Abstract
The adherens junctions (AJs) and tight junctions (TJs) provide critical adhesive contacts between neighboring epithelial cells and are crucial for epithelial adhesion, integrity, and barrier functions in a wide variety of tissues and organisms. The striatin protein family, which are part of the striatin interaction phosphatases and kinases complex, are multidomain scaffolding proteins that play important biologic roles. We have previously shown that striatin colocalizes with the tumor suppressor protein adenomatous polyposis coli in the TJs of epithelial cells. Here we show that striatin affects junction integrity and cell migration, probably through a mechanism that involves the adhesion molecule E-cadherin. Cells engaged in cell–cell adhesion expressed a high MW-modified form of striatin that forms stable associations with detergent-insoluble, membrane-bound cellular fractions. In addition, striatin has recently been suggested to be a target of the poly (ADP-ribose) polymerases Tankyrase 1, and we have found that striatin interacts with Tankyrase 1 and is subsequently poly-ADP-ribosylated. Taken together, our results suggest that striatin is a novel cell–cell junctional protein that functions to maintain correct cell adhesion and may have a role in establishing the relationship between AJs and TJs that is fundamental for epithelial cell–cell adhesion.—Lahav-Ariel, L., Caspi, M., Nadar-Ponniah, P. T., Zelikson, N., Hofmann, I., Hanson, K. K., Franke, W. W., Sklan, E. H., Avraham, K. B., Rosin-Arbesfeld, R. Striatin is a novel modulator of cell adhesion.
Keywords: cell–cell junctions, Wnt signaling, epithelial cells, tight junctions
Cell–cell adhesion is of fundamental importance to the organization and maintenance of multicellular organs. The lateral surface of epithelial cells is characterized by the formation of intercellular junctions, such as the tight junctions (TJs) and adherens junctions (AJs), which are both associated with the actin cytoskeleton (1). TJs are thought to fulfill 2 mutually exclusive functions in that they control the paracellular passage of ions and solutes between cells as well as maintain the polarization of apical and basolateral membrane proteins. These functions are mediated by occludins and claudins, 2 types of transmembrane proteins that are characteristic of TJs, as well as by cytoplasmic proteins, including members of the membrane-associated guanylate kinase homologs protein family and ZO-1-ZO-3, among others (2). Together, these proteins form a complex network that links TJs to the actin-cytoskeleton and the AJs (1).
AJs provide the main mechanical link with neighboring epithelial cells by initiation and stabilization of cell–cell adhesion. They are also responsible for additional functions such as regulation of the actin cytoskeleton, intracellular signaling, and transcriptional control. At the core of the AJs are transmembrane glycoproteins of the classic cadherin superfamily, such as E-cadherin (3). However numerous other members of the armadillo (arm)-family of catenin proteins are also located in the cell junctions. These include β-catenin, plakoglobin, and members of the p120-catenin subfamily, such as p120-catenin itself, p0071-catenin (also known as PKP4), arm repeat gene deleted in Velo-Cardio-Facial syndrome, δ-catenin (also known as neurojungin or neural plakophilin-related protein), and the plakophilins (which are often associated with desmosomes) (1, 4). In addition to its role in AJs, β-catenin is also the key factor of the canonical Wnt signaling pathway that is implicated in a wide variety of important biologic processes, ranging from early development to cell behavior, stem cell maintenance, and the development and progression of several diseases including cancer (5, 6). Adenomatous polyposis coli (APC) is a scaffolding protein involved in the regulation of β-catenin (6). In addition, APC has various functions, including the regulation of the microtubule/actin cytoskeletons and control of apical-basal polarity. These critical roles (7–10) indicate the importance of APC in the maintenance of correct epithelial structures. We have previously found that APC interacts with striatin protein members in areas of cell–cell contact (11).
Striatin, SG2NA, and zinedin are a 3-member subfamily of the calmodulin-binding WD-40 repeat proteins that are thought to have scaffolding functions. These proteins are core components of the striatin-interacting phosphatase and kinase (STRIPAK) complex, where they function as regulatory subunits of protein phosphatase 2A (PP2A) (12). The STRIPAK complex contains a large number of proteins, including members of the cortactin-binding family that regulate cortical actin cytoskeleton dynamics, and sarcolemmal membrane–associated protein, which is a centrosome-associated protein (13–15). Striatin has also been shown to regulate the hippo signaling pathway (16, 17).
In the present study, we show that APC and striatin interact with components of the TJs and that a specific APC point mutation that hinders cell–cell adhesion also disrupts the striatin-APC association. Our results show that cells with well-developed cell–cell contacts express a high MW form of striatin that is yet to be characterized. Striatin is visible at sites of cell–cell contacts before the junctions are actually formed and the expression levels and subcellular localization of striatin are tightly connected to those of E-cadherin. Taken together, these results suggest that striatin plays an important role in establishing the cell–cell contacts that are required for correct cell–cell adhesion and polarization.
MATERIALS AND METHODS
Cell culture and transfection
HEK293T, COS-7, SW480, MDCK, Caco-2, Huh7, MCF7, and HepG2 cells were maintained in DMEM supplemented with 10% fetal calf serum and 100 U/ml penicillin/streptomycin and incubated at 37°C in a humidified 5% carbon dioxide atmosphere. Huh-7 medium was supplemented with 1% nonessential amino acids solution. Transfection of HEK293T cells was performed by either calcium phosphate precipitation or by using Polyethyleneimine Max (Polysciences, Warrington, PA, USA) following manufacturer’s protocols. All other cells were transfected using the DNA transfection reagents jetPEI (Polyplus Transfection, Illkirch, France) following the manufacturer’s protocols. For Caco2 striatin stably-depleted cell lines, Lipofectamin 2000 (Thermo Fisher Scientific, Waltham, CA, USA) was used for transfection, and then 48 h later, puromycin antibiotics (1 µg) were added to the growing medium until visible colonies could be isolated.
Plasmids and reagents
pcDNA-FLAG-APCarm encodes the human APC arm repeat region spanning amino acids 188–774 (11). This FLAG APCarm expression vector was used as a template in the QuikChange site-directed mutagenesis kit (Stratagene California, San Diego, CA, USA) in order to replace asparagine 507 in APC with a lysine, generating FLAG-N507K-APCarm. The construct pEHN2striatin [pIG 1803-23, provided by Dr. U. Kück (Bochum University, Bochum, Germany)] was used to prepare pKS-HA-Striatin. HA–striatin and FLAG-striatin were constructed by inserting the human striatin coding region from the KS-striatin plasmid. Caco-2 cells stably depleted of striatin were previously described (11). Specific RNA oligonucleotides for silencing striatin were purchased from Thermo Scientific Dharmacon (Lafayette, CO, USA) (only sense strand shown); striatin, 5′-GCACAGAGGCUGAAGUUAA; striatin 3 (SG2NA), 5′-GCAAAAGGGACAAGAAAUA; and striatin 4 (zinedin), 5′-GAUCAAGAUGCUAGAGUAU. Dharmafect-4 was used as the transfection reagent for introducing the small interfering RNAs (siRNAs). Transfections were performed according to the manufacturer’s protocol using 100 nM siRNA oligonucleotides. Nontargeting RNA oligonucleotides (Thermo Scientific Dharmacon) were used as controls. To generate the pACT and pBIND plasmids for the mammalian 2 hybrid system, striatin, APCarm, and occludin were amplified by PCR and subcloned into the SalI/NotI sites of pACT or pBIND). Cytochalasin D (1 μg/ml, 30 min; AG Scientific, San Diego, CA, USA) was used to inhibit actin polymerization.
Antibodies
The following antibodies were used: mouse anti-FLAG (1:5000; MilliporeSigma, Burlington, MA, USA) rabbit anti-occludin (IB: 1:1000, IF: 1; 200; Thermo Fisher Scientific), rabbit antristriatin (IP:1:500; MilliporeSigma), mouse antristriatin (IB:1:2000, IF:1:500; BD Biosciences, San Jose, CA, USA), mouse antistriatin 3 (SG2NA IB:1:500; Novus Biologicals, Centennial, CO, USA), mouse anti-VP-16 (IB: 1:1000; Santa Cruz Biotechnology, Dallas, TX, USA), mouse anti-GALA4 (IB:1:1000; Santa Cruz Biotechnology), mouse E-cadherin (IB: 1:5000; BD Biosciences), rat anti–E-cadherin (IF:1:100; MilliporeSigma), mouse anti-PP2A (IB:1:2000; Thermo Fisher Scientific), mouse anti-actin (1:10,000; MP Biomedicals, Santa Ana, CA, USA), rabbit anti-poly (ADP-ribose)(PAR) (IB:1:1000; BD Biosciences), mouse anti-occludin (IB: 1:1000, OC-3F10; Thermo Fisher Scientific), mouse anti-cingulin (1:1000, 365264; Santa Cruz Biotechnology), and Tankyrase-1 (IB:1:1000; Santa Cruz Biotechnology). Mouse anti-tubulin (1:10,000; MilliporeSigma) was used as a loading control. Guinea pig sera specific for cingulin or hp0071 were both from Progen Biotechnik (Heidelberg, Germany). Anti-rat horseradish peroxidase-conjugated secondary antibodies (1:5000) were obtained from Santa Cruz Biotechnology. Anti-mouse and anti-rabbit secondary antibodies were obtained from (1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Alexa red and green (1:500; Molecular Probes, Eugene, OR, USA) or Alexa488-conjugated (MoBiTec, Göttingen, Germany) or Cy3-conjugated (BioTrend Chemicals, Destin, FL, UDA) secondary antibodies were used for the immunofluorescence (IF) analysis.
Western blot analysis and immunoprecipitation
Forty-eight hours following transfection, cells were washed with PBS and solubilized in reporter lysis buffer (luciferase buffer; Promega, Madison, WI, USA) or M2 lysis buffer (100 mM NaCl, 50 mM Tris, pH 7.5, 1% Triton X-100, 2 mM EDTA) containing a protease inhibitor cocktail (MilliporeSigma). Extracts were clarified by centrifugation at 12,000 g for 15 min at 4°C. Following SDS-PAGE separation, proteins were transferred to nitrocellulose membranes and blocked with 5% low-fat milk. The membranes were then incubated with specific primary antibodies, washed with PBS containing 0.001% Tween-20, and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody. After washing in the PBS solution, membranes were subjected to ECL detection analysis. For immunoprecipitation (IP) analysis, cells were solubilized in lysis buffer (see above). Cell lysates were incubated at 4°C for 2–18 h with anti-FLAG M2-agarose affinity gel (MilliporeSigma), with rotation. Alternatively, cell lysates were incubated with the relevant specific antibody for 1–2 h at 4°C prior to 2–18 h rotated incubation with protein A/G agarose (Santa Cruz Biotechnology) at 4°C. Beads were collected by slow centrifugation, washed 4 times with lysis buffer, and analyzed by SDS-PAGE followed by detection with specific antibody.
Mammalian 2-hybrid experiments
Striatin, APCarm, and occludin were subcloned into pACT and pBIND plasmids (CheckMate; Promega). The appropriate plasmids (500 ng of each) were transfected together with the reporter plasmid pG5luc into HEK293T cells. The cells were lysed with luciferase reporter lysis buffer (Promega), and luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega).
Soluble and insoluble cell triton X-100 fractionation
Cells cultured in 6 well plates were extracted at 25°C with 200 μl of 0.5% Triton X-100, 2.5 mM EGTA, 5 mM MgCl2, and 50 mM MES (pH 6.0) for 2 min. The Triton-soluble fraction was collected, and the plates were washed twice with the same buffer. The insoluble fraction was scraped into 200 μl of the same buffer. Equal volumes of these fractions were analyzed by SDS-PAGE (18).
Immunofluorescence microscopy
Cells grown on glass coverslips were fixed for 20 min in PBS containing 3.7% formaldehyde. The fixed cells were then washed 3 times with PBS, permeabilized with 0.1% Triton X-100 for 10 min, and blocked in PBS containing 1% bovine serum albumin and 0.1% Triton X-100 for 1 h. Subsequently, cells were incubated at room temperature with primary and secondary antibodies for 60 and 30 min, respectively. Cells were stained with 10 μg/ml (DAPI; MilliporeSigma) for 5 min to stain the cell nuclei. In order to visualize actin stress fibers, cells were stained for 30 min with Phalloidin Tritc (1:10,000; MilliporeSigma). Alexa red and green (1:500; Molecular Probes) were used as secondary antibodies. Where indicated, the cells were extracted with Triton X-100 buffer (50 mM Nacl2, 10 mM PIPES pH 6.8, 3 mM sucrose) on ice for 10 min before fixation. Immunofluorescence microscopy was performed using a confocal laser microscopy system (LSM 510; Carl Zeiss, Oberkochen, Germany, and Leica TCS SP5; Leica Microsystems, Buffalo Grove, IL, USA) or wide field microscope (Axio Observer Z1; Carl Zeiss).
Migration assay
Cell migration was assayed in 24-well, 8-μm pore membrane Transwell cell culture chambers (MilliporeSigma). Cells (2 × 105) from each of the Caco-2 striatin depleted stable cell lines were seeded in the upper chamber in DMEM deprived of fetal calf serum. Growth medium was added to the lower chamber after 2 h. Twenty-four hours postseeding, the cells were washed twice with PBS and then fixed with ice-cold methanol for 5 min and stained (Hema 3 Stain System, Thermo Fisher Scientific). The nonmigrating cells were scraped away with a cotton swab, and cells that had migrated to the lower surface of the membrane were imaged using a Nikon TE2000E inverted microscope integrated with a Nikon DS5 cooled CCD camera by ×10 objective, bright-field illumination (Nikon, Tokyo, Japan).
E –cadherin inhibition assay
To disrupt cell adhesion, SW480 cells were incubated for 48 h in the presence of a 1:50 dilution of rat anti-E-cadherin (MilliporeSigma) or a control antibody as previously described (19, 20).
Identification of interacting proteins using mass spectrometry
HEK293T cells were transfected with pcDNA3.1-FLAG striatin or pCMV-FLAG as a control. Two days later, the cells were washed with ice-cold PBS and lysed in lysis buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) containing complete EDTA-free protease inhibitor mixture (Roche Applied Science, Penzberg, Germany). Extracted proteins were immunoprecipitated with anti-FLAG M2 agarose (MilliporeSigma) at 4°C overnight. Following SDS-PAGE and colloidal Coomassie blue staining, the gel was analyzed by tandem mass spectrometry (Smoler Proteomic Center, Technion, Israel Institute of Technology).
Statistical analysis
Statistical analysis was performed with Prism (GraphPad Software, La Jolla, CA, USA) using 1-way ANOVA and Dunnett’s multiple comparison tests.
RESULTS
Striatin forms a complex with APC and TJ proteins
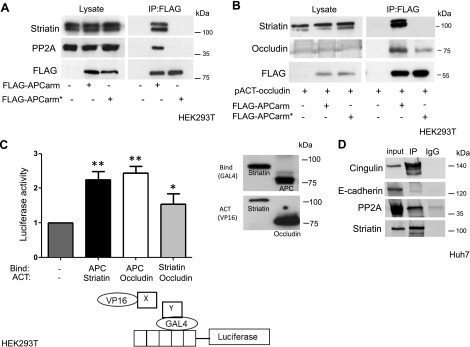
We have previously found that APC binds striatin through the arm repeat region (APCarm) and that these proteins colocalize in the junctions of epithelial cells, presumably in TJs (11). A specific point mutation, N507K, within the APCarm that has been shown to reduce cell–cell adhesion (9) also prevents the interaction of APC with several proteins, including APC-stimulated guanine nucleotide exchange factor (21), APC membrane recruitment 1 (22), and APC membrane recruitment 2 (23). Similarly, in HEK293T cells, this APCarm mutation also disrupted the APC-striatin complex that includes PP2A (Fig. 1A), a vital serine/threonine protein phosphatase that has been shown to bind both striatin (24), and APCarm (25). IP experiments conducted in HEK293T cells revealed that the APC N507K mutation also decreased the association between APC and occludin, another TJ protein found in this complex (Fig. 1B).
Figure 1.
Striatin forms a complex with APC and TJ proteins. A) HEK293T cells were cotransfected with plasmids encoding FLAG-APCarm or the N507K FLAG-APCarm mutated (FLAG-APCarm*) vector. Forty-eight hours later, the cells were harvested and lysates were coimmunoprecipitated using anti-FLAG M2 affinity beads and subjected to Western blot analysis as indicated. B) HEK293T cells were cotransfected with plasmids encoding FLAG-APCarm or the N507K FLAG-APCarm mutated (FLAG-APCarm*) and pACT occludin vectors. Forty-eight hours later, the cells were harvested and lysates were coprecipitated using anti-FLAG M2 affinity beads and subjected to Western blot analysis as indicated. C) HEK293T cells were cotransfected with the indicated plasmid combination and the pG5luc plasmid. Forty-eight hours following transfection, the cells were lysed and the levels of Renilla luciferase and firefly luciferase were determined using the Dual-Luciferase Reporter Assay System (Promega). Values (means ± sd) are from triplicate wells (left panel). The graph presents a representative result from 3 independent experiments. Asterisks denote statistical significance in an unpaired Student’s t test. *P = 0.05, **P = 0.01. A schematic representation of the Mammalian 2-Hybrid System (lower panel). Western blot analysis using VP16 and GAL4 antibodies showed comparable levels of expression. D) Huh7 cells were subjected to IP experiments using the antristriatin antibody, or IgG control. Antibodies used for detection are as indicated.
The mammalian 2 hybrid system CheckMate (Promega) was used to verify these findings, and the results indicated that both APC and striatin interact with the TJ protein-occludin (Fig. 1C). Huh7, hepatocellular carcinoma epithelial cells, are able to polarize and form cell–cell junctions. Thus, we conducted additional IP experiments with other known junction proteins using this cell line. Our results demonstrate that both PP2A and the TJ protein cingulin form a complex with striatin (Fig. 1D). The striatin-cingulin interaction was further confirmed by mass spectrometry analysis using FLAG-striatin as bait (Supplemental Table S1). Figure 1D shows that E-cadherin, although associated with both the AJs and TJs, did not participate in the striatin-PP2A complex. This is consistent with our previous results showing that striatin interacts with TJ but not AJ proteins (11).
Striatin colocalizes with TJ markers in polarized cells and tissues
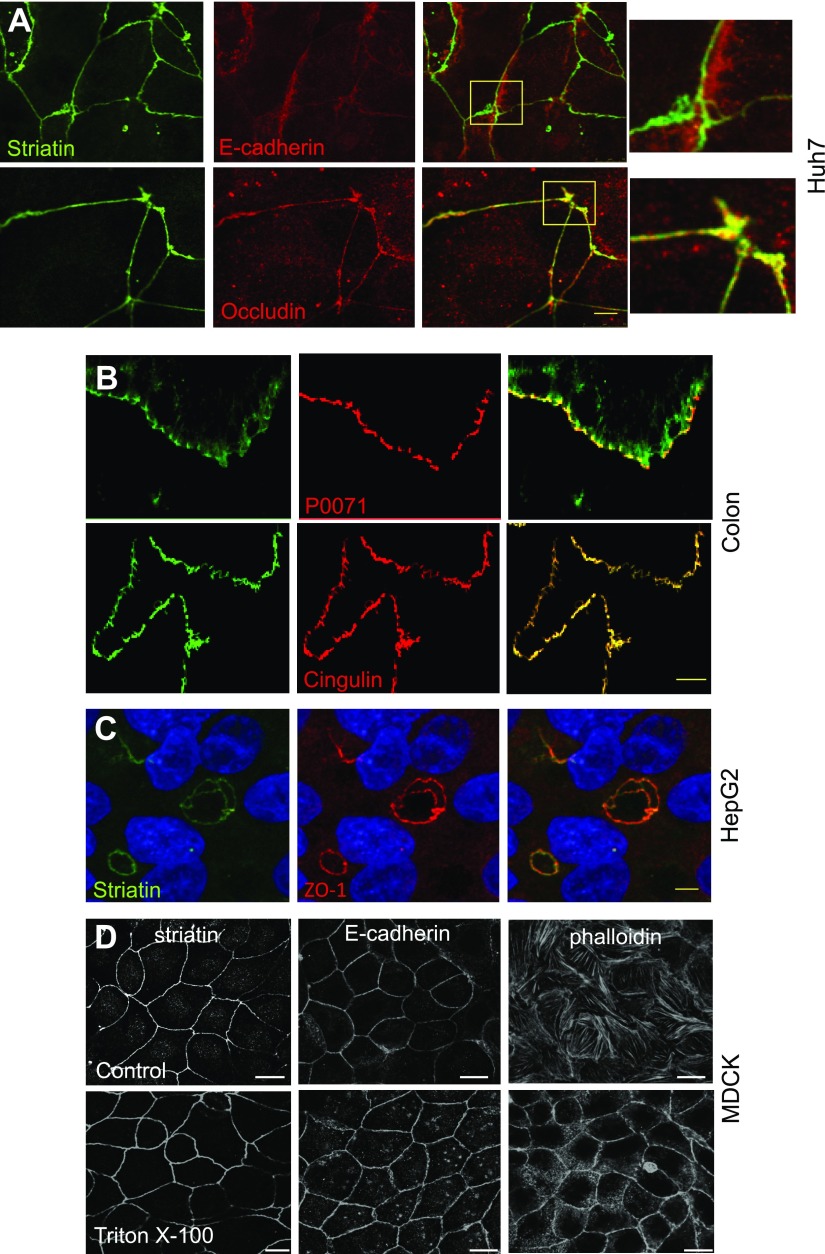
To reinforce our previous observations that striatin is localized to TJs of epithelial cells (11), we examined the localization of striatin in different polarized epithelial systems. As shown previously, in Huh7 cells, striatin colocalized with the TJ protein occludin but not with the AJ marker E-cadherin (Fig. 2A). Colonic epithelium stained for striatin, the TJ protein cingulin, and the AJ protein p0071 confirmed the colocalization of striatin with the former, whereas the latter is observed in close proximity (Fig. 2B). Next, we examined the localization of striatin in HepG2 hepatoblastoma cells. The polarity of HepG2 cells in culture is more complex than that of most epithelial cells, with several apical and basolateral poles. The apical poles of front-facing and adjacent hepatocytes form a continuous network of bile canaliculi, highly polarized structures that form typical “ovoid” shaped structures between adjacent cells (26). Our results show that striatin colocalized with ZO-1 in these typical bile canaliculi structures (Fig. 2C). To confirm that striatin is associated with the cytoskeletal fraction at cell–cell junctions, we performed an in situ extraction with Triton X-100 (27, 28). When MDCK cells were treated with 0.5% Triton X-100 before fixation, the expression pattern of striatin did not change, suggesting that, like E-cadherin, it is expressed in the Triton-insoluble compartment. Cytosolic actin, on the other hand, was clearly decreased following Triton X-100 extraction, leaving only the cell–cell contacts intact (Fig. 2D).
Figure 2.
The expression pattern of striatin in polarized cells and tissues. A) Huh7 hepatocellular carcinoma epithelial cells were grown on glass coverslips to high confluency. The cells were fixed and stained using the indicated antibodies. Although striatin was found in close proximity to E-cadherin, the colocalization seen with occludin was not present. B) Comparison of the localization of striatin (green), cingulin (red), and p0071 (red) in frozen sections of human colon epithelium. C) Highly polar hepatoma HepG2 cells were grown to confluency then fixed and stained as indicated. D) MDCK cells were overlaid with PBS (control) or PBS containing 1% Triton (Triton X-100) on ice for 10 min. After removing solubilized proteins, the residual proteins were fixed in 4% formaldehyde and immunostained with antibodies as indicated. Scale bars, 5 µM.
Striatin sequestering to cell–cell contact areas occur prior to junctional maturation
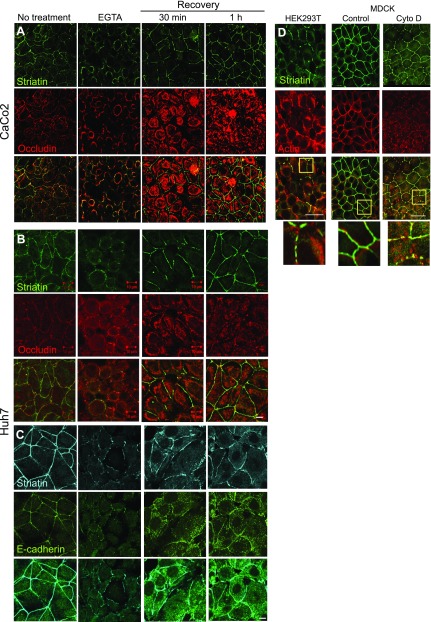
Epithelial junctions depend on Ca2+, and numerous studies have reported that Ca2+ is required for maintaining proper TJ integrity. In this context, incubation of epithelial cells with EGTA disrupts the TJs, resulting in epithelial depolarization (29, 30). Treatment of Caco2 cells with EGTA disrupted the junctional organization of occludin and striatin and resulted in redistribution of these proteins to intracellular compartments (Fig. 3A). This experiment was repeated in Huh7 cells, and both occludin and E-caderin were monitored (Fig. 3B, C). Our results indicate that E-cadherin, similarly to striatin, is recruited to the junctional location earlier than occludin and is detected in the junctions 30 min following recovery (Fig. 3C). This result is in line with previous evidence showing that AJs initiate cell–cell contact (1). Previous results indicated that a partial reorganization of occludin at the intercellular junctions was achieved around 3 h after restoring cellular calcium (31). Our results demonstrate that striatin was redistributed into characteristic junctional structures within 30 min of supplementing the cells with complete medium (Fig. 3A–C). This observation indicates that striatin recruitment to the junctional compartment occurs prior to junction formation because the occludin kinetics show that at this time point, occludin is only partly junctional (Fig. 3A, B). Comparing the subcellular localization of striatin in polarized and nonpolarized cells without TJs (32, 33) revealed a puncta pattern with a partial localization to the cell cortex in confluent nonpolarized HEK293T. Interestingly, in cells that expressed some cortical striatin, the protein was colocalized with actin (Fig. 3D). In contrast, the polarizable MDCK cells exhibit a uniform expression of striatin at all cell–cell junctions. This expression pattern is similar to that of actin, although the 2 proteins do not entirely overlap (Fig. 3D, right panels). Treatment of MDCK cells with cytochalasin D leads to actin depolymerization, disruption of TJs, and formation of actin puncta that include additional proteins (34). Under these conditions, striatin’s expression pattern was also disrupted and displayed partial colocalization with actin. Taken together, these results suggest that striatin localizes to areas of cell–cell junctions before the junctions have completed functional assembly.
Figure 3.
Striatin sequestering to cell–cell contact areas occurs prior to junctional maturation. A–C) CaCo2 (A) or Huh7 (B, C) cells were treated with 4 mM EGTA in the growth medium for 45 min and then fixed or transferred to normal growth medium for 30 or 60 min before fixation. Fixed cells were stained for occludin or E-cadherin and striatin and examined using a confocal microscope. D) HEK293T and MDCK cells were coimmunostained for actin and striatin. MDCK cells were treated with cytochalasin D (1 μg/ml for 30 min) to depolymerize the actin fibers. Scale bars, 10 µM.
Cells that form adhesive cell–cell contacts express a modified form of striatin
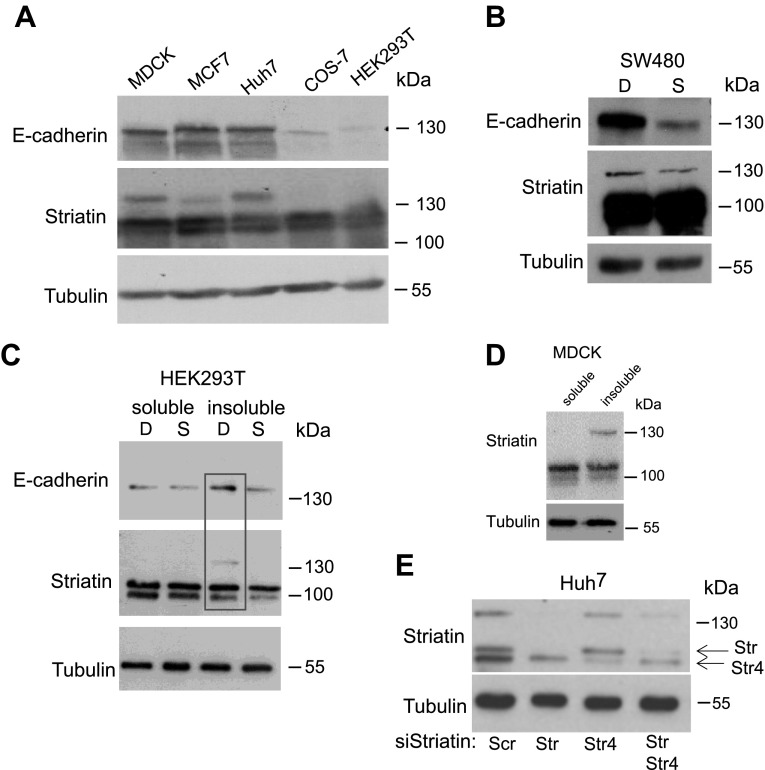
Although MDCK, Huh7, and MCF7 cells originate from different tissues and species, they are all able to polarize and form cell–cell junctions (35–37). In contrast, COS-7 and HEK293T cells do not form stable adhesive contact between neighboring cells (33, 38). Figure 4A shows that, as expected, MDCK, Huh7, and MCF7 cells express higher levels of E-cadherin than COS-7 and HEK293T. Interestingly, a high molecular band of striatin was detected by Western blot analysis in cells expressing high levels of E-cadherin (Fig. 4A). The colorectal cancer cell line SW480 was used to examine this modified form of striatin more closely. It was previously shown that sparse SW480 cells do not express E-cadherin, whereas dense SW480 cells display high levels of the protein (19). Our results show that in addition to the increase in E-cadherin levels, dense SW480 cells also expressed higher levels of the modified striatin band, although detected only at longer exposure (Fig. 4B). This form of striatin was also detected in the same insoluble, membrane-bound fractions of dense HEK293T cells as increased levels of E-cadherin (Fig. 4C) and in the membrane insoluble pools of MDCK cells (Fig. 4D) (18). In order to confirm that this high molecular band is indeed striatin, we designed a specific striatin siRNA and introduced this oligo into Huh7 cells (Fig. 4E). The results clearly show the elimination of both the expected size (100 kDa) and the larger striatin band.
Figure 4.
Cell–cell contact leads to expression of a high MW form of striatin. A) Immunoblot analysis of total cell lysates from MDCK, MCF7, Huh7, COS-7, and HEK293T grown to high confluency and reacted with the indicated antibodies. B) SW480 cells were seeded in a semiconfluent culture dish at 6 × 103 cells/cm2 (Sparse-S) and 6 × 104 cells/cm2 (Dense-D) and analyzed by Western blot for the expression of striatin, E-cadherin, and tubulin. C) HEK293T cells grown in sparse (S) or dense (D) cultures were separated into Triton X-100 soluble and insoluble fractions and were analyzed by Western blot to assess the expression levels of E-cadherin, striatin, and tubulin. D) MDCK cells grown as dense cultures were fractionated into Triton X-100 soluble and insoluble fractions, and equal amounts of lysate were analyzed by Western blot for striatin and tubulin levels. E) Huh7 cells were transfected with the indicated siRNAs (100 nM) for 72 h, and the lysates were analyzed by SDS-PAGE.
Loss of E-cadherin leads to cytoplasmic accumulation of striatin
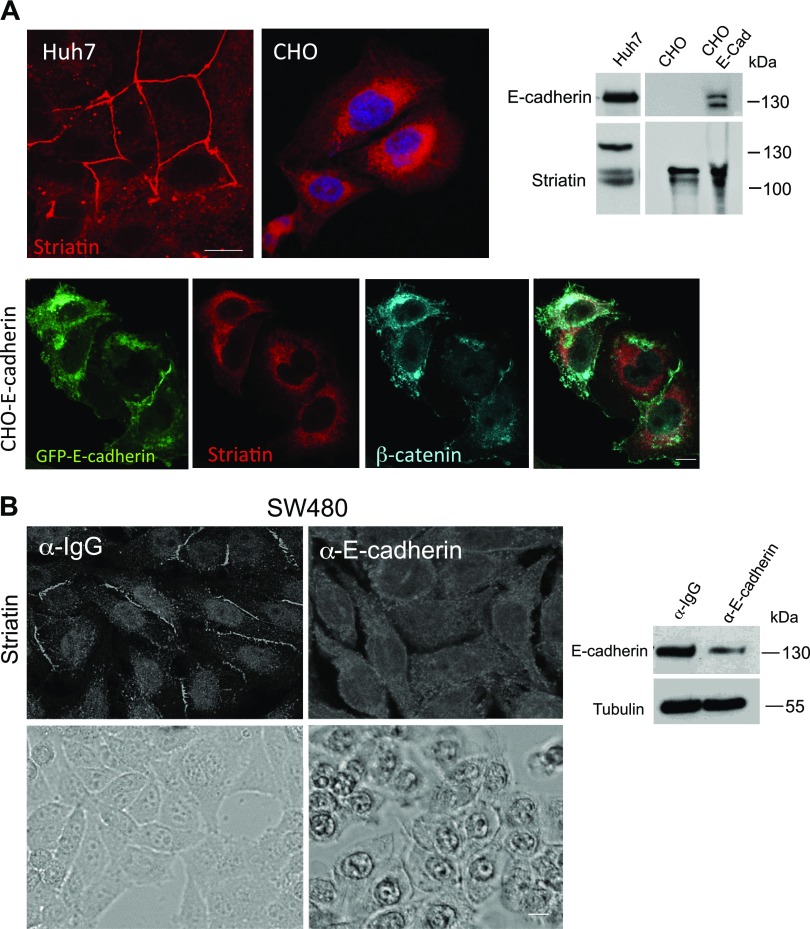
CHO cells are nonadhesive cells that completely lack cadherin expression (39, 40). Examining the expression pattern of striatin in these cells revealed that, in contrast to Huh7 cells, striatin expression pattern is cytoplasmic and the high molecular striatin form is absent (Fig. 5A). Reintroducing E-cadherin into CHO cells by establishing a stable CHO-E-Cad green fluorescent protein (GFP) cell line did not restore striatin junctional distribution, and the modified striatin isoform could not be detected even in the presence of E-cadherin (Fig. 5A). This observation is consistent with our notion that striatin is involved in the formation of TJs and thus cannot obtain junctional pattern in cells that do not form them. As mentioned earlier, dense SW480 cells express high levels of E-cadherin and are able to form both TJs and AJs. E-cadherin–dependent AJ assembly can be inhibited by incubating cells with a pAb against the extracellular domain of E-cadherin. The treated cells exhibit reduced levels of endogenous E-cadherin (Fig. 5B, right panel) and acquire an altered colony morphology compared with cells cultured with control antibody (19) (Fig. 5B, lower panel). Interestingly, the decrease in E-cadherin levels was accompanied by the sequestering of striatin from the membranes contact sites into the cytoplasm (Fig. 5A, upper panel).
Figure 5.
Loss of E-cadherin leads to cytoplasmic accumulation of striatin. A) A CHO-GFP-E-cadherin stable cell line (CHO-E-Cad) was established. Lysates from Huh7, CHO, and CHO-E-Cad were subjected to Western blot analysis. The cells were also plated and fixed for IF analysis using antristriatin and anti–β-catenin antibodies. E-cadherin was detected by the GFP tag. B) SW480 cells were seeded as dense cultures in the presence of an anti–E-cadherin antibody, or a control antibody and incubated for 48 h. Striatin expression pattern (upper panel) was determined by antristriatin IF. Cell morphology was observed by light microscopy (lower panel). The levels of E-cadherin were determined by Western blot analysis of lysates from SW480 cell cultures incubated with an anti–E-cadherin antibody or a control antibody. Scale bars, 10 µM.
Striatin functions in cell–cell adhesion
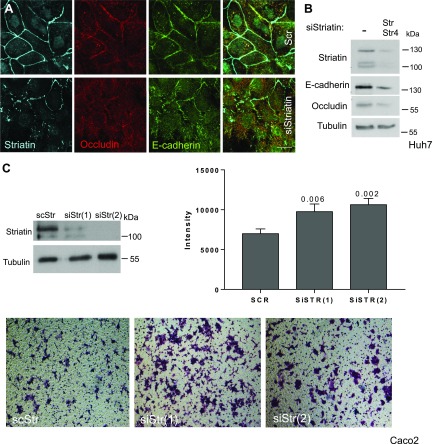
The apparent relationship between striatin and cell density prompted us to examine the connection between striatin and cell–cell adhesion. Depletion of striatin from Huh7 cells resulted in reduced levels of junctional E-cadherin and occludin, as depicted both by immunostaining (Fig. 6A) and Western blot analysis (Fig. 6B). Next, we performed migration assays in Caco2 cells transfected with siStr-harboring plasmids. Loss of striatin decreased cell adhesion as evidenced by the larger number of cells depleted of striatin that migrated to the bottom chamber of a Transwell insert as compared with control cells (Fig. 6C). Statistical analysis was performed on multiple fields with Prism using ordinary 1-way ANOVA and Dunnett’s multiple comparisons tests.
Figure 6.
Striatin and cell adhesion. Huh7 cells were transfected with the indicated siRNAs (100 nM) for 72 h. A) The siRNA-treated cells were fixed and immunostained as indicated. Scale bar, 10 µM. B) The lysates were analyzed by Western blot using the indicated antibodies. C) Stable depletion of striatin was observed in Caco2 cell lines using 2 different siRNA sequences (siStr1 or siStr2) and siRNA-scrambled striatin sequence (11). The 3 different Caco2 stable line clones were seeded in the top chamber of the Transwell migration chambers. After 24 h, cells that had not migrated to the lower chamber were removed from the upper surface of the Transwell membrane. Migrating cells on the lower membrane surface were fixed, stained, and photographed.
The poly(ADP-ribose)polymerase tankyrase regulates the expression pattern of striatin
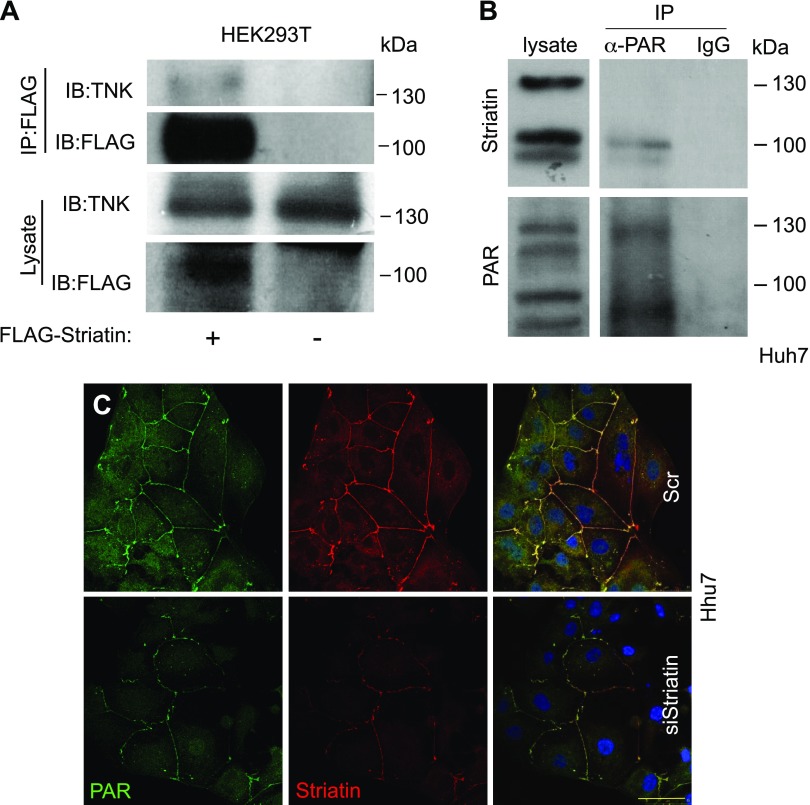
Recent reports demonstrated that striatin is a target of the poly(ADP-ribose)polymerase Tankyrase (41). Coimmunoprecipitation experiments in striatin-transfected HEK293T cells confirmed that striatin and Tankyrase are part of the same cellular complex (Fig. 7A). To test whether Tankyrase can PARylate striatin, we performed an IP experiment with the anti-PAR antibody (Fig. 7B). This experiment revealed that although striatin seems to be poly-ADP-ribosylated (PARylated) in Huh7 cells, the high molecular striatin band is not, and the specific modification has yet to be identified. Because Huh7 cells treated with siStriatin presented decreased striatin expression accompanied by reduced PAR staining (Fig. 7C), we propose that striatin may be PARylated in the contact sites. However, PARylation could be also attributed to other junctional proteins, such as ZO-2, and the membrane-bound axin (42, 43). Alternatively, other striatin splice variants may be PARylated.
Figure 7.
Tankyrase interacts and PARylates striatin. A) Lysates from HEK293T cells transfected with FLAG-striatin or control FLAG vector were immunoprecipitated using anti-FLAG antibodies and analyzed by immunoblotting with the indicated antibodies. B) Huh7 cell lysates were immunoprecipitated using the anti-PAR antibody, or IgG control, and then probed with antristriatin and anti-PAR antibodies. C) Huh7 cells were subjected to IF assay as previously described, using the anti-PAR and antistriatin antibodies. Scale bar, 50 µM.
DISCUSSION
Cell polarization is of fundamental importance in normal development and function. Thus, impaired cellular polarization is implicated in a large number of human disorders and in most cancer types. In epithelial cells, distinct apical, baso-lateral, and basal membrane domains are exposed to different environments. Between these domains are junctional complexes such as the TJs and AJs (44) that provide important adhesive contacts between neighboring epithelial cells. Although the different functions and the importance of these junctions have been well appreciated, the concept of the junctions as a complex, multiprotein structure with roles in other cellular processes (such as cell polarity, proliferation, and differentiation) has only recently been recognized (45). Currently, more than 50 TJ-associated proteins have been described and these can be classified as integral membrane proteins, scaffolding proteins, or signaling proteins. Striatins are multidomain molecules that are thought to function as scaffold proteins because they contain several domains known to be involved in protein-protein interactions. Striatin has also recently been shown to be part of the large evolutionally conserved STRIPAK complex where it functions as the B regulatory subunit of PP2A to assemble the catalytic C and structural A subunits. In this complex, striatin interacts with a large number of additional proteins to regulate numerous different biologic processes ranging from apoptosis to cardiac function (13–15, 46, 47). Our previous work has shown that striatin is expressed in epithelial cells and colocalizes with the colorectal cancer tumor suppressor protein APC in the TJs of epithelial cells (11). Here we further characterized the role of APC and striatin in cell adhesion and report that a point mutation in the arm repeats of APC, previously shown to disrupt cell adhesion (9), also abolishes the APC-striatin interaction. Both striatin and APCarm are PP2A binding proteins (25, 48), and PP2A has been shown to interact with occludin to negatively regulate TJ formation (31, 49). The B subunit of PP2A determines the substrate specificity as well as the subcellular localization of PP2A. Because it has been suggested that striatin affects the subcellular localization of APC (50) and APCarm has been shown to bind the B subunit of PP2A, striatin may connect APC with the PP2A heterodimer and may thus regulate the activity of PP2A in the TJs and in the Wnt signaling cascade. In this study, we report that in cells that form functional cell–cell junctions, striatin is expressed as a high-MW protein in the insoluble, cell membrane fraction. The process of cell–cell adhesion formation in epithelial cells occurs in sequential steps from the formation of new cell–cell contacts, through stabilization of these contacts, to junction maturation. In all of these steps, actin structures are dynamically reorganized in response to the formation of the junctions (51). Our study provides evidence suggesting that striatin is important for correct cell–cell adhesion, as we show that striatin is localized to the cell–cell junctional area during the first stages of junctional assemble, before the mature junctions are formed, and at these stages striatin may bind actin. Interestingly, mass spectrometry analysis suggests that striatin forms a complex with different junctional proteins, such as Cingulin, Desmoplakin, and Plakoglobin, that are all involved in cell–cell adhesion. These proteins are part of different cellular junctions that were shown to include striatin (52). Importantly, loss of striatin in TJs leads to reduced levels of E-cadherin and thus impaired cell–cell adhesion, whereas loss of E-cadherin leads to striatin cytoplasmic sequestering, and cells that do not express E-cadherin do not possess the large MW striatin form. It has been shown that depletion of E-cadherin disrupts the establishment but not maintenance of cell junctions (53), suggesting that striatin might function in cell–cell junction formation. As an additional property, striatin has recently been shown to be a substrate of the poly(ADP-ribose)polymerases Tankyrase (41). Tankyrase PARsylation of substrates usually leads to their ubiquitination and degradation. Our results show that striatin is PARsylated and localizes with PAR at cell–cell junctions and may suggest that Tankyrase is recruited to lateral membranes and cell–cell junctions in polarized epithelial cells and leads to PARsylation of striatin at the TJs.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by grants from the German-Israeli Foundation (1098), the Israel Science Foundation (829/14), the Rising Tide Foundation for Clinical Cancer Research, the Gateway for Cancer Research Foundation (to R.R.-A.), the United States–Israel Binational Science Foundation (2017173, to R.R.-A. and K.B.A.), and the U.S. National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grant R01DC011835 (to K.B.A.). The authors declare no conflicts of interest.
Glossary
- AJ
adherens junction
- APC
adenomatous polyposis coli
- arm
armadillo
- GFP
green fluorescent protein
- IF
immunofluorescence
- IP
immunoprecipitation
- PAR
poly(ADP-ribose)
- PARylated
poly-ADP-ribosylated
- PP2A
protein phosphatase 2A
- siRNA
small interfering RNA
- STRIPAK
striatin-interaction phosphatases and kinase
- TJ
tight junction
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. Lahav-Ariel, M. Caspi, P. T. Nadar-Ponniah, N. Zelikson, I. Hofmann, K. K. Hanson, and E. H. Sklan performed research; W. W. Franke and R. Rosin-Arbesfeld designed research; M. Caspi, K. B. Avraham, and R. Rosin-Arbesfeld wrote the manuscript; and K. B. Avraham contributed reagents.
REFERENCES
- 1.Hartsock A., Nelson W. J. (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneeberger E. E., Lynch R. D. (2004) The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286, C1213–C1228 [DOI] [PubMed] [Google Scholar]
- 3.Gumbiner B. M. (2005) Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622–634 [DOI] [PubMed] [Google Scholar]
- 4.Green K. J., Getsios S., Troyanovsky S., Godsel L. M. (2010) Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb. Perspect. Biol. 2, a000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nusse R., Varmus H. (2012) Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 31, 2670–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon R. T., Bowerman B., Boutros M., Perrimon N. (2002) The promise and perils of Wnt signaling through beta-catenin. Science 296, 1644–1646 [DOI] [PubMed] [Google Scholar]
- 7.Akiyama T., Kawasaki Y. (2006) Wnt signalling and the actin cytoskeleton. Oncogene 25, 7538–7544 [DOI] [PubMed] [Google Scholar]
- 8.Bahmanyar S., Nelson W. J., Barth A. I. (2009) Role of APC and its binding partners in regulating microtubules in mitosis. Adv. Exp. Med. Biol. 656, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada F., Bienz M. (2002) A Drosophila APC tumour suppressor homologue functions in cellular adhesion. Nat. Cell Biol. 4, 208–213 [DOI] [PubMed] [Google Scholar]
- 10.Olmeda D., Castel S., Vilaró S., Cano A. (2003) Beta-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol. Biol. Cell 14, 2844–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitman M., Zilberberg A., Caspi M., Rosin-Arbesfeld R. (2008) The armadillo repeat domain of the APC tumor suppressor protein interacts with Striatin family members. Biochim. Biophys. Acta 1783, 1792–1802 [DOI] [PubMed] [Google Scholar]
- 12.Benoist M., Gaillard S., Castets F. (2006) The striatin family: a new signaling platform in dendritic spines. J. Physiol. Paris 99, 146–153 [DOI] [PubMed] [Google Scholar]
- 13.Goudreault M., D’Ambrosio L. M., Kean M. J., Mullin M. J., Larsen B. G., Sanchez A., Chaudhry S., Chen G. I., Sicheri F., Nesvizhskii A. I., Aebersold R., Raught B., Gingras A. C. (2009) A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteomics 8, 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kean M. J., Ceccarelli D. F., Goudreault M., Sanches M., Tate S., Larsen B., Gibson L. C., Derry W. B., Scott I. C., Pelletier L., Baillie G. S., Sicheri F., Gingras A. C. (2011) Structure-function analysis of core STRIPAK proteins: a signaling complex implicated in Golgi polarization. J. Biol. Chem. 286, 25065–25075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceccarelli D. F., Laister R. C., Mulligan V. K., Kean M. J., Goudreault M., Scott I. C., Derry W. B., Chakrabartty A., Gingras A. C., Sicheri F. (2011) CCM3/PDCD10 heterodimerizes with germinal center kinase III (GCKIII) proteins using a mechanism analogous to CCM3 homodimerization. J. Biol. Chem. 286, 25056–25064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribeiro P. S., Josué F., Wepf A., Wehr M. C., Rinner O., Kelly G., Tapon N., Gstaiger M. (2010) Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 39, 521–534 [DOI] [PubMed] [Google Scholar]
- 17.Couzens A. L., Knight J. D., Kean M. J., Teo G., Weiss A., Dunham W. H., Lin Z. Y., Bagshaw R. D., Sicheri F., Pawson T., Wrana J. L., Choi H., Gingras A. C. (2013) Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal. 6, rs15 [DOI] [PubMed] [Google Scholar]
- 18.Sadot E., Simcha I., Shtutman M., Ben-Ze’ev A., Geiger B. (1998) Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc. Natl. Acad. Sci. USA 95, 15339–15344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conacci-Sorrell M., Simcha I., Ben-Yedidia T., Blechman J., Savagner P., Ben-Ze’ev A. (2003) Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, slug, and MAPK. J. Cell Biol. 163, 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vestweber D., Kemler R. (1985) Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 4 (13A), 3393–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe T., Wang S., Noritake J., Sato K., Fukata M., Takefuji M., Nakagawa M., Izumi N., Akiyama T., Kaibuchi K. (2004) Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell 7, 871–883 [DOI] [PubMed] [Google Scholar]
- 22.Grohmann A., Tanneberger K., Alzner A., Schneikert J., Behrens J. (2007) AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J. Cell Sci. 120, 3738–3747 [DOI] [PubMed] [Google Scholar]
- 23.Pfister A. S., Tanneberger K., Schambony A., Behrens J. (2012) Amer2 protein is a novel negative regulator of Wnt/β-catenin signaling involved in neuroectodermal patterning. J. Biol. Chem. 287, 1734–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castets F., Rakitina T., Gaillard S., Moqrich A., Mattei M. G., Monneron A. (2000) Zinedin, SG2NA, and striatin are calmodulin-binding, WD repeat proteins principally expressed in the brain. J. Biol. Chem. 275, 19970–19977 [DOI] [PubMed] [Google Scholar]
- 25.Seeling J. M., Miller J. R., Gil R., Moon R. T., White R., Virshup D. M. (1999) Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science 283, 2089–2091 [DOI] [PubMed] [Google Scholar]
- 26.Decaens C., Durand M., Grosse B., Cassio D. (2008) Which in vitro models could be best used to study hepatocyte polarity? Biol. Cell 100, 387–398 [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki D., Oikawa T., Takenawa T. (2007) Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J. Cell Sci. 120, 86–100 [DOI] [PubMed] [Google Scholar]
- 28.Hori K., Konno D., Maruoka H., Sobue K. (2003) MALS is a binding partner of IRSp53 at cell-cell contacts. FEBS Lett. 554, 30–34 [DOI] [PubMed] [Google Scholar]
- 29.Mandel L. J., Bacallao R., Zampighi G. (1993) Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature 361, 552–555 [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Palomo A., Meza I., Beaty G., Cereijido M. (1980) Experimental modulation of occluding junctions in a cultured transporting epithelium. J. Cell Biol. 87, 736–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seth A., Sheth P., Elias B. C., Rao R. (2007) Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 282, 11487–11498 [DOI] [PubMed] [Google Scholar]
- 32.Ciarlet M., Crawford S. E., Estes M. K. (2001) Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J. Virol. 75, 11834–11850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Vecchio G., Tscheik C., Tenz K., Helms H. C., Winkler L., Blasig R., Blasig I. E. (2012) Sodium caprate transiently opens claudin-5-containing barriers at tight junctions of epithelial and endothelial cells. Mol. Pharm. 9, 2523–2533 [DOI] [PubMed] [Google Scholar]
- 34.Mortensen K., Larsson L. I. (2003) Effects of cytochalasin D on the actin cytoskeleton: association of neoformed actin aggregates with proteins involved in signaling and endocytosis. Cell. Mol. Life Sci. 60, 1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Mariscal L., Chávez de Ramírez B., Cereijido M. (1985) Tight junction formation in cultured epithelial cells (MDCK). J. Membr. Biol. 86, 113–125 [DOI] [PubMed] [Google Scholar]
- 36.Sainz B., Jr., TenCate V., Uprichard S. L. (2009) Three-dimensional Huh7 cell culture system for the study of hepatitis C virus infection. Virol. J. 6, 103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou Z. Z., Petersen O. W., van Deurs B. (1989) Polarized expression of an apical membrane glycoprotein is established before functional tight junctions have developed in MCF-7 cells. J. Histochem. Cytochem. 37, 15–24 [DOI] [PubMed] [Google Scholar]
- 38.Nunes F. D., Lopez L. N., Lin H. W., Davies C., Azevedo R. B., Gow A., Kachar B. (2006) Distinct subdomain organization and molecular composition of a tight junction with adherens junction features. J. Cell Sci. 119, 4819–4827 [DOI] [PubMed] [Google Scholar]
- 39.Liwosz A., Lei T., Kukuruzinska M. A. (2006) N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J. Biol. Chem. 281, 23138–23149 [DOI] [PubMed] [Google Scholar]
- 40.Petrova Y. I., Schecterson L., Gumbiner B. M. (2016) Roles for E-cadherin cell surface regulation in cancer. Mol. Biol. Cell 27, 3233–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guettler S., LaRose J., Petsalaki E., Gish G., Scotter A., Pawson T., Rottapel R., Sicheri F. (2011) Structural basis and sequence rules for substrate recognition by Tankyrase explain the basis for cherubism disease. Cell 147, 1340–1354 [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Yuan Q., Ling X., Tan Q., Liang H., Chen J., Lin L., Xiao Y., Chen W., Liu L., Tang H. (2017) PARP-1 may be involved in hydroquinone-induced apoptosis by poly ADP-ribosylation of ZO-2. Mol. Med. Rep. 16, 8076–8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fearon E. R. (2009) PARsing the phrase “all in for Axin”- Wnt pathway targets in cancer. Cancer Cell 16, 366–368 [DOI] [PubMed] [Google Scholar]
- 44.Dejana E., Breviario F., Caveda L. (1994) Leukocyte-endothelial cell adhesive receptors. Clin. Exp. Rheumatol. 12 (Suppl 10), S25–S28 [PubMed] [Google Scholar]
- 45.Shin K., Fogg V. C., Margolis B. (2006) Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22, 207–235 [DOI] [PubMed] [Google Scholar]
- 46.Glatter T., Wepf A., Aebersold R., Gstaiger M. (2009) An integrated workflow for charting the human interaction proteome: insights into the PP2A system. Mol. Syst. Biol. 5, 237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang J., Pallas D. C. (2014) STRIPAK complexes: structure, biological function, and involvement in human diseases. Int. J. Biochem. Cell Biol. 47, 118–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno C. S., Park S., Nelson K., Ashby D., Hubalek F., Lane W. S., Pallas D. C. (2000) WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem. 275, 5257–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunbhakdi-Craig V., Machleidt T., Ogris E., Bellotto D., White C. L., III, Sontag E. (2002) Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 158, 967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran H., Bustos D., Yeh R., Rubinfeld B., Lam C., Shriver S., Zilberleyb I., Lee M. W., Phu L., Sarkar A. A., Zohn I. E., Wertz I. E., Kirkpatrick D. S., Polakis P. (2013) HectD1 E3 ligase modifies adenomatous polyposis coli (APC) with polyubiquitin to promote the APC-axin interaction. J. Biol. Chem. 288, 3753–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valnickova Z., Christensen T., Skottrup P., Thøgersen I. B., Højrup P., Enghild J. J. (2006) Post-translational modifications of human thrombin-activatable fibrinolysis inhibitor (TAFI): evidence for a large shift in the isoelectric point and reduced solubility upon activation. Biochemistry 45, 1525–1535 [DOI] [PubMed] [Google Scholar]
- 52.Stern J. A., Lahmers S., Meurs K. M. (2015) Identification of striatin, a desmosomal protein, in the canine corneal epithelium. Res. Vet. Sci. 102, 182–183 [DOI] [PubMed] [Google Scholar]
- 53.Capaldo C. T., Macara I. G. (2007) Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 18, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.