Abstract
Deregulation of innate immune TLR4 signaling contributes to various diseases including neuropathic pain and drug addiction. Naltrexone is one of the rare TLR4 antagonists with good blood-brain barrier permeability and showing no stereoselectivity for TLR4. By linking 2 naltrexone units through a rigid pyrrole spacer, the bivalent ligand norbinaltorphimine was formed. Interestingly, (+)-norbinaltorphimine [(+)-1] showed ∼25 times better TLR4 antagonist activity than naltrexone in microglial BV-2 cell line, whereas (−)-norbinaltorphimine [(−)-1] lost TLR4 activity. The enantioselectivity of norbinaltorphimine was further confirmed in primary microglia, astrocytes, and macrophages. The activities of meso isomer of norbinaltorphimine and the molecular dynamic simulation results demonstrate that the stereochemistry of (+)-1 is derived from the (+)-naltrexone pharmacophore. Moreover, (+)-1 significantly increased and prolonged morphine analgesia in vivo. The efficacy of (+)-1 is long lasting. This is the first report showing enantioselective modulation of the innate immune TLR signaling.—Zhang, X., Peng, Y., Grace, P. M., Metcalf, M. D., Kwilasz, A. J., Wang, Y., Zhang, T., Wu, S., Selfridge, B. R., Portoghese, P. S., Rice, K. C., Watkins, L. R., Hutchinson, M. R., Wang, X. Stereochemistry and innate immune recognition: (+)-norbinaltorphimine targets myeloid differentiation protein 2 and inhibits toll-like receptor 4 signaling.
Keywords: norbinaltorphimine, enantioselective modulation, TLR4, MD-2, morphine analgesia
The innate immune TLR4, combined with its accessory protein myeloid differentiation protein 2 (MD-2), is responsible for recognizing pathogen-associated, damage-associated, and xenobiotic-associated molecular patterns (1–6). The signaling via TLR4’s TLR/IL-1 receptor domain results in the induction of proinflammatory factors (7–9). TLR4 signaling malfunctions contribute to the development and progression of various diseases including neuropathic pain and drug addiction (3, 9–14). Therefore, there is great interest in the development of TLR4 small-molecule modulators (3, 10, 15). A variety of TLR4 signaling antagonists targeting MD-2 have been reported (3, 10, 15–18). Among them, (+)-naltrexone is one of the rare TLR4 antagonists with good blood-brain barrier (BBB) permeability. However, the TLR4 antagonist activity of (+)-naltrexone was moderate [half maximal inhibitory concentration (IC50) = 105.5 ± 10.1 μM], and it is not long acting (19), which severely prevents its wide in vivo application. Structure-activity relationship studies of (+)-naltrexone showed that the substituted group at N-17 affected the binding with MD-2 and TLR4 antagonistic activity, increasing the hydrophobicity of the substituent at N-17 and improving its TLR4 antagonistic activity (19, 20). However, it should be noted that increasing the hydrophobicity of substituted groups meanwhile compromised the drug likeness of (+)-naltrexone–inspired TLR4 antagonists.
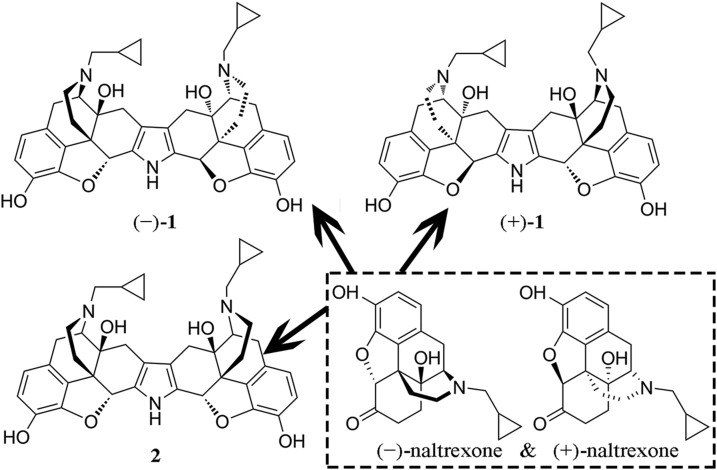
Naltrexone nonstereoselectively targets the LPS binding pocket of MD-2 (21, 22), which accommodates 5 of the 6 lipid chains of LPS (23). Compared with classic TLR4 ligand LPS (lipid A, MW: ∼1900 Da), naltrexone (MW: ∼300 Da) is much smaller. In order to design potent TLR4 antagonists, a bivalent ligand approach using naltrexone-derived antagonist pharmacophore was proposed. The enhanced potency and selectivity could be conferred by the simultaneous occupation of proximal recognition sites within the LPS binding pocket of MD-2 by both units of a single bivalent ligand (24). To eliminate the possibility of the simultaneous interaction of both naltrexone-derived pharmacophores and 2 proximal MD-2 coreceptors, a conformationally rigid pyrrole spacer was used (24, 25). (−)-Norbinaltorphimine [(−)-1], which is a classic κ-opioid receptor antagonist (24, 25), contains 2 pharmacophores derived from (−)-naltrexone. (+)-Norbinaltorphimine [(+)-1] has 2 pharmacophores derived from (+)-naltrexone, whereas the meso isomer 2 of norbinaltorphimine contains a combination of the pharmacophores derived from (−)-naltrexone and (+)-naltrexone. Interestingly, (+)-1 showed ∼25 times better TLR4 antagonist activity than (+)-naltrexone, whereas (−)-1 lost TLR4 activity. The meso isomer 2 has similar TLR4 antagonistic activity as (+)-1. The results show that the stereochemistry of (+)-1 is derived from the (+)-naltrexone pharmacophore. Molecular dynamics simulations support that the binding site of the pharmacophore derived from (+)-naltrexone within the LPS binding pocket of MD-2 is responsible for the stereoselectivity. In vivo animal testing showed that (+)-1 significantly increased and prolonged morphine analgesia. Further, the effect of (+)-1 is long lasting.
MATERIALS AND METHODS
In vitro assays
Chemicals
(−)-1 was obtained from Drug Supply Program of National Institute of Drug Abuse (Bethesda, MD, USA). (+)-1 and 2 were prepared according to the method described by Portoghese et al. (24, 25).
BV-2 cell culture
BV-2 murine microglia were grown in supplemented DMEM including 10% fetal bovine serum (FBS), 50 U/ml penicillin, and 50 μg/ml streptomycin. BV-2 cells were detached from the flask by a cell lifter when confluence was reached. Cells were seeded at a density of 4 × 104 cells per well in 96-well plates. After overnight incubation, medium was aspirated and changed to DMEM without FBS. Cells were then treated with 200 ng/ml LPS and various concentrations of (−)-1, (+)-1, or 2. After 24 h, supernatant was collected for NO analysis and TNF-α ELISA. Meanwhile, cells were stained by crystal violet for viability analysis or collected for IL-1β ELISA.
Primary microglia and astrocyte isolation and culture
Brain cortices from Passage 0/1 neonatal Sprague-Dawley rat pups were separately and carefully dissected, and the overlying meninges were removed. The cortical tissue was then minced with a scalpel blade and digested for 30 min in Liberase Blendzyme III (1.4 mWunsch units/brain; Roche, Basel, Switzerland) and DNAse (0.1 U/brain; MilliporeSigma, Burlington, MA, USA) at 37°C with agitation. The cells were triturated with a 21-gauge and a 23-gauge hypodermic needle. Modified Eagle medium (MEM) supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, 0.6% glucose, and 2 mM l-glutamine was added, and the cells were centrifuged. The supernatant was discarded, and the cells were resuspended in 10 ml of MEM with supplements/4 brains. The cells were filtered through a 70-μm and then a 40-μm filter. Cells were plated in 75-cm2 tissue culture flasks at 4 brains/flask. Cells were incubated at 37°C and 5% CO2 until confluence was reached (about 10 d). Medium was changed every 3–4 d, with the first change being a complete medium change and subsequent changes being 50% medium changes. Once confluence was reached, microglial cells were shaken from the remaining astrocytes for 90 min on an orbital shaker at 160 rpm. The medium containing the microglia were removed and centrifuged, and the pellet was resuspended in 1 ml fresh MEM. A small aliquot of cells was counted with trypan exclusion and plated in 96-well v-bottom tissue culture plates at 40,000 cells/well in 100 μl medium. At 48 h after plating, medium was changed to MEM without FBS. In total, 200 ng/ml LPS and various concentrations of (−)-1, (+)-1, or 2 were added. After 24 h of treatment, medium was harvested for NO assay and TNF-α ELISA. Meanwhile, cells were stained by crystal violet for viability analysis.
Once the microglia were removed from the flask, the astrocytes were treated for 2 h with 10 ml DMEM (10% FBS and 0.6% glucose) containing 5 mM l-leucine methyl ester to deplete any remaining microglia. The cells were washed twice with DPBS. A single cell suspension was obtained by 5 min of incubation with 0.05% trypsin/EDTA. The cells were centrifuged, and the pellet was reconstituted with DMEM/F12 supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2 mM l-glutamine at 40,000 cells/well of a 96-well plate. At 48 h after plating, the medium was changed to DMEM without FBS. In total, 200 ng/ml LPS and various concentrations of (−)-1, (+)-1, or 2 were added. After 24 h treatment, the medium was harvested for NO assay and TNF-α ELISA assay. Meanwhile, cells were stained by crystal violet for viability analysis.
NO assay
A 100-µl portion of supernatant medium was removed after cells were treated for 24 h and added to flat, black 96-well Microfluor plates (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, 10 µl of 2, 3-diaminonaphthalene (0.05 mg/ml in 0.62 M HCl) was added to each well and incubated for 15 min. The reaction was quenched by addition of 5 µl of 3 M NaOH, and the plate was read on a Synergy [1H] Microplate Reader (BioTek Instruments, Winooski, VT, USA) with excitation at 360 nm and emission at 430 nm. The NO of the LPS (200 ng/ml)-treated group was set as 100%.
TNF-α and IL-1β ELISA assays
TNF-α and IL-1β proteins were measured using commercially available ELISA kits (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions.
Cell viability assay
Crystal violet staining was used to determine cell viability as previously described (21). After treatment, cells were fixed with 3.7% paraformaldehyde for 5 min and then stained with 0.05% crystal violet for 15 min. The plates were subsequently washed twice with tap water and dried for 30 min at room temperature; 200 μl of methanol was added to each well, and the plates were shaken for 15 min at room temperature to dissolve the dye. Absorbance at 540 nm was measured using a Synergy [1H] Microplate Reader.
MD-2 binding
MD-2 expression and purification was performed as previously described (4, 5, 22). Fluorescence measurements were performed on a Cary Eclipse spectrofluorometer (Agilent Technologies, Santa Clara, CA, USA). All measurements were carried out under room temperature in a 2-×-10-mm quartz cell (Starna Cells, Atascadero, CA, USA). Two hundred and ninety nanometers was chosen as the excitation wavelength of MD-2 intrinsic Trp fluorescence, and emission at 310–450 nm was measured. Appropriate controls were subtracted from spectra obtained on the samples. In total, 0.5 μM MD-2 was titrated with different concentrations of ligand. The fluorescence intensity at 337 nm was plotted against compound concentration. The raw data were fitted by nonlinear least square method using the following equation: F=0.5×<2×F0-FPL×{Kd+[LT]+[PT]-[(Kd+[LT]+[PT])2-4×[LT]×[PT]]0.5}>, where F is the observed fluorescence; F0 is the initial fluorescence of protein in the absence of ligand; FPL is the adjustable parameter for protein-ligand complex molar fluorescence; Kd is the dissociation constant; [LT] is the total concentration of the ligand; and [PT] is the total protein concentration.
In silico simulations
System preparation and molecular docking
MD-2 was extracted from the X-ray crystal structure of TLR4–MD-2–lipid IVa complex (Protein Data Bank identifier: 3VQ1) (26). The missing residues and hydrogen atoms were added under pH 7.0 using Maestro (27). (−)-1, (+)-1, and 2 were obtained from PubChem and refined by GaussView 5 (28). All compounds were optimized by the Gaussian 09 program with B3LYP density functional method and 6-31+G (d, p) basis set (29–31). The docking poses were determined by AutoDock Vina 1.1.2 (32). Optimal binding sites were searched in a box of 51 Å × 48 Å × 56 Å, in which the top 20 poses were picked up based on the Iterated Local Search Globule Optimizer (33, 34). The semiflexible molecular docking was carried out, and MD-2 was treated as a rigid body.
Molecular dynamics simulation and free-energy calculation
Based on the docking results, the best binding models of (−)-1, (+)-1, and 2 with MD-2 were refined using molecular dynamics simulation with the NAMD 2.11 (35) program. The Amber 03 force field (36, 37) was used for MD-2 protein. Atomic charges of (−)-1, (+)-1, and 2 were fitted by R.E.D. tools (38) based on the quantum mechanics calculations. All systems were solvated in a cubic box with TIP3P water molecules, with a minimum distance of 10 Å between the solute and the edge of the box. Na+ and Cl− atoms were added to neutralize the system and mimic the physiologic conditions. All directions were performed under periodic boundary conditions. All of the angles and bonds involving hydrogen were constrained by using the SHAKE algorithm (39). The particle mesh Ewald method (40) was used to deal with the long-range electrostatic interactions. Pressure was scaled at 1 atm with the Nosé-Hoover-Langevin piston method (41).
All systems were first minimized with 10,000 steps using the conjugate gradient algorithm followed by gradual heating to 310 K in 310 ps. After that, temperature was kept at 310 K using Langevin dynamics with the collision frequency of 5 1/ps. Then, the volume of each system was adjusted under a constant number, pressure, and temperature ensemble for 2 ns. Subsequently, 3 independent molecular dynamics productions with 100-ns lengths were performed under a constant number, volume, and temperature ensemble for each system.
Based on the 300 snapshots extracted from the last 30-ns equilibrated molecular dynamics trajectory, the binding free energies of binary naltrexone derivatives in complex with MD-2 were calculated using the molecular mechanics Poisson-Boltzmann solvent accessible surface area (MM-PBSA) method (42)
where binding free energy (ΔGbinding) consists of the molecular mechanical energy in the gas phase (ΔEMM), the solvation free energy (ΔGsolvation), and the penalty of entropy (−TΔS). The entropy contributions were ignored because of the high computational cost and the low reliability (20, 43–45). ΔEMM can be divided into the van der Waals interactions (ΔEvdw), electrostatic interactions (ΔEele). ΔGsolvation contains polar solvation free energy (ΔGsol-polar) and nonpolar solvation free energy (ΔGsol-nonpolar). In addition, per-residue decomposition of free energy (ΔGdecomp) was calculated.
Potential of mean force calculation
Umbrella-sampling (US) (46) simulation was performed to explore the free-energy profile of the binding process of binary naltrexone derivatives. The reaction coordinate was defined as the distance between the center of mass of the nonhydrogen atoms of ligands and the center of mass of the stable part of the so-called β-cup fold (47) of MD-2. The biasing harmonic potential with a force constant of 10 kcal/(mol × Å2) was performed along the reaction coordinate. The initial complex structure was adopted from the most stable model during a 100-ns molecular dynamics simulation. The sampling windows were spaced every 0.5 Å from 3.5 to 33 Å, resulting in 60 windows for each system. All windows were adjusted under an unrestrained equilibration for 0.2 ns, followed by a 5-ns US simulation. Subsequently, the unbiased potential of mean force (PMF) was recovered using the weighted histogram analysis method (48) from the US probability distributions. PMF errors were performed using bootstrap error analysis with 60 bootstraps and 4000 random number seeds.
In vivo study
Animals
Pathogen-free, adult male Sprague-Dawley rats were used (300–325 g on arrival) and housed in temperature (23 ± 3°C)- and light (12-h light/dark cycle; lights on at 07:00 am)-controlled rooms with standard rodent chow and water available ad libitum and allowed to habituate to the holding facility for at least 1 wk prior to experimentation.
Intrathecal catheter implantation and drug delivery
The method of acute intrathecal drug administration and the construction and implantation of the indwelling intrathecal catheters were based on work previously described by Manning et al. (49). In brief, the day prior to experimentation, intrathecal operations were conducted under isoflurane anesthesia by threading sterile Polyethylene-10 Intramedic Tubing [Becton Dickinson (BD), San Diego, CA, USA] guided by an 18-gauge needle between the L5 and L6 vertebrae. The catheter was inserted such that the proximal catheter tip lay over the lumbosacral enlargement. The needle was removed, and the catheter was sutured to the superficial musculature of the lower back. The catheters were preloaded with 60 μg of (+)-1 in 0.9% saline + 15 μg of morphine in 0.9% saline or vehicle (0.9% saline + 15 μg morphine in 0.9% saline) at the distal end in a total volume of 10 μl. The catheters were 90 cm in length, allowing remote drug delivery without touching or otherwise disturbing the rats during the testing. A second intrathecal dose of morphine (15 μg) was administered 24 h later. On the day of experimentation, drugs were delivered over 20–30 s.
Hargreaves test for analgesia
Rats received at least three 60 min habituations over successive days to the test environment prior to behavioral testing. Latencies for behavioral response to radiant heat stimuli applied to the tail were assessed using a modified Hargreaves test (50). All testing was conducted blind with respect to group assignment. Briefly, baseline withdrawal values were calculated from an average of 3 consecutive withdrawal latencies of the tail, measured at 10 min intervals. A short baseline latency (2–3 s) was used to allow quantification of analgesia (lengthening of the latency relative to baseline in response to analgesia). A cutoff time of 10 s was imposed to avoid tissue damage. Nociceptive assessments for acute administration experiments were then made at 0 min (immediately following remote drug delivery) and every 10 min thereafter for 180 min. The results are expressed as percent maximum potential effect. Data are presented as means ± sd, and significance was set at P < 0.05.
Data analysis
Data are presented as means ± sd, unless otherwise stated. Origin 2016 (OriginLab, Northampton, MA, USA) was used for plotting the data and statistical analysis. Statistical significance was evaluated by unpaired Student’s t test or 1-way ANOVA. Probability values were 2-tailed, and the statistical significance criterion P value was 0.05.
RESULTS
(+)-1 enantioselectively inhibits TLR4 signaling
Naltrexone nonstereoselectively targets the LPS binding pocket of MD-2. It is a moderate TLR4 antagonist (21, 51). To improve its activity, a bivalent ligand approach was employed. (+)-1 was formed by coupling 2 pharmacophores derived from (+)-naltrexone through a conformationally rigid pyrrole spacer. Similarly, (−)-1 has 2 pharmacophores derived from (−)-naltrexone (Fig. 1).
Figure 1.
Structures of naltrexone and its binary derivatives, (+)-1, (−)-1, and the meso isomer 2.
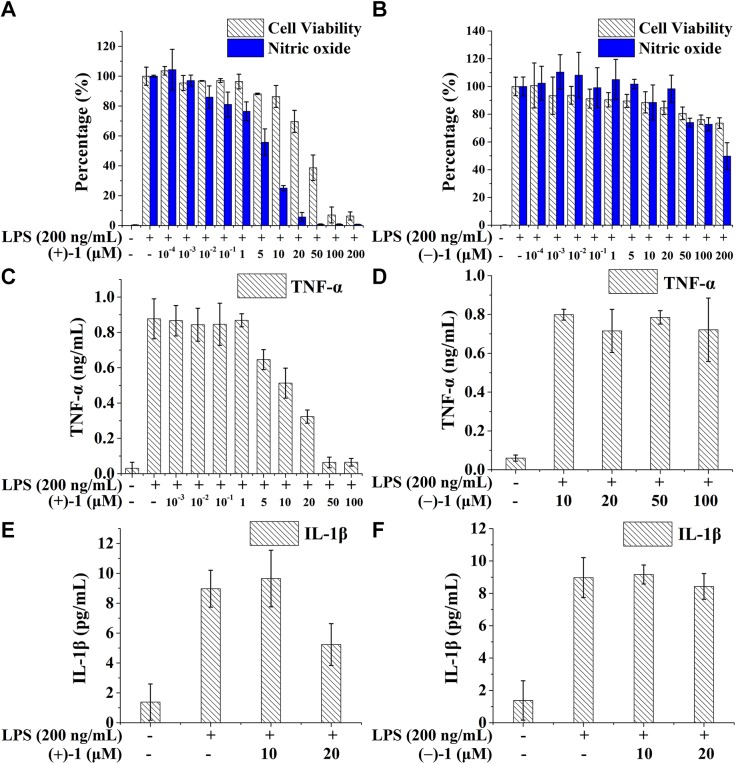
Microglia are the resident cells of the innate immune system in the CNS, where TLR4 is primarily expressed (52). Naltrexone nonstereoselectively inhibits microglial activation and proinflammatory factor overproduction (21). Therefore, the BV-2 microglial cell line was used for testing the activities of norbinaltorphimine, as these cells reproduce many of the responses of primary microglia (53). As shown in Fig. 2A, (+)-1 inhibited LPS-induced TLR4 signaling of NO in a concentration-dependent manner, with an IC50 of 4.7 ± 1.8 μM, which possesses ∼25 times better TLR4 antagonist activity than (+)-naltrexone (105.5 ± 10.1 μM) (19, 21). In addition to NO inhibition, (+)-1 also inhibited the LPS-induced TLR4 signaling of proinflammatory factors TNF-α (IC50 = 12.1 ± 2.0 μM, Fig. 2B) and IL-1β (Fig. 2C) in a concentration-dependent manner. The effect of (+)-1 on BV-2 cell viability was also tested by crystal violet staining assay, and an IC50 of 44.1 ± 11.2 μM was obtained. (+)-1 showed no apparent cellular toxicity at concentrations <20 μM, which eliminates the possibility of the observed inhibition of TLR4 signaling by (+)-1 due to an artifact like cell death. Unlike (+)-1, enantiomer (−)-1 showed no apparent inhibition toward LPS-induced NO (Fig. 2D), TNF-α (Fig. 2E), or IL-1β (Fig. 2F) overproduction in BV-2 cells. The IC50 value of (−)-1 was >200 μM in the cell viability testing.
Figure 2.
Norbinaltorphimine enantioselectively inhibits TLR4 signaling in the microglial BV-2 cell line. (+)-1 inhibited LPS-induced NO (A), TNF-α (B), and IL-1β (C) overproduction in BV-2 cell line, whereas (−)-1 showed no apparent inhibition toward LPS-induced NO (D), TNF-α (E), and IL-1β (F) overproduction in BV-2 cells.
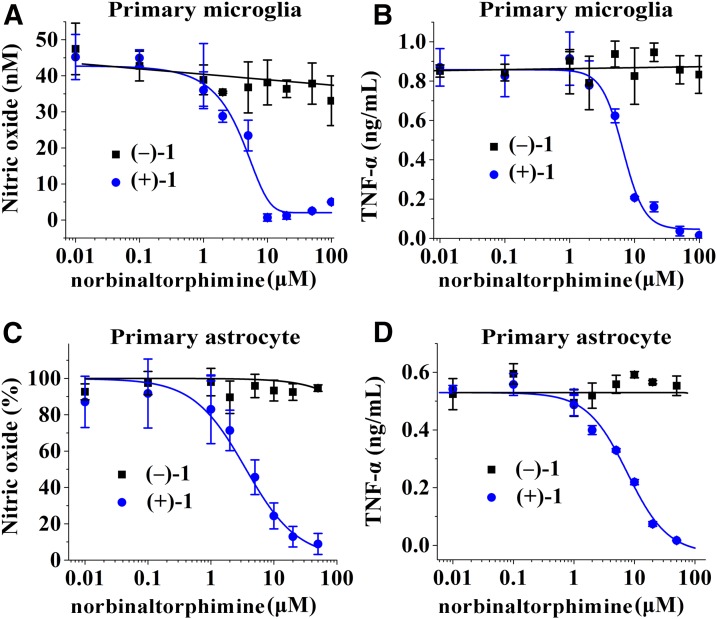
The effects of norbinaltorphimine were also investigated in primary microglial cells to demonstrate the enantioselectivity of (+)-1 in inhibiting TLR4 signaling. (+)-1 inhibited LPS-induced NO (IC50 = 2.3 ± 0.8 μM, Fig. 3A) and TNF-α (IC50 = 6.4 ± 0.8 μM, Fig. 3B) in primary microglial cells. The therapeutic ratio of cytotoxicity (136.8 ± 39.8 μM, Supplemental Fig. S1) to TLR4 antagonism for (+)-1 in primary microglia was ∼20–60. In contrast, (−)-1 did not inhibit LPS-induced NO and TNF-α overproduction.
Figure 3.
Norbinaltorphimine enantioselectively inhibits TLR4 signaling in the primary microglial cells and primary astrocytes. (+)-1 inhibited LPS-induced NO (A, C) and TNF-α (B, D) overproduction in primary microglia (A, B) and primary astrocytes (C, D), whereas (−)-1 showed no apparent effect.
In addition to microglia, astrocytes also express TLR4 (6, 54). To further confirm the enantioselectivity of (+)-1 in modulating TLR4 signaling, the effects of norbinaltorphimine were tested in primary astrocytes. (+)-1 inhibited LPS-induced NO (IC50 = 4.1 ± 0.4 μM, Fig. 3C) and TNF-α (IC50 = 7.7 ± 0.8 μM, Fig. 3D) in astrocytes in a concentration-dependent manner and did not show apparent cellular toxicity toward primary astrocytes at concentrations <20 μM (Supplemental Fig. S2). As observed in BV-2 cell line and primary microglia, (−)-1 showed no apparent inhibition of TLR4 signaling in astrocytes.
In addition to CNS immunocompetent cells, the effects of norbinaltorphimine on TLR4 signaling in peripheral macrophages were also measured. (+)-1 inhibits LPS-induced NO overproduction in peritoneal macrophages with an IC50 = 4.6 ± 1.5 μM, whereas (−)-1 shows no apparent effect in inhibiting LPS-induced NO overproduction (Supplemental Fig. S3). Both (+)-1 and (−)-1 showed no apparent cellular toxicity toward primary macrophages under the tested concentrations. These results show that (+)-1 could also modulate peripheral TLR4 innate immune signaling.
In order to investigate the TLR4 specificity of (+)-1, the effects of (+)-1 on TLR signaling of NO were measured because TLRs share common structures and signaling that lead to the overproduction of NO. (+)-1 did not affect TLR1-TLR2, TLR2-TLR6, and TLR7/8 signaling of NO overproduction (Supplemental Fig. S4). It should be noted that BV-2 cells are not responsive to TLR3, TLR5, or TLR9 agonist. Among all members of the TLR protein family, TLR4 is the only one that requires the coreceptor (MD-2) responsible for ligand recognition. It is not surprising that (+)-1, which targets MD-2, shows TLR4 signaling specificity.
Together, these results demonstrate that (+)-1 enantioselectively inhibited TLR4 signaling, whereas (−)-1 had no effect on TLR4. These data highlight the stereochemistry of TLR4 recognition and modulation.
Biophysical binding of norbinaltorphimine with MD-2
Naltrexone binds to the LPS binding pocket of MD-2 (21, 22, 51, 55). In order to improve TLR4 antagonistic activity, 2 naltrexone molecules were linked by a conformationally rigid pyrrole spacer. The increased activity and selectivity could be conferred by the simultaneous occupation of proximal recognition sites within the LPS binding pocket of MD-2 by both pharmacophores of a single bivalent ligand (24).
In order to understand how the stereochemistry of norbinaltorphimine affects TLR4 signaling, the interactions of norbinaltorphimine with MD-2 were investigated. (+)-1 bound to MD-2 with a dissociation constant (Kd) of 0.60 ± 0.10 μM (Supplemental Fig. S5), which is ∼20 times more potent than (+)-naltrexone (Kd = 13.7 ± 0.3 μM) (22). The Kd of (−)-1 with MD-2 was too weak to fit (Supplemental Fig. S5). The binding of MD-2 with the meso isomer 2 of norbinaltorphimine, which contains a combination of the pharmacophores derived from (−)-naltrexone and (+)-naltrexone, was also investigated. Isomer 2 bound to MD-2 with a similar affinity (Kd = 0.70 ± 0.14 μM) as (+)-1. Further activity testing showed that isomer 2 had similar TLR4 antagonistic activity (IC50 = 6.1 ± 1.9 μM) as (+)-1 in inhibiting LPS-induced NO overproduction in BV-2 cells (Supplemental Fig. S6). These results indicate that the stereochemistry of norbinaltorphimine in modulating TLR4 is derived from the (+)-naltrexone pharmacophore.
Molecular dynamics simulations of norbinaltorphimine binding to MD-2
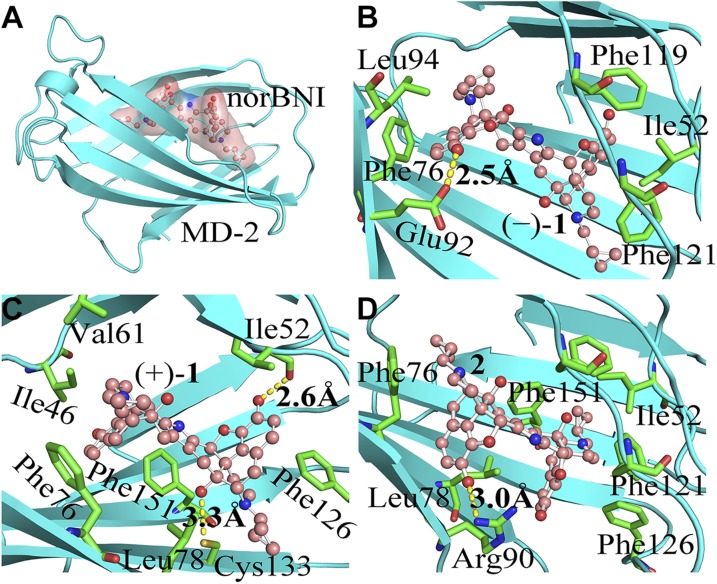
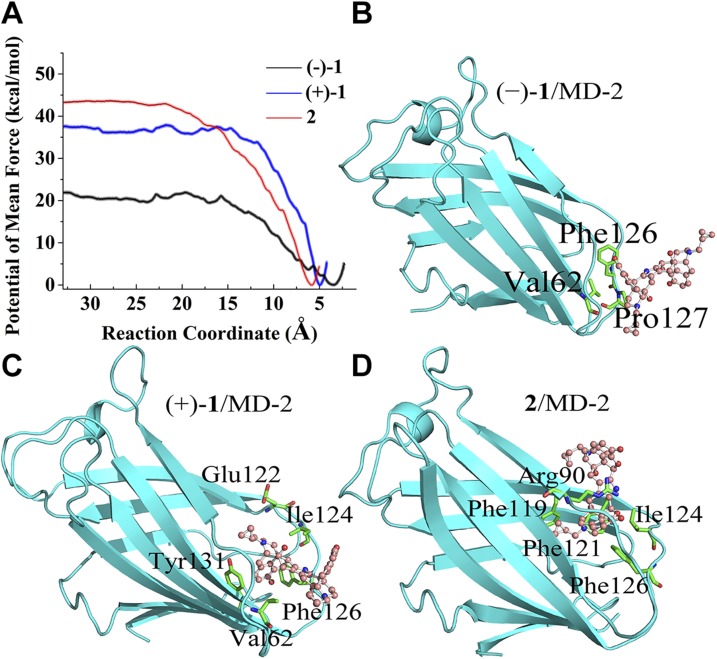
To further explore stereochemistry in the innate immune recognition of TLR4, molecular dynamics simulations of norbinaltorphimine binding to MD-2 were performed. (−)-1, (+)-1, and 2 (Fig. 1) were first docked into the conserved hydrophobic cavity of MD-2. They occupied a large portion of the LPS binding pocket. The best docking pose of each molecule in MD-2 is given in Supplemental Fig. S7. Molecular dynamics simulations were subsequently performed. According to the root-mean square deviation values of MD-2 (Supplemental Fig. S8), each system reached stable state during 100-ns simulations. The binding free energies were evaluated by using the MM-PBSA method. Figure 4A shows the view of the overall binding mode of norbinaltorphimine/MD-2 with the lowest energy during molecular dynamics simulations. The calculated binding free energies are shown in Table 1. (+)-1 (ΔGbinding = −32.7 ± 0.3 kcal/mol) and 2 (ΔGbinding = −32.0 ± 0.2 kcal/mol) have similar binding free energies, whereas ΔGbinding of (−)-1 (−22.2 ± 0.3 kcal/mol) was much higher. The calculated binding free energies were in good agreement with biophysical binding results that (+)-1 and 2 had similar binding affinity with MD-2, whereas (−)-1 showed poor MD-2 binding affinity. Energy decomposition (Table 1) showed that the binding of (+)-1 and its derivatives to MD-2 are primarily driven by hydrophobic interactions (ΔEvdw + ΔGsol-nonpolar).
Figure 4.
The calculated binding mode of norbinaltorphimine/MD-2 complex. A) View of the overall binding structure of norbinaltorphimine/MD-2 with the lowest energy during molecular dynamics simulations. B–D) Enlarged view of the interactions of (−)-1 (B), (+)-1 (C), and 2 (D) binding with MD-2. Ligands are shown as a ball-and-stick model, and MD-2 is shown as a cyan cartoon. Key residues of MD-2 in interacting with small-molecule antagonists are shown as green sticks. The yellow dash line represents a hydrogen bond.
TABLE 1.
MM-PBSA derived binding free energies (kcal/mol), experimentally determined Kd with MD-2 and IC50 values of inhibiting TLR4 induced NO overproduction in BV-2 cells for (−)-1, (+)-1, and 2
| ID | ΔEvdw | ΔEele | ΔGsol-polar | ΔGsol-nonpolar | ΔGbinding | Kd (μM) | IC50 (μM) |
|---|---|---|---|---|---|---|---|
| (−)-1 | −64.6 ± 0.1 | −2.6 ± 0.3 | 4.6 ± 0.1 | 37.8 ± 0.3 | −22.2 ± 0.3 | N.A. | 208.6 ± 46.9 |
| (+)-1 | −75.8 ± 0.3 | −6.1 ± 0.1 | 4.4 ± 0.1 | 38.7 ± 0.3 | −32.7 ± 0.3 | 0.6 ± 0.1 | 4.7 ± 1.8 |
| 2 | −74.3 ± 0.2 | −2.3 ± 0.1 | 5.4 ± 0.1 | 36.9 ± 0.2 | −32.0 ± 0.2 | 0.7 ± 0.1 | 6.1 ± 1.9 |
ΔEvdw, van der Waals interactions; ΔEele, electrostatic interactions; ΔGbinding, binding free energy; ΔGsol-polar, polar solvation free energy; ΔGsol-nonpolar, nonpolar solvation free energy; ID, identifier; N.A., not applicable.
To further investigate the detailed binding mode of (−)-1, (+)-1, and 2 in complex with MD-2, the interactions were analyzed by per-residue free-energy decomposition. For (−)-1, the hydroxyl oxygen atoms formed hydrogen bonds with the benzene hydroxyl of the side chain of Glu92. The backbone of (−)-1 formed hydrophobic interactions with the residues Ile52, Phe76, Phe119, and Phe121 (Fig. 4B). Compared with (−)-1, (+)-1/2 with the (+)-naltrexone–derived portion showed better interaction (Supplemental Table S1) with MD-2. The backbone of 1 (+)-naltrexone–derived pharmacophore of (+)-1 was sandwiched by Phe126 and Phe151 through π stacking interaction. As the donor, 3′- and 6'-benzene hydroxyls of this (+)-naltrexone–derived pharmacophore formed 2 hydrogen bonds with the oxygen of backbone of Ile52 (2.6 Å) and the sulfur of side chain of Cys133 (3.3 Å), respectively. The other portion of (+)-1 was stabilized by the inside residue Ile46, Val61, Phe76, Leu78, and Phe121 of MD-2 through hydrophobic interactions (Fig. 4C). For the meso isomer 2, 1 hydrogen bond was formed between 3′-benzene hydroxyl oxygen atoms of (−)-naltrexone–derived portion and the nitrogen atoms of side chain of Arg90 (3.0 Å). The backbone of (+)-naltrexone–derived portion of isomer 2 was surrounded by Ile52, Phe76, Leu78, Phe119, Phe121, Phe126, and Phe151 through strong hydrophobic interactions (Fig. 4D and Supplemental Table S1).
In order to explore the binding process of norbinaltorphimine with MD-2, PMF calculations were performed. The corresponding binding free-energy profile along the reaction coordinate for each ligand is shown in Fig. 5A. (+)-1 and 2 had similar dissociation energy barriers. In contrast, the dissociation energy barrier of (−)-1 with MD-2 was much less than that of (+)-1 and 2. These results agree with the data of measured Kd (Supplemental Fig. S5) and binding free energies calculated by MM-PBSA (Table 1).
Figure 5.
The calculated binding process of norbinaltorphimine with MD-2. A) The free-energy profiles for (−)-1 (black), (+)-1 (blue), and 2 (red) binding with MD-2. The reaction coordinate was defined as the distance between the center of mass of the nonhydrogen atoms of ligands and that of the C-α atoms of the stable part of the so-called β-cup fold of MD-2. B–D) The binding snapshot for (−)-1 (B), (+)-1 (C), and 2 (D) at the entrance of MD-2 (cavity A). Key residues of MD-2 in recognizing norbinaltorphimine are shown as green sticks. Ligands are shown as a ball-and-stick model, and MD-2 is shown as a cyan cartoon.
The binding pocket of MD-2 could be divided into cavity A and cavity B (56). As shown in Supplemental Fig. S7A, cavity A is located around the gating loop, whereas cavity B is deep and inaccessible to solvent molecules. Along the binding process, the (+)-naltrexone–derived pharmacophore of (+)-1 (Fig. 5C) and 2 (Fig. 5D) were recognized by the residues of cavity A, which facilitates (+)-1 and 2 entering toward the inner cavity B of MD-2 and prompts the formation of stable state (Supplemental Table S2). However, the (−)-naltrexone–derived pharmacophore showed poor interactions with the residues at the entrance of MD-2 (cavity A, Fig. 5B), which disfavored (−)-1 moving into the cavity B and the formation of the stable complex with MD-2 (Supplemental Table S2). These in silico simulation results clearly explain that the (+)-naltrexone pharmacophore determined the stereochemistry of naltrexone-derived bivalent ligands in the MD-2 recognition as well as TLR4 signaling modulation.
The in vivo efficacy of (+)-1
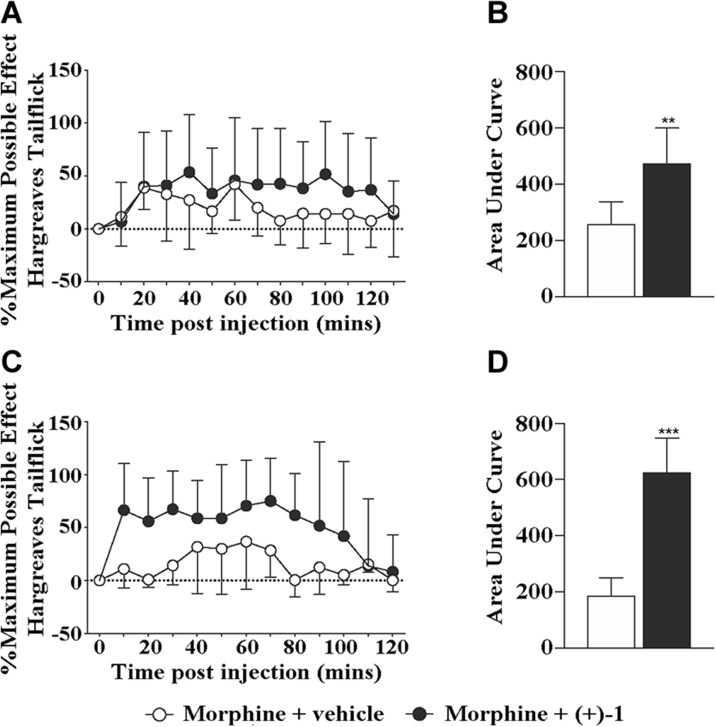
(+)-Naltrexone acts as a TLR4 antagonist and has been shown to potentiate acute intrathecal morphine analgesia in vivo (57). To investigate the in vivo efficacy of (+)-1, the Hargreaves thermal hyperalgesia assays were performed to test whether (+)-1 was able to potentiate the analgesic effect of morphine. Coadministration of (+)-1 (60 μg, intrathecal) significantly increased and prolonged intrathecal morphine analgesia (Fig. 6A, B; P < 0.01). Interestingly, (+)-1 could even boost the analgesic effect of a second dose of morphine 24 h after (+)-1 administration (Fig. 6C, D; P < 0.001). (+)-1 was active in vivo; it significantly increased and prolonged morphine analgesia. Unlike (+)-naltrexone, the in vivo efficacy of (+)-1 on potentiating morphine analgesia is long lasting.
Figure 6.
The in vivo efficacy of (+)-1 on potentiating morphine analgesia. A, B) Intrathecal coadministration of morphine (15 μg) with (+)-1 (60 μg) produced a significant potentiation of morphine tail flick analgesia (A), as measured by area under the curve (B). C, D) (+)-1 even potentiated a second dose of morphine 24 h after (+)-1 administration (C) as measured by area under the curve (D); n = 5–6/group. **P < 0.01, ***P < 0.001.
DISCUSSION
Opioid isomers (−)-naltrexone and (+)-naltrexone nonstereoselectively target the LPS binding pocket of MD-2 (21, 22), the key coreceptor of TLR4. Via this binding, (−)-naltrexone and (+)-naltrexone inhibit TLR4 signaling with equipotency (21). Unlike other TLR4 antagonists, (−)-naltrexone and (+)-naltrexone show good BBB permeability owing to its opioid-like structure. It should be noted that the molecular recognition of opioids by classic opioid receptors shows stereoselectivity. (−)-Opioid isomers but not the (+)-isomers show good affinity toward classic opioid receptors. Therefore, (+)-opioid isomers are inactive toward classic opioid receptors and will not interfere with the reward system of endogenous opiates. To prevent the side effects of being a classic opioid receptor antagonist, (+)-naltrexone but not (−)-naltrexone was used as the template for the development of TLR4 antagonist. (+)-Naltrexone potentiates acute intrathecal morphine analgesia (57), prevents cocaine self-administration (22), and decreases incubated cue-induced heroin seeking. However, (+)-naltrexone shows moderate TLR4 antagonist activity and is not long acting in vivo. Because of this, we have explored the possibility of improving TLR4 antagonist activity through a bivalent ligand approach, which had been successfully used by Portoghese et al. (24, 25) to generate bivalent ligand (−)-1 as a κ-opioid antagonist. The increased TLR4 antagonistic potency could be conferred by the simultaneous occupation of proximal recognition sites within the large binding pocket of MD-2 by both units of a single bivalent ligand (24). A conformationally rigid pyrrole spacer was used to connect 2 naltrexone-derived pharmacophores to eliminate the possibility of simultaneously interacting with 2 MD-2 coreceptors (24, 25). Bivalent ligand (+)-1 enantioselectively inhibited TLR4 signaling in the microglial BV-2 cell line, primary microglial cells, and primary astrocytes, whereas (−)-1 showed no apparent TLR4 inhibition. Compared with naltrexone, (+)-1 showed an ∼25-fold increase in activity and much better window of inhibiting TLR4 activity without apparent cellular toxicity. Most gratifying, the bivalent ligand approach generated chemical probes for enantioselectively modulating the innate immune TLR4 signaling. Biophysical binding and molecular dynamics simulations showed (+)-1 had much better MD-2 binding affinity than (−)-1. The meso isomer 2 of norbinaltorphimine was also investigated to understand how the stereochemistry of norbinaltorphimine affects TLR4 innate immune recognition. The results showed that the (+)-naltrexone pharmacophore determined the stereochemistry of naltrexone-derived bivalent ligands in the MD-2 recognition as well as TLR4 signaling modulation. In vivo animal testing showed that (+)-1 significantly increased and prolonged morphine analgesia. Further, the effect of (+)-1 is long lasting. This enhancement of potency and duration of (+)-1 is related to the reduced CNS sensitization as a consequence of TLR4 inhibition in both microglia and astrocytes (58). Therefore, (+)-1 may have great translational potential. However, it should be noted that the therapeutic window of (+)-1 is narrow. Further structure-activity relationship studies would be required for improving potency and reducing toxicity in order to advance (+)-naltrexone–based bivalent ligand into preclinical development.
Opioid derivatives are mainly used as chemical probes or drugs in the CNS, owing to their good BBB permeability. Herein, TLR4 antagonist (+)-1 was found to potentiate acute intrathecal morphine analgesia. Deregulation of innate immune TLR4 signaling contributes to various diseases (3), most of which are not within the CNS. Systemic administration of (+)-1 would affect both central and peripheral TLR4 innate immune signaling. Therefore, (+)-1 might have potential for treating TLR4-driven non-CNS diseases such as acute and chronic peripheral inflammatory diseases.
Stereochemistry is vital for pharmaceutical development and determines drug effects. Numerous TLR small-molecule modulators have been developed (3). However, none of them shows enantioselectivity toward TLRs. Herein, (+)-1 but not (−)-1 was found to target MD-2 and inhibit TLR4 signaling. It should be noted that (−)-1 is a classic κ-opioid receptor antagonist, whereas (+)-1 has no apparent κ-opioid receptor activity (24, 25). Using (+)-1 instead of racemic norbinaltorphimine as TLR4 antagonist has the advantage of not interfering with endogenous κ-opioid receptor activity, therefore reducing the off-target side effects.
In summary, this study demonstrates that the bivalent ligand approach is a novel strategy for modulating ligand’s biologic chiral recognition. This study provides a novel example of an opioid derivative enantioselectively targeting and modulating nonclassic opioid receptors. To the best of our knowledge, this is the first report showing enantioselective modulation of the innate immune TLR signaling.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the Drug Supply Program of National Institute of Drug Abuse (Bethesda, MD, USA) for providing (−)-norbinaltorphimine. The authors also thank the Network and Coumputing Center, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, National Supercomputer Center of China in Guangzhou and the Computing Center of Jilin Province for the supply of computational resources. This work was supported by the National Key Research and Development Program of China (2016YFC0800907), the National Natural Science Foundation of China (21850410455, 21750110432, 21877106), the 100 Talents Program of Chinese Academy of Sciences, Young Talents Program of Chinese Academy of Agricultural Sciences, Central Public-interest Scientific Institution Basal Research Fund (NO.1610342016013), Natural Science Foundation of Jilin Province (20180101021JC), Open Fund of State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University (Grant No. KF-GN-201601), and U.S. National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research Grant R01 DE017782 (to L.R.W.). The authors declare no conflicts of interest.
Glossary
- BBB
blood-brain barrier
- FBS
fetal bovine serum
- IC50
half maximal inhibitory concentration
- Kd
dissociation constant
- MD-2
myeloid differentiation protein 2
- MEM
modified Eagle medium
- MM-PBSA
molecular mechanics Poisson-Boltzmann solvent accessible surface area
- ΔGbinding
binding free energy
- (+)-1
(+)-norbinaltorphimine
- (−)-1
(−)-norbinaltorphimine
- PMF
potential of mean force
- US
umbrella sampling
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Wang designed the research; X. Zhang and Y. Wang performed in silico simulations; X. Zhang, Y. Peng, T. Zhang, and S. Wu performed the cell culture, nitric oxide assay, ELISA assay, and cell viability assay; X. Wang performed the fluorometric titration assay; P. M. Grace, L. R. Watkins, and M. R. Hutchinson and A. J. Kwilasz performed the in vivo study; M. D. Metcalf, B. R. Selfridge, P. S. Portoghese, and K. C. Rice prepared the compounds; and X. Zhang, P. M. Grace, L. R. Watkins, M. R. Hutchinson, and X. Wang analyzed the data and wrote the manuscript.
REFERENCES
- 1.Takeuchi O., Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–820 [DOI] [PubMed] [Google Scholar]
- 2.Yang H., Hreggvidsdottir H. S., Palmblad K., Wang H., Ochani M., Li J., Lu B., Chavan S., Rosas-Ballina M., Al-Abed Y., Akira S., Bierhaus A., Erlandsson-Harris H., Andersson U., Tracey K. J. (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA 107, 11942–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Smith C., Yin H. (2013) Targeting Toll-like receptors with small molecule agents. Chem. Soc. Rev. 42, 4859–4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X., Loram L. C., Ramos K., de Jesus A. J., Thomas J., Cheng K., Reddy A., Somogyi A. A., Hutchinson M. R., Watkins L. R., Yin H. (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. USA 109, 6325–6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson M. R., Northcutt A. L., Hiranita T., Wang X., Lewis S. S., Thomas J., van Steeg K., Kopajtic T. A., Loram L. C., Sfregola C., Galer E., Miles N. E., Bland S. T., Amat J., Rozeske R. R., Maslanik T., Chapman T. R., Strand K. A., Fleshner M., Bachtell R. K., Somogyi A. A., Yin H., Katz J. L., Rice K. C., Maier S. F., Watkins L. R. (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci. 32, 11187–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Grace P. M., Pham M. N., Cheng K., Strand K. A., Smith C., Li J., Watkins L. R., Yin H. (2013) Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J. 27, 2713–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill L. A., Bowie A. G. (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 [DOI] [PubMed] [Google Scholar]
- 8.Jerala R. (2007) Structural biology of the LPS recognition. Int. J. Med. Microbiol. 297, 353–363 [DOI] [PubMed] [Google Scholar]
- 9.Zhang T. S., Peng Y. H., Wang X. H. (2016) Drug discovery for treating drug addiction by targeting glia. Prog. Pharm. Sci. 39, 56–61 [Google Scholar]
- 10.Xiaozheng Z., Tianshu Z., Fengchao C., Yunqi L., Yinghua P., Xiaohui W. (2016) Toll-like receptor 4 small molecule modulators. Chin. J. of Appl. Chem. 33, 876–886 [Google Scholar]
- 11.Wang X., Cochran T. A., Hutchinson M. R., Yin H., Watkins L. R. (2014) Drug addiction. In Microglia in Health and Disease (Tremblay M.-È., and Sierra A., eds.), pp. 299–317, Springer, New York [Google Scholar]
- 12.Watkins L. R., Hutchinson M. R., Rice K. C., Maier S. F. (2009) The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 30, 581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis S. S., Hutchinson M. R., Rezvani N., Loram L. C., Zhang Y., Maier S. F., Rice K. C., Watkins L. R. (2010) Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience 165, 569–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacagnina M. J., Watkins L. R., Grace P. M. (2018) Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 184, 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peri F., Piazza M. (2012) Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol. Adv. 30, 251–260 [DOI] [PubMed] [Google Scholar]
- 16.Coller J. K., Hutchinson M. R. (2012) Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol. Ther. 134, 219–245 [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Chen G., Chen L., Liu X., Fu W., Zhang Y., Li C., Liang G., Cai Y. (2015) Insights into the binding mode of curcumin to MD-2: studies from molecular docking, molecular dynamics simulations and experimental assessments. Mol. Biosyst. 11, 1933–1938 [DOI] [PubMed] [Google Scholar]
- 18.Fu W., Chen L., Wang Z., Zhao C., Chen G., Liu X., Dai Y., Cai Y., Li C., Zhou J., Liang G. (2016) Determination of the binding mode for anti-inflammatory natural product xanthohumol with myeloid differentiation protein 2. Drug Des. Devel. Ther. 10, 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selfridge B. R., Wang X., Zhang Y., Yin H., Grace P. M., Watkins L. R., Jacobson A. E., Rice K. C. (2015) Structure-activity relationships of (+)-naltrexone-inspired Toll-like receptor 4 (TLR4) antagonists. J. Med. Chem. 58, 5038–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Cui F., Chen H., Zhang T., Yang K., Wang Y., Jiang Z., Rice K. C., Watkins L. R., Hutchinson M. R., Li Y., Peng Y., Wang X. (2018) Dissecting the innate immune recognition of opioid inactive isomer (+)-naltrexone derived Toll-like receptor 4 (TLR4) antagonists. J. Chem. Inf. Model. 58, 816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Zhang Y., Peng Y., Hutchinson M. R., Rice K. C., Yin H., Watkins L. R. (2016) Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol. 173, 856–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northcutt A. L., Hutchinson M. R., Wang X., Baratta M. V., Hiranita T., Cochran T. A., Pomrenze M. B., Galer E. L., Kopajtic T. A., Li C. M., Amat J., Larson G., Cooper D. C., Huang Y., O’Neill C. E., Yin H., Zahniser N. R., Katz J. L., Rice K. C., Maier S. F., Bachtell R. K., Watkins L. R. (2015) DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol. Psychiatry 20, 1525–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 [DOI] [PubMed] [Google Scholar]
- 24.Portoghese P. S., Nagase H., Lipkowski A. W., Larson D. L., Takemori A. E. (1988) Binaltorphimine-related bivalent ligands and their kappa opioid receptor antagonist selectivity. J. Med. Chem. 31, 836–841 [DOI] [PubMed] [Google Scholar]
- 25.Portoghese P. S., Nagase H., Takemori A. E. (1988) Only one pharmacophore is required for the kappa opioid antagonist selectivity of norbinaltorphimine. J. Med. Chem. 31, 1344–1347 [DOI] [PubMed] [Google Scholar]
- 26.Ohto U., Fukase K., Miyake K., Shimizu T. (2012) Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl. Acad. Sci. USA 109, 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sastry G. M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 27, 221–234 [DOI] [PubMed] [Google Scholar]
- 28.Roy D., Todd K., John M. (2009) GaussView, Version 5, Semichem Inc., Shawnee Mission, KS [Google Scholar]
- 29.Becke A. D. (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 38, 3098–3100 [DOI] [PubMed] [Google Scholar]
- 30.Lee C., Yang W., Parr R. G. (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 37, 785–789 [DOI] [PubMed] [Google Scholar]
- 31.Miehlich B., Savin A., Stoll H., Preuss H. (1989) Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 157, 200–206 [Google Scholar]
- 32.Trott O., Olson A. J. (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxter J. (1981) Local optima avoidance in depot location. J. Oper. Res. Soc. 32, 815–819 [Google Scholar]
- 34.Blum C., Aguilera M. J. B., Roli A., Sampels M. (2008) Hybrid Metaheuristics: An Emerging Approach to Optimization, Springer, Berlin: [Google Scholar]
- 35.Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kalé L., Schulten K. (2005) Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan Y., Wu C., Chowdhury S., Lee M. C., Xiong G., Zhang W., Yang R., Cieplak P., Luo R., Lee T., Caldwell J., Wang J., Kollman P. (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 24, 1999–2012 [DOI] [PubMed] [Google Scholar]
- 37.Wang J., Wolf R. M., Caldwell J. W., Kollman P. A., Case D. A. (2004) Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 [DOI] [PubMed] [Google Scholar]
- 38.Dupradeau F. Y., Pigache A., Zaffran T., Savineau C., Lelong R., Grivel N., Lelong D., Rosanski W., Cieplak P. (2010) The R.E.D. tools: advances in RESP and ESP charge derivation and force field library building. Phys. Chem. Chem. Phys. 12, 7821–7839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryckaert J.-P., Ciccotti G., Berendsen H. J. C. (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 [Google Scholar]
- 40.Darden T., York D., Pedersen L. (1993) Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 [Google Scholar]
- 41.Feller S. E., Zhang Y. H., Pastor R. W., Brooks B. R. (1995) Constant pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 103, 4613–4621 [Google Scholar]
- 42.Massova I., Kollman P. A. (2000) Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to predict ligand binding. Perspect. Drug Discov. Des. 18, 113–135 [Google Scholar]
- 43.Sun H., Li Y., Tian S., Xu L., Hou T. (2014) Assessing the performance of MM/PBSA and MM/GBSA methods. 4. Accuracies of MM/PBSA and MM/GBSA methodologies evaluated by various simulation protocols using PDBbind data set. Phys. Chem. Chem. Phys. 16, 16719–16729 [DOI] [PubMed] [Google Scholar]
- 44.Genheden S., Ryde U. (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 10, 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spacková N., Cheatham T. E., III, Ryjácek F., Lankas F., Van Meervelt L., Hobza P., Sponer J. (2003) Molecular dynamics simulations and thermodynamics analysis of DNA-drug complexes. Minor groove binding between 4′,6-diamidino-2-phenylindole and DNA duplexes in solution. J. Am. Chem. Soc. 125, 1759–1769 [DOI] [PubMed] [Google Scholar]
- 46.Torrie G. M., Valleau J. P. (1974) Monte Carlo free energy estimates using non-Boltzmann sampling: application to the sub-critical Lennard-Jones fluid. Chem. Phys. Lett. 28, 578–581 [Google Scholar]
- 47.Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., Lee J. O. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130, 906–917 [DOI] [PubMed] [Google Scholar]
- 48.Kumar S., Bouzida D., Swendsen R. H., Kollman P. A., Rosenberg J. M. (1992) The weighted histogram analysis method for free-energy calculations on biomolecules. 1. The method. J. Comput. Chem. 13, 1011–1021 [Google Scholar]
- 49.Manning T. J., Jr., Sontheimer H. (1999) Recording of intracellular Ca2+, Cl-, pH and membrane potential in cultured astrocytes using a fluorescence plate reader. J. Neurosci. Methods 91, 73–81 [DOI] [PubMed] [Google Scholar]
- 50.Hargreaves K., Dubner R., Brown F., Flores C., Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 [DOI] [PubMed] [Google Scholar]
- 51.Bachtell R., Hutchinson M. R., Wang X., Rice K. C., Maier S. F., Watkins L. R. (2015) Targeting the toll of drug abuse: the translational potential of Toll-like receptor 4. CNS Neurol. Disord. Drug Targets 14, 692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumann H., Wekerle H. (2013) Brain microglia: watchdogs with pedigree. Nat. Neurosci. 16, 253–255 [DOI] [PubMed] [Google Scholar]
- 53.Henn A., Lund S., Hedtjärn M., Schrattenholz A., Pörzgen P., Leist M. (2009) The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 26, 83–94 [DOI] [PubMed] [Google Scholar]
- 54.Gorina R., Font-Nieves M., Márquez-Kisinousky L., Santalucia T., Planas A. M. (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59, 242–255 [DOI] [PubMed] [Google Scholar]
- 55.Theberge F. R., Li X., Kambhampati S., Pickens C. L., St Laurent R., Bossert J. M., Baumann M. H., Hutchinson M. R., Rice K. C., Watkins L. R., Shaham Y. (2013) Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol. Psychiatry 73, 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah M., Anwar M. A., Yesudhas D., Krishnan J., Choi S. (2016) A structural insight into the negative effects of opioids in analgesia by modulating the TLR4 signaling: an in silico approach. Sci. Rep. 6, 39271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hutchinson M. R., Zhang Y., Shridhar M., Evans J. H., Buchanan M. M., Zhao T. X., Slivka P. F., Coats B. D., Rezvani N., Wieseler J., Hughes T. S., Landgraf K. E., Chan S., Fong S., Phipps S., Falke J. J., Leinwand L. A., Maier S. F., Yin H., Rice K. C., Watkins L. R. (2010) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 24, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ikeda H., Kiritoshi T., Murase K. (2012) Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol. Pain 8, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.