Abstract
Sarcopenic obesity, the combination of skeletal muscle mass and function loss with an increase in body fat, is associated with physical limitations, cardiovascular diseases, metabolic stress, and increased risk of mortality. Cannabinoid receptor type 1 (CB1R) plays a critical role in the regulation of whole-body energy metabolism because of its involvement in controlling appetite, fuel distribution, and utilization. Inhibition of CB1R improves insulin secretion and insulin sensitivity in pancreatic β-cells and hepatocytes. We have now developed a skeletal muscle–specific CB1R-knockout (Skm-CB1R−/−) mouse to study the specific role of CB1R in muscle. Muscle-CB1R ablation prevented diet-induced and age-induced insulin resistance by increasing IR signaling. Moreover, muscle-CB1R ablation enhanced AKT signaling, reducing myostatin expression and increasing IL-6 secretion. Subsequently, muscle-CB1R ablation increased myogenesis through its action on MAPK-mediated myogenic gene expression. Consequently, Skm-CB1R−/− mice had increased muscle mass and whole-body lean/fat ratio in obesity and aging. Muscle-CB1R ablation improved mitochondrial performance, leading to increased whole-body muscle energy expenditure and improved physical endurance, with no change in body weight. These results collectively show that CB1R in muscle is sufficient to regulate whole-body metabolism and physical performance and is a novel target for the treatment of sarcopenic obesity. —González-Mariscal, I., Montoro, R. A., O’Connell, J. F., Kim, Y., Gonzalez-Freire, M., Liu, Q.-R., Alfaras, I., Carlson, O. D., Lehrmann, E., Zhang, Y., Becker, K. G., Hardivillé, S., Ghosh, P., Egan, J. M. Muscle cannabinoid 1 receptor regulates Il-6 and myostatin expression, governing physical performance and whole-body metabolism.
Keywords: CB1R, insulin sensitivity, myokines, skeletal muscle
Obesity and obesity-related diseases, sedentary behavior, and aging are associated with sarcopenia in adulthood, which is a degenerative loss of muscle mass (1, 2). Westernized diets and a sedentary lifestyle also result in obesity and associated metabolic disorders such as insulin resistance in muscle and other peripheral tissues and lead to an accumulation of fat in liver, muscle, and adipose tissue. When sarcopenia is accompanied by high–body fat content (obesity), it is known as sarcopenic obesity, which increases the risk for cardiovascular and metabolic diseases (3). Known inducers of myogenesis in adulthood are exercise and growth factors that induce changes in myokine secretion from muscle. Myokines comprise cytokines and other proteins that are secreted from muscle and modify whole-body metabolism by regulating glucose uptake, β-oxidation, lipolysis, glucose production, and myogenesis (4). In the late 1990s, the first myokines, MSTN (an inhibitor of muscle growth) (5, 6) and IL-6 (an inducer of muscle growth) (4), were described. These were soon followed by other myokines, such as irisin (FNDC5) (4). They function in an autocrine, paracrine, and endocrine fashion to control muscle biology, and they have other effects, such as inducing fat browning (4). At present, there are no treatments for sarcopenia or sarcopenic obesity besides lifestyle and nutritional interventions, which are difficult to implement, especially in the elderly and in patients with disabilities. Current therapeutic options for sarcopenia include β-adrenergic receptor blockers, ghrelin, and testosterone, but these treatments do not improve muscle performance or control body weight, and they have negative effects on prostate and the cardiovascular system (2). There are data suggesting that inhibitors of Mstn expression or MSTN action are promising therapies (7).
Cannabinoid receptor type 1 (CB1R) is a master regulator of whole-body and cell metabolism and plays a critical role in food intake, lipogenesis, glucose uptake, insulin secretion, and gluconeogenesis (8). In obesity, CB1R becomes overactive due to an increase of the circulating endogenous ligands, the endocannabinoids (8). CB1R overactivity in liver co-occurs with insulin and leptin resistance and dyslipidemia, and it may be a contributing factor to the low-grade, chronic inflammatory state seen in obesity (9–12). Rimonabant, a CB1R inverse agonist, improves insulin sensitivity and increases glucose uptake of lean and obese Zucker rats (13); these findings may be a consequence of improvements in total body metabolism and reduced food intake. CB1R is expressed in murine and human skeletal muscle (14), but the relevance, if any, of muscle-CB1R to whole-body metabolism and muscle biology remains to be defined. For that purpose, we developed a skeletal muscle–specific CB1R-knockout (Skm-CB1R−/−) mouse model, and here, we determine the role of muscle-CB1R and its impact in whole-body performance and metabolism.
MATERIALS AND METHODS
Mice
Mice expressing Cre under the Acta1 gene promoter (ACTA-Cre; The Jackson Laboratory, Bar Harbor, ME, USA) and cannabinoid receptor type 1 gene -Cnr1- flanked by LoxP sites (CNR1LoxP/LoxP) mice (12) were bred to obtain CNR1LoxP/LoxP-Cre+ (Skm-CB1R−/−) mice and CNR1LoxP/LoxP-Cre- control (Skm-CB1R+/+) littermates. Mice were housed in groups of 4 using 12-h light/dark cycles, provided with water, and fed ad libitum. Age- and sex- (male) matched littermate mice were randomly assigned to vehicle or S961 and to standard diet (SD) or high-fat–high-sugar diet (HFHS) groups. Male mice were fed an SD (16.7% kJ fat, 12.4% kJ sugar, w/w) or HFHS (49.2% kJ fat, 32.2% kJ sugar, w/w) (Dyets, Bethlehem, PA, USA) to induce obesity. Body weight and food intake were measured weekly in the diet studies. After 15 wk of diet, in vivo tests were performed. Tissues were collected, weighed, and flash frozen or fixed for immunohistochemistry.
Microarray analysis
Skeletal muscles (gastrocnemius) were dissected, and total RNAs were isolated using Trizol (Thermo Fisher Scientific, Waltham, MA, USA). Microarray analysis was performed as previously described (12). Total RNA concentrations and integrity numbers (RIN) were 6.0–8.0 [measured by Agilent Bioanalyzer 2000 Microchips (Agilent Technologies, Santa Clara, CA, USA)]. Two hundred nanograms total RNA was labeled using the Low-Input QuickAmp Labeling Kit (Agilent Technologies) and were purified and quantified per the manufacturer’s recommendations. Six hundred nanograms cyanine 3–labeled cRNA was hybridized for 17 h to SurePrint G3 8 × 60 K mouse v1 oligo microarrays (Agilent Technologies). Arrays were scanned using an Agilent SureScan Microarray Scanner (Agilent Technologies) at 3-μm resolution, and hybridization intensity data were extracted using Agilent Feature Extraction Software (Agilent Technologies). Raw microarray hybridization intensity data (n = 4 separate biologic experiments) were log transformed. Raw microarray data were log transformed to yield z scores. The z ratio was calculated as the difference between the observed gene z scores for the experimental and control comparisons divided by the standard deviation. Z-ratio values of ±1.5 were used as cut-off values and calculated using a 5% false discovery rate threshold. The complete set was tested for gene set enrichment using parametric analysis of gene set enrichment. For each pathway z score, a P value was computed using JMP v.6.0 software (SAS Institute, Cary, NC, USA). Significant genes were selected by the z test (P ≤ 0.05, false discovery rate ≤0.30, and z ratio ≥1.5). Gene regulatory network and canonic pathway analysis were performed by using Ingenuity Pathway Analysis (IPA) (Qiagen, Germantown, MD, USA) and gene heatmap by JMP program. The data have been deposited into the Gene Expression Omnibus (National Center for Biotechnology Information, Bethesda, MD, USA; https://www.ncbi.nlm.nih.gov/geo/).
IR antagonism by S961
Mini-osmotic pumps (7 d, 1 µl/h; Alzet, Cupertino, CA, USA) were filled with 10 nmol of S961, and the pumping rate was 0.05 nmol/h (15). Implantation of pumps was performed as previously described by Wang et al. (16). Tail vein blood was collected daily until d 5, and animals were euthanized.
Blood glucose and hormone measurements
Mice were unfed for 4 h [insulin tolerance test (ITT)] or overnight [(intraperitoneal glucose tolerance test (IPGTT)] and given free access to water. For ITTs mice were injected IP with 1–1.5 IU/kg of insulin (Eli Lilly and Co., Indianapolis, IN, USA) (SD-fed or HFHS-fed, respectively), and blood glucose was measured at 0, 15, 30, 60, and 90 min. For IPGTTs, mice were given a bolus of 1.5 g/kg glucose IP, and tail vein blood was collected at similar time points. Area under the curve was calculated using Prism (GraphPad Software, La Jolla, CA, USA). Tail vein blood glucose was measured utilizing an Easygluco blood glucose meter (Abbott Laboratories, Chicago, IL, USA). Plasma insulin and IL-6 were quantified by ELISA (Crystal Chem, Elk Grove Village, IL, USA). Triglyceride levels in plasma were quantified using the Triglyceride Assay Kit (Abcam, Cambridge, MA, USA).
Membrane protein isolation
For membrane and cytosol protein extraction from muscle, Mem-PER Plus Membrane Protein Extraction Kit (Thermo Fisher Scientific) was used following the manufacturer’s instructions.
Immunoblotting
Protein samples were extracted from muscle using RIPA buffer (Boston BioProducts, Ashland, MA, USA) containing protease and phosphatase inhibitor cocktails (MilliporeSigma, Burlington, MA, USA). IR was immunoprecipitated from 500 µg of protein using anti-IR (1:50; Cell Signaling Technology, Danvers, MA, USA). Samples were subjected to Tris-glycine PAGE; immunoblotted with anti–phosphorylated (phospho-) IR (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA), AKT, phospho-AKT(Ser473), phospho-AKT(Thr308) (1:1000; Cell Signaling Technology), IR (1:1000; Santa Cruz Biotechnology), β-Actin (1:1000; Abcam), phospho-Tyr (1:1000; MilliporeSigma), β-tubulin, and calveolin-1 (1:3000; Cell Signaling Technology); and visualized by ECL (Thermo Fisher Scientific). Densitometry of bands was quantified using ImageJ [National Institutes of Health (NIH), Bethesda, MD, USA].
Phosphoprotein array
Protein phosphorylation and protein cleavage in dissected muscles was performed using the PathScan Array Kit (Cell Signaling Technology) following the manufacturer’s instructions. Briefly, muscle samples were lysed and diluted up to 0.2 mg/ml. Samples were incubated for 2 h at room temperature, and images were captured using the Odyssey Sa Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). Densitometry was analyzed using Odyssey Sa Imaging Software.
Cell culture and cell transfection, quantitative PCR, and reagents
C2C12 [CRL-1772; American Type Culture Collection (ATCC), Manassas, VA, USA] were grown in DMEM complemented with 10% fetal calf serum at 37°C and under 5% CO2. Cells were washed and serum starved for 2 h; medium was then changed for serum-free medium containing increasing concentrations of JD-5037 (Cayman Chemical, Ann Arbor, MI, USA). After 24 h, cells were counted. For transfection, cells were seeded at 0.25 × 106 cells per well in 6-well plates. Transfections were performed using Lipofectamine 2000 (Thermo Fisher Scientific) using 1.5 µg of either pcDNA3.1, peGFP, or pMuLE ENTR CMV CreERT2 following the manufacturer’s instructions. Cells were then treated with 1 µM of 4-hydroxytamoxifen or vehicle for 24 h. Total RNA was extracted using RNeasy Plus Kit (Qiagen) following the manufacturer’s instructions. RNA was reverse transcribed using GoScript Reverse Transcription System (Promega, Madison, WI, USA) using random primer according to the manual’s instructions. Real-time PCR was conducted on the thermal cycler Mx3005P (Stratagene, San Diego, CA, USA) using Sybr Green/ROX Master Mix (Thermo Fisher Scientific). Il-6, Mstn, and Hprt (housekeeping gene) were amplified using the following set of primers: 5′-TAGTCCTTCCTACCCCAATTTCC-3′ and 5′-TTGGTCCTTAGCCACTCCTTC-3′; 5′-AGTGGATCTAAATGAGGGCAGT-3′ and 5′-GTTTCCAGGCGCAGCTTAC-3′; and 5′-GCCAGACTTTGTTGGATTTG-3′ and 5′-CGCTCATCTTAGGCTTTGTATTTGG-3′, respectively. Data were processed following the 2−ΔΔCt method and represent 3 technical replicates of 3 independent biologic replicates (N = 3, n = 3).
Real-time RT-PCR analysis
Total RNAs were isolated using Trizol reagent from dissected skeletal muscles. RNA concentration and quality were measured by NanoDrop (Thermo Fisher Scientific). Reverse transcription was performed using SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific). Relative expression of selected genes was assayed using TaqMan Fast Advanced Master Mix and FAM-labeled TaqMan Gene Expression Assays on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). Duplex reactions were performed using VIC-labeled β-actin for endogenous control.
Quantification of total muscle cAMP
Gastrocnemius was weighed and crushed with a mortar and pestle on dry ice; total cAMP was quantified using Direct cAMP ELISA Kit (Enzo Life Sciences, Farmingdale, NY, USA) following manufacturer’s instructions.
Immunohistochemistry of muscle and cross-sectional area quantification
Immunohistochemistry was carried out as previously described by Fiori et al. (17) with slight modifications. Skeletal muscles were dissected, frozen in methyl butane, and sectioned at 8 µm. Hematoxylin and eosin staining was performed following the manufacturer’s protocol. Imaging was performed at ×20 and 40 using an inverted Olympus IX51 light microscope (Olympus, Tokyo, Japan). Cross-sectional area was measured using ImageJ.
Comprehensive metabolic analysis
Animals were placed in metabolic cages [Columbus Instruments Comprehensive Lab Monitoring System (CLAMS), Columbus, OH, USA] with ad libitum access to food and water and controlled light. Following acclimatization for 48 h, metabolic measurements taken included the respiratory exchange ratio (RER; RER = CO2 produced/O2 consumed), activity levels, sleeping time, and heat, food, and water intake.
Assessment of body composition using NMR spectroscopy
Anesthetized mice were weighed and immediately placed on a Bruker minispec LF90 NMR (Bruker, Billerica, MA, USA). Readouts were lean, fat, and fluid mass. Data are presented as lean-to-fat ratio and percent body weight.
Physical performance measurements
Results from grip strength, rotarod, and treadmill were presented as previously described by Martin-Montalvo et al. (18). Briefly, grip strength of front limbs was tested using a grip strength meter with a bar (Harvard Apparatus, Holliston, MA, USA), and the final score was the average of 3 trials per mouse. The rotarod test was performed at increasing speed from 4 to 40 rpm over 5 min, and the final score is the average of 3 trials per mouse with 30 min rest periods between trials. The treadmill test was performed until exhaustion at increasing speed from 9 to 23 m/min. Mice were given a habituation trial 24 h before each test at constant speed (4 rpm for 1 min for rotarod, 4 m/min for 5 min for treadmill).
High-resolution respirometry
Skeletal muscle fiber bundles from muscles were immediately placed in ice-cold preservation buffer and prepared for high-resolution respirometry measurements (Oxygraph 2K; Oroboros Instruments, Innsbruck, Austria) as previously described (19). Briefly, muscle bundles were dissected and permeabilized in saponin and washed with cold mitochondrial respiration medium. The oxygen flux levels were recorded using DatLab 4 software (Oroboros Instruments). A substrate-uncoupler–inhibitor titration protocol was used to assess mitochondrial function: malate (5 mM), glutamate (10 mM), and succinate (10 mM) were added initially to stimulate respiration after the addition of malate, glutamate, and succinate (LEAK respiration). ADP (5 mM) was added to stimulate oxphos capacity. Oligomycin (2.5 μM) was added to inhibit ATP synthase, followed by carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (1 μM) to determine maximal uncoupled respiration. Rotenone (0.5 μM) and antimycin A (2.5 μM) were added to terminate respiration by inhibiting mitochondrial complex I and complex III. Cytochrome c (10 μm) was used to confirm the integrity of the outer mitochondrial membrane. Data were normalized to wet fibers weight.
Blinding
In vivo physiologic measurements and ex vivo analysis, including immunoblotting and high-resolution respirometry, were blinded to the testers.
Statistics
Data are presented as means ± sem. Differences between mean values for variables within individual experiments were compared statistically by Student’s t test or ANOVA; Bonferroni’s and Fisher’s least significant difference post hoc tests were used. Comparisons were performed using GraphPad Prism v.6.0. A value of P < 0.05 was considered statistically significant.
Study approval
All animal experiments and care were performed following NIH guidelines and were approved by the National Institute on Aging Animal Care and Use Committee (Animal Study Proposal 443-LCI-2016).
RESULTS
Transcription profiling of skeletal muscle from Skm-CB1R−/− mice
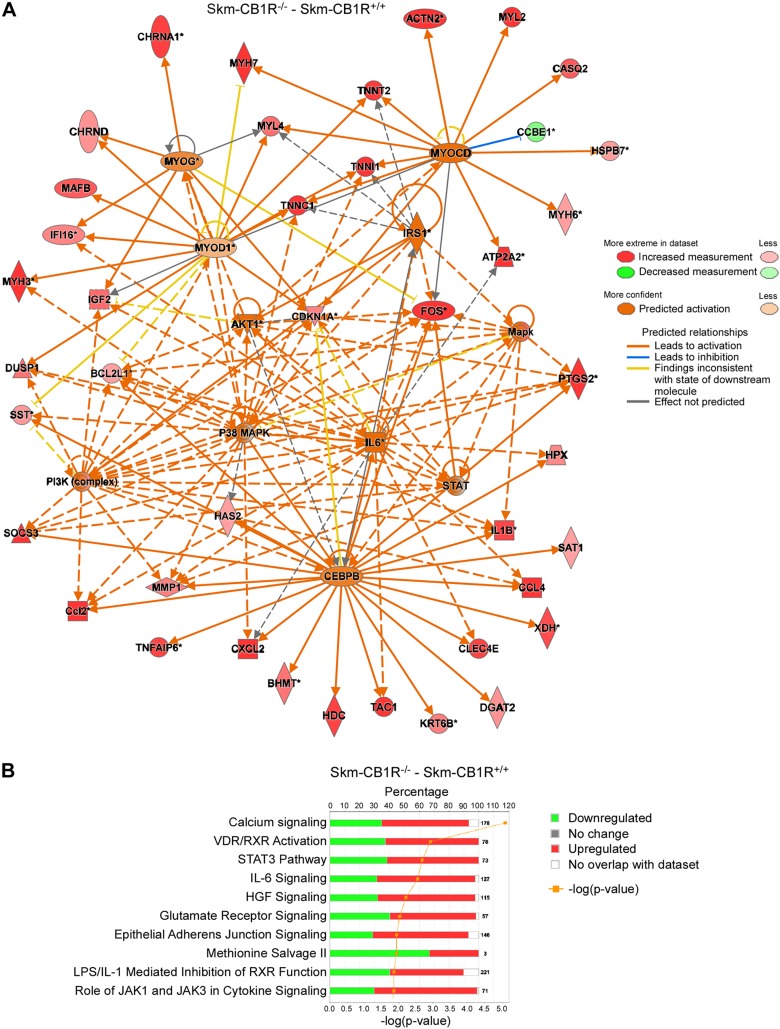
Skm-CB1R−/− and control littermate (Skm-CB1R+/+) mice were developed by crossing a LoxP-flanked CB1R gene (Cnr1) mouse with an ACTA-Cre (Cre under control of Acta1 promoter) (Supplemental Fig. S1A). Ablation of CB1R in skeletal muscle but not in brain was confirmed by PCR from genomic DNA (Supplemental Fig. S1A). To first characterize the impact of ablating CB1R in muscle, we extracted mRNA from quadriceps of Skm-CB1R−/− and Skm-CB1R+/+ mice and performed a microarray analysis. IR downstream pathways, as shown by activation of the pathways under IRS-1, AKT, and PI3K regulation (Fig. 1A, Supplemental Fig. S1B, and Table 1), were up-regulated in Skm-CB1R−/− mice based on IPA (20). The analysis also uncovered activation of MAPKs and myogenesis by activation of the upstream regulators MAPKs, p38, STAT, CEBP-β, IL-6, and the myogenic factors MYOCD, MYOG, and MYOD1 (Fig. 1A, Supplemental Fig. S1C, and Table 1). The canonical pathways predicted to be up-regulated with a –log (P value) >2 were calcium signaling, which is required for muscle contraction, VDR, STAT3, IL-6, and hepatocyte growth factor pathways (Fig. 1B), which are involved in myogenesis and metabolism. However, phenotypically, both mouse strains were similar. Skm-CB1R−/− mice (25 wk old) had similar body weights and fasting circulating insulin and glucose levels to Skm-CB1R+/+ mice (Supplemental Fig. S1D–F), and blood glucose during an IPGTT and an ITT were similar (Supplemental Fig. S1F, G).
Figure 1.
Analysis of gene expression array by IPA of skeletal muscle from Skm-CB1R+/+ and Skm-CB1R−/− mice. A) Microarray analysis of RNA extracted from Skm-CB1R+/+ and Skm-CB1R−/− muscle (gastrocnemius). Changes were analyzed by IPA software and Ingenuity Upstream Regulator Analysis is shown. B) Canonical pathway expression changes in a microarray of RNA from Skm-CB1R+/+ and Skm-CB1R−/− muscle (n = 4 unique mice/group). HGF, human growth factor.
TABLE 1.
Analysis of gene expression array by IPA of skeletal muscle from Skm-CB1R+/+ and Skm-CB1R−/− mice
| Upstream regulator | Z score | P |
|---|---|---|
| IRS1 | 0.42 | 7.3.10-2 |
| PI3K (complex) | 3.35 | 9.1.10-6 |
| Akt1 | 2.38 | 1.5.10-3 |
| Mapk (group) | 2.46 | 9.8.10-5 |
| P38 MAPK (group) | 4.22 | 1.6.10-11 |
| STAT (group) | 2.40 | 4.1.10-5 |
| IL-6 | 3.40 | 1.4.10-13 |
| CEBP | 1.51 | 2.6.10-6 |
| MYOCD | 2.23 | 9.8.10-7 |
| MYOG | 1.25 | 6.9.10-5 |
| MYOD1 | 0.71 | 4.3.10-4 |
Analysis of up-regulators in Skm-CB1R−/− compared to Skm-CB1R+/+ muscle (n = 4 unique mice/group).
Skeletal muscle–CB1R ablation protects from insulin resistance in adult mice
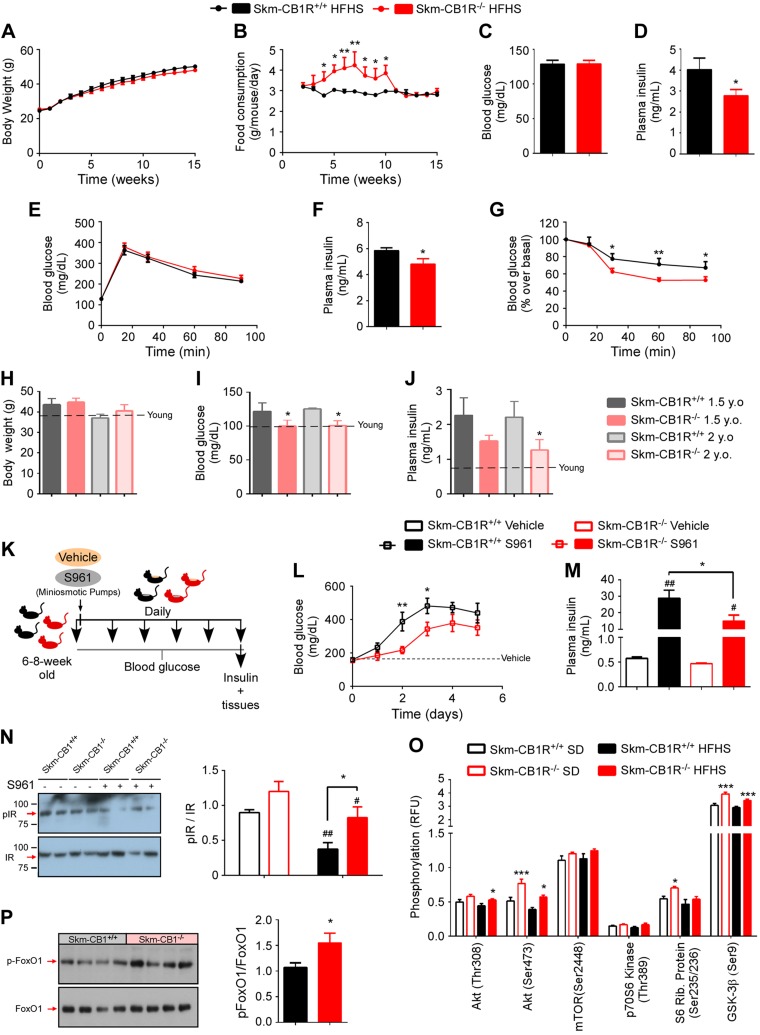
Skm-CB1R−/− and Skm-CB1R+/+ male mice (6–8 wk old) were fed an HFHS (HFHS-mice) to induce obesity. Body weights were similar in both strains over the course of 15 wk (Fig. 2A), although HFHS–Skm-CB1R−/− mice consumed significantly more food compared with HFHS–Skm-CB1R+/+ mice during the first 2 mo of the study (Fig. 2B). At week 15, fasting blood glucose was 128 ± 17 mg/dl in both mouse strains (Fig. 2C), but fasting plasma insulin was significantly lower in HFHS-fed Skm-CB1R−/− mice (Fig. 2D). Although both strains had similar blood glucose levels during an IPGTT (Fig. 2E), HFHS–Skm-CB1R−/− mice had lower circulating insulin (Fig. 2F) 15 min after a glucose bolus and were more insulin sensitive than HFHS–Skm-CB1R+/+ mice at wk 15, based on an ITT (Fig. 2G). Insulin resistance also increases with age (21). Body weight of 18- and 24-mo-old Skm-CB1R−/− male mice were similar to their age-matched Skm-CB1R+/+ mice (Fig. 2H), but they had significantly lower fasting blood glucose at 18 and 24 mo of age (Fig. 2I) and lower fasting plasma insulin at 24 mo of age (Fig. 2J).
Figure 2.
Skm-CB1R−/− mice are protected from insulin resistance compared with Skm-CB1R+/+ mice. Skm-CB1R−/− and Skm-CB1R+/+ male mice (6–8 wk old) were fed an HFHS for 3 mo. A–G) Body weight (A) and food consumption (B) over the course of 15 wk. Fasting blood glucose (C) and fasting plasma insulin (D) levels after 15 wk. Blood glucose during an IPGTT (E), plasma insulin 15 min after glucose administration (F), and ITT after 15 wk of HFHS (G) (n = 9 mice). Data show means ± sem. F = 9.6, F = 25.8, F = 10.1. *P ≤ 0.05, **P ≤ 0.01 (ANOVA or Student’s t test). H–J) Skm-CB1R+/+ and Skm-CB1R−/− mice were aged up to 2 yr. Body weight (H), fasting blood glucose (I), and fasting plasma insulin (J) levels were measured at 1.5 and 2 yr of age. Dotted line shows levels at 6 mo of age (n = 6–17 mice). Data show means ± sem. *P ≤ 0.05 compared with Skm-CB1R+/+ mice (Student’s t test). K) Male mice (6–8 wk old) were continuously infused with S961, an IR antagonist, for 5 d. L) Circulating blood glucose was measured daily. M) Circulating plasma insulin at d 5 of S961 or vehicle infusion. N) Postmortem analysis (d 5 of S961 or vehicle infusion) of insulin signaling in Skm-CB1R+/+ and Skm-CB1R−/− quadriceps by Western blot for phospho-IR and total IR (n = 6–7 mice). Data show means ± sem. F = 5.6. *P ≤ 0.05 compared with Skm-CB1R+/+ mice, #P ≤ 0.05, ##P ≤ 0.01 compared with vehicle (ANOVA or Student’s t test). O) Quantification of protein phosphorylation downstream of insulin in skeletal muscle (gastrocnemius) from SD- (empty bars) or HFHS-fed (filled bars) Skm-CB1R+/+ and Skm-CB1R−/− mice as measured by phosphoprotein array. P) Western blot for phospho-FoxO1 and total FoxO1 and densitometry quantification (n = 6–9 mice each run in duplicates). P, phosphorylated; Rib, ribosomal; RFU, relative fluorescence units. Data show means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 compared with Skm-CB1R+/+ mice (Student’s t test).
Male mice (6–8 wk old) were continuously infused with S961, a peptide-based competitive antagonist of endogenous insulin binding to its receptor (22, 15), or vehicle for up to 5 d (Fig. 2K). S961 increases circulating levels of insulin as insulin secretion increases in response to rising blood glucose levels (12, 15). Blood glucose levels were measured daily, and circulating plasma insulin levels were measured on d 5 (Fig. 2K). Glucose levels in S961–Skm-CB1R+/+ mice by d 2 were 387 ± 55 and <400 mg/dl by d 3–5 (Fig. 2L). Blood glucose levels in S961–Skm-CB1R−/− mice remained low until d 3 and did not raise above 400 mg/dl (Fig. 2L), which can be interpreted as increased affinity of insulin for its receptor (i.e., improved insulin sensitivity). Plasma insulin levels increased up to 28.8 ± 4.8 ng/ml in S961–Skm-CB1R+/+ mice, 1.9-fold higher than S961–Skm-CB1R−/− mice (Fig. 2M).
Postmortem analysis of insulin signaling in skeletal muscle showed that blockade of insulin action by S961 reduced phospho-IR by 58% in Skm-CB1R+/+ mice, whereas it reduced phospho-IR by just 31% in Skm-CB1R−/− mice (Fig. 2N). Thus, the reduced glucose (Fig. 2L) and insulin (Fig. 2M) levels result from an improved insulin sensitivity in muscle, the largest insulin-sensitive organ. This improvement in insulin sensitivity was not due to improvement in liver IR sensitivity (Supplemental Fig. S2A); in fact, the reduced phosphorylation of IR in S961-treated Skm-CB1R−/− liver likely reflects the reduced circulating insulin levels (Fig. 2M). We examined IR downstream signaling gastrocnemius from SD– and HFHS–Skm-CB1R+/+ and Skm-CB1R−/− mice. A chemiluminescent phosphoprotein array uncovered an increase in phospho-AKT in SD– and HFHS–Skm-CB1R−/− compared with Skm-CB1R+/+ mice (Fig. 2O). Data were validated by Western blot analysis (Supplemental Fig. S2B–D). There were no changes in phospho-mTOR or phospho-p70S6K. Phospho-S6 was increased in SD–Skm-CB1R−/− compared with SD–Skm-CB1R+/+ mice. GSK-3β was significantly more phosphorylated in SD– and HFHS–Skm-CB1R−/− mice compared with Skm-CB1R+/+ mice (Fig. 2O). FoxO1 is a downstream target of AKT that, when dephosphorylated, induces Mstn genetic transcription (23). Phospho-FoxO1 was higher in HFHS–Skm-CB1R−/− than in HFHS–Skm-CB1R+/+ muscle (Fig. 2P).
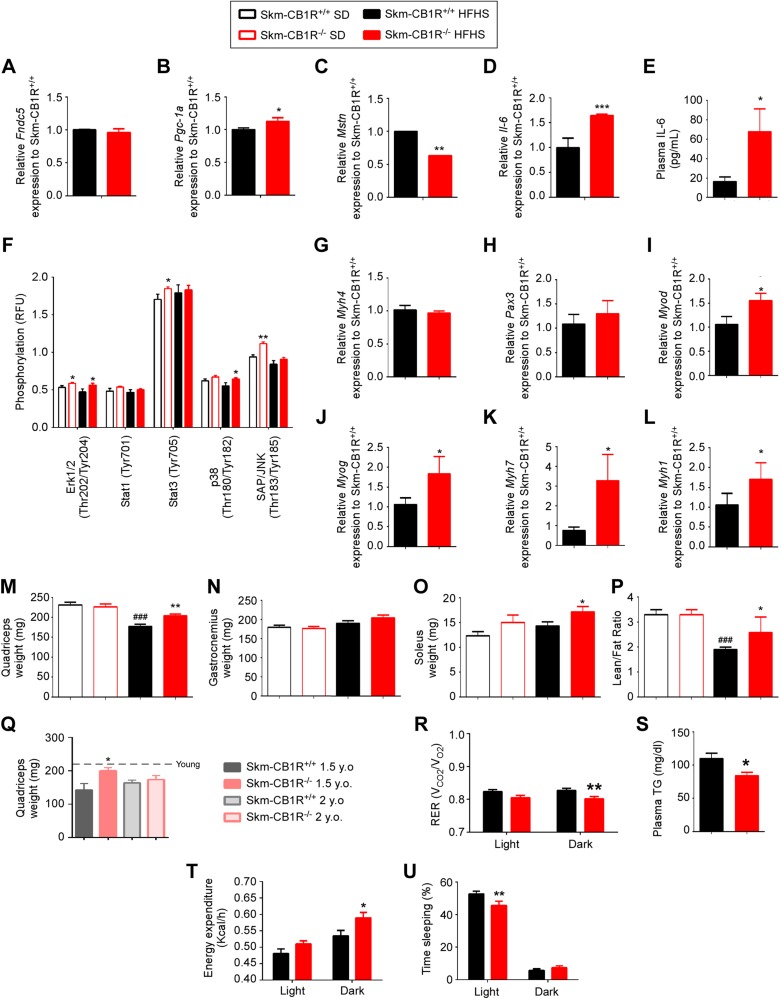
CB1R regulates myokines and myogenesis in muscle
There was no change in Fndc5 expression in HFHS–Skm-CB1R−/− skeletal muscle compared with HFHS–Skm-CB1R+/+ (Fig. 3A), despite a small increase in the expression of Pgc-1a (Fig. 3B), a known inducer of Fndc5 expression (24). Mstn expression was significantly lower in muscle from HFHS–Skm-CB1R−/− mice compared with HFHS–Skm-CB1R+/+ mice (Fig. 3C), in agreement with the phospho-FoxO1 data (Fig. 2P). Il-6 expression in skeletal muscle (Fig. 3D) and its plasma protein levels (Fig. 3E) were significantly increased in HFHS–Skm-CB1R−/− compared with HFHS–Skm-CB1R+/+ mice, in agreement with the microarray data (Fig. 1A and Supplemental Fig. S1C); there was no increase in Tnfa expression (Supplemental Fig. S3C), indicating a myokine role and not a proinflammatory role. Both Mstn and Il-6 expression were similarly altered in SD–Skm-CB1R−/− compared with SD–Skm-CB1R+/+ skeletal muscle (Supplemental Fig. S3A, B). IL-6 receptor signals through IR/AKT, JAK/STAT, and MAPKs to induce glucose uptake, β-oxidation, and myogenesis (25, 26). Phosphoprotein analysis of SD- and HFHS–Skm-CB1R+/+ and Skm-CB1R−/− gastrocnemius showed that phospho-ERK1/2 was increased in SD- and HFHS–Skm-CB1R−/− mice compared with Skm-CB1R+/+ mice (Fig. 3F). Phospho-SAP/JNK and phospho-STAT3 were higher in SD–Skm-CB1R−/− mice, whereas phospho-p38 was increased only in HFHS–Skm-CB1R−/− mice when compared with Skm-CB1R+/+ mice (Fig. 3F). Expression of early/embryonic myogenic genes (Myh4 and Pax3) in muscle was comparable in both strains (Fig. 3G, H). Expression levels of Myod (Fig. 3I) and Myog (Fig. 3J) were significantly increased in Skm-CB1R−/− compared with Skm-CB1R+/+ muscle. The protein product of Myh7, MHC, is found in type 1 slow-twitch (oxidative) skeletal muscle fibers. Its expression level was 3.2-fold higher (Fig. 3K) in Skm-CB1R−/− compared with Skm-CB1R+/+ muscle. The protein product of Myh1, MHC1, is found in type II fast-twitch glycolytic fibers. Its expression level was 1.7-fold higher in Skm-CB1R−/− compared with Skm-CB1R+/+ muscle (Fig. 3L). There were no significant changes in Ppard or Myh2 (type IIA fibers) expression (Supplemental Fig. S3D, E).
Figure 3.
Skeletal muscle–CB1R ablation impacts myokine expression and induces myogenesis and whole-body energy expenditure. A–C) Analysis of relative mRNA expression of myokines by real-time PCR in skeletal muscle (gastrocnemius) from HFHS-fed Skm-CB1R−/− and Skm-CB1R+/+ mice. Irisin (Fndc5) (A), Pgc1a (B), and Mstn (C) relative mRNA expression to Skm-CB1R+/+ mice. D) Il-6 relative mRNA expression to Skm-CB1R+/+ mice. E) Plasma levels of Il-6. F) Quantification of protein phosphorylation (MAPK) in skeletal muscle (gastrocnemius) from SD- (empty bars) or HFHS-fed (filled bars) Skm-CB1R+/+ and Skm-CB1R−/− mice as measured by phosphoprotein array (n = 6–9 mice, each run in duplicates). G–L) Analysis of relative mRNA expression of myogenic genes. Expression data was corrected to β-actin; N = 9 mice. Data show means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 compared with Skm-CB1R+/+ mice (Student’s t test). M–O) Weight of SD-fed and HFHS-fed quadriceps (M), gastrocnemius (N), and soleus (O) from Skm-CB1R−/− and Skm-CB1R+/+ mice. P) Lean and fat mass ratio were calculated by NMR in SD-fed and HFHS-fed Skm-CB1R+/+ and Skm-CB1R−/− mice, after which mice were euthanized (n = 9 mice). Data show means ± sem. F = 1.9, F = 12, F = 3.5; *P ≤ 0.05, **P ≤ 0.01, compared with Skm-CB1R+/+ mice, ###P ≤ 0.001 compared with SD (ANOVA). Q) Weight of quadriceps in 1.5- and 2-yr-old Skm-CB1R+/+ and Skm-CB1R−/− mice. Dotted line shows weight at 6 mo of age (n = 5–11). R) RER was measured during light (resting) and dark (active) cycles at wk 15 of diet. S) Plasma levels of triglycerides (TG) in HFHS-fed Skm-CB1R+/+ and Skm-CB1R−/− mice. T, U) Energy expenditure (T) and sleeping time (U) during light and dark cycles (n = 9 mice). RFU, relative fluorescence units. Data show means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (Student’s t test).
Skeletal muscles were dissected and weighed. Quadriceps from SD-mice weighed 228 ± 7 mg each in both strains; however, in HFHS–Skm-CB1R+/+ mice, quadriceps weighed 178 ± 6 mg, which is a 22% reduction, whereas no reduction occurred in the HFHS–Skm-CB1R−/− mice (Fig. 3M). Gastrocnemius, a highly glycolytic muscle, from SD-mice weighed the same in both strains and tended to weigh more in HFHS–Skm-CB1R−/− than in HFHS–Skm-CB1R+/+ mice (P = 0.09; Fig. 3N). Soleus, the more oxidative muscle, weighed 12.3 ± 0.8 and 15 ± 1.5 mg in SD–Skm-CB1R+/+ and SD–Skm-CB1R−/− mice, respectively (Fig. 3O; P = 0.06); however, soleus weight in HFHS–Skm-CB1R−/− mice was 17.2 ± 1 mg (Fig. 3O; P < 0.05 compared with HFHS–Skm-CB1R+/+). These data show an increase in muscle mass in HFHS–Skm-CB1R−/− mice compared with HFHS–Skm-CB1R+/+, despite no change in body weight (Fig. 2A). The changes in muscle weight were noticeable as early as 8 wk of age (Supplemental Fig. S3F, G), as was an increase in Il-6 expression (Supplemental Fig. S3H), and were not due to Cre expression (Supplemental Fig. S3F–I). We quantified lean and fat mass by NMR. Although lean/fat mass ratio was similar in both strains when fed an SD, HFHS–Skm-CB1R−/− mice had higher lean/fat mass ratio than HFHS–Skm-CB1R+/+ mice (Fig. 3P). Quadriceps mass was protected from age-related loss in Skm-CB1R−/− mice up until 1.5 yr old (Fig. 3Q). Because of the contribution of cAMP to muscle hypertrophy, we measured muscle total cAMP. There were no significant differences in cAMP levels of SD-fed Skm-CB1R−/− compared with Skm-CB1R+/+ muscle, and when fed an HFHS, there was a trend (P = 0.1) to greater cAMP accumulation in Skm-CB1R−/− than Skm-CB1R+/+ muscle (Supplemental Fig. S3J). CB1R is coupled to both Gαo/i and Gq in muscle (27–29); the effects observed in the skeletal muscle–specific CB1R knockout are therefore a pleiotropic effect combining Gq and Gαo/i protein signaling. Moreover, JD-5037, a CB1R inverse agonist, stimulated C2C12 cell proliferation in a dose-dependent manner (Supplemental Fig. S3K).
The changes in myokine levels and lean mass were reflected in a reduction of the RER (RER = ratio of carbon dioxide production and oxygen consumption) (Fig. 3R), which indicates a preference for fat as an energy source in place of glucose. Circulating triglyceride levels were indeed significantly lower in HFHS–Skm-CB1R−/− compared with HFHS–Skm-CB1R+/+ mice (Fig. 3S). HFHS–Skm-CB1R−/− mice had significantly higher energy expenditure during dark cycles (Fig. 3T) and fewer sleeping hours during light cycles (Fig. 3U) than HFHS–Skm-CB1R+/+ mice, although spontaneous total activity was comparable (Supplemental Fig. S4). Altogether, skeletal muscle–CB1R ablation increased myogenesis and muscle mass and a change in the proportion of fat to lean mass, leading to an increase in whole-body metabolism and energy expenditure, which may further contribute to reduction of fat mass (30.4 ± 0.4 vs. 29.4 ± 0.3% of body weight in HFHS–Skm-CB1R+/+ and HFHS–Skm-CB1R−/− mice, respectively).
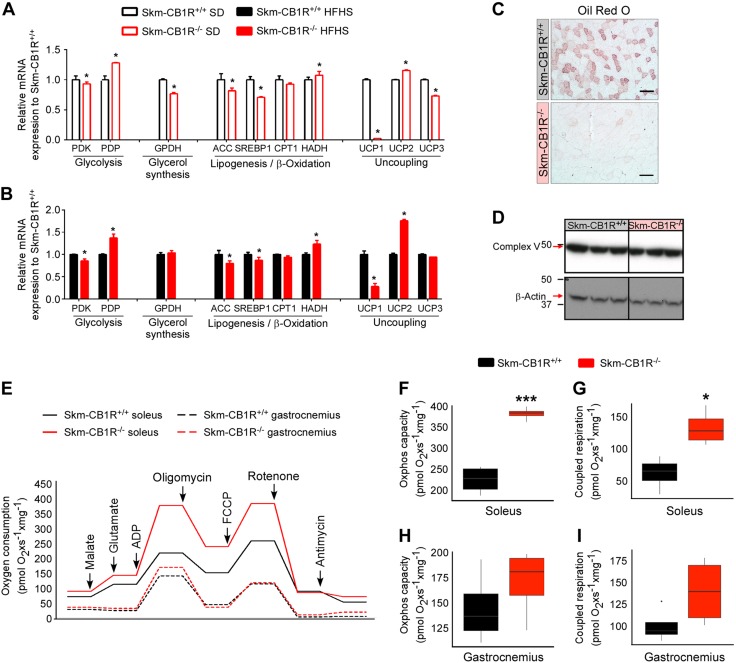
CB1R ablation impacts on muscle metabolism
Muscle metabolism is highly impacted by myokines (4). We analyzed the expression of genes related to glucose and lipid metabolism in gastrocnemius from SD- and HFHS–Skm-CB1R+/+ and Skm-CB1R−/− mice (Fig. 4A, B). In SD- (Fig. 4A) and HFHS-mice (Fig. 4B), muscle-CB1R ablation reduced pyruvate dehydrogenase kinase expression and increased pyruvate dehydrogenase phosphatase, meaning higher pyruvate activation, and SD–Skm-CB1R−/− mice had lower expression of glycerol-3-phosphate dehydrogenase. Muscle-CB1R ablation also reduced lipogenic gene expression (acetyl-coA carboxylase and SREBP1), whereas hydroxyacyl-coenzyme A dehydrogenase (β-oxidation) expression was increased. No significant changes were observed in carnitine palmitoyl transferase-1 expression. The expression of the genes encoding for the uncoupling proteins UCP1 and UCP3 was significantly down-regulated in SD– and HFHS–Skm-CB1R−/− mice, whereas UCP2 expression was significantly higher in Skm-CB1R−/− mice (Fig. 4A, B). Biopsies of gastrocnemius were stained with Oil Red O to determine the accumulation of neutral lipids. Skm-CB1R−/− muscle was almost absent of lipid droplets, whereas Skm-CB1R+/+ had fibers with a high accumulation of lipid droplets (Fig. 4C). Complex V-subunit protein was equal in both strains (Fig. 4D). Soleus and gastrocnemius from Skm-CB1R+/+ and Skm-CB1R−/− mice were dissected, and oxygen consumption was measured in monolayers of myofibers (Fig. 4E). Soleus (more oxidative muscle) from Skm-CB1R−/− mice had increased maximal oxidative phosphorylation capacity (Fig. 4F), and the respiration was more coupled to ATP production (Fig. 4G) than Skm-CB1R+/+ soleus. The differences in gastrocnemius (more glycolytic muscle than soleus) were not significant (Fig. 4H, I).
Figure 4.
Ablation of CB1R induces a metabolic change in muscle and improves mitochondrial performance. A, B) Analysis of relative mRNA expression of metabolic genes in muscle from SD-fed (A) and HFHS-fed (B) Skm-CB1R+/+ and Skm-CB1R−/− mice (n = 9 mice). Data show means ± sem. *P ≤ 0.05 compared with Skm-CB1R+/+ mice (Student’s t test). C) Oil Red O staining of skeletal muscle from Skm-CB1R+/+ and Skm-CB1R−/− mice. Scale bars, 50 µm. D) Western blot of the mitochondrial complex V of the respiratory chain. E) Analysis of oxygen consumption in Skm-CB1R+/+ and Skm-CB1R−/− digitonized myofibers. F–I) Analysis of maximum oxidative phosphorylation capacity (F, H) and oxygen consumption coupled to ATP production (G, I) in soleus (F, G) and gastrocnemius (H, I) (n = 4 mice). ACC, acetyl-coA carboxylase; CPT1, carnitine palmitoyl transferase-1; HADH, hydroxyacyl-coenzyme A dehydrogenase; GPDH, glycerol-3-phosphate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PDP, pyruvate dehydrogenase phosphatase. Data show medians ± range. *P ≤ 0.05, ***P ≤ 0.001 compared with Skm-CB1R+/+ mice (Student’s t test).
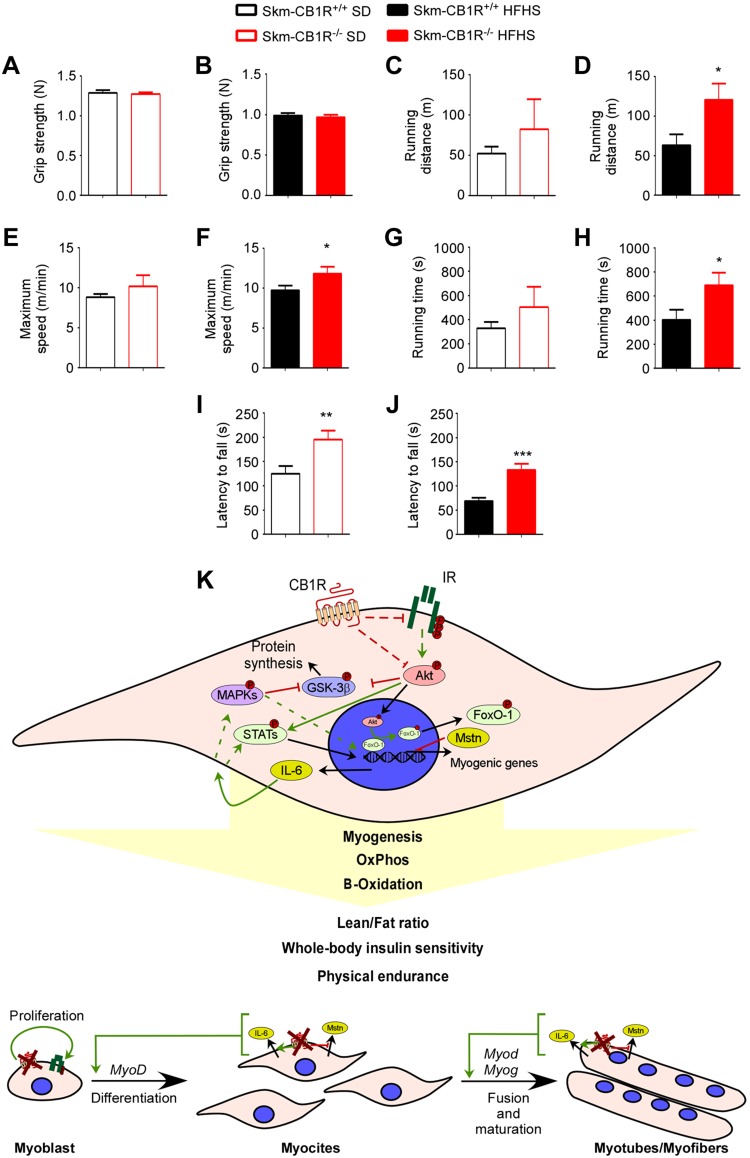
Skeletal muscle–CB1R ablation improves endurance but not strength
To ascertain if the observed increase in mass and metabolism in the Skm-CB1R−/− skeletal muscle had any biologic significance, we performed strength and endurance tests in SD- and HFHS-mice. Grip strength was similar in both strains under both dietary conditions (Fig. 5A, B), and indeed, analysis of the cross-sectional area of the muscle fibers was similar in all groups (Supplemental Fig. S5A–D). However, when mice were placed on a treadmill, running distances in both strains were similar in SD-mice (Fig. 5C), but in contrast, HFHS–Skm-CB1R−/− mice ran twice the distance compared with HFHS–Skm-CB1R+/+ mice in 15 min (Fig. 5D). Maximum running speed was approximately the same in both SD-strains (Fig. 5E) but was significantly higher in HFHS–Skm-CB1R−/− mice (Fig. 5F). Total running time on the treadmill was also similar in SD-mice (Fig. 5G) but 1.75-fold higher in HFHS–Skm-CB1R−/− mice compared with HFHS–SkmCB1R+/+ (Fig. 5H). Mice were placed on a rotarod, and time to fall was measured in seconds. SD– (Fig. 5I) and HFHS–Skm-CB1R−/− mice (Fig. 5J) had 1.56- and 1.93-fold longer latency to fall, respectively, compared with Skm-CB1R+/+ mice. The increase in running distance, running time, and latency to fall time all reflect increased endurance most likely due to increased lean muscle mass and improved capability of type 1 fibers.
Figure 5.
Skm-CB1R−/− mice have more physical endurance than Skm-CB1R+/+ mice. A–H) Grip strength was measured in SD-fed (A) and HFHS-fed (B) Skm-CB1R+/+ and Skm-CB1R−/− mice. Maximum running distance (C, D), maximum running speed (E, F), and maximum running time (G, H) in SD-fed and HFHS-fed Skm-CB1R+/+ and Skm-CB1R−/− mice measured in a treadmill test. I, J) Mice were placed on a rotarod and time to fall in seconds was measured (n = 9 mice). Data show means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (Student’s t test). K) Schema of CB1R function in skeletal muscle. CB1R down-regulates IR/AKT signaling, impacting the transcriptional regulation of the myokines Mstn and Il-6. IL-6, when secreted, activates via MAPKs signaling the myogenesis. IL-6 also impacts muscle metabolism by inducing β-oxidation and oxidative phosphorylation. Therefore, ablation of CB1R in muscle would lead to an increase in muscle mass (induce proliferation, differentiation, and fusion/maturation) and lean/fat ratio, improved whole-body insulin sensitivity, and physical endurance.
DISCUSSION
Atrophy of muscle and fatigue in adulthood due to sedentary lifestyles, obesity, and aging are common metabolic phenomena. Obesity epidemic is on the rise, and so is sarcopenic obesity. Current pharmacological treatments for sarcopenia do not help to control body weight or improve physical performance. The most effective treatment for sarcopenic obesity is exercise and changes in nutrition, which fail due to the mobility disability or advanced age of patients (30). Our study demonstrates that absence of CB1R specifically in muscle has significant benefits to whole-body metabolism, muscle mass, physical endurance, insulin action, and mitochondrial function in diet-induced obese and aged mice.
CB1R expression was reported to increase during myoblast differentiation (28), and activation of CB1R was shown to prevent differentiation of satellite cells (28). CB1R-specific synthetic agonists inhibit proliferation in a rat muscle cell line by blocking insulin signaling (31). Indeed, despite weighing less than wild type mice, global CB1R−/− mice fed regular chow were shown to have an increased number of muscle fibers (28). We do not see such changes in our mouse model. We only found an increase in muscle mass in aged– or HFHS–Skm-CB1R−/− mice, which was accompanied by an increased expression of Myod and Myog, required for differentiation of myoblasts into myocytes and in myocyte maturation to myotubes, respectively. Our mouse model expresses Cre under the regulation of Acta1 gene promoter, and Acta1 is expressed in myocytes and myotubes but not in undifferentiated myoblasts (32). Therefore, the changes observed in muscle size in Skm-CB1R−/− mice in comparison to global CB1R−/− mice do not result from a direct impact on satellite cell differentiation by lack therein of CB1R. AKT was more phosphorylated in Skm-CB1R−/− compared with Skm-CB1R+/+ muscle, which blocks Mstn expression by phosphorylating FoxO1 (23). In fact, HFHS–Skm-CB1R−/− muscle had significantly more phospho-FoxO1, lower Mstn expression, and increased Il-6 than HFHS–Skm-CB1R+/+ muscle. IL-6 stimulates myogenesis through STAT3, which was more phosphorylated in Skm-CB1R−/− than in Skm-CB1R+/+ muscle, whereas MSTN blocks myoblast proliferation and differentiation; thus, CB1R also regulates myogenesis by inducing crosstalk between myocytes/myotubes and myoblasts through myokines such as MSTN and IL-6.
We further found that ablation of CB1R in muscle allowed a greater activation of MAPKs. In brain, CB1R activation positively regulates MAPK signaling, inducing the phosphorylation of p38, JNK, and ERK1/2 (33). Activation of CB1R in a rat muscle cell line has an inhibitory effect on insulin-mediated ERK1/2 activation, which is reversed by Rimonabant (31). We found an activation of the MAPKs in the muscle of our unfed Skm-CB1R−/− mice. Activation of p38 as well as AKT induces the expression of Myod and Myog through activation of the ribosomal subunit S6 (34), which was also more phosphorylated in Skm-CB1R−/− muscle, leading to myogenesis. The activation observed in MAPK signaling could be a result of increased IR signaling and/or increased IL-6 signaling, which is known to activate not only JAK and STAT but also MAPKs (25).
The presence of CB1R in mitochondria of skeletal muscle was recently described by Mendizabal-Zubiaga et al. (35), and activation of CB1R by tetrahydrocannabinol reduced oxygen consumption in purified mitochondria from cardiac muscle. Treatment of myofibers with tetrahydrocannabinol reduced the rate of oxygen consumption during state 3 respiration (35), although no difference in basal respiration was found between myofibers of wild type and global CB1R−/− mice (35). However, we show that skeletal muscle–CB1R ablation increases mitochondrial respiration in isolated myofibers compared with Skm-CB1R+/+ myofibrils. This increase in respiration was coupled to ATP production. Discrepancies in our data with those previously reported for the global CB1R−/− mice may be explained by the presence of CB1R in the motor-end plate (36) because motor-end plates are rich in mitochondria (37), which may alter the outcome. In obese humans, the reduced expression of Ucp2 but not Ucp3 has been suggested to be the cause of impaired mitochondrial activity in skeletal muscle (38). Skm-CB1R−/− mice had increased Ucp2 expression, whereas Ucp3 and Ucp1 were reduced or virtually absent, respectively. Ucp3 is more expressed in glycolytic fibers (type II) than in oxidative fibers (type I). Skm-CB1R−/− muscle had 3.2-fold higher expression of Myh7 than Skm-CB1R+/+ muscle, which is enriched in type I fibers, further suggesting that Skm-CB1R−/− muscles are enriched in oxidative fibers. These data describe muscle-CB1R as a regulator of muscle composition and muscle fiber respiration and emphasize the need of tissue-specific CB1R knockouts to study the role of this receptor.
Physical endurance and muscle fatigue highly depend on which fiber type forms the muscle as well as the metabolic profile of each fiber (39, 40). Skm-CB1R−/− mice showed increased physical endurance compared with Skm-CB1R+/+ mice in several physical tests. This improvement was accompanied by an increase of type I fiber MHC isoform expression and type I fiber markers. Type II fibers have a faster twitch than type I fibers and are more glycolytic; they are also more prone to fatigue due to exhaustion of energy (39). Furthermore, Skm-CB1R−/− fibers had increased expression of pyruvate dehydrogenase phosphatase and hydroxyacyl-coenzyme A dehydrogenase and lower expression of pyruvate dehydrogenase kinase, indicating a higher usage of glucose for oxidative phosphorylation rather than lactate production and increased lipid oxidation. Indeed, Skm-CB1R−/− mice showed increased fiber maximal respiratory capacity, which is positively related to endurance (40), and lower intrafiber lipid content. Additionally, Skm-CB1R−/− fibers had higher oxidative phosphorylation coupled to ATP production than Skm-CB1R+/+ fibers, which further leads to increased physical endurance. The proportion of type I fibers is also positively related to phospho-FoxO1 levels, whereas MSTN favors type II fibers (41). Thus, CB1R also governs endurance not only by regulating the oxidative metabolism of the fibers but also by altering the fiber muscle composition.
Furthermore, skeletal muscle–CB1R ablation also induced a dramatic change in whole-body metabolism. Although HFHS–Skm-CB1R−/− mice had similar body weights to HFHS–Skm-CB1R+/+ mice, they ate considerably more food. Comprehensive metabolic analysis of these mice showed that HFHS–Skm-CB1R−/− mice had increased energy expenditure and slept significantly less, which would account for the extra calories consumed. The RER was significantly lower during dark cycle, which indicates that when eating an HFHS Skm-CB1R−/− mice metabolize more fat. Recently, it has been reported that ablation of CB1R in adipocytes leads to a radical reduction in adipose tissue formation (11). Ablation of CB1R in muscle did not affect total body weight, but it induced a change in body composition; therefore, the weight loss observed with peripheral CB1R blockade (42) is not due to loss of muscle CB1R but most probably due to, at least partially, blockade of CB1R in adipose tissue.
We also show that CB1R regulates IR sensitivity, as it has been previously described in hepatocytes and pancreatic β-cells (9, 12, 43, 44). Skeletal muscle–CB1R ablation improved whole-body glucose homeostasis when mice were fed an HFHS or treated with IR antagonist (S961). The ablation of CB1R in skeletal muscle improves IR activity in muscle (the largest insulin-sensitive organ in the body), thereby enhancing insulin sensitivity and reducing the S961-induced glycemia and hyperinsulinemia. Thus, periphery-restricted CB1R antagonists can prevent hyperinsulinemia in both skeletal muscle and liver. The bulk of the glucose uptake in humans, unlike mice, is into muscle after eating, and therefore, modulating CB1R activity in humans would be expected to have a greater impact on glucose uptake.
In summary, genetic ablation of CB1R in muscle up-regulates IR signaling and reduces activation of transcription factors that regulate myokine expression, such as Mstn, while increasing Il-6 expression. These changes impact muscle metabolism by reducing fat accumulation, inducing oxidative phosphorylation, increasing muscle mass and, changing muscle composition, which eventually leads to improved physical performance and whole-body metabolism. Therefore, chronic activation of CB1R in muscle, as may occur in obesity, would be expected to negatively impact body composition, favor fat formation, and reduce whole-body insulin sensitivity and physical endurance (Fig. 5K). Thus, peripheral blockade of CB1R would not only control body weight (11, 42) but also increase muscle mass and improve physical performance; we propose CB1R as a novel target for the treatment of sarcopenia and sarcopenic obesity.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health, National Institute on Aging Intramural Research Program. S961 was donated by Novo Nordisk. The authors declare no conflicts of interest.
Glossary
- CB1R
cannabinoid receptor type 1
- HFHS
high-fat–high-sugar diet
- ITT
insulin tolerance test
- IPA
Ingenuity Pathway Analysis
- IPGTT
intraperitoneal glucose tolerance test
- phospho-
phosphorylated
- RER
respiratory exchange ratio
- SD
standard diet
- Skm-CB1R+/+
control
- Skm-CB1R−/−
skeletal muscle–specific CB1R knockout
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
I. González-Mariscal designed, performed, and analyzed most of the experiments; R. A. Montoro, J. F. O’Connell, Y. Kim, M. Gonzalez-Freire, I. Alfaras, O. D. Carlson, and P. Ghosh performed and analyzed experiments; Q.-R. Liu, E. Lehrmann, Y. Zhang, and K. G. Becker performed the microarray; S. Hardivillé performed the in vitro cell study; I. González-Mariscal and J. M. Egan wrote the manuscript; J. M. Egan designed experiments and is the guarantor of the study; and all authors approved the final version of the manuscript.
REFERENCES
- 1.Gonzalez-Freire M., Semba R. D., Ubaida-Mohien C., Fabbri E., Scalzo P., Højlund K., Dufresne C., Lyashkov A., Ferrucci L. (2017) The human skeletal muscle proteome project: a reappraisal of the current literature. J. Cachexia Sarcopenia Muscle 8, 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali S., Garcia J. M. (2014) Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology 60, 294–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waters D. L., Baumgartner R. N. (2011) Sarcopenia and obesity. Clin. Geriatr. Med. 27, 401–421 [DOI] [PubMed] [Google Scholar]
- 4.Pedersen B. K., Febbraio M. A. (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 [DOI] [PubMed] [Google Scholar]
- 5.Langley B., Thomas M., Bishop A., Sharma M., Gilmour S., Kambadur R. (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 277, 49831–49840 [DOI] [PubMed] [Google Scholar]
- 6.Guo W., Flanagan J., Jasuja R., Kirkland J., Jiang L., Bhasin S. (2008) The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/β-catenin signaling pathways. J. Biol. Chem. 283, 9136–9145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed S. A., Sandesara P. B., Senf S. M., Judge A. R. (2012) Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J. 26, 987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazier W., Saucisse N., Gatta-Cherifi B., Cota D. (2015) The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol. Metab. 26, 524–537 [DOI] [PubMed] [Google Scholar]
- 9.Cinar R., Godlewski G., Liu J., Tam J., Jourdan T., Mukhopadhyay B., Harvey-White J., Kunos G. (2014) Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology 59, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jourdan T., Godlewski G., Cinar R., Bertola A., Szanda G., Liu J., Tam J., Han T., Mukhopadhyay B., Skarulis M. C., Ju C., Aouadi M., Czech M. P., Kunos G. (2013) Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 19, 1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz de Azua I., Mancini G., Srivastava R. K., Rey A. A., Cardinal P., Tedesco L., Zingaretti C. M., Sassmann A., Quarta C., Schwitter C., Conrad A., Wettschureck N., Vemuri V. K., Makriyannis A., Hartwig J., Mendez-Lago M., Bindila L., Monory K., Giordano A., Cinti S., Marsicano G., Offermanns S., Nisoli E., Pagotto U., Cota D., Lutz B. (2017) Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Invest. 127, 4148–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Mariscal I., Montoro R. A., Doyle M. E., Liu Q. R., Rouse M., O’Connell J. F., Santa-Cruz Calvo S., Krzysik-Walker S. M., Ghosh S., Carlson O. D., Lehrmann E., Zhang Y., Becker K. G., Chia C. W., Ghosh P., Egan J. M. (2018) Absence of cannabinoid 1 receptor in beta cells protects against high-fat/high-sugar diet-induced beta cell dysfunction and inflammation in murine islets. Diabetologia 61, 1470–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindborg K. A., Jacob S., Henriksen E. J. (2011) Effects of chronic antagonism of endocannabinoid-1 receptors on glucose tolerance and insulin action in skeletal muscles of lean and obese zucker rats. Cardiorenal Med. 1, 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavuoto P., McAinch A. J., Hatzinikolas G., Cameron-Smith D., Wittert G. A. (2007) Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell. Endocrinol. 267, 63–69 [DOI] [PubMed] [Google Scholar]
- 15.Vikram A., Jena G. (2010) S961, an insulin receptor antagonist causes hyperinsulinemia, insulin resistance and depletion of energy stores in rats. Biochem. Biophys. Res. Commun. 398, 260–265 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Perfetti R., Greig N. H., Holloway H. W., DeOre K. A., Montrose-Rafizadeh C., Elahi D., Egan J. M. (1997) Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J. Clin. Invest. 99, 2883–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiori J. L., Shin Y. K., Kim W., Krzysik-Walker S. M., González-Mariscal I., Carlson O. D., Sanghvi M., Moaddel R., Farhang K., Gadkaree S. K., Doyle M. E., Pearson K. J., Mattison J. A., de Cabo R., Egan J. M. (2013) Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes 62, 3500–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Montalvo A., Mercken E. M., Mitchell S. J., Palacios H. H., Mote P. L., Scheibye-Knudsen M., Gomes A. P., Ward T. M., Minor R. K., Blouin M. J., Schwab M., Pollak M., Zhang Y., Yu Y., Becker K. G., Bohr V. A., Ingram D. K., Sinclair D. A., Wolf N. S., Spindler S. R., Bernier M., de Cabo R. (2013) Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Freire M., Scalzo P., D’Agostino J., Moore Z. A., Diaz-Ruiz A., Fabbri E., Zane A., Chen B., Becker K. G., Lehrmann E., Zukley L., Chia C. W., Tanaka T., Coen P. M., Bernier M., de Cabo R., Ferrucci L. (2018) Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: the Baltimore Longitudinal Study of Aging. Aging Cell 17, e12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krämer A., Green J., Pollard J., Jr., Tugendreich S. (2014) Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30, 523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink R. I., Kolterman O. G., Griffin J., Olefsky J. M. (1983) Mechanisms of insulin resistance in aging. J. Clin. Invest. 71, 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schäffer L., Brand C. L., Hansen B. F., Ribel U., Shaw A. C., Slaaby R., Sturis J. (2008) A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 376, 380–383 [DOI] [PubMed] [Google Scholar]
- 23.Allen D. L., Unterman T. G. (2007) Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am. J. Physiol. Cell Physiol. 292, C188–C199 [DOI] [PubMed] [Google Scholar]
- 24.Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Boström E. A., Choi J. H., Long J. Z., Kajimura S., Zingaretti M. C., Vind B. F., Tu H., Cinti S., Højlund K., Gygi S. P., Spiegelman B. M. (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano T., Ishihara K., Hibi M. (2000) Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19, 2548–2556 [DOI] [PubMed] [Google Scholar]
- 26.Holmes A. G., Watt M. J., Carey A. L., Febbraio M. A. (2004) Ionomycin, but not physiologic doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metabolism 53, 1492–1495 [DOI] [PubMed] [Google Scholar]
- 27.Liao Y., Bin J., Luo T., Zhao H., Ledent C., Asakura M., Xu D., Takashima S., Kitakaze M. (2013) CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. Int. J. Cardiol. 167, 1936–1944 [DOI] [PubMed] [Google Scholar]
- 28.Iannotti F. A., Silvestri C., Mazzarella E., Martella A., Calvigioni D., Piscitelli F., Ambrosino P., Petrosino S., Czifra G., Bíró T., Harkany T., Taglialatela M., Di Marzo V. (2014) The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proc. Natl. Acad. Sci. USA 111, E2472–E2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oláh T., Bodnár D., Tóth A., Vincze J., Fodor J., Reischl B., Kovács A., Ruzsnavszky O., Dienes B., Szentesi P., Friedrich O., Csernoch L. (2016) Cannabinoid signalling inhibits sarcoplasmic Ca2+ release and regulates excitation-contraction coupling in mammalian skeletal muscle. J. Physiol. 594, 7381–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trouwborst I., Verreijen A., Memelink R., Massanet P., Boirie Y., Weijs P., Tieland M. (2018) Exercise and nutrition strategies to counteract sarcopenic obesity. Nutrients 10, e605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipina C., Stretton C., Hastings S., Hundal J. S., Mackie K., Irving A. J., Hundal H. S. (2010) Regulation of MAP kinase-directed mitogenic and protein kinase B-mediated signaling by cannabinoid receptor type 1 in skeletal muscle cells. Diabetes 59, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Stern-Straeter J., Bonaterra G. A., Kassner S. S., Zügel S., Hörmann K., Kinscherf R., Goessler U. R. (2011) Characterization of human myoblast differentiation for tissue-engineering purposes by quantitative gene expression analysis. J. Tissue Eng. Regen. Med. 5, e197–e206 [DOI] [PubMed] [Google Scholar]
- 33.Turu G., Hunyady L. (2010) Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 44, 75–85 [DOI] [PubMed] [Google Scholar]
- 34.Williamson D., Gallagher P., Harber M., Hollon C., Trappe S. (2003) Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J. Physiol. 547, 977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendizabal-Zubiaga J., Melser S., Bénard G., Ramos A., Reguero L., Arrabal S., Elezgarai I., Gerrikagoitia I., Suarez J., Rodríguez De Fonseca F., Puente N., Marsicano G., Grandes P. (2016) Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 7, 476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trujillo X., Sánchez-Pastor E., Andrade F., Huerta M. (2014) Presence and colocalization of type-1 cannabinoid receptors with acetylcholine receptors in the motor end-plate of twitch skeletal muscle fibers in the frog. J. Membr. Biol. 247, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 37.Chouhan A. K., Zhang J., Zinsmaier K. E., Macleod G. T. (2010) Presynaptic mitochondria in functionally different motor neurons exhibit similar affinities for Ca2+ but exert little influence as Ca2+ buffers at nerve firing rates in situ. J. Neurosci. 30, 1869–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordfors L., Hoffstedt J., Nyberg B., Thörne A., Arner P., Schalling M., Lönnqvist F. (1998) Reduced gene expression of UCP2 but not UCP3 in skeletal muscle of human obese subjects. Diabetologia 41, 935–939 [DOI] [PubMed] [Google Scholar]
- 39.Bogdanis G. C. (2012) Effects of physical activity and inactivity on muscle fatigue. Front. Physiol. 3, 142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogdanis G. C., Nevill M. E., Boobis L. H., Lakomy H. K. (1996) Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J. Appl. Physiol. 80, 876–884 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Pessin J. E. (2013) Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 16, 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T., Szanda G., Mukhopadhyay B., Chedester L., Liow J. S., Innis R. B., Cheng K., Rice K. C., Deschamps J. R., Chorvat R. J., McElroy J. F., Kunos G. (2012) Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 16, 167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim W., Lao Q., Shin Y. K., Carlson O. D., Lee E. K., Gorospe M., Kulkarni R. N., Egan J. M. (2012) Cannabinoids induce pancreatic β-cell death by directly inhibiting insulin receptor activation. Sci. Signal. 5, ra23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maccarrone M., Bab I., Bíró T., Cabral G. A., Dey S. K., Di Marzo V., Konje J. C., Kunos G., Mechoulam R., Pacher P., Sharkey K. A., Zimmer A. (2015) Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 36, 277–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.