Abstract
Nonsteroidal anti-inflammatory drugs interfere with the metabolism of arachidonic acid to proinflammatory prostaglandins and leukotrienes by targeting cyclooxygenases (COXs), 5-lipoxygenase (LOX), or the 5-LOX–activating protein (FLAP). These and related enzymes act in conjunction with marked crosstalk within a complex lipid mediator (LM) network where also specialized proresolving LMs (SPMs) are formed. Here, we present how prominent LM pathways can be differentially modulated in human proinflammatory M1 and proresolving M2 macrophage phenotypes that, upon exposure to Escherichia coli, produce either abundant prostaglandins and leukotrienes (M1) or SPMs (M2). Targeted liquid chromatography–tandem mass spectrometry–based metabololipidomics was applied to analyze and quantify the specific LM profiles. Besides expected on-target actions, we found that: 1) COX or 15-LOX-1 inhibitors elevate inflammatory leukotriene levels, 2) FLAP and 5-LOX inhibitors reduce leukotrienes in M1 but less so in M2 macrophages, 3) zileuton blocks resolution-initiating SPM biosynthesis, whereas FLAP inhibition increases SPM levels, and 4) that the 15-LOX-1 inhibitor 3887 suppresses SPM formation in M2 macrophages. Conclusively, interference with discrete LM biosynthetic enzymes in different macrophage phenotypes considerably affects the LM metabolomes with potential consequences for inflammation-resolution pharmacotherapy. Our data may allow better appraisal of the therapeutic potential of these drugs to intervene with inflammatory disorders.—Werner, M., Jordan, P. M., Romp, E., Czapka, A., Rao, Z., Kretzer, C., Koeberle, A., Garscha, U., Pace, S., Claesson, H.-E., Serhan, C. N., Werz, O., Gerstmeier, J. Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome.
Keywords: resolution, macrophages, inflammation, leukotrienes, prostaglandins
Arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are precursor substrates for lipoxygenases (LOXs) and cyclooxygenases (COXs) that initiate the biosynthesis of potent bioactive lipid mediators (LMs) that regulate the initiation and resolution of inflammation (1, 2). Unresolved, chronic inflammation with elevated levels of proinflammatory prostaglandins (PGs) and leukotrienes (LTs) contributes to numerous widespread diseases, including arthritis, atherosclerosis and cardiovascular diseases, type 2 diabetes, asthma, and Alzheimer’s disease that require therapeutic targeting of the inflammatory process and its resolution (3, 4). For pharmacological intervention with chronic inflammation, drugs that block the formation of PGs and LTs by inhibition of COX-1/2 and 5-LOX or 5-LOX–activating protein (FLAP), respectively, are commonly used (5, 6). Specifically, the so-called nonsteroidal anti-inflammatory drugs (NSAIDs, e.g., ibuprofen) that inhibit PG formation provoke their beneficial effects mainly by alleviating pain and by blocking acute inflammation (5) but are essentially inefficient at terminating inflammation or in promoting resolution and tissue repair.
A novel superfamily of LMs that are called specialized proresolving mediators (SPMs), including lipoxins (LXs), resolvins (Rvs), maresins (MaRs), and protectins (PDs), that actively terminate inflammation and promote tissue regeneration are biosynthesized by COX and LOX pathways as well (Fig. 1A) (2, 7, 8). Thus, COX and 5-LOX pathway inhibitors may interfere with beneficial SPM formation too. Moreover, NSAIDs are frequently associated with adverse on-target side effects by suppressing homeostatic prostanoids (5) and by redirecting LM biosynthesis toward proinflammatory LTs (9). The 15-LOX-1 appears to be involved in inflammation in the respiratory tract (10, 11) but also in the formation of SPM, particularly in anti-inflammatory macrophage phenotypes (12).
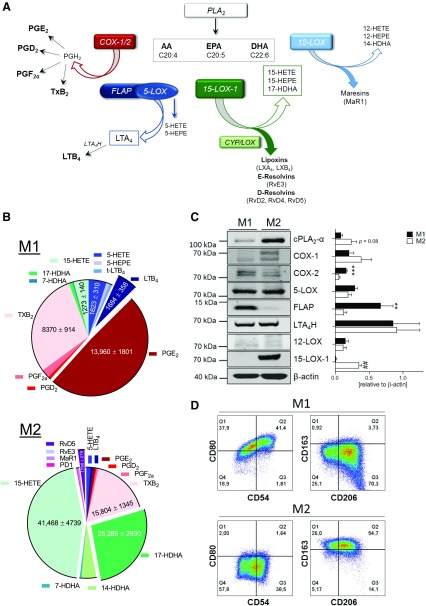
Figure 1.
LM biosynthetic pathways in human M1 and M2 macrophages. A) Schematic overview on the bioactive eicosanoid and docosanoid pathways in inflammation and resolution. B) LM profiles of M1 and M2 macrophages upon exposure to pathogenic E. coli. Human monocyte–derived macrophages were polarized for 48 h to M1 and M2 (2 × 106 cells/ml) and incubated for 180 min with E. coli (O6:K2:H1; ratio = 1:50) at 37 °C. Formed LMs were isolated by SPE and analyzed by UPLC-MS-MS, shown as a pie chart. Values are means ± sem of n = 24–34 for M1 and n = 33–39 for M2. C) Protein expression of LM biosynthetic enzymes and β-actin by Western blot and densitometric analysis thereof; n = 5. Data were log-transformed for paired Student’s t test; M1 vs. M2. **P=0.01, ***P=0.001, ##P=0.01. D) Expression of surface polarization markers for M1 (CD54, CD80) and M2 (CD206, CD163), polarized for 48 h, was analyzed by flow cytometry, and representative histograms are shown from 3 independent experiments with separate donors.
LM biosynthesis inhibitors (including NSAIDs) have been evaluated in cell-based studies with limited read-out, mainly addressing inflammation-promoting PGs and LTs in proinflammatory immune cells (i.e., neutrophils, monocytes, and M1-like macrophages), whereas SPMs have not yet been essentially studied because they are the newest mediators uncovered with novel proresolving functions (7, 13). Thus, modulation of SPM biosynthesis by COX and LOX inhibitors and other potential anti-inflammatory drugs on the cellular level is elusive. Moreover, because LM biosynthesis is organized within connected cascades that can crosstalk, pharmacological interference with one pathway may redirect toward other LM routes within competent cells (9, 14, 15).
Macrophages are innate immune cells that are crucial for initiation, maintenance, and resolution of inflammation depending on their phenotypes, namely, proinflammatory M1-like and proresolving, anti-inflammatory M2-like subtypes (16). Human M1 and M2 macrophages exposed to pathogenic bacteria produce differential LMs that distinguish their inflammatory or proresolving phenotypes: M1-like mainly generate 5-LOX– and COX-2–derived PGs and LTs, whereas M2-like produce predominantly 15-LOX–derived SPMs, including LX, Rv, PD, and MaR1 (12). Such bacteria-stimulated human macrophage phenotypes represent a pathophysiologically relevant, cell-based approach that enables the assessment of a broad spectrum of bioactive LMs by metabololipidomics. Therefore, we made use of this convenient experimental system for a comprehensive analysis of LM pathway inhibitors to reveal their ability to affect the complex network of proinflammatory and proresolving LMs.
MATERIALS AND METHODS
Cell isolation and polarization of macrophages
Leukocyte concentrates from freshly withdrawn peripheral blood of healthy adult human donors were provided by the Institute of Transfusion Medicine, Jena University Hospital (Jena, Germany). The experimental protocol was approved by the ethical committee of the Jena University Hospital. All methods were performed in accordance with the relevant guidelines and regulations. Peripheral blood mononuclear cells were isolated using dextran sedimentation and Ficoll-Histopaque 1077-1 (MilliporeSigma, Burlington, MA, USA) centrifugation. For differentiation and polarization toward M1 and M2 macrophages, criteria published by Werz et al. (12) were used. Thus, M1 macrophages were generated by incubating monocytes with 20 ng/ml granulocyte-macrophage colony-stimulating factor (Peprotech, Rocky Hill, NJ, USA) for 6 d in Roswell Park Memorial Institute medium 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine (Merck, Kenilworth, NJ, USA), and penicillin-streptomycin (Merck), followed by 100 ng/ml LPS (MilliporeSigma) and 20 ng/ml INF-γ (Peprotech) treatment for another 48 h. M2 macrophages were incubated with 20 ng/ml M-CSF (Peprotech) for 6 d of differentiation plus 20 ng/ml IL-4 (Peprotech) for an additional 48 h of polarization.
Incubations of macrophages and LM metabololipidomics
Macrophages (2 × 106/ml) were incubated in PBS containing 1 mM CaCl2. Compounds or vehicle control (0.1% DMSO) were applied 15 min prior to stimulation with E. coli (serotype O6:K2:H1) at a ratio of 1:50 (M1/M2:E. coli) for 180 min at 37 °C. The 15-LOX-1 inhibitor 3887 was synthesized as described by Han et al. (17). Ibuprofen and celecoxib were purchased from MilliporeSigma, MK886 from Cayman Chemicals (Ann Arbor, MI, USA), zileuton from Sequoia Research Products (Pangbourne, United Kingdom), and RSC-3388 from Merck. Supernatants were transferred to 2 ml of ice-cold methanol containing 10 µl of deuterium-labeled internal standards [200 nM d8-5S-hydroxyeicosatetraenoic acid (HETE), d4-LTB4, d5-LXA4, d5-RvD2, d4-PGE2 and 10 µM d8-AA] to facilitate quantification. Deuterated and nondeuterated LM standards were purchased from Cayman Chemicals. Sample preparation was conducted by adapting criteria published by Colas et al. (18). In brief, samples were kept at −20°C for 60 min to allow protein precipitation. After centrifugation (1200 g, 4°C, 10 min), 8 ml acidified H2O (pH 3.5) was added and subjected to solid phase extraction (SPE). Solid phase cartridges (Sep-Pak Vac 6cc 500 mg/6 ml C18; Waters, Milford, MA, USA) were equilibrated with 6 ml methanol and 2 ml H2O before samples were loaded onto columns. After washing with 6 ml H2O and an additional 6 ml n-hexane, LMs were eluted with 6 ml methyl formate. Finally, the samples were brought to dryness using an evaporation system (TurboVap LV; Biotage, Uppsala, Sweden) and resuspended in 100 µl methanol water (50/50, v/v) for ultraperformance liquid chromatography–tandem mass spectrometry (UPLC-MS-MS) automated injections. LM profiling was analyzed with an Acquity UPLC system (Waters) and a QTrap 5500 Mass Spectrometer (Sciex, Framingham, MA, USA) equipped with a Turbo V Source and electrospray ionization. LMs were eluted using an Acquity UPLC BEH C18 column (1.7 µm, 2.1 × 100 mm; Waters) at 50°C with a flow rate of 0.3 ml/min and a mobile phase consisting of methanol, water, and acetic acid at a ratio of 42:58:0.01 (v/v/v) that was ramped to 86:14:0.01 (v/v/v) over 12.5 min and then to 98:2:0.01 (v/v/v) for 3 min (Supplemental Table S1). The QTrap 5500 was operated in negative-ionization mode using scheduled multiple reaction monitoring (MRM) coupled with information-dependent acquisition. The scheduled MRM window was 60 s, optimized LM parameters (CE; Collision Energy, EP; Entrance Potential, DP; Declustering Potential, CXP; Collision Cell Exit Potential) were adopted (19), and the curtain gas pressure was set to 35 psi. The retention time and at least 6 diagnostic ions for each LM were confirmed by means of an external standard (Cayman Chemicals). Quantification was achieved by calibration curves for each LM. Linear calibration curves were obtained for each LM and gave r2 values of 0.998 or higher (for fatty acids, 0.95 or higher). Additionally, the limit of detection for each targeted LM was determined (Supplemental Table S3).
LM coregulation network analysis
LM circular correlation network was generated with the Cytoscape 3.6.0. software. In brief, percentage changes vs. vehicle control (100%) for each LM obtained by treatment with ibuprofen, celecoxib, indomethacin, diflapolin [kind gift by Dr. Barbara Matuszczak (University of Innsbruck, Innsbruck, Austria)], zileuton, MK886, 3887, and RSC-3388 were determined in M1 and M2 macrophages. With these values, a Bravais-Pearson correlation was performed to enlighten positively correlated LM species with a correlation coefficient of 0.7 or higher. Coregulated LM species appear in close proximity to each other, forming specific clusters where the distance and connection lines visualize their proximity. The size of nodes reflects the LM abundance (in picograms) of DMSO controls produced from 2 × 106 macrophages.
SDS-PAGE and Western blot
Cell lysates of macrophages (2 × 106 cells) were separated on 8% [cytosolic phospholipase A2 (cPLA2)-α)], 10% (5-LOX, 12-LOX, 15-LOX-1, COX-1, COX-2, and LTA4H), and 16% (FLAP) polyacrylamide gels and blotted onto nitrocellulose membranes (Amersham Protran Supported 0.45 µm nitrocellulose; GE Healthcare, Chicago, IL, USA). The membranes were incubated with the following primary antibodies: polyclonal rabbit anti-cPLA2-α, 1:200 (2832; Cell Signaling Technology, Danvers, MA, USA); rabbit polyclonal anti–5-LOX, 1:1000 (by Genscript, Piscataway, NJ, USA, to a peptide with the C-terminal 12 aa of 5-LOX: CSPDRIPNSVA; kindly provided by Dr. Marcia Newcomer, Louisiana State University, Baton Rouge, LA, USA); polyclonal rabbit anti–12-LOX, 1:200 (NBP2-29941; Novus Biologicals, Centennial, CO, USA); mouse monoclonal anti–15-LOX-1, 1:500 (ab119774; Abcam, Cambridge, United Kingdom); rabbit polyclonal anti–COX-1, 1:500 (4841; Cell Signaling Technology); rabbit polyclonal anti–COX-2, 1:500 (4842; Cell Signaling Technology); rabbit polyclonal anti-LTA4H, 1:1000 (ab133512; Abcam); rabbit polyclonal anti-FLAP, 0.1 µg/ml (ab85227; Abcam), and rabbit polyclonal anti–β-actin, 1:1000 (4967S; Cell Signaling Technology). Immunoreactive bands were stained with IRDye 800CW goat anti-mouse IgG (H+L), 1:10,000 (926-32210; Li-Cor Biosciences, Lincoln, NE, USA), IRDye 800CW goat anti-rabbit IgG (H+L), 1:15,000 (926 32211; Li-Cor Biosciences) and IRDye 680LT goat anti-mouse IgG (H+L), 1:40,000 (926-68020; Li-Cor Biosciences), and visualized by an Odyssey infrared imager (Li-Cor Biosciences). Data from densitometric analysis were background corrected.
Flow cytometry
Fluorescent staining for flow cytometric analysis of M1 or M2 macrophages after 48 h polarization was performed in flow cytometry buffer (PBS with 0.5% bovine serum albumin, 2 mM EDTA, and 0.1% sodium azide). Nonspecific antibody binding was blocked using mouse serum for 10 min at 4°C prior to antibody staining. Subsequently, macrophages were stained with fluorochrome-labeled antibody mixtures at 4°C for 30 min. The following antibodies were used: FITC anti-human CD14 (2 µg/test, clone M5E2), PE anti-human CD54 (1 µg/test, clone HA58), APC-H7 anti-human CD80 (0.25 µg/test, clone L307.4; BD Biosciences, San Jose, CA, USA), PE-Cy7 anti-human CD163 (2 µg/test, clone RM3/1; BioLegend, San Diego, CA, USA), PerCP-eFluor710 anti-human CD206 (0.06 µg/test, clone 19.2; BD Biosciences, San Diego, CA, USA). Upon staining, M1 or M2 macrophages were analyzed using a Canto Plus flow cytometer (BD Biosciences), and data were analyzed using FlowJo X Software (BD Biosciences).
Statistical analysis
The sample size for experiments was chosen empirically based on previous studies (12, 20) to ensure adequate statistical power. Results are expressed as means ± sem of n observations, where n represents the number of experiments with cells from separate donors and performed on different days in simplicates, as indicated. For the different treatments of cells with compounds, experiments were performed with n ≥ 5 unless otherwise mentioned; for some experiments, n <5 but ≥3 where highly consistent results were obtained. Analysis of data was conducted using Prism 7 software (GraphPad, La Jolla, CA, USA). Data were log-transformed to generate stronger Gaussian-distributed data sets amenable to parametric analysis. A paired Student’s t test was used for comparison between 2 groups. The criterion for statistical significance is a value of P < 0.05. The Bravais-Pearson correlation was analyzed with Microsoft Excel 2016 (Redmond, WA, USA) and Cytoscape 3.6.0 software (https://cytoscape.org/).
RESULTS
Differential bioactive LM pathways in human M1 and M2 macrophage phenotypes
Proinflammatory M1-like macrophages were obtained by differentiation of human peripheral blood monocytes with granulocyte-macrophage colony-stimulating factor (6 d) and 48 h polarization with LPS plus INF-γ, whereas anti-inflammatory M2-like cells were made from monocytes by macrophage colony-stimulating factor–induced differentiation (6 d) followed by 48 h treatment with IL-4 (21). Metabololipidomics was applied to monitor broad-spectrum LM profiles of M1 and M2 macrophages. Adapting criteria published by Dalli and Serhan (19) for LM detection and quantitative analysis, we established a targeted lipidomics approach based on UPLC-MS-MS (Supplemental Tables S1–S3). This method allows simultaneous profiling and high-throughput (16 min) quantitative analysis of 33 LMs from M1 and M2 macrophages, identified based on published criteria (e.g., matching fragmentation patterns and 6 characteristic diagnostic ions) by Colas et al. (18). UPLC-specific retention time and separation of each LM was validated by chemical standards (Supplemental Table S3), and signature ion pairs obtained via MRM were used for quantification (18).
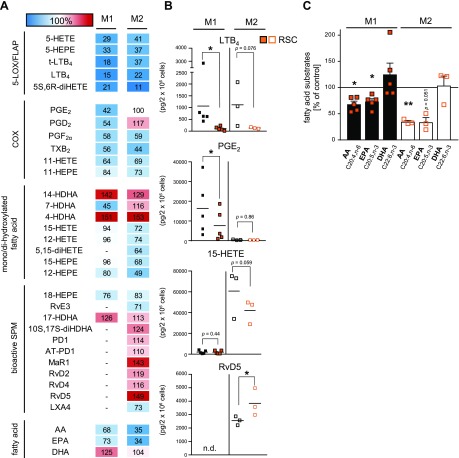
The phenotype-specific LM profiles of E. coli–challenged M1 and M2 macrophages (ratio 1:50; M1/M2:E. coli, 180 min) is reported in Fig. 1B and Supplemental Table S4; a comprehensive overview of the investigated LM pathways is shown in Fig. 1A. In agreement with the overall superior fatty acid substrate release (Supplemental Table S4) in M2 macrophages, the protein level of cPLA2-α was higher than that in M1 macrophages (Fig. 1C). The marked differences in the LM metabolome (Fig. 1B) is a consequence of the distinct expression pattern of LM biosynthetic enzymes in M1 vs. M2 macrophages (Fig. 1C). The M1 phenotype generated predominantly 5-LOX–related LTs and COX-related PGs as well as 11-HETE and 11-HEPE from AA and EPA, respectively, surpassing the respective capacities of M2 macrophages. Although 5-LOX and LTA4H were equally expressed in both phenotypes, FLAP dominated in M1 macrophages (Fig. 1C), presumably accounting for higher LTB4 levels. Consequent to higher COX-2 expression, PGE2 biosynthesis was more than 20-fold higher in M1 vs. M2 cells, whereas the expression of COX-1 and formation of TXB2 was superior in M2 macrophages. Control experiments without E. coli revealed marginal LM formation in either M1 or M2 macrophages that were below the detection limit in these incubations (Supplemental Table S4) but are still relevant during several pathophysiological chronic diseases (18). Correct macrophage polarization was assured by flow cytometry analysis of surface markers CD54 and -80 for M1 and CD163 and -206 for M2 (Fig. 1D). Alterations of the phenotype during 180 min incubations of M1 and M2 with E. coli (i.e., CD54, -80 for M1 and CD163, -206 for M2) were not observed, and E. coli incubation without cells failed to produce appreciable amounts of LM (unpublished results, see ref. 12).
Challenge of M1 macrophages by E. coli failed to produce abundant SPM below the detection limit of our analytical method (Supplemental Table S4), and the production of monohydroxylated SPM precursors was also minute as compared with M2 macrophages that generated substantial amounts of bioactive SPM including LXA4, RvD2, -D4, -D5, Mar1, PD1, AT-PD1, and RvE3 as well as respective precursors (Fig. 1B and Supplemental Table S4). Accordingly, and in agreement with our previous study (12), protein expression of human 15-LOX-1, one of the key enzymes for SPM formation (22, 23), was not detectable in polarized M1 cells but abundantly expressed in the M2 phenotype (Fig. 1C). However, human 15-LOX-2 was reported by Snodgrass et al. (24) to be equally expressed in both macrophage phenotypes and to be capable of producing 15-HETE and 15-HEPE as well as DHA–derived 17-hydroxy DHA (HDHA). Note that isolated human 15-LOX-2 lacks the dual reaction specificity that is unique for human 15-LOX-1 that also generates 12-HETE, 12-HEPE, and 14-HDHA (in an ∼10:1 ratio, at least for 15-HETE:12-HETE) (25). The dual reaction specificity of human 15-LOX-1 might be advantageous for SPM biosynthesis in M2 and may explain why all detectable 12-LOX products, including MaR1, were much higher in M2 vs. M1 macrophages, although 12-LOX levels did not differ between the phenotypes, suggesting that 15-LOX-1 can contribute to the biosynthesis of these LMs as well.
To evaluate how blockade of COX-1 or COX-2 affects the LM profiles in bacteria-challenged macrophages, the clinically used COX-1/2 inhibitor ibuprofen and the COX-2–selective celecoxib were studied. Pretreatment with both drugs for 15 min at 37 °C efficiently suppressed the formation of PGD2, PGE2, PGF2, and TXB2 in M1 and M2 phenotypes to a similar degree (Fig. 2A), even though the absolute capacities to biosynthesize PGE2 were strikingly higher in M1 over M2 cells (Fig. 2B). Moreover, formation of the COX products 11-HETE and 11-HEPE (26) was effectively reduced by ibuprofen or celecoxib in M1 and to a minor extent also in M2 macrophages. Interestingly, both COX inhibitors significantly reduced 15-HETE biosynthesis in M1 (by ∼70%) but not in M2 cells (Fig. 2).
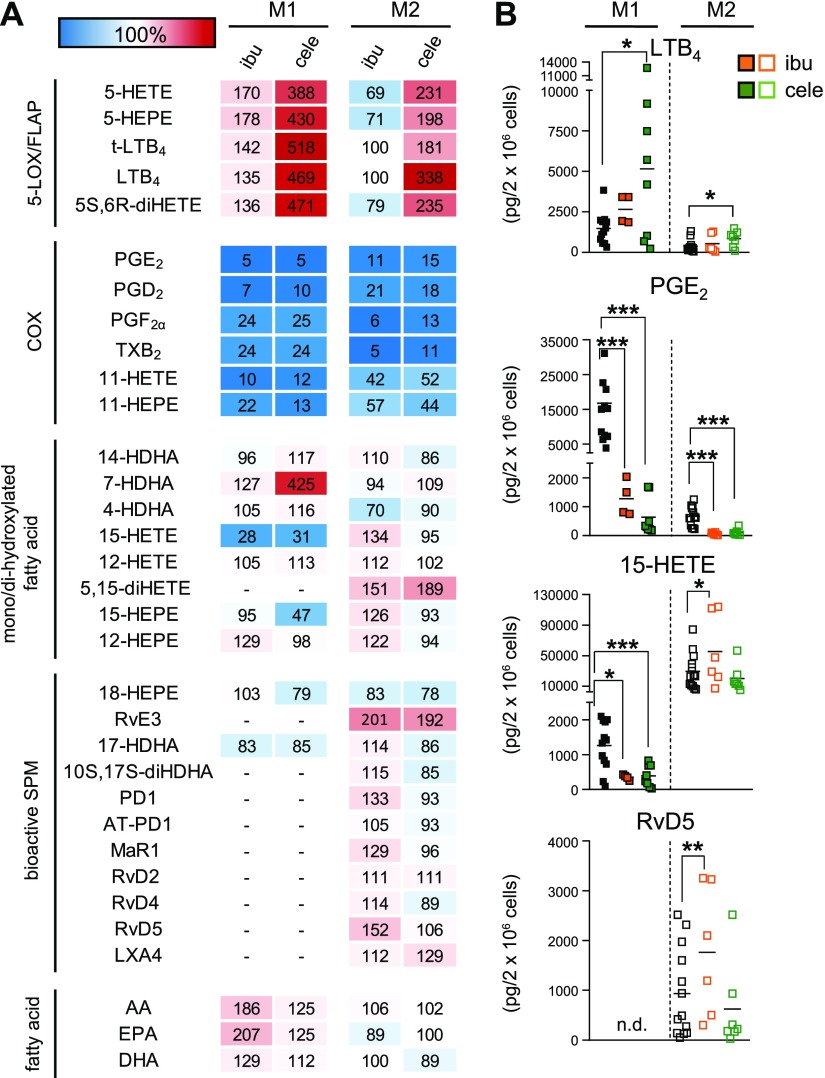
Figure 2.
Effects of the COX inhibitors ibuprofen and celecoxib on LM biosynthesis in M1 and M2 macrophages. A) Human M1 or M2 (2 × 106 cells/ml) were preincubated with 30 µM ibuprofen (ibu), 3 µM celecoxib (cele), or vehicle (0.1% DMSO) for 15 min at 37 °C before incubation with E. coli (O6:K2:H1; ratio 1:50) for another 180 min. Formed LMs were extracted by SPE and analyzed by UPLC-MS-MS; means are shown in a heat map as percentage of vehicle-treated cells (= 100% control, white). For ibu, n = 4 in M1 (n = 3 for 17-HDHA), and n = 6 in M2 (n = 4 for RvD2; n = 5 for RvD4 and LXA4). For cele, n = 8 in M1 (n = 5 for 14-HDHA, 15-HEPE, 12-HEPE, and 17-HDHA; n = 7 for 12-HETE) and n = 7 in M2 (n = 6 for RvE3, MaR1, RvD4). B) Effects of ibu (orange) and cele (green) on LTB4, PGE2, 15-HETE, and RvD5 biosynthesis in M1 and M2 cells, shown as picogramm (pg)/2 × 106 cells. N.d., not detectable. Data were log-transformed for Student’s paired t test. Ibu vs. vehicle in M1: LTB4, P = 0.1675; PGE2, ***P = 0.00016; 15-HETE, *P = 0.0118, and in M2: LTB4, P = 0.8733; PGE2, ***P = 0.000011; 15-HETE, *P = 0.0360; RvD5, **P = 0.0089. Cele vs. vehicle in M1: LTB4, *P = 0.0242; PGE2, ***P = 0.000001; 15-HETE, ***P = 0.000002, and in M2: LTB4, *P = 0.0139; PGE2, ***P = 0.000119; 15-HETE, P = 0.4608; RvD5, P = 0.6133.
In both macrophage phenotypes, particularly in M1, the biosynthesis of the 5-LOX products LTB4 and its trans-isomers, 5-HETE, 5-HEPE, and 5S,6R-diHETE was strongly increased by celecoxib and, to a minor degree, by ibuprofen (at least in M1, Fig. 2), probably as a result of AA substrate shunting from the COX toward the 5-LOX and FLAP pathway as reported by Mazaleuskaya et al. (26). Notably, also 7-HDHA levels were increased by celecoxib in M1 (Fig. 2A) but not in M2 macrophages, which suggests an involvement of the 5-LOX pathway in M1 as well. SPM biosynthesis in the M2 phenotype was not suppressed by the two COX inhibitors and rather slightly increased by ibuprofen. The levels of AA and EPA were strongly elevated by ibuprofen in M1 with minor impact on DHA, whereas in M2 macrophages such alterations were not observed (Fig. 2). Conclusively, the prominent blockade of both COX isoforms results in elevated free AA and EPA levels in M1 macrophages because AA and EPA cannot be converted to the respective COX products that actually dominate the LM profile of M1 and in shunting of LM formation toward proinflammatory 5-LOX products as observed by Mazaleuskaya et al. (26), whereas in M2 macrophages, the COX isoforms are less expressed with consequent little elevation for SPM levels.
Differential effects of 5-LOX and FLAP inhibitors on LM biosynthesis
Because inhibitors of 5-LOX and of FLAP are uniformly used to suppress the biosynthesis of LT and of other 5-LOX products (6), we next studied the clinically validated 5-LOX inhibitor zileuton (27) and the well-recognized FLAP inhibitor MK886 (28) in E. coli–induced LM biosynthesis in M1 and M2 macrophages side by side. In M1, low concentrations of zileuton (300 nM) and MK886 (30 nM) efficiently blocked the formation of LTB4 and its trans-isomers by >50% with similar or somewhat minor efficiency for 5-HETE and 5-HEPE; at 3 µM, both inhibitors suppressed 5-LOX products by >85% in M1 macrophages (Fig. 3A, B). This is in sharp contrast to the M2 phenotype, in which 300 nM zileuton and 30 nM MK886 failed to significantly inhibit 5-LOX product formation; LTB4 and 5-HETE levels remained >80%. Intriguingly, even at high inhibitor concentrations (3 µM), inhibition of 5-LOX product biosynthesis was much less pronounced in M2 macrophages with only 30–40% inhibition (Fig. 3A, B). In contrast, when the classic 5-LOX stimulus Ca2+-ionophore A23187 was used instead of E. coli, the 5-LOX inhibitory potencies of zileuton and MK888 were not different between M1 and M2 macrophages (Fig. 3C), with IC50 values in the low nM range. Because A23187 failed to induce appreciable amounts of SPM and 15-LOX-1 products in both cell types (unpublished results), it was not further used in this study.
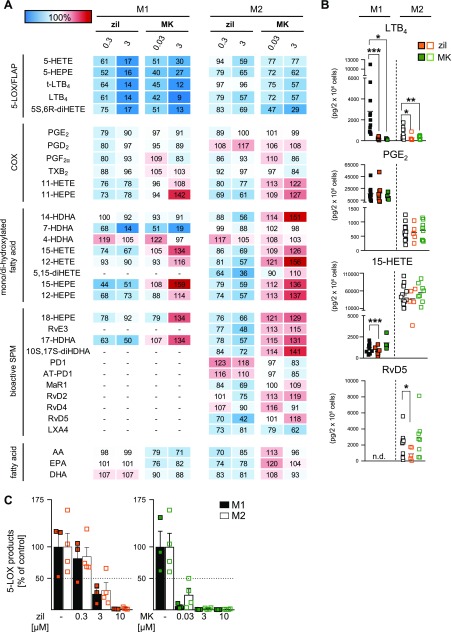
Figure 3.
Effects of the 5-LOX inhibitor zileuton (zil) and the FLAP inhibitor MK886 (MK) on LM biosynthesis in M1 and M2. A) Human M1 and M2 (2 × 106 cells/ml) were preincubated with 0.3 or 3 µM zileuton, 0.03 or 3 µM MK886, or vehicle (0.1% DMSO) for 15 min at 37 °C and then incubated with E. coli (O6:K2:H1; ratio 1:50) for another 180 min. Formed LMs were extracted by SPE and analyzed by UPLC-MS-MS, and means are shown in a heat map as percentage of vehicle-treated cells (= 100% control, white). For zil, n = 8 in M1 (n = 4 for 14-HDHA, 7-HDHA, 12-HETE, and 17-HDHA; n = 6 for 15-HEPE and 12-HEPE; n = 7 for 4-HDHA, 18-HEPE, and DHA) and n = 5 in M2 (n = 4 for LXA4). For MK, n = 4 in M1 and n = 8 in M2 (n = 6 for RvD2; n = 7 for MaR1; n = 4 for LXA4). B) Effects of 3 µM zileuton (zil, orange) and 3 µM MK886 (MK, green) on LTB4, PGE2, 15-HETE, and RvD5 biosynthesis in M1 and M2, shown as pg/2 × 106 cells. Data were log-transformed for paired Student’s t test. N.d., not detectable. Zil vs. vehicle in M1: LTB4, ***P = 0.000006; PGE2, P = 0.274; 15-HETE, ***P = 0.00016, and in M2: LTB4, *P = 0.0475; PGE2, P = 0.896; 15-HETE, P = 0.0797; RvD5, *P = 0.0125. MK vs. vehicle in M1: LTB4, *P = 0.0234; PGE2, P = 0.329; 15-HETE, P = 0.1324, and in M2: LTB4, **P = 0.0083; PGE2, P = 0.706; 15-HETE, P = 0.0719; RvD5, P = 0.2800. C) M1 and M2 (2 × 106 cells/ml) were preincubated with zileuton, MK886, or vehicle (0.1% DMSO) for 15 min at 37 °C and then incubated with A23187 (2.5 µM) for another 10 min. Formed 5-LOX products (LTB4, its isomers, 5-HETE, 5S,6R-diHETE, and 5-HEPE) were extracted by SPE and analyzed by UPLC-MS-MS. Data are given as means + sem, n = 4 independent experiments, shown as percentage of vehicle control (= 100%).
COX product formation was essentially not affected by zileuton or MK886, neither in M1 nor in M2 macrophages after E. coli stimulation. However, striking differential effects of zileuton and MK886 were obvious for the modulation of SPM biosynthesis and their precursors, particularly in M2 cells, which is in line with our previous study (12): 1) zileuton (3 µM), but not MK886, consistently suppressed (30–40%) the formation of 12-LOX– and 15-LOX–derived monohydroxylated products (i.e., DHA-derived 17-HDHA and 14-HDHA, AA-derived 15-HETE and 12-HETE, and EPA-derived 15-HEPE and 12-HEPE); 2) zileuton (3 µM) blocked the formation of DHA-derived RvD2, RvD5, and Mar1 as well as the SPM derivatives 5,15-diHETE and 10S,17S-diHDHA, which were all elevated by low-dose MK886 (30 nM) and even more pronounced upon 3 µM MK886 treatment; 3) formation of EPA-derived RvE3 and its precursor 18-HEPE was inhibited by zileuton but not by MK886 (3 µM each, Fig. 3A, B). In contrast, AA-derived LXA4 formation in M2 macrophages was reduced by both inhibitors, similar to LTB4 formation. Moreover, whereas zileuton and MK886 strongly (>80%) blocked 7-HDHA formation in M1 macrophages, both inhibitors failed in this respect in the M2 phenotype, suggesting that 7-HDHA in M2 macrophages is formed independent of 5-LOX or FLAP. The product 4-HDHA and the PDs PD1 and AT-PD1 were hardly affected by 5-LOX and FLAP inhibition. Alterations of AA, EPA, and DHA levels that were due to the inhibitors were moderate without conclusive tendencies. Together, both zileuton and MK886 strongly inhibit LT biosynthesis primarily in M1 macrophages, whereas only the 5-LOX inhibitor zileuton suppresses also 12-LOX– and 15-LOX–derived products (including SPM) in M2 cells, in which the FLAP inhibitor MK886 elevates formation of SPMs and their precursors, especially those that are derived from DHA.
Targeting 15-LOX-1 activity in M2 macrophages suppresses SPM formation and increases proinflammatory 5-LOX product biosynthesis
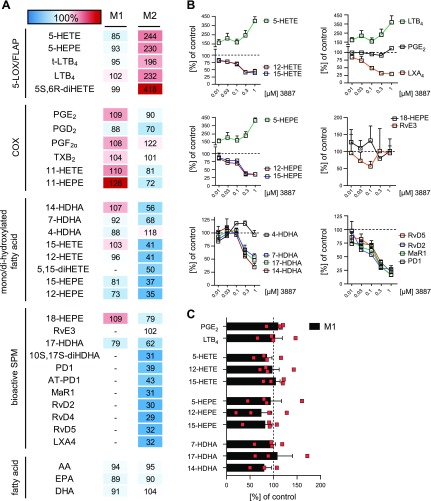
To study the role of 15-LOX-1 in LM formation in E. coli–activated M1 and M2 macrophages, the 15-LOX-1-selective inhibitor 3887 (17, 29) (Supplemental Fig. S2A) was used. In line with the moderate protein expression of 15-LOX-1 in M1, LM formation was essentially unaffected by 300 nM 3887 in this macrophage subtype (alterations <25%), regardless of the biosynthetic LM pathway (i.e., COX, 5-LOX, 12-LOX, and 15-LOX-1; Fig. 4A, C). In M2, however, the formation of 15-LOX-1–related monohydroxylated products, such as 12- and 15-HETE, 12- and 15-HEPE, as well as 14- and 17-HDHA, was concentration-dependently inhibited with consistent IC50 ≈ 200–300 nM (Fig. 4B and Supplemental Fig. S2B). We confirmed the dual reaction specificity of human 15-LOX-1 at position C15 and C12 for AA and EPA and C17 and C14 for DHA in a ratio of 10:1 (Supplemental Fig. S2C). Inhibitory effects of 3887 against 5-LOX (Supplemental Fig. S2D) or against 15-LOX-2 (29) were not evident. The formation of the 15-LOX–derived SPM—that is, LXA4, RvD2, -D4, -D5, PD1, and AT-PD1, their derivatives 5,15-diHETE and 10S,17S-diHDHA, as well as MaR1—were efficiently suppressed by 3887 (IC50 ≈ 100–200 nM, Fig. 4B and Supplemental Fig. S2B). In parallel, 5-LOX–derived LTB4, 5-HETE, and 5-HEPE concentration-dependently increased up to about 4-fold, presumably because of substrate redirection from the 15-LOX-1 to the 5-LOX pathway. Although 15-LOX-1 was shown to be involved in EPA-derived RvE3 biosynthesis in eosinophils (30), exposure of M2 cells to 3887 had only minor effects of EPA-derived LM, such as 18-HEPE and RvE3 (Fig. 4 and Supplemental Fig. S2B), whereas 15-HEPE levels were strongly suppressed, as expected. In general, LMs derived from EPA were less produced compared with AA or DHA products (Supplemental Fig. S2B). Interestingly, 3887 inhibited the formation of 7-HDHA in M2 (Fig. 4A, B) but not in M1 macrophages (Fig. 4A, C); 4-HDHA was not reduced but increased (Fig. 4B), and COX product formation as well as fatty acid substrate release was hardly affected by 3887 (Fig. 4A). Together, 3887 effectively suppresses 15-LOX-1–derived product formation including AA- and DHA-derived SPMs while increasing 5-LOX–related LM biosynthesis.
Figure 4.
Effects of the 15-LOX-1 inhibitor 3887 on LM biosynthesis in M1 and M2. A) Human M1 and M2 (2 × 106 cells/ml) were preincubated with 300 nM 3887 or vehicle (0.1% DMSO) for 15 min at 37 °C before incubation with E. coli (O6:K2:H1; ratio 1:50) for another 180 min. Formed LMs were extracted by SPE and analyzed by UPLC-MS-MS, and results are given as percentages of vehicle-treated cells (= 100% control, white) shown as means in a heat map. B) Concentration-dependent inhibition of LM formation derived from AA (top), EPA (middle), and DHA (bottom) by 3887 in M2. C) Effects of 300 nM 3887 on LM formation in M1. Data are expressed as means + sem of n = 4 in M1 (n = 3 for 14-HDHA and 17-HDHA) and n = 3 in M2, independent experiments, shown as percentage of uninhibited control (= 100%).
The cPLA2 inhibitor RSC-3388 blocks the release of AA and EPA but not of DHA
To investigate how cPLA2 inhibition affects the release of fatty acid substrates and in turn LM formation, we analyzed the LM profile of E. coli–stimulated M1 and M2 macrophages that were pretreated with RSC-3388, a commonly used cPLA2 reference inhibitor (31). Although AA and EPA levels were significantly lowered by RSC-3388 (10 µM), particularly in the M2 subtype, the amounts of free DHA were rather increased (Fig. 5A, C). Along these lines, the substantial formation of AA- and EPA-derived LMs (PG and LT in M1 cells and 15-LOX–derived LM in M2 cells) was effectively decreased, but DHA-derived LMs (including D-series of SPM and their precursors) were elevated by RSC-3388 (Fig. 5A, B). These data suggest that cPLA2 contributes to AA and EPA release and respective LM biosynthesis, but the enzyme is dispensable for DHA liberation and DHA-derived SPM generation.
Figure 5.
Effects of the cPLA2 inhibitor RSC-3388 on LM biosynthesis in M1 and M2. A) Human M1 and M2 (2 × 106 cells/ml) were preincubated with 10 µM RSC-3388 or vehicle (0.1% DMSO) for 15 min at 37 °C before incubation with E. coli (O6:K2:H1; ratio 1:50) for another 180 min. Formed LMs were extracted by SPE and analyzed by UPLC-MS-MS, and means are shown in a heat map as percentages of vehicle-treated cells (= 100% control, white) of n = 5 in M1 (n = 2 for 14-HDHA; n = 3 for 7-HDHA, 12-HETE, and 17-HDHA; n = 4 for 15-HEPE and 12-HEPE) and n = 3 in M2. B) Effects of RSC-3388 on LTB4, PGE2, 15-HETE, and RvD5 biosynthesis in M1 and M2 shown as pg/2 × 106 cells. C) Effects of 10 µM RSC-3388 on free AA, EPA, and DHA levels in M1 and M2 shown as percentage of vehicle control. N.d., not detectable. Data were log-transformed for statistical analysis, paired Student’s t test; RSC vs. vehicle in M1: LTB4, *P = 0.0105; PGE2, *P = 0.0161; 15-HETE, P = 0.4427; AA, *P = 0.0102; EPA, *P = 0.0165; DHA, P = 0.3332 and in M2: LTB4, P = 0.0764; PGE2, P = 0.8617; 15-HETE, P = 0.0590; RvD5, *P = 0.0377; AA, **P = 0.0047; EPA, P = 0.0507; DHA, P = 0.9974.
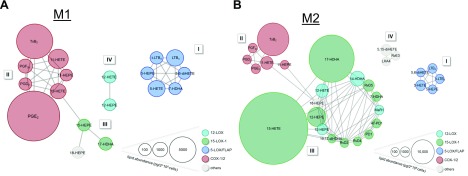
M1 and M2 phenotype–specific network of coregulated LM
Given the broad diversity in LMs produced from bacteria-activated M1 and M2 macrophages, we analyzed the coregulation of specific LMs to enzyme-specific LM pathways in the two phenotypes under pharmacological modulation (for overview, see Supplemental Table S4). Unbiased analysis confirmed PG and LT clusters for the M1 phenotype (Fig. 6A), whereas SPMs and their 12-LOX– and 15-LOX–derived precursors associated with M2 macrophages (Fig. 6B). Thus, positively correlated LMs clustered in close proximity to each other, whereas distant LMs and their clusters are not coregulated. For M1, 11- and 15-HETE and 15-HEPE positively correlated with prostanoids, reflecting their proximity to the COX pathway (Fig. 6A). Another prominent cluster in M1 cells grouped the typical 5-LOX and FLAP products, and 7-HDHA represents a 5-LOX– and FLAP-derived product in M1. For M2, however, 7-HDHA was associated with the 15-LOX-1 cluster (Fig. 6B), suggesting that 7-HDHA is biosynthesized by 5-LOX in M1 macrophages but mainly by 15-LOX-1 in M2 cells. As a result of abundant 15-LOX-1 expression in M2 macrophages, 7 SPMs and 6 SPM precursors grouped together (Fig. 6B), a cluster that is absent in the M1 phenotype. The 15-HETE and 17-HDHA biosynthesis clearly dominated the M2 phenotype. Of note, solely DHA-derived SPMs coregulated in a 15-LOX-1–dependent expression manner. AA-derived 5,15-diHETE, LXA4, and EPA-derived RvE3 grouped together in a separate cluster distinct from the prominent 15-LOX-1 and 5-LOX and FLAP cluster.
Figure 6.
Network of coregulated eicosanoids and docosanoids in M1 and M2 macrophages. M1 (A) and M2 cells (B), corresponding to 2 × 106 cells/ml, were treated with vehicle (0.1% DMSO) or test compounds 15 min before challenge with E. coli (O6:K2:H1; ratio 1:50) for 180 min at 37 °C. LMs were isolated by SPE and analyzed by UPLC-MS-MS. Visualization of positive LM/LM correlations as circular network with r ≥ 0.7. Nodes visualize the LM; for color and size meanings see legend.
DISCUSSION
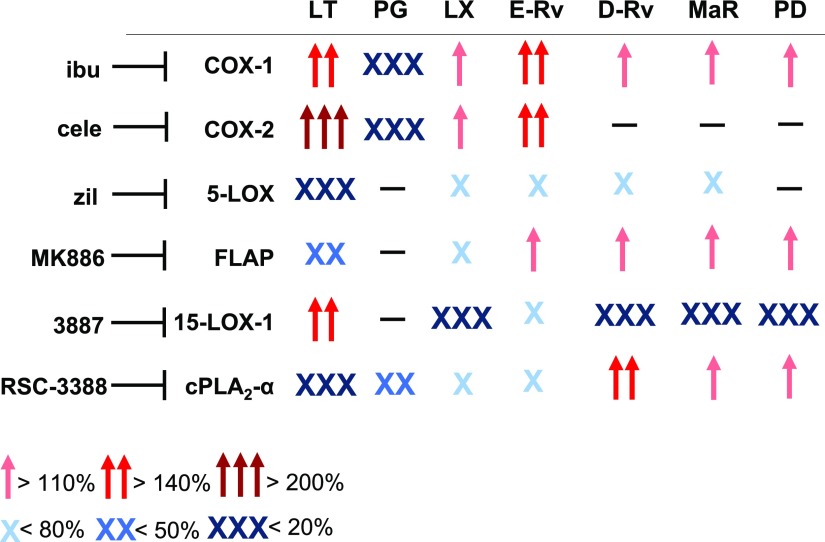
Here we present how clinically relevant anti-inflammatory drugs and prominent LM biosynthesis inhibitors modulate the formation of proinflammatory and proresolving LMs in bacteria-stimulated human M1 and M2 macrophages (for an overview, see Fig. 7). Earlier studies on such agents almost exclusively assessed their potential to suppress proinflammatory PGs or LTs, applying test systems, such as stimulated whole blood or isolated neutrophils and monocytes, that reflect solely inflammatory conditions (26, 32, 33). We present here a new experimental, cell-based approach exploiting LM lipidomics profiling with UPLC-MS-MS to comprehensively evaluate these inhibitors for their impact on the biosynthesis of various LMs that determine not only the promotion of inflammation (i.e., PG and LT) but also its resolution (i.e., SPM). Our results broaden the knowledge about the pharmacological profile of different LM biosynthesis inhibitors and disclose for the first time their ability to manipulate SPM biosynthesis—insights that can allow better appraisal of their therapeutic potential to intervene with inflammatory disorders.
Figure 7.
Schematic overview of inhibitory and stimulatory effects of LM biosynthesis inhibitors on the LM profile in human M1 and M2 macrophages upon exposure to pathogenic E. coli. Cells, corresponding to 2 × 106 cells/ml, were treated with vehicle (0.1% DMSO) or test compounds 15 min before challenge with E. coli (O6:K2:[1H]; ratio 1:50) for 180 min at 37 °C. LMs were isolated by SPE and analyzed by UPLC-MS-MS. Modulation (increase or decrease) of the LM profile by each compound is shown in a simplified scheme. Cele, celecoxib; ibu, ibuprofen; zil, zileuton.
The motivation to conduct this study originated from various reasoning. First, the discovery of differential bacteria-induced formation of proinflammatory and proresolving LMs in human macrophage phenotypes (12) enables us for the first time to assess the effects of LM biosynthesis inhibitors under pathophysiologically relevant conditions that reflect either inflammation-promoting (M1-like) or inflammation-resolving (M2-like) potential. Second, COX and 5-LOX or FLAP inhibitors are considered beneficial in inflammation therapy because of the blockade of the formation of proinflammatory PGs and LTs (34), but how these drugs influence SPM biosynthesis, and thus resolution of inflammation is elusive (35, 36). Third, the biosynthetic pathways of LMs are organized within connected cascades that can crosstalk within a complex network (14, 15), and therefore, pharmacological interference with one pathway may redirect toward other LM routes. In fact, COX inhibitors and the 15-LOX-1 inhibitor 3887 each increased formation of proinflammatory 5-LOX products. Fourth, proinflammatory and proresolving LMs might be partially biosynthesized by common enzymes, such as 5-LOX or FLAP, that mediate formation of LTs but possibly also of LXs and Rvs (37). This implies that 5-LOX and FLAP inhibitors block inflammation but also may hamper resolution. Indeed, zileuton suppressed SPM formation, whereas FLAP inhibition failed in this respect and instead elevated SPM levels. Together, our data highlight the contribution of key enzymes in LM biosynthesis under pathophysiologically relevant conditions, and they suggest that drugs that act on these key enzymes may affect the overall LM network with potential consequences for the pharmacotherapy of inflammation.
LMs produced via the COX-1/2 pathway display proinflammatory but also protective actions depending on stimulus, cell type, and status that program the ability to resolve inflammation (5, 38). Although beneficial effects of NSAIDs are well established for alleviating acute inflammation and pain (26, 39), they cause severe on-target side effects and may negatively impact inflammation resolution (35, 40). Thus, COX-2–deficient mice failed to resolve from inflammation, and the COX inhibitor indomethacin exacerbated inflammation because of reduced proresolving PGD2 levels (41). Also, COX-2–derived PGD2 ameliorated colonic inflammation in rats (42). In our study, both COX inhibitors efficiently reduced all prostanoids and caused a strong shift to the 5-LOX– and FLAP-mediated LT biosynthesis pathway in M1 and M2 macrophages but, interestingly, not to the 15-LOX and DHA pathway and SPM formation in the M2 phenotype. Elevated LT levels that were due to COX-1/2 inhibition are well known and are seemingly causative for increased risk for asthma, and more frequent gastrointestinal lesions might be due to LTB4-elicited neutrophil infiltration (9, 43).
The proinflammatory functions of LTs are reflected by LTB4 as potent chemo-attractant for neutrophils (44) and by cysteinyl-LTs that increase microvascular permeability and immune cell recruitment (45). Suppression of LT formation by targeting the 5-LOX pathway is a therapeutic strategy to treat inflammatory disorders, neglecting, however, the role of 5-LOX in the biosynthesis of proresolving mediators, such as LXA4 (46, 47). 5-LOX and FLAP are essential for LT biosynthesis (45) and apparently also for LX and Rv formation (12, 37, 48). However, our data with the 5-LOX inhibitor zileuton (27) and the FLAP inhibitor MK886 (28) in M1 and M2 macrophages reveal differential and more complex functions for 5-LOX and FLAP. Thus, zileuton blocked the formation of LT and SPM (LXA4 and Rv), that supports the role of 5-LOX in LT and in LX and Rv formation. This suggests that zileuton and other 5-LOX inhibitors could interfere with inflammation resolution by inhibiting SPM biosynthesis. We also observed suppression of 15-LOX products by zileuton in M2 cells that were possibly due to direct interference with 15-LOX-1 that shares structural features with 5-LOX in the active site (49). In contrast, MK886 selectively suppressed LT biosynthesis, whereas, in agreement with our previous report (12), 15-LOX-1 products are increased in M2 macrophages, especially d-series Rv and MaR1. Obviously, zileuton and MK886 were much less efficient to inhibit E. coli–induced LT formation in M2 than in M1 cells, which is not readily understood. In control experiments with A23187, however, this bias was abolished, excluding unequal inhibitor availability in M1 and M2 macrophages or differential 5-LOX and FLAP expression as reasons. Possibly, the potency of zileuton and MK886 may depend on the signaling pathways that lead to activation of 5-LOX (e.g., phosphorylation and oxidative tone), which may differ for activation by A23187 and by pathogenic E. coli in M1 and M2 subtypes. Indeed, differential potencies for other 5-LOX inhibitors depending on kinase signaling and oxidative tone were observed before (50, 51).
We propose that 5-LOX subcellular localization and differential access to AA vs. DHA determines whether LTs or DHA-derived SPMs are preferably formed by 5-LOX, depending on the interaction with FLAP. Experimental evidence suggests that when activated 5-LOX interacts with FLAP at the nuclear membrane, AA is presented by FLAP and 5-LOX converts it to LT (52). But when 5-LOX is activated in a nonnuclear compartment and distant from FLAP [e.g., if 5-LOX phosphorylation at Ser271 is blocked (53) or FLAP inhibitors like MK886 prevent the interaction with FLAP (12)] then 5-LOX may preferably access DHA-derived intermediates to biosynthesize specific SPMs. In fact, in MK886-treated M2 cells, formation of the AA-derived SPM LXA4 is reduced as LTB4, but DHA-derived SPMs are still produced (Fig. 3A). We showed before that targeting of FLAP by MK886 in M2 macrophages precludes the interaction with 5-LOX at the nuclear membrane (12), supporting the hypothesis by other researchers (53) about a differentially regulated nonnuclear 5-LOX favoring the biosynthesis of proresolving LMs. In murine liver injury, the FLAP inhibitor BAY X-1005 reduced cysteinyl-LT formation but elevated SPM levels (54). Therefore, FLAP might be negligible for the biosynthesis of d-series Rv in M2 macrophages, and hence FLAP inhibitors may be more beneficial as anti-inflammatory drugs than direct 5-LOX inhibitors because they suppress proinflammatory LTs but might maintain SPM biosynthesis to enable resolution of inflammation, such as PDs and MaRs that do not rely on 5-LOX for their biosynthesis (55, 56).
Expression of 15-LOX-1 is characteristic for the M2 phenotype, as well as its high capacity to biosynthesize SPM compared with the M1 subset (12). However, 15-LOX-1 is a versatile enzyme that plays a role in both the onset and the termination of immune responses (10, 11, 57, 58). Recently, an anti-inflammatory role of 15-LOX and a related SPM in mouse skin was reported with implications for the maintenance of dermal integrity (59). In fact, current developments focus on small molecule 15-LOX activators to foster SPM formation and abrogate LT and PG production (60). On the other hand, 3887 inhibited dendrite and podosome formation in human dendritic cells, indicating an anti-inflammatory effect of 15-LOX-1 inhibitors (17). Targeting 15-LOX-1 by the selective inhibitor 3887 (17, 29) in M2 macrophages blocked the formation of all detectable SPMs. The inhibitor 3887, like COX inhibitors, redirected AA toward the 5-LOX pathway in the M2 phenotype with increased LTB4 and 5-HETE levels, indicating that both 15-LOX-1 and COX inhibitors have a complex effect mechanism of action and influence both inflammation and resolution. This suggests careful consideration of application and use of these inhibitors as potential therapeutics where LTs promote the disease and SPMs beneficially contribute to resolution and tissue regeneration (48).
cPLA2 provides fatty acid substrates for COX and LOX to generate LMs (61). Results with the cPLA2 inhibitor RSC-3388 support an involvement of cPLA2 in AA and EPA release, particularly in M2 cells, in which cPLA2 protein levels were higher than in M1 macrophages, accompanied by reduced levels of AA- and EPA-derived 5-LOX and 15-LOX products. Remarkably, RSC-3388 increased DHA levels and promoted the formation of DHA-derived SPMs as well as the respective precursors. Discrimination of DHA from AA release was reported earlier by Shikano et al. (62), and it was suggested that DHA supply in macrophages for SPM biosynthesis is mediated by secreted PLA2 group IID (63).
Together, metabololipidomics of broad-spectrum LMs produced in bacteria-challenged human proinflammatory M1 and anti-inflammatory M2 macrophages led to important, partially unexpected insights into the pharmacological manipulation of bioactive LMs that determine initiation or resolution of inflammation. The pharmacological profiles of these drugs and agents that act on discrete LM biosynthetic enzymes can impact the overall LM network with potential consequences for inflammation pharmacotherapy. In search of more efficient and safer anti-inflammatory drugs, our data prompt for better consideration of the effects of such agents on SPM formation that may translate into resolution-based pharmacology as alternative strategies to resolve chronic inflammation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (SFB1127, ChemBioSys, and SFB1278, Polytarget). J.G. received a Carl-Zeiss stipend, and C.N.S. was supported by U.S. National Institutes of Health, National Institute of General Medical Sciences Grant P01GM095467. The authors declare no conflicts of interest.
Glossary
- AA
arachidonic acid
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FLAP
5-LOX–activating protein
- HDHA
hydroxy DHA
- HEPE
hydroxyeicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- LM
lipid mediator
- LOX
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- MaR
maresin
- MRM
multiple reaction monitoring
- NSAID
nonsteroidal anti-inflammatory drug
- PD
protectin
- PG
prostaglandin
- pg
picogram
- Rv
resolvin
- SPM
specialized proresolving mediator
- UPLC-MS-MS
ultraperformance liquid chromatography–tandem mass spectrometry
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Werner performed the ibuprofen, celecoxib, zileuton, RSC-3388 experiments and performed the network analysis; P. M. Jordan performed the 3887 experiments, flow cytometry analysis, and A23187 studies; E. Romp performed MK886 experiments; A. Czapka, Z. Rao, and C. Kretzer performed Western blots; M. Werner, P. M. Jordan, A. Czapka, S. Pace, and J. Gerstmeier performed data analysis and prepared graphs; M. Werner, A. Koeberle, and J. Gerstmeier developed the ultraperformance liquid chromatography method; H.-E. Claesson supplied 3887; C. N. Serhan, O. Werz, and J. Gerstmeier designed the study; O. Werz and J. Gerstmeier wrote the manuscript; and all authors contributed to manuscript preparation.
REFERENCES
- 1.Chiang N., Fredman G., Bäckhed F., Oh S. F., Vickery T., Schmidt B. A., Serhan C. N. (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spite M., Clària J., Serhan C. N. (2014) Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19, 21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabas I., Glass C. K. (2013) Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339, 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rainsford K. D. (2007) Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 42, 3–27 [DOI] [PubMed] [Google Scholar]
- 6.Werz O., Gerstmeier J., Garscha U. (2017) Novel leukotriene biosynthesis inhibitors (2012-2016) as anti-inflammatory agents. Expert Opin. Ther. Pat. 27, 607–620 [DOI] [PubMed] [Google Scholar]
- 7.Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte M. S., Desai T. A., Wu B., Schaller M., Werlin E. (2018) Pro-resolving lipid mediators in vascular disease. J. Clin. Invest. 128, 3727–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rainsford K. D. (1993) Leukotrienes in the pathogenesis of NSAID-induced gastric and intestinal mucosal damage. Agents Actions 39, C24–C26 [DOI] [PubMed] [Google Scholar]
- 10.Andersson C. K., Claesson H. E., Rydell-Törmänen K., Swedmark S., Hällgren A., Erjefält J. S. (2008) Mice lacking 12/15-lipoxygenase have attenuated airway allergic inflammation and remodeling. Am. J. Respir. Cell Mol. Biol. 39, 648–656 [DOI] [PubMed] [Google Scholar]
- 11.Claesson H. E. (2009) On the biosynthesis and biological role of eoxins and 15-lipoxygenase-1 in airway inflammation and Hodgkin lymphoma. Prostaglandins Other Lipid Mediat. 89, 120–125 [DOI] [PubMed] [Google Scholar]
- 12.Werz O., Gerstmeier J., Libreros S., De la Rosa X., Werner M., Norris P. C., Chiang N., Serhan C. N. (2018) Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 9, 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalli J., Colas R. A., Serhan C. N. (2013) Novel n-3 immunoresolvents: structures and actions. Sci. Rep. 3, 1940; erratum: 4, 6726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang K., Ma W., Liang H., Ouyang Q., Tang C., Lai L. (2007) Dynamic simulations on the arachidonic acid metabolic network. PLOS Comput. Biol. 3, e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He C., Wu Y., Lai Y., Cai Z., Liu Y., Lai L. (2012) Dynamic eicosanoid responses upon different inhibitor and combination treatments on the arachidonic acid metabolic network. Mol. Biosyst. 8, 1585–1594 [DOI] [PubMed] [Google Scholar]
- 16.Motwani M. P., Gilroy D. W. (2015) Macrophage development and polarization in chronic inflammation. Semin. Immunol. 27, 257–266 [DOI] [PubMed] [Google Scholar]
- 17.Han H., Liang X., Ekberg M., Kritikou J. S., Brunnström Å., Pelcman B., Matl M., Miao X., Andersson M., Yuan X., Schain F., Parvin S., Melin E., Sjöberg J., Xu D., Westerberg L. S., Björkholm M., Claesson H. E. (2017) Human 15-lipoxygenase-1 is a regulator of dendritic-cell spreading and podosome formation. FASEB J. 31, 491–504 [DOI] [PubMed] [Google Scholar]
- 18.Colas R. A., Shinohara M., Dalli J., Chiang N., Serhan C. N. (2014) Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307, C39–C54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalli J., Serhan C. N. (2012) Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pace S., Pergola C., Dehm F., Rossi A., Gerstmeier J., Troisi F., Pein H., Schaible A. M., Weinigel C., Rummler S., Northoff H., Laufer S., Maier T. J., Rådmark O., Samuelsson B., Koeberle A., Sautebin L., Werz O. (2017) Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J. Clin. Invest. 127, 3167–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., Natoli G., Saeij J. P., Schultze J. L., Shirey K. A., Sica A., Suttles J., Udalova I., van Ginderachter J. A., Vogel S. N., Wynn T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aursnes M., Tungen J. E., Colas R. A., Vlasakov I., Dalli J., Serhan C. N., Hansen T. V. (2015) Synthesis of the 16S,17S-epoxyprotectin intermediate in the biosynthesis of protectins by human macrophages. J. Nat. Prod. 78, 2924–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Primdahl K. G., Tungen J. E., De Souza P. R. S., Colas R. A., Dalli J., Hansen T. V., Vik A. (2017) Stereocontrolled synthesis and investigation of the biosynthetic transformations of 16(S),17(S)-epoxy-PDn-3 DPA. Org. Biomol. Chem. 15, 8606–8613 [DOI] [PubMed] [Google Scholar]
- 24.Snodgrass R. G., Zezina E., Namgaladze D., Gupta S., Angioni C., Geisslinger G., Lütjohann D., Brüne B. (2018) A novel function for 15-lipoxygenases in cholesterol homeostasis and CCL17 production in human macrophages. Front. Immunol. 9, 1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kutzner L., Goloshchapova K., Heydeck D., Stehling S., Kuhn H., Schebb N. H. (2017) Mammalian ALOX15 orthologs exhibit pronounced dual positional specificity with docosahexaenoic acid. Biochim Biophys Acta Mol Cell Biol Lipids 1862, 666–675 [DOI] [PubMed] [Google Scholar]
- 26.Mazaleuskaya L. L., Lawson J. A., Li X., Grant G., Mesaros C., Grosser T., Blair I. A., Ricciotti E., FitzGerald G. A. (2016) A broad-spectrum lipidomics screen of antiinflammatory drug combinations in human blood. JCI Insight 1, e87031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter G. W., Young P. R., Albert D. H., Bouska J., Dyer R., Bell R. L., Summers J. B., Brooks D. W. (1991) 5-lipoxygenase inhibitory activity of zileuton. J. Pharmacol. Exp. Ther. 256, 929–937 [PubMed] [Google Scholar]
- 28.Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H., Piechuta H., Riendeau D., Rouzer C. A., Rokach J., Young R., MacIntyre D. E., Peterson L., Bach T., Eiermann G., Hopple S., Humes J., Hupe L., Luell S., Metzger J., Meurer R., Miller D. K., Opas E., Pacholok S. (1989) L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can. J. Physiol. Pharmacol. 67, 456–464 [DOI] [PubMed] [Google Scholar]
- 29.Archambault A. S., Turcotte C., Martin C., Provost V., Larose M. C., Laprise C., Chakir J., Bissonnette É., Laviolette M., Bossé Y., Flamand N. (2018) Comparison of eight 15-lipoxygenase (LO) inhibitors on the biosynthesis of 15-LO metabolites by human neutrophils and eosinophils. PLoS One 13, e0202424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isobe Y., Arita M., Matsueda S., Iwamoto R., Fujihara T., Nakanishi H., Taguchi R., Masuda K., Sasaki K., Urabe D., Inoue M., Arai H. (2012) Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 287, 10525–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seno K., Okuno T., Nishi K., Murakami Y., Watanabe F., Matsuura T., Wada M., Fujii Y., Yamada M., Ogawa T., Okada T., Hashizume H., Kii M., Hara S., Hagishita S., Nakamoto S., Yamada K., Chikazawa Y., Ueno M., Teshirogi I., Ono T., Ohtani M. (2000) Pyrrolidine inhibitors of human cytosolic phospholipase A(2). J. Med. Chem. 43, 1041–1044 [DOI] [PubMed] [Google Scholar]
- 32.Khanapure S. P., Garvey D. S., Janero D. R., Letts L. G. (2007) Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr. Top. Med. Chem. 7, 311–340 [DOI] [PubMed] [Google Scholar]
- 33.Werz O., Steinhilber D. (2005) Development of 5-lipoxygenase inhibitors--lessons from cellular enzyme regulation. Biochem. Pharmacol. 70, 327–333 [DOI] [PubMed] [Google Scholar]
- 34.Funk C. D. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 35.Fukunaga K., Kohli P., Bonnans C., Fredenburgh L. E., Levy B. D. (2005) Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 174, 5033–5039 [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto M. A., Sousa L. P., Pinho V., Perretti M., Teixeira M. M. (2016) Resolution of inflammation: what controls its onset? Front. Immunol. 7, 160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehmann C., Homann J., Ball A. K., Blöcher R., Kleinschmidt T. K., Basavarajappa D., Angioni C., Ferreirós N., Häfner A. K., Rådmark O., Proschak E., Haeggström J. Z., Geisslinger G., Parnham M. J., Steinhilber D., Kahnt A. S. (2015) Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein. FASEB J. 29, 5029–5043 [DOI] [PubMed] [Google Scholar]
- 38.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 39.Dubois R. N., Abramson S. B., Crofford L., Gupta R. A., Simon L. S., Van De Putte L. B., Lipsky P. E. (1998) Cyclooxygenase in biology and disease. FASEB J. 12, 1063–1073 [PubMed] [Google Scholar]
- 40.Gilroy D. W., Colville-Nash P. R., Willis D., Chivers J., Paul-Clark M. J., Willoughby D. A. (1999) Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 [DOI] [PubMed] [Google Scholar]
- 41.Wallace J. L., McKnight W., Reuter B. K., Vergnolle N. (2000) NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119, 706–714 [DOI] [PubMed] [Google Scholar]
- 42.Ajuebor M. N., Singh A., Wallace J. L. (2000) Cyclooxygenase-2-derived prostaglandin D(2) is an early anti-inflammatory signal in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G238–G244 [DOI] [PubMed] [Google Scholar]
- 43.Burnett B. P., Levy R. M. (2012) 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv. Ther. 29, 79–98 [DOI] [PubMed] [Google Scholar]
- 44.Lämmermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmüller W., Parent C. A., Germain R. N. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rådmark O., Werz O., Steinhilber D., Samuelsson B. (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta 1851, 331–339 [DOI] [PubMed] [Google Scholar]
- 46.Levy B. D., De Sanctis G. T., Devchand P. R., Kim E., Ackerman K., Schmidt B. A., Szczeklik W., Drazen J. M., Serhan C. N. (2002) Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat. Med. 8, 1018–1023 [DOI] [PubMed] [Google Scholar]
- 47.Bhavsar P. K., Levy B. D., Hew M. J., Pfeffer M. A., Kazani S., Israel E., Chung K. F. (2010) Corticosteroid suppression of lipoxin A4 and leukotriene B4 from alveolar macrophages in severe asthma. Respir. Res. 11, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 49.Gilbert N. C., Bartlett S. G., Waight M. T., Neau D. B., Boeglin W. E., Brash A. R., Newcomer M. E. (2011) The structure of human 5-lipoxygenase. Science 331, 217–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer L., Steinhilber D., Werz O. (2004) Molecular pharmacological profile of the nonredox-type 5-lipoxygenase inhibitor CJ-13,610. Br. J. Pharmacol. 142, 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer L., Szellas D., Rådmark O., Steinhilber D., Werz O. (2003) Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 17, 949–951 [DOI] [PubMed] [Google Scholar]
- 52.Gerstmeier J., Weinigel C., Rummler S., Rådmark O., Werz O., Garscha U. (2016) Time-resolved in situ assembly of the leukotriene-synthetic 5-lipoxygenase/5-lipoxygenase-activating protein complex in blood leukocytes. FASEB J. 30, 276–285 [DOI] [PubMed] [Google Scholar]
- 53.Fredman G., Ozcan L., Spolitu S., Hellmann J., Spite M., Backs J., Tabas I. (2014) Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc. Natl. Acad. Sci. USA 111, 14530–14535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Titos E., Clària J., Planagumà A., López-Parra M., González-Périz A., Gaya J., Miquel R., Arroyo V., Rodés J. (2005) Inhibition of 5-lipoxygenase-activating protein abrogates experimental liver injury: role of Kupffer cells. J. Leukoc. Biol. 78, 871–878 [DOI] [PubMed] [Google Scholar]
- 55.Serhan C. N., Dalli J., Colas R. A., Winkler J. W., Chiang N. (2015) Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 1851, 397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serhan C. N., Yang R., Martinod K., Kasuga K., Pillai P. S., Porter T. F., Oh S. F., Spite M. (2009) Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 206, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feltenmark S., Gautam N., Brunnström A., Griffiths W., Backman L., Edenius C., Lindbom L., Björkholm M., Claesson H. E. (2008) Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc. Natl. Acad. Sci. USA 105, 680–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S. N., Akindehin S., Kwon H. J., Son Y. H., Saha A., Jung Y. S., Seong J. K., Lim K. M., Sung J. H., Maddipati K. R., Lee Y. H. (2018) Anti-inflammatory role of 15-lipoxygenase contributes to the maintenance of skin integrity in mice. Sci. Rep. 8, 8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng H., McClendon C. L., Dai Z., Li K., Zhang X., He S., Shang E., Liu Y., Lai L. (2016) Discovery of novel 15-lipoxygenase activators to shift the human arachidonic acid metabolic network toward inflammation resolution. J. Med. Chem. 59, 4202–4209 [DOI] [PubMed] [Google Scholar]
- 61.Leslie C. C. (2015) Cytosolic phospholipase A2: physiological function and role in disease. J. Lipid Res. 56, 1386–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shikano M., Masuzawa Y., Yazawa K., Takayama K., Kudo I., Inoue K. (1994) Complete discrimination of docosahexaenoate from arachidonate by 85 kDa cytosolic phospholipase A2 during the hydrolysis of diacyl- and alkenylacylglycerophosphoethanolamine. Biochim. Biophys. Acta 1212, 211–216 [DOI] [PubMed] [Google Scholar]
- 63.Miki Y., Yamamoto K., Taketomi Y., Sato H., Shimo K., Kobayashi T., Ishikawa Y., Ishii T., Nakanishi H., Ikeda K., Taguchi R., Kabashima K., Arita M., Arai H., Lambeau G., Bollinger J. M., Hara S., Gelb M. H., Murakami M. (2013) Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 210, 1217–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.