyPGs signal in a similar manner.
Here we hypothesized that endogenous CyPGs produced during inflammation act through a feedback loop via Crth2 to suppress the expression of proinflammatory genes in murine macrophages. In our studies, we observed increased expression of mRNA and protein of Cox2 (Ptgs2), iNOS (Nos2), and microsomal prostaglandin E synthase 1 (mPGES1; Pges) in Crth2 knockout (KO) cells upon treatment with LPS. Targeting the endogenous biosynthetic pathway of CyPGs or antagonizing Crth2 recapitulated the phenotype that was similar to Crth2 KOs generated via clustered regularly interspaced short palindromic repeats (CRISPR)–mediated mutation or by genetic recombination. Peritoneal lavage immune cell infiltrates from zymosan-treated Crth2−/− mice also showed increased expression of Cox2 and iNOS. Interestingly, Crth2 signals were required to repress Carhsp1 via miR-155 to dampen inflammatory response triggered through the TNF-α–NF-κB axis. Crth2 signaling was mediated via the activation of SOCE upon Crth2 receptor stimulation. In summary, these studies present a novel regulatory proresolving role of Crth2 signals in LPS-induced inflammatory response in murine macrophages.
MATERIALS AND METHODS
Mice
C57BL/6 male age-matched mice were purchased from Taconic Biosciences (Renssalaer, NY, USA) and maintained on an American Institute of Nutrition-76–based semipurified diet from Envigo (East Millstone, NJ, USA). All studies were preapproved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee at Penn State University.
Establishment of Crth2 (heterozygous) KO mice
The C57BL/6 background embryoic stem (ES) cells used to generate the Crth2 heterozygous KO mice (Crth2+/−) were obtained from the Knockout Mouse Project Repository (http://www.komp.org/). In the targeted allele, exon 3 of Crth2 was replaced with the targeting vector, including a β-galactosidase cDNA inserted in the translation start site of the Crth2 locus. Thus, the targeted alleles are fully devoid of coding exon 3 of Crth2, whereas the locus expresses the β-galactosidase reporter gene. Targeting of Crth2 was confirmed by long-range PCR analysis followed by sequencing. ES cell clones were injected into blastocysts of C57BL/6 female mice for chimeric mouse production, and the resultant chimeric male mice were bred with female C57BL/6 mice to test germ-line transmission and to establish the colony. PCR of tail-derived genomic DNA with primer pairs for wild-type (WT) allele (forward, 5′-CTTCCTGCTCAGTGCCATTAGC-3′; reverse, 5′-ACGTGGCTCGAGGCAATTATGG-3′) and for the targeted allele (after Cre) (forward, 5′-ACTTGCTTTAAAAAACCTCCCACA-3′; reverse, 5′-CACAAAGCAGGGAGTTATGAGATGC-3′) were used to genotype the mice.
Bone marrow–derived macrophage culture
Femurs were harvested, and bone marrow was collected from C57BL/6 male and female age-matched mice. Bone marrow was separated into a single cell suspension. Cells were plated in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) with 5% (v/v) fetal bovine serum (R&D Systems, Minneapolis, MN, USA), 2 mM l-glutamine, 100 IU/ml of penicillin, 100 μg/ml of streptomycin (Corning, Corning, NY, USA), 250 nM sodium selenite (MilliporeSigma, Burlington, MA, USA), and 10% (v/v) L929 fibroblast-conditioned DMEM. L929 fibroblasts were purchased from the American Type Culture Collection (Manassas, VA, USA).
Zymosan-induced peritonitis model
Zymosan-induced peritonitis was induced in WT and Crth2−/− (on a C57BL/6 background) mice as previously described in ref. 37. Briefly, mice were injected intraperitoneally with zymosan A (10 mg/kg body weight). After 24 h, mice were euthanized by carbon dioxide exposure, and peritoneal cavities were lavaged with 5 ml of sterile PBS containing 3 mM EDTA. Aliquots of the lavage fluid were then centrifuged at 400 g for 5 min, and cell pellets were prepared for RNA extraction and quantitative PCR analysis.
Treatments
Various compounds such as antagonists, agonists, and inhibitors were used at the following concentrations: 100 ng/ml LPS (Escherichia coli serotype 0128:B12; MilliporeSigma); 10 mg/kg i.p. zymosan A (MilliporeSigma); 10 ng/ml Tnf-α (mouse Tnf-α; GoldBio, St. Louis, MO, USA); 5 μM CAY10595, 25 µM HQL79, 30 mM H89, and 20 nM 13,14-dihydro-15-keto-PGD2 (DKPGD2) (all from Cayman Chemicals, Ann Arbor, MI, USA); and 1 µg/ml pertussis toxin (PTX; List Biological Laboratories, Campbell, CA, USA). LPS, PTX, and DKPGD2 were reconstituted in PBS, whereas cell culture–grade DMSO (MilliporeSigma) was used to reconstitute all other compounds. The final concentration of DMSO never exceeded 0.1% (v/v). Appropriate vehicle controls were used where appropriate. HQL79 and H89 were added to cultures 6 h prior to LPS stimulation, whereas PTX was added to cultures 16 h prior to stimulation. The above-mentioned compounds were added to bone marrow–derived macrophage (BMDM) cultures and collected for analysis. Cell viability was confirmed by trypan blue staining. All experiments were conducted in technical replicates in each of the biologic triplicates.
Transfection and transduction
Lentiviral vectors containing CRISPR-associated protein 9 (Cas9) and single-guide RNA (sgRNA) were generated as described earlier in Kutner et al. (38). Briefly, human embryonic kidney 293TN cells (3 × 106 cells/10 ml; System Biosciences, Palo Alto, CA, USA) were plated in the above-mentioned macrophage medium in a 100-mm plate and allowed to adhere overnight. Cells were then transfected with 6.85 μg of lentiviral CRISPR guide RNA plasmid (Ampr/tRFPs) and Cas9 plasmid (Ampr/Purr) (Transomic Technologies, Huntsville, AL, USA) in a ratio of 1:3, 6.85 µg of packaging plasmid; psPAX2 and 2.3 µg of envelope plasmid; pMD2.G (plasmid #12259; Addgene, Cambridge, MA, USA) with 48 µl of TransIT 293 transfection reagent (Mirus, Madison, WI, USA), in 800 μl of serum-free DMEM. The guide RNA sequences for nontargeting control (NTC) (5′-GGAGCGCACCATCTTCTTCA-3′) and Crth2 (5′-TGTTCAGAGACACCATCCCG-3′, 5′-GTGGGCACAGAACCACCGCA-3′, and 5′-CGGTGGCGGTCGCGCGCACA-3′) were used to generate lentiviral particles. Viruses were harvested and passed through a 0.45-μm filter and concentrated using polyethylene glycol 6000. RAW264.7 cells (5 × 105) were infected with lentiviral particles with a multiplicity of infection of 50 with 8 µg/ml of polybrene (MilliporeSigma) for 12 h and cultured for 4 d after a medium change with 5 µg/ml puromycin (GoldBio). Puromycin-selected turbo red fluorescent protein (tRFP)+ cells were sorted to obtain single cell colonies and screened for indels using a surveyor assay kit as per the manufacturer’s protocol (Integrated DNA Technologies, Coralville, IA, USA). Homozygous Crth2 KO (Crth2−/−) cells thus obtained were used in cultures alongside NTC RAW264.7 cells.
RNA extraction and real-time quantitative PCR
Cell pellets were stored in 1 ml of Tri Reagent (Thermo Fisher Scientific) at −80°C until RNA extraction. RNA extraction was performed according to the manufacturer’s protocol as previously described in Shay et al. (39). RNA was reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). cDNA was used in quantitative PCR with specific TaqMan probes for Cox2, iNOS, mPges1, miR-155, Tnf-α, Il-1β, Carhsp1, and 18S (Thermo Fisher Scientific) in a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). Transcript abundance was analyzed, and 2−ΔΔCt was calculated to represent changes in gene expression relative to controls (40).
Protein extraction and immunoblotting
Cells were washed with cold PBS twice and scraped into Mammalian Protein Extraction Reagent (Thermo Fisher Scientific) containing protease inhibitor mixture (Roche, Basel, Switzerland) and 5 mM sodium orthovanadate (MilliporeSigma), vortexed for 30 s, and incubated on ice for 30 min. Lysates were then centrifuged at 12,000 g for 10 min at 4°C. Clear supernatants were collected, and protein amounts were quantified using a Bicinchoninic Acid Protein Assay Kit (Thermo Fisher Scientific). Protein was loaded onto SDS-PAGE gel (T = 12.5%). Nitrocellulose blotting membranes were blocked with a 5% (w/v) skim milk solution made with Tris-buffered saline containing 0.1% Tween 20 (MilliporeSigma) for 1 h prior to the addition of primary antibodies. Primary antibodies were added to the blots for 12 h at 4°C at the following dilutions: 1:20000 Cox2 (Cayman Chemicals), 1:2000 iNOS (Cayman Chemicals), 1:1000 Orai1 (MilliporeSigma), 1:200 Stim1 (Santa Cruz Biotechnology, Dallas, TX, USA), 1:1000 Cas9 (Abcam, Cambridge, MA, USA), 1:1000 Carhsp1 (GeneTex, Irvine, CA, USA), 1:1000 Crth2 (Santa Cruz Biotechnology), 1:40,000 β-actin (Fitzgerald Industries, Acton, MA, USA), and 1:80,000 glyceraldehyde 3-phosphate dehydrogenase (Fitzgerald Industries). Appropriate secondary antibodies conjugated to horseradish peroxidase were used at the dilutions 1:20,000 for goat anti-rabbit (Thermo Fisher Scientific) and 1:5000 for goat anti-mouse (Thermo Fisher Scientific) and developed using West Pico reagent (Thermo Fisher Scientific). Autoradiograms densities were evaluated using ImageJ (National Institutes of Health, Bethesda, MD, USA).
ELISAs
Levels of Tnf-α (BioLegend, San Diego, CA, USA), prostaglandin E2 (PGE2; Cayman Chemicals), and intracellular cAMP (Enzo Life Sciences, Farmingdale, NY, USA) were measured using specific ELISA kits according to the manufacturer’s instructions. A 4-parameter fit for the standard curve was used, and the values were normalized to total cellular protein content.
Nitrite measurement
Production of NO was assessed through the accumulation of nitrite in the supernatants using a Griess Reagent Kit (MilliporeSigma) as previously described in Prabhu et al. (41). Nitrite concentrations were determined using a calibration curve with sodium nitrite (1–100 µM).
Chromatin immunoprecipitation
To examine binding of NF-κB (p65) to the NF-κB response element (NRE) on the Ptgs2 (COX2) and Nos2 (iNOS) gene promoters, chromatin immunoprecipitation assay (ChIP) was performed as described in Finch et al. (42). Briefly, NTC and Crth2−/− RAW264.7 cells (1 × 107) were harvested after stimulation with LPS for 8 h and used for cross-linking ChIP. Cells were treated with 1% formaldehyde for 10 min, followed by 125 mM glycine to terminate the cross-linking reaction. The cells were washed with ice-cold PBS and harvested. Approximately 10 × 106 cells were lysed with ChIP lysis buffer (50 mM Tris, pH 8.0, 10 mM EDTA, 1% SDS) and subjected to sonication [Diagenode Bioruptor (Diagenode, Liège, Belgium); 30 s on, 60 s off for 28 cycles]. Lysates were precleared using 20 μl of protein A/G Plus Agarose beads and subjected to immunoprecipitation with anti-rabbit polyclonal NF-κB p65 antibody (3 μg; Cell Signaling Technology, Danvers, MA, USA) or IgG control with 20 μl of protein A/G Plus Agarose overnight at 4°C. Immune complexes were washed 4 times each with low-salt wash buffer (20 mM Tris-HCl, pH 8.00 containing 0.1% SDS, 1% Triton X-100, 2 mM EDTA, and 150 mM NaCl), high-salt wash buffer (above buffer with 500 mM NaCl), and twice with 10 mM Tris (1 mM) EDTA. Immune complexes were eluted, and cross-links were reversed. DNA was extracted and used in quantitative PCR with the following primers: Ptgs2 NRE forward, 5′-GTAGCTGTGTGCGTGCTCTG-3′, reverse, 5′-CTCCGGTTTCCTCCCAGT-3′; Nos2 NRE forward, 5′-GTCCCAGTTTTGAAGTGACTACG-3′, reverse, 5′-GTTGTGACCCTGGCAGCAG-3′.
Preparation of membranes
Membranes from NTC and Crth2−/− RAW264.7 cells were isolated for radioligand binding experiments as described earlier in Hata et al. (3). Cells were rinsed with PBS, detached using dissociation buffer (MilliporeSigma), and collected by centrifugation at 500 g for 10 min at 4°C. The final cell pellet was resuspended in 10 mM HEPES-KOH (pH 7.4) containing 1 mM EDTA with protease inhibitor mixture (Roche, Basel, Switzerland) at ∼107 cells per ml and disrupted with a Dounce homogenizer. Cell membranes were isolated by differential centrifugation at 1000 g for 10 min followed by 100,000 g for 30 min. The pellet was resuspended in 10 mM HEPES-KOH pH 7.4, 1 mM EDTA, using Dounce homogenization (10 strokes) and stored at −80°C. Sample was also used for immunoblotting and ligand binding assays.
Radioligand binding assay
Isolated membranes were incubated with [3H]PGD2 at 4°C for 1.5 h in binding buffer [25 mM HEPES (pH7.4), 1 mM EDTA, 5 mM MgCl2, 140 mM NaCl, 5 mM KCl]. The binding reaction was terminated by the addition of 3 ml of ice-cold binding buffer and rapidly filtered under vacuum over polyethylenimine preblocked Filtermat A (PerkinElmer, Waltham, MA, USA). The filtermat was washed 3 times with 3 ml of ice-cold binding buffer, dried, and sealed in a sample bag with liquid scintillation cocktail for counting on a MicroBeta microplate counter (PerkinElmer). For saturation binding experiments, nonspecific binding was determined in the presence of 50 μM DKPGD2. Specific binding experiments were performed in the presence of 3 nM [3H]PGD2.
[Ca2+]i measurements
For multicell fluorescence Ca2+ measurements, an imaging workstation interfaced with a Leica DMi8 inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Hamamatsu Flash 4 camera (Hamamatsu, Hamamatsu, Japan) and Leica Application Suite X was used for data acquisition and analysis. Briefly, NTC and Crth2−/− RAW264.7 cells were seeded onto 25-mm round glass coverslips at a density of 2 × 105 cells per coverslip. Once attached, cells were incubated with complete culture medium containing the ratiometric Ca2+ indicator Fura2–acetoxymethyl ester (Fura2-AM; 2 µM) for 30 min at 37°C, 5% CO2 in an incubator. Fura2-AM–loaded cells were subsequently washed 3 times with a HEPES-buffered salt solution; 120 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 20 mM HEPES, 10 mM glucose adjusted to pH 7.4 with NaOH) as previously described in Desai et al. (43). Glass coverslips with cells were kept for 10 min at room temperature, then placed into an Attofluor coverslip holder and imaged with a fluorescence ×20 objective lens. Fura2-emitted fluorescence was collected at 510 nm every 2 s for a total of 15 min after alternately exciting at 340 and 380 nm using a fast shutter wheel (Sutter Instrument, Novato, CA, USA). Emitted fluorescence was collected from individual cells on a pixel-by-pixel basis, and the fluorescence (F)340:F380 ratio was determined. After an initial recording to establish baseline, cells were treated with 50 nM DKPGD2 (in nominally Ca2+-free HBSS) at 2 min to determine Ca2+ release from internal stores, followed by restoration of HBSS supplemented with 2 mM CaCl2 at 8 min to determine the extent of Ca2+ entry across the plasma membrane. Cells were then incubated in HBSS with Ca2+ containing gadolinium (III) chloride (Gd3+; 5 µM) at 11 min. Gd3+ at these relatively low concentrations is a selective inhibitor of SOCE channels (44). Ionomycin (10 µM) was added at the end of recordings at 14 min to determine the maximal Fura2 signal. Ca2+ traces were plotted as means ± sem from ≥150 individual cells from 3 independent coverslips per condition.
Statistical analysis
Experiments were repeated at least 3 times or as indicated. Data were analyzed by Prism 6.0 (GraphPad Software, La Jolla, CA, USA) and expressed as means ± sem. Either an unpaired, 1- or 2-tailed Student’s t test, or a 1- or 2-way ANOVA with Fisher’s least significant difference test was used where appropriate, using Prism (GraphPad Software). Values of P ≤ 0.05 were considered significant.
RESULTS
Antagonism of Crth2 up-regulates LPS-induced expression of inflammatory genes in BMDMs
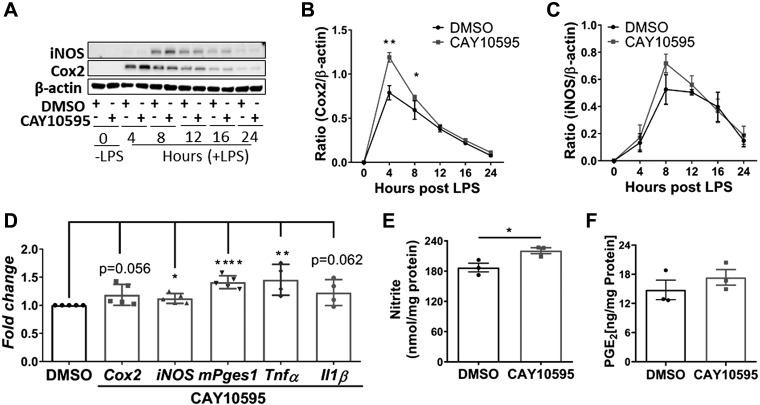
BMDMs pretreated with Crth2 antagonist CAY10595 (5 μM), DMSO vehicle (0.05%), or both were harvested between 0 and 24 h after LPS stimulation to assess the role of Crth2 signaling in LPS-induced inflammatory mediator expression. Harvested cells were processed and analyzed for expression of iNOS and Cox2 by Western immunoblot. The expression of iNOS at 8 h and Cox2 expression at 4 h after LPS treatment in antagonist-treated BMDMs showed an increased trend compared with vehicle-treated controls (Fig. 1A). Blocking Crth2 with CAY10595 significantly increased LPS-induced expression of Cox2 by 32.95 and 21.02% at 4 and 8 h time points, respectively, in comparison with expression in vehicle-treated BMDMs at indicated time points (Fig. 1B). Similarly, the expression of iNOS was increased by 39.86% in CAY10595-treated BMDMs compared with vehicle-treated cells at 8 h after LPS stimulation (Fig. 1C). Transcripts of key inflammatory genes were up-regulated in Crth2 signal–impeded BMDMs (Fig. 1D). Levels of Cox2, iNOS, mPges1, Tnf-α, and Il-1β mRNAs were increased 18.6, 12.2, 45.1, 45.4, and 22.6% in CAY10595-treated BMDMs, respectively (Fig. 1D). As shown in Fig. 1E, nitrite levels were significantly increased (18.4%) in culture supernatants at 16 h after LPS treatment in BMDMs that were treated with CAY10595. Further, Crth2-antagonized BMDMs produced higher extracellular levels of PGE2, in line with increased expression of Cox2 and mPges1 (Fig. 1F). Surprisingly, BMDMs and RAW264.7 macrophages only expressed Crth2 mRNA, but not DP1 (DP; Supplemental Fig. S1), in agreement with previous findings (5), indicating preferential Crth2-mediated signaling in these cells.
Figure 1.
The Crth2 antagonist CAY10595 up-regulates inflammatory gene expression during LPS stimulation of BMDMs. A–C) Representative immunoblot (A) and quantitation of Cox2 (B) and iNOS (C) levels in BMDMs treated with DMSO or CAY10595 (5 µM) after 4–24 h LPS stimulation (100 ng/ml) or no stimulation (0 h). β-actin is used as loading control; n = 3 independent experiments. D) Mean fold change of mRNA levels of Cox2 (4 h), iNOS (4 h), mPges1 (8 h), Tnf-α (4 h), and Il-1β (4 h) in BMDMs treated with CAY10595 relative to BMDMs treated with 0.1% DMSO after LPS stimulation (100 ng/ml); n = 4–5 independent experiments. E) PGE2 production 8 h after LPS stimulation; n = 3 independent experiments. F) Nitrite production 4–24 h after LPS stimulation; n = 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with DMSO (Student’s t test).
Deletion of Crth2 in RAW264.7 cells
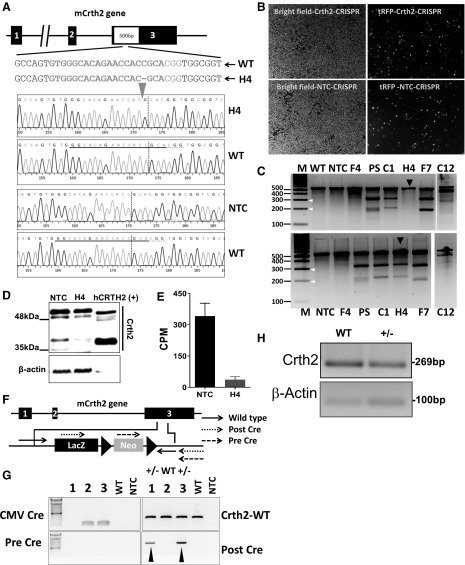
To induce a mutation in the coding region of Crth2, 3 sgRNAs targeting exon 3 were generated as shown in Fig. 2A. Modular lentiviral sgRNA and Cas9 vectors were generated and transduced into RAW264.7 cells. Such lentiviral-transduced cells expressing tRFP (Fig. 2B) were sorted into a 96-well plate, and 7 single cell-derived clones were tested for insertion and deletions (indels) at the Crth2 locus using a surveyor assay (Fig. 2C). Of the 7 clones, clone 4 (H4) showed homozygous mutation resulting in no fragmentation upon surveyor endonuclease digestion of homoduplexes. In contrast, treatment of heteroduplexes (1:1 amplicons of WT and H4 clone) with surveyor endonuclease–generated fragments. DNA sequence analysis revealed deletion of a cytosine residue 4 bases upstream of the protospacer-adjacent motif sequence, leading to effective disruption of coding frame (Fig. 2A). Immunoblot analysis for Crth2 expression in membrane fraction of H4 clone showed little or no expression of the receptor when compared with NTC (Fig. 2D). Furthermore, the ability of the membranes isolated from H4 clone to bind to [3H]PGD2 was greatly reduced in comparison with membranes isolated from NTC (Fig. 2E), confirming the deletion of Crth2 in the H4 clone of RAW264.7 cells. To study the role of Crth2 in mediating the effect of LPS on BMDMs, we generated Crth2 heterozygous (Crth2+/−) KO mice using ES cells that lack Crth2 allele with targeting vector as shown in Fig. 2F. The Crth2+/− mice showed a decreased expression of Crth2 in BMDMs (Fig. 2G, H).
Figure 2.
Generation and validation of Crth2 KO cell line and deletion of the Crth2 gene in C57BL/6 mouse. A) Mouse Crth2 (mCrth2) gene structure composed of 3 exons and nonhomologous end joining–mediated gene editing design that targets coding region in exon 3 of the Crth2 gene, resulting in the deletion of a cytosine 4 nt upstream of the protospacer-adjacent motif sequence generating frame shift mutation. B) Representative microscopic pictures of RAW264.7 cells after 4 d of lentiviral transduction under bright field and tetramethyl rhodamine iso-thiocyabate (TRITC) filter for visualization of tRFP+ cells. C) Mismatch-specific endonuclease assay. Genomic PCR products spanning exon 3 of the Crth2 gene were amplified from WT and RAW264.7 cell mutants such as NTC, PS, F4, C1, H4, F7, and C12. White arrows indicate the size of fragments formed after the surveyor endonuclease treatment of rehybridized homoduplexes (top) or heteroduplexes (bottom). Black arrows indicate the formation of fragments upon digestion of homoduplexes (top) and heteroduplexes (bottom) of genomic PCR amplicons of H4 clone and 1:1 ratio of H4 clone and WT, respectively. NTC clones are primary sorted tRFP+ RAW264.7 cells transduced with nontargeting sgRNA and Cas9 nuclease–containing lentiviral particles. PS clones are tRFP+ RAW264.7 cells transduced with Crth2 gene–targeting sgRNA and Cas9 nuclease–containing lentiviral particles. F4, C1, H4, F7, and C12 clones are single cell clones of tRFP+ RAW264.7 cells. D) Representative immunoblot of Crth2 protein with human recombinant CRTH2 as control. E) Percent specific binding of 5 nM [3H]PGD2 in a radioligand binding assay using membranes isolated from NTC and H4 clones. Data are means ± semof technical duplicate. F) Schematic illustration of the murine Crth2 gene and design of targeting vector used for Crth2 gene deletion in ES cells to generate a Crth2 KO C57BL6 mouse colony. G) Representative genotyping PCR of WT, after-Cre, pre-Cre, and CMV-Cre BMDMs. H) Representative PCR amplicon of cDNA isolated from WT and Crth2 heterozygous BMDMs. CPM, counts per minute; CMV, cytomegalovirus; LacZ, β-galectosidase coding sequence from the E.Coli LacZ gene; Neo, coding sequences for neomycin.
Deletion of Crth2 exacerbates inflammatory markers in murine macrophages and peritoneal cells
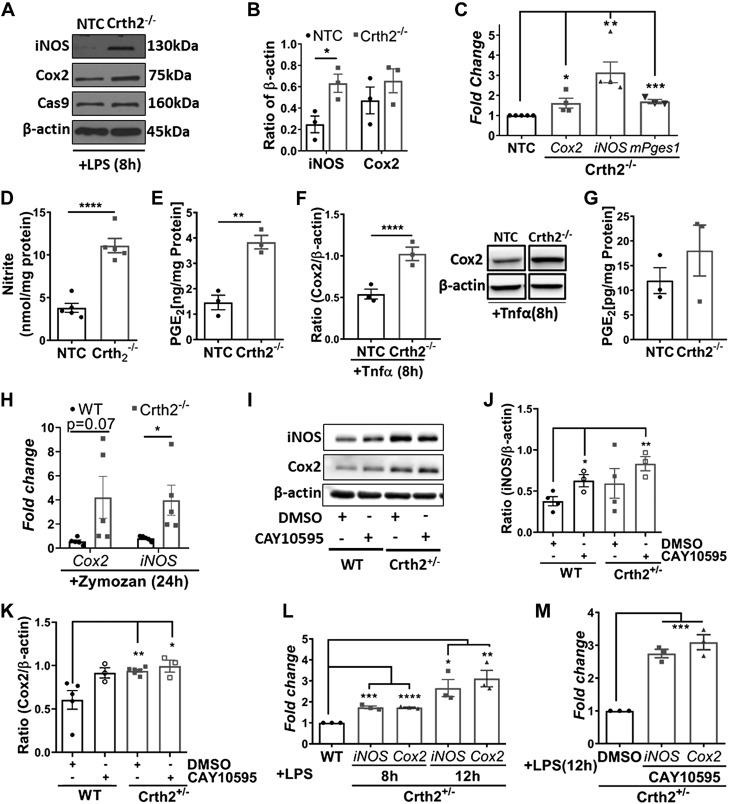
Based on the temporal regulation of inflammatory gene expression in the presence of CAY10595 (Fig. 1), we selected the 8-h time point to examine the effect of Crth2 deletion. Upon 8 h of LPS stimulation of Crth2−/− RAW264.7 cells, we saw increased expression of iNOS and Cox2 by 209.1 and 48.3% respectively, in comparison with NTC RAW264.7 cells (Fig. 3A, B). NTC and Crth2−/− RAW264.7 cells stably expressed Cas9 enzyme as a part of the CRISPR gene editing machinery (Fig. 3A). Increased protein expression was corroborated by significantly up-regulated mRNA levels of Cox2, iNOS, and mPges1 in Crth2−/− RAW264.7 cells relative to NTC RAW264.7 cells (Fig. 3C). The amount of nitrite was significantly increased to 11.29 ± 0.7 nmol/mg protein in Crth2−/− RAW264.7 cells from 4.49 ± 0.8 nmol/mg protein in NTC RAW264.7 cells (Fig. 3D), and a similar trend was observed in the case of PGE2 (Fig. 3E) in the culture medium supernatants of these cells. The Tnf-α–mediated activation of Crth2−/− RAW264.7 cells showed significant increase in expression of Cox2 (Fig. 3F) in addition to increased levels of PGE2 (Fig. 3G) in the culture supernatants compared with NTC RAW264.7 cells. Using a zymosan-induced peritonitis model, we noticed increased expression of Cox2 and iNOS in the peritoneal lavage cells (a mixture of macrophages and neutrophils) isolated from Crth2−/− mice compared with those from WT mice subjected to zymosan (Fig. 3H). Given the low breeding rate of Crth2−/− mice, to assess the Crth2 gene dosage effect, we used Crth2 heterozygous (Crth2+/−) primary BMDMs and Crth2+/− BMDMs pretreated with CAY10595 to emulate a complete KO model. Expression of Cox2 and iNOS was analyzed at 8 h after LPS stimulation in Crth2 heterozygous (Crth2+/−) primary BMDMs compared with BMDMs from age-matched littermate WT controls. Fig. 3I, J show a 56.9% increase in the expression of iNOS at 8 h after LPS in Crth2+/− BMDMs compared with its expression in WT BMDMs treated with vehicle. Pretreatment of Crth2+/− BMDMs with CAY10595 further increased the expression of iNOS by 63.4% at 8 h after LPS when compared with its expression in WT controls (Fig. 3I, J). We observed a similar trend in increased expression of Cox2 (55.4% increase at 8 h after LPS, compared with WT BMDM) in Crth2+/− BMDMs (Fig. 3I–K). The expression of Cox2 was further up-regulated by 9% in LPS-stimulated (8 h) Crth2+/− BMDMs pretreated with CAY10595. The transcript levels of iNOS and Cox2 were also significantly up-regulated in Crth2+/− BMDMs compared with their expression levels in WT counterparts (Fig. 3L). A similar trend was observed even at 12 h after LPS treatment in CAY10595-pretreated WT and Crth2+/− BMDMs (Fig. 3M).
Figure 3.
Deletion of Crth2 in murine macrophages increases inflammatory gene expression. A, B) Representative immunoblot (A) and quantitation (B) of iNOS and Cox2 in Crth2−/− and NTC RAW264.7 cells after 8 h of LPS stimulation (100 ng/ml). β-Actin is used as loading control. P value is in comparison with NTC RAW264.7 cells; n = 3 independent experiments. C) Mean fold change of mRNA levels of Cox2, iNOS, and mPges1 in Crth2−/− RAW264.7 cells relative to NTC RAW264.7 cells after LPS stimulation for 8 h. P value is in comparison with NTC RAW264.7 cells; n = 3–5 independent experiments. D) Nitrite production after 8 h after LPS stimulation. n = 5 independent experiments. E) PGE2 production after 8 h of LPS stimulation; n = 3 independent experiments. F) Densitometry of expression of Cox2 in NTC and Crth2−/− RAW264.7 cells after 8 h of Tnf-α stimulation (10 ng/ml). Representative immunoblot is in the inset. β-actin was used as loading control. P value is in comparison with NTC RAW264.7 cells; n = 3 independent experiments. G) PGE2 production after 8 h of Tnf-α stimulation; n = 3 independent experiments. WT and Crth2−/− mice were treated with zymosan A (10 mg/kg body weight) for 24 h. Peritoneal lavage fluid was obtained from mice after zymosan treatment, and cells were isolated by centrifugation. Pelleted cells were used for mRNA as described in Materials and Methods. H) Mean fold change in mRNA levels of Cox2 and iNOS in peritoneal lavage cells isolated from WT and Crth2−/− mice. P value is in comparison with the expression recorded in WT lavage cells; n = 5 independent experiments. I–K) Immunoblot (I) and quantitation of iNOS (J) and Cox2 (K) in Crth2+/− and WT BMDMs after 8 h of LPS stimulation. β-actin is used as loading control. P value is in comparison with WT BMDMs; n = 3–5 independent experiments. L) Mean fold change of mRNA levels of iNOS and Cox2 in Crth2+/− BMDMs after 8 and 12 h of LPS stimulation relative to WT. P value is in comparison with WT BMDMs. M) Mean fold change of mRNA levels of iNOS and Cox2 in Crth2+/− BMDMs treated with CAY10595 after 12 h of LPS stimulation relative to Crth2+/− BMDMs treated with 0.1% DMSO. P value is in comparison with DMSO-treated Crth2+/− BMDMs. Technical triplicate of RNA pooled from 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (Student’s t test).
Depletion of endogenous ligands of Crth2 up-regulates the expression of iNOS and Cox2 in LPS-induced macrophages
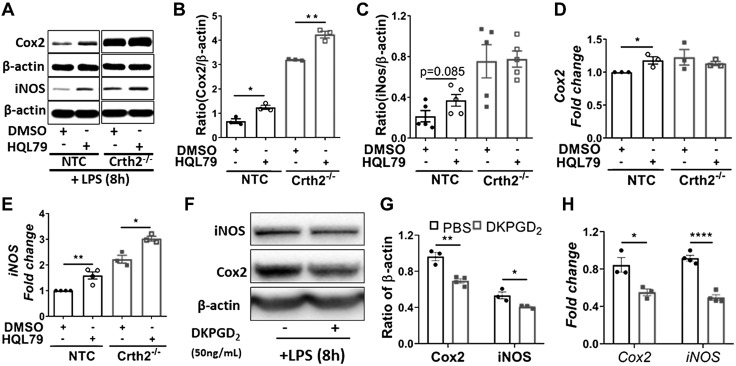
Although our results indicated the role of Crth2 receptor activation in repressing LPS-induced expression of iNOS and Cox2 in murine macrophages, we further evaluated the effect of blocking the production of endogenous ligands of Crth2 (Δ12-PGJ2 and 15-dPGJ2) by treatment of cells with HQL79, a specific inhibitor of hematopoietic PGDS, a key enzyme that acts on COX-derived PGH2 to form PGD2, which converts to CyPGs. Treatment of NTC RAW264.7 cells with HQL79 increased the expression of iNOS and Cox2 by 123 and 90% compared with vehicle-treated cells (Fig. 4A–C). In HQL79-treated NTC RAW264.7 cells, increased transcript levels of Cox2 (17.9% increase) (Fig. 4D) and iNOS (58.95% increase) (Fig. 4E) compared with vehicle-treated NTC RAW264.7 cells corroborated with protein levels. Furthermore, exogenous treatment of RAW264.7 cells with 50nM DKPGD2, a specific agonist of Crth2, prior to LPS activation significantly down-regulated the expression of Cox2 and iNOS at the protein (Fig. 4F, G) and mRNA levels (Fig. 4H). As expected, LPS-induced up-regulation of Cox2 was unaffected in Crth2−/− RAW264.7 cells pretreated with DKPGD2 (Supplemental Fig. S2).
Figure 4.
Inhibition of biosynthesis of Crth2 endogenous ligands increases iNOS and Cox2 expression in RAW264.7 cells. A–C) Representative immunoblot (A) and quantitation of Cox2 (B) and iNOS (C) in NTC and Crth2−/− RAW264.7 cells treated with DMSO or HQL79 (25 µM) after 8 h of LPS stimulation (100 ng/ml). β-actin is used as loading control. P value is in comparison with NTC RAW264.7 cells; n = 3 independent experiments. D, E) Real-time PCR of Cox2 (D) and iNOS (E) expression in NTC and Crth2−/− RAW264.7 cells treated with DMSO or HQL79 (25 µM) after 8 h of LPS stimulation. P value is in comparison with NTC RAW264.7 cells; n = 3 independent experiments. F, G) Representative immunoblot (F) and quantitation (G) of iNOS and Cox2 in RAW264.7 cells lysates prepared after stimulation with LPS (100 ng/ml) for 1 h with or without DKPGD2 (50 nM) after 1 h cells were washed and replenished with fresh medium containing either PBS or DKPGD2 (50 nM) up to 8 h. β-actin is used as loading control. P value is in comparison with expression in cells incubated with PBS; n = 3–4 independent experiments. H) Mean fold change of mRNA levels of Cox2 (8 h) and iNOS (4 h) normalized to 18S RNA. P value is in comparison with PBS-treated group; n = 3–4 independent experiments. *P < 0.05, **P < 0.01, ****P < 0.0001 (Student’s t test).
Ca2+ signaling and regulation of cAMP metabolism are abrogated in Crth2−/− RAW264.7 cells
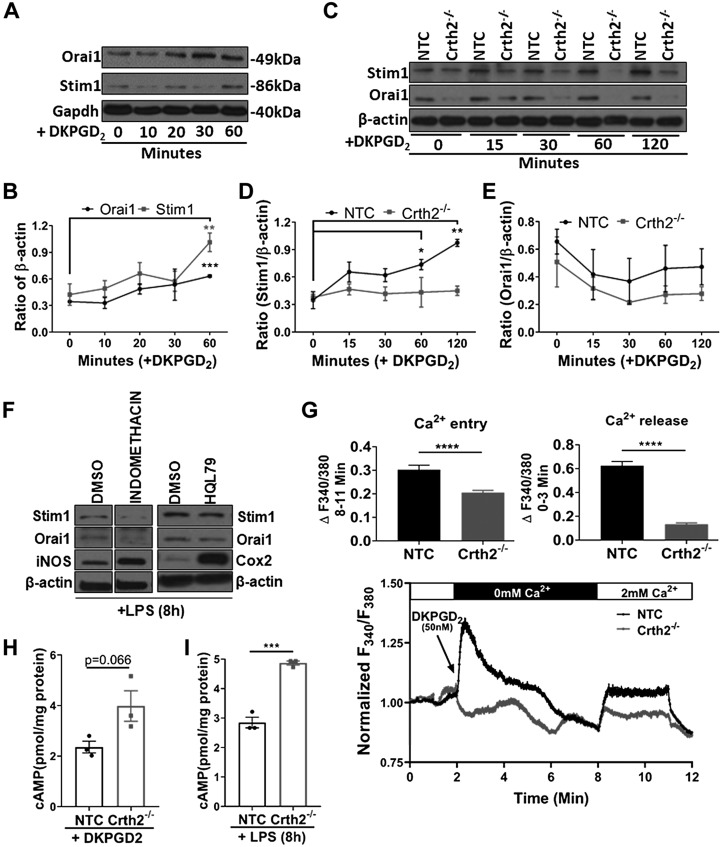
To understand the mechanism of Crth2-dependent anti-inflammatory mechanisms with an emphasis on cytosolic Ca2+ mobilization, we examined ligand-induced expression of SOCE proteins (STIM1 and Orai1) as surrogate markers with DKPGD2, a synthetic and specific ligand of Crth2. DKPGD2-treated BMDMs consistently showed increased expression of Orai1 (from 41.3% increase at 20 min to 83.72% increase at 60 min after treatment with 50 nM DKPGD2 compared with its expression in untreated BMDMs). Similarly, basal expression of Stim1 was increased by 16.1, 56.1, 35.6, and 139.6% at 10, 20, 30, and 60 min after DKPGD2 addition (Fig. 5A, B), respectively, indicating that the changes in [Ca2+]i levels were likely mediated by SOCE in response to ligand-dependent activation of Crth2. Interestingly, the expression of Stim1 was either unchanged or decreased in BMDMs pretreated with CAY10595 (Supplemental Fig. S3A–C). We further investigated DKPGD2-induced expression of Stim1 and Orai1 in NTC and Crth2−/− RAW264.7 cells as a function of time (0–120 min). In NTC RAW264.7 cells, DKPGD2 treatment induced a steady increase in expression of Stim1 (2.3, 72.5, 104.7, and 170.9% increase at 15, 30, 60, and 120 min, respectively), which was significant at 60 and 120 min when compared with untreated cells (Fig. 5C, D). DKPGD2-induced expression of Stim1 in NTC RAW264.7 cells was abolished when these cells were pretreated with PTX, which blocks Gαi (Supplemental Fig. S4). However, the expression of Stim1 in ligand-induced Crth2−/− RAW264.7 cells showed either little or no change in its expression (Fig. 5C, D). Overall, the expression of Orai1 did not change significantly upon DKPGD2 treatment of NTC and Crth2−/− RAW264.7 cells. It was interesting to note that the expression of Orai1 was higher in NTC compared with Crth2−/− RAW264.7 cells at all time points tested (Fig. 5C, E). Furthermore, pretreatment of BMDMs with either indomethacin or HQL79 followed by stimulation with LPS decreased the expression of Stim1 and Orai1, compared with vehicle-treated control cells, while increasing the expression of iNOS and Cox2, suggesting that the COX- and PGDS-derived metabolites modulate the expression of SOCE proteins (Fig. 5F).
Figure 5.
DKPGD2-mediated Ca2+ release and entry are abrogated in Crth2−/− RAW264.7 cells. A, B) Representative immunoblot (A) and quantitation (B) of Stim1 and Orai1 at 0, 10, 20, 30, and 60 min after DKPGD2 (50 nM) treatment of BMDMs. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) is used as loading control. C–E) Representative immunoblot (C) and quantitation of Stim1 (D) and Orai1 (E) in NTC and Crth2−/− RAW264.7 cells after DKPGD2 stimulation for 0–120 min. β-actin is used as loading control. P value is in comparison with expression in unstimulated cells. F) Representative blot showing expression of Stim1, Orai1, iNOS, and Cox2 in BMDMs pretreated with indomethacin (1 μM) or HQL79 (25 μM) followed by LPS stimulation for 8 h. G) Original traces show cytosolic Ca2+ signals after stimulation of NTC or Crth2−/− RAW264.7 cells by DKPGD2. These cells were loaded with Fura2-AM and stimulated with 50 nM DKPGD2 in HBSS (without extracellular calcium) to assess Ca2+ release followed by the addition of CaCl2 (2 mM extracellular calcium) to determine the extent of Ca2+ entry through SOCE. SOCE was also monitored after addition of the SOCE inhibitor Gd3+ (5 µM). Results are represented as means ± sem from 3 independent measurements, and the magnitude of Ca2+ release and Ca2+ entry signals was quantified (see inset). H) Levels of intracellular cAMP concentrations in response to DKPGD2 treatment for 15 min in NTC and Crth2−/− RAW264.7 cells. I) Intracellular cAMP levels measured at 8 h after LPS stimulation in NTC and Crth2−/− RAW264.7 cells. P value is in comparison with levels of cAMP in NTC RAW264.7 cells. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test; n = 3 independent experiments).
Stimulation of Crth2 receptor activates the Gαi-PLC pathway to produce IP3, which increases [Ca2+]i through IP3R-mediated ER Ca2+ release, which activates Stim1 and Orai1 to induce Ca2+ influx across the plasma membrane. To examine the role of Gαi protein in coupling to IP3-mediated Ca2+ release from the ER and subsequent Ca2+ entry, we used multicell fluorescence microscopy with the Ca2+ dye Fura2 to visualize [Ca2+]i dynamics in NTC and Crth2−/− RAW264.7 cells. In Crth2−/− RAW264.7 cells, DKPGD2-induced Ca2+ release assessed in nominally Ca2+-free bath solutions was significantly reduced compared with NTC RAW264.7 cells (Fig. 5G). Furthermore, Ca2+ entry measured upon restoration of 2 mM Ca2+ to the external milieu was significantly affected in Crth2−/− RAW264.7 cells compared with NTC RAW264.7 cells (Fig. 5G).
Another mechanism of the Gαi signal transduction pathway is via inhibition of cAMP. NTC RAW264.7 cells treated with DKPGD2 for 15 min exhibited low intracellular cAMP levels compared with Crth2−/− RAW264.7 cells (Fig. 5H), indicating dysregulation of the Gαi-mediated signaling pathway in Crth2−/− RAW264.7 cells. Furthermore, intracellular cAMP levels remained significantly higher at 8 h after LPS stimulation in Crth2−/− RAW264.7 cells in comparison with NTC RAW264.7 cells (Fig. 5I).
Inhibition of PKA in LPS-treated RAW264.7 cells down-regulates the expression of iNOS and Cox2
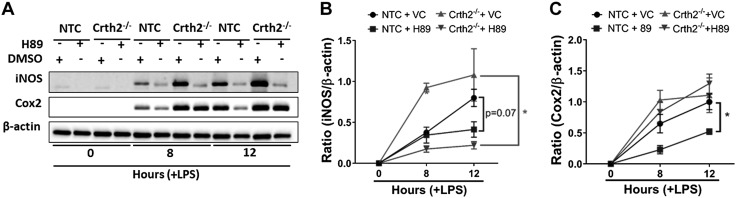
cAMP activates PKA, which phosphorylates the p65 subunit of NF-κB, among other proteins, to activate NF-κB target genes such as iNOS and Cox2 (18, 20). Therefore, we inhibited the activity of PKA with H89, a widely used synthetic inhibitor of PKA (45). H89 treatment significantly reduced (48.3% decrease) the expression of iNOS in NTC RAW264.7 cells at 12 h after LPS, whereas in Crth2−/− RAW264.7 cells, the decrease was even more substantial (81.1 and 79.6% decrease at 8 and 12 h after LPS, respectively) in comparison with vehicle-treated cells in each group (Fig. 6A, B). Similarly, the expression of Cox2 was down-regulated in H89-pretreated NTC RAW264.7 cells by 64.7 and 47.5% decrease at 8 and 12 h after LPS, respectively, whereas in Crth2−/− RAW264.7 cells, H89 decreased Cox2 expression by 18.6% at 8 h, but not at 12 h after LPS treatment (Fig. 6A–C).
Figure 6.
Inhibition of PKA in LPS-stimulated RAW264.7 cells down-regulates the expression of iNOS and Cox2. Representative immunoblot (A) and quantitation of iNOS (B) and Cox2 (C) levels in NTC and Crth2−/− RAW264.7 cells treated with DMSO or H89 (30 µM) after 8 and 12 h of LPS stimulation (100 ng/ml) or no stimulation (0 h). β-Actin is used as loading control. *P < 0.05 compared with DMSO (Student’s t test; n = 3 independent experiments). VC, vehicle.
LPS stimulation increased NF-κB occupancy of promoters of Nos2 and Ptgs2 in Crth2−/− RAW264.7 cells
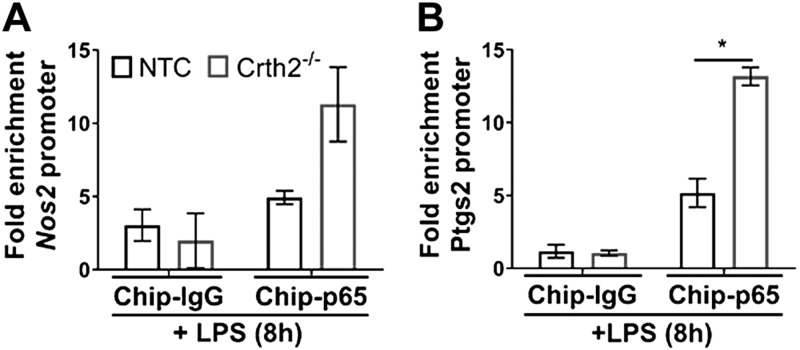
Based on the above experiments, we hypothesized that signals originating from Crth2 negatively affected the NF-κB–dependent activation of Cox2 and iNOS. Therefore, we investigated the promoter occupancy of p65 on the proximal promoters of iNOS (Nos2) and Cox2 (Ptgs2) after LPS stimulation using ChIP followed by quantitative PCR. As shown in Fig. 7A, LPS treatment significantly increased p65 binding to the proximal NRE on Nos2 promoter in Crth2−/− RAW264.7 cells (129.3% increase at 8 h after LPS) compared with NTC RAW264.7 cells. Similarly, binding of p65 was increased by 154.7% on Ptgs2 promoter in Crth2−/− RAW264.7 cells in comparison with NTC RAW264.7 cells (Fig. 7B).
Figure 7.
Increased binding of NF-κB to iNOS and Cox2 promoter in Crth2−/− RAW264.7 cells. ChIP of NF-κB p65 on iNOS (A) and Cox2 (B) promoters is compared with immunoprecipitation of IgG (negative control) in cells treated with LPS (100 ng/ml) for 8 h. Data represent mean fold enrichments. Data are means ± sem of technical triplicate. *P < 0.05 (Student’s t test).
Tnf-α mRNA is stabilized by the miR-155–Carhsp1 axis in Crth2−/− RAW264.7 cells
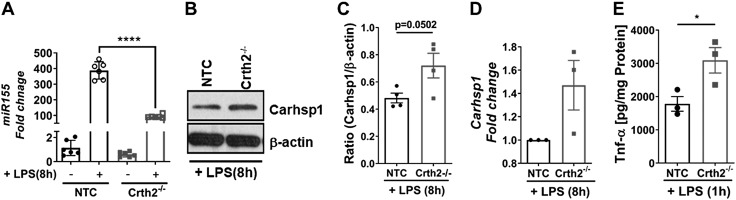
It is reported in ref. 33 that miR-155 suppresses chronic inflammation by signaling via the miR-155–Carhsp1–Tnf-α pathway. miR-155 targets Carhsp1, which regulates the stability of Tnf-α mRNA. To examine this paradigm, we analyzed the expression of miR-155 in LPS-treated NTC and Crth2−/− RAW264.7 cells. As shown in Fig. 8A, the expression of miR-155 was significantly reduced in Crth2−/− RAW264.7 cells (75.5% decrease) with a corresponding increase in expression of Carhsp1 protein and mRNA levels at 8 h after LPS treatment when compared with NTC RAW264.7 cells (Fig. 8B–D). Furthermore, increased extracellular levels of Tnf-α were observed in Crth2−/− RAW264.7 cells compared with NTC RAW264.7 cells at 1 h after LPS treatment (Fig. 8E).
Figure 8.
TNF-α is up-regulated by the miR-155–Carhsp1 axis in Crth2−/− RAW264.7 cells. A) Mean fold change of mRNA levels of miR-155 in NTC and Crth2−/− RAW264.7 cells after 8 h of LPS stimulation (100 ng/ml); n = 6 independent experiments. B, C) Representative immunoblot (B) and densitometric quantitation (C) of Carhsp1 levels in NTC and Crth2−/− RAW264.7 cells after 8 h LPS stimulation (100 ng/ml). β-Actin is used as loading control; n = 4 independent experiments. D) Tnf-α production 1 h after LPS stimulation. n = 3 independent experiments; n = 3 independent experiments. E) Mean fold change of mRNA levels of Carhsp1 8 h after LPS stimulation relative to NTC RAW264.7 cells; n = 3 independent experiments. *P < 0.05, ****P < 0.0001 compared with NTC (Student’s t test).
DISCUSSION
In this study, we report a previously unknown regulatory circuit involving the Crth2 receptor–based signal transduction pathway in the modulation of NF-κB–mediated gene expression during LPS-induced inflammatory response in murine macrophages. Crth2 receptor is expressed in a variety of cells and tissues, including macrophages (46). The downstream signaling pathways of Crth2 are known to be nonredundant across different cell types (11). For example, in Crth2-transfected cells, Th2 cells, and peripheral blood eosinophils, mobilization of [Ca2+]i and inhibition of cAMP upon receptor activation are PTX sensitive (3, 4). Similarly, in our studies, DKPGD2-induced Crth2-dependent activation of Gαi mobilization followed by [Ca2+]i mobilization was associated with decreased cAMP levels (Fig. 5C, I, J). Interestingly, the activation of Crth2-dependent up-regulation of Stim1 expression in a ligand-dependent manner (by DKPGD2) and in a PTX-sensitive manner suggested a Gαi mechanism underlying Ca2+ mobilization. Treatment of cells with PGE2 (50 nM) failed to up-regulate Stim1 expression, in contrast to that seen with DKPGD2 (unpublished results), suggesting a mechanism in response to the CyPGs that are produced during the resolution phase. Though the mechanism is not clear, the regulation of STIM1 expression by Crth2-dependent activation is noteworthy and warrants further investigation.
Studies have shown that LPS stimulation of macrophages in vitro leads to the accumulation of Δ12-PGJ2 and 15d-PGJ2, particularly in cells that express redox-active selenoproteins (14, 47), which can bind to both human (16) and mouse CRTH2 (3) with an affinity several orders of magnitude greater than that observed for the intracellular PPAR-γ. As a result, ligand binding to Crth2 and subsequent activation of Gαi leads to the inhibition of adenylate cyclase to decrease cAMP and concomitantly mobilize Ca2+ from intracellular stores via IP3Rs on the ER. Thus, Crth2 signaling appears to be essential for homeostasis during LPS-induced inflammation, which is required to downgrade the inflammatory response in a temporal manner. In addition to modulation of LPS-induced activation of murine macrophages, Crth2 signals are also essential to regulate inflammatory response to diverse signals, including TNFa and zymosan (Fig. 3F–H). Furthermore, an observation of 3–4-fold increases in mRNA levels of Cox2 and iNOS upon zymosan-induced peritonitis in Crth2 KO mice compared with WT mice (Fig. 3H) reiterates the role of Crth2 signals in regulation of multimodal inflammation.
Early in an inflammatory response, when Crth2 signals are impeded by increased PGE2 production, the level of intracellular cAMP builds up because of increased adenylate cyclase activity induced by PGE2 15d-receptor (EP) signals. As result, this leads to the activation of PKA and phosphorylation of NF-κB to increase transcription of its target genes. The IκB-associated catalytic subunit of PKA phosphorylates p65 (Ser276) to activate the transcription complex with cAMP-response element binding protein (CREB)–p300, for which cAMP-mediated activation of PKA is pivotal (17, 18). PKA can also affect NF-κB activation via negative regulation of protein phosphatase-2Cβ (19), as well as the activation of PKC and p38 MAPK, which further drives IKK-dependent NF-κB activation to increase iNOS and Il-6 expression (21). Therefore, increased adenylate cyclase activity by Gαs activation through EP receptors drives LPS-induced inflammation of macrophages, whereas Crth2 signals inhibit adenylate cyclase activity to suppress PKA-dependent activation of NF-κB, as seen in cells treated with PKA inhibitor (Fig. 7).
There exists a key difference in targeting inflammatory mediator generation in macrophage-mediated chronic inflammatory conditions such as rheumatoid arthritis and chronic venous ulcers and acute self-limiting inflammation such as peritonitis (48). LPS-induced inflammatory response involves classic activation of macrophages comparable to inappropriately activated macrophages that correlate with disease activity in rheumatoid arthritis and chronic venous ulcers (49, 50). Under such chronic inflammatory conditions, mechanisms that alter inflammatory response of macrophages via the production of endogenous mediators and associated mechanisms, such as Crth2-mediated inhibition of NF-κB activation in resolution of inflammation, could be most relevant. Although Cox2 is considered proinflammatory during the early phase of an acute inflammation, its role in mediating resolution at the later phase by generating an alternative set of anti-inflammatory prostaglandins makes Crth2-dependent signaling even more critical (51). That said, PGE2, being a prominent eicosanoid during chronic inflammatory and neoplastic disorders, including rheumatoid arthritis (52, 53) and many forms of cancer (54), could exert immunosuppressive properties to affect resolution of inflammation and help restore tissue homeostasis (55, 56). Furthermore, the PGE2-cAMP pathway has been shown to inhibit expression of inflammatory genes (57). Although several studies show that activation of the cAMP-PKA pathway may up-regulate NF-κB–mediated inflammatory response via up-regulation of iNOS in macrophages, it is possible that PGE2-mediated events lead to increased CyPGs that act in a feedback loop to abrogate NF-κB signaling events. This is currently being tested in the laboratory and will be reported in the future.
Studies have shown that miRs are key determinants of macrophage activation, controlling the expression of variety of molecules involved in pattern recognition receptor signaling and NF-κB activation (58). It is also known that miR-155 is elevated by Gαi activation via GPCRs such as leukotriene receptor, leukotriene B4 receptor 1 (BLT1) (59). In the present study, LPS stimulation significantly increased the expression of miR-155, but this effect was lost in the absence of Crth2 expression. Further studies are necessary to elucidate the molecular mechanisms underlying this effect. Recent studies have reported that anti-inflammatory activity of miR-155 is mediated through the inhibition of Carhsp1 expression by the binding of miR-155 to the 3'UTR of Carhsp1 mRNA (33, 60). In fact, Carhsp1 was originally identified as the physiologic substrate for calcineurin (61). Interestingly, the expression of Carhsp1 in NTC RAW264.7 cells, even in the unstimulated condition, was low compared with that in Crth2−/− RAW264.7 cells, which increased upon stimulation with LPS in Crth2−/− RAW264.7 cells (Fig. 8B, C). These observations suggest the importance of signals from Crth2 in keeping Carhsp1 levels under control during LPS stimulation. Further studies are necessary to test if Crth2 regulates calcineurin-mediated dephosphorylation of Carhsp1 and if this affects its RNA binding activity.
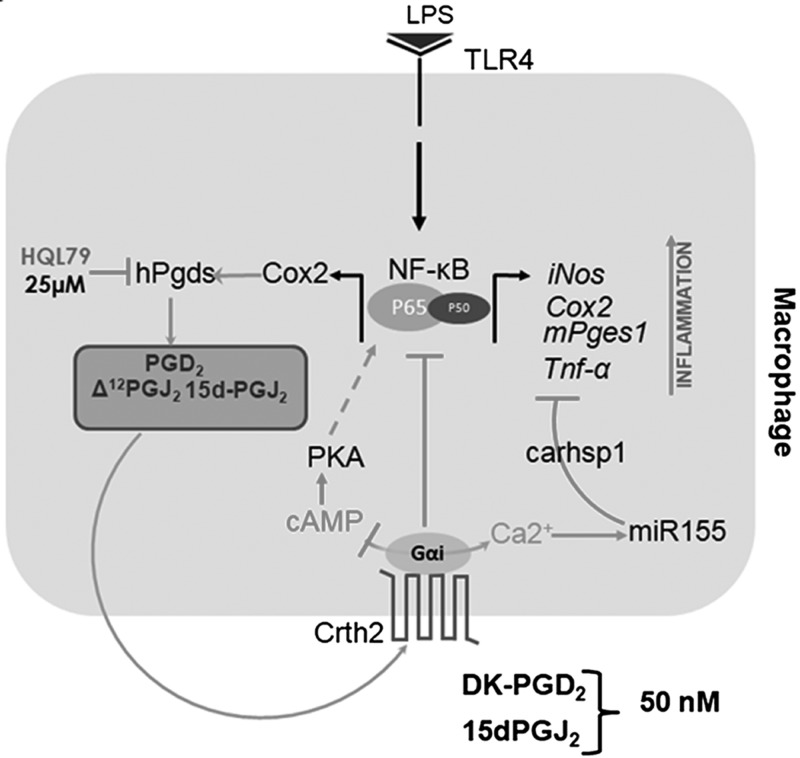
As summarized in Fig. 9, our study presents a novel regulatory mechanism of control of inflammatory gene expression by signals generated via the activation of the Crth2 receptor that are key to restoring second messenger homeostasis and PKA-mediated activation of NF-κB. Crth2 signals decreased PKA-mediated NF-κB transcription while affecting miR-155–mediated repression of Carhsp1 to decrease Tnf-α expression. In addition, this study also led to a novel finding of a crosstalk between Crth2 signaling and the activation of SOCE via the modulation of Stim1 expression. These studies present a novel proresolving role of Crth2 signals in LPS-induced inflammatory response in murine macrophages. Given the role of Crth2 in allergic responses, it remains to be seen if the effect on Stim1 and Ca2+ homeostasis is restricted to only macrophages or if a similar mechanism is operative in other cells, particularly in the lung epithelium.
Figure 9.
Schematic representation of the Crth2-dependent control of NF-κB gene expression in macrophages. Cyclopentenone metabolites of PGD2, Δ12-PGJ2 and 15d-PGJ2, are produced during LPS-induced inflammation in murine macrophages. Crth2 activation by CyPGs or synthetic ligands such as DKPGD2 increases Ca2+ influx through SOCE. The Gαi signals down-regulate Carhsp1, which stabilizes Tnf-α mRNA via increased miR-155 expression to decrease inflammatory responses triggered through the TNF-α–NF-κB axis. Additionally, Crth2 signals inhibit adenylate cyclase activity to suppress PKA-dependent activation of NF-κB to down-regulate LPS-induced inflammatory response. hPgds, hematopoietic prostaglandin D synthase.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Drs. Connie Rogers and Ramesh Ramachandran (Pennsylvania State University, State College, PA, USA) for their help with the MicroBeta microplate counter and fluorescent microscope, respectively. ES cells were from the Trans-U.S. National Institutes of Health (NIH) Knock-Out Mouse Project (KOMP) Repository. NIH National Human Genome Research Institute grants to Velocigene at Regeneron, Inc. (U01HG004085), the Complementary Sex Determination (CSD) Consortium (U01HG004080), and KOMP Repository at University of California–Davis (Davis, CA, USA) and Children’s Hospital Oakland Research Institute (CHORI) (U42RR024244) funded the program to generate the gene-targeted ES cells. These studies were funded, in part, by Public Health Service (PHS) grants from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (DK077152) and Office of Dietary Supplements, and U.S. Department of Agriculture (USDA) Hatch Funds (PENO 4605, Accession 1010021 to K.S.P., DK080040; PENO 4581, Accession 1005468 to R.F.P.). The authors declare no conflicts of interest.
Glossary
- [Ca2+]i
intracellular calcium
- Δ12-PGJ2
Δ12prostaglandin J2
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- BMDM
bone marrow–derived macrophage
- Carhsp1
Ca2+-regulated heat stable protein 1
- Cas9
CRISPR-associated protein 9
- ChIP
chromatin immunoprecipitation
- COX
cyclooxygenase
- CRISPR
clustered regularly interspaced short palindromic repeats
- Crth2
chemoattractant receptor homologous molecule expressed on type 2 T-helper cells
- CyPG
cyclopentenone prostaglandin
- DKPGD2
13,14-dihydro-15-keto-PGD2
- DP
d-type prostanoid
- ER
endoplasmic reticulum
- ES
embryonic stem
- Fura2-AM
Fura2–acetoxymethyl ester
- Gd3+
gadolinium (III) chloride
- IP3
inositol 1,4,5-triphosphate
- IP3R
IP3 receptor
- KO
knockout
- mPges1
microsomal prostaglandin E synthase 1
- NRE
NF-κB response element
- NTC
nontargeting control
- Orai1
calcium release–activated calcium modulator 1
- PGD2
prostaglandin D2
- PGDS
prostaglandin D synthase
- PGE2
prostaglandin E2
- PPAR-γ
peroxisome proliferator–activated receptor-γ
- PTX
pertussis toxin
- sgRNA
single-guide RNA
- SOCE
store-operated Ca2+ entry
- Stim1
stromal interaction molecule-1
- Th2
type 2 T helper
- tRFP
turbo red fluorescent protein
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. T. Diwakar, M. Trebak, R. F. Paulson, and K. S. Prabhu designed research, analyzed data, and wrote the paper; B.T. Diwakar, R. Yoast, S. Nettleford, F. Qian, T.-J. Lee, S. Berry, and I. Huffnagle performed research; and R. M. Rossi contributed new reagents.
REFERENCES
- 1.Schuligoi R., Sturm E., Luschnig P., Konya V., Philipose S., Sedej M., Waldhoer M., Peskar B. A., Heinemann A. (2010) CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology 85, 372–382 [DOI] [PubMed] [Google Scholar]
- 2.Pettipher R. (2008) The roles of the prostaglandin D(2) receptors DP(1) and CRTH2 in promoting allergic responses. Br. J. Pharmacol. 153 (Suppl 1), S191–S199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hata A. N., Zent R., Breyer M. D., Breyer R. M. (2003) Expression and molecular pharmacology of the mouse CRTH2 receptor. J. Pharmacol. Exp. Ther. 306, 463–470 [DOI] [PubMed] [Google Scholar]
- 4.Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., Nagata K. (2001) Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajima T., Murata T., Aritake K., Urade Y., Hirai H., Nakamura M., Ozaki H., Hori M. (2008) Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). J. Pharmacol. Exp. Ther. 326, 493–501 [DOI] [PubMed] [Google Scholar]
- 6.Tsubosaka Y., Nakamura T., Hirai H., Hori M., Nakamura M., Ozaki H., Murata T. (2014) A deficiency in the prostaglandin D2 receptor CRTH2 exacerbates adjuvant-induced joint inflammation. J. Immunol. 193, 5835–5840 [DOI] [PubMed] [Google Scholar]
- 7.Wojno E. D., Monticelli L. A., Tran S. V., Alenghat T., Osborne L. C., Thome J. J., Willis C., Budelsky A., Farber D. L., Artis D. (2015) The prostaglandin D2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 8, 1313–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal A. K., Zhang Z., Ray R., Choi M. S., Chowdhury B., Pattabiraman N., Mukherjee A. B. (2004) Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J. Exp. Med. 199, 1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M. C., Bleecker E. R., Lichtenstein L. M., Kagey-Sobotka A., Niv Y., McLemore T. L., Permutt S., Proud D., Hubbard W. C. (1990) Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am. Rev. Respir. Dis. 142, 126–132 [DOI] [PubMed] [Google Scholar]
- 10.Fujitani Y., Kanaoka Y., Aritake K., Uodome N., Okazaki-Hatake K., Urade Y. (2002) Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J. Immunol. 168, 443–449 [DOI] [PubMed] [Google Scholar]
- 11.Jandl K., Heinemann A. (2017) The therapeutic potential of CRTH2/DP2 beyond allergy and asthma. Prostaglandins Other Lipid Mediat. 133, 42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chevalier E., Stock J., Fisher T., Dupont M., Fric M., Fargeau H., Leport M., Soler S., Fabien S., Pruniaux M. P., Fink M., Bertrand C. P., McNeish J., Li B. (2005) Cutting edge: chemoattractant receptor-homologous molecule expressed on Th2 cells plays a restricting role on IL-5 production and eosinophil recruitment. J. Immunol. 175, 2056–2060 [DOI] [PubMed] [Google Scholar]
- 13.Ishii M., Asano K., Namkoong H., Tasaka S., Mizoguchi K., Asami T., Kamata H., Kimizuka Y., Fujiwara H., Funatsu Y., Kagawa S., Miyata J., Ishii K., Nakamura M., Hirai H., Nagata K., Kunkel S. L., Hasegawa N., Betsuyaku T. (2012) CRTH2 is a critical regulator of neutrophil migration and resistance to polymicrobial sepsis. J. Immunol. 188, 5655–5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vunta H., Davis F., Palempalli U. D., Bhat D., Arner R. J., Thompson J. T., Peterson D. G., Reddy C. C., Prabhu K. S. (2007) The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J. Biol. Chem. 282, 17964–17973 [DOI] [PubMed] [Google Scholar]
- 15.Gandhi U. H., Kaushal N., Ravindra K. C., Hegde S., Nelson S. M., Narayan V., Vunta H., Paulson R. F., Prabhu K. S. (2011) Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. J. Biol. Chem. 286, 27471–27482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawyer N., Cauchon E., Chateauneuf A., Cruz R. P., Nicholson D. W., Metters K. M., O’Neill G. P., Gervais F. G. (2002) Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br. J. Pharmacol. 137, 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong H., SuYang H., Erdjument-Bromage H., Tempst P., Ghosh S. (1997) The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89, 413–424 [DOI] [PubMed] [Google Scholar]
- 18.Zhong H., Voll R. E., Ghosh S. (1998) Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1, 661–671 [DOI] [PubMed] [Google Scholar]
- 19.Choi H. K., Park S. Y., Oh H. J., Han E. J., Lee Y. H., Lee J., Jun W. J., Choi K. C., Yoon H. G. (2013) PKA negatively regulates PP2Cβ to activate NF-κB-mediated inflammatory signaling. Biochem. Biophys. Res. Commun. 436, 473–477 [DOI] [PubMed] [Google Scholar]
- 20.Chen C. C., Chiu K. T., Sun Y. T., Chen W. C. (1999) Role of the cyclic AMP-protein kinase A pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J. Biol. Chem. 274, 31559–31564 [DOI] [PubMed] [Google Scholar]
- 21.Chio C. C., Chang Y. H., Hsu Y. W., Chi K. H., Lin W. W. (2004) PKA-dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell. Signal. 16, 565–575 [DOI] [PubMed] [Google Scholar]
- 22.Lee S., Lee H. C., Kwon Y. W., Lee S. E., Cho Y., Kim J., Lee S., Kim J. Y., Lee J., Yang H. M., Mook-Jung I., Nam K. Y., Chung J., Lazar M. A., Kim H. S. (2014) Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 19, 484–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon Y. J., Yang K. H., Pulaski J. T., Kaminski N. E. (1996) Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor- kappa B/Rel activation. Mol. Pharmacol. 50, 334–341 [PubMed] [Google Scholar]
- 24.Nüsing R. M., Klein T., Pfeilschifter J., Ullrich V. (1996) Effect of cyclic AMP and prostaglandin E2 on the induction of nitric oxide- and prostanoid-forming pathways in cultured rat mesangial cells. Biochem. J. 313, 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirokawa K., O’Shaughnessy K., Moore K., Ramrakha P., Wilkins M. R. (1994) Induction of nitric oxide synthase in cultured vascular smooth muscle cells: the role of cyclic AMP. Br. J. Pharmacol. 112, 396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trebak M., Putney J. W., Jr (2017) ORAI calcium channels. Physiology (Bethesda) 32, 332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakriya M., Lewis R. S. (2015) Store-operated calcium channels. Physiol. Rev. 95, 1383–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391, 79–82 [DOI] [PubMed] [Google Scholar]
- 29.Cernuda-Morollón E., Pineda-Molina E., Cañada F. J., Pérez-Sala D. (2001) 15-Deoxy-Delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 276, 35530–35536 [DOI] [PubMed] [Google Scholar]
- 30.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M. G. (2000) Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 403, 103–108 [DOI] [PubMed] [Google Scholar]
- 31.Fullerton J. N., Gilroy D. W. (2016) Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 [DOI] [PubMed] [Google Scholar]
- 32.Ma X., Becker Buscaglia L. E., Barker J. R., Li Y. (2011) MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 3, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Kong D., Chen H., Liu S., Hu H., Wu T., Wang J., Chen W., Ning Y., Li Y., Lu Z. (2016) miR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci. Rep. 6, 21789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker R., Sheth U. (2007) P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646 [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer J. R., McAvoy B. L., Fecteau R. E., Deleault K. M., Brooks S. A. (2011) CARHSP1 is required for effective tumor necrosis factor alpha mRNA stabilization and localizes to processing bodies and exosomes. Mol. Cell. Biol. 31, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C. N. (2011) MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 25, 544–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajuebor M. N., Virág L., Flower R. J., Perretti M., Szabó C. (1998) Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology 95, 625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutner R. H., Zhang X. Y., Reiser J. (2009) Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 4, 495–505 [DOI] [PubMed] [Google Scholar]
- 39.Shay A. E., Diwakar B. T., Guan B. J., Narayan V., Urban J. F., Jr., Prabhu K. S. (2017) IL-4 up-regulates cyclooxygenase-1 expression in macrophages. J. Biol. Chem. 292, 14544–14555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 41.Prabhu K. S., Zamamiri-Davis F., Stewart J. B., Thompson J. T., Sordillo L. M., Reddy C. C. (2002) Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: role of nuclear factor-kappaB in up-regulation. Biochem. J. 366, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finch E. R., Tukaramrao D. B., Goodfield L. L., Quickel M. D., Paulson R. F., Prabhu K. S. (2017) Activation of PPARγ by endogenous prostaglandin J2 mediates the antileukemic effect of selenium in murine leukemia. Blood 129, 1802–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai P. N., Zhang X., Wu S., Janoshazi A., Bolimuntha S., Putney J. W., Trebak M. (2015) Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci. Signal. 8, ra74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trebak M., Bird G. S., McKay R. R., Putney J. W., Jr (2002) Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J. Biol. Chem. 277, 21617–21623 [DOI] [PubMed] [Google Scholar]
- 45.Murray A. J. (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci. Signal. 1, re4 [DOI] [PubMed] [Google Scholar]
- 46.Abe H., Takeshita T., Nagata K., Arita T., Endo Y., Fujita T., Takayama H., Kubo M., Sugamura K. (1999) Molecular cloning, chromosome mapping and characterization of the mouse CRTH2 gene, a putative member of the leukocyte chemoattractant receptor family. Gene 227, 71–77 [DOI] [PubMed] [Google Scholar]
- 47.Shibata T., Kondo M., Osawa T., Shibata N., Kobayashi M., Uchida K. (2002) 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 277, 10459–10466 [DOI] [PubMed] [Google Scholar]
- 48.Sica A., Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., Weiss J. M., Wlaschek M., Sunderkötter C., Scharffetter-Kochanek K. (2011) An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 121, 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haringman J. J., Gerlag D. M., Zwinderman A. H., Smeets T. J., Kraan M. C., Baeten D., McInnes I. B., Bresnihan B., Tak P. P. (2005) Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 64, 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilroy D. W., Colville-Nash P. R., Willis D., Chivers J., Paul-Clark M. J., Willoughby D. A. (1999) Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 [DOI] [PubMed] [Google Scholar]
- 52.Kojima F., Kapoor M., Yang L., Fleishaker E. L., Ward M. R., Monrad S. U., Kottangada P. C., Pace C. Q., Clark J. A., Woodward J. G., Crofford L. J. (2008) Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. J. Immunol. 180, 8361–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westman M., Korotkova M., af Klint E., Stark A., Audoly L. P., Klareskog L., Ulfgren A. K., Jakobsson P. J. (2004) Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis Rheum. 50, 1774–1780 [DOI] [PubMed] [Google Scholar]
- 54.Greenhough A., Smartt H. J., Moore A. E., Roberts H. R., Williams A. C., Paraskeva C., Kaidi A. (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30, 377–386 [DOI] [PubMed] [Google Scholar]
- 55.Kalinski P. (2012) Regulation of immune responses by prostaglandin E2. J. Immunol. 188, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roulis M., Nikolaou C., Kotsaki E., Kaffe E., Karagianni N., Koliaraki V., Salpea K., Ragoussis J., Aidinis V., Martini E., Becker C., Herschman H. R., Vetrano S., Danese S., Kollias G. (2014) Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc. Natl. Acad. Sci. USA 111, E4658–E4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chinen T., Komai K., Muto G., Morita R., Inoue N., Yoshida H., Sekiya T., Yoshida R., Nakamura K., Takayanagi R., Yoshimura A. (2011) Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat. Commun. 2, 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Neill L. A., Sheedy F. J., McCoy C. E. (2011) MicroRNAs: the fine-tuners of toll-like receptor signalling. Nat. Rev. Immunol. 11, 163–175 [DOI] [PubMed] [Google Scholar]
- 59.Wang Z., Filgueiras L. R., Wang S., Serezani A. P., Peters-Golden M., Jancar S., Serezani C. H. (2014) Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote MyD88-dependent macrophage activation. J. Immunol. 192, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu J., Chen T., Yang L., Li Z., Wong M. M., Zheng X., Pan X., Zhang L., Yan H. (2012) Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One 7, e46551; erratum: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schäfer C., Steffen H., Krzykowski K. J., Göke B., Groblewski G. E. (2003) CRHSP-24 phosphorylation is regulated by multiple signaling pathways in pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G726–G734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.