Abstract
Hematopoietic stem cells (HSCs) have the capacity for self-renewal to maintain the HSCs’ pool and the ability for multilineage differentiation, which are responsible for sustained production of multiple blood lineages. The regulation of HSC development is controlled precisely by complex signal networks and hematopoietic microenvironment, which has been termed the HSCs’ niche. The Wnt signaling pathway is one of a variety of signaling pathways that have been involved in HSC self-renewal and maintenance. Previous studies are indeterminant on the regulation of adult HSCs upon canonical Wnt signaling pathways because of the different experimental systems and models used. In this study, we generated the conditional knockout Wnt coreceptor low-density lipoprotein receptor–related protein 5 (Lrp5) and low-density lipoprotein receptor–related protein 6 (Lrp6) mice in adult hematopoiesis via Vav-Cre Loxp system. Inactivation of Lrp5 and -6 in a hematopoietic system diminished the pool of HSCs, but there were no obvious defects in mature immune cells. Lrp5 and -6 double deficiency HSCs showed intrinsic defects in self-renewal and differentiation due to reduced proliferation and increased quiescence of the cell cycle. Analysis of HSC gene expression suggested that the quiescence regulators were significantly up-regulated, such as Egr1, Cdkn1a, Nr4a1, Gata2, Junb and Btg2, and the positive cell cycle regulators were correspondingly down-regulated, such as Ccna2 and Ranbp1. Taken together, we investigated the roles of Lrp5 and -6 in HSCs by functional and bioinformatic assays, and we demonstrated that Lrp5 and -6 are required for the self-renewal and differentiation of adult HSCs. The canonical Wnt pathway may contribute to maintaining the HSC pool and regulate the differentiation of adult HSCs by controlling cell cycle gene regulatory module.—Liu, J., Cui, Z., Wang, F., Yao, Y., Yu, G., Liu, J., Cao, D., Niu, S., You, M., Sun, Z., Lian, D., Zhao, T., Kang, Y., Zhao, Y., Xue, H.-H., Yu, S. Lrp5 and Lrp6 are required for maintaining self-renewal and differentiation of hematopoietic stem cells.
Keywords: Wnt signaling, reconstitution, development, lineage, cell cycle
Hematopoietic stem cells (HSCs) can renew themselves and differentiate to all types of blood and immune cells (1, 2). The regulation of HSC development and function is precisely controlled by a number of transcription factors and molecules appertaining to different signaling pathways (3). Recent studies have revealed that the Wnt signaling pathway is involved in the regulation of HSC function, but its role has not been thoroughly understood and is somewhat controversial (4).
The Wnt signaling pathway is subdivided into canonical (also known as the Wnt/β-catenin pathway) and noncanonical pathways, which are involved in embryonic development, tissue homeostasis, and severe diseases (5, 6). The binding of Wnt proteins to membrane-associated receptors triggers the activation of the canonical Wnt pathway, which leads to an accumulation of β-catenin in the cytoplasm and eventually translocation into the nucleus to regulate transcription factors TCF/LEF-dependent transcriptional programs (6). Low-density lipoprotein receptor–related protein 5 (Lrp5) and low-density lipoprotein receptor–related protein 6 (Lrp6), which are important coreceptors in the Wnt/β-catenin pathway, belong to the low-density lipoprotein receptor family with a high homology (7). They are type I single transmembrane proteins consisting of an intracellular domain, a transmembrane domain, and a large extracellular domain, which is a Wnt ligands binding site (8). Previous studies have shown that Lrp5 and -6 are not only associated with a variety of bone development–related diseases (9, 10) but also connected with various cancers (11), such as abnormal development of the eyes (12), neurodegenerative diseases (13), congenital heart development diseases (14), and metabolic diseases (15). Recent works have suggested that Lrp5 and -6 play a critical role in the development and differentiation of mammary stem cells (16), indicating Lrp5 and -6 might be essential regulators of stem cells.
Canonical Wnt signaling is well characterized in T-cell development; its roles in the regulation of HSCs also have been depicted by different approaches. Ablation of Wnt3a (17) or overexpression of Wnt inhibitor DKK1 (18) impaired normal HSC functions. Giving its crucial role in Wnt signaling, the function of β-catenin in HSCs has been a profoundly disputatious issue. Because of different experimental systems researchers have adopted, β-catenin has been reported to have beneficial, destructive, or even no effects on HSCs. Conditional deletion of β-catenin by Vav-Cre impairs HSC self-renewal (19); however, inducible ablation of β-catenin (20) or β-catenin and its homologous γ-catenin (21, 22) simultaneously has no effect on hematopoiesis. In contrast, forced expression of β-catenin leads to conflicting results that Wnt signaling positively regulates HSC abilities. Subsequently, Ruiz-Herguido et al. (23) demonstrated deletion of β-catenin in embryonic endothelium compromises long-term HSCs (LT-HSCs) generation in the AGM region and adult hematopoiesis. These results collectively indicated that a delicate balance of canonical Wnt signaling is required to maintain HSC integrity (24). Altogether, there are still many unknowns that need to be revealed because the mechanism of the canonical Wnt pathway on regulating HSC self-renewal and maintenance is complex and only partially understood. In the present study, we generated conditional knockout (KO) Lrp5 and -6 mice to investigate the roles of the canonical Wnt pathway in the hematopoietic system.
MATERIALS AND METHODS
Mice
Lrp5fl/fl and Lrp6fl/fl mice were generously provided by Bart Williams (Van Andel Research Institute, Grand Rapids, MI, USA). The targeting strategy was previously described in Joeng et al. (25), and the genotyping primers are shown in Supplemental Table S1. All of the mice were bred and maintained in the Animal Care Facility of China Agricultural University. All animal experiments were performed according to the legal regulations approved by the China Agricultural University Institutional Animal Care and Use Committee.
Western blot
Cells were solubilized in 1× Protein Loading Buffer (Beyotime Biotechnology, Beijing, China) with protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA). Western blot analysis was performed as previously described in Zhang et al. (26). Membranes were incubated (overnight at 4°C) with the following primary antibodies at the dilutions indicated: anti-Lrp5 (D5G4, 1:1000; Cell Signaling Technology, Danvers, MA, USA); anti-Lrp6 (C47E12, 1:1000; Cell Signaling Technology) and anti-β-Tubulin (1:4000; MilliporeSigma, Burlington, MA, USA).
Cell isolation, staining, and flow cytometry
Single-cell suspensions were prepared from bone marrow (BM), spleen, or thymus and stained with fluorochrome-conjugated antibodies. The following antibodies were used: CD34 (RAM34), CD135 (A2F10), CD48 (HM48-1), CD150 (TC15-12F12.2), Sca1 (D7), c-Kit (2B8), CD45.1 (A20), CD45.2 (104), CD16/32 (93), CD3e (145-2C11), CD4 (RM4-5), CD8a (53-6.7), TCRγδ (GL-3), B220 (RA3-6B2), Gr.1 (RB6-8C5), TER119 (TER-119), Mac1 (M1/70), CD11c (N418), NK1.1 (N418), and APC-eFluor 780–labeled or eFluor 450–labeled Streptavidin. All fluorochrome-conjugated antibodies were from Thermo Fisher Scientific (Waltham, MA, USA) or BD Biosciences (San Jose, CA, USA), except for CD150 from BioLegend (San Diego, CA, USA). Data were collected on an LSR Fortessa and a FACSVerse (BD Biosciences) and analyzed with FlowJo software (BD Biosciences). All cell sorting was done on a FACSAria II (BD Biosciences).
Primary BM cell culture
Lineage− BM cells were isolated by depleting lineage-positive cells from total BM cells using biotinylated antibodies and Dynabeads M280 Streptavidin (Thermo Fisher Scientific). The Lin− BM cells were cultured in Iscove's Modified Dulbecco's Medium with 15% fetal bovine serum (Thermo Fisher Scientific), 50 µM 2-ME (Stemcell Technologies, Vancouver, BC, Canada), 100 µg/ml streptomycin and penicillin (Thermo Fisher Scientific), 50 ng/ml stem cell factor (Peprotech, Rocky Hill, NJ, USA), and 20 ng/ml thrombopoietin (Peprotech) in a 96-well flat-bottom plate at the density of 0.5 × 106/ml with 40 ng/ml rmWnt3a (R&D Systems, Minneapolis, MN, USA) (27).
Colony-forming unit–spleen12 assay
For the colony-forming units (CFU) in the spleen on d 12 (CFU-Spleen12) assay, BM cells were isolated from Lrp5KO, -6KO, double KO (dKO), and wild-type (WT) littermate control. We intravenously injected 1 × 105 BM cells into lethally irradiated recipients. After 12 d, spleens from the recipients were harvested and colonies were counted.
Competitive repopulation assay and serial transplantation assay
For competitive transplantations, 1 × 106 BM cells from WT, Lrp5KO, -6KO, and dKO (CD45.2+) mice were transplanted with the same number of BM cells from B6.SJL (CD45.1+) into each of the lethally irradiated B6.SJL (CD45.1+) recipients. Repopulation capacity was assessed by surface staining CD45.1 and -45.2 8 and 16 wk posttransplantation using fluorescence activated cell sorter (FACS) analysis of peripheral blood. For serial transplantation, 2 × 106 whole BM cells from WT and dKO (CD45.2+) littermates were intravenously injected into lethally irradiated B6.SJL (CD45.1+) recipients at the dose of 8.5 Gray. Hematopoietic reconstitution was monitored by FACS analysis of peripheral blood 8 and 16 wk posttransplantation. For the second to fourth transplantations, total BM cells from the original transplant recipients were transplanted at a dose by 2 × 106 cells per mouse.
Analysis of apoptosis, BrdU incorporation, and cell cycle
Apoptosis assays were performed by staining cells with Annexin V and 7-aminoactinomycin D (7-AAD) (BD Biosciences) following surface staining. For BrdU incorporation analysis, the mice were intraperitoneally injected with BrdU (1 mg/10 g body weight) 12 h before the experiment. Cells were surface stained, fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences), and intracellularly stained with anti-BrdU antibody using the APC BrdU Flow Kit (BD Biosciences). Cell cycle analyses were performed by intracellularly stained with PE-Ki67 or corresponding isotype control together with 20 μg/ml Hoechst 33342 (MilliporeSigma) for 30 min at room temperature.
RNA isolation, reverse transcription, and quantitative RT-PCR
Total RNA was extracted using a modified protocol with the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) and RNeasy Micro Kit (Qiagen) (28). RNA was reverse transcribed using QuantiTech Reverse Transcription Kit (Qiagen) following the manufacturer’s instructions. Relative gene expression levels were analyzed by quantitative RT-PCR (qRT-PCR) with the SYBR Green Master Mix on the Roche LightCycler 480 System (Roche, Basel, Switzerland). Gapdh or Hprt1 was used to normalize the expression of other genes.
RNA sequencing
Total RNA was extracted from CD34−Flt3− Lin− Sca-1+c-Kithi (LSK) cells sorted from dKO mice or their WT littermates and subjected to RNA sequencing. The pair-end fastq reads were assessed for quality using FastQC [Babraham Institute (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/)]; adaptor and low-quality regions were trimmed using Trimmomatic [The Usadel Laboratory, Germany (http://www.usadellab.org/cms/index.php?page=trimmomatic)] (29). Reads were then aligned to the mm9 mouse reference sequence using TopHat2 [The Center for Computational Biology, Johns Hopkins University (http://ccb.jhu.edu/software/tophat)], and read counts were obtained using htseq-count. Differential expression was determined with DESeq2 [Department of Computational Molecular Biology, Germany (http://bioconductor.org/packages/DESeq2/)] (30) using a custom R script. RNA sequencing (RNA-Seq) data were submitted to the Gene Expression Omnibus under accession number GSE122635.
Gene ontology enrichment analysis
Gene ontology term enrichment analysis was performed by the Database for Annotation, Visualization and Integrated Discovery (DAVID)(https://david.ncifcrf.gov) Bioinformatics Resources 6.7 (31) with the default mouse genome as background.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA) was performed with the javaGSEA (http://www.broadinstitute.org/gsea/downloads.jsp) application (v.2.2.4) available online with default settings (32). The gene sets (DNA repair, cell cycle, DNA replication) were acquired from the Gene Set Knowledgebase in Mouse database. The hematopoietic signature gene set was retrieved from Jason C. Tsang (Wellcome Trust Sanger Institute, Hinxton, Cambridge, United Kingdom) (33). Enrichment was considered significant if the false discovery rate is <0.1 and the nominal significance value is P < 0.05. Note that a reported P = 0 indicates an actual P < 1 per number of permutations. In our analysis, the number of permutations was set at 1000, and thus the reported “P = 0” is equivalent to P < 0.001.
Statistical analysis
The Student’s t test was used to analyze the significance between Lrp5KO and WT control, Lrp6KO and WT control, and dKO and WT control. A value of P < 0.05 was considered statistically significant.
RESULTS
Lrp5 and -6 dKO leads to a decrease of the HSCs pool in mice
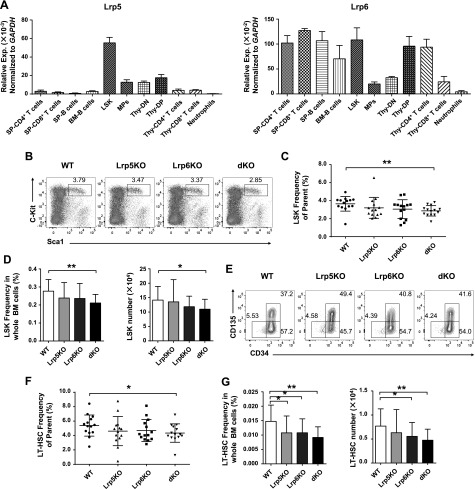
To investigate the functional importance of Lrp5 and -6 in the murine hematopoietic systems, we first detected the relative expression of Lrp5 and -6 in HSCs and progenitor cells and differentiated blood cells by qRT-PCR. We confirmed that Lrp5 and -6 were expressed in LSK cells (Fig. 1A). To explore the roles of Lrp5 and -6 in the hematopoietic system, we crossed mice with loxp-flanked alleles encoding Lrp5 (Lrp5fl/fl) and Lrp6 (Lrp6fl/fl) (25) with mice carrying a Cre recombinase under the control of the Vav1 enhancer and promoter (Vav-Cre). We confirmed Lrp5 and -6 were deleted effectively by both qRT-PCR and Western blotting (Supplemental Fig. S1A–C). We next performed the activation analysis of canonical Wnt pathway in the absence of both Lrp5 and -6. Lin− BM cells were treated with Wnt3a for 6 h, and RNA was then extracted and analyzed. We found the expression level of Axin2 and c-Myc was reduced significantly (Supplemental Fig. S1D) compared with the WT controls, indicating that the canonical Wnt pathway was partially blocked in dKO cells.
Figure 1.
Loss of Lrp5 and -6 impairs HSC pool. A) Relative expression of Lrp5 and -6, respectively, in different cell subsets. Indicated cells were isolated from thymus, spleen and BM from WT C57B6/L and analyzed the relative Lrp5 and -6 expression normalized to Gapdh. Data are from 1 experiment with 3 mice and 2 biologic replicates. B–D) Analysis of LSK cells in lineage-negative BM cells by flow cytometry. BMs were surface stained and the percentages of LSK cells from parent gate (B) are shown in representative pseudocolor plots. Cumulative data (C) of indicated cells are shown. The absolute frequency (D) in total BM cells (left) and numbers (right) of LSK cells are shown. E–G) Analyses of LT-HSC, ST-HSC, and MPP populations in LSK cells based on CD34 and -135 staining. The percentages of LT-HSC, ST-HSC and MPP populations (E) are shown in representative contour plots. Cumulative data (F) of indicated cells are shown. The absolute frequency (G) in total BM cells (left) and numbers (right) of LT-HSCs are shown. Data are means ± sd from 7 independent experiments (n ≥ 14). *P < 0.05, **P < 0.01 by Student’s t test.
We next analyzed whether ablation of Lrp5 and -6 led to defects in the pool of HSCs. We found that the frequency and cell numbers of LSK cells were slightly changed in Lrp5 or -6 single KO mice, but significantly reduced in dKO mice (Fig. 1B–D). LSK cells are heterogeneous and can be subdivided into LT-HSCs, short-term HSCs (ST-HSCs) and multipotent progenitors (MPPs) by the expression level of CD34 and -135. The frequency of LT-HSCs (CD34−CD135− LSK cells) from parent gate is significantly decreased in dKO but slightly reduced in Lrp5KO and -6KO mice (Fig. 1E, F), whereas the percentages of LT-HSCs in total BM cells were remarkably decreased in Lrp5KO, -6KO, and dKO mice, and the absolute numbers of LT-HSCs were statistically decreased in Lrp6KO and dKO mice but not significant in Lrp5KO mice (Fig. 1G). In contrast to decreased LT-HSCs, no obvious differences were detected in common lymphoid progenitors, common myeloid progenitors, granulocyte-macrophage progenitors, and megakaryocyte erythrocyte progenitors among all genotype mice (Supplemental Fig. S2A–D). Furthermore, the mature lineage cells were not affected in Lrp5KO, -6KO, and dKO mice (Supplemental Fig. S3A–H). Thus, these data demonstrated that the loss of both Lrp5 and -6 caused a specific reduction of the pool of HSCs, but no effects on progenitors and mature immune cells.
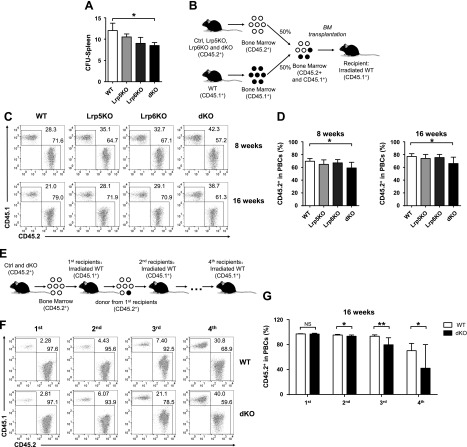
Lrp5 and -6 dKO reduces the self-renewal and repopulation capacities of HSCs
To address the impact of double deficiency of Lrp5 and -6 on HSCs, we performed a series of classic functional experiments in vivo. The splenic CFUs assay showed that colonies derived from dKO HSCs in the recipient spleens were significantly diminished, indicating Lrp5 and -6 ablation impaired HSCs ability of repopulating blood cells (Fig. 2A). We next explored the reconstitution capacity of HSCs by competitive repopulation assay. We transplanted the whole BM cells (CD45.2+) from different genotype mice as donors and competitor (CD45.1+) BM cells at a ratio of 1:1 to the lethally irradiated recipients (CD45.1+) (Fig. 2B). We then determined the contribution of donor BM cells to peripheral blood nucleated cells (PBCs) reconstitution in recipients at short- and long-term (8 and 16 wk) (Fig. 2C, D). We found that a reduced proportion of donor-derived CD45.2+ cells transplanted with dKO donor cells, indicating that Lrp5 and -6 double deficiency impaired the repopulation capacity of HSCs. Based on the results from competitive repopulation assay, there are no significant reconstitution defects from either the Lrp5KO or -6KO donor. Therefore, we performed serial transplantations assay to detect the self-renewal of HSCs by using BM cells from dKO and littermate controls as donors (Fig. 2E). After 16 wk hematopoietic reconstitution, we found that donor BM cells from dKO and control mice contributed similarly to PBCs in the primary recipients but significantly decreased in second, tertiary and quaternary recipients (Fig. 2F, G). Taken together, these results revealed that Lrp5 and -6 double deficiency in HSCs leads to defects on self-renewal and reconstitution capacities.
Figure 2.
Ablating Lrp5 and -6 compromises HSC functions. A) CFU-Spleen12 assays. 1 × 105 BM cells isolated from WT, Lrp5KO, -6KO, and dKO mice were injected into irradiated recipients. After 12 d, spleens from recipients were harvested and colonies were counted. B) Experimental design for competitive repopulation assay. Whole BM cells from test (CD45.2+) and competitor (CD45.1+) were mixed at a 1:1 ratio and transplanted into irradiated recipients. The contributions to PBCs were analyzed at 8 and 16 wk post-transplantation. C, D) Representative pseudocolor plots (C) and cumulative data (D) are shown (n ≥ 4). E) Experimental design for serial transplantation assay. 2 × 106 BM cells from test mice (CD45.2+) were transplanted into irradiated primary recipients (CD45.1+). After 16 wk, BM cells from the primary recipients were transplanted into secondary recipients (CD45.1+), and this process was repeated to obtain quaternary recipients. The contributions to PBCs from original donors were determined at 16 wk after each transplantation. F, G) Representative pseudocolor plots (F) and cumulative data (G) are shown. Data are representative of at least 2 independent experiments (mean ± sd) (n ≥ 7). *P < 0.05, **P < 0.01 by Student’s t test.
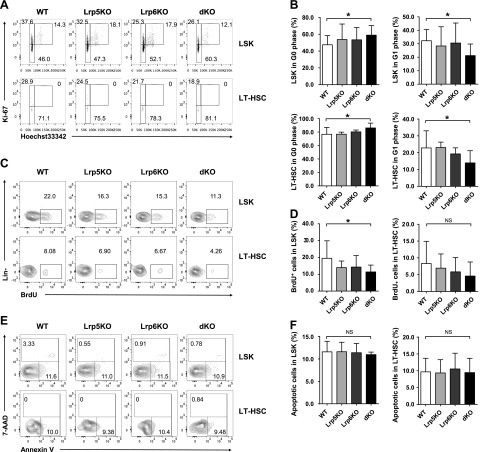
Increased HSC quiescence in Lrp5 and -6 dKO mice
We next focused on the proliferation, apoptosis and cell cycle analysis. The cell cycle of LSK cells and LT-HSCs were analyzed in the BM by intracellular staining of Hoechst33342 and Ki67. The LSK cells and LT-HSCs in dKO mice were significantly increased of G0 phase (Hoechst33342loKi67−), with corresponding reduction of G1 phase (Hoechst33342loKi67+) (Fig. 3A, B), but no obvious changes in either Lrp5KO or -6KO mice. We also performed in vivo BrdU uptake assay to determine the proliferation of HSCs. Consistent with the results of the cell cycle, dKO LSK cells and LT-HSCs also exhibited a pronounced decreased incorporation of BrdU, indicating the increase of quiescence, but no changes in either Lrp5 or -6 single KO cells (Fig. 3C, D). However, the proportion of Annexin V+ 7-AAD− apoptotic cells in LSK cells and LT-HSCs from all genotype mice showed no significant difference from those in control mice, indicating the mutation of Lrp5 and -6 did not alter the survival status of HSCs (Fig. 3E, F). Collectively, these data suggested that Lrp5 and -6 affect the differentiation and self-renewal of HSCs through inhibiting the proliferation and decelerating the cell cycle of HSCs.
Figure 3.
Lrp5 and -6 are essential for maintaining HSC quiescence. A, B) Cell cycle analysis of LSK cells and LT-HSCs. BM cells were surface stained followed by intracellular staining of Ki67 and Hoechst33342. A) The percentages of G0 (HoechstloKi67−), G1 (HoechstloKi67+), and S/G2/M (Hoechstmed-hiKi67+) phase cells in LSK cells (top row) and LT-HSCs (bottom row) are shown in representative dot plots. B) Cumulative data of G0 and G1 status in LSK cells and LT-HSC subsets (n ≥ 6). C, D) BrdU incorporation assay of LSK cells and LT-HSCs. BM cells were surface stained first and intracellularly stained with BrdU. The percentages of BrdU+ cells in LSK cells (top row) and LT-HSCs (bottom row) are shown in representative contour plots (C). Cumulative data (D) of BrdU+ cells in LSK cells and LT-HSC subsets (n ≥ 8). E, F) Analysis of apoptosis in LSK cells and LT-HSCs. BM cells were surface stained and were further stained with 7-AAD and Annexin V. The percentage of apoptotic cells (Annexin V+7-AAD−) and dead cells (Annexin V+7-AAD+) in LSK cells (top row) and LT-HSCs (bottom row) are shown in representative contour plots (E). Cumulative data (F) of apoptotic cells in LSK cells and LT-HSC subsets are shown. Data are representative of 3 (A, B) or 4 (C–F) independent experiments (mean ± sd) (n ≥ 9). NS, not significant. *P < 0.05, by Student’s t test.
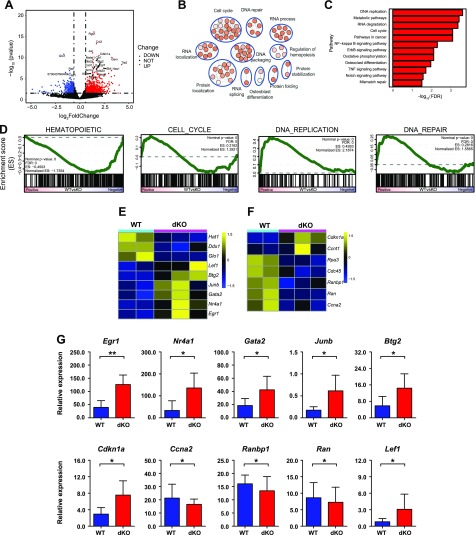
Lrp5 and -6 dKO alters the expression levels of cell cycle genes in HSCs
The above data suggested the critical roles of Lrp5 and -6 in hematopoietic system. To investigate the molecular mechanisms, RNA-seq was performed using HSC cells isolated from WT and dKO mice. We further analyzed the gene expression between normal and Lrp5- and Lrp6-deleted HSC cells. In total, 788 and 503 genes were up-regulated and down-regulated in dKO HSCs (Fig. 4A), respectively. Functional annotation of differential expression genes (Fold >1.5, P < 0.05) using DAVID and the Kyoto Encyclopedia of Genes and Genomes analysis showed significant enrichment in cell cycle, DNA replication, and regulation of hematopoiesis-signaling pathways (Fig. 4B, C). We next performed GSEA using gene sets, which were downloaded from ge-lab’s database (32). Consistently, gene sets related to cell cycle and DNA replication were significantly enriched, as expected. In addition, we also documented significant enrichment of gene sets involved in DNA damage repair. This finding is in accordance with previous reports that quiescent adult HSCs accumulate DNA damage, and this damage is repaired upon entry into the cell cycle. Meanwhile, we analyzed the hematopoietic signature gene sets, which revealed that Lrp5 and -6 might have correlations in maintaining the HSC pool and activities (Fig. 4D).
Figure 4.
Distinct transcriptional profile of Lrp5 and -6 dKO HSCs. A) RNA-Seq was performed on WT and dKO mice HSCs (CD34−Flt3− LSK cells) isolated from BM. A volcano plot of log2(fold change) (dKO-WT) vs. nominal P value (−log10) is shown. Differentially expressed genes (>1.5 fold, P <0.05) are highlighted in light blue (down-regulated) and red (up-regulated) dots. B) Representative gene ontology biologic process categories enrichment map of differentially expressed genes (>1.5 fold, P <0.05). C) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of differentially expressed genes in dKO HSCs compared to WT cells in normal physiologic system and disease. D) GSEA showing significant enrichment of the self-renewal gene signature, cell cycle, DNA repair, DNA replication, and hematopoietic signature [False discovery Rate (FDR) < 0.25]. Note that a reported P = 0 indicates an actual P < 1 per number of permutations. In our analysis, the number of permutations was set at 1000, and thus the reported “P = 0” is equivalent to P < 0.001. E) Heat map showing the differentially expressed genes of hematopoiesis signaling pathways. F) Heat map showing the differentially expressed genes of cell cycle. G) qRT-PCR analysis of selected differentially expressed genes in RNA-seq. Relative gene expression levels were normalized to Gapdh. Data are from 1 experiment with biologic duplicates (A–F) or pooled with 2 experiments with duplicates from at least 5 mice per group (G); mean ± sd. *P < 0.05, **P < 0.01 by Student’s t test.
Based on the above results, we analyzed differentially expressed genes using functional annotation tools in the DAVID bioinformatic resources, which revealed that the differentially regulated genes included transcription factors and genes involved in regulation of cell cycle progression and DNA repair in dKO HSCs. We observed elevated expression of several molecules in the cell cycle pathway, including Egr1 and Cdkn1a. Wnt signaling has been implicated in promoting HSC expansion as well as maintaining HSCs quiescence and self-renewal, and the expressions of a number of molecules in this pathway were changed in dKO HSCs. A number of key difference genes were shown in the heat map (Fig. 4E, F), and we further verified these differentially expressed genes by qRT-PCR (Fig. 4G). We noted that the expression of genes involved in the regulation of HSCs (Egr1, Cdkn1a, Lef1, Nr4a1, Gata2, Junb, and Btg2) was up-regulated, whereas the expression of Ccna2, Ranbp1, and Ran was down-regulated in dKO HSCs. Collectively, these results suggested that Lrp5 and -6 contribute to maintaining HSC quiescence by directly regulating the cell cycle–associated genes.
DISCUSSION
Lrp5 and -6 are highly homologous proteins that play vital roles in the canonical Wnt signaling pathway as coreceptors (34). Mutations in these 2 genes can lead to altered bone metabolism (35), abnormal eye development (36), neurodegenerative disorders (37), and metabolic disorders (15). In this study, we explored the roles of the Lrp5 and -6 in the regulation of HSCs. Our results indicated essential roles of Lrp5 and -6 in maintaining self-renewal and differentiation of HSCs.
We detected that Lrp5 is specifically expressed in LSK cells, and Lrp6 is expressed in several kinds of progenitors and mature immune cells besides LSK cells. Conditional deletion of Lrp5 and -6 by the Vav-cre loxp system in hematopoietic lineage did not affect the development and cell numbers of mature lymphoid and myeloid cells in mice. We found the numbers of dKO LSK cells and LT-HSCs were significantly diminished than that of their littermate WT mice, which indicated that ablation of Lrp5 and -6 together specifically impaired the HSC pool in adult mice. Although Lrp5 is more specifically expressed in LSK cells and Lrp6 is widely expressed in several hematopoietic lineages, the results showed Lrp6 KO had a more profound effect in reduced frequency and numbers of LSK cells and LT-HSCs. However, both Lrp5KO and -6KO mice exhibited much slighter phenotype than dKO mice due to their redundant roles. To analyze the differentiation ability of HSCs, we performed competitive repopulation assay using donors from all genotype mice. The results showed that dKO mice have significant defect during lineage reconstitution, but the slight effect was detected from Lrp5 or -6 single KO mice. Using serial transplantation assay as a golden standard test for long-term self-renewal of HSCs, we found reduced hematopoietic reconstitution ability from dKO donors. The self-renewal and differentiation capacities of dKO HSCs were significantly lower than that of WT HSCs in the recipients of secondary, tertiary, and quaternary transplants. Honestly, we thought the functional defect was not strong enough because the defect started from secondary transplantation, not from the first round. The possible reason may be that Lrp5 and -6 double deficiency did not directly lead to the severe effects on the self-renewal of HSCs. Meanwhile, we cannot exclude that other factors compensate the Lrp5 or -6 function through canonical Wnt singling pathway based on our current experimental system.
To address cell cycle, proliferation, and apoptosis, which are 3 crucial factors for HSC maintenance and expansion, we performed relative experiments using all genotype mice. We found dKO LT-HSCs showed significant increase of G0 phase, with corresponding reduction of that in G1 phase, indicating that Lrp5 and -6 together contribute to maintaining the homeostasis of HSCs. Consistently, dKO LT-HSCs showed decreased BrdU incorporation, indicating a slower cell proliferation in double deficiency HSCs. However, the quiescence of cell cycle and decreased proliferation were not detected in either Lrp5 or -6 single KO mice, revealing redundant functions of these 2 factors in regulating HSC pool maintenance. Using Annexin V analysis, we found the unalterable apoptosis rates of LSK cells and LT-HSCs from either single KO or dKO mice compared with WT controls. These data demonstrated that Lrp5 and -6 did not contribute to the apoptosis regulation in adult HSCs.
High-throughput gene expression profiling of HSCs following genetic ablation of Lrp5 and -6 resulted in alteration of transcriptional programs responsible for cell cycle, regulation of hemostasis, and DNA repair. We confirmed that the expressions of Egr1, Cdkn1a, Nr4a1, Gata2, Junb, and Btg2 (genes related to cell cycle of HSCs) were up-regulated, whereas the expression of Ccna2, Ranbp1, and Ran was down-regulated. Egr1 is a member of the early growth response family and plays essential roles in cell growth, development, and stress responses in many tissues. Previous studies have shown that Egr1 plays an indispensable role in regulating the homeostasis of HSCs (38). The decreased expression of cell cycle–related gene Cdkn1a in Egr1−/− LT-HSCs suggested that Egr1 normally targets the regulation of cell cycle machinery and core components to block HSC proliferation (38). The Cdkn1a protein binds to and inhibits the activity of the cyclin-CDK2, -CDK1, and -CDK4/6 complexes, thereby regulating the cell cycle progression in G1 and S phases (39). Cdkn1a high-expressing cells enter G0 quiescence, whereas Cdkn1a low-expressing cells continue to proliferate (40). Our previous work showed Lef1 directly binds to 5′-regulatory regions of Egr1 to maintain HSC quiescence (41). We also found the expression of Lef1 was significantly increased in dKO HSCs. Nr4a1, Gata2, Junb, and Btg2 as up-regulated genes in dKO mice may also contribute to the decelerated cell cycle of HSCs. Nr4a1 and Nr4a2 are preferentially expressed in HSCs and control HSC quiescence by acting upstream of key HSC transcriptional networks (42). GATA2 is essential for HSC survival (43). Junb inactivation deregulates the cell cycle machinery and increases the LT-HSCs (44). We also observed that the expression of Ccna2, which encodes cyclin A2, that is essential for cell cycle progression and proliferation of HSCs (45, 46) was much lower in dKO HSCs than in WT HSCs. Moreover, dKO HSCs exhibited much lower expression of Ran, which regulates nuclear protein import and cell cycle progression (47). Of particular note, the expression of Ranbp1, which is a partner of Ran and regulates cell cycle progression, was higher in WT HSCs than that in dKO HSCs (48). Collectively, Lrp5 and -6 regulate the self-renewal and maintenance of HSCs by targeting the genes relative to cell cycle and certain key genes of HSCs.
Previous studies have shown that the canonical Wnt signaling pathway plays a critical role in the development and differentiation of HSCs. However, due to the gain- or loss-of-function approaches and different experimental conditions, whether Wnt signaling pathways play a positive or negative regulatory role in HSCs is still controversial. The stabilization and nuclear translocation of β-catenin are important events of the canonical Wnt-β-catenin signaling pathway, but the function of β-catenin in regulating HSCs is in dispute. Reya et al. (49) found forced expression of β-catenin in HSCs of Bcl2 transgenic mice could promote their explosive expansion, indicating β-catenin may positively regulate HSCs pool. Overexpression of β-catenin in hematopoietic cells leads to the defects of HSCs and multilineages differentiation (50, 51). However, induced deletion of β-catenin alone (20) or both its homolog γ-catenin had no obvious defects of HSCs (21, 22). Subsequent study showed the TCF/LEF-dependent transcription still continued in the absence of β- and γ-catenin (21). In contrast, Zhao et al. (19) subsequently used Vav-Cre to ablate β-catenin in fetal liver and found β-catenin–null BM cells showed impaired ability of all blood lineages’ reconstitution. In agreement with the requirement of Wnt signaling pathway for normal HSC expansion and function, the deletion of Wnt3a (17) or overexpression of Wnt inhibitor Dkk1 in osteoblastic stem cell niche (18), both contributing to the blockade of canonical Wnt signaling, led to a deficiency of LT-HSC reconstitution capacity. In accordance with these results, we observed that the deletion of both Lrp5 and -6 in hematopoietic cells partially blocked the canonical Wnt signaling and exhibited a similar effect, with a slight impact on HSCs at the steady state but impairing their repopulation capacity under competitive stress conditions. Our new findings on Lrp5 and -6 deficiency reveal the intrinsic requirements of canonical Wnt signaling pathway in HSCs.
In summary, our study has uncovered a novel functional role of Lrp5 and -6 in self-renewal maintenance and differentiation of HSCs through alteration of molecules responsible for the cell cycle of HSCs. Our findings provide new evidence to support the standpoint that canonical Wnt signaling pathway exerts positive regulation in maintaining the pool of adult HSCs and the ability of LT-HSC self-renewal.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Bart Williams (Center for Cancer and Cell Biology, Van Andel Research Institute, Grand Rapids MI, USA) for providing Lrp5- and Lrp6-floxed mice. The authors also thank Dr. Jinping Song for her assistance with cell sorting at China Astronaut Research and Training Center, Beijing, China. This work is supported by National Key R&D Program of China (2017YFA0104401 to S.Y.) and the National Natural Science Foundation of China (31571522, 31630038, and 31422037 to S.Y.). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
- BM
bone marrow
- CFU
colony-forming unit
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- dKO
double KO
- GSEA
gene set enrichment analysis
- HSC
hematopoietic stem cell
- KO
knockout
- Lrp5
low-density lipoprotein receptor–related protein 5
- Lrp6
low-density lipoprotein receptor–related protein 6
- LSK
Lin− Sca-1+c-Kithi
- LT-HSC
long-term hematopoietic stem cell
- MPP
multipotent progenitor
- PBC
peripheral blood nucleated cell
- qRT-PCR
quantitative RT-PCR
- RNA-seq
RNA sequencing
- ST-HSC
short-term hematopoietic stem cell
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Juanjuan Liu, Z. Cui, and S. Yu performed the major experiments and analyzed the data; Juanjuan Liu, Z. Cui, Y. Yao, and S. Yu wrote the manuscript; F. Wang and M. You analyzed the high throughput data; G. Yu, Jingjing Liu, D. Cao, S. Niu, Z. Sun, D. Lian, T. Zhao, Y. Kang, and Y. Zhao assisted with the overall experiments; and H.-H. Xue and S. Yu designed and supervised the experiments.
REFERENCES
- 1.Huang X., Cho S., Spangrude G. J. (2007) Hematopoietic stem cells: generation and self-renewal. Cell Death Differ. 14, 1851–1859 [DOI] [PubMed] [Google Scholar]
- 2.Song K., Li L., Wang Y., Liu T. (2016) Hematopoietic stem cells: multiparameter regulation. Hum. Cell 29, 53–57 [DOI] [PubMed] [Google Scholar]
- 3.Zon L. I. (2008) Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature 453, 306–313 [DOI] [PubMed] [Google Scholar]
- 4.Blank U., Karlsson G., Karlsson S. (2008) Signaling pathways governing stem-cell fate. Blood 111, 492–503 [DOI] [PubMed] [Google Scholar]
- 5.Wodarz A., Nusse R. (1998) Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88 [DOI] [PubMed] [Google Scholar]
- 6.Staal F. J., Luis T. C., Tiemessen M. M. (2008) WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 8, 581–593 [DOI] [PubMed] [Google Scholar]
- 7.Williams B. O., Insogna K. L. (2009) Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J. Bone Miner. Res. 24, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herz J., Chen Y., Masiulis I., Zhou L. (2009) Expanding functions of lipoprotein receptors. J. Lipid Res. 50 (Suppl), S287–S292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babij P., Zhao W., Small C., Kharode Y., Yaworsky P. J., Bouxsein M. L., Reddy P. S., Bodine P. V., Robinson J. A., Bhat B., Marzolf J., Moran R. A., Bex F. (2003) High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 18, 960–974 [DOI] [PubMed] [Google Scholar]
- 10.Kubota T., Michigami T., Sakaguchi N., Kokubu C., Suzuki A., Namba N., Sakai N., Nakajima S., Imai K., Ozono K. (2008) Lrp6 hypomorphic mutation affects bone mass through bone resorption in mice and impairs interaction with Mesd. J. Bone Miner. Res. 23, 1661–1671 [DOI] [PubMed] [Google Scholar]
- 11.Polakis P. (2007) The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 17, 45–51 [DOI] [PubMed] [Google Scholar]
- 12.De Iongh R. U., Abud H. E., Hime G. R. (2006) WNT/Frizzled signaling in eye development and disease. Front. Biosci. 11, 2442–2464 [DOI] [PubMed] [Google Scholar]
- 13.De Ferrari G. V., Moon R. T. (2006) The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene 25, 7545–7553 [DOI] [PubMed] [Google Scholar]
- 14.Tzahor E. (2007) Wnt/beta-catenin signaling and cardiogenesis: timing does matter. Dev. Cell 13, 10–13 [DOI] [PubMed] [Google Scholar]
- 15.Mani A., Radhakrishnan J., Wang H., Mani A., Mani M. A., Nelson-Williams C., Carew K. S., Mane S., Najmabadi H., Wu D., Lifton R. P. (2007) LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315, 1278–1282; erratum 341, 959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goel S., Chin E. N., Fakhraldeen S. A., Berry S. M., Beebe D. J., Alexander C. M. (2012) Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. J. Biol. Chem. 287, 16454–16466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luis T. C., Weerkamp F., Naber B. A., Baert M. R., de Haas E. F., Nikolic T., Heuvelmans S., De Krijger R. R., van Dongen J. J., Staal F. J. (2009) Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood 113, 546–554 [DOI] [PubMed] [Google Scholar]
- 18.Fleming H. E., Janzen V., Lo Celso C., Guo J., Leahy K. M., Kronenberg H. M., Scadden D. T. (2008) Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2, 274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C., Blum J., Chen A., Kwon H. Y., Jung S. H., Cook J. M., Lagoo A., Reya T. (2007) Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 12, 528–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobas M., Wilson A., Ernst B., Mancini S. J., MacDonald H. R., Kemler R., Radtke F. (2004) Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199, 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeannet G., Scheller M., Scarpellino L., Duboux S., Gardiol N., Back J., Kuttler F., Malanchi I., Birchmeier W., Leutz A., Huelsken J., Held W. (2008) Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood 111, 142–149 [DOI] [PubMed] [Google Scholar]
- 22.Koch U., Wilson A., Cobas M., Kemler R., Macdonald H. R., Radtke F. (2008) Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood 111, 160–164 [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Herguido C., Guiu J., D’Altri T., Inglés-Esteve J., Dzierzak E., Espinosa L., Bigas A. (2012) Hematopoietic stem cell development requires transient Wnt/β-catenin activity. J. Exp. Med. 209, 1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra S., Kincade P. W. (2009) Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell 4, 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joeng K. S., Schumacher C. A., Zylstra-Diegel C. R., Long F., Williams B. O. (2011) Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev. Biol. 359, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L., Cui Z., Zhou L., Kang Y., Li L., Li J., Dai Y., Yu S., Li N. (2016) Developing a triple transgenic cell line for high-efficiency porcine reproductive and respiratory syndrome virus infection. PLoS One 11, e0154238; erratum: e0160325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma F., Ye H., He H. H., Gerrin S. J., Chen S., Tanenbaum B. A., Cai C., Sowalsky A. G., He L., Wang H., Balk S. P., Yuan X. (2016) SOX9 drives WNT pathway activation in prostate cancer. J. Clin. Invest. 126, 1745–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu S., Cui K., Jothi R., Zhao D. M., Jing X., Zhao K., Xue H. H. (2011) GABP controls a critical transcription regulatory module that is essential for maintenance and differentiation of hematopoietic stem/progenitor cells. Blood 117, 2166–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolger A. M., Lohse M., Usadel B. (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love M. I., Huber W., Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang D. W., Sherman B. T., Tan Q., Kir J., Liu D., Bryant D., Guo Y., Stephens R., Baseler M. W., Lane H. C., Lempick R. A. (2007) DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 35, W169–W175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsang J. C., Yu Y., Burke S., Buettner F., Wang C., Kolodziejczyk A. A., Teichmann S. A., Lu L., Liu P. (2015) Single-cell transcriptomic reconstruction reveals cell cycle and multi-lineage differentiation defects in Bcl11a-deficient hematopoietic stem cells. Genome Biol. 16, 178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joiner D. M., Ke J., Zhong Z., Xu H. E., Williams B. O. (2013) LRP5 and LRP6 in development and disease. Trends Endocrinol. Metab. 24, 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher C. A., Joiner D. M., Less K. D., Drewry M. O., Williams B. O. (2016) Characterization of genetically engineered mouse models carrying Col2a1-cre-induced deletions of Lrp5 and/or Lrp6. Bone Res. 4, 15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong Y., Slee R. B., Fukai N., Rawadi G., Roman-Roman S., Reginato A. M., Wang H., Cundy T., Glorieux F. H., Lev D., Zacharin M., Oexle K., Marcelino J., Suwairi W., Heeger S., Sabatakos G., Apte S., Adkins W. N., Allgrove J., Arslan-Kirchner M., Batch J. A., Beighton P., Black G. C., Boles R. G., Boon L. M., Borrone C., Brunner H. G., Carle G. F., Dallapiccola B., De Paepe A., Floege B., Halfhide M. L., Hall B., Hennekam R. C., Hirose T., Jans A., Jüppner H., Kim C. A., Keppler-Noreuil K., Kohlschuetter A., LaCombe D., Lambert M., Lemyre E., Letteboer T., Peltonen L., Ramesar R. S., Romanengo M., Somer H., Steichen-Gersdorf E., Steinmann B., Sullivan B., Superti-Furga A., Swoboda W., van den Boogaard M. J., Van Hul W., Vikkula M., Votruba M., Zabel B., Garcia T., Baron R., Olsen B. R., Warman M. L.; Osteoporosis-Pseudoglioma Syndrome Collaborative Group (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 [DOI] [PubMed] [Google Scholar]
- 37.Liu C. C., Tsai C. W., Deak F., Rogers J., Penuliar M., Sung Y. M., Maher J. N., Fu Y., Li X., Xu H., Estus S., Hoe H. S., Fryer J. D., Kanekiyo T., Bu G. (2014) Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer’s disease. Neuron 84, 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min I. M., Pietramaggiori G., Kim F. S., Passegué E., Stevenson K. E., Wagers A. J. (2008) The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell 2, 380–391 [DOI] [PubMed] [Google Scholar]
- 39.Gartel A. L., Radhakrishnan S. K. (2005) Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 65, 3980–3985 [DOI] [PubMed] [Google Scholar]
- 40.Abbas T., Dutta A. (2009) p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 9, 400–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu S., Li F., Xing S., Zhao T., Peng W., Xue H. H. (2016) Hematopoietic and leukemic stem cells have distinct dependence on Tcf1 and Lef1 transcription factors. J. Biol. Chem. 291, 11148–11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freire P. R., Conneely O. M. (2018) NR4A1 and NR4A3 restrict HSC proliferation via reciprocal regulation of C/EBPα and inflammatory signaling. Blood 131, 1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Pater E., Kaimakis P., Vink C. S., Yokomizo T., Yamada-Inagawa T., van der Linden R., Kartalaei P. S., Camper S. A., Speck N., Dzierzak E. (2013) Gata2 is required for HSC generation and survival. J. Exp. Med. 210, 2843–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santaguida M., Schepers K., King B., Sabnis A. J., Forsberg E. C., Attema J. L., Braun B. S., Passegué E. (2009) JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell 15, 341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanakkanthara A., Jeganathan K. B., Limzerwala J. F., Baker D. J., Hamada M., Nam H. J., van Deursen W. H., Hamada N., Naylor R. M., Becker N. A., Davies B. A., van Ree J. H., Mer G., Shapiro V. S., Maher L. J., III, Katzmann D. J., van Deursen J. M. (2016) Cyclin A2 is an RNA binding protein that controls Mre11 mRNA translation. Science 353, 1549–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalaszczynska I., Geng Y., Iino T., Mizuno S., Choi Y., Kondratiuk I., Silver D. P., Wolgemuth D. J., Akashi K., Sicinski P. (2009) Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell 138, 352–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guarguaglini G., Battistoni A., Pittoggi C., Di Matteo G., Di Fiore B., Lavia P. (1997) Expression of the murine RanBP1 and Htf9-c genes is regulated from a shared bidirectional promoter during cell cycle progression. Biochem. J. 325, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battistoni A., Guarguaglini G., Degrassi F., Pittoggi C., Palena A., Di Matteo G., Pisano C., Cundari E., Lavia P. (1997) Deregulated expression of the RanBP1 gene alters cell cycle progression in murine fibroblasts. J. Cell Sci. 110, 2345–2357 [DOI] [PubMed] [Google Scholar]
- 49.Reya T., Duncan A. W., Ailles L., Domen J., Scherer D. C., Willert K., Hintz L., Nusse R., Weissman I. L. (2003) A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 [DOI] [PubMed] [Google Scholar]
- 50.Kirstetter P., Anderson K., Porse B. T., Jacobsen S. E., Nerlov C. (2006) Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 7, 1048–1056 [DOI] [PubMed] [Google Scholar]
- 51.Scheller M., Huelsken J., Rosenbauer F., Taketo M. M., Birchmeier W., Tenen D. G., Leutz A. (2006) Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat. Immunol. 7, 1037–1047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.