Abstract
Glucocorticoids (GCs) are frequently used to treat chronic disorders in children, including inflammation and cancer. Prolonged treatment with GCs is well known to impair bone growth, an effect linked to increased apoptosis and suppressed proliferation in growth plate chondrocytes. We hypothesized that the endogenous antiapoptotic protein humanin (HN) may prevent these effects. Interestingly, GC-induced bone growth impairment and chondrocyte apoptosis was prevented in HN overexpressing mice, HN-treated wild-type mice, and in HN-treated cultured rat metatarsal bones. GC-induced suppression of chondrocyte proliferation was also prevented by HN. Furthermore, GC treatment reduced Indian Hedgehog expression in growth plates of wild-type mice but not in HN overexpressing mice or HN-treated wild-type animals. A Hedgehog (Hh) antagonist, vismodegib, was found to suppress the growth of cultured rat metatarsal bones, and this effect was also prevented by HN. Importantly, HN did not interfere with the desired anti-inflammatory effects of GCs. We conclude that HN is a novel regulator of Hh signaling preventing GC-induced bone growth impairment without interfering with desired effects of GCs. Our data may open for clinical studies exploring a new possible strategy to prevent GC-induced bone growth impairment by cotreating with HN.—Zaman, F., Zhao, Y., Celvin, B., Mehta, H. H., Wan, J., Chrysis, D., Ohlsson, C., Fadeel, B., Cohen, P., Sävendahl, L. Humanin is a novel regulator of Hedgehog signaling and prevents glucocorticoid-induced bone growth impairment.
Keywords: glucocorticoids, inflammation, chondrocytes, apoptosis
Systemic treatment with glucocorticoids (GCs) is given to children suffering from a multitude of chronic disorders, such as leukemia, rheumatoid arthritis, ulcerative colitis, Crohn’s disease, asthma, and nephrotic syndrome. However, negative side effects of GCs remain an important concern, and it is recommended to carefully monitor children who are treated with high doses of systemic steroids (1, 2). In children, bone growth impairment is probably the most significant of these side effects (3). Clinical studies have shown that long-term treatment with high doses of GCs often leads to pronounced growth failure, an effect that has been linked to negative effects on the growth plate (4–6). Today, there is no therapy available for the prevention of GC-induced bone growth impairment in treated children. A new strategy to rescue bone growth in these patients is therefore highly desired. Obviously, it is important to ensure that any approach to achieve this rescue does not interfere with the desired anti-inflammatory effect of GCs.
Longitudinal bone growth takes place in the growth plate, which is a layer of cartilage present at both ends of long bones and where chondrocytes play a key role, being tightly regulated by several hormones (7). Inhibition of chondrocyte proliferation/differentiation or increased cell death can disturb the normal activity of the growth plate and thereby impair longitudinal bone growth (8, 9). Numerous in vitro and in vivo studies have shown that GC treatment not only suppresses systemic growth hormone levels but also alters the process of chondrocyte proliferation and differentiation in the growth plate (10–12). We have previously shown that dexamethasone (Dexa) triggers undesired cell death in growth plate chondrocytes through activation of the caspase cascade (caspase 8, 9, and 3) and suppression of PI3K-PKB signaling (8). Furthermore, we have recently reported that mice lacking the proapoptotic protein Bax are resistant to GC-induced bone growth retardation (13). The Hedgehog (Hh) pathway is known to play a key role in the regulation of bone growth. Indian Hedgehog (Ihh) is secreted by chondrocytes (14), and Ihh signaling regulates proliferation and differentiation of chondrocytes and is essential for bone growth (15). Recently, it has been reported that vismodegib, the first Hh-targeting agent to gain approval by the U.S. Food and Drug Administration, caused growth plate fusion, resulting in bone growth impairment in treated children (16).
Humanin (HN) is a 24 aa peptide that was originally discovered as a neuroprotective factor (17), having multiple modes of action in different cell types (18). For instance, HN has been reported to be a key regulator of peripheral insulin action (19). The cell rescuing activity of HN in pheochromocytoma cells seems to be mediated by the formyl peptide receptor-like 1, a GPCR (20), and ciliary neurotrophic factor receptor α (21). HN has also been reported to exert anti-inflammatory effects (21) as well as antiapoptotic effects (22, 23) by blocking activation of the proapoptotic proteins Bax (24) and Bak (25). HN treatment has shown very promising results in preclinical models of diabetes (22), stroke (26), atherosclerosis (27), and Alzheimer disease (28), but so far, there are no data available in bone growth disorders. Based on our recent findings that mice lacking the proapoptotic protein Bax are resistant to GC-induced bone growth impairment (13), we hypothesized that HN can prevent GC-induced bone growth impairment. In this study, we used the HN analog [Gly14]-HN (HNG) and HN overexpressing HN transgenic (HNtg) mice to investigate if HN may prevent GC-induced bone growth impairment and, if so, possible underlying mechanisms focusing on the regulation of apoptosis and Hh signaling.
MATERIALS AND METHODS
Reagents
Dexa, HNG (Met-Ala-Pro-Arg-Gly-Phe-Ser-Cys-Leu-Leu-Leu-Leu-Thr-Gly-Glu-Ile-Asp-Leu-Pro-Val-Lys-Arg-Arg-Ala), and IGF-I (MilliporeSigma, Steinheim, Germany and GenScript, Piscataway, NJ, USA) were dissolved in an appropriate solvent (ethanol or saline, according to the manufacturer’s instructions). Saline was injected in all controls (FVB and C57BL/6 animals) as both Dexa and HNG were dissolved in saline. Trypsin, PBS, EDTA, fetal bovine serum, minimum essential medium α, and DMEM/F12 were all purchased from Thermo Fisher Scientific (Paisley, United Kingdom).
Quantitative histology of the growth plate and X-rays
Four-week-old female FVB mice (purchased from Charles River Laboratories, Wilmington, MA, USA) received Dexa (2.5 mg/kg body weight/d; s.c. neck injection) or HNG (100 µg/kg body weight/d; intraperitoneal injection), or both, for 28 d. To measure longitudinal bone growth, animals were mildly anesthetized using isoflurane, placed on a digital X-ray cassette, and femur X-ray images (GE AMX-4; Soma Technology, Bloomfield, CT, USA, with the settings: 50 kV, 2.5 mAs) were captured before start of treatment (at d 0) and thereafter every week as previously reported (13). To investigate any direct effects of Dexa and HNG on bone growth, an organ culture system of fetal rat metatarsal bones was used as previously described (29). Histologic evaluations of the growth plates (×20 or ×40, or both) were done by using Image J software [National Institutes of Health (NIH), Bethesda, MD, USA] as previously reported (13).
HN overexpressing HNtg mice
Cytomegalovirus-driven expression of HN in C57BL6 HNtg mice resulted in stable high expression of native HN levels globally. A construct that includes the HN–open reading frame driven by a Cytomegalovirus promoter injected into the pronucleus of fertilized B6D2F1 mouse ova (Transgenic/Knockout Rodent Core Facility, University of Southern California, Los Angeles, CA, USA). Mice harboring the HN transgene are viable and fertile. To obtain a congenic line, the transgene was backcrossed into the C57BL6 strain. The overexpression of HN was confirmed with ELISA (Thermo Fisher Scientific). Four-week-old wild-type and HNtg male mice were treated with Dexa (2.5 mg/kg body weight/d, s.c. neck injection) or saline for 28 d. Seven days before the euthanasia, the mice were injected with calcein (200 mg/kg body weight) and plasma, and femur and tibia were collected.
HN ELISA
Circulating HN was measured from wild-type control and HNtg mouse plasma by an in-house sandwich ELISA, as previously described (30). Prior to assay, plasma was extracted with 90% acetonitrile and 10% 1 N HCl. Briefly, 200 μl of extraction reagent was added to 100 μl of plasma, gently mixed and incubated at room temperature. The supernatant was removed and dried by SpeedVac (Thermo Fisher Scientific) after centrifuge. The dried extracts were reconstituted with PBS and then used for ELISA. Synthetic HN was used as a standard within a range of 0.1–50 ng/ml. A 96-well microtiter plate was coated with capture antibody in 50 mM sodium bicarbonate buffer on a shaker. The plates were washed and blocked with Superblock buffer. Standards, controls, or extracted samples and pretitered detection antibody are added to the appropriate wells and incubated overnight. The absorbance was read at 490 nm on a plate spectrophotometer followed by streptavidin-horseradish peroxidase and o-phenylenediamine dihydrochloride steps.
Human growth plate cartilage and fetal rat bone organ cultures
Human growth plate biopsies were collected from the femur and tibia growth plates of children (boys, 13–15 yr old) who underwent epiphyseal surgery (epiphysiodesis) to reduce their bone growth. Immediately after collection, the biopsies were sliced and cultured as previously reported (31). Fetal rat metatarsal bones were cultured and monitored as previously described (32). Briefly, bone length was measured on d 2, 5, 7 or as indicated, and the increase in length is expressed as percent increase from d 0. Image J software (NIH) was used to measure bone growth.
Immunohistochemistry
Growth plate sections were incubated with anti-Bax antibody (clone 6A7; BD Biosciences, San Jose, CA, USA), anti-Bax (sc-6236; Santa Cruz Biotechnology, Dallas, TX, USA) and Ihh (33, 34) (sc-1196; Santa Cruz Biotechnology). Similarly, growth plate sections were incubated with HN antibody (35) (1:100, H2414; MilliporeSigma) to detect HN in the growth plate cartilage. The specificity of Ihh and HN antibodies used in this study has previously been validated and well characterized by using with negative control normal IgG isotype (33–35). Rabbit anti-proliferating cell nuclear antigen (PCNA) pAb (36) (ab-18197; Abcam, Cambridge, United Kingdom) was used to assess proliferation in mouse growth plates. The sections were incubated at 4°C overnight. Next, sections were washed with 1× Tris-buffered saline with Tween for 15 min and further incubated with corresponding fluorescent or horseradish peroxidase secondary antibody (Santa Cruz Biotechnology) or for 1 h at room temperature as reported previously (13). On each slide, sections from all groups were mounted, allowing all samples to be treated similarly during immunohistochemistry. Image J software (NIH) was used to quantify positive area in growth plates.
Chondrocyte and human macrophage cell cultures
The human chondrocytic cell line, HCS-2/8 (chondrosarcoma-derived) (37), was maintained as previously described (8). To investigate if HNG may interfere with the desired anti-inflammatory effect of Dexa, mononuclear cells were isolated from buffy coats obtained from healthy adult blood donors (Karolinska University Hospital, Stockholm, Sweden) by density gradient centrifugation as reported previously (38). Briefly, cells were resuspended in 24-well tissue-culture plates in RPMI-1640 medium (Thermo Fisher Scientific). Thereafter, monocytes were separated by adhesion to tissue-culture plastic for 1 h at 37°C. Human monocyte-derived macrophages were then stimulated with 50 ng/ml M-CSF (R&D Systems, Minneapolis, MN, USA) for an additional 3 d in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. TNF-α levels in culture media were measured as previously described (39).
LPS-induced inflammation and cytokine detection in mice
To mimic systemic inflammation (40), 4-wk-old female C57BL6 mice were treated with LPS (30 mg/kg). Animals also received intraperitoneal injection of HNG (100 μg/kg) and Dexa (2.5 mg/kg), 30 min before the injection of LPS. Three hours after injecting LPS, all mice were euthanized; their blood was collected and serum IL-6 levels were measured as previously described (41).
Western immunoblotting
Western immunoblotting was performed as previously described (31). The primary antibodies against Bax (1:1000) were from Santa Cruz Biotechnology. The antibody against Bid (P955957) was purchased from Cell Signaling Technology (Danvers, MA, USA). Image J (NIH) software was used to quantify Western blots.
Histone-associated DNA fragmentation and mitochondrial potential
To investigate cell death in cultured HCS-2/8 cells, cytoplasmic histone-associated DNA fragments (mono- and oligonucleasomes) were quantified using a Cell Death ELISA kit as previously described (8). In growth plate cartilage from humans and mice, apoptotic cells were identified by TUNEL assay by using a DNA fragmentation kit (EMD Millipore, Billerica, MA, USA). Mitochondrial membrane potential was measured in Dexa- and HNG-treated chondrocytes as previously reported (13).
Cell proliferation analysis
In fetal rat metatarsal bones, cell proliferation was analyzed using a BrdU cell proliferation kit (Amersham Biosciences, Buckinghamshire, United Kingdom), whereas digital automatic cell counting was performed as previously described (42). For human proliferative HCS-2/8 chondrocytes, BrdU was added to the cell culture medium for 3 h, and the proliferation rate was analyzed using an ELISA kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions.
Study approval
All animal experiments were approved by the local ethical committee (Stockholm North Animal Ethics Committee and University of Southern California, Los Angeles, CA, USA). The human tissue collection was approved by the local ethical committee, (Karolinska Institutet Research Ethics Committee North at the Karolinska University Hospital, Stockholm, Sweden). According to this approval, informed consent was obtained from each subject and his/her parents, which was documented in the original hospital records.
Statistical analysis
For statistical analysis, 2-tailed Student’s t test or 1-way ANOVA was applied to compare differences between groups (SigmaPlot 11.0; Systat Software, San Jose, CA, USA). Pairwise comparisons were performed using the Holm-Sidak procedure. All values are shown as means ± sd, and a value of P < 0.05 was considered to indicate a significant difference. The statistical tests used are stated in the figure legends.
RESULTS
HNtg mice are resistant to GC-induced bone growth impairment
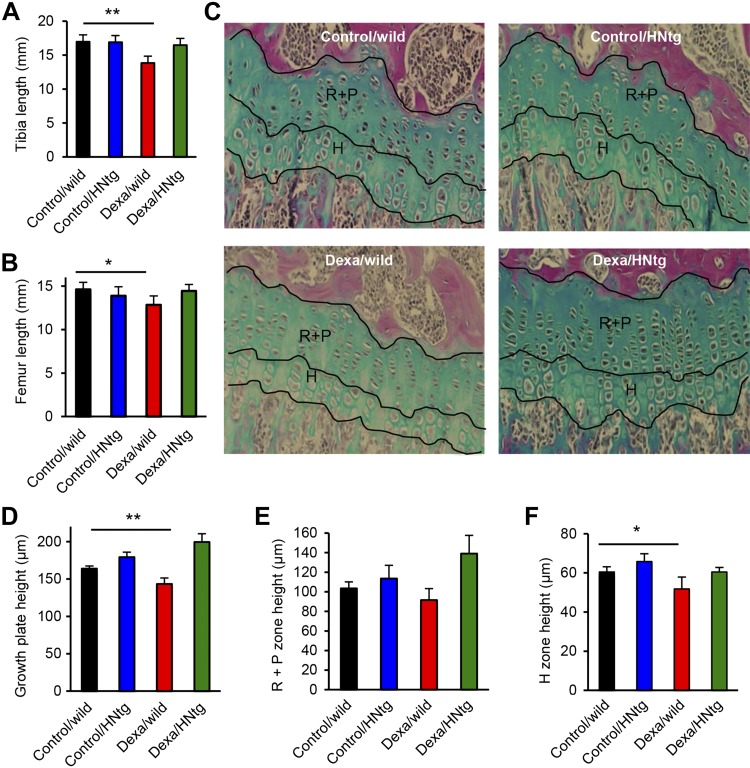
Based on our previous findings that mice lacking the proapoptotic protein Bax are resistant to GC-induced bone growth impairment (13) and that HN reduces the proapoptotic activity of Bax (43), we hypothesized that overexpression of HN may rescue young mice from GC-induced bone growth impairment. To validate this, 4-wk-old wild-type mice as well as HNtg male mice were treated with Dexa (2.5 mg/kg body weight, s.c.) or saline for 28 d. We observed that in wild-type mice, Dexa treatment reduced tibia growth by 18% (Fig. 1A; P < 0.01 Dexa/wild vs. control/wild). In contrast, HNtg mice were found to be resistant, and tibia growth in these animals was only suppressed by 3% when treated with Dexa (Fig. 1A; not significant, Dexa/HNtg vs. control/HNtg). Similarly, Dexa treatment decreased femur growth in wild-type (P < 0.05) but not in HNtg mice (Fig. 1B). Histomorphometric analyses of growth plates revealed that Dexa treatment reduced the total growth plate height by 14% in wild-type mice (Fig. 1C, D; P < 0.01 Dexa/wild vs. control/wild), whereas HNtg mice were found to be resistant against GC treatment as there was no significant difference between control/HNtg and Dexa/HNtg animals (Fig. 1C, D). No significant differences were found in resting+proliferative (R+P) zone height (Fig. 1E), but the hypertrophic zone was significantly reduced in Dexa-treated wild-type mice (Fig. 1F; Dexa/wild vs. control/wild, P < 0.05). In HNtg mice, plasma HN levels measured by ELISA were slightly increased by 16% when compared with those in wild-type mice (1825 ± 151 vs. 1566 ± 122 pg/ml, respectively; P = 0.12, n = 3–5). HNtg animals were slightly smaller than wild type but their overall health was unaffected.
Figure 1.
HNtg mice are resistant to GC-induced bone growth impairment. A, B) Four-week-old male wild-type and HNtg (C57BL6 background) mice were treated with/without Dexa (2.5 mg/kg body weight/d) for 28 consecutive days (n = 3–6/group). At end point, tibia (A) and femur (B) bone length were measured by digital caliper. C) Representative images indicating the different zones in the tibia growth plate in each group. Images stained with Alcian blue. Original magnification, ×20. D–F) The total height of the growth plate (D) as well as the height of R+P zone (E) and hypertrophic H zone (F) were analyzed in histologic sections of tibia growth plates. All error bars indicate sd. For statistical analysis, Student’s t test or Mann-Whitney Rank Sum Test was performed. *P < 0.05, **P < 0.01.
HN prevents GC-induced growth impairment in mice and cultured bones
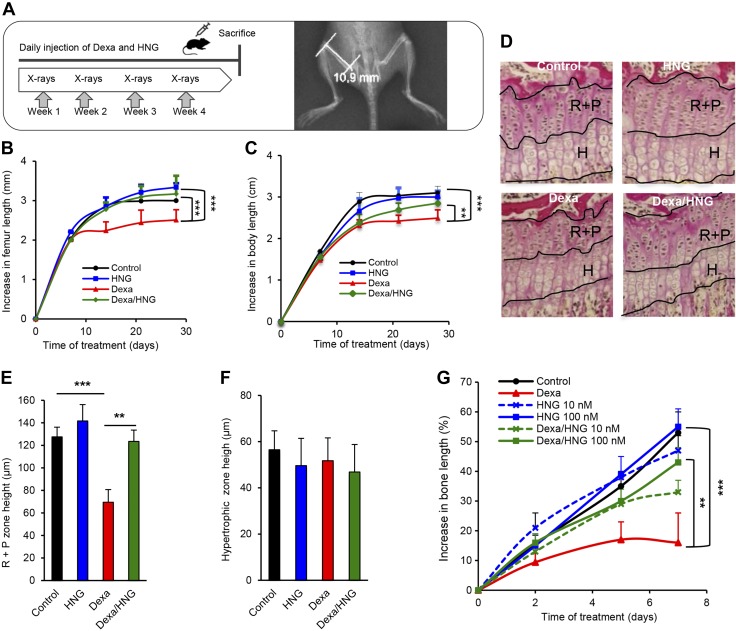
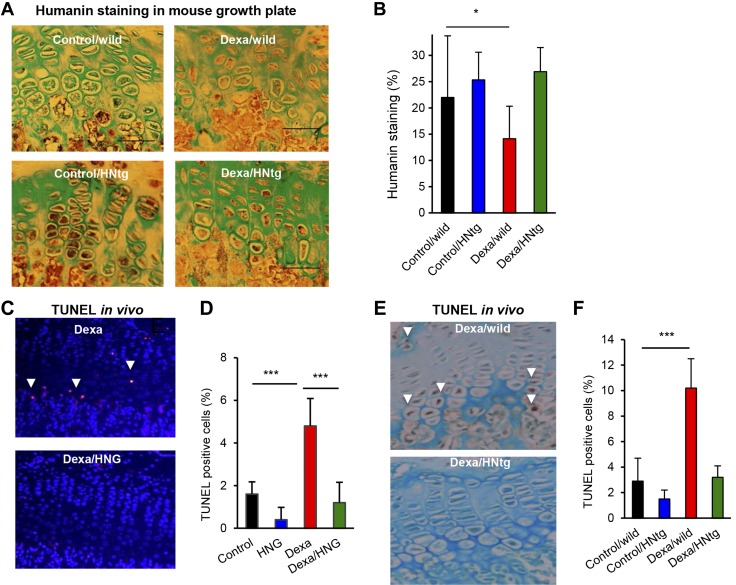
We next asked whether systemic administration of the HN analog HNG can rescue 4-wk-old FVB female mice from GC-induced bone growth impairment. Dexa (2.5 mg/kg body weight/d, s.c., as shown in Fig. 2A) treatment was confirmed to reduce femur growth by 16% (Fig. 2B; P < 0.001 Dexa vs. control), whereas HNG (100 μg/kg body weight, intraperitoneal) treatment rescued the growth when administered in combination with Dexa (Fig. 2B; P < 0.001 Dexa/HNG vs. Dexa) for 28 d. Dexa also reduced total body growth by 20% (Fig. 2C; P < 0.001 Dexa vs. control), whereas cotreatment with HNG entirely prevented this effect (Fig. 2C; P < 0.01 Dexa vs. Dexa/HNG). Total body growth was slightly reduced in wild-type mice treated with HNG alone (Fig. 2C; HNG vs. control, not significant), similarly as seen in HNtg animals. Histomorphometric growth plate analyses showed decreased height of the R+P zone in Dexa-treated wild-type mice (Fig. 2D, E; P < 0.001 Dexa vs. control), an effect that was completely blocked upon cotreatment with HNG (Fig. 2D, E; P < 0.01 Dexa vs. Dexa/HNG). The hypertrophic zone height was not affected by any of the treatments (Fig. 2F). Any local effects of HN were explored in cultured fetal rat metatarsal bones exposed to Dexa or HNG, or both, for 7 d. Dexa treatment impaired bone growth (Fig. 2G; 17% growth in Dexa vs. 53% in control, P < 0.001), whereas cotreatment with HNG, both at 10 and 100 nM, rescued bone growth (Fig. 2G; 43% growth in Dexa/HNG 100 nM vs. 17% in Dexa alone, P < 0.01), confirming a local action. This led us to hypothesize that Dexa impairs endogenous HN expression within the growth plate. Indeed, immunostaining showed that Dexa decreased HN expression in growth plates of treated wild-type mice (Fig. 3A, B; P < 0.05 Dexa/wild vs. control/wild), whereas HNtg animals were protected from this effect (Fig. 3A, B).
Figure 2.
Long-term treatment with HNG prevents GC-induced bone growth impairment in vivo and ex vivo. A) Schematic representation of experimental procedure, showing 4-wk-old FVB female mice treated with Dexa (2.5 mg/kg body weight/d; n = 8), HNG (100 µg/kg body weight), or both, for 28 consecutive days, and a representative image of X-rays taken to measure femur length. B, C) Longitudinal bone growth (B) was measured by performing weekly X-rays, and nose-anus body length (C) was measured manually with a caliper. Student’s t test was used to compare Dexa and Dexa/HNG groups. D) Representative images of tibia growth plates indicating the R+P and hypetrophic H zones in each group. Images processed with Van Gieson staining. Original magnification, ×20. E, F) Height measurements of R+P (E) and H (F) zone. G) Fetal rat metatarsal bones were cultured ex vivo and treated with Dexa (1 µM), HNG (10 or 100 nM), or both, for 7 d. Bone length was measured on d 0, 2, 5 and 7 (n = 15). All error bars indicate sd. For statistical analysis, 1-way ANOVA was performed. **P < 0.01, ***P < 0.001.
Figure 3.
Local (ex vivo) and systemic antiapoptotic effects of HN. A, B) Quantitative immunohistochemistry of HN expression (brown staining) with Alcian blue counter staining in the growth plate of wild-type and HNtg mice (C57BL6 background) treated with/without Dexa (2.5 mg/kg body weight/d) for 28 consecutive days. n = 3–6; scale bars, 50 µm; original magnification, ×40. C, D) Quantitative TUNEL analysis in growth plate of FVB mice treated with HNG (n = 5), fluorescent staining of apoptotic cells is shown in red and DAPI-staining of nuclei in blue. E, F) Quantitative TUNEL analysis in growth plate of HNtg animals (n = 3–6) treated with Dexa (2.5 mg/kg body weight), HNG (100 µg/kg body weight), or both, for 28 consecutive days. Apoptotic cells stained brown (DAB) and marked with white arrows. All error bars indicate sd. For statistical analysis, 1-way ANOVA was performed. Original magnification (C, E), ×20. *P < 0.05, ***P < 0.001.
HN prevents GC-induced chondrocyte apoptosis in mice and cultured rat bones
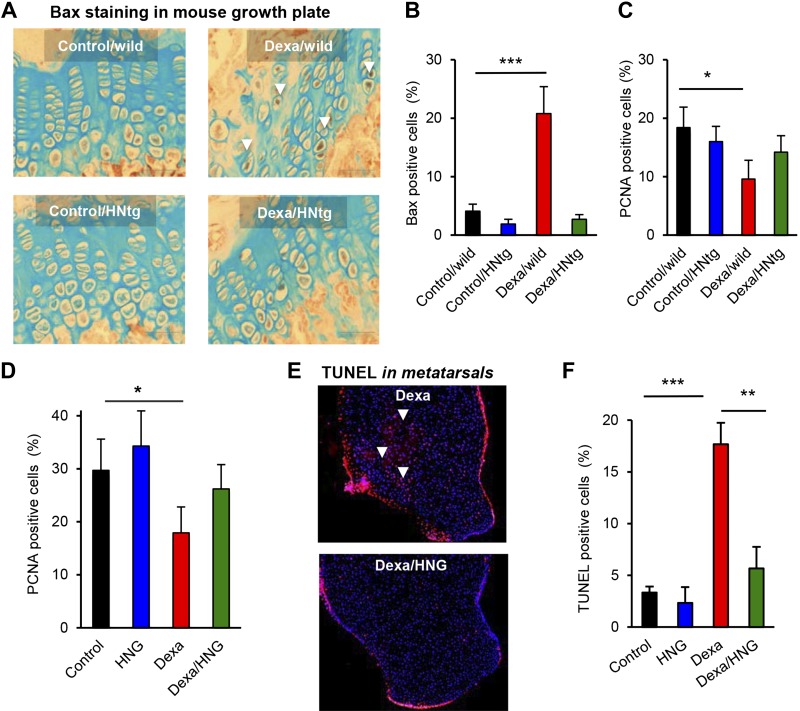
Because HN is known to exert strong antiapoptotic effects (24), we next explored if Dexa-induced suppression of HN levels is associated with increased chondrocyte apoptosis. Apoptosis was indeed confirmed to be significantly increased in growth plates of Dexa-treated wild-type mice (Fig. 3C, D; P < 0.001 Dexa/wild vs. saline/wild), an effect that was prevented in HN-treated wild-type (Fig. 3C, D; P < 0.001 Dexa/HNG vs. Dexa) as well as HNtg mice (Fig. 3E, F). Furthermore, Dexa increased the proapoptotic protein Bax in wild-type mice (Fig. 4A, B; P < 0.001 Dexa/wild vs. control/wild), whereas HNtg mice were resistant to this effect (Fig. 4A, B). Cell proliferation was decreased in wild-type mice (Fig. 4C; P < 0.05 Dexa/wild vs. control/wild) but not in HNtg mice. Pharmacological treatment with HNG showed similar effects, suppressed chondrocyte proliferation was observed in wild-type mice (Fig. 4D; P < 0.05 Dexa vs. control), and there was a trend for HNG to prevent this effect (Fig. 4D). Ex vivo studies in cultured rat metatarsal bones confirmed that local treatment with Dexa increased apoptosis by 17%, mainly in the R+P zone (Fig. 4E, F; P < 0.001 Dexa vs. control), an effect that was completely abolished when cotreating with Dexa and HNG (Fig. 4E, F; P < 0.01 Dexa vs. Dexa/HNG).
Figure 4.
Local and systemic effects on Bax, proliferation and apoptosis. A, B) Four-week-old male wild-type and HNtg mice (C57BL6 background, n = 3–6) were treated with Dexa (2.5 mg/kg body weight) for 28 consecutive days. Quantitative analysis for active Bax (brown staining, white arrows, original magnification, ×20) expression by using immunohistochemistry. C) PCNA analysis for cell proliferation in the growth plate of HNtg mice. D) PCNA analysis for cell proliferation in the growth plate of FVB mice (n = 5) treated with Dexa (2.5 mg/kg body weight/d), HNG (100 µg/kg body weight), or both, for 28 consecutive days. E, F) Fluorescent quantitative TUNEL in growth plates of fetal rat metatarsal bones (n = 5) treated with Dexa (1 µM), HNG (100 nM), or both, for 7 d. Apoptotic cells stained in red (white arrows) and nuclei in blue (DAPI). Original magnification (E), ×10. All error bars indicate sd. For statistical analysis, 1-way ANOVA was performed. *P < 0.05, **P < 0.01, ***P < 0.001.
HN rescues human chondrocytes from GC-induced apoptosis
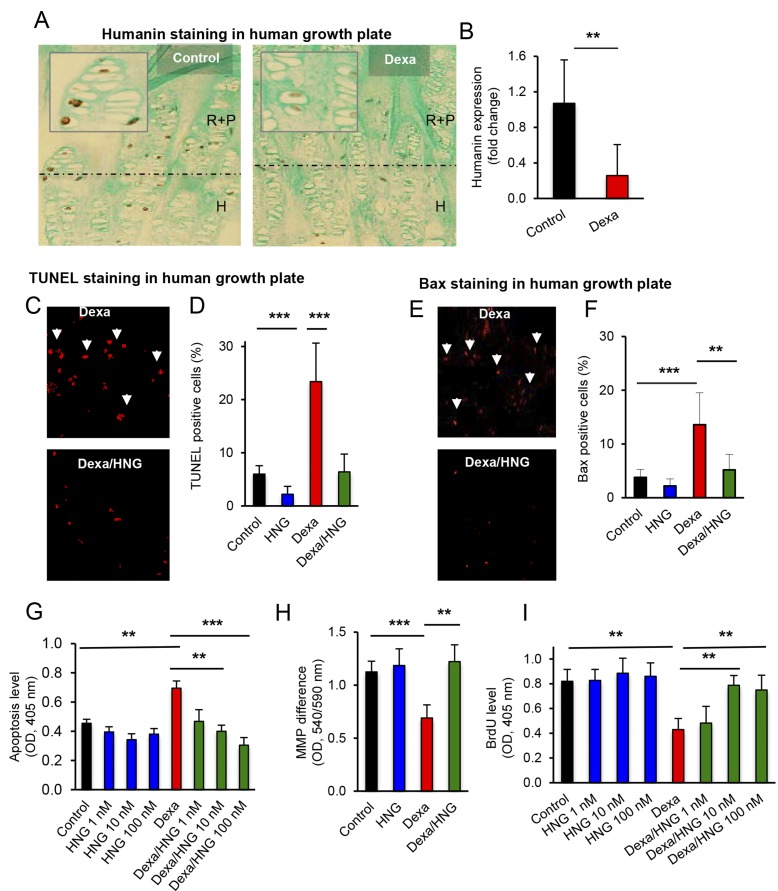
To verify if the data obtained in mice and cultured fetal rat metatarsal bones also applies to humans, we performed studies in a human chondrocytic cell line and in rare tissue samples of human growth plates collected from extremely tall adolescents undergoing epiphysiodesis surgery to limit their growth (31). Immunohistochemistry revealed that HN is widely expressed in the human growth plate, and, when cultured with Dexa for 24 h, the HN expression was markedly decreased (Fig. 5A, B; P < 0.01 control vs. Dexa). Chondrocyte apoptosis was markedly increased by Dexa (Fig. 5C, D; P < 0.001 Dexa vs. control), and cotreatment with HNG completely abolished this effect (Fig. 5C, D; P < 0.001 Dexa/HNG vs. Dexa).
Figure 5.
HN expression and protective effects of HN in cultured human growth plate cartilage and cultured human proliferative chondrocytes. A, B) Studies of endogenous levels of HN in human growth plate cartilage treated with Dexa (A; 1 µM) for 24 h and analyzed for changes in endogenous HN expression (brown staining; matrix stained blue with Alcian blue, n = 4) shown in R+P zone and hypertrophic H zone. Quantitative analysis of HN (B), expressed as fold change. C–F) Quantitative analysis of apoptosis and Bax levels in cultured human growth plate biopsies. Human growth plate cartilage was treated with Dexa (1 µM), HNG (100 nM), or both, for 24 h and analyzed for changes in apoptosis level (red staining, white arrows) by using TUNEL assay (C, D) and Bax expression (red staining, white arrows) using immunohistochemistry (E, F) (n = 4). G–I) HCS-2/8 chondrocytes were used to study cell death, proliferation, and changes in mitochondrial membrane potential (MMP). The cells were treated with Dexa (25 µM), HNG (1, 10, and 100 nM), or both, for 72 h. Apoptosis (G) was measured by Cell Death ELISA. MMP difference (H) by using a commercially available kit. Cell proliferation (I) by BrdU incorporation analyses applying a BrdU-ELISA (n = 4). All error bars indicate sd. For statistical analysis, 1-way ANOVA was performed. Original magnification (A, C, E), ×20. **P < 0.01, ***P < 0.001.
The expression of the proapoptotic protein Bax was also increased by Dexa (Fig. 5E, F; P < 0.001 Dexa vs. control), and cotreatment of the human growth plate tissues with HNG prevented this effect (Fig. 5E, F; P < 0.01 Dexa/HNG vs. Dexa). Mechanistic studies were performed in human HCS-2/8 chondrocytes in which Dexa treatment first was confirmed to increase apoptosis (Fig. 5G; P < 0.01 Dexa vs. control) and cell death to be completely abolished upon cotreatment with HNG at all tested concentrations (Fig. 5G; P < 0.001 Dexa/HNG 100 nM vs. Dexa). Dexa treatment also decreased mitochondrial membrane potential by 38% (Fig. 5H; P < 0.001 Dexa vs. control), an effect that was completely prevented by cotreatment with HNG (Fig. 5H, P < 0.01 Dexa/HNG vs. Dexa). Furthermore, HNG blocked activation of the proapoptotic protein Bax (Supplemental Fig. S1A, B; P < 0.05 Dexa/HNG vs. Dexa) and prevented formation of proapoptotic truncated Bid (tBid); (Supplemental Fig. S1A).
HN prevents GC-induced suppression of chondrocyte proliferation
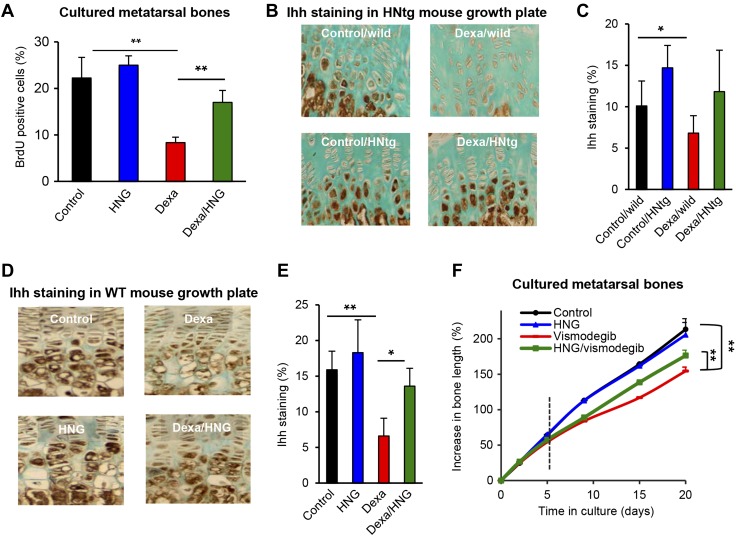
Dexa treatment decreased chondrocyte proliferation in cultured human HCS-2/8 chondrocytes (Fig. 5I; P < 0.01 Dexa vs. control) as well as in ex vivo cultured rat metatarsal bones (Fig. 6A; P < 0.01 Dexa vs. control). This effect was prevented when cotreating with Dexa and HNG in both HCS-2/8 chondrocytes (Fig. 6A; P < 0.01 Dexa/HNG 100 nM vs. Dexa) and cultured metatarsal bones (Fig. 6A; P < 0.01 Dexa/HNG vs. Dexa).
Figure 6.
Effects of HN on Hh signaling. A) BrdU proliferation analysis in fetal rat metatarsal bones cultured ex vivo and treated with Dexa (10 µM), HNG (100 nM), or both, for 7 d (n = 5). B) Four-week-old male wild-type and HNtg mice (C56BL6 background, n = 3–6) were treated with Dexa (2.5 mg/kg body weight/d) for 28 consecutive days. Immunohistochemistry was performed to detect any changes in Ihh expression (brown staining). C) Quantitative analysis of Ihh, staining expressed as percent of growth plate area. D) Four-week-old FVB wild-type mice (n = 5) treated with Dexa (2.5 mg/kg body weight/d), HNG (100 µg/kg body weight), or both, for 28 consecutive days. Immunohistochemistry was performed to detect any changes in Ihh expression (brown staining) after pharmacological treatment with HNG. E) Quantitative analysis of Ihh, staining expressed as percent of growth plate area (n = 5). F) Fetal rat metatarsal bones cultured ex vivo were treated with the Hh inhibitor vismodegib (100 nM), HNG (38 nM), or both, for 5 d and thereafter followed for 15 d as indicated by the dotted vertical line without vismodegib or HNG (n = 9). All error bars indicate sd. One-way ANOVA was performed. Original magnification (B, D), ×20. *P < 0.05, **P < 0.01.
HN prevents GC-induced suppression of Ihh in growth plate chondrocytes
Immunostaining showed that the expression of Ihh was suppressed in the growth plates of Dexa-treated wild-type mice (Fig. 6B, C; P < 0.05 Dexa/wild vs. control/wild) but not in HNtg animals (Fig. 6B, C). Pharmacological treatment with HNG showed similar effects; Dexa significantly suppressed Ihh levels (Fig. 6D, E; P < 0.01 Dexa vs. control), whereas cotreatment with HNG significantly restored Ihh levels (Fig. 6D, E; P < 0.05 Dexa/HNG vs. Dexa). Gli1 is a transcription factor involved in the Hh pathway (44), and we noted a similar trend for Gli1 expression, although the difference did not reach statistical significance (Supplemental Fig. S2A, B). We further evaluated whether impaired Hh signaling could be linked to bone growth impairment by treating cultured fetal rat metatarsal bones with vismodegib (GDC-0449), a Hh antagonist. The bones were cultured with vismodegib, alone or in combination with HNG for 5 d and thereafter followed for another 15 d without any treatment. Decreased growth was observed in vismodegib-treated bones (Fig. 6F; P < 0.01 vismodegib vs. control, and Supplemental Fig. S3A), whereas when cotreated with HNG, bone growth was rescued (Fig. 6F; P < 0.01 vismodegib/HNG vs. vismodegib). Treatment with vismodegib was confirmed to impair Ihh also in cultured fetal rat metatarsal bones (Supplemental Fig. S3B).
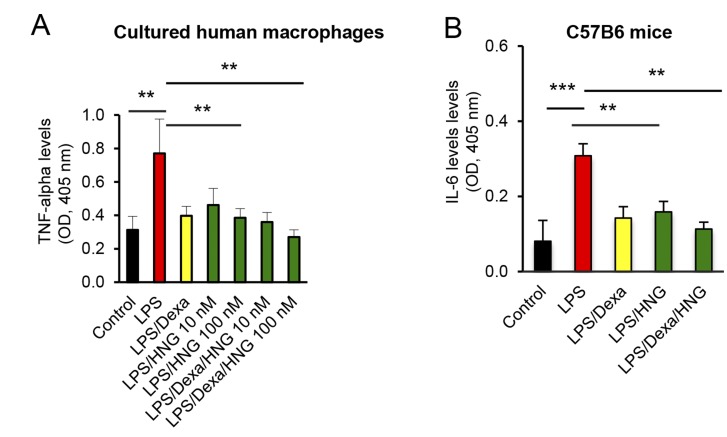
HN does not interfere with the desired anti-inflammatory effects of GCs
To explore whether HN interferes with the desired anti-inflammatory effects of GCs, cultured human macrophages were treated with LPS. In human macrophages, TNF-α was strongly up-regulated when challenged with LPS (Fig. 7A; P < 0.01 LPS vs. control), and, as expected, Dexa prevented this effect (Fig. 7A). HNG by itself also prevented LPS-induced up-regulation of TNF-α (Fig. 7A; P < 0.01 LPS/HNG 100 nM vs. LPS) and, importantly, did not interfere with Dexa-induced suppression of TNF-α (Fig. 7A; P < 0.01 LPS/Dexa/HNG 100 nM vs. LPS). Similarly, when 4-wk-old female C57BL6 mice were injected with LPS, serum levels of IL-6 increased by 26% (Fig. 7B; P < 0.001 LPS vs. control), and, as expected, this effect was suppressed by Dexa. HNG by itself suppressed LPS-induced IL-6 to the same extent as Dexa alone (Fig. 7B; P < 0.01 LPS/HNG vs. LPS), and HNG in combination with Dexa did not interfere with the anti–IL-6 effects of Dexa (Fig. 7B; P < 0.01 LPS/Dexa/HNG vs. LPS).
Figure 7.
Anti-inflammatory effects of HNG in LPS-treated mice and cultured human macrophages. A) Human macrophages were treated with Dexa (10 µM), LPS (100 ng/ml), or HNG (10 or 100 nM), or a combination thereof. The cell culture medium was collected and TNF-α levels were measured by ELISA (n = 4). B) Four-week-old C57BL6 male mice were treated with LPS (30 mg/kg body weight), Dexa (2 mg/kg body weight), or HNG (100 µg/kg body weight), or a combination thereof, for 3 h followed by blood collection and euthanasia. Serum levels of IL-6 were analyzed by ELISA (n = 8). All error bars indicate sd. For statistical analysis, 1-way ANOVA was used. **P < 0.01, ***P < 0.001.
DISCUSSION
We have previously shown that Dexa activates caspase-8 in proliferative chondrocytes (8) and that both Bax and Bid are potential mediators of the proapoptotic effects of GCs in chondrocytes (31). Caspase-8 activation is known to cleave Bid, and truncated Bid initiates mitochondrial apoptosis (45). We have also shown that ablation of Bax protects young mice from GC-induced bone growth impairment (13). This led us to the mitochondrial-derived peptide HN, reported to bind with Bax in human embryonic kidney cells (24) and to neutralize Bax in the testis (46). We report herein that the HN derivative HNG prevents Dexa-induced bone growth impairment and chondrocyte apoptosis in young mice, effects that were verified in HNtg mice and ex vivo cultured rat metatarsal bones. Furthermore, our data suggest that HN is a novel regulator of Hh signaling and that targeting this pathway may rescue from GC-induced bone growth impairment without interfering with the desired anti-inflammatory effects of GCs.
Expression of endogenous HN has previously been reported in several tissues, including liver, heart, colon, brain, and neurons (47, 48). By studying rare human tissues, we show here that HN is expressed also in the growth plate and that human growth plate chondrocytes are highly sensitive to GC-induced apoptosis. In vivo studies showed that endogenous HN levels were decreased by Dexa treatment in wild-type mice but not in HNtg animals. Exogenous administration of HNG not only restored HN levels but also prevented Dexa-induced apoptosis, suggesting that suppressed endogenous HN production sensitizes growth plate chondrocytes to undergo apoptosis. Antiapoptotic effects of HN have been reported on earlier in a variety of cell types, including pancreatic β cells (22), germ cells (46) and neurons (49). A clear growth plate phenotype with significant differences in R+P zone height was observed in wild-type mice treated with HNG. In contrast, no clear growth plate phenotype was observed in HNtg mice, which could be due to the fact that HN levels were only slightly increased. A recent clinical study showed that only marginally increased HN levels are associated with improved blood glucose levels after resistance training in men with impaired glucose metabolism, suggesting that even a modest increase in circulating HN levels can exert positive effects (50).
We here report that GCs suppress growth plate expression of Ihh and that pharmacological treatment with HNG not only restored Ihh levels within the growth plate but also prevented GC-induced growth retardation in treated mice. Suppression of Ihh expression in postnatal chondrocytes has previously been reported to cause growth plate fusion and impaired bone growth (51). To study whether HN can overcome impaired Hh signaling and restore bone growth, we used vismodegib, an Hh inhibitor developed to treat malignancies and that unfortunately also has been reported to causes early fusion of growth plates in children, leading to short stature (16). Interestingly, we observed that HNG treatment rescued from vismodegib-induced bone growth impairment, although the effect remained partial, suggesting potential involvement of other signaling pathways. Vismodegib inhibits Hh signaling via Smo (52), a putative G protein–coupled protein involved in transducing Ihh signaling (53). Vismodegib has also been reported to suppress Gli1, a transcription factor of Ihh (54). Similarly, increase in primary cilium length is also known to inhibit Hh signaling (55) and Dexa increases cilium length (56). This may explain the possible connection between the use of GCs and decreased Hh signaling. Ihh is a critical factor secreted by chondrocytes, but its expression is restricted mainly to the hypertrophic zone (14). It is well known that increased activity of the Ihh signaling pathway promotes chondrocyte proliferation (57) and mutation or disruption of Ihh signaling results in reduction of the height of the columnar layer in the growth plate (58). GCs are also known to suppresses Hh activity in neurons (59). All taken together, disruption of Hh signaling, whether it is through inhibition of Smo, using inhibitors or targeted deletion of Ihh, leads to growth plate fusion and bone growth impairment. We have shown previously that HNG not only prevents bone growth impairment without interfering with the desired anticancer effects of anticancer agents but also prevents cancer cell proliferation, though Hh inhibitors were not studied (43).
Although Hh signaling plays a crucial role in the regulation of chondrocyte proliferation and differentiation, it has so far been unclear whether suppression of Ihh may sensitize chondrocytes to apoptosis. We show here that GCs induce growth plate apoptosis and that cotreatment with HN restores Hh signaling, which in turn blocks apoptotic signaling by preventing activation of Bax and mitochondrial injury. Thus, our observations suggest that HN not only modulates Hh signaling, but also promotes chondrocytes survival. Indeed, Hh stimulation has been reported to act as a potent survival signal for neural progenitor cells, as the inhibition of Hh signaling by the Hh antagonist vismodegib triggers massive apoptotic reaction, which is Bax/Bak dependent but p53 independent (60). Similarly, HN has been shown to directly bind with active Bax (24) and Bak (25). Our data are in line with these observations as we observed decreased levels of active Bax after treatment with HN, both in mouse and human growth plates. Altogether, these findings suggest that GC-induced loss of Hh signaling not only inhibits chondrocyte proliferation but also triggers apoptotic signaling in which activation of Bax plays a key role. Therefore, targeting Hh signaling could be attractive when aiming to develop a novel treatment preventing GC-induced growth impairment. Our data suggest that HN has the potential to achieve this.
The HN analog HNG was found to effectively protect human growth plate cartilage from GC-induced cytotoxicity, suggesting that HNG potentially could be used to rescue bone growth in GC-treated children. The clinical relevance of our data is underscored by the fact that no effective treatment is currently available for rescuing bone growth during prolonged GC exposure (61). Recombinant human growth hormone has been reported to improve the growth in some children treated with GCs, although the effect is moderate and the cost high (62). Furthermore, the use of recombinant human growth hormone to promote GC-induced growth failure has not been approved by medical authorities, emphasizing the need to explore alternative treatment options. Our studies in mice and human macrophages showed that HNG does not interfere with the desired anti-inflammatory effects of Dexa, suggesting that the combined treatment with Dexa and HNG is a feasible approach. We also found that HNG, by itself, decreased LPS-induced production of the proinflammatory cytokines TNF-α and IL-6 in mice. LPS is known to activate monocytes and macrophages involving TLR-4 and triggers the production of TNF-α (63), thereby up-regulating systemic inflammation. This is noteworthy, as our previous work has disclosed that both TNF-α and IL-6 suppress bone growth acting in synergy (29, 64). Consequently, the anti-inflammatory effects of HNG could potentially alleviate bone growth impairment in patients with chronic inflammatory disorders.
We conclude that HN is a novel regulator of Hh signaling that prevents GC-induced bone growth impairment without interfering with the desired actions of GCs; in fact, HNG alone displayed anti-inflammatory effects. Based on our present experimental data obtained in several model systems, including ex vivo cultured human growth plate cartilage, we propose a novel strategy using a HN analog to rescue bone growth in children in need of prolonged treatment with high doses of GCs or Hh inhibitors. To date, no HN analog is approved for clinical use, but our data support the clinical development of such compounds.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This study was supported by the Swedish Research Council (Grant 2015-02406, to L.S.), the Swedish Childhood Cancer Foundation (Grants PR2014-0167, TJ2017-0089, and PR2017-0167, to L.S. and F.Z.), Stiftelsen Frimurare Barnhuset Stockholm (to F.Z., L.S., and B.C.), HKH Kronprinsessan Lovisas Förening för Barnasjukvård/Stiftelsen Axel Tielmans Minnesfond (to F.Z. and L.S.), and Karolinska Institutet (to L.S.); and by an AFAR BIG Award and by Grant 1P01AG034906 from the U.S. National Institutes of Health, National Institute on Aging (to P.C.). The authors declare no conflicts of interest.
Glossary
- Dexa
dexamethasone
- GC
glucocorticoid
- Hh
Hedgehog
- HN
humanin
- HNG
[Gly(14)]-humanin
- HNtg
humanin transgenic
- Ihh
Indian Hedgehog
- PCNA
proliferating cell nuclear antigen
- R+P
resting+proliferative
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
F. Zaman performed most of the experiments, analyzed and interpreted the results, and prepared the draft manuscript; Y. Zhao and B. Celvin performed experiments, reviewed data, and contributed to manuscript preparation; H. H. Mehta, J. Wan, and P. Cohen provided genetically modified mice and contributed to manuscript preparation; and D. Chrysis, C. Ohlsson, B. Fadeel, and L. Sävendahl provided advice on project planning, data interpretation, and contributed to manuscript preparation.
REFERENCES
- 1.Silva I. N., Kater C. E., Cunha C. F., Viana M. B. (1997) Randomised controlled trial of growth effect of hydrocortisone in congenital adrenal hyperplasia. Arch. Dis. Child. 77, 214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boon L. M., MacDonald D. M., Mulliken J. B. (1999) Complications of systemic corticosteroid therapy for problematic hemangioma. Plast. Reconstr. Surg. 104, 1616–1623 [DOI] [PubMed] [Google Scholar]
- 3.Yeh T. F., Lin Y. J., Lin H. C., Huang C. C., Hsieh W. S., Lin C. H., Tsai C. H. (2004) Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N. Engl. J. Med. 350, 1304–1313 [DOI] [PubMed] [Google Scholar]
- 4.Loeb J. N. (1976) Corticosteroids and growth. N. Engl. J. Med. 295, 547–552 [DOI] [PubMed] [Google Scholar]
- 5.Lai H. C., FitzSimmons S. C., Allen D. B., Kosorok M. R., Rosenstein B. J., Campbell P. W., Farrell P. M. (2000) Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N. Engl. J. Med. 342, 851–859 [DOI] [PubMed] [Google Scholar]
- 6.Baron J., Huang Z., Oerter K. E., Bacher J. D., Cutler G. B., Jr (1992) Dexamethasone acts locally to inhibit longitudinal bone growth in rabbits. Am. J. Physiol. 263, E489–E492 [DOI] [PubMed] [Google Scholar]
- 7.Sävendahl L. (2005) Hormonal regulation of growth plate cartilage. Horm. Res. 64 (Suppl 2), 94–97 [DOI] [PubMed] [Google Scholar]
- 8.Chrysis D., Zaman F., Chagin A. S., Takigawa M., Sävendahl L. (2005) Dexamethasone induces apoptosis in proliferative chondrocytes through activation of caspases and suppression of the Akt-phosphatidylinositol 3′-kinase signaling pathway. Endocrinology 146, 1391–1397 [DOI] [PubMed] [Google Scholar]
- 9.Mushtaq T., Farquharson C., Seawright E., Ahmed S. F. (2002) Glucocorticoid effects on chondrogenesis, differentiation and apoptosis in the murine ATDC5 chondrocyte cell line. J. Endocrinol. 175, 705–713 [DOI] [PubMed] [Google Scholar]
- 10.Chrysis D., Ritzen E. M., Sävendahl L. (2003) Growth retardation induced by dexamethasone is associated with increased apoptosis of the growth plate chondrocytes. J. Endocrinol. 176, 331–337 [DOI] [PubMed] [Google Scholar]
- 11.Mushtaq T., Bijman P., Ahmed S. F., Farquharson C. (2004) Insulin-like growth factor-I augments chondrocyte hypertrophy and reverses glucocorticoid-mediated growth retardation in fetal mice metatarsal cultures. Endocrinology 145, 2478–2486 [DOI] [PubMed] [Google Scholar]
- 12.Rooman R. P., Kuijpers G., Gresnigt R., Bloemen R., Koster J. G., van Buul-Offers S. C. (2003) Dexamethasone differentially inhibits thyroxine- or growth hormone-induced body and organ growth of Snell dwarf mice. Endocrinology 144, 2553–2558 [DOI] [PubMed] [Google Scholar]
- 13.Zaman F., Chrysis D., Huntjens K., Fadeel B., Sävendahl L. (2012) Ablation of the pro-apoptotic protein Bax protects mice from glucocorticoid-induced bone growth impairment. PLoS One 7, e33168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vortkamp A., Lee K., Lanske B., Segre G. V., Kronenberg H. M., Tabin C. J. (1996) Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613–622 [DOI] [PubMed] [Google Scholar]
- 15.St-Jacques B., Hammerschmidt M., McMahon A. P. (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson G. W., Kaste S. C., Chemaitilly W., Bowers D. C., Laughton S., Smith A., Gottardo N. G., Partap S., Bendel A., Wright K. D., Orr B. A., Warner W. C., Onar-Thomas A., Gajjar A. (2017) Irreversible growth plate fusions in children with medulloblastoma treated with a targeted hedgehog pathway inhibitor. Oncotarget 8, 69295–69302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto Y., Niikura T., Ito Y., Sudo H., Hata M., Arakawa E., Abe Y., Kita Y., Nishimoto I. (2001) Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J. Neurosci. 21, 9235–9245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S. J., Guerrero N., Wassef G., Xiao J., Mehta H. H., Cohen P., Yen K. (2016) The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget 7, 46899–46912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzumdar R. H., Huffman D. M., Atzmon G., Buettner C., Cobb L. J., Fishman S., Budagov T., Cui L., Einstein F. H., Poduval A., Hwang D., Barzilai N., Cohen P. (2009) Humanin: a novel central regulator of peripheral insulin action. PLoS One 4, e6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi Y., Hashimoto Y., Niikura T., Nishimoto I. (2003) Identification of essential amino acids in Humanin, a neuroprotective factor against Alzheimer’s disease-relevant insults. Peptides 24, 585–595 [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto Y., Kurita M., Aiso S., Nishimoto I., Matsuoka M. (2009) Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol. Biol. Cell 20, 2864–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoang P. T., Park P., Cobb L. J., Paharkova-Vatchkova V., Hakimi M., Cohen P., Lee K. W. (2010) The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism 59, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H., Liu T., Wang W. X., Xu J. H., Yang P. B., Lu H. X., Sun Q. R., Hu H. T. (2010) Protective effects of [Gly14]-Humanin on beta-amyloid-induced PC12 cell death by preventing mitochondrial dysfunction. Neurochem. Int. 56, 417–423 [DOI] [PubMed] [Google Scholar]
- 24.Guo B., Zhai D., Cabezas E., Welsh K., Nouraini S., Satterthwait A. C., Reed J. C. (2003) Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423, 456–461 [DOI] [PubMed] [Google Scholar]
- 25.Zhai D., Luciano F., Zhu X., Guo B., Satterthwait A. C., Reed J. C. (2005) Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J. Biol. Chem. 280, 15815–15824 [DOI] [PubMed] [Google Scholar]
- 26.Xu X., Chua C. C., Gao J., Hamdy R. C., Chua B. H. (2006) Humanin is a novel neuroprotective agent against stroke. Stroke 37, 2613–2619 [DOI] [PubMed] [Google Scholar]
- 27.Oh Y. K., Bachar A. R., Zacharias D. G., Kim S. G., Wan J., Cobb L. J., Lerman L. O., Cohen P., Lerman A. (2011) Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis 219, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W., Zhang W., Li Z., Hao J., Zhang Z., Liu L., Mao N., Miao J., Zhang L. (2012) S14G-humanin improves cognitive deficits and reduces amyloid pathology in the middle-aged APPswe/PS1dE9 mice. Pharmacol. Biochem. Behav. 100, 361–369 [DOI] [PubMed] [Google Scholar]
- 29.Mårtensson K., Chrysis D., Sävendahl L. (2004) Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J. Bone Miner. Res. 19, 1805–1812 [DOI] [PubMed] [Google Scholar]
- 30.Chin Y. P., Keni J., Wan J., Mehta H., Anene F., Jia Y., Lue Y. H., Swerdloff R., Cobb L. J., Wang C., Cohen P. (2013) Pharmacokinetics and tissue distribution of humanin and its analogues in male rodents. Endocrinology 154, 3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaman F., Chrysis D., Huntjens K., Chagin A., Takigawa M., Fadeel B., Sävendahl L. (2014) Dexamethasone differentially regulates Bcl-2 family proteins in human proliferative chondrocytes: role of pro-apoptotic Bid. Toxicol. Lett. 224, 196–200 [DOI] [PubMed] [Google Scholar]
- 32.Zaman F., Menendez-Benito V., Eriksson E., Chagin A. S., Takigawa M., Fadeel B., Dantuma N. P., Chrysis D., Sävendahl L. (2007) Proteasome inhibition up-regulates p53 and apoptosis-inducing factor in chondrocytes causing severe growth retardation in mice. Cancer Res. 67, 10078–10086 [DOI] [PubMed] [Google Scholar]
- 33.Wei F., Zhou J., Wei X., Zhang J., Fleming B. C., Terek R., Pei M., Chen Q., Liu T., Wei L. (2012) Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis Cartilage 20, 755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adolphe C., Narang M., Ellis T., Wicking C., Kaur P., Wainwright B. (2004) An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development 131, 5009–5019 [DOI] [PubMed] [Google Scholar]
- 35.Janzen C., Lei M. Y. Y., Jeong I. S. D., Ganguly A., Sullivan P., Paharkova V., Capodanno G., Nakamura H., Perry A., Shin B. C., Lee K. W., Devaskar S. U. (2018) Humanin (HN) and glucose transporter 8 (GLUT8) in pregnancies complicated by intrauterine growth restriction. PLoS One 13, e0193583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan L., Wu R., Zhang X., Wang D., You Y., Zhang Y., Zhou L., Chen W. (2018) HBx-induced S100A9 in NF-κB dependent manner promotes growth and metastasis of hepatocellular carcinoma cells. Cell Death Dis. 9, 629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takigawa M., Tajima K., Pan H. O., Enomoto M., Kinoshita A., Suzuki F., Takano Y., Mori Y. (1989) Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 49, 3996–4002 [PubMed] [Google Scholar]
- 38.Farrera C., Fadeel B. (2013) Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 191, 2647–2656 [DOI] [PubMed] [Google Scholar]
- 39.Konduru N. V., Tyurina Y. Y., Feng W., Basova L. V., Belikova N. A., Bayir H., Clark K., Rubin M., Stolz D., Vallhov H., Scheynius A., Witasp E., Fadeel B., Kichambare P. D., Star A., Kisin E. R., Murray A. R., Shvedova A. A., Kagan V. E. (2009) Phosphatidylserine targets single-walled carbon nanotubes to professional phagocytes in vitro and in vivo. PLoS One 4, e4398; erratum [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juskewitch J. E., Knudsen B. E., Platt J. L., Nath K. A., Knutson K. L., Brunn G. J., Grande J. P. (2012) LPS-induced murine systemic inflammation is driven by parenchymal cell activation and exclusively predicted by early MCP-1 plasma levels. Am. J. Pathol. 180, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinbicker A. U., Sachidanandan C., Vonner A. J., Yusuf R. Z., Deng D. Y., Lai C. S., Rauwerdink K. M., Winn J. C., Saez B., Cook C. M., Szekely B. A., Roy C. N., Seehra J. S., Cuny G. D., Scadden D. T., Peterson R. T., Bloch K. D., Yu P. B. (2011) Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood 117, 4915–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chagin A. S., Karimian E., Zaman F., Takigawa M., Chrysis D., Sävendahl L. (2007) Tamoxifen induces permanent growth arrest through selective induction of apoptosis in growth plate chondrocytes in cultured rat metatarsal bones. Bone 40, 1415–1424 [DOI] [PubMed] [Google Scholar]
- 43.Eriksson E., Wickström M., Perup L. S., Johnsen J. I., Eksborg S., Kogner P., Sävendahl L. (2014) Protective role of humanin on bortezomib-induced bone growth impairment in anticancer treatment. J. Natl. Cancer Inst. 106, djt459 [DOI] [PubMed] [Google Scholar]
- 44.Das S., Harris L. G., Metge B. J., Liu S., Riker A. I., Samant R. S., Shevde L. A. (2009) The hedgehog pathway transcription factor GLI1 promotes malignant behavior of cancer cells by up-regulating osteopontin. J. Biol. Chem. 284, 22888–22897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481–490 [DOI] [PubMed] [Google Scholar]
- 46.Jia Y., Lue Y. H., Swerdloff R., Lee K. W., Cobb L. J., Cohen P., Wang C. (2013) The cytoprotective peptide humanin is induced and neutralizes Bax after pro-apoptotic stress in the rat testis. Andrology 1, 651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charununtakorn S. T., Shinlapawittayatorn K., Chattipakorn S. C., Chattipakorn N. (2016) Potential roles of humanin on apoptosis in the heart. Cardiovasc. Ther. 34, 107–114 [DOI] [PubMed] [Google Scholar]
- 48.Tajima H., Niikura T., Hashimoto Y., Ito Y., Kita Y., Terashita K., Yamazaki K., Koto A., Aiso S., Nishimoto I. (2002) Evidence for in vivo production of Humanin peptide, a neuroprotective factor against Alzheimer’s disease-related insults. Neurosci. Lett. 324, 227–231 [DOI] [PubMed] [Google Scholar]
- 49.Wang T., Huang Y., Zhang M., Wang L., Wang Y., Zhang L., Dong W., Chang P., Wang Z., Chen X., Tao L. (2013) [Gly14]-Humanin offers neuroprotection through glycogen synthase kinase-3β inhibition in a mouse model of intracerebral hemorrhage. Behav. Brain Res. 247, 132–139 [DOI] [PubMed] [Google Scholar]
- 50.Gidlund E. K., von Walden F., Venojärvi M., Risérus U., Heinonen O. J., Norrbom J., Sundberg C. J. (2016) Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol. Rep. 4, e13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda Y., Nakamura E., Nguyen M. T., Suva L. J., Swain F. L., Razzaque M. S., Mackem S., Lanske B. (2007) Indian hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc. Natl. Acad. Sci. USA 104, 6382–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekulic A., Von Hoff D. (2016) Hedgehog pathway inhibition. Cell 164, 831 [DOI] [PubMed] [Google Scholar]
- 53.Zhang X. M., Ramalho-Santos M., McMahon A. P. (2001) Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell 105, 781–792 [PubMed] [Google Scholar]
- 54.Yang H., Cong W. N., Yoon J. S., Egan J. M. (2015) Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 4, 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson C. L., Wiles A., Poole C. A., Knight M. M. (2016) Lithium chloride modulates chondrocyte primary cilia and inhibits hedgehog signaling. FASEB J. 30, 716–726 [DOI] [PubMed] [Google Scholar]
- 56.Forcioli-Conti N., Lacas-Gervais S., Dani C., Peraldi P. (2015) The primary cilium undergoes dynamic size modifications during adipocyte differentiation of human adipose stem cells. Biochem. Biophys. Res. Commun. 458, 117–122 [DOI] [PubMed] [Google Scholar]
- 57.Long F., Schipani E., Asahara H., Kronenberg H., Montminy M. (2001) The CREB family of activators is required for endochondral bone development. Development 128, 541–550 [DOI] [PubMed] [Google Scholar]
- 58.Maeda Y., Schipani E., Densmore M. J., Lanske B. (2010) Partial rescue of postnatal growth plate abnormalities in Ihh mutants by expression of a constitutively active PTH/PTHrP receptor. Bone 46, 472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gulino A., De Smaele E., Ferretti E. (2009) Glucocorticoids and neonatal brain injury: the hedgehog connection. J. Clin. Invest. 119, 243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noguchi K. K., Cabrera O. H., Swiney B. S., Salinas-Contreras P., Smith J. K., Farber N. B. (2015) Hedgehog regulates cerebellar progenitor cell and medulloblastoma apoptosis. Neurobiol. Dis. 83, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sävendahl L. (2012) The effect of acute and chronic stress on growth. Sci. Signal. 5, pt9 [DOI] [PubMed] [Google Scholar]
- 62.Simon D., Alberti C., Alison M., Le Henaff L., Chevenne D., Boizeau P., Canal A., Ollivier G., Decostre V., Jacqz-Aigrain E., Carel J. C., Czernichow P., Hogrel J. Y. (2013) Effects of recombinant human growth hormone for 1 year on body composition and muscle strength in children on long-term steroid therapy: randomized controlled, delayed-start study. J. Clin. Endocrinol. Metab. 98, 2746–2754 [DOI] [PubMed] [Google Scholar]
- 63.Beutler B., Rietschel E. T. (2003) Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3, 169–176 [DOI] [PubMed] [Google Scholar]
- 64.Fernandez-Vojvodich P., Zaman F., Sävendahl L. (2013) Interleukin-6 acts locally on the growth plate to impair bone growth. Ann. Rheum. Dis. 72, e24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.