Abstract
Current fructose consumption levels often overwhelm the intestinal capacity to absorb fructose. We investigated the impact of fructose malabsorption on intestinal endocrine function and addressed the role of the microbiota in this process. To answer this question, a mouse model of moderate fructose malabsorption [ketohexokinase mutant (KHK)−/−] and wild-type (WT) littermate mice were used and received a 20%-fructose (KHK-F and WT-F) or 20%-glucose diet. Cholecystokinin (Cck) mRNA and protein expression in the ileum and cecum, as well as preproglucagon (Gcg) and neurotensin (Nts) mRNA expression in the cecum, increased in KHK-F mice. In KHK-F mice, triple-label immunohistochemistry showed major up-regulation of CCK in enteroendocrine cells (EECs) that were glucagon-like peptide-1 (GLP-1)+/Peptide YY (PYY−) in the ileum and colon and GLP-1−/PYY− in the cecum. The cecal microbiota composition was drastically modified in the KHK-F in association with an increase in glucose, propionate, succinate, and lactate concentrations. Antibiotic treatment abolished fructose malabsorption-dependent induction of cecal Cck mRNA expression and, in mouse GLUTag and human NCI-H716 cells, Cck mRNA expression levels increased in response to propionate, both suggesting a microbiota-dependent process. Fructose reaching the lower intestine can modify the composition and metabolism of the microbiota, thereby stimulating the production of CCK from the EECs possibly in response to propionate.—Zhang, X., Grosfeld, A., Williams, E., Vasiliauskas, D., Barretto, S., Smith, L., Mariadassou, M., Philippe, C., Devime, F., Melchior, C., Gourcerol, G., Dourmap, N., Lapaque, N., Larraufie, P., Blottière, H. M., Herberden, C., Gerard, P., Rehfeld, J. F., Ferraris, R. P., Fritton, J. C., Ellero-Simatos, S., Douard, V. Fructose malabsorption induces cholecystokinin expression in the ileum and cecum by changing microbiota composition and metabolism.
Keywords: CCK, KHK, propionate
Fructose intake increased substantially over the past few decades, rising to the current per capita consumption of 50–80 g/d in the United States (1) and increasing in most continents (2). Meanwhile, >50% of the adult population cannot fully absorb a 25-g fructose load (3). Moreover, despite being the age group that consumes the highest amount of fructose (4), children display the greatest susceptibility to fructose malabsorption (5). Fructose malabsorption is commonly associated with bloating, diarrhea, and visceral hypersensitivity as well as depression (6). However, the fundamental mechanisms by which fructose leads to these pathologies remain unknown.
Under conditions of normal fructose intake, fructose absorption occurs mainly in the proximal intestine, in the duodenum and proximal jejunum. Transepithelial fructose transport is mediated by 2 members of the facilitative glucose transporter family, facilitated glucose/fructose transporter (GLUT) 5 and GLUT2, which are expressed in the enterocytes and are localized to their apical and basal membranes, respectively (7, 8). GLUT5 expression is strongly up-regulated by luminal fructose in a ketohexokinase (KHK)-dependent manner and is essential for intestinal fructose transport (9, 10). Yet, intestinal transport of fructose is less efficient than glucose transport (11), and a substantial fraction of fructose reaches the distal regions of the gut (ileum to colon) following excessive fructose intake (12).
Enteroendocrine cells (EECs) distributed along the gastrointestinal (GI) tract release gut peptides in response to luminal stimuli and regulate GI and peripheral physiologic functions. Numerous studies have shown that changes in nutrient flow in the intestine can modify the endocrine function along the GI tract through modifications in the distribution or transcriptional activity of EECs. For instance, the number of glucagon-like peptide-1 (GLP-1)-, glucose-dependent insulinotropic polypeptide (GIP)-, and peptide YY (PYY)-positive cells as well as the density of cholecystokinin (CCK)- and neurotensin (NTS)-positive cells increased in rodent models or in human patients after Roux-en-Y gastric bypass (13–16). Animal models of short bowel syndrome, a strong malabsorption disorder resulting from the resection of the small intestine, exhibited major endocrine adaptations in the GI tract including elevated Pyy and Gcg transcription levels in the colon (17). Interestingly, the transfer of short bowel syndrome patient microbiota into germ-free rats increased the density of GLP-1-positive cells in the colon of recipient animals when compared with rats colonized with conventional microbiota, emphasizing the role of the intestinal ecosystem in intestinal EEC proliferation and differentiation (18). Numerous studies have highlighted the ability of microbiota-derived products to regulate Gcg expression, GLP-1 or PYY secretion, or L-cell (EECs secreting GLP-1 and PYY) transcriptional activities (19–21). However, although it is now well established that most of the EECs secrete more than 1 peptide (22), the ability of the microbiota to modulate specifically the panel of secreted hormones in individual EECs remains unclear.
Recently, the use of the KHK-knockout (KHK−/−) mouse model demonstrated that although basal GLUT5 expression permits low levels of fructose absorption, the KHK−/− mice cannot adapt to increased luminal fructose concentration (9, 23). Therefore, they represent a good model for moderate fructose malabsorption. Here, we used the KHK−/− mice to investigate the impact of fructose malabsorption on the endocrine function of the lower intestine regions (ileum and cecum as well as colon) and to study the role of the intestinal microbiota in mediating fructose effects. We found that fructose reaching the distal regions of the GI tract is able to modify the panel of peptides secreted by the EECs of the ileum, cecum, and colon (normally dedicated to the secretion of PYY and GLP-1), particularly by stimulating the transcription and secretion of CCK. We showed that this effect is, at least in part, mediated by the fructose-induced changes in the microbiota populations and metabolism in the lower intestine.
MATERIALS AND METHODS
Animals
The present protocol received written agreement from the local animal ethics committee [Comité d’Ethique en Expérimentation Animale (COMETHEA) at Jouy-en-Josas, France, APAFIS 1620-2015102618572930v2]. KHK−/− (background: C57/BL6) mice were obtained from Ronaldo P. Ferraris (Rutgers University, Newark, NJ, USA) with permission from Richard J. Johnson (Division of Renal Diseases and Hypertension, University of Colorado–Denver, Aurora, CO, USA) (24). The mice were a global KHK knockout and were lacking both isoforms (A and C) of the KHK as previously described by Diggle et al. (25). All KHK−/− and wild-type (WT) mice were littermates. Only males were used, and all feeding experiments started with mice aged from 4 to 7 wk.
Experimental design
In the first experiment, WT and KHK−/− mice (6–8 mice/group) received for 8 wk either 20% of fructose (WT-F and KHK-F groups, respectively) or 20% of glucose (WT-G and KHK-G groups) experimental isocaloric diets based on a standard American Institute of Nutrition 93G formula (Research Diets, New Brunswick, NJ, USA) and containing in addition to starch either 20% fructose or 20% glucose, respectively.
In the second experiment, same-aged chow-fed WT and KHK−/− mice were divided into 2 groups (5–7 mice/group) of similar body weight and received for 7 d either plain water or antibiotic (AB) mix in their bottle. The AB mix was composed of vancomycin (45 µg/ml), streptomycin (1 mg/ml), colistin (1 mg/ml), and ampicillin (1 mg/ml). The mice were individually housed. Body weight and food and drink intake measurements as well as AB mix and litter changes were done every 2 d. After the first 7 d, the AB treatment was maintained, and both AB and control water groups received 20% fructose diet for 3 wk. The shortening of this fructose feeding period to 3 wk was first validated by showing that the effects on peptide expression levels and the microbiota composition were similar after 3 or 8 wk of fructose feeding (Supplemental Fig. S1A, B).
For all experiments, fed mice were euthanized under anesthesia. Blood was collected at the level of the portal vein. Cecum was sampled and weighed. The entire small intestine and colon were sampled and flushed with cold PBS. Sections of 1 cm of duodenum, midjejunum, ileum, and proximal colon were stored with RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) at −80°C until RNA extraction or fixed in 4% paraformaldehyde for immunohistology. The cecal content was sampled and stored at −80°C for bacterial DNA and metabolite analysis. The cecum epithelium was flushed, sectioned, and stored as described above for RNA extraction, immunohistology, or CCK content analysis.
Tissue RNA extraction and real-time quantitative PCR
mRNA from duodenum, jejunum, cecum, and colon sections was extracted using MirVana Isolation Kit (Thermo Fisher Scientific). Total RNA (2 µg) was reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). To quantify gut peptide mRNA, real-time quantitative PCR (qPCR) using SYBR Green chemistry was performed (Thermo Fisher Scientific). The β-actin housekeeping gene was used to normalize the mRNA abundance. Primers are listed in Table 1. For the EEC differentiation markers [atonal bHLH transcription factor (Math) 1, neurogenin (Neurog) 3, neuronal differentiation (NeuroD) 1, paired box (Pax) 4, and Pax6], a preamplification was performed with predesigned TaqMan primers [Mm00476035_s1, Mn00437606_s1, Mm01946604_s1, Mm01159036_m1, and Mm00443081_m1 (Assays-on-Demand Gene Expression; Thermo Fisher Scientific)] using TaqMan PreAmp Master Mix (Thermo Fisher Scientific). The quantification of the preamplified targeted genes was achieved using real-time qPCR based on TaqMan gene expression assays with the same predesigned TaqMan primers. The β-actin (Mm00607939_s1) gene was used to normalize the mRNA abundance.
TABLE 1.
Primers used for real-time qRT-PCR
| Gene bank accession number | Primers, 5′–3′ |
||
|---|---|---|---|
| Gene name (abbreviation) | Forward | Reverse | |
| Mouse primers | |||
| β Actin (β-actin) | NM_007393 | CTAAGGCCAACCGTGAAAAG | ACCAGAGGCATACAGGGACA |
| Gastric inhibitory polypeptide (Gip) | NM_008119 | CAGGTAGGAGGAGAAGACCTCAT | CCTAGATTGTGTCCCCTAGCC |
| Cholecystokinin (Cck) | NM_031161 | GCTGATTTCCCCATCCAAA | GCTTCTGCAGGGACTACCG |
| Peptide YY (Pyy) | NM_145435 | CCTACCCTGCCAAACCAG | GGACATCTCTTTTTCCATACCG |
| Preproglucagon (Gcg) | AF276754 | CACGCCCTTCAAGACACAG | GTCCTCATGCGCTTCTGTC |
| Leptin (Lep) | NM_008493 | CAGGATCAATGACATTTCACACA | GCTGGTGAGGACCTGTTGAT |
| Neurotensin (Nts) | NM_024435 | AGCTGGTGTGCCTGACTCTC | CCAGGGCTCTCACATCTTCT |
| Somatostatin (Sst) | NM_009215 | CCCAGACTCCGTCAGTTTCT | GGGCATCATTCTCTGTCTGG |
| Secretin (Sct) | NM_001309439 | CGATGCTACTGCTGTTGCTG | TCTGAGTGTCTTGGGGTCCT |
| Tryptophan hydroxylase 1 (Tph1) | NM_009414 | CACAGTTCAGATCCCCTCTACA | GAACGTGGCCTAGGAGTTCA |
| Human primers | |||
| β Actin (β-actin) | NM_001101 | ATTGGCAATGAGCGGTTC | GGATGCCACAGGACTCCA |
| Preproglucagon (Gcg) | NM_002054 | TCTGTTCTACAGCACACTACCAGA | AGCTGCCTTGTACCAGCATT |
| Neurotensin (Nts) | NM_006183 | TGACCAATATGCATACATCAAAGA | CTTCATGAACTTCTCCTGTTTCC |
| Cholecystokinin (Cck) | NM_000729 | AGAGAACGGATGGCGAGTC | CATTCGTCCAGAAGGAGCTT |
Immunohistochemistry
Ileum, cecum, and colon were fixed in fresh 4% paraformaldehyde in PBS (pH 7.3) overnight at 4°C and then embedded in paraffin. The 5-µm sections were obtained. After paraffin removal, heat-induced antigen retrieval was performed in 10 mM trisodium citrate buffer (pH 6.0) for 40 min at 96°C. The sections were rinsed in PBS and blocked in 1/100 mouse IG blocking reagent (Vector Laboratories, Burlingame, CA, USA) diluted in 1% normal donkey serum for 1 h at room temperature. The sections were then incubated with primary antibodies: rabbit anti-CCK (sc2037, 1:100; Santa Cruz Biotechnology, Dallas, TX, USA), mouse anti–GLP-1 (ab23472, 1:400, Abcam, Cambridge, United Kingdom, USA), and goat anti-PYY (sc47318, 1:50; Santa Cruz Biotechnology) for 16 h at 4°C in 1% donkey serum in PBS. After PBS rinses, the slides were incubated with the appropriate secondary antibodies (Thermo Fisher Scientific): Alexa Fluor 555-conjugated donkey anti-rabbit IgG (1:750), Alexa Fluor 488y, facilitated glucose/fructose transporter conjugated donkey anti-mouse IgG (1:700), and Alexa Fluor 647y, facilitated glucose/fructose transporter conjugated donkey anti-goat IgG (1:500) for 1 h at room temperature. Stained sections were imaged using laser scanning confocal microscopy (SP8; Leica Microsystems, Wetzlar, Germany). All sections stained with the same marker combination were imaged using the same settings. Nonspecific staining with secondary antibodies was consistently negligible. For counting cells, the immune-stained sections were scanned using a high-capacity digital slide scanner (Pannoramic Scan; 3DHistech, Budapest, Hungary) and images were analyzed using 3DHistech CaseViewer software. The counts were performed blind. Counts were based on averages of 2–4 intestinal cross-sections from each limb for each animal. For each section, the surface of the tissue section was measured, and the data are presented as the number of cells per square millimeter.
Cecal content analysis
CCK peptide concentrations in cecal tissue extracts were measured using a specific in-house radioimmunoassay as previously detailed by Rehfeld (26).
The cecal contents from WT and KHK−/− mice fed fructose with or without ABs were analyzed for short-chain fatty acids (SCFAs) and branched-chain fatty acids by gas chromatography as previously described by Lan et al. (27).
Cecal contents were analyzed by NMR spectroscopy (1H-NMR). Briefly 80–100 mg of the cecal content was extracted with 500 μl phosphate buffer (0.2 M, pH 7.4) in D2O containing 1% (w/v) of sodium 3-(trimethylsilyl) propionate. After vortexing, each sample was subjected to a freeze-thaw cycle in liquid nitrogen and subsequently homogenized with a tissue lyser (Qiagen, Hilden, Germany) at 20 Hz for 40 s followed by centrifugation at 10,000 g for 10 min at 4°C. The supernatants were collected, and the remaining pellet was extracted once more as described above. Supernatants obtained from the 2 extractions were combined and centrifuged at 10,000 g for 10 min at 4°C. A total of 600 μl supernatant was transferred into an NMR tube (outer diameter, 5 mm) pending NMR analysis. All 1H-NMR spectra were obtained on a Bruker DRX-600-Avance NMR spectrometer (Bruker, Billerica, MA, USA) on the Axiom metabolomics platform (MetaToul, Toulouse, France) as previously described in detail by Lukowicz et al. (28).
Microbiota DNA extraction and real-time qPCR quantification
All bacteria DNA was extracted from cecum content using Gnome DNA Isolation Kit (MP Biomedicals, Santa Ana, CA, USA). Quantifications of the total bacteria DNA and specific phyla were performed on 10 ng of DNA by real-time qPCR following the procedure previously described by Furet et al. (29). Real-time qPCR were performed using the following probes specific of the 4 main phyla: Firmicutes (928F-Firm-5′-TGAAACTYAAAGGAATTGACG-3′ and 1040-FirmR-5′-ACCATGCACCACCTGTC-3′), Bacteroidetes (MIBF-5′-GGCGACCGGCGCACGGG-3′ and MIBR-5′-GRCCTTCCTCTCAGAACCC-3′), Proteobacteria (1080γF-5′-TCGTCAGCTCGTGTYGTGA-3′ and γ1202R-5′-CGTAAGGGCCATGATG-3′) and Actinobacteria (Act920F3-5′-TACGGCCGCAAGGCTA-3′ and Act1200R-5′ -TCRTCCCCACCTTCCTCCG-3′) with SYBR Green PCR 2× Master Mix (Thermo Fisher Scientific) as previously described. All real-time qPCR reactions were conducted in a final volume of 25 μl with 0.2 μM final concentration of each primer and 5 μl of appropriate dilutions of DNA samples. Amplifications were carried out using the following ramping profile: 1 cycle at 95°C for 10 min followed by 40 cycles of 95°C for 30 s and 61°C for 1 min. The total number of bacteria was inferred from averaged standard curves as previously described by refs. 29–31. For the quantification of Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, standard curves were generated from serial dilutions of a known concentration of genomic DNA from Lactobacillus acidophilus, Bacteroides fragilis, Bifidobacterium adolescentis, and Escherichia coli, respectively.
16S rRNA sequencing
The V3-V4 region of the 16S rRNA genes was amplified using MolTaq (Molzym, Bremen, Germany) and the primers V3F: 5′-TACGGRAGGCAGCAG-3′ and V4R: 5′-ATCTTACCAGGGTATCTAATCCT-3′ (32). Purified amplicons were sequenced using the MiSeq sequencing technology (Illumina, San Diego, CA, USA) at the GeT-PLaGe platform (Genotoul, Toulouse, France). Paired-end reads obtained from MiSeq sequencing were analyzed using the Galaxy-supported pipeline named Find, Rapidly, Operational Taxonomic Units (OTUs) with Galaxy Solution (FROGS) (33). For the preprocessing, reads with length ≥380 bp were kept. The clustering and chimera removal tools followed the guidelines of FROGS (33). Assignment was performed using the Silva database (https://www.arb-silva.de/). OTUs with abundances lower than 0.005% were removed from the analysis (34).
GLUTag and NCI-H716 cell culture and peptide mRNA expression
The GLUTag cell line provided by Daniel Drucker (Mount Sinai Hospital, Toronto, ON, Canada) and NCI-H716 (CCL-251; ATCC, Manassas, VA, USA) were kept at 37°C and 5% CO2 at 95% humidity until 80% confluent, then split and transferred to fresh T75 flasks. GLUTag cell maintenance medium composition was DMEM with 1 g/L glucose (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS), 1% penicillin (10,000 U/ml) and streptomycin (10,000 µg/ml). NCI-H716 cell maintenance medium composition was RPMI-1640 with 2 g/L glucose (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS), 1% penicillin (10,000 U/ml) and streptomycin (10,000 µg/ml) and 2 mM l-glutamine. For the assay, 24–48 h before the study, GLUTag cells were plated onto 6-well plates precoated with matrigel basement membrane (BD Biosciences, San Jose, CA, USA) and NCI-H716 cells were seeded onto 12-well standard plates, both with the same medium as for their maintenance. At ∼80% confluence, GLUTag cells were incubated in 1 mM glucose DMEM and NCI-H716 cells were incubated in 1 mM glucose and 1% glutamine RPMI. Each medium was supplemented with 1% FBS and 1% penicillin and streptomycin. For the experiments, the medium was used as a control 1 mM glucose or were completed to reach 25 mM glucose, 25 mM fructose, 10 mM lactate, 2 mM propionate, 2 mM succinate, or 25 mM glucose + 10 μM forskolin + 10 μM 3-isobutyl-1-methylxanthine (positive control) for 24 h. Then, the cell pellets were used for mRNA extraction with Trizol (Thermo Fisher Scientific) following the manufacturer’s protocol. These experiments were repeated 3 times for the GLUTag (for each experiment, each condition was done in triplicate) and 4 times for the NCI-H716 cells. Reverse transcription and real-time qPCR were performed as previously described and the primers are listed in Table 1.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software v.5 (GraphPad Software, La Jolla CA, USA), and data were expressed as means ± sem. For all the means obtained from in vivo experiments with more than 2 groups, 1-way ANOVA was performed followed by Tukey’s post hoc test after initial ANOVA showed significant effects. For means of 2 groups, a Student’s t test was performed. Means of peptide expression data from GLUTag and NCI-H716 were compared by Kruskal-Wallis test followed by Dunn’s multiple comparison test.
16S sequencing data were analyzed using the Phyloseq (29) and ggplot2 (35) R packages in addition to custom scripts. Samples were rarefied to even sampling depths before computing within-samples compositional diversities (observed richness and inverse Simpson) and between-samples compositional diversity (Bray-Curtis). Principal Coordinate Analysis was also performed on Bray-Curtis dissimilarities to obtain a 2-dimensional representation of the samples. Alpha diversity data were analyzed using 1-way ANOVA. A permutational multivariate ANOVA test was performed on the Bray-Curtis matrices using 9999 random permutations and at a significance level of 0.01. Phylum relative abundances were compared using Kruskal-Wallis test followed by Dunn’s test because they did not satisfy the normal assumption of standard ANOVA. Family-level abundances of KHK-F group were compared with all other groups using Wilcoxon test, and P values were adjusted using the Holm correction. Raw, unrarefied OTU counts were used to produce relative abundance graphs and to find taxa with significantly different abundances in KHK-F and WT-F. A negative binomial model was fit to each OTU, using DESeq2 (36) with default parameters, to estimate abundance log-fold changes (FCs). Values of P were corrected for multiple testing using the BH procedure to control the false-discovery rate and significant OTUs were selected based on effect size (FC >8 or FC <1/8), adjusted P value (<0.001), and prevalence (relative abundance >0.1% in at least half the samples).
1H-NMR data were mean-centered prior to analysis using principal component analysis (PCA) and orthogonal projection on latent structure-discriminant analysis (O-PLS-DA) as previously described by Lukowicz et al. (28).
RESULTS
Fructose malabsorption impacts intestinal parameters
As expected, in the WT mice, fructose diet (WT-F) induced a significant up-regulation of Glut5 expression in the jejunum compared with the mice fed glucose diet (WT-G) (Supplemental Fig. S2A). The deletion of KHK gene prevented this induction of Glut5 expression by fructose. In the KHK−/− mice, fructose feeding (KHK-F) was associated with a significant increase in the cecum weight and an enlargement of cecum epithelium relative to the glucose-fed KHK mutants (KHK-G) or the WT-F or WT-G groups (Supplemental Fig. S2B, C). Morphologic changes in the ileum and cecum tissues were also observed, such as significant increase in villus lengths in the ileum and crypt + villus lengths in the cecum of the KHK-F compared with the WT-F mice (Supplemental Table S1). No differences in the colon crypt depth were observed. At the physiology level, neither the deletion of KHK nor the fructose diet affected body weight, daily food intake, or epididymal adiposity (Supplemental Table S2).
Fructose malabsorption impacts gut peptide expression pattern along the GI tract
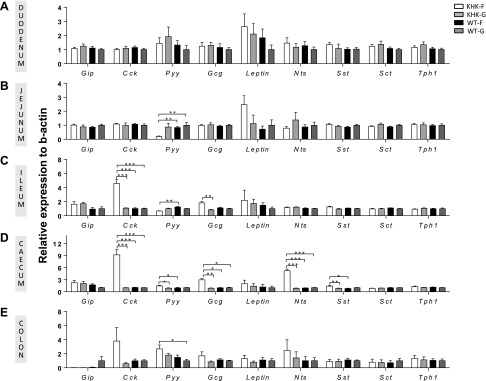
Previous studies demonstrated that changes in nutrient flux could modify gut peptide expression in specific areas of the gut (13–16). Thus, we investigated the impact of fructose malabsorption on the expression pattern of the main gut peptides along the GI tract. In the duodenum and jejunum, no significant changes in the gut peptide expression were observed among the 4 groups except for a decrease in Pyy mRNA expression in the jejunum of KHK-F when compared with WT-F and WT-G mice (Fig. 1A, B). In contrast, in the ileum and the cecum, fructose malabsorption was associated with major changes in peptide mRNA expression (Fig. 1C, D). The expression levels of Cck mRNA was 5-fold and 9-fold greater in the ileum and cecum, respectively, of KHK-F than in the 3 other groups of mice. The expression of Gcg was 1.8-fold and 3-fold higher in the ileum and cecum, respectively, of KHK-F compared with the 3 other groups. Although Nts transcript levels were similar in the ileum in the 4 groups, its expression was up-regulated 6-fold in the cecum of KHK-F when compared with the 3 other groups. More modest increases in Pyy (1.6-fold) and Sst (encoding for somatostatin) (1.7-fold) mRNA expression were also measured in the cecum of KHK-F mice compared with the KHK-G and WT-F group. In the colon, a more variable expression was observed for most of the peptides (Fig. 1E) and only Pyy mRNA expression was significantly higher in the KHK-F when compared with WT-G mice. GIP, Sct (encoding for secretin), and Tph1 (encoding for tryptophan hydroxylase 1, an enzyme of the serotonin synthesis pathway) mRNA expression levels did not change in response to fructose or fructose malabsorption in any of the GI segments.
Figure 1.
KHK−/− and WT mice were fed 20% fructose (KHK-F or WT-F) or 20% glucose diet (KHK-G or WT-G) for 8 wk. The mRNA expression levels of the main entero-hormones were measured in KHK-F, KHK-G, WT-F, and WT-G mice in the duodenum (A), the jejunum (B), the ileum, (C), the cecum (D), and the proximal colon (E). The relative values were normalized to WT-G levels (n = 6–8/group). All values are means ± sem. Means were compared by 1-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001.
Fructose malabsorption impacts CCK-positive cell type population in the lower intestine
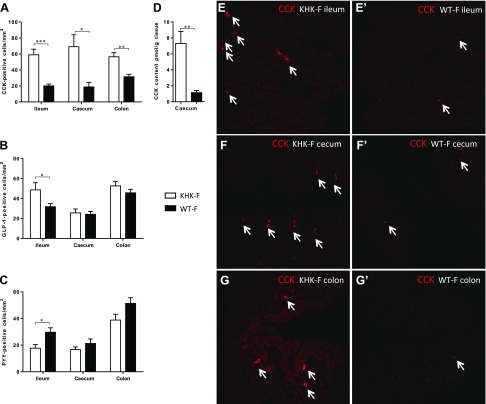
The l-cells can synthesize GLP-1 (expressed from Gcg gene) and PYY. These cells are mainly localized in the lower intestine (ileum, cecum, and colon), whereas CCK is normally synthesized by other EECs, the I-cells mainly localized in the duodenum and the proximal jejunum. However, recent studies demonstrated that more than 1 peptide can be expressed in each L- or I-cells (37). To determine whether the changes in peptide expression were supported by an increase in EEC number or a shift in the pattern of peptide expression in existing cells, we quantified the number of CCK-, GLP-1-, and PPY-positive cells (CCK+, GLP-1+ and PYY+) in the ileum, cecum, and colon of the KHK-F and WT-F mice. Triple labeling by immunohistochemistry of CCK, GLP-1, and PYY was also performed to determine which cell type supports the drastic CCK increase in KHK-F. The density of CCK+ cells increased significantly in the ileum, cecum, and colon of KHK-F relative to WT-F mice (Fig. 2A). In the ileum, fructose malabsorption was also associated with a significant increase in GLP-1+ cell density (Fig. 2B), whereas the PYY+ cell density significantly decreased (Fig. 2C). No change in density was observed in the cecum and colon for GLP-1+ and PYY+ cells. Importantly, the increased Cck mRNA levels and CCK+ cell density translate to a 7-fold increase in the CCK peptide content in the cecal tissue of the KHK-F when compared to the WT-F (Fig. 2D). The increase of CCK+ cell density in the ileum, cecum, and colon in the KHK-F compared with WT-F is illustrated in Fig. 2E–G’.
Figure 2.
KHK−/− and WT mice were fed 20% fructose (KHK-F and WT-F, respectively) for 8 wk. A–C) Distribution of CCK- (A), GLP-1- (B), and PYY-positive EECs (C) per section area (square millimeters) of mucosa in the ileum, cecum, and colon of KHK-F and WT-F. D) CCK peptide content in cecum tissue of KHK-F and WT-F. E–G′) Representative immunofluorescence staining of CCK-positive cells (red) in ileum (E, E′), cecum (F, F′), and colon (G, G′) of KHK-F (E, F, G) and WT-F (E′, F′, G′) mice (n = 5–6/group). Original magnification value, ×158. All values are means ± sem. Means were compared with Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
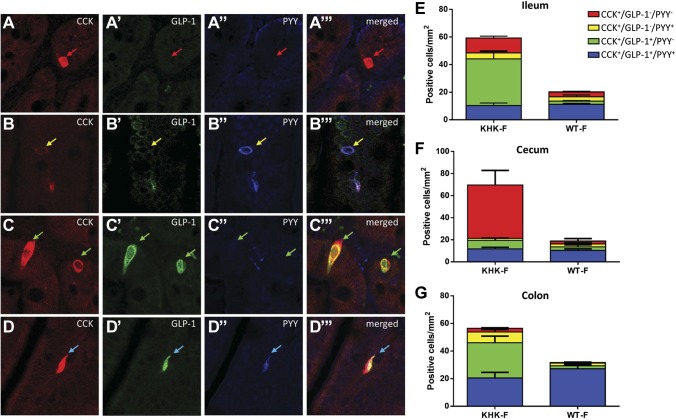
Triple staining used to investigate the colocalization of CCK, GLP-1, and PYY showed 4 cell types of CCK-immunoreactive cells in the distal GI tract: CCK+ cells coexpressing neither GLP-1 nor PYY (CCK+/GLP-1−/PYY−) (Fig. 3A–A‴), CCK+ cells coexpressing PYY but not GLP-1 (CCK+/GLP-1−/PYY+) (Fig. 3B–B‴), CCK+ cells coexpressing GLP-1 but not PPY (CCK+/GLP-1+/PYY−) (Fig. 3C–C‴), and CCK+ cells coexpressing both GLP-1 and PYY (CCK+/GLP-1+/PYY+) (Fig. 3D–D‴).
Figure 3.
KHK−/− and WT mice were fed 20% fructose (KHK-F or WT-F) for 8 wk. A–D‴) Representative triple-immunofluorescence staining of CCK (red), GLP-1 (green), and PYY (blue) in the ileum of KHK-F mice. Each set of panels (e.g., A–A‴) shows the same field of view with CCK, GLP-1, and PYY channels separately and a merged image. Red arrows show EECs in which CCK only is expressed (CCK+/GLP-1−/PYY−), yellow arrows show EECs in which CCK and PYY are both expressed (CCK+/GLP-1−/PYY+), green arrows show EECs in which CCK and GLP-1 are both expressed (CCK+/GLP-1+/PYY−), and blue arrows show EECs in which CCK, GLP-1, and PYY (CCK+/GLP-1+/PYY+) are expressed. E–G) Quantification of the density of the different populations of CCK+ cells in the ileum (E), cecum (F), and colon (G) of KHK-F and WT-F (n = 5–6/group). All values are means ± sem. The counts and statistics are presented in Supplemental Table S3.
In the WT-F group, the predominant CCK+ cell population in the ileum, cecum, and colon was the triple-positive cells (CCK+/GLP-1+/PYY+) (Fig. 3E–G and Supplemental Table S3). In the KHK-F group, the density of this cell type remained the same as in WT-F (Fig. 3E–G). Rather, the expansion of the CCK+ cell population was due to density changes in other types of CCK+-EEC types. In the ileum and the colon, this expansion was primarily due to the increased density of CCK+/GLP-1+/PYY− cells, whereas in the cecum, it was almost entirely due to a drastic increase in density of CCK+/GLP-1−/PYY− cells (Fig. 3E–G).
Differentiation of intestinal progenitor cells in the enteroendocrine lineage involves coordinated expression of various transcription factors. We tested a number of them for changes in expression (Supplemental Fig. S3). Neurod1, a late marker for the differentiation of intestinal progenitor cells in the EEC lineage, was significantly up-regulated in the cecum of the KHK-F when compared with WT-G mice. In addition, Pax6, which acts downstream of Neurod1 and is a key transcription factor for GLP-1-positive cell differentiation, was significantly up-regulated in the cecum of the KHK-F when compared with the other 3 groups.
Fructose malabsorption impacts the cecal microbiota composition and metabolism
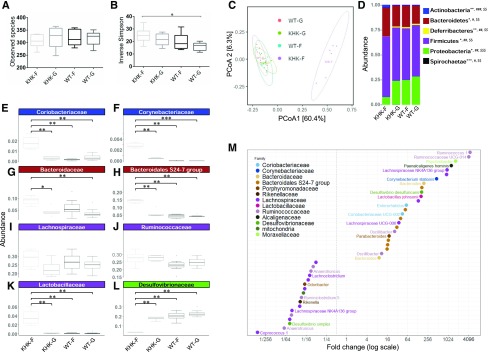
Our data supports the idea that malabsorbed fructose reaches the lower intestinal tract. This could potentially affect the local microbiota populations. To address this possibility, we investigated the cecal microbial composition based on the sequencing of V3–V4 regions of microbial 16S rRNA. At the OTU level, no significant change in richness was observed among the 4 groups of mice (Fig. 4A), although the inverse Simpson index indicated a mildly significantly higher number of effective taxa in the KHK-F mice compared with WT-G (Fig. 4B). Bray-Curtis–derived dissimilarity matrices indicated that fructose malabsorption (KHK-F) contributed significantly (P <0.01) to the different compositions observed in microbial communities of the cecum (Fig. 4C). The relative abundance of the 5 main phyla, Actinobacteria, Bacteroidetes, Deferribacteres, Firmicutes, and Proteobacteria, was significantly affected by fructose malabsorption (Fig. 4D). At the phylum level, the impact of fructose malabsorption was striking at the level of minor phyla with a marked relative enrichment of Actinobacteria and a major decrease of the relative abundance of the Proteobacteria and the Deferribacteres (Fig. 4D). The differences in Actinobacteria and Proteobacteria were validated by real-time qPCR (Supplemental Fig. S1B). At lower taxonomic levels, main differences were also observed when comparing KHK-F mice with the 3 other groups. The Fig. 4E–L represent the most abundant families from the 4 major phyla described above. Within Actinobacteria, at the family level, KHK-F mice displayed a major significant increase in the relative abundance of Coriobacteriaceae and Corynebacteriaceae, the latter family being almost undetectable in the 3 other groups (Fig. 4E, F). Within Bacteroidetes, we observed a higher abundance of the Bacteroidales S24-7 group in KHK-F when compared with the 3 other groups and of Bacteroidacae in KHK-F when compared with KHK-G and WT-G mice (Fig. 4G, H). Within Firmicutes, despite no overall statistically significant change in relative abundance of the 2 major families, Lachnospiraceae and Ruminococcaceae, at the genera and species level, the relative abundance of some members within both families were strongly increased in the KHK-F when compared with WT-F (Fig. 4I, J, M). In addition, relative abundance of Lactobacillaceae increased drastically in KHK-F (Fig. 4K) when compared with the 3 other groups. Differential abundance analysis at the OTU level revealed that this enhancement in the family of Lactobacillaceae is largely supported by increase in the relative abundance of Lactobacillus johnsonii (Fig. 4M). In line with the observations at the level of Proteobacteria phylum, the abundance of Desulfovibrionaceae family was significantly reduced in the KHK-F as the result of a major decrease in Desulfovibrio simplex and despite greater D. desulfuricans abundance (Fig. 4L, M). Differential abundance analysis identified 25 highly significant species or OTUs (adjusted P < 0.001) that were both strongly (FC >8) positively differentially abundant between KHK-F and WT-F (Fig. 4M) and highly prevalent (abundance >0.1% in at least half of the samples). Among them, 6 species or OTUs exhibited a FC >1000, meaning that they were almost exclusively found in KHK-F mice. Similarly, 17 species or OTUs had highly reduced abundance (FC <1/8) in KHK-F relative to WT-F.
Figure 4.
Microbiota composition of the cecum content collected from KHK−/− or WT mice fed 20% fructose (KHK-F or WT-F) or 20% glucose (KHK-G or WT-G) diet for 8 wk (n = 6–8/group). Analysis was based on 16S rDNA sequencing (region V3-V4). A, B) Observed species richness (A) and inverse Simpson index (B) as indicators of α-diversity. C) Principal coordinates analysis (PCoA) of Bray-Curtis compositional dissimilarity at the OTU level. D) Mean relative abundance at phylum level in cecum content of each group. E–L) The most abundant families from Actinobacteria (E, F), Bacteroidetes (G, H), Firmicutes (I–K), and Proteobacteria (L) phyla. M) Graphic representation of differentially abundant OTUs between KHK-F and WT-F having a large (FC >8 or FC <1/8) and significant (P < 0.001) effect size in addition to high relative abundances (>0.1% in at least half the samples). Each OTU is represented by a dot and colored according to its taxonomic classification at the family level. Taxonomy at the genus or species level is also indicated, when available, next to each OTU. A logarithmic scale (log-2) was used for the x axis. Observed species richness and inverse Simpson index values are means ± sem. Means were compared by 1-way ANOVA followed by Tukey’s post hoc test. *P < 0.05. For D, the mean of each group is represented along the x axis and the y axis refers to relative normalized abundances. Phylum relative abundance data were compared using Kruskal-Wallis test. *P < 0.05, **P < 0.01, ***P < 0.001 indicating significantly different phyla abundances between KHK-F and KHK-G, #P < 0.05, ##P < 0.01, ###P < 0.001 between KHK-F and WT-F, and $$P < 0.01, $$$P < 0.001 between KHK-F and WT-G (significance adjusted for false-discovery rate of P < 0.05). For E–L, the y axis refers to relative normalized abundances, and the lines indicate median and the boxes indicate first and third quartiles. Family abundance data were compared using the Wilcoxon test. *P < 0.05, **P < 0.01 when compared with KHK-F.
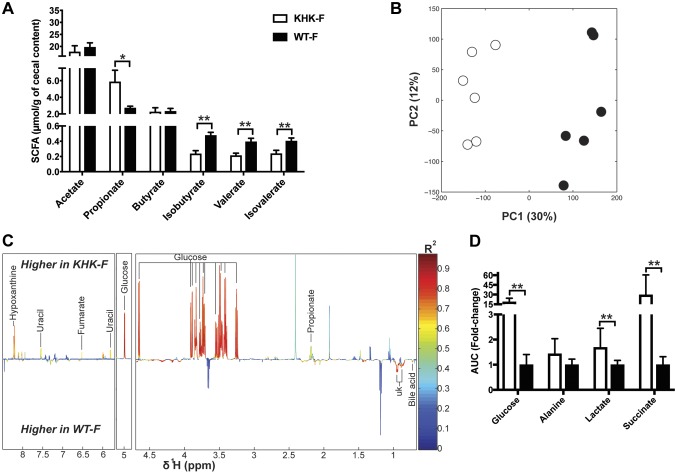
These changes in the composition of the bacteria community were associated with changes in the metabolic activity of the gut microbiota, as first assessed using gas chromatography (Fig. 5A). Among the major SCFA detected, the amount of propionate increased by 50% in the cecum content of the KHK-F when compared with the WT-F. Otherwise, only minor SCFAs (e.g., valerate) or branched SCFAs such as isobutyrate and isovalerate displayed a lower concentration in KHK-F relative to WT-F. 1H-NMR–based metabolic profiling of the same cecal extracts confirmed a very strong shift in the metabolic activity of the microbiota upon fructose malabsorption as illustrated by the separation of the cecal metabolic profiles of WT-F vs. KHK-F in the PCA plot (Fig. 5B). A supervised PLS-DA model was then fitted to identify the metabolites significantly changed between the 2 groups (Fig. 5C). The most prominent discriminating signals belong to glucose, which was strongly increased in KHK-F when compared with WT-F mice (Fig. 5C, D). 1H-NMR also confirmed the significant increase in propionate levels detected by gas chromatography and revealed the increase in succinate in the KHK-F when compared with WT-F mice (Fig. 5C, D). A number of other metabolites were also higher in KHK-F mice than in WT-F, such as lactate, alanine, uracil, fumarate, and hypoxanthine. One bile acid signal and additional signals belonging to unidentified metabolites were lower in KHK-F than in WT-F mice (Fig. 5D). Of note, fructose itself was not detected in the cecum of KHK-F or WT-F.
Figure 5.
Metabolite composition of the cecum content collected from KHK−/− or WT mice fed 20% fructose (KHK-F or WT-F) diet for 8 wk (n = 5–6/group). A) SCFA quantification in cecum content by gas chromatography. B) PCA score plots obtained from 1H-NMR spectra of cecum content extracts of KHK-F or WT-F. C) Plot of O-PLS-DA coefficients related to the discrimination between 1H-NMR spectra from cecum content extracts of WT-F vs. KHK-F. D) Bar graph representation of the relative integral in arbitrary units (a.u) for different metabolites (glucose, lactate, alanine, and succinate) in cecum content. AUC, area under the curve; UK, unknown. All values are means ± sem; means were compared with Student’s t test. *P < 0.05, **P < 0.001.
ABs partially reverse the fructose malabsorption effects on peptide expression in the lower intestine
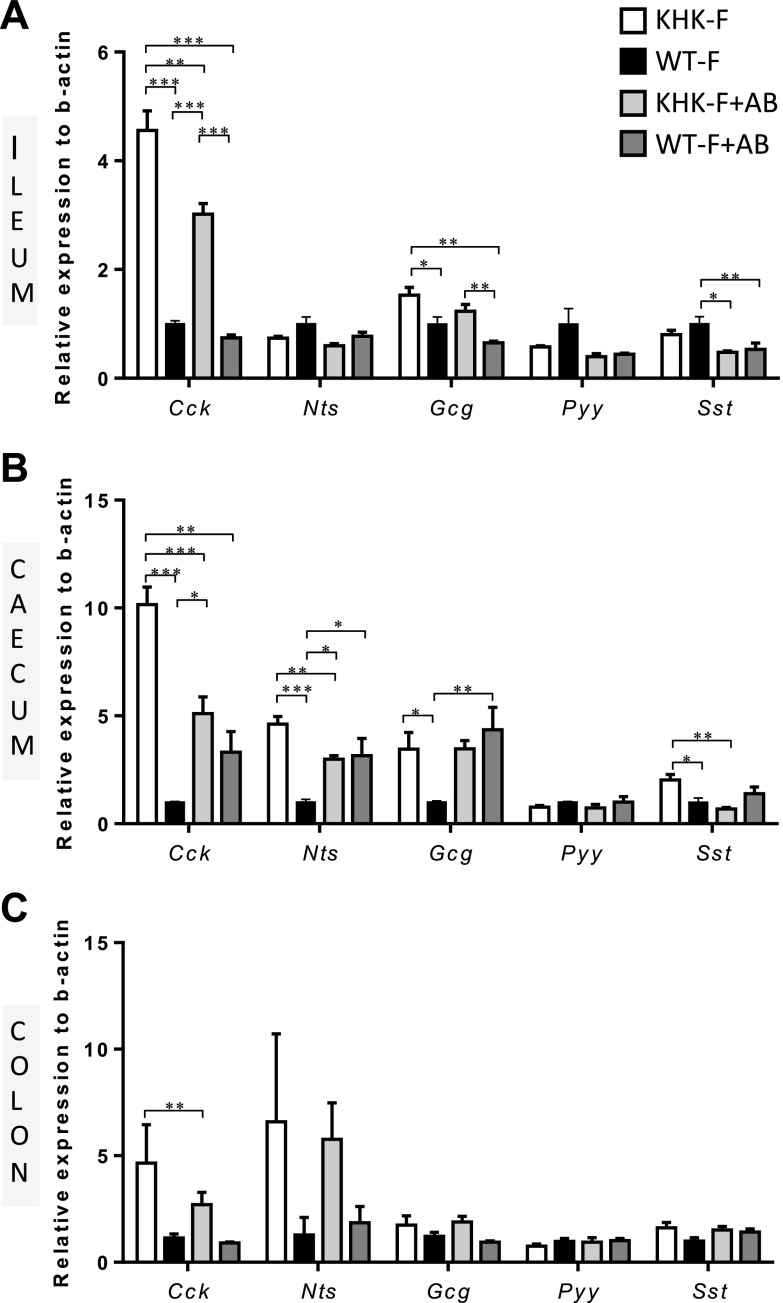
Because fructose feeding alters the gut microbiota in KHK−/− mice, we treated them with ABs to test whether a reduced or modified bacterial population would prevent or cause further changes in expression of peptides in the distal GI tract in response to fructose malabsorption. The AB treatment did not affect body weight, food consumption or drink intake in the WT or KHK−/− mice fed fructose when compared with water (no AB) controls (Supplemental Fig. S4A–C). The 4 wk of fructose feeding significantly enlarged the cecum in the KHK−/− mice when compared with the WT as shown by the increase in the weight of the full and empty ceca (Supplemental Fig. S4D, E). The 4 wk of AB treatment exacerbated the enlargement of the empty cecum in the KHK−/− mice fed fructose and also increased the weight of the full and empty cecum of the WT mice. However, 4 wk of AB treatment drastically reduced the total amount of bacteria in the cecum as the result of a major decrease in the main 4 phyla (Supplemental Fig. S4F). In the ileum, the AB treatment, independently of the KHK status, had no significant effect on the expression of any of the peptides except for a mild decrease in Sst mRNA levels (Fig. 6A). In the KHK-F + AB mice, AB treatment partially prevented the KHK mutation-dependent increase in Cck expression in the ileum but had no effect on Gcg expression when compared with KHK-F mice (Fig. 6A). In the cecum, the AB treatment by itself (WT-F + AB) had a significant positive effect on the expression of Nts and Gcg (Fig. 6B). AB treatment reduced, by 50 and 40%, respectively, the up-regulation of Cck and Nts in the cecum of KHK-F mice, leading to no significant differences between WT-F + AB and KHK-F + AB. The AB treatment in KHK-F + AB abolished completely the increase in Sst expression observed in the KHK-F group but had no effect on Gcg expression level. In the colon, expression demonstrated greater variability and no differences were found (Fig. 6C).
Figure 6.
KHK−/− and WT mice were fed 20% fructose for 3 wk with (KHK-F + AB or WT-F + AB) or without (KHK-F or WT-F) AB treatment (n = 5–7/group). The mRNA expression levels of gut peptides were measured in WT-F, KHK-F, WT-F + AB, and KHK-F + AB mice in the ileum (A), the cecum (B), and the proximal colon (C). Data were normalized to levels in WT-F. All values are means ± sem. Means were compared by 1-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001.
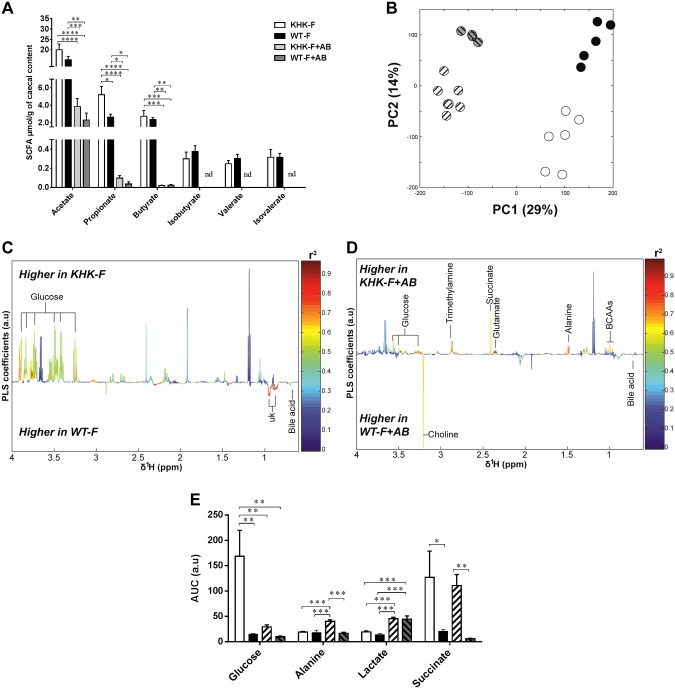
AB treatment partially prevented fructose malabsorption–induced changes in metabolites of the cecal content
Both AB groups (KHK-F + AB and WT-F + AB) had almost undetectable SCFA contents except for acetate, for which the cecal content was reduced in the same proportion relative to KHK-F and WT-F (Fig. 7A). The AB treatment created a shift in the metabolic profiles of the cecum content obtained by 1H-NMR analysis as shown by PCA results (Fig. 7B) and by 2 supervised PLS-DA models (Fig. 7C, D). ABs almost completely prevented the fructose-induced increase in cecal glucose levels in the KHK-F + AB mice when compared with KHK-F mice (Fig. 7E). Conversely, AB treatment did not reduce the increase in succinate observed in the cecum of the KHK-F mice. In both genotypes, AB treatment led to greater lactate levels.
Figure 7.
Metabolite composition of the cecum content collected from KHK−/− or WT mice fed 20% fructose diet for 3 wk with (KHK-F + AB or WT-F + AB) or without (KHK-F or WT-F) AB treatment (n = 5–7/group). A) SCFA quantification in cecum content by gas chromatography. B) PCA score plots obtained from 1H-NMR spectra of cecum content extracts from KHK-F, KHK-F + AB, WT-F, and WT-F + AB. C) Plots of O-PLS-DA coefficients related to the discrimination between 1H-NMR spectra of cecum content extracts from WT-F vs. KHK-F. D) Plots of O-PLS-DA coefficients related to the discrimination between 1H-NMR spectra of cecum content extracts from WT-F + AB vs. KHK-F + AB. E) Bar graph representation of the relative integral for different metabolites (glucose, lactate, alanine, and succinate) in cecum content. AUC, area under the curve. All values are means ± sem. Means were compared by 1-way ANOVA followed by Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
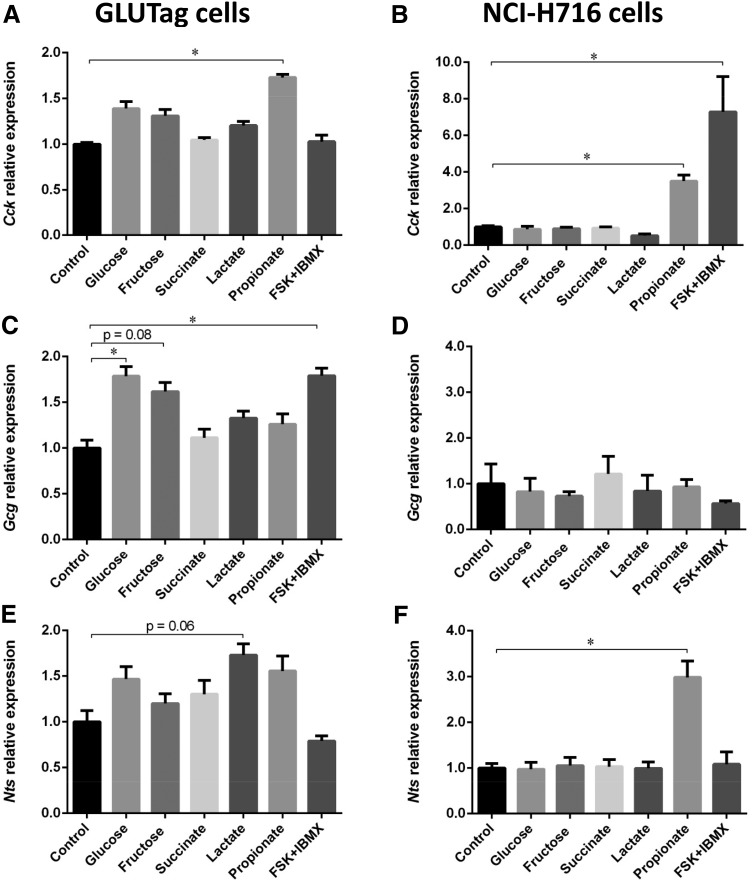
Activation of peptide expression by microbial metabolites in GLUTag and NCI-H716 cell lines
In order to determine whether the metabolites (glucose, lactate, succinate, and propionate) identified as increased in the cecum of KHK-F mice could regulate Cck, Gcg, and Nts mRNA expression, we tested the impact of those metabolites on enteroendocrine peptide expression in mouse and human EECs, GLUTag, and NCI-H716 cells, respectively. Exposure of GLUTag and NCI-H716 cells to 2 mM propionate increased significantly mRNA expression of Cck when compared with the control, whereas glucose, fructose, succinate, and lactate had no effect (Fig. 8A, B). GLUTag cells cultured with 25 mM glucose significantly increased Gcg mRNA expression when compared with the control, whereas cells cultured with 25 mM fructose also increased Gcg mRNA level, but not significantly (P = 0.08) (Fig. 8C). None of the metabolite tested were able to increase Gcg expression in NCI-H716 cells (Fig. 8D). In GLUTag cells, Nts mRNA expression remained unchanged after exposure to any of the metabolites tested (Fig. 8E), except for 10 mM lactate, in which a tendency for up-regulation (P = 0.06) was observed. However, in NCI-H716, Nts expression was significantly activated by propionate (Fig. 8F).
Figure 8.
Cck (A, B), Gcg (C, D), and Nts (E, F) mRNA expression in GLUTag and NCI-H716 cells, respectively, incubated for 24 h with 1 mM glucose (control) or 25 mM glucose, 25 mM fructose, 2 mM succinate, 10 mM lactate, 2 mM propionate, or 25 mM glucose + 10 μM forskolin + 10 μM 3-isobutyl-1-methylxanthine (FSK/IBMX) (n = 3/group for GLUTag and n = 4/group for NCI-H716). The relative values were normalized to control levels. All values are means ± sem. Means were compared by Kruskal-Wallis test followed by Dunn’s multiple comparison test. *P < 0.05.
DISCUSSION
In our model of KHK mutant mice fed fructose diet, fructose malabsorption had a significant impact on the lower intestine (ileum, cecum, and colon), inducing major changes in the intestinal ecosystem composition, microbiota metabolism, and intestinal endocrine response. We found that fructose malabsorption regulates the functional identity of the specific subtype of EECs by stimulating the expression of Cck (and to a lesser extend Nts and Gcg) in the ileum and cecum. The signals (e.g., propionate) able to activate peptide expressions in the EECs of the lower intestine likely originate from byproducts of the fructose metabolism by the microbiota that is drastically modified by fructose malabsorption.
Changes in CCK expression, CCK+ cell density, and CCK+ EEC subtypes in response to fructose malabsorption
It has long been known that Cck expression and secretion takes place in the upper intestinal EECs. However, recent results (38) have highlighted a potential secretion of this hormone also in the lower intestine. We confirmed the presence of CCK+ cells in the lower intestine of WT mice, and using triple labeling with anti-CCK, anti-GLP-1, and anti-PYY antibodies, we identified several subtypes of CCK+ cells. Thus, under normal fructose absorption conditions (WT-F, Fig. 3), the predominant EEC subtype present in the lower intestine was CCK+/GLP-1+/PYY+, although we also detected a number of minority subtypes (Fig. 3 and Supplemental Table S3). Other peptides, not tested here, could also be coexpressed with CCK in the lower intestine. Nevertheless, our data clearly corroborates the new paradigm that each EEC can express multiple gut peptides (37, 39–42).
The change in the CCK+ cell number plays a significant role in the intestinal response to fructose malabsorption without excluding the possible increase in CCK production per cell. Interestingly, the density of the normally predominant CCK-expressing cell type, the triple-positive CCK+/GLP-1+/PYY+ cells, did not increase in the KHK-F mice. In fact, the 3 regions of the lower intestine responded differently through expansion of different CCK+ cell subtypes. Whether this is due to intrinsic differences in the 3 intestinal regions or different environments created by fructose or fructose-microbiota interactions is an important question that remains to be addressed.
Our results further support the idea of the plasticity of the intestinal endocrine system (43). Although it remains undetermined which stages of EEC differentiation (stem cells, progenitor cells, or differentiated cells) are affected by the luminal environment, in the cecum the changes in peptide pattern expression were associated with an increase in Neurod1 and Pax6 but not in Neurog3 and Math1 expression. Both Neurod1 and Pax6 are transcription factors known to regulate the differentiation of CCK- and GLP-1-expressing cells, respectively, and they are both considered late markers for the differentiation of intestinal progenitor cells in the EEC lineage (44–46). In contrast, Neurog3 is an early marker that controls the commitment of Math1-positive progenitors to the endocrine cell fate (47). Thus, the changes in the luminal environment resulting from fructose malabsorption may regulate the differentiation process downstream of Neurog3.
The role of the microbiota in the regulation of the gut peptide expression in the ileum, cecum, and colon
It is unlikely that fructose itself changes the gut peptide expression in the ileum, cecum, and colon. First, the fructose content is likely substantially increased in the duodenum and jejunum in KHK-F mice (23). Yet, no changes in Cck or other peptide expression were detected in these regions. Second, fructose was not detected in the cecum content of KHK-F mice, suggesting its rapid metabolism by the bacteria of the lower intestine. On the other hand, the drastic changes in gut microbiota composition in KHK-F mice and the effect of AB treatment suggest that the microbiota, and more likely the compounds originating from the bacterial metabolism of fructose, are involved in the EEC response to fructose malabsorption. Several studies have showed that changes in the intestinal ecosystem can affect L-cell density and function (48, 49), and more specifically, the lack of bacteria was associated with an increase in L-cell density and Gcg expression levels (20, 21). Interestingly, in our study, bacterial depletion alone resulted in a moderate up-regulation of Cck, Gcg, and Nts expression in the cecum independently of the genotype. This confirms the previous studies focusing on the regulation of Gcg expression (20, 21) and raises a possibility that there are bacterial metabolites that can exert a repressive effect on the expression of a number of peptides including Cck. In the cecum, this effect may interfere with our ability to detect a potentially complete reversal of the fructose malabsorption-induced Cck expression by AB treatment. However, our study uncovered the potential of the gut microbiota to regulate Cck expression. This role of the microbiota is also supported by our observations that propionate, a SCFA produced by bacteria, can activate specifically the expression of Cck in mouse and human GLUTag and NCI-H716 cell lines, respectively.
The potential role of propionate, glucose, and lactate in regulating gut peptides in response to fructose malabsorption
The concomitant increase in Cck, Gcg, and Nts expression in the cecum raises the idea of a possible common activation mechanism. Because propionate, lactate, glucose, and succinate were the main microbial metabolites that increased in the cecum in response to fructose malabsorption, they were plausible candidates for activation of Cck, Gcg, and Nts expression. Interestingly, our in vitro results indicated that the expression of Nts and Cck is regulated by propionate but also that each peptide might be regulated by different metabolites that are nonetheless all resulting from the fructose metabolism by the gut microbiota.
From our metabolite analysis and the in vitro experiment using GLUTag and NCI-H716 cells, propionate is the strongest candidate for the activation of Cck expression. It has been shown to regulate GLP-1 and PYY secretion in vivo and in vitro (50, 51) and here we show for the first time that it can also up-regulate Cck expression. In NCI-H716 cells, propionate also activates the expression of Nts. Propionate effects on intestinal cells are mediated primarily through 2 G-protein-coupled receptors, free fatty acid receptor (FFAR) 2, and FFAR3 (51, 52). Interestingly, FFAR3 was found in CCK-positive cells in the duodenum and in NTS-positive cells in the ileum and colon (53). Propionate is also an inhibitor of histone deacetylases (54); therefore, it can also regulate gene expression through the increase in histone acetylation (55). To our knowledge, no study has focused on the regulation of CCK by SCFAs, likely because, until recently, the abundance of CCK in the ileum, cecum, and colon was thought to be insignificant (56).
We also found that fructose malabsorption is associated with a dramatic increase in glucose content in the cecum of the KHK-F, which is likely to participate to the increase in Gcg expression and GLP-1+ cell density in the ileum and cecum. Indeed, even in the lower intestine, L-cells seem to be able to sense glucose (37), and as shown by others and confirmed here in GLUTag cells, glucose is a potent activator of Gcg expression. Whether glucose participates in changing CCK expression and CCK+ cell density in the lower intestine of KHK-F remains to be clarified. In vivo, duodenal I-cells secreting CCK did not seem to be equipped to sense sugar (57). Based on this and on the lack of increase in Cck expression in response to glucose in GLUTag and NCI-H716 cells, the regulation of CCK by glucose in the lower intestine appears unlikely. However, the regulation of CCK+ cells in the lower intestine may differ from that in the upper small intestine as strongly conjectured for the L-cells (37).
The lactate, which was also increased in the cecum of the KHK-F mice, may stimulate Nts expression in GLUTag cells. Thus, it might be the regulatory component activating Nts expression in the cecum of the KHK-F. Lactate signals though the G-protein-coupled receptor GPR81 (58) or is transported into intestinal cells by the monocarboxylate transporters (59). Expression of GPR81 in the intestine is not well documented (60). Members of the monocarboxylate transporter family are broadly expressed along the intestine; however, their specific presence and potential function in EECs has not been explored yet. The succinate, which after glucose showed the strongest increase in the cecum of the KHK-F mice, did not stimulate Cck, Gcg, or Nts mRNA expression in either GLUTag or NCI-H716 cells. Succinate signals via GPR91 receptor (61), and it is not known whether GPR91 is expressed in the GLUTag and NCI-H716 cells, thus the signaling role of succinate in vivo cannot be excluded.
Changes in microbiota composition and metabolites in response to fructose malabsorption
We propose that glucose, lactate, propionate, and succinate are byproducts of the fructose metabolism by the intestinal bacteria populations that are expanded in the distal intestine in response to fructose malabsorption. The most striking and significant effect we observed was the increase in abundance of Lactobacillaceae family, especially Lactobacillus johnsonii, in the cecum of the KHK-F. Because carbohydrates, including sucrose and fructose, are the major energy source favoring growth of Lactobacillaceae (62), these results support the hypothesis that unabsorbed fructose was moving into the cecum to become a key carbon source for the bacteria of the lower intestine. Fructose itself was not detected in the cecum of the KHK-F mice and was likely converted to glucose (12), which increased drastically. A total of 3 metabolic pathways can convert fructose into glucose in bacteria (Supplemental Fig. S5), and all 3 can be deployed by the bacteria that increased in relative abundance in the KHK-F group.
Among the various SCFAs of the cecal content, only propionate increased in response to fructose malabsorption. Normally, SCFAs originate from fermentation of nondigestible carbohydrates by the gut bacteria (63). In the KHK-F mice, the increase in propionate concentration likely arises from the fermentation of the malabsorbed fructose via glucose (Supplemental Fig. S5). A total of 2 pathways could contribute to bacterial propionate formation from carbohydrates: the succinate pathway and the acrylate pathway (Supplemental Fig. S5) (64). In the cecum of KHK-F mice, the level of some intermediates of the succinate pathway (i.e., fumarate and succinate) or acrylate pathway (i.e., lactate) were higher than in the WT-F, confirming that both synthetic pathways were stimulated by the fructose malabsorption. However, the accumulation of succinate in the cecal content of KHK-F suggests that the part of the succinate pathway acting downstream of succinate was inefficient. On the other hand, the Lachnospiraceae, which increased in the KHK-F group (Fig. 4J), have the lactoyl-CoA dehydratase subunit α gene (64), and may be the main bacteria supporting the propionate production through the lactate pathway.
Even though the AB treatment almost completely removed propionate, the Cck expression was only partially prevented in the KHK-F + AB mice, especially in the ileum. Therefore, in addition to propionate, there could be other fructose malabsorption-responsive compounds, perhaps less sensitive to AB treatment, that are also able to induce Cck expression.
Interestingly, the role of CCK in mediating visceral pain has been suggested in mice and humans (65–67). In humans, fructose malabsorption had been associated with high serum amylase and lipase concentrations (68). Interestingly, the release of both lipase and amylase is known to be stimulated by CCK (69), supporting the idea that CCK may also be elevated in the plasma of patients diagnosed with fructose malabsorption condition. Further characterization of the plasma and cecal content of fructose malabsorption patients are needed to test this hypothesis. However, our results provide a possible explanation for the pain experienced by fructose mal-absorbing patients and identify a number of possible therapeutic entry points to alleviate this condition.
CONCLUSIONS
We showed that, in response to fructose malabsorption, CCK is the highest up-regulated gut hormone in the ileum and cecum. We also demonstrated for the first time that microbial metabolites such as propionate can regulate CCK expression. Our results confirmed the plasticity of the EECs in response to changes in the luminal environment. Importantly, we showed that this response to the changes in the luminal environment can be EEC subtype specific. In our system, only some CCK+-EEC subtypes, as defined by the combination of hormone that they express, increased in their density in response to fructose malabsorption. Whether the CCK+/GLP-1− or CCK+/GLP-1+ cells differ markedly in their functional responsiveness and physiologic roles will be interesting topics for future evaluation. Thus, the nature of the EEC response to specific luminal environment changes may be one of the defining characteristics of EEC subtypes with distinct functional identities.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank J. P. Furet (Micalis Institute, INRA, Jouy-en-Josas, France), F. Rouyer (Paris-Saclay Institute of Neuroscience, Université Paris Sud, CNRS, Université Paris–Saclay, Gif-sur-Yvette, France), and S. P. Shirazi-Beechey (Institute of Integrative Biology, University of Liverpool, United Kingdom) for sharing reagents and equipment. The authors are grateful to the INRA Migale bioinformatics platform (http://migale.jouy.inra.fr/) for providing computational resources, the Genotoul high-throughput sequencing platform (http://bioinfo.genotoul.fr/), and the histology facility of UMR 1313 Génétique Animale et Biologie Intégrative (GABI). This work was supported by grants from Institut Benjamin Delessert and by INRA. The China Scholarship Council (CSC) and INRA funded PhD fellowships to X.Z. The authors declare no conflicts of interest.
Glossary
- AB
antibiotic
- CCK
cholecystokinin
- EEC
enteroendocrine cell
- FC
fold change
- FFAR
free fatty acid receptor
- FROGS, Find, Rapidly
OTUs with Galaxy Solution
- GCG
preproglucagon
- GIP
gastric inhibitory polypeptide
- GI
gastrointestinal
- GLP-1
glucagon-like peptide-1
- GLUT
facilitated glucose/fructose transporter
- KHK
ketohexokinase
- Math1
atonal bHLH transcription factor 1
- NeuroD
neuronal differentiation
- Neurog
neurogenin
- NTS
neurotensin
- O-PLS-DA
orthogonal projection on latent structure-discriminant analysis
- OTU
operational taxonomic unit
- Pax
paired box
- PCA
principal component analysis
- PYY
peptide YY
- qRT-PCR
quantitative PCR
- SCFA
short-chain fatty acid
- SCT
secretin
- SST
somatostatin
- TPH1
tryptophan hydroxylase 1
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Zhang, A. Grosfeld, E. Williams, R. P. Ferraris, J. C. Fritton, and V. Douard designed the research; X. Zhang, A. Grosfeld, E. Williams, S. Barretto, L. Smith, C. Philippe, F. Devime, N. Dourmap, P. Larraufie, J. F. Rehfeld, S. Ellero-Simatos, and V. Douard conducted the research; X. Zhang, A. Grosfeld, D. Vasiliauskas, M. Mariadassou, C. Philippe, J. F. Rehfeld, S. Ellero-Simatos, and V. Douard analyzed the data; A. Grosfeld, D. Vasiliauskas, C. Herberden, S. Ellero-Simatos, and V. Douard wrote the manuscript; D. Vasiliauskas, C. Melchior, G. Gourcerol, N. Lapaque, P. Larraufie, H. M. Blottière, P. Gerard, R. P. Ferraris, J. C. Fritton, and V. Douard contributed to the discussion; V. Douard has primary responsibility for the final content; and all authors read and approved the final manuscript.
REFERENCES
- 1.Marriott B. P., Cole N., Lee E. (2009) National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 139, 1228S–1235S [DOI] [PubMed] [Google Scholar]
- 2.Tappy L., Lê K. A. (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 90, 23–46 [DOI] [PubMed] [Google Scholar]
- 3.Jones H. F., Butler R. N., Brooks D. A. (2011) Intestinal fructose transport and malabsorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G202–G206 [DOI] [PubMed] [Google Scholar]
- 4.Douard V., Ferraris R. P. (2013) The role of fructose transporters in diseases linked to excessive fructose intake. J. Physiol. 591, 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones H. F., Burt E., Dowling K., Davidson G., Brooks D. A., Butler R. N. (2011) Effect of age on fructose malabsorption in children presenting with gastrointestinal symptoms. J. Pediatr. Gastroenterol. Nutr. 52, 581–584 [DOI] [PubMed] [Google Scholar]
- 6.Putkonen L., Yao C. K., Gibson P. R. (2013) Fructose malabsorption syndrome. Curr. Opin. Clin. Nutr. Metab. Care 16, 473–477 [DOI] [PubMed] [Google Scholar]
- 7.Manolescu A. R., Witkowska K., Kinnaird A., Cessford T., Cheeseman C. (2007) Facilitated hexose transporters: new perspectives on form and function. Physiology (Bethesda) 22, 234–240 [DOI] [PubMed] [Google Scholar]
- 8.Davidson N. O., Hausman A. M., Ifkovits C. A., Buse J. B., Gould G. W., Burant C. F., Bell G. I. (1992) Human intestinal glucose transporter expression and localization of GLUT5. Am. J. Physiol. 262, C795–C800 [DOI] [PubMed] [Google Scholar]
- 9.Patel C., Douard V., Yu S., Tharabenjasin P., Gao N., Ferraris R. P. (2015) Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R499–R509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel C., Sugimoto K., Douard V., Shah A., Inui H., Yamanouchi T., Ferraris R. P. (2015) Effect of dietary fructose on portal and systemic serum fructose levels in rats and in KHK-/- and GLUT5-/- mice. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G779–G790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douard V., Ferraris R. P. (2008) Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 295, E227–E237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang C., Hui S., Lu W., Cowan A. J., Morscher R. J., Lee G., Liu W., Tesz G. J., Birnbaum M. J., Rabinowitz J. D. (2018) The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 27, 351–361.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nergård B. J., Lindqvist A., Gislason H. G., Groop L., Ekelund M., Wierup N., Hedenbro J. L. (2015) Mucosal glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide cell numbers in the super-obese human foregut after gastric bypass. Surg. Obes. Relat. Dis. 11, 1237–1246 [DOI] [PubMed] [Google Scholar]
- 14.Cavin J. B., Couvelard A., Lebtahi R., Ducroc R., Arapis K., Voitellier E., Cluzeaud F., Gillard L., Hourseau M., Mikail N., Ribeiro-Parenti L., Kapel N., Marmuse J. P., Bado A., Le Gall M. (2016) Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology 150, 454–464.e9 [DOI] [PubMed] [Google Scholar]
- 15.Rhee N. A., Wahlgren C. D., Pedersen J., Mortensen B., Langholz E., Wandall E. P., Friis S. U., Vilmann P., Paulsen S. J., Kristiansen V. B., Jelsing J., Dalbøge L. S., Poulsen S. S., Holst J. J., Vilsbøll T., Knop F. K. (2015) Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia 58, 2254–2258 [DOI] [PubMed] [Google Scholar]
- 16.Mumphrey M. B., Patterson L. M., Zheng H., Berthoud H. R. (2013) Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil. 25, e70–e79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillard L., Billiauws L., Stan-Iuga B., Ribeiro-Parenti L., Jarry A. C., Cavin J. B., Cluzeaud F., Mayeur C., Thomas M., Freund J. N., Lacorte J. M., Le Gall M., Bado A., Joly F., Le Beyec J. (2016) Enhanced ghrelin levels and hypothalamic orexigenic AgRP and NPY neuropeptide expression in models of Jejuno-colonic short bowel syndrome. Sci. Rep. 6, 28345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillard L., Mayeur C., Robert V., Pingenot I., Le Beyec J., Bado A., Lepage P., Thomas M., Joly F. (2017) Microbiota is involved in post-resection adaptation in humans with short bowel syndrome. Front. Physiol. 8, 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plovier H., Cani P. D. (2017) Enteroendocrine cells: metabolic relays between microbes and their host. Endocr. Dev. 32, 139–164 [DOI] [PubMed] [Google Scholar]
- 20.Wichmann A., Allahyar A., Greiner T. U., Plovier H., Lundén G. O., Larsson T., Drucker D. J., Delzenne N. M., Cani P. D., Bäckhed F. (2013) Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14, 582–590 [DOI] [PubMed] [Google Scholar]
- 21.Arora T., Akrami R., Pais R., Bergqvist L., Johansson B. R., Schwartz T. W., Reimann F., Gribble F. M., Bäckhed F. (2018) Microbial regulation of the L cell transcriptome. Sci. Rep. 8, 1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gribble F. M., Reimann F. (2016) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277–299 [DOI] [PubMed] [Google Scholar]
- 23.Patel C., Douard V., Yu S., Gao N., Ferraris R. P. (2015) Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. FASEB J. 29, 4046–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishimoto T., Lanaspa M. A., Le M. T., Garcia G. E., Diggle C. P., Maclean P. S., Jackman M. R., Asipu A., Roncal-Jimenez C. A., Kosugi T., Rivard C. J., Maruyama S., Rodriguez-Iturbe B., Sánchez-Lozada L. G., Bonthron D. T., Sautin Y. Y., Johnson R. J. (2012) Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 109, 4320–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diggle C. P., Shires M., McRae C., Crellin D., Fisher J., Carr I. M., Markham A. F., Hayward B. E., Asipu A., Bonthron D. T. (2010) Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiol. Genomics 42A, 235–243 [DOI] [PubMed] [Google Scholar]
- 26.Rehfeld J. F. (1998) How to measure cholecystokinin in tissue, plasma and cerebrospinal fluid. Regul. Pept. 78, 31–39 [DOI] [PubMed] [Google Scholar]
- 27.Lan A., Bruneau A., Bensaada M., Philippe C., Bellaud P., Rabot S., Jan G. (2008) Increased induction of apoptosis by Propionibacterium freudenreichii TL133 in colonic mucosal crypts of human microbiota-associated rats treated with 1,2-dimethylhydrazine. Br. J. Nutr. 100, 1251–1259 [DOI] [PubMed] [Google Scholar]
- 28.Lukowicz C., Ellero-Simatos S., Régnier M., Polizzi A., Lasserre F., Montagner A., Lippi Y., Jamin E. L., Martin J. F., Naylies C., Canlet C., Debrauwer L., Bertrand-Michel J., Al Saati T., Théodorou V., Loiseau N., Mselli-Lakhal L., Guillou H., Gamet-Payrastre L. (2018) Metabolic effects of a chronic dietary exposure to a low-dose pesticide cocktail in mice: sexual dimorphism and role of the constitutive androstane receptor. Environ. Health Perspect. 126, 067007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furet J. P., Firmesse O., Gourmelon M., Bridonneau C., Tap J., Mondot S., Doré J., Corthier G. (2009) Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol. Ecol. 68, 351–362 [DOI] [PubMed] [Google Scholar]
- 30.Bacchetti De Gregoris T., Aldred N., Clare A. S., Burgess J. G. (2011) Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods 86, 351–356 [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi Y., Murashima K., Ohara H., Suzuki T., Hayashi H., Sakamoto M., Fukasawa T., Kubota H., Hosono A., Kono T., Kaminogawa S., Benno Y. (2006) Increase in terminal restriction fragments of Bacteroidetes-derived 16S rRNA genes after administration of short-chain fructooligosaccharides. Appl. Environ. Microbiol. 72, 6271–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., Schloss P. D. (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escudié F., Auer L., Bernard M., Mariadassou M., Cauquil L., Vidal K., Maman S., Hernandez-Raquet G., Combes S., Pascal G. (2018) FROGS: find, rapidly, OTUs with galaxy solution. Bioinformatics 34, 1287–1294 [DOI] [PubMed] [Google Scholar]
- 34.Bokulich N. A., Subramanian S., Faith J. J., Gevers D., Gordon J. I., Knight R., Mills D. A., Caporaso J. G. (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickham H. (2009) ggplot2: Elegant Graphics for Data Analysis, Springer-Verlag, New York, NY, USA [Google Scholar]
- 36.Love M. I., Huber W., Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habib A. M., Richards P., Cairns L. S., Rogers G. J., Bannon C. A., Parker H. E., Morley T. C., Yeo G. S., Reimann F., Gribble F. M. (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153, 3054–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fakhry J., Wang J., Martins P., Fothergill L. J., Hunne B., Prieur P., Shulkes A., Rehfeld J. F., Callaghan B., Furness J. B. (2017) Distribution and characterisation of CCK containing enteroendocrine cells of the mouse small and large intestine. Cell Tissue Res. 369, 245–253 [DOI] [PubMed] [Google Scholar]
- 39.Aiken K. D., Kisslinger J. A., Roth K. A. (1994) Immunohistochemical studies indicate multiple enteroendocrine cell differentiation pathways in the mouse proximal small intestine. Dev. Dyn. 201, 63–70 [DOI] [PubMed] [Google Scholar]
- 40.Egerod K. L., Engelstoft M. S., Grunddal K. V., Nøhr M. K., Secher A., Sakata I., Pedersen J., Windeløv J. A., Füchtbauer E. M., Olsen J., Sundler F., Christensen J. P., Wierup N., Olsen J. V., Holst J. J., Zigman J. M., Poulsen S. S., Schwartz T. W. (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153, 5782–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grunddal K. V., Ratner C. F., Svendsen B., Sommer F., Engelstoft M. S., Madsen A. N., Pedersen J., Nøhr M. K., Egerod K. L., Nawrocki A. R., Kowalski T., Howard A. D., Poulsen S. S., Offermanns S., Bäckhed F., Holst J. J., Holst B., Schwartz T. W. (2016) Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology 157, 176–194 [DOI] [PubMed] [Google Scholar]
- 42.Lopez M. J., Upchurch B. H., Rindi G., Leiter A. B. (1995) Studies in transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. J. Biol. Chem. 270, 885–891 [DOI] [PubMed] [Google Scholar]
- 43.Richards P., Pais R., Habib A. M., Brighton C. A., Yeo G. S., Reimann F., Gribble F. M. (2016) High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides 77, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naya F. J., Stellrecht C. M., Tsai M. J. (1995) Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev. 9, 1009–1019 [DOI] [PubMed] [Google Scholar]
- 45.Hill M. E., Asa S. L., Drucker D. J. (1999) Essential requirement for Pax6 in control of enteroendocrine proglucagon gene transcription. Mol. Endocrinol. 13, 1474–1486 [DOI] [PubMed] [Google Scholar]
- 46.Schonhoff S. E., Giel-Moloney M., Leiter A. B. (2004) Minireview: development and differentiation of gut endocrine cells. Endocrinology 145, 2639–2644 [DOI] [PubMed] [Google Scholar]
- 47.Jenny M., Uhl C., Roche C., Duluc I., Guillermin V., Guillemot F., Jensen J., Kedinger M., Gradwohl G. (2002) Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21, 6338–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cani P. D., Hoste S., Guiot Y., Delzenne N. M. (2007) Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br. J. Nutr. 98, 32–37 [DOI] [PubMed] [Google Scholar]
- 49.Delmée E., Cani P. D., Gual G., Knauf C., Burcelin R., Maton N., Delzenne N. M. (2006) Relation between colonic proglucagon expression and metabolic response to oligofructose in high fat diet-fed mice. Life Sci. 79, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 50.Psichas A., Sleeth M. L., Murphy K. G., Brooks L., Bewick G. A., Hanyaloglu A. C., Ghatei M. A., Bloom S. R., Frost G. (2015) The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39, 424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tolhurst G., Heffron H., Lam Y. S., Parker H. E., Habib A. M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F. M. (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown A. J., Goldsworthy S. M., Barnes A. A., Eilert M. M., Tcheang L., Daniels D., Muir A. I., Wigglesworth M. J., Kinghorn I., Fraser N. J., Pike N. B., Strum J. C., Steplewski K. M., Murdock P. R., Holder J. C., Marshall F. H., Szekeres P. G., Wilson S., Ignar D. M., Foord S. M., Wise A., Dowell S. J. (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 [DOI] [PubMed] [Google Scholar]
- 53.Nøhr M. K., Pedersen M. H., Gille A., Egerod K. L., Engelstoft M. S., Husted A. S., Sichlau R. M., Grunddal K. V., Poulsen S. S., Han S., Jones R. M., Offermanns S., Schwartz T. W. (2013) GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154, 3552–3564 [DOI] [PubMed] [Google Scholar]
- 54.Sealy L., Chalkley R. (1978) The effect of sodium butyrate on histone modification. Cell 14, 115–121 [DOI] [PubMed] [Google Scholar]
- 55.Larraufie P., Martin-Gallausiaux C., Lapaque N., Dore J., Gribble F. M., Reimann F., Blottiere H. M. (2018) SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 8, 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akiba Y., Inoue T., Kaji I., Higashiyama M., Narimatsu K., Iwamoto K., Watanabe M., Guth P. H., Engel E., Kuwahara A., Kaunitz J. D. (2015) Short-chain fatty acid sensing in rat duodenum. J. Physiol. 593, 585–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daly K., Al-Rammahi M., Moran A., Marcello M., Ninomiya Y., Shirazi-Beechey S. P. (2013) Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G271–G282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun S., Li H., Chen J., Qian Q. (2017) Lactic acid: no longer an inert and end-product of glycolysis. Physiology (Bethesda) 32, 453–463 [DOI] [PubMed] [Google Scholar]
- 59.Gill R. K., Saksena S., Alrefai W. A., Sarwar Z., Goldstein J. L., Carroll R. E., Ramaswamy K., Dudeja P. K. (2005) Expression and membrane localization of MCT isoforms along the length of the human intestine. Am. J. Physiol. Cell Physiol. 289, C846–C852 [DOI] [PubMed] [Google Scholar]
- 60.Ranganathan P., Shanmugam A., Swafford D., Suryawanshi A., Bhattacharjee P., Hussein M. S., Koni P. A., Prasad P. D., Kurago Z. B., Thangaraju M., Ganapathy V., Manicassamy S. (2018) GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J. Immunol. 200, 1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diehl J., Gries B., Pfeil U., Goldenberg A., Mermer P., Kummer W., Paddenberg R. (2016) Expression and localization of GPR91 and GPR99 in murine organs. Cell Tissue Res. 364, 245–262 [DOI] [PubMed] [Google Scholar]
- 62.Gänzle M. G., Follador R. (2012) Metabolism of oligosaccharides and starch in lactobacilli: a review. Front. Microbiol. 3, 340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pouteau E., Nguyen P., Ballèvre O., Krempf M. (2003) Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc. Nutr. Soc. 62, 87–93 [DOI] [PubMed] [Google Scholar]
- 64.Reichardt N., Duncan S. H., Young P., Belenguer A., McWilliam Leitch C., Scott K. P., Flint H. J., Louis P. (2014) Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8, 1323–1335; erratum: 1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harvey R. F., Read A. E. (1973) Effect of cholecystokinin on colonic motility and symptoms in patients with the irritable-bowel syndrome. Lancet 1, 1–3 [DOI] [PubMed] [Google Scholar]
- 66.Chua A. S., Keeling P. W. (2006) Cholecystokinin hyperresponsiveness in functional dyspepsia. World J. Gastroenterol. 12, 2688–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao B., Zhang X., Yan N., Chen S., Li Y. (2012) Cholecystokinin enhances visceral pain-related affective memory via vagal afferent pathway in rats. Mol. Brain 5, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ledochowski M., Murr C., Lass-Flörl C., Fuchs D. (2001) Increased serum amylase and lipase in fructose malabsorbers. Clin. Chim. Acta 311, 119–123 [DOI] [PubMed] [Google Scholar]
- 69.Barrett T. D., Yan W., Freedman J. M., Lagaud G. J., Breitenbucher J. G., Shankley N. P. (2008) Role of CCK and potential utility of CCK1 receptor antagonism in the treatment of pancreatitis induced by biliary tract obstruction. Br. J. Pharmacol. 153, 1650–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.