Abstract
Sepsis remains a significant health care burden, with high morbidities and mortalities. Patients with sepsis often require general anesthesia for procedures and imaging studies. Knowing that anesthetic drugs can pose immunomodulatory effects, it would be critical to understand the impact of anesthetics on sepsis pathophysiology. The volatile anesthetic sevoflurane is a common general anesthetic derived from ether as a prototype. Using a murine sepsis model induced by cecal ligation and puncture surgery, we examined the impact of sevoflurane on sepsis outcome. Different from volatile anesthetic isoflurane, sevoflurane exposure significantly improved the outcome of septic mice. This was associated with less apoptosis in the spleen. Because splenic apoptosis was largely attributed to the apoptosis of neutrophils, we examined the effect of sevoflurane on FasL-induced neutrophil apoptosis. Sevoflurane exposure significantly attenuated apoptosis. Sevoflurane did not affect the binding of FasL to the extracellular domain of Fas receptor. Instead, in silico analysis suggested that sevoflurane would bind to the interphase between Fas death domain (DD) and Fas-associated DD (FADD). The effect of sevoflurane on Fas DD–FADD interaction was examined using fluorescence resonance energy transfer (FRET). Sevoflurane attenuated FRET efficiency, indicating that sevoflurane hindered the interaction between Fas DD and FADD. The predicted sevoflurane binding site is known to play a significant role in Fas DD–FADD interaction, supporting our in vitro and in vivo apoptosis results.—Koutsogiannaki, S., Hou, L., Babazada, H., Okuno, T., Blazon-Brown, N., Soriano, S. G., Yokomizo, T., Yuki, K. The volatile anesthetic sevoflurane reduces neutrophil apoptosis via Fas death domain–Fas-associated death domain interaction.
Keywords: Sepsis, splenic apoptosis, anesthesia
Sepsis is characterized by an overwhelming immune response to infection that leads to a systemic inflammation followed by tissue damage and organ failure. Although significant efforts have been made to understand its pathophysiology and tailor the clinical management strategies for patients with this detrimental syndrome, it continues to be associated with high morbidity and mortality and is a leading cause of death in hospitals worldwide. No specific therapy or drug is still available for sepsis, and the mainstay treatment is to control the source of infection. Patients often undergo imaging studies for source detection and surgical procedures for evacuation, for which anesthesia is often indicated. Ether-based volatile anesthetics such as isoflurane and sevoflurane are among the popular drugs of choice. In addition to their anesthetic effect, off-target effects of anesthetics have been increasingly recognized. We and others have demonstrated that volatile anesthetics may negatively affect immune functions and surgical outcomes (1–7). Regarding sepsis, we have previously shown that isoflurane worsened the outcome of septic mice (8). Understanding the impact of existing medical treatment on sepsis outcome and optimizing it is critical, particularly because there is no targeted therapy at this point.
Recently, many studies point out a major role of apoptotic cell death in the immune and organ dysfunction as well as in the mortality observed in sepsis (9–12). Apoptosis represents an inherent process through which cells are deleted in a control manner to protect the surrounding environment from excessive damage. Thus, apoptosis can be beneficial to the host by eliminating many immune cells that produce proinflammatory cytokines and protect the tissues from damage on one hand (13). On the other hand, however, apoptosis could be detrimental in sepsis by depleting immune cells, which results in compromising the host defense against the invading organisms (12). The latter theory is supported by studies showing that immune cell apoptosis contributes to the development of the immunosuppressive pathophysiology of sepsis (14, 15). Immune cell apoptosis not only leads to the condition of the host overwhelmed by pathogenic organisms and opportunistic pathogens but also causes immune tolerance, mainly induced by the surviving immune cells taking up apoptotic cells. Antiapoptosis therapy such as the administration of antiapoptotic cytokines, caspase inhibitors, and death receptor antagonists have been shown to improve survival in sepsis in preclinical studies, further supporting the idea that immune cell apoptosis can be detrimental in sepsis (16).
Sepsis-associated apoptosis has been reported in many different immune cells (17). Most of innate immune cells have been shown to undergo apoptosis in sepsis, including dendritic cells, immature macrophages, NK cells, and neutrophils (9, 18). Regarding the adaptive immune system, both T helper (Th) and T cytotoxic cells as well as B cells show apoptosis (19, 20). The Fas-FasL complex has been identified as a major pathway involved in the induction of apoptotic cell death in most of those cell types (21). Fas [cell differentiation antigen 95 (CD95)] is a cell‐surface protein belonging to the TNF superfamily of membrane receptors and is responsible for the extrinsic apoptotic signaling pathway. Fas is expressed on numerous cell types, including thymocytes, activated B cells, T cells, monocytes, macrophages, neutrophils, as well as on a variety of nonimmune cells in the liver, lung, and heart (22). Upon binding of TNF family receptors, such as Fas to their respective ligand FasL, trimerization and subsequent death‐induced signaling complex (DISC) formation is initiated. An adaptor molecule containing a death domain (DD) called Fas-associated DD (FADD) binds to these activated DDs and to procaspase 8 through death effector domains to form the DISC. The death signal is then transduced from the DISC to a downstream caspase pathway that cleaves and activates downstream effector caspases, such as caspase-3, leading to apoptosis (23). FasL-mediated apoptosis in sepsis plays a significant role. Blocking Fas signaling by Fas fusion protein or Fas small interfering RNA, FasL knockout mice reduced organ damage and improved mortality from sepsis induced by cecal ligation and puncture (CLP) (9, 10, 24).
In this study, we investigated the role of sevoflurane in sepsis outcome and compared it with isoflurane. The association between sepsis outcome and apoptosis was examined. To further investigate the effect of sevoflurane on sepsis-induced apoptosis, we tested the effect of sevoflurane in vitro including testing on Fas-FasL and Fas-FADD interactions.
MATERIALS AND METHODS
Mice
Wild-type (WT) mice on the C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and inbred in our animal facility. They were housed under specific pathogen-free condition, with 12-h light/dark cycles. Male mice at 8–10 wk of age were used for the experiments.
CLP model
All the experimental procedures complied with institutional and federal guidelines regarding the use of animals in research. Polymicrobial abdominal sepsis was induced by CLP, as we previously performed (8). In brief, mice were anesthetized with ketamine (60 mg/kg) and xylazine (5 mg/kg) given intraperitoneally. Following exteriorization, the cecum was ligated at 1.0 cm from its tip and subjected to a single through and through puncture using an 18-gauge needle. A small amount of fecal material was expelled with gentle pressure to maintain the patency of puncture sites. The cecum was reinserted into the abdominal cavity. Warmed saline (0.1 ml/g) was administered subcutaneously. Buprenorphine was given subcutaneously to alleviate postoperative surgical pain. A group of mice was fitted with nose cones to be continuously exposed to 0.9% isoflurane using an isoflurane vaporizer (VetEquip, Livermore, CA, USA) or exposed to 2% sevoflurane using sevoflurane vaporizer (VetEquip). Mice were continuously observed during anesthetic exposure and until they were fully recovered from anesthesia. Tail blood pressure was measured during isoflurane or sevoflurane exposure using tail cuff (Harvard Apparatus, Holliston, MA, USA) (25).
Quantitative organ culture for bacterial load examination
To determine the bacterial loads in the organs, peritoneal cavity, and blood in mice after CLP surgery, tissue homogenates, peritoneal lavage fluid, and blood were loaded on 5% blood agar plates (Teknova, Hollister, CA, USA) after serial dilutions and were incubated for 18 h as previously described in refs. 8 and 26. Colonies of all morphologies on plates were counted.
Whole blood flow cytometry
Following incubation with Fc blocking antibody, surface expressions of CD11a, CD11b, and CD11c were probed using M17/4 (CD11a), M1/70 (CD11b), and N418 (CD11c) antibodies, respectively. Erythrocytes were lyzed using lysis buffer (BD Biosciences, Billerica, MA, USA). Neutrophil population was gated by anti-Ly6G antibody. All the antibodies were purchased from BioLegend (San Diego, CA, USA).
Serum and peritoneal fluid cytokine measurement
The level of TNF-α, IFN-γ, IL-1β, and IL-12 in the serum and peritoneal lavage fluid was measured using mouse Th1/Th2 9-Plex Ultra-Sensitive Kit (Meso Scale Discovery, Rockville, MD, USA).
Lipoxin A4 and aresin 1 measurement
Reverse-phase mass spectrometry-based quantitation technique was used for lipoxin A4 (LXA4) and maresin 1 (MaR1) measurement as previously described by Okuno et al. (27). The samples were diluted with 2 ml of methanol and 7 ml of water containing 0.1% formic acid containing a mixture of deuterium-labeled eicosanoids as internal standards, and then loaded on Oasis HLB cartridge (Waters, Milford, MA, USA). The column was washed with 1 ml of water, 1 ml of 15% methanol, 1 ml of petroleum ether, and then eluted with 0.2 ml of methanol containing 0.1% formic acid. LXA4 and MaR1 were quantified by reverse-phase HPLC-electrospray ionization-tandem mass spectrometry method.
In vitro neutrophil apoptosis assay by FasL
Bone marrow neutrophils were prepared as we previously described (28). Neutrophils (1 × 105 cells) were measured in aliquots for each well and cultured in RPMI 1640/10% FBS for neutrophil apoptosis experiment as previously described Croker et al. (29). Briefly, 100 ng/ml Flag-FasL (Axxora, Farmingdale, NY, USA) cross-linked with anti-Flag antibody (M2; MilliporeSigma, Burlington, MA, USA) was incubated with neutrophils in the presence or absence of isoflurane or sevoflurane in an airtight chamber as we previously performed (30). At the end of incubation, neutrophils were collected and subjected to apoptosis analysis by Western blot.
Apoptosis analysis using Western blot
The total protein from mouse tissues or primary neutrophils was extracted in a lysis buffer containing proteinase inhibitors (Roche Applied Science, Indianapolis, IN, USA). Total protein concentrations for mouse tissue protein were determined using bicinchoninic acid protein assay (Fischer Scientific, Morris Plains, NJ, USA). Equal amount of protein was resolved by 4–20% SDS-PAGE and transferred to nitrocellulose membranes. After blocking in 5% nonfat dry milk, the membranes were incubated with anticleaved caspase-3, caspase-8, caspase-9, or β actin antibody. Membranes were then incubated with anti-rabbit IgG horseradish peroxidase (HRP) conjugate. All the antibodies used were obtained from Cell Signaling Technology (Danvers, MA, USA). Immunoreactive proteins were visualized by ECL (Thermo Fisher Scientific, Waltham, MA, USA).
Flow cytometric apoptosis analysis of mouse splenocytes
Mice subjected to CLP procedures were euthanized at 24 h after CLP. Apoptosis of splenocytes was assessed either by cleaved caspase-3 expression or Annexin V expression. For cleaved caspase-3 staining, single splenic cells were first stained with CD11b-allophycocyanin, Ly6G-phycoerythrin (PE), Ly6C-PE-cyanine 7 for myeloid cell population, and then intracellular staining of cleaved caspase-3-FITC was performed after permeabilization. Lymphocytes were probed with CD3-PE, CD4-Pacific Blue, CD8-PE-cyanine 7, and CD19-allophycocyanin (BioLegend). For Annexin V staining, single splenic cells were stained with Annexin V-FITC in addition to myeloid and lymphocyte markers as above. All the antibodies used were from BioLegend. Flow cytometry analysis was performed using FACS Canto (BD Biosciences, San Diego, CA, USA). Data were analyzed using FlowJo software (Ashland, OR, USA).
In vitro Fas-FasL binding assay
In vitro Fas-FasL binding assay was performed as previously described with a minor modification (31). Ninety-six-well plates were coated with human Fas-Fc (1 µg/ml) (R&D Systems, Minneapolis, MN, USA) overnight. Wells were washed with PBST (0.05% Tween-20) and blocked with PBS containing 2% bovine serum albumin. Then wells were incubated with FasL for 1 h with or without isoflurane or sevoflurane in an airtight chamber. FasL was detected by mouse anti-FLAG antibody followed by rabbit anti-mouse IgG-HRP (Cell Signaling Technology). HRP was detected by 3,3′,5,5′-tetramethylbenzidine substrate. Absorbance was measured at 490 nm with the plate reader (Molecular Devices, Sunnyvale, CA, USA).
Docking analysis of volatile anesthetic sevoflurane on Fas receptor and its associated protein
Upon FasL engagement to the extracellular domain of Fas receptor, the intracellular interaction between Fas DD and FADD is critical for caspase-3 activation (32). In silico prediction of potential sevoflurane binding sites on Fas DD–FADD complex [Protein Data Bank (PDB): 3EZQ] was performed using SiteMap (Schrödinger, New York, NY, USA). The protein structure was prepared using the built-in protein preparation wizard. “Site” is defined as the protein surface suitable for binding of a ligand to the receptor. SiteMap was set as follows: 15 site points per reported site, report up to 5 sites, using a restrictive definition of hydrophobicity, standard grids and cropping site maps at 4 Å from the nearest site point. The OPLS_2005 force field was used for calculation. Estimated binding site with the highest score was then embedded into the grid with the size of 20 × 20 × 20 Å3 for docking with sevoflurane using Autodock Vina (Scripps Research, San Diego, CA, USA).
Fluorescence resonance energy transfer experiment for Fas DD–FADD interaction
The effect of sevoflurane on the interaction between Fas DD and FADD was examined using fluorescence resonance energy transfer (FRET) experiment as follows. pEYFP-N1-human Fas [Fas-yellow fluorescent protein (YFP)] and pECFP-C1-human FADD [FADD- cyan fluorescent protein (CFP)] plasmids were previously used for Fas DD–FADD FRET experiment and kindly provided by Dr. Richard Siegel and Dr. Anthony Cruz (NIH, Bethesda, MD, USA) (33). Fas-YFP and FADD-CFP plasmids were transiently transfected into HEK293T cells using Lipofectamine 3000 (Thermo Fisher Scientific) per the company protocol. At 48 h after the transfection, transfected cells were pre-exposed to isoflurane (1%) or sevoflurane (2%) in an airtight chamber for 30 min. Then, cells were immediately subjected to FRET examination using Synergy plate reader (BioTek Instruments, Winooski, VT, USA) as previously described by Skeldal et al. (34). Three filter sets [filter A- excitation 430 nm, emission 460 nm; filter B- excitation 485 nm, emission 535 nm; filter C (FRET filter)- excitation 430 nm, emission 535 nm] were used. The raw FRET signal obtained from the plate reader is contaminated by overlap of the emission spectrum of the donor with that of the acceptor (donor spectral bleed-through; DSBT) and by direct excitation of the acceptor by the donor excitation light (acceptor spectral bleed-through; ASBT). DSBT and ASBT were calculated as follows. Cells expressing both Fas DD and FADD, cells expressing only Fas DD and cells expressing only FADD were called Fas-FADD, Fas, and FADD, respectively.
 |
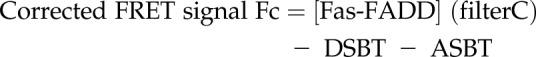 |
then,
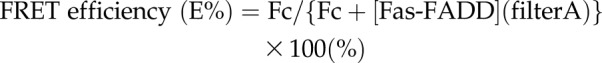 |
Statistical analysis
Statistical analysis method was shown in each figure legend. Statistical significance was defined as P < 0.05. All statistical calculations were performed using Prism 5 software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Sevoflurane exposure improved the survival of septic mice
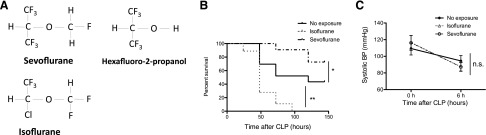
Previously we have shown that prolonged exposure (∼6 h) of isoflurane at the clinical relevant concentration (∼1%) worsened the outcome of septic mice (8). The minimum alveolar concentration (MAC) of volatile anesthetics (Fig. 1A) is defined as the concentration at which 50% of mice do not respond to tail clamping and used as a gauge of the depth of anesthesia. One MAC is 1.3% for isoflurane (35) and 2.8% for sevoflurane in mice (36). Volatile anesthetics with MAC >1 are typically required for surgical anesthesia when they are solely given. 2% of sevoflurane (∼0.7 MAC) corresponds to 0.93% of isoflurane. To our surprise, 2% of sevoflurane exposure for 6 h significantly improved the outcome of septic mice, whereas the equipotent isoflurane (0.9%) isoflurane exposure for the same duration worsened it (Fig. 1B). We measured the tail blood pressure at 6 h after CLP. There was no statistically significant difference in tail blood pressure between volatile anesthetic exposure and nonexposure groups (Fig. 1C), suggesting that hemodynamics in mice were not an explanation for the survival difference.
Figure 1.
Sevoflurane exposure improved the outcome of murine polymicrobial abdominal sepsis. A) Chemical structure of sevoflurane, sevoflurane metabolite (HFIP), and isoflurane. B) The outcomes of polymicrobial abdominal sepsis induced by CLP surgery in WT mice with sevoflurane (2%) exposure (n = 15), with isoflurane (0.9%) exposure (n = 10) for 6 h, and without exposure (n = 15). Statistical significance was evaluated using log-rank test. **P < 0.01. C) Systolic blood pressure of mice at the time of CLP surgery and 6 h after CLP was shown in volatile anesthetic nonexposure group (n = 6), isoflurane group (n = 6), and sevoflurane group (n = 6). Statistical analysis was performed using 2-way ANOVA; n.s, not significant.
Sevoflurane exposure attenuated apoptosis of spleen
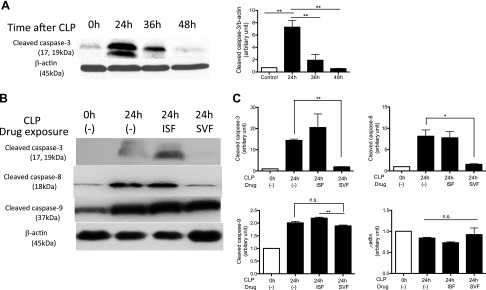
Previously, Hotchkiss et al. (16, 19) demonstrated that the severity of splenic apoptosis correlated with the mortality of septic mice induced by CLP surgery. We examined the degree of splenic apoptosis at 24, 36, and 48 h after CLP surgery. Because we observed the highest cleaved caspase-3 expression at 24 h after CLP (Fig. 2A), we performed the subsequent experiments at this time point. Splenic apoptosis was significantly attenuated by sevoflurane exposure, not by isoflurane exposure (Fig. 2B), which was compatible with the relationship between the survival and the severity of splenic apoptosis shown by Hotchkiss et al. (16, 19).
Figure 2.
The effect of volatile anesthetics on splenic apoptosis after polymicrobial abdominal sepsis. A) Splenic cleaved caspase-3 expression at different time points after CLP surgery. Representative image of 3 independent experiments was shown. B) The degree of splenic apoptosis was probed at 24 h after CLP exposed to different anesthetics [0.9% isoflurane (ISF), 2% sevoflurane (SVF)]. Cleaved caspase-3, -8, and -9 were probed on different blots. Representative image of 3 independent experiments was shown. C) The density of bands was determined using ImageJ and plotted. The density at CLP 0 h, no exposure was considered as 1. Data represent means ± sd of 3 independent experiments. Statistical analysis was performed using 1-way ANOVA with Bonferroni post hoc analysis; n.s., not significant. *P < 0.05, **P < 0.01.
Apoptosis is induced by both extrinsic and intrinsic pathways. Extrinsic and intrinsic pathways are probed with activation of caspase 8 and caspase 9, respectively. The expression of cleaved caspase-8 was significantly attenuated by sevoflurane exposure (Fig. 2B), suggesting that sevoflurane largely affected the extrinsic pathway.
Sevoflurane exposure reduced bacterial loads at 12 and 24 h after CLP
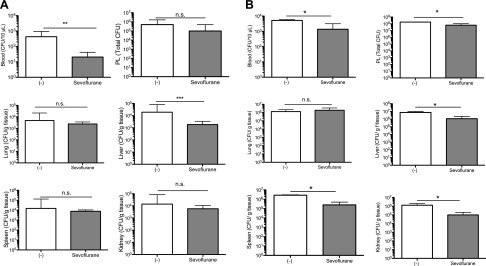
The degree of bacteremia is associated with sepsis survival (37). Thus, we examined the effect of sevoflurane exposure on bacterial loads (Fig. 3). Bacterial loads in liver, lung, spleen, kidney, blood, and peritoneal cavity were examined after CLP surgery. At 12 h, sevoflurane-exposed mice had significantly less bacterial loads in blood and liver, although we did not observe any difference in peritoneal cavity, lung, liver, and kidney (Fig. 3A). At 24 h, sevoflurane-exposed mice had significantly less bacterial loads in blood, peritoneal cavity, liver, spleen, and kidney (Fig. 3B). Because we previously reported that sevoflurane did not affect bacterial growth (38), we speculated that this result would be explained by the effect of sevoflurane on the host immune system. Hotchkiss et al. (16) showed that the inhibition of apoptosis led to the reduction in bacterial loads and better sepsis survival. Our data were in line with this finding.
Figure 3.
The effect of sevoflurane on bacterial loads in sepsis. Bacterial loads were examined at 12 h (A) and 24 h (B) after CLP procedure (n = 4/group). Organs (lung, liver, spleen, and kidney), blood, and peritoneal lavage fluid (PL) were collected. Homogenized organs, blood, and peritoneal fluid were serially diluted and plated on blood agar plates. Plates were cultured at 37°C for 18 h and subjected to counting. Bacterial loads were shown as median + interquartile ranges. For organs, colony forming unit (CFU)/gm; for PL, total CFU; and for blood, CFU/10 µl blood were shown. Statistical analysis was performed using Mann-Whitney U test; n.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Sevoflurane exposure attenuated neutrophil apoptosis
Our data so far showed that sevoflurane exposure was associated with less splenic apoptosis, less bacterial loads, and better survival in the CLP model. However, the mechanism of how sevoflurane attenuated splenic apoptosis and improved survival is not known. In the study by Hotchkiss et al. (16), caspase inhibitors mitigated apoptosis of T cells, leading to the preservation of their cytokine production and innate immune cell function. Sevoflurane-exposed mice showed higher IFN-γ levels in the peritoneal cavity, but not in the blood (Supplemental Fig. S1A, B), suggesting that sevoflurane might have also mitigated T-cell apoptosis, although T cells are not the only source for IFN-γ.
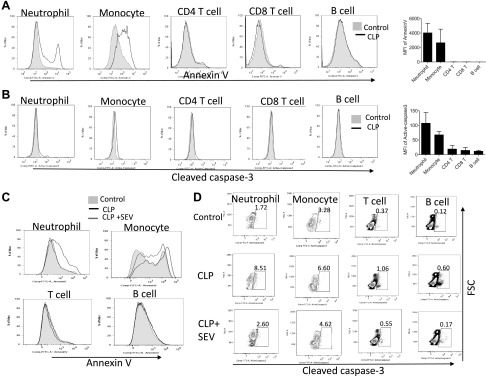
Here we focused on studying the mechanism of how sevoflurane affected apoptosis in sepsis. First, we have examined the type of apoptotic cells in spleen. Because the majority of splenic cells are leukocytes (CD45 positive) (39), we examined the apoptosis of splenic leukocytes by Annexin V and cleaved caspase-3 expression. Based on flow cytometry analysis, neutrophils and monocytes showed higher Annexin V and cleaved caspase-3 expression among them (Fig. 4A, B). CD4 T cells also showed positive cleaved caspase-3 staining (Fig. 4A, B). The number of apoptotic leukocytes probed by cleaved caspase-3 expression is shown in Supplemental Table S1. We observed apoptotic B cells > T cells > neutrophils. The data of apoptotic T cells supported our IFN-γ results and were also in line with the reports by Hotchkiss et al. (16, 19). However, the degree of cleaved caspase-3 expression was the most robust in neutrophils, and the number of apoptotic neutrophils was only slightly lower than that of apoptotic T cells (Supplemental Table S1). We also examined the effect of sevoflurane in this method. Neutrophils were the major cell type whose apoptosis was affected by sevoflurane (Fig. 4C, D). Thus, we focused on the effect of sevoflurane on neutrophil apoptosis.
Figure 4.
Apoptosis of splenic leukocytes in polymicrobial abdominal sepsis. A, B) The apoptosis of splenic leukocytes was probed at 24 h after CLP surgery using Annexin V (A) or cleaved caspase-3 (B). Representative histogram and the mean fluorescence intensity (MFI) of CLP and control mice are shown. Data are shown as means ± sd of 6 replicates. C) The effect of sevoflurane on Annexin V expression in representative histogram. D) The effect of sevoflurane on cleaved caspase 3 expression shown in representative dot plot. Cleaved caspase 3 positive population is shown in percentage.
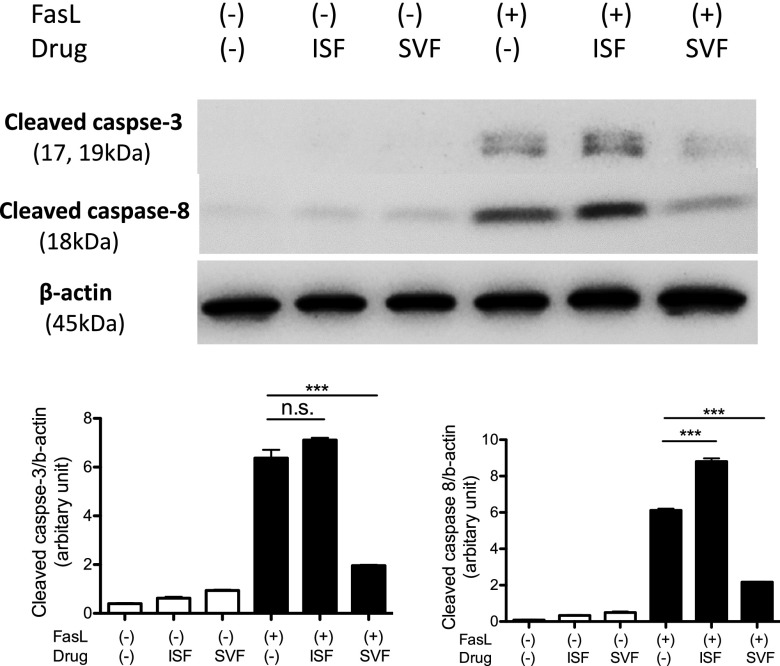
Because sevoflurane significantly attenuated splenic apoptosis via extrinsic pathway in our Western blot analysis (Fig. 2B), we focused on the extrinsic pathway-mediated apoptosis in the subsequent investigation. Ligands such as FasL and TNF-α bind to death receptors and induce apoptosis in neutrophils (40, 41). In contrast, the engagement of β2 integrins to its ligands inhibits apoptosis. Sevoflurane did not affect β2 integrin surface expression on neutrophils (Supplemental Fig. S2). TNF-α level was not affected by sevoflurane (Supplemental Fig. S1A, B). IL-1β, also implicated in apoptosis (42), was not affected by sevoflurane. Thus, we sought a different explanation for the attenuation of splenic apoptosis under sevoflurane anesthesia. Because FasL is a critical player in apoptosis in sepsis (9, 10, 24), we tested the effect of isoflurane and sevoflurane on neutrophil apoptosis induced by FasL in vitro. As in line with the result of splenic apoptosis in CLP sepsis, sevoflurane attenuated FasL-induced neutrophil apoptosis, but isoflurane did not (Fig. 5). Cleaved caspase-8 level was also attenuated by sevoflurane exposure.
Figure 5.
The effect of volatile anesthetics on FasL mediated apoptosis in neutrophils. Bone marrow neutrophils were stimulated with FasL with or without 1% isoflurane (ISF) or 2% sevoflurane (SVF). Representative image of 3 independent experiments was shown. The density of bands was determined 3 times using ImageJ and plotted. Statistical analysis was performed using 1-way ANOVA with Bonferroni post hoc analysis; n.s., not significant. ***P < 0.001.
The effect of volatile anesthetics on Fas receptor–FasL interaction
Because cleaved caspase-8 and cleaved capase-3 expression was similarly attenuated by sevoflurane, we hypothesized that sevoflurane would affect Fas signaling pathway at steps before caspase 8 activation in the extrinsic pathway. The engagement of FasL to the extracellular domain of Fas receptor results in Fas receptor–FasL assembly. Then, the cytoplasmic domain of Fas DD undergoes conformational changes and binds to the adaptor FADD (33). We first tested the effect of volatile anesthetics on the binding of FasL to Fas receptor. Both isoflurane and sevoflurane did not affect the binding of FasL to Fas receptor (Supplemental Fig. S3).
Sevoflurane attenuated the interaction between Fas DD and FADD
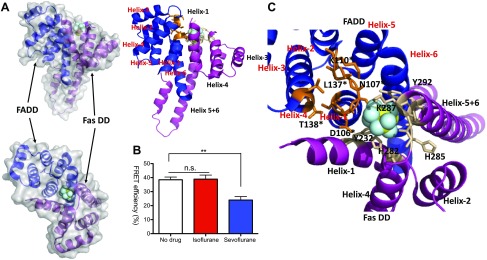
The interaction between Fas DD and FADD is a critical step in assembling the DISC following the binding of FasL to Fas receptor (43). Our structural analysis using SiteMap software predicted that sevoflurane would bind to the interphase between Fas DD and FADD complex (Fig. 6A). Based on this result, we decided to examine the effect of isoflurane and sevoflurane on the interaction between Fas DD and FADD using FRET. We found that sevoflurane significantly attenuated the FRET efficiency, whereas isoflurane did not (Fig. 6B), suggesting that sevoflurane reduced the interaction between Fas DD and FADD. Nearby, amino acids from the docked sevoflurane were shown in Fig. 6C and Supplemental Table S2. Tyr-292 in Fas DD interacted with Asn-107 in FADD via polar interaction to form Fas DD–FADD complex, but sevoflurane was found between these 2 amino acids (Fig. 6C). The functional role of some of the amino acids near the docked sevoflurane on the Fas DD–FADD complex was previously reported in mutagenesis studies. The mutation of Tyr-232 in Fas DD causes autoimmune lymphoproliferative syndrome, characterized by genetic defect in apoptosis (33). The mutation of Asp-106 in FADD hinders the binding between Fas DD and FADD (44). Helix-1 and helix-6 in WT FADD bound to Fas DD (Fig. 6C). The predicted structure of FADD D106A mutant showed that helix-6 shifted significantly away from Fas DD (Supplemental Fig. S4), explaining the mechanism of impaired binding between Fas DD WT and FADD D106A. The presence of sevoflurane might have similarly affected the interaction between Fas DD and FADD.
Figure 6.
Fas DD–FADD interaction with sevoflurane. A) Predicted sevoflurane binding site on FADD–Fas DD complex. B) Using HEK cells transiently transfected with Fas WT-YFP and FADD WT-CFP, FRET experiment was performed with or without 1% isoflurane or 2% sevoflurane. Data were shown as means ± sd of triplicates. Two independent experiments were performed. Statistical analysis was performed using 1-way ANOVA with Bonferroni post hoc analysis; n.s., not significant. **P < 0.01. C) Nearby residues from docked sevoflurane site were shown. Amino acids from FADD are denoted with an asterisk.
Sevoflurane exposure group showed earlier resolution of inflammation
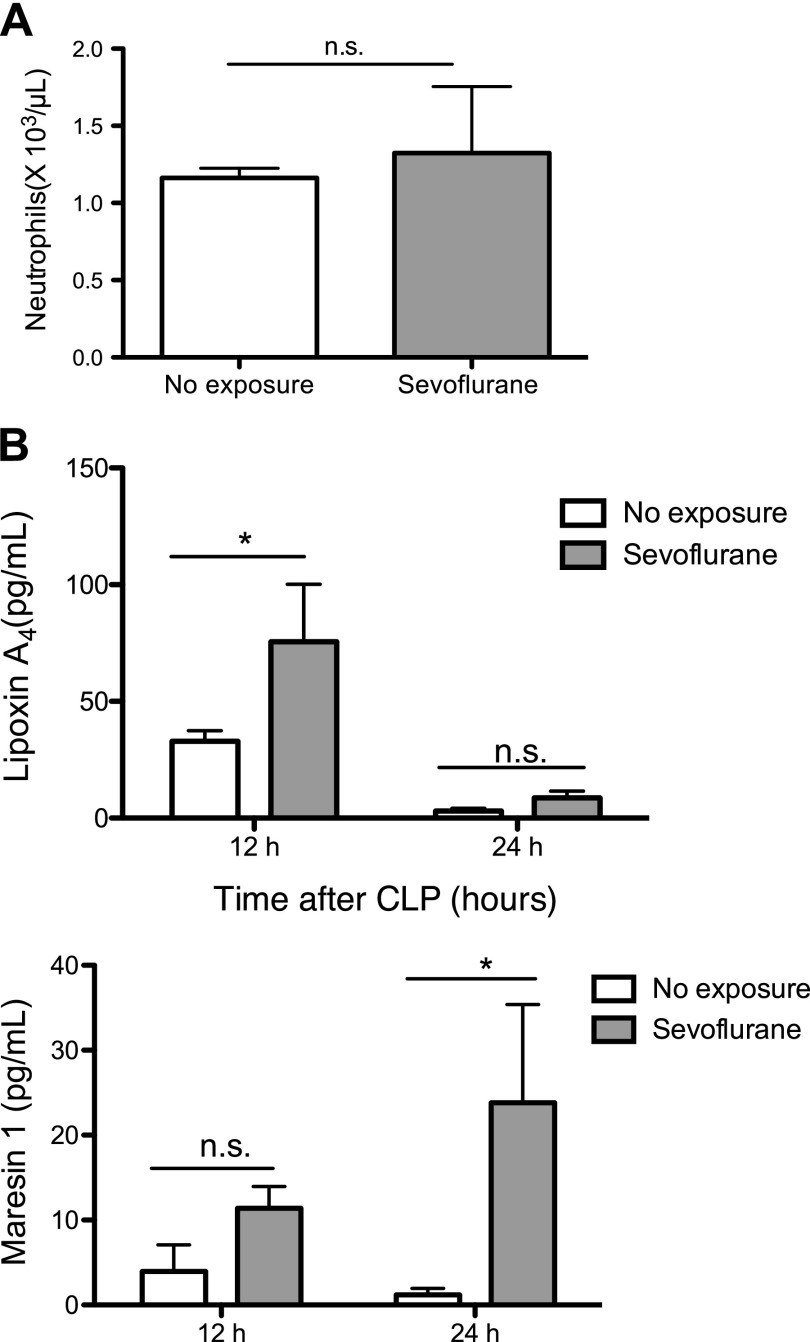
In the previous report of bacterial sepsis, significant neutrophil apoptosis and bone marrow neutrophil depletion were associated with mortality (45). In our study, sevoflurane group attenuated neutrophil apoptosis and less bacteremia in sepsis. This might be explained by the number of neutrophils available for bacterial eradication being larger in the sevoflurane group. We examined if sevoflurane exposure affected the number of circulating neutrophils in the blood. We did not observe statistically significant differences between sevoflurane-exposed and -nonexposed group (Fig. 7A). Examining the number of neutrophils in different organs in the future will clarify the effect of sevoflurane on total neutrophil number.
Figure 7.
The effect of sevoflurane exposure on resolution of inflammation. A) Peripheral blood neutrophil counts at 24 h after CLP. Data were shown as means ± sd of 4 mice. Statistical analysis was performed using Student’s t test; n.s., not significant. B) Peritoneal lavage fluid was collected at 12 and 24 h after CLP for lipidomics. Resolution lipid mediator LXA4 and MaR1 were measured. Data were shown as means ± sd of 4 mice. Statistical analysis was performed using 1-way ANOVA with Bonferroni post hoc analysis; n.s., not significant. *P < 0.05.
Because sevoflurane exposure was associated with better survival with less bacterial loads, we expected that inflammation might have started to resolve earlier in the sevoflurane arm. To test this hypothesis, we examined resolution mediators LXA4 and MaR1 (46). The levels of both mediators were higher in the peritoneal fluid of sevoflurane-exposed mice (Fig. 7B), supporting our hypothesis. IL-10 level was not statistically higher in sevoflurane group, but there was a trend to be higher in sevoflurane group. This result matches with the fact that the interaction of LXA4 to lipoxin receptor (ALX) receptor induces IL-10 (47).
Sevoflurane metabolite hexafluoro-2-propanol did not affect Fas DD–FADD interaction
In contrast to isoflurane, sevoflurane is highly metabolized in body. Its metabolite hexafluoro-2-propanol (HFIP) is detected in blood within 5 min after sevoflurane exposure. Its concentration ranges up to ∼50 µM (48). HFIP is also reported as immunomodulatory (49). HFIP did not affect Fas DD–FADD interaction (Supplemental Fig. S5), suggesting that antiapoptotic effect we observed was due to sevoflurane, not HFIP.
DISCUSSION
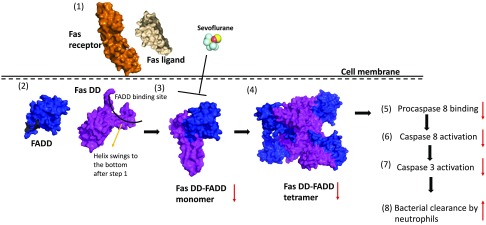
In this study, we have shown that volatile anesthetic sevoflurane exposure significantly improved the survival of septic mice, and sevoflurane attenuated splenic apoptosis in vivo and neutrophil apoptosis in vitro, but isoflurane did not. Furthermore,) sevoflurane attenuated Fas signaling via hindering the interaction between Fas DD and FADD (Fig. 8).
Figure 8.
Scheme of sevoflurane interaction with Fas-mediated apoptosis pathway. FasL (PDB: 4MSV) binds to the extracellular domain of Fas receptor (PDB: 3TJE) (1). Then, Fas DD changes its conformation (2) to bind to FADD (PDB: 3EZQ) (3). Fas-FADD multimers will be more stable than monomer (4) to facilitate procaspace 8 binding (5) that leads to caspase 8 activation (6) followed by caspase-3 activation (7). Red arrows indicate predicated responses by sevoflurane exposure.
The effect of volatile anesthetics on apoptosis was largely studied in the developing brain. A landmark study by Ikonomidou et al. (50) suggested that N-methyl-d-aspartic acid (NMDA) receptor antagonists increased apoptosis in the neonatal rat brain up to postnatal d 14. The same group reported that GABA receptor agonists also increased apoptosis in the neonatal rat brain (51). Although the mechanisms of volatile anesthetics are yet to be determined, NMDA and GABAA receptors are believed to be prime targets for anesthesia in the CNS. Jevtovic-Todorovic et al. (52) showed that early exposure to common anesthetic agents (a combination of midazolam, isoflurane, and nitrous oxide) induced widespread apoptosis in the neonatal brain, along with functional impairments. The study was alarming enough to facilitate worldwide anesthesia neurotoxicity research. Despite this concern, the recently published clinical trial showed that general anesthetics, often provided by volatile anesthetics, did not change the outcome of neurodevelopment in young patients undergoing hernia repair (53). Although the study was conducted in hernia surgery, requiring only a short duration of anesthesia, nonetheless the study suggested that it would be safe to use volatile anesthetics from neurologic perspective (53). Of note, in vivo preclinical studies have been frequently performed using models without pain stimulation or stress condition, and it is known that pain itself induces neuroapoptosis (54). The effect of volatile anesthetics on the apoptosis of immune system was studied on lymphocytes in vitro. Volatile anesthetics only at supra-clinical concentrations or for extremely long durations induced lymphocyte apoptosis (55, 56). In addition, in these studies lymphocytes were cultured without any proinflammatory mediators, which may be different from clinical cases. Our study showed that sevoflurane at the clinically relevant concentration significantly attenuated neutrophil apoptosis in sepsis model. Although we also observed splenic lymphocyte apoptosis, we did not examine the effect of sevoflurane on splenic lymphocyte apoptosis in vitro.
In general, isoflurane and sevoflurane have similar properties as small molecules. Isoflurane and sevoflurane are very similar in structure and size (isoflurane-144 Å3 and sevoflurane-154 Å3) (Fig. 1A) (57). But their minute structural differences may determine their compatibility to binding sites (30, 58) and account for the variable effects of isoflurane and sevoflurane on the clinical outcomes of patients (59). These 2 drugs showed different interaction with Fas DD–FADD. In our recent investigation, we have shown that sevoflurane bound to and inhibited small GTPase Rap1 (60), but isoflurane did not. In contrast, adhesion molecule macrophage-1 antigen was directly inhibited by isoflurane, not by sevoflurane (61). We also showed that HFIP did not contribute to Fas DD–FADD interaction. Not only CF3 chain but also CF chain of sevoflurane must play some role in hindering Fas DD–FADD interaction. The underlying mechanism of difference between isoflurane and sevoflurane is unclear and requires future investigation.
The effect of isoflurane and sevoflurane on the outcome of septic mice induced by CLP surgery was previously examined by Herrmann et al. (62). In the study, authors had used very severe sepsis model induced by 4-time puncture of the cecum with the mortality rate of 75% within the first 48 h. The survival rates of mice exposed to isoflurane (1.5%) and sevoflurane (4.0%) for 2 h were 42 and 83%, respectively, whereas the group without volatile anesthetic exposure (ketamine/xylazine anesthesia) showed the survival rate of 17%. The better survival of isoflurane- or sevoflurane-exposed group was presumably explained by the significant reduction of proinflammatory mediator levels by these volatile anesthetics. In contrast, our model used 2-time puncture of the cecum and was not associated with the mortality within the first 48 h in WT mice (8). Our proinflammatory cytokine levels were not as elevated as in the aforementioned study (8). In our model, isoflurane exposure (1%) for 6 h attenuated bacterial clearance and worsened the outcome of septic mice by attenuating neutrophil function (8). However, isoflurane exposure (1%) for 2 h did not affect the outcome of sepsis, and we postulated that an exposure for 2 h was not long enough for bacteria to amplify to affect the sepsis outcome, although the doubling time for some bacteria can be extremely short (∼20–30 min). The study using isoflurane implies that exposure dose, duration, and severity of the sepsis model may play a significant role in sepsis outcome. In our CLP model using 2-time cecal puncture, however, sevoflurane (2%) exposure for 6 h improved the survival of septic mice. In the study by Herrmann et al., sevoflurane also offered much better survival than isoflurane. This may indicate that sevoflurane does more than attenuating proinflammatory responses. We showed that sevoflurane significantly attenuated neutrophil apoptosis. Although apoptosis is immunologically silent, attenuation of neutrophil apoptosis may help to reserve neutrophils necessary to eradicate infection, and the previous study by Navarini et al. (45) supported this idea. Given we did not examine the kinetics of neutrophil behaviors in various organs and blood, this needs to be clarified in the future. Resolution mediators LXA4 and MaR1 levels were higher in the peritoneal fluid in sevoflurane group, suggesting that mice exposed to sevoflurane demonstrated earlier resolution from inflammation. LXA4 facilitated peritoneal neutrophil phagocytic activity without apoptosis in CLP model (63), and MaR1 attenuated proinflammatory mediators and organ injury (64, 65), which is in line with our survival result. The studies from Herrmann and us are very promising to suggest sevoflurane as a preferred anesthetic drug in the setting of sepsis. So far, no studies have examined the effect of isoflurane vs. sevoflurane on sepsis outcomes in a clinical setting. Although the sepsis surviving campaign has stressed the importance of early intervention against sepsis by fluid resuscitation, antibiotic administration, and infection source removal (66), patients may undergo general anesthesia to control infectious source. These procedures might be performed after established sepsis rather than at the initiation of sepsis, but we did not examine the use of sevoflurane exposure in established sepsis from CLP. Often patients suffering from sepsis may have underlying comorbidities, but we did experiment using mice without comorbidities. Knowing these inherent limitations, it is urgently needed to perform this type of clinical study. Using anesthetic regimens that would potentially improve the outcome of sepsis is critical and novel. In addition, such studies will help us to better understand the complex pathophysiology of sepsis and will possibly lead to the design of more effective therapies. Another consideration is to develop sevoflurane-mimetic drugs devoid of anesthetic effects. Drugs without anesthetic effects would pose significant advantage for wider application. One of important aspects of sevoflurane is that it has an extremely short half-life. Thus, it can be administered when indicated, and it is no longer effective once administration is stopped.
In summary, we have shown that sevoflurane exposure improved the outcome of septic mice. Furthermore, sevoflurane attenuated splenic apoptosis by hindering Fas DD–FADD interaction. Clinical relevance of this observation remains to be determined and earlier clinical investigation is warranted.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported, in part, by Children’s Hospital Medical Center (CHMC) Anesthesia Foundation (to K.Y.), and U.S. National Institutes of Health, National Institute of General Medical Sciences Grant R01GM118277 (to K.Y.). The authors declare no conflicts of interest.
Glossary
- CFP
cyan fluorescent protein
- CLP
cecal ligation and puncture
- DD
death domain
- DISC
death-induced signaling complex
- FADD
Fas-associated death domain
- FRET
fluorescence resonance energy transfer
- HFIP
hexafluoro-2-propanol
- HRP
horseradish peroxidase
- LXA4
lipoxin A4
- MAC
minimum alveolar concentration
- MaR1
maresin 1
- PDB
Protein Data Bank
- PE
phycoerythrin
- Th
T helper
- WT
wild type
- YFP
yellow fluorescent protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Koutsogiannaki. L. Hou, and K. Yuki designed the study, performed experiments, analyzed data, and wrote the manuscript; H. Babazada and T. Okuno designed the study, performed experiments, and analyzed data; N. Blazon-Brown designed the study and performed experiments; and S. G. Soriano and T. Yokomizo wrote the manuscript.
REFERENCES
- 1.Chang C. C., Lin H. C., Lin H. W., Lin H. C. (2010) Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology 113, 279–284 [DOI] [PubMed] [Google Scholar]
- 2.Choi H. J., Adiyani L., Sung J., Choi J. Y., Kim H. B., Kim Y. K., Kwak Y. G., Yoo H., Lee S. O., Han S. H., Kim S. R., Kim T. H., Lee H. M., Chun H. K., Kim J. S., Yoo J. D., Koo H. S., Cho E. H., Lee K. W.; Korean Nosocomial Infections Surveillance System (KONIS) (2016) Five-year decreased incidence of surgical site infections following gastrectomy and prosthetic joint replacement surgery through active surveillance by the Korean Nosocomial Infection Surveillance System. J. Hosp. Infect. 93, 339–346 [DOI] [PubMed] [Google Scholar]
- 3.Hiller J. G., Perry N. J., Poulogiannis G., Riedel B., Sloan E. K. (2018) Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 15, 205–218 [DOI] [PubMed] [Google Scholar]
- 4.Jun I. J., Jo J. Y., Kim J. I., Chin J. H., Kim W. J., Kim H. R., Lee E. H., Choi I. C. (2017) Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci. Rep. 7, 14020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koutsogiannaki S., Bernier R., Tazawa K., Yuki K. (2019) Volatile anesthetic attenuates phagocyte function and worsens bacterial loads in wounds. J. Surg. Res. 233, 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z. F., Lee M. S., Wong C. S., Lu C. H., Huang Y. S., Lin K. T., Lou Y. S., Lin C., Chang Y. C., Lai H. C. (2018) Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology 129, 932–941 [DOI] [PubMed] [Google Scholar]
- 7.Yuki K., Eckenhoff R. G. (2016) Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth. Analg. 123, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutsogiannaki S., Schaefers M. M., Okuno T., Ohba M., Yokomizo T., Priebe G. P., DiNardo J. A., Sulpicio S. G., Yuki K. (2017) From the cover: prolonged exposure to volatile anesthetic isoflurane worsens the outcome of polymicrobial abdominal sepsis. Toxicol. Sci. 156, 402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung C. S., Song G. Y., Lomas J., Simms H. H., Chaudry I. H., Ayala A. (2003) Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J. Leukoc. Biol. 74, 344–351 [DOI] [PubMed] [Google Scholar]
- 10.Chung C. S., Yang S., Song G. Y., Lomas J., Wang P., Simms H. H., Chaudry I. H., Ayala A. (2001) Inhibition of Fas signaling prevents hepatic injury and improves organ blood flow during sepsis. Surgery 130, 339–345 [DOI] [PubMed] [Google Scholar]
- 11.Ayala A., Evans T. A., Chaudry I. H. (1998) Does hepatocellular injury in sepsis involve apoptosis? J. Surg. Res. 76, 165–173 [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss R. S., Swanson P. E., Freeman B. D., Tinsley K. W., Cobb J. P., Matuschak G. M., Buchman T. G., Karl I. E. (1999) Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27, 1230–1251 [DOI] [PubMed] [Google Scholar]
- 13.Elmore S. (2007) Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss R. S., Karl I. E. (2003) The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss R. S., Nicholson D. W. (2006) Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 6, 813–822 [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss R. S., Chang K. C., Swanson P. E., Tinsley K. W., Hui J. J., Klender P., Xanthoudakis S., Roy S., Black C., Grimm E., Aspiotis R., Han Y., Nicholson D. W., Karl I. E. (2000) Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1, 496–501 [DOI] [PubMed] [Google Scholar]
- 17.Wesche D. E., Lomas-Neira J. L., Perl M., Chung C. S., Ayala A. (2005) Leukocyte apoptosis and its significance in sepsis and shock. J. Leukoc. Biol. 78, 325–337 [DOI] [PubMed] [Google Scholar]
- 18.Sônego F., Castanheira F. V., Ferreira R. G., Kanashiro A., Leite C. A., Nascimento D. C., Colón D. F., Borges V. F., Alves-Filho J. C., Cunha F. Q. (2016) Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front. Immunol. 7, 155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotchkiss R. S., Swanson P. E., Knudson C. M., Chang K. C., Cobb J. P., Osborne D. F., Zollner K. M., Buchman T. G., Korsmeyer S. J., Karl I. E. (1999) Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162, 4148–4156 [PubMed] [Google Scholar]
- 20.Oberholzer C., Oberholzer A., Bahjat F. R., Minter R. M., Tannahill C. L., Abouhamze A., LaFace D., Hutchins B., Clare-Salzler M. J., Moldawer L. L. (2001) Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc. Natl. Acad. Sci. USA 98, 11503–11508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Squier M. K., Sehnert A. J., Cohen J. J. (1995) Apoptosis in leukocytes. J. Leukoc. Biol. 57, 2–10 [DOI] [PubMed] [Google Scholar]
- 22.Krammer P. H. (1999) CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv. Immunol. 71, 163–210 [DOI] [PubMed] [Google Scholar]
- 23.Janeway C. A., Jr. (2001) How the immune system works to protect the host from infection: a personal view. Proc. Natl. Acad. Sci. USA 98, 7461–7468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wesche-Soldato D. E., Chung C. S., Lomas-Neira J., Doughty L. A., Gregory S. H., Ayala A. (2005) In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood 106, 2295–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamim C. F., Canetti C., Cunha F. Q., Kunkel S. L., Peters-Golden M. (2005) Opposing and hierarchical roles of leukotrienes in local innate immune versus vascular responses in a model of sepsis. J. Immunol. 174, 1616–1620 [DOI] [PubMed] [Google Scholar]
- 26.Liu J. R., Han X., Soriano S. G., Yuki K. (2014) The role of macrophage 1 antigen in polymicrobial sepsis. Shock 42, 532–539 [DOI] [PubMed] [Google Scholar]
- 27.Okuno T., Koutsogiannaki S., Ohba M., Chamberlain M., Bu W., Lin F. Y., Eckenhoff R. G., Yokomizo T., Yuki K. (2017) Intravenous anesthetic propofol binds to 5-lipoxygenase and attenuates leukotriene B4 production. FASEB J. 31, 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbo C., Yuki K., Demers M., Wagner D. D., Shimaoka M. (2013) Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J. Anesth. 27, 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croker B. A., O’Donnell J. A., Nowell C. J., Metcalf D., Dewson G., Campbell K. J., Rogers K. L., Hu Y., Smyth G. K., Zhang J. G., White M., Lackovic K., Cengia L. H., O’Reilly L. A., Bouillet P., Cory S., Strasser A., Roberts A. W. (2011) Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc. Natl. Acad. Sci. USA 108, 13135–13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuki K., Astrof N. S., Bracken C., Yoo R., Silkworth W., Soriano S. G., Shimaoka M. (2008) The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 22, 4109–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider P., Bodmer J. L., Holler N., Mattmann C., Scuderi P., Terskikh A., Peitsch M. C., Tschopp J. (1997) Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J. Biol. Chem. 272, 18827–18833 [DOI] [PubMed] [Google Scholar]
- 32.Strasser A., Jost P. J., Nagata S. (2009) The many roles of FAS receptor signaling in the immune system. Immunity 30, 180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Yang J. K., Kabaleeswaran V., Rice A. J., Cruz A. C., Park A. Y., Yin Q., Damko E., Jang S. B., Raunser S., Robinson C. V., Siegel R. M., Walz T., Wu H. (2010) The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat. Struct. Mol. Biol. 17, 1324–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skeldal S., Kjaergaard M. M., Alwasel S., Nyengaard J. R. (2015) Establishing a cellular FRET-based fluorescence plate reader assay to monitor proNGF-induced cross-linking of sortilin and the neurotrophin receptor p75(NTR). Int. J. Biochem. Mol. Biol. 6, 17–25 [PMC free article] [PubMed] [Google Scholar]
- 35.Sonner J. M., Gong D., Li J., Eger E. I., II, Laster M. J. (1999) Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth. Analg. 89, 1030–1034 [DOI] [PubMed] [Google Scholar]
- 36.Dahan A., Sarton E., Teppema L., Olievier C., Nieuwenhuijs D., Matthes H. W., Kieffer B. L. (2001) Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology 94, 824–832 [DOI] [PubMed] [Google Scholar]
- 37.Yagupsky P., Nolte F. S. (1990) Quantitative aspects of septicemia. Clin. Microbiol. Rev. 3, 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chamberlain M., Koutsogiannaki S., Schaefers M., Babazada H., Liu R., Yuki K. (2017) The differential effects of anesthetics on bacterial behaviors. PLoS One 12, e0170089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butts C. L., Shukair S. A., Duncan K. M., Harris C. W., Belyavskaya E., Sternberg E. M. (2007) Evaluation of steroid hormone receptor protein expression in intact cells using flow cytometry. Nucl. Recept. Signal. 5, e007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wesche-Soldato D. E., Chung C. S., Gregory S. H., Salazar-Mather T. P., Ayala C. A., Ayala A. (2007) CD8+ T cells promote inflammation and apoptosis in the liver after sepsis: role of Fas-FasL. Am. J. Pathol. 171, 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Kebir D., Filep J. G. (2013) Modulation of neutrophil apoptosis and the resolution of inflammation through β2 integrins. Front. Immunol. 4, 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen J., Xu S., Zhou H., Liu H., Jiang W., Hao J., Hu Z. (2017) IL-1β induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci. Rep. 7, 41067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hymowitz S. G., Dixit V. M. (2010) Unleashing cell death: the Fas-FADD complex. Nat. Struct. Mol. Biol. 17, 1289–1290 [DOI] [PubMed] [Google Scholar]
- 44.Hill J. M., Morisawa G., Kim T., Huang T., Wei Y., Wei Y., Werner M. H. (2004) Identification of an expanded binding surface on the FADD death domain responsible for interaction with CD95/Fas. J. Biol. Chem. 279, 1474–1481 [DOI] [PubMed] [Google Scholar]
- 45.Navarini A. A., Lang K. S., Verschoor A., Recher M., Zinkernagel A. S., Nizet V., Odermatt B., Hengartner H., Zinkernagel R. M. (2009) Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc. Natl. Acad. Sci. USA 106, 7107–7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza D. G., Fagundes C. T., Amaral F. A., Cisalpino D., Sousa L. P., Vieira A. T., Pinho V., Nicoli J. R., Vieira L. Q., Fierro I. M., Teixeira M. M. (2007) The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J. Immunol. 179, 8533–8543 [DOI] [PubMed] [Google Scholar]
- 48.Kharasch E. D., Karol M. D., Lanni C., Sawchuk R. (1995) Clinical sevoflurane metabolism and disposition. I. Sevoflurane and metabolite pharmacokinetics. Anesthesiology 82, 1369–1378 [DOI] [PubMed] [Google Scholar]
- 49.Urner M., Schläpfer M., Herrmann I. K., Hasler M., Schimmer R. R., Booy C., Roth Z’graggen B., Rehrauer H., Aigner F., Minshall R. D., Stark W. J., Beck-Schimmer B. (2015) Insight into the beneficial immunomodulatory mechanism of the sevoflurane metabolite hexafluoro-2-propanol in a rat model of endotoxaemia. Clin. Exp. Immunol. 181, 468–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikonomidou C., Bosch F., Miksa M., Bittigau P., Vöckler J., Dikranian K., Tenkova T. I., Stefovska V., Turski L., Olney J. W. (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283, 70–74 [DOI] [PubMed] [Google Scholar]
- 51.Ikonomidou C., Bittigau P., Ishimaru M. J., Wozniak D. F., Koch C., Genz K., Price M. T., Stefovska V., Hörster F., Tenkova T., Dikranian K., Olney J. W. (2000) Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287, 1056–1060 [DOI] [PubMed] [Google Scholar]
- 52.Jevtovic-Todorovic V., Hartman R. E., Izumi Y., Benshoff N. D., Dikranian K., Zorumski C. F., Olney J. W., Wozniak D. F. (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 23, 876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCann M. E., de Graaff J. C., Dorris L., Disma N., Withington D., Bell G., Grobler A., Stargatt R., Hunt R. W., Sheppard S. J., Marmor J., Giribaldi G., Bellinger D. C., Hartmann P. L., Hardy P., Frawley G., Izzo F., von Ungern Sternberg B. S., Lynn A., Wilton N., Mueller M., Polaner D. M., Absalom A. R., Szmuk P., Morton N., Berde C., Soriano S., Davidson A. J.; GAS Consortium (2019) Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393, 664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shu Y., Zhou Z., Wan Y., Sanders R. D., Li M., Pac-Soo C. K., Maze M., Ma D. (2012) Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol. Dis. 45, 743–750 [DOI] [PubMed] [Google Scholar]
- 55.Loop T., Dovi-Akue D., Frick M., Roesslein M., Egger L., Humar M., Hoetzel A., Schmidt R., Borner C., Pahl H. L., Geiger K. K., Pannen B. H. (2005) Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 102, 1147–1157 [DOI] [PubMed] [Google Scholar]
- 56.Matsuoka H., Kurosawa S., Horinouchi T., Kato M., Hashimoto Y. (2001) Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology 95, 1467–1472 [DOI] [PubMed] [Google Scholar]
- 57.Jenkins A., Greenblatt E. P., Faulkner H. J., Bertaccini E., Light A., Lin A., Andreasen A., Viner A., Trudell J. R., Harrison N. L. (2001) Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J. Neurosci. 21, RC136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuki K., Astrof N. S., Bracken C., Soriano S. G., Shimaoka M. (2010) Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology 113, 600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebert T. J., Robinson B. J., Uhrich T. D., Mackenthun A., Pichotta P. J. (1998) Recovery from sevoflurane anesthesia: a comparison to isoflurane and propofol anesthesia. Anesthesiology 89, 1524–1531 [DOI] [PubMed] [Google Scholar]
- 60.Zha H., Matsunami E., Blazon-Brown N., Koutsogiannaki S., Hou L., Bu W., Babazada H., Odegard K. C., Liu R., Eckenhoff R. G., Yuki K. (2019) Volatile anesthetics affect macrophage phagocytosis. PLoS One 14, e0216163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jung S., Yuki K. (2016) Differential effects of volatile anesthetics on leukocyte integrin macrophage-1 antigen. J. Immunotoxicol. 13, 148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrmann I. K., Castellon M., Schwartz D. E., Hasler M., Urner M., Hu G., Minshall R. D., Beck-Schimmer B. (2013) Volatile anesthetics improve survival after cecal ligation and puncture. Anesthesiology 119, 901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu B., Walker J., Spur B., Rodriguez A., Yin K. (2015) Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot. Essent. Fatty Acids 94, 55–64 [DOI] [PubMed] [Google Scholar]
- 64.Abdulnour R. E., Dalli J., Colby J. K., Krishnamoorthy N., Timmons J. Y., Tan S. H., Colas R. A., Petasis N. A., Serhan C. N., Levy B. D. (2014) Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA 111, 16526–16531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li R., Wang Y., Ma Z., Ma M., Wang D., Xie G., Yin Y., Zhang P., Tao K. (2016) Maresin 1 mitigates inflammatory response and protects mice from sepsis. Mediators Inflamm. 2016, 3798465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukherjee V., Evans L. (2017) Implementation of the Surviving Sepsis Campaign guidelines. Curr. Opin. Crit. Care 23, 412–416 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.