ABSTRACT
Drosophila melanogaster has recently been developed as a simple, in vivo, genetic model of chemotherapy-induced peripheral neuropathy. Flies treated with the chemotherapy agent cisplatin display both a neurodegenerative phenotype and cell death in rapidly dividing follicles, mimicking the cell specific responses seen in humans. Cisplatin induces climbing deficiencies and loss of fertility in a dose dependent manner. Drosophila sensitivity to cisplatin in both cell types is affected by genetic background. We show that mutation or RNAi-based knockdown of genes known to be associated with CIPN incidence in humans affect sensitivity of flies to CIPN. Drosophila is a promising model with which to study the effect of genetics on sensitivity to CIPN.
KEYWORDS: Drosophila, chemotherapy-induced peripheral neuropathy, cisplatin, neurodegeneration, ABC transporter, glutathione
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a critical side effect of platinum-based cancer therapeutics such as cisplatin, affecting approximately 30% of patients treated.1–3 CIPN is an acute concern for cancer patients as the neuropathic pain is often severe enough to cause suspension of chemotherapy treatments.2 CIPN is also a quality of life concern for cancer survivors because it is a life-long side effect that often worsens after chemotherapy is suspended.2,4 Importantly, there are no preventive therapies or treatments for CIPN currently in use and patients are generally limited to use of topical analgesics to manage the associated pain.5,6 In addition, there are few reliable predictive measures to identify those patients most likely to be susceptible to CIPN.1,7,8 In order to treat or prevent CIPN, there is a strong need to understand the underlying mechanisms causing peripheral nerve damage and to identify high risk patients for possible alternative therapy approaches.
Cisplatin causes DNA damage by forming platinum-DNA adducts (Pt-DNA) leading to apoptosis in rapidly dividing cancer cells and dorsal root ganglion (DRG) neurons.9,10 Cisplatin forms adducts with mitochondrial DNA11 leading to mitochondria vacuolization and membrane depolarization.12,13 Cisplatin leads to increased reactive oxygen species (ROS), inducing oxidative stress and cellular damage, including lipid peroxidation and changes to glutathione and catalase activity.14–16 The damage caused by cisplatin can be mitigated by the nucleotide excision repair pathway, which can remove nuclear Pt-DNA adducts,17 and by the glutathione pathway, which can conjugate Pt and remove it from the cell via ABC transporters.18,19
Little is known about what causes peripheral nerve sensitivity in humans. Known risk factors that increase susceptibility to CIPN include existing co-morbidities such as HIV, diabetes, and inherited peripheral neuropathy as well as drug dosage and co-treatments.8 However, a significant percentage of patients with no known co-morbidities develop CIPN.8 Recently, genetic background has become a focus to better understand CIPN susceptibility. Genome-wide association studies and more targeted approaches analyzing specific pathways (for example, glutathione and DNA repair) in patients have identified individual SNPs and genetic variants associated with relatively small changes in susceptibility to CIPN.8,20 Genes identified in these studies include those involved with known CIPN mechanistic pathways glutathione (GPX, ABCC4) and DNA repair (MGMT).8 However, no genetic screen to assess patient risk of CIPN is currently in practice highlighting the need for further advancement in this area.7
To study the effect of genetics on sensitivity to CIPN, we established Drosophila melanogaster as a simple genetic model.21 Drosophila have been used as a model for neurodegenerative disorders for many years22 and show great promise for the study of CIPN. Drosophila treated with cisplatin develop dose-dependent climbing deficiencies in a negative geotaxis climbing assay, and an automated climbing apparatus23 makes this approach amenable to semi-high throughput genetic screening. As this is an in vivo model, the potential effects of genetic changes on other cell types, including rapidly dividing cells in the ovary, can also be easily assessed.21
We demonstrate that Drosophila strains have different sensitivity to cisplatin treatment establishing a role for genetic background in susceptibility to CIPN in the Drosophila model. In addition, we show that genetic background can have a differential effect on the sensitivity of different cell types and that conserved genes in pathways associated with cisplatin toxicity in humans affect cisplatin sensitivity in flies highlighting the translational potential of this model system.
Materials and methods
Fly stocks
All fly stocks were maintained on standard molasses-agar food (Archon Scientific) at 25°C in a 12-hour light/dark cycle. Oregon-R flies were a generous gift from Amy Tang. The following Drosophila stocks were obtained from the Bloomington Drosophila Stock Center: Canton-S, w1118 (FBal0018186), y1w1 (FBst0001495), bw1 (FBal0001342), v1 (FBal0017656), yv; attP2 (FBti0040535), GPX RNAi (FBti0140596), white RNAi (FBti0140096), elavGal4 (FBti0072910), Actin5CGal4 (FBst0004414).
Cisplatin treatment
1 mg/mL cisplatin stock solution (Fresenius Kabi) was diluted in 10% sucrose in DPBS (Life Technologies) and fed to flies in empty plastic vials (40 flies per vial). Flies were fed 125 uL of sucrose/cisplatin solution per day for 3 days and maintained in a 25°C incubator throughout the cisplatin treatment. All flies used for cisplatin treatments and experiments began treatment at four to five days old.
For delivery of cisplatin in solid food, cisplatin stock solution was first diluted in 1X PBS to the specified concentration. 2.0 mL of diluted cisplatin was added to 0.5 g instant food (Carolina Biological Supply Company). Flies were flipped to the instant food/cisplatin vials and maintained at 25°C for 5 days.
Negative geotaxis climbing assay
The negative geotaxis climbing assay was performed in an automated climbing apparatus as described previously.23 After a 3 or 5 day treatment with cisplatin, flies were scored for survival and moved to clean empty vials (30 flies per vial). The climbing vials were loaded into racks holding 10 vials each, and flies were allowed to recover and acclimate to their environment for one hour. The climbing racks were then placed in the climbing apparatus and again allowed to acclimate for 5 minutes. The climbing program was then initiated for a standard climbing assay: 4 successive taps, one picture taken after 15 seconds, 45 seconds of recovery time, and 4 more tapping cycles. Image analysis and quantification of climbing was performed using an automated ImageJ (National Institutes of Health) macro. Each assay was performed a minimum of 3 times.
Fertility assay
Female Drosophila treated with the indicated doses of cisplatin (3 days, in 10% sucrose) were mated to untreated Oregon-R males (3 females and 3 males), allowed to lay eggs on standard food for 3 days and maintained at 25°C. Progeny per female were counted 12 days later. Assay was performed a minimum of 3 times for each genotype and treatment.
Fecundity assay
Female Drosophila were treated with cisplatin and mated to untreated Oregon-R males as above in the fertility assays. Flies were placed on grape agar plates with a dab of wet yeast paste and allowed to lay eggs for 48 hours. Grape agar plates were replaced every 24 hours. The number of laid eggs on each plate was counted immediately after the plate was replaced, and the number of hatched eggs was determined 48 hours later by counting cuticles left behind. All assays were performed at 25°C and were done in triplicate.
Platinum quantification
Measurement of platinum was performed as previously described.21 Flies were treated with the indicated doses of cisplatin in 10% sucrose as above and were then frozen at −80°C in 1.5 mL Eppendorf tubes. Total DNA was extracted using the tissue extraction protocol for the Wizard Genomic DNA Purification Kit (Promega), diluted to 6 M HCl and analyzed via Inductively Coupled Plasma Mass Spectrometry (ICP-MS) in the Mayo Clinic Metals Laboratory. Analysis was done in duplicate.
Results
Drosophila wild-type strains have different sensitivity to cisplatin
We established Drosophila as a model for CIPN primarily utilizing one common wild-type strain, Oregon-R.21 However, Drosophila mutants and transgenic flies are produced using a variety of genetic background strains, and many of these strains are known to exhibit different behaviors or altered sensitivity to neurodegeneration.24 In addition, because DRG from multiple rodent strains have different sensitivity to cisplatin,25 it was necessary to understand the effect of genetic background on Drosophila sensitivity to cisplatin. We first compared three Drosophila strains commonly used as wild-type or background controls: Oregon-R, Canton-S, and w1118. We treated adult flies (four to five days old) with 0–500 μg/mL cisplatin in solid instant food for 5 days. The treated flies were then subjected to a negative geotaxis climbing assay to assess neurologic damage. We found that the wild-type strains respond differently to cisplatin. Canton-S flies were less sensitive than Oregon-R flies in the climbing assay. We also found that w1118 flies were significantly more sensitive to cisplatin than either of the wild-type strains. This result was consistent whether the cisplatin was delivered via solid instant food (Figure 1a, Table 1) or 10% sucrose (data not shown).
Figure 1.
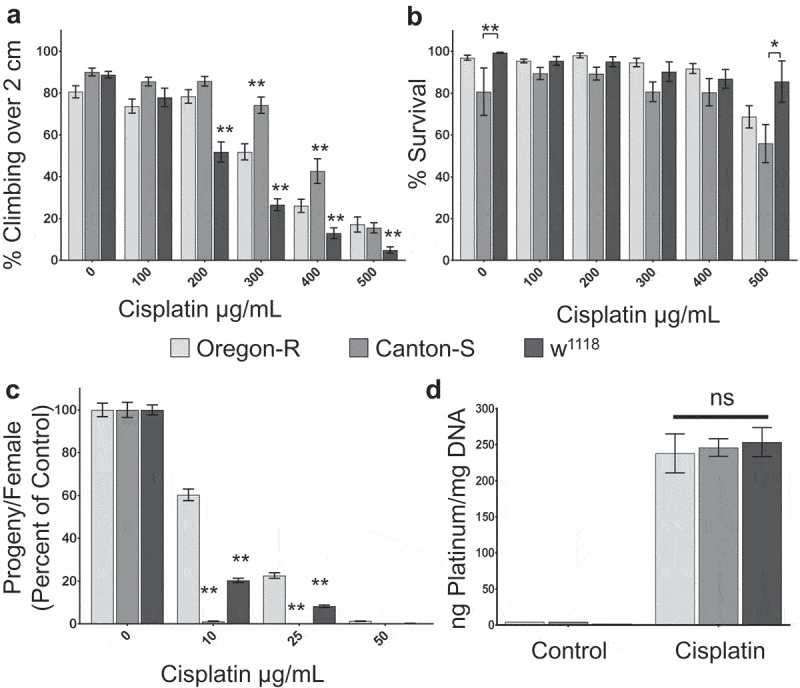
Wild-type strains have different sensitivity to cisplatin. a. Graph displaying the average percent of flies able to climb over a height of 2 cm in an automated climbing assay. b. Graph showing the percent survival of the flies tested in a. c. Graph quantifying fertility of female flies, displayed as the number of progeny per female, normalized to untreated controls for each genotype. d. Graph of the average ng of platinum per mg of total DNA isolated from flies of the indicated genotypes treated with control media or 75 ug/mL cisplatin. Wild-type strains have different sensitivity to dose-dependent climbing deficiencies (a), lethality (b), and fertility defects (c), despite acquiring the same levels of Pt-DNA adducts (d). *p < .05, **p < .01.
Table 1.
Climbing assay cisplatin IC50 values.
| Strain | IC50 |
|---|---|
| OregonR | 346.6 ± 10.59 |
| CantonS | 394.9 ± 8.39 |
| w1118 | 223.7 ± 9.99 |
| yw | 230 ± 15.05 |
| bw | 317.3 ± 30.19 |
| v | 510.7 ± 41.58 |
Cisplatin causes dose-dependent lethality in Drosophila. We examined whether wild-type strains displayed any difference in cisplatin lethality. The difference in climbing ability was not reflected in fly survival. We observed no significant difference in survival of the cisplatin treatment when the drug was delivered in solid food, though w1118 flies trended towards the strongest survival and Canton-S trended lower (Figure 1b). However, when cisplatin was delivered in 10% sucrose, Canton-S flies experienced higher lethality than Oregon-R flies at lower doses of cisplatin, while w1118 flies survived the treatment at higher levels than either of the wild-type strains (data not shown). These results suggest that genetic background may affect sensitivity to cisplatin differently in different cell types.
To follow up on this interesting observation in Drosophila background strains, we wanted to determine whether differential sensitivity to cisplatin extended to a rapidly dividing cell type. We therefore analyzed the sensitivity of female ovaries to cisplatin using a fertility assay. We found that Canton-S and w1118 flies were more sensitive to cisplatin than Oregon-R flies in the fertility assay (Figure 1c). Canton-S females produced fewer progeny per female in control untreated flies, but even very low dose cisplatin (10 μg/mL) caused sterility in Canton-S females while 50 μg/mL cisplatin was required to cause sterility in Oregon-R females. The differences in female fertility were reflected in both the number of eggs laid per female and the percent of eggs hatched (Supplemental Table 1). The differential results observed in the climbing, fertility, and survival assays demonstrate that Drosophila may be useful as a model to determine whether genetic variation affects the sensitivity of certain cell types to cisplatin more than others.
Platinum leads to apoptosis by causing DNA damage in the form of Pt-DNA adducts in both nuclear and mitochondrial DNA. We expected strains that were more sensitive to cisplatin in the climbing assay to have increased Pt-DNA as an indication of increased DNA damage. In order to determine whether the differences in sensitivity to cisplatin in wild-type strains correlate with the amount of Pt-DNA adducts, we quantified the amount of platinum in total DNA isolated from cisplatin-treated flies. We found no significant difference in Pt-DNA adducts between Oregon-R, Canton-S, and w1118 flies (Figure 1d). Therefore, the sensitivity of Drosophila neurons to cisplatin, as measured through negative geotaxis, does not correlate with total Pt-DNA levels.
Eye color mutants are highly sensitive to cisplatin
Transgenic Drosophila are commonly made in white mutant backgrounds, and transgenes often re-insert a mini-white gene as an easy phenotypic marker. Therefore, we followed up on the important observation than w1118 flies appear to be more sensitive to cisplatin than other wild-type controls using a second white mutant strain. We found that compared to wild-type Oregon-R controls, white mutants (w1118 and y1w1) were highly sensitive to cisplatin in our climbing assay (Figure 2a, Table 1) but showed no difference in survival (w1118) or were only slightly more sensitive (y1w1) (Figure 2b). Knockdown of white via RNAi also sensitized flies to cisplatin in the climbing assay (Figure 2c), but had no effect on survival (Figure 2d). Knockdown of white via RNAi had a relatively mild effect on fly climbing compared to genetic mutation, likely because the Gal4 line necessary for expression of the RNAi also contains its own mini-white gene and the knockdown achieved is only partial.
Figure 2.
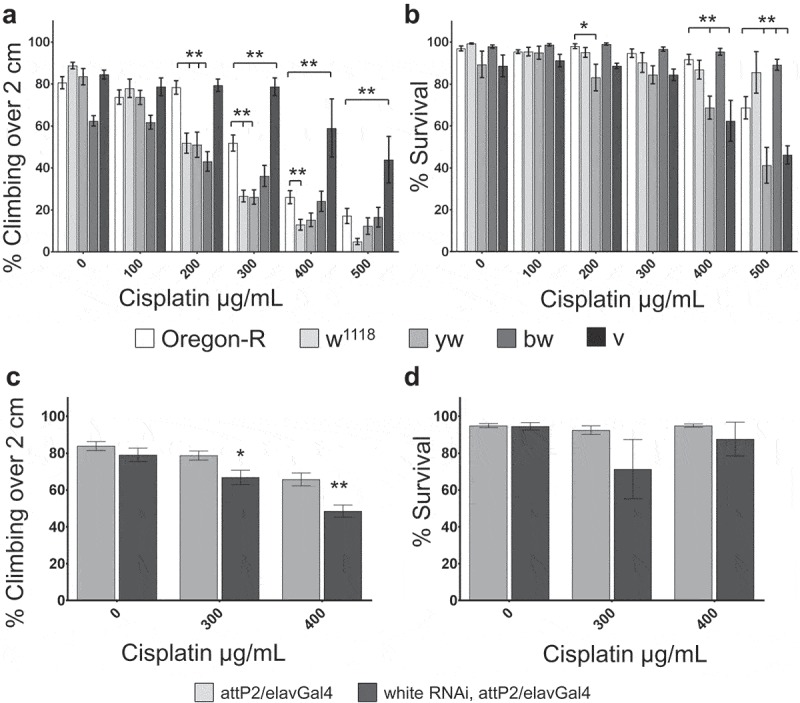
Eye color mutants have increased sensitivity to cisplatin. a,c. Climbing assay results for flies of the indicated genotypes and cisplatin treatments. b,d. Graph of the percent survival for flies analyzed in the climbing assay in panels a and c, respectively. White mutant flies (w1118 and y1w1) and brown (bw1) mutant flies are more sensitive to cisplatin-induced climbing deficiencies, while vermillion (v1) mutant flies are less sensitive to cisplatin-induced climbing deficiencies. Knockdown of white via RNAi also causes increased sensitivity to cisplatin-induced climbing deficiencies (c). *p < .05, **p < .01.
White is an ABC class transporter26,27 associated with export of glutathione-platinum conjugates and multi-drug resistance transporters.28,29 This class of transporters has been linked to cisplatin sensitivity in the context of both efficacy of cancer treatments and CIPN.8 Understanding the effect of these mutants is important not only for study of Drosophila genetic backgrounds, but also for the translational potential of this model. We further analyzed the effects of ABC transporters on sensitivity to cisplatin in our model. Another Drosophila eye color gene, brown, is an ABC transporter. Mutations in brown also caused increased sensitivity to cisplatin in a climbing assay (Figure 2a, Table 1). As a control, we also tested the sensitivity of vermilion mutants (v1) to cisplatin. Vermilion is an eye color gene involved in pigment metabolism, and is not an ABC transporter. We observed that vermilion mutants did not have increased sensitivity to cisplatin, and responded similarly to Canton-S flies in both the climbing assay (less sensitive than Oregon-R, Figure 2a, Table 1) and in survival (more sensitive than Oregon-R, Figure 2b). These data demonstrate that ABC transporters affect sensitivity of Drosophila neurons to cisplatin similar to effects seen in humans.
Knockdown of glutathione genes increases sensitivity to cisplatin
Our long-term goal is to utilize the Drosophila model of CIPN is to uncover conserved genes which affect sensitivity to cisplatin. Our results with ABC transporter mutants white and brown suggest that there are indeed conserved genes that affect cisplatin sensitivity in both Drosophila and humans. We developed an RNAi-based approach as a means to screen for conserved genes affecting cisplatin sensitivity. To control for genetic background, we chose the TRiP RNAi collection.30,31 In order to determine whether this approach was viable, we assessed the effect of glutathione peroxidase (PHGPx) knockdown. PHGPx is known to be associated with human patient sensitivity to CIPN and known mechanisms of cellular clearance of cisplatin. We knocked down PHGPx specifically in neurons using a TRiP RNAi line crossed to the neuron-specific elavGal4 driver. PHGPx knockdown in neurons caused increased sensitivity to cisplatin in a climbing assay compared to genetic background control (attP2/elavGal4, Figure 3a) but did not affect survival compared to controls (Figure 3b). We knocked down PHGPx globally (using Actin5CGal4) in order to assess its effect on ovary sensitivity to cisplatin. Reduced PHGPx caused increased sensitivity to cisplatin in a fertility assay compared to controls (Figure 3c). Interestingly, PHGPx RNAi females laid as many eggs as attP2 controls, but the percent hatching of those eggs is significantly reduced with increasing cisplatin dose (Supplemental Table 2). These experiments demonstrate that RNAi-based knockdown of conserved genes affect sensitivity to cisplatin supporting the translational potential of this model.
Figure 3.
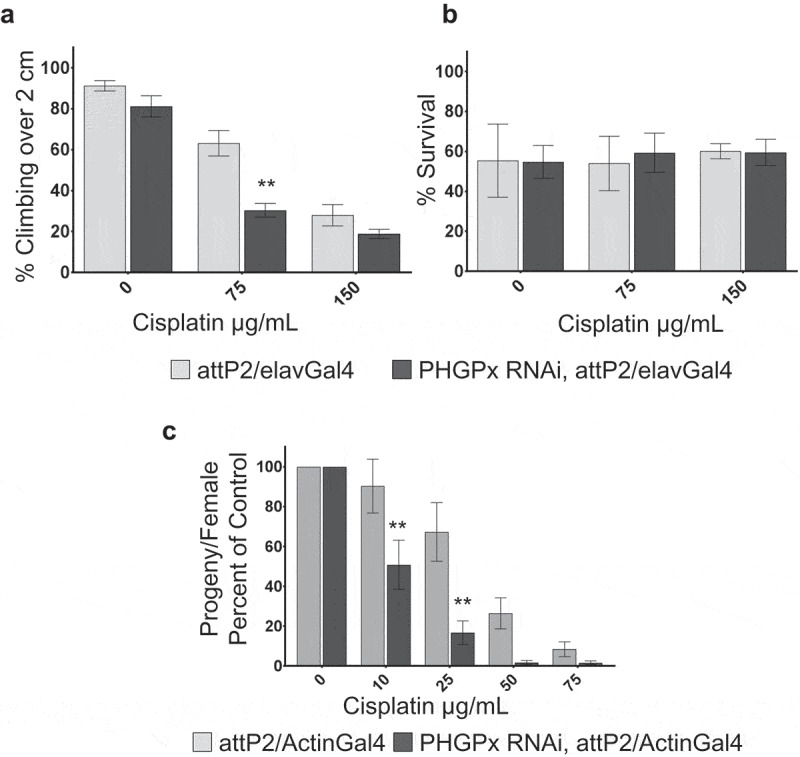
Knockdown of glutathione peroxidase affects cisplatin sensitivity. a. Climbing assay results for flies of the indicated genotypes and cisplatin treatments. b. Graph of the percent survival for flies analyzed in the climbing assay in panel a. c. Graph displaying fertility of female flies of the indicated genotypes and cisplatin treatments. Fertility is displayed the number of progeny per female, normalized to untreated controls for each genotype. Knockdown of glutathione peroxidase via RNAi causes increased sensitivity to cisplatin-induced climbing deficiencies (a) and fertility defects (b). **p < .01.
Discussion
We have shown that Drosophila melanogaster is an excellent genetic model system for uncovering the genetic underpinnings of sensitivity to chemotherapy-induced peripheral neuropathy. Negative geotaxis climbing assays have long been used in flies to assess locomotor function linked to neurodegenerative phenotypes.32 Drosophila exhibit a dose-dependent response to cisplatin in a negative geotaxis climbing assay. Different wild-type strains of Drosophila have differing sensitivity to cisplatin in our assays. These results are similar to previous observations in rodent models of CIPN25 and add to the growing literature that suggests a genetic component to CIPN susceptibility.8,33
The results reported highlight a strength of Drosophila as an in vivo model of CIPN: the ability to specifically identify drug effects on neural cells compared with other cells. By studying different wild-type strains we were able to determine the cisplatin sensitivity of neurons compared to rapidly dividing ovarian cells and overall lethality. These results demonstrate that genetic background differences can have opposite effects in different cell types. One of the major roadblocks in CIPN prevention and treatment is the concern that interventions may reduce drug efficacy in cancer cells.7 Our model has the potential to address this issue in a simple assay before attempting lengthy and expensive vertebrate animal studies.
The accumulation of Pt-DNA adducts is one of the primary mechanisms by which cisplatin causes cell death. We have shown that three Drosophila strains accumulate similar levels of Pt-DNA adducts despite differences in sensitivity to cisplatin as measured by three different assays. This indicates that the amount of platinum bound to total DNA is not indicative of the level of cell death caused by the cisplatin treatment and suggests that other mechanisms affect sensitivity to cisplatin. Cisplatin is known to cause damage to the mitochondria, and to increase cellular ROS. Either of these mechanisms may be more critical to understanding the specific sensitivity of neurons to cisplatin damage.
White and brown are ABC transporters and their closest homologs, ABCG2 and ABCG1 respectively, are linked to glutathione-Pt transport or altered sensitivity to platinum drugs.28,34 While the ABC transporters most commonly linked to CIPN are the ABCC family of transporters,8 ABCG transporters have been connected to cisplatin efficacy and multi-drug resistance.34 Our results in Drosophila suggest that ABC transporters affect the sensitivity of neurons and rapidly dividing cells to cisplatin treatment and show that conserved Drosophila genes affect sensitivity to cisplatin similarly in flies and humans. This finding highlights the potential of the fruit fly as a model to find conserved genes that are relevant to human sensitivity to CIPN.
The glutathione pathway has long been linked to platinum drugs as a mechanism of cellular resistance. Specific glutathione S transferases conjugate glutathione to platinum, and these conjugates are exported from the cell via ABC transporters. SNPs in glutathione pathway genes (GPX, Gst) are associated with increased patient susceptibility to CIPN.8 We have shown that knockdown of a key glutathione pathway gene, glutathione peroxidase, affects Drosophila sensitivity to CIPN. This work demonstrates that genes uncovered in Drosophila are often conserved in humans. The availability of large numbers of mutant strains facilitates detailed genetic manipulation and dissection of mechanistic cellular pathways.
The data we have shown here demonstrates that Drosophila is an excellent genetic model system for understanding sensitivity to cisplatin-induced peripheral neuropathy. It highlights the critical importance of background control selection, especially when using a white mutant background and/or transgenic lines that include a mini-white insert. In conclusion, we have shown the unique translational potential of Drosophila as a model of CIPN that can be used to assess sensitivity in multiple cell types relevant to clinical implications of cisplatin treatment.
Funding Statement
This work was supported by the Mayo Clinic Center for Regenerative Medicine and the Bowen Foundation;Mayo Clinic [Center for Regenerative Medicine Bowen Foundation].
Acknowledgments
Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
We would like to thank the Mayo Metals Laboratory for their work quantifying platinum-DNA levels. We also thank Jane Meyer for administrative assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M.. Incidence, prevalence, and predictors of chemotherapy induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Shah A, Hoffman EM, Mauermann ML, Loprinzi CL, Windebank AJ, Klein CJ, Staff NP. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatr. 2018;89(6):636–641. doi: 10.1136/jnnp-2017-317215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–666. doi: 10.1038/nrneurol.2010.160. [DOI] [PubMed] [Google Scholar]
- 5.Piccolo J, Kolesar JM. Prevention and treatment of chemotherapy-induced peripheral neuropathy. Am J Health Syst Pharm. 2014;71(1):19–25. doi: 10.2146/ajhp130126. [DOI] [PubMed] [Google Scholar]
- 6.Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2014;(3):CD005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017. doi: 10.1002/ana.v81.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson C, Pankratz VS, Velazquez AI, Aakre JA, Loprinzi CL, Staff NP, Windebank AJ, Yang P. Candidate pathway-based genetic association study of platinum and platinum–taxane related toxicity in a cohort of primary lung cancer patients. J Neurol Sci. 2015;349(1–2):124–128. doi: 10.1016/j.jns.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer SJ, McDonald ES, Gross L, Windebank AJ. Alterations in cell cycle regulation underlie cisplatin induced apoptosis of dorsal root ganglion neurons in vivo. Neurobiol Dis. 2001;8(6):1027–1035. doi: 10.1006/nbdi.2001.0426. [DOI] [PubMed] [Google Scholar]
- 10.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18(2):305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41(3):661–668. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, Taroni F, Lauria G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008;214(2):276–284. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Podratz JL, Lee H, Knorr P, Koehler S, Forsythe S, Lambrecht K, Arias S, Schmidt K, Steinhoff G, Yudintsev G, et al. Cisplatin induces mitochondrial deficits in Drosophila larval segmental nerve. Neurobiol Dis. 2017;97(Pt A):60–69. doi: 10.1016/j.nbd.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3(4):294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 16.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Guo C, Thompson EL, Jiang Y, Kelley MR, Vasko MR, Lee S-H. APE1, the DNA base excision repair protein, regulates the removal of platinum adducts in sensory neuronal cultures by NER. Mutat Res. 2015;779:96–104. doi: 10.1016/j.mrfmmm.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312–318. [PubMed] [Google Scholar]
- 19.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travis LB, Fossa SD, Sesso HD, Caberto CP, Kocarnik JM, Han Y, Love S-A, Young A, Dumitrescu L, Lin Y, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst. 2014;106(5). doi: 10.1093/jnci/dju061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podratz JL, Staff NP, Froemel D, Wallner A, Wabnig F, Bieber AJ, Tang A, Windebank AJ. Drosophila melanogaster: a new model to study cisplatin-induced neurotoxicity. Neurobiol Dis. 2011;43(2):330–337. doi: 10.1016/j.nbd.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirth F. Drosophila melanogaster in the study of human neurodegeneration. CNS & Neurol Disord Drug Targets. 2010;9:504–523. doi: 10.2174/187152710791556104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podratz JL, Staff NP, Boesche JB, Giorno NJ, Hainy ME, Herring SA, Klennert MT, Milaster C, Nowakowski SE, Krug RG, et al. An automated climbing apparatus to measure chemotherapy-induced neurotoxicity in Drosophila melanogaster. Fly. 2013;7(3):187–192. doi: 10.4161/fly.24789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambegaokar SS, Jackson GR. Interaction between eye pigment genes and tau-induced neurodegeneration in Drosophila melanogaster. Genetics. 2010;186(1):435–442. doi: 10.1534/genetics.110.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Podratz JL, Kulkarni A, Pleticha J, Kanwar R, Beutler AS, Staff NP, Windebank AJ. Neurotoxicity to DRG neurons varies between rodent strains treated with cisplatin and bortezomib. J Neurol Sci. 2016;362:131–135. doi: 10.1016/j.jns.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreesen TD, Johnson DH, Henikoff S. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol Cell Biol. 1988;8:5206–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borycz J, Borycz JA, Kubow A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol. 2008;211(Pt 21):3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 29.Brechbuhl HM, Gould N, Kachadourian R, Riekhof WR, Voelker DR, Day BJ. Glutathione transport is a unique function of the ATP-binding cassette protein ABCG2. J Biol Chem. 2010;285(22):16582–16587. doi: 10.1074/jbc.M109.090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu L-P, Villalta C, Booker M, Perkins L, Perrimon N. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5(1):49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni JQ, Zhou R, Czech B, Liu L-P, Holderbaum L, Yang-Zhou D, Shim H-S, Tao R, Handler D, Karpowicz P, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011;8(5):405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 33.Cavaletti G, Alberti P, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity in the era of pharmacogenomics. Lancet Oncol. 2011;12:1151–1161. doi: 10.1016/S1470-2045(11)70131-0. [DOI] [PubMed] [Google Scholar]
- 34.Moyer AM, Sun Z, Batzler AJ, Li L, Schaid DJ, Yang P, Weinshilboum RM. Glutathione pathway genetic polymorphisms and lung cancer survival after platinum-based chemotherapy. Cancer Epidemiol Biomarkers Prev. 2010;19(3):811–821. doi: 10.1158/1055-9965.EPI-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


