ABSTRACT
Background: Delayed vaccinations at 2, 4, and 6 months are associated with a higher probability of delayed age-appropriate vaccination during childhood. This study aimed to assess the effectiveness of an information session on immunization during infancy.
Methods: An individual educational information session with motivational interview techniques for immunization of infants was conducted (experimental group) or not conducted (control group) during postpartum stay in a quasi-experimental cohort study. Immunization data were collected from the Eastern Townships Public Health registry at 3, 5, 7, 13, 19, and 24 months of age. Logistic regressions with repeated measures were performed to assess the intervention’s impact. Relative risks (RR) were estimated. A multivariate model was obtained adjusted for confounding factors.
Results: The experimental and control groups included 1140 and 1249 families, respectively. In per protocol analysis, a significant increase in VC of 3.2, 4.9, 7.3, 6.7, 10.6, and 5.1% was observed at 3, 5, 7, 13, 19, and 24 months. Children from experimental group had 9% more chance at a complete vaccination status between 3 and 24 months compared to children from control group (RR (95% CI): 1.09 (1.05-1.13), p < .001). Children with complete vaccination status at 3 months were more likely to have a complete vaccination status at 24 months (82.3 vs. 48.1%, RR (95% CI): 2.72 (2.28-3.24), p < .001). After adjustment, the estimated RR of the intervention’s impact was 1.05 (1.02-1.07), p < .001.
Conclusions: An educational information session about immunization based on motivational interview techniques conducted during postpartum hospitalization could improve immunization during infancy.
KEYWORDS: Motivational interviewing, vaccine coverage, infants, health promotion intervention, maternity wards
Introduction
Vaccination is considered one of the greatest public health achievements of the 20th century.1 One of the objectives of the Quebec Public Health Program is reaching and maintaining high VC rates (95%) during infancy.2 However, this objective has not been met so far. In fact, the VC in Quebec remains suboptimal.3 In 2008, in the Quebec region of Eastern Townships, VC was 83% at 3 months of age and decreased to 62% at 24 months.4 Confirmed by the most recent childhood National Immunization Coverage Survey, vaccination uptake by vaccine type at two years in 2014 varied from 71% to 85%.5 Among the suggested course of action, one of them was using or developing tools that provide proper vaccination information to support parents and healthcare providers. At the same time, a Quebecers plan to promote vaccination has been elaborated.6 Two of the main objectives of this plan were to foster (1) compliance with the vaccination calendar particularly for the infants; and (2) positive attitudes toward immunization in the community. To this end, an effective intervention to increase community demand for vaccination was necessary. Furthermore, several studies showed that the observed delays in the first vaccination at 2, 4, and 6 months of life are associated with a higher probability of incomplete vaccination status during infancy.7-13 Therefore, an effective and early intervention to promote the first vaccination is required.
However, among the few studies that addressed parental vaccine hesitancy and refusal, no effective strategies were suggested;14,15 particularly, no studies found the effectiveness of education-only interventions for improving VC.16 Moreover, more parents are ambivalent about the effectiveness and safety of vaccines; several studies showed that one-third of parents are vaccine-hesitant.17-22 With the increasing proportion of vaccine-hesitant parents, developing effective vaccination promotion strategies is important.
In this context, we developed a vaccination promotion intervention (15–20 minutes per session) for newborn parents at postpartum stay in the maternity ward. As traditional educational methods have often failed,14-16 we used motivational interview (MI) techniques in our educational session. Described as a promising tool for health promotion strategy,23 MI is a patient-centered communication style used to enhance patient’s internal motivation to change by exploring and addressing their ambivalences.24 Originally developed in the context of substance abuse, MI was also used for behavior change in several health-related fields such as nutrition, physical activity, and smoking cessation.25-27 This effective approach is used for ambivalent and hesitant clients.28
This new vaccination promotion strategy, called “PromoVac,” was implemented and tested through a pre-experimental study at the Centre Hospitalier Universitaire de Sherbrooke (CHUS).29,30 This strategy has especially revealed that this intervention positively influenced the determinants of vaccination leading to a global increase of 15% in mother’s vaccination intention.29 Also, these results were confirmed by a significant overall VC increase for the 2-, 4-, and 6-month vaccinations.30
The short-term impact of this new strategy is very important, particularly in the context of suboptimal VC and delays in the first vaccination at 2, 4, and 6 months of life. This led to the following question: Does the intervention’s impact on coverage rate persist in time and subsequent doses in infancy? Therefore, this study essentially aimed to evaluate the longer-term effects of the tested intervention on infant’s VC at 13, 19, and 24 months of age.
Results
Patients’ characteristics
During the study period, 1225 mothers were not approached; children of these mothers were assigned to the control group, with a total of 1249 children (twin births included) (Figure 1). Among the approached mothers, 1128 mothers agreed to participate and received the tested intervention; children of these mothers were assigned to the experimental group (1140 children). Finally, two other groups were also considered. First, children whose mothers refused to participate in the study were assigned to the “primary refusal group” (167 children). Second, children whose mothers agreed to participate but refused to receive intervention or those not available to receive it due to fatigue or breastfeeding issues were assigned to the “secondary refusal or impossible intervention group” (203 children). These 203 children were included in the experimental group for the ITT analysis. Thus, 1,140 and 1,343 newborns of the experimental group were included in PP and ITT analyses, respectively.
Figure 1.
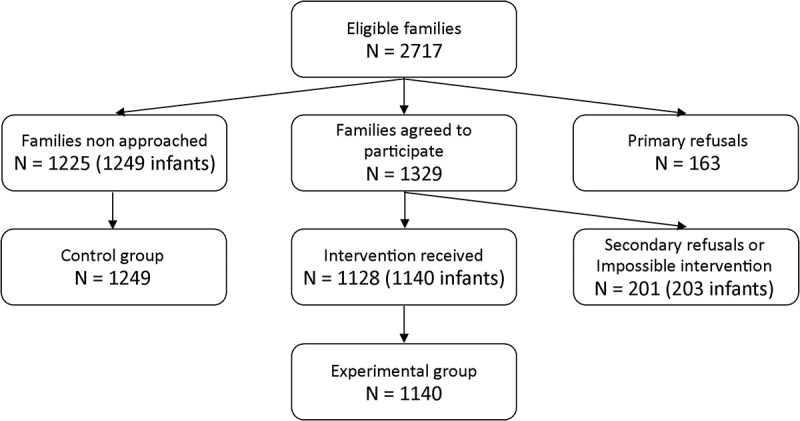
Flowchart of inclusions
There was no statistically significant difference in the mothers’ ages and the length of postpartum hospitalization between the experimental and control groups in the PP analyses (Table 1). However, statistically significant differences were observed for the following variables: “At least one another child in the family,” “Cesarean birth”, and “Newborn hospitalized in the neonatology ward during postpartum stay”. Similar results were obtained by ITT analyses, except for “Cesarean birth,” for which non-significant differences were observed. Primary and secondary refusal groups had similar characteristics compared to the control group (data not shown).
Table 1.
Characteristics of mothers.
| Experimental group n = 1140 |
Control group n = 1249 |
p | |
|---|---|---|---|
| Mother’s age (Mean ± S.D.) | 28,4 ± 5 | 28,5 ± 5 | .593 |
| Postpartum hospitalization length (h) (Méd.(EIQ)) | 48 (48–72) | 48 (48–72) | .328 |
| Ceasarean delivery (n (%)) | 187/1138 (16,4) | 267/1248 (21,4) | .002 |
| Neonatology hospitalization (n (%)) | 51/1140 (4,5) | 172/1249 (13,8) | .001 |
| More than one child (n (%)) | 553/1140 (48,5) | 524/1249 (42,0) | .001 |
Numbers in parenthesis shows percentages in each group
Long-term effect on VC
VC of children from experimental and control groups during infancy in PP analysis are presented in Figure 2. VC increased significantly at 13, 19, and 24 months of life in PP (6.7%, 10.6%, and 5.1%, respectively) and in ITT (5.6%, 8.6%, and 4.6 %) in the experimental group compared to control group (Table 2). When considering the complete period of vaccination (i.e., 0–2 years), the chance of having a complete vaccination status during infancy was higher in children from experimental group in PP (RR (95% CI) = 1.09 (1.05–1.13), p < .001) and ITT (RR (95% CI) = 1.08 (1.04–1.11), p < .001). After excluding twins, the effectiveness on the intervention for single infants was identical in PP (RR(95%CI) = 1.09 (1.06–1.13), p < 0.001) and ITT (RR(95%CI) = 1.08 (1.04–1.11), p < 0.001).
Figure 2.
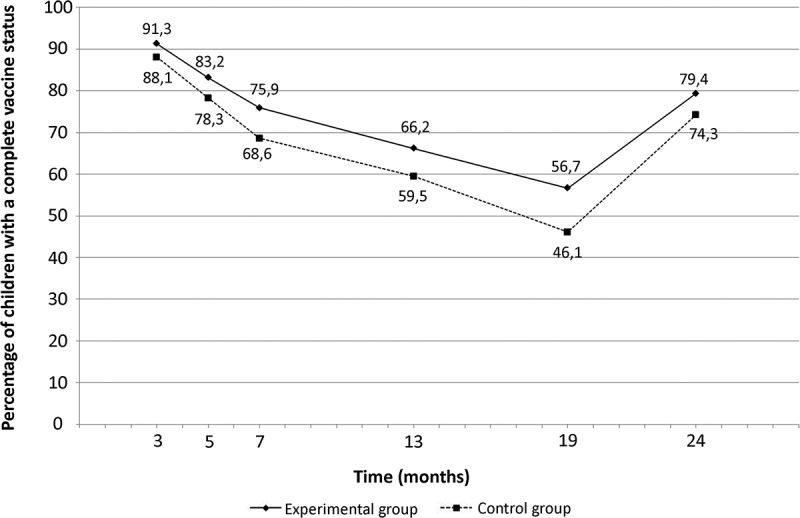
Percentage of children with a complete vaccine status during infancy (per protocol analysis)
Table 2.
Comparison of proportions of children aged from 3 to 24 months with a complete vaccine status (PP and ITT analysis).
| PP analysis |
ITT analysis |
||||||
|---|---|---|---|---|---|---|---|
| Vaccine coverage (VC) | Control Group (n = 1249) | Experimental group (n = 1140) | Absolute differences of VC (%) | Relative Risk (95% CI) |
Experimental group (n = 1343) | Absolute differences of VC (%) | Relative Risk (95% CI) |
| 13 months | 743 (59.5) | 755 (66.2) | +6.7 | 1.10 (1.04–1.17)*** | 874 (65,1) | 5,6 | 1,09 (1,03–1,16)** |
| 19 months | 576 (46.1) | 646 (56.7) | +10.6 | 1.23 (1.14–1.33)*** | 735 (54,7) | 8,6 | 1,19 (1,01–1,28)*** |
| 24 months | 928 (74.3) | 905 (79.4) | +5.1 | 1.07 (1.02–1.12)** | 1060 (78,9) | 4,6 | 1,06 (1,02–1,11)** |
Numbers in parenthesis shows percentages in each group * p < .05, ** p < .01, ***p < .001
Impact of complete vaccination status at 3 months on vaccination status at 24 months of life
There was a statistically significant association between complete vaccination status at 3 and 24 months of life when infants had a complete vaccination status at 3 months, wherein 82.3% had complete vaccination status at 24 months compared to 48.1% without complete vaccination status (Table 3). The estimated relative risk for an infant with complete vaccination status at 3 months of having complete vaccination status at 24 months during infancy was RR (95% CI) = 2.72 (2.20–3.37), p < .001.
Table 3.
Intervention adjusted impact on vaccine status between 3 and 24 months of age.
| Univariate analyses |
multivariate PP analyses |
multivariate ITT analyses |
|
|---|---|---|---|
| Variables | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Adjusted RR (95% CI) |
| Intervention PP | 1.09 (1.05–1.13)*** | 1.05 (1.02–1.07)*** | – |
| Intervention ITT | 1.08 (1.04–1.11)*** | – | 1.04 (1.01–1.06)** |
| Complete vaccine status at 3 month of life | 2.72 (2.20–3.37)*** | 6.81 (5.58–8.30)*** | 6.76 (5.59–8.17)*** |
| Time (month) | 0.99 (0.99–0.99)*** | 0.99 (0.99–0.99)*** | 0.99 (0.99–0.99)*** |
| Mother’s age | 1.00 (1.00–1.00) | 1.00 (1.00–1.01)** | 1.00 (1.00–1.01)* |
| More than one child | 0.90 (0.88–0.93)*** | 0.91 (0.88–0.93)*** | 0.91 (0.88–0.93)*** |
| Ceasarean | 0.97 (0.93–1.01) | 0.99 (0.96–1.02) | 0.99 (0.96–1.02) |
| Neonatology | 0.90 (0.83–0.95)*** | 0.96 (0.92–1.01) | 0.96 (0.92–1.01) |
*p < .05, ** p < .01, ***p < .001
Adjusted impact of intervention on vaccination status during infancy (between 3 and 24 months of life)
After adjusting for immunization status at 3 months, time, mother’s age, number of children, cesarean delivery, and hospitalization in neonatology, the experimental group still had a significant higher chance of having complete vaccination status during infancy (between 3 and 24 months of age) in PP (RR (95% CI) = 1.05 (1.02–1.07), p < .001) and ITT (1.04 (1.01–1.06), p < .01) (Table 3). There was also a strong significant association between complete vaccination status at 3 months and vaccination status during the rest of infancy (RR (95% CI) = 6.81 (5.58–8.30), p < .001) in PP and 6.76 (5.59–8.17), p < .001) in ITT). There was no significant association of cesarean delivery and neonatology stay with vaccination status during infancy.
Discussion
This study aimed to test whether the impact of the tested intervention of the PromoVac study on the VC persists through time. This study revealed that the observed impact on VC at 3, 5, and 7 months of life persists until 24 months. GEE’s findings allow us to conclude that 9% and 7% of the children whose parents received the intervention were more likely to have complete vaccination status throughout the infancy (0–2 yrs) in PP and in ITT analyses, respectively. The latter finding is important because it allows us to conclude that this intervention is effective even in real life situation. Sensitive analysis (excluded twins) showed identical results from the PP and ITT analyses, which demonstrate the robust effectiveness of the intervention for single infants.
The PromoVac study was the first to demonstrate that parental education intervention using MI techniques improves immunization status at 3, 5, and 7 months of life.29,30 The present study is the first to show that such promotion strategies improve immunization status during whole infancy. So far, systematic reviews of interventions to improve VC in children, adolescents, and adults concluded that available studies provide insufficient evidence to assess the effectiveness of educational interventions regarding improving knowledge or attitudes toward vaccinations or improving vaccination provision14,35 Another systematic review suggested that face-to-face interventions to inform or educate parents about childhood vaccination have little impact on immunization status or knowledge or understanding of vaccination.36 In fact, traditional educational methods have not been shown to be effective in addressing vaccine hesitancy.37 Some studies have even shown that trying to convince vaccine-hesitant parents to vaccinate their child by giving them more information could backfire and make them even more hesitant.38 On the contrary, high level of acceptability and effectiveness of the PromoVac strategy could be related to the use of MI techniques. First, the use of the MI approach calls for a respectful and empathetic discussion of vaccination and helps build a strong relationship between parents and the healthcare practitioner. Parents can freely discuss their concerns and ask questions about vaccination without the feeling of being judged. The intervention is adapted to parents’ needs and is based on their own concerns and questions. Healthcare practitioners can, therefore, avoid providing unnecessary or unwanted information. Using MI techniques, healthcare professionals can help parents explore their ambivalence and recognize their arguments for change in order to make an informed decision about their child’s vaccination. Using the MI strategy, healthcare practitioners can identify and target parental concerns or misconceptions about vaccination and provide tailored information. When faced with unsolicited information on a complex issue, people can interpret the information in a way that supports their initial position or beliefs; instead of stimulating people to question their initial position, it is, in fact, reinforced.38-40 The MI approach ensures that the information provided by healthcare practitioners is tailored to parents’ specific concerns and is transmitted in a way that helps them resolve their ambivalence about vaccination. Moreover, in a study about the decisional process in vaccination, Paulussen et al. showed that most parents did not actively process information about the benefits and drawbacks before deciding whether to have their child vaccinated.41 This might indicate that the positive attitude towards vaccination and high vaccination intention expressed by some parents are not very stable and are, therefore, susceptible to counterarguments. By eliciting and exploring parents’ reasons for vaccination, the MI approach enhances personal motivation to vaccinate via a more robust decisional process.
In conclusion, MI was described as a promising tool for strategies for the promotion of health42 and vaccination against Hepatitis A and B among patients undergoing methadone maintenance.43 In the field of vaccination, only two studies used MI techniques to promote immunization among adults.42,43 Although their results were promising, they were not significant, mostly because of the small sample size43 and the specific targeted population (adults undergoing methadone maintenance treatment.42
In addition, those results are interesting because this early intervention addressed first vaccination at 2, 4, and 6 months of life. As was shown in numerous studies, first vaccinations realized on time were associated with better vaccination status during infancy,7-13 and this should be targeted by immunization promotion strategies. Indeed, according to our results, children with complete vaccination status at 3 months were almost 3 times more likely to have complete vaccination status at 24 months of age. Moreover, multivariate analyses showed that the intervention is an independent factor which explains the increase in VC. In previous survey of VC in Quebec, the complete vaccination status at 3 months was the main predictor of a complete vaccination status at 24 months.5 We demonstrated that our intervention increase vaccine status at 3 months and by this way indirectly increase vaccination status at 24 months. The impact of the intervention on both VC at 3 and 24 months are probably linked but multivariate analysis indicated that the intervention have an independent effect on VC at 24 months. In conclusion, that strategy had an impact on VC at 3 months that indirectly increase VC at 24 months and also a direct impact but it will be difficult to quantify exactly the respective part of this effect.
The main limitation of this study is that participants were not recruited randomly, as mothers were approached according to delivery chronology, and therefore, conclusions should be made carefully. However, no statistically significant difference was observed between VC of children of families that did not receive the intervention (control group, primary refusal, and secondary refusal groups), suggesting the absence of selection bias. Furthermore, no significant difference was observed between the vaccination site (nurses or medical centers) of the children of the experimental and control groups.
The control group had also a higher proportion of mothers whose newborn was not the first child. This difference between the two groups may have had an impact on the results of this study because having more than one child is an independent factor explaining low VC in infancy. Thus, it probably minimized the effect of the educational session. Further, the immunization registry does not contain vaccination data of children who move out of the region after birth. Thus, VC calculated in this study could be underestimated. However, this bias was non differential as relocations could have occurred both in the experimental and the control groups.
Conclusions
Finally, our results indicated that our 20-minute intervention based on MI techniques administered during postpartum seems to be an encouraging tool to address suboptimal VC during infancy. These results should be considered when choosing effective strategies aimed at promoting infant immunization. A multisite randomized controlled trial is currently being conducted in the Province of Quebec to confirm these results. A good communication strategy involves understanding people, establishing a respectful partnership, and helping them change their behavior according to their capacities. Only with a better understanding of the underlying causes of vaccine hesitancy among hesitant parents can effective tailored information be delivered. This is what MI offers.
Patients and methods
The methods section adheres to the Transparent Reporting of Evaluations with non-randomized designs (TREND) statements checklist guidelines.31
Participants
This study is an extension of the quasi-experimental cohort study, the PromoVac study. Detailed PromoVac design, eligibility criteria, and recruitment methods have been previously published.29,30 Briefly, during a one-year period, eligible mothers (aged 18 or over, speaking French or English, and living in the Eastern Townships region) who gave birth at the CHUS and the respective newborn infants (twins included) were included in the study. Mothers or newborns requiring acute care were excluded from the study. Births occurring at the CHUS represent 95% of the total births in the region.
Mothers were screened during their postpartum stay in the maternity ward, over regular business hours (8AM to 5PM), in chronological order of delivery. In practical terms, this meant that mothers who had delivered first and who had not been approached by the research team were screened first. This approach was adopted in order to optimize recruitment given the short duration of postpartum maternity ward stays (mean duration = 48 hours). Mothers who agreed to participate provided written informed consent prior to their participation, as per applicable law. All children whose mothers received the study intervention were assigned to the experimental group, while the static control group included children of mothers who were not approached to participate in the study. Two other groups were considered in this study: the primary refusals group, with children of approached mothers who refused to participate in the study; and the secondary refusal or impossible intervention group comprising children of mothers who agreed to participate but withdrew their consent before receiving the intervention because of fatigue or breastfeeding issues.
The longer-term effects of the tested intervention on VC were evaluated by the static group comparison study with repeated measures. The study population comprised the children of mothers who received the PromoVac intervention (experimental group) and those who did not (control group).29,30
Intervention
The educational session was delivered to mothers during their postpartum stay (24-48h after delivery) at the maternity ward by research nurses trained in MI theory and techniques. This intervention (approximately 15 to 20 minutes) is carried out in simple and understandable language in order to allow discussion and questions from parents rather than providing prescriptive and direct information. The MI intervention is oriented according to Prochaska’s stages of change,32 a model proposing that people go through several stages when wanting to change a behavior. Thus, each MI intervention was adapted to parents’ readiness to vaccinate their child. Overall, this procedure aimed to administer a standardized intervention, adapted to each mother according to her current stage of change regarding vaccination intention. This approach aimed to help each woman progress through the later stages of change at her own pace, ultimately enabling her to self-mobilize toward vaccination on her own. Using MI techniques, five points were discussed during this session1) summary of the six VPDs at 2, 4, and 6 months of life; 2) vaccines administered at 2, 4, and 6 months and their effectiveness; 3) importance of the routine immunization schedule at 2, 4, and 6 months; 4) fears and side effects related to vaccination; and 5) organization of local vaccination services in the Eastern Townships. During the study period, the Quebec routine immunization schedule recommended vaccines at 2, 4 and 6 months to protect against diphtheria, tetanus, poliomyelitis, whooping cough, infections from hemophilus influenza B and pneumococcus.33
Objectives
We hypothesized that an individualized educational information session regarding immunization and given during postpartum hospitalization would improve short-term but also the entire 0 to 2 years period. The aim of the study was to assess the impact of this novel educational strategy based on MI techniques, on the VC of infants at 13, 19, and 24 months of age.
Outcomes and vaccination data source
The main outcome measures were the VC of infants at 13, 19, and 24 months of age. Indicators of VC at 13, 19 and 24 months were chosen according to indicators evaluated in the provincial survey of VC in Quebec.5 To evaluate the VC of infants, vaccination data were obtained from LOGIVAC, the immunization registry of the Eastern Townships region. This exhaustive registry contains all births that have occurred in the region and records all vaccines administered to residents of the Eastern Townships since 1998, including data for those born outside the region. Thus, all children born in the region, regardless of their vaccination status, are included in the LOGIVAC registry. Vaccination data were extracted by the Eastern Townships Public Health Department for all the participant infants (experimental group), children of mothers who were not approached (control group), and for children of mothers who refused the intervention (primary and secondary refusal groups). Because we had access to nominal data for all the mothers who gave birth at the CHUS, the extraction of vaccination data of infants for all eligible mothers in the study was possible. The extraction of nominal vaccination data and data pairing with the study data was performed by a research agent of the Eastern Townships Public Health Authority, who was not involved with our study and was blinded to the assignation groups of the study.
Variables
VC was considered the dependent variable. Before computing the study group’s VC, immunization status was determined at 13, 19, and 24 months for each study subject. A child was considered having a complete vaccination status if he/she received all vaccines or antigens recommended in 2010 by Quebec Immunization protocol.33 This one-month delay to assess VC corresponds to the national standards established by the Canadian Immunization Registry Network.34
Assignment method and sample size
Once the immunization status was determined for all children, the main outcome measures (VC at 13, 19, and 24 months) were computed for each study group as the proportion of children with a complete vaccination status among the total number of children in each group. The independent variables, such as mother’s age, length of postpartum hospitalization, cesarean birth, infant’s rank in the family, and hospitalization of the newborn in the neonatology ward during the postpartum stay, were used to assess the comparability of groups and to control for potential confounding factors. In order to identify a statistically significant amelioration of 5% in the VC of infants, and taking into account a VC of 80%6, a risk of alpha error of 0.05 and a power of 80%, a total of 943 mothers per group should be recruited accordingly with the 3000 annual births at the maternity ward of the CHUS.
Data analysis
First, normally distributed continuous variables are presented using means and standard deviations; abnormally distributed variables are presented with median and interquartile range. Student t-test and U Mann-Whitney test were used to compare the characteristics of the experimental and control groups according to data distribution. For categorical variables, they are presented with frequencies and percentages.
Thereafter, VC at 13, 19, and 24 months of life in the experimental and control groups were compared using Pearson’s chi-square test. An estimate of relative risk (RR) and 95% confidence interval (CI) were calculated for each VC. These analyses were carried out in “per protocol” (PP) but also in “intention-to-treat” (ITT). To detect the presence of means of monitoring related to the vaccination site, and in particular, how the patients are monitored in each of these places, subjects’ vaccination sites in both groups were compared using Pearson’s chi-square test. In addition, VC of the population that did not receive the intervention (control group, primary, and secondary denial) were compared using the same test to verify the absence of selection bias. Univariate logistic regressions with repeated measures were also performed according to the generalized estimating equations (GEE) procedure with Poisson distribution to estimate the chance for a child to have a complete vaccination status during early childhood (i.e., from 0 to 2 years), conducted in PP and ITT analyses. Univariate logistic regression was also performed to evaluate the impact of the complete vaccination status at 3 months of life on the vaccination status at 24 months of age. Finally, multivariate GEE models with repeated measures with Poisson distribution were used in PP and ITT analyses to estimate the chance of a child having complete immunization status at 24 months depending on whether or not parents have received the intervention, adjusting for immunization status at three months, time, mother’s age, the number of children, cesarean delivery, and hospitalization in neonatology. Sensitive analysis excluding twins was also conducted to estimate the robust of the results for single infants.
Analyses were performed with IBM SPSS version 22.0 (Armonk, NY) and SAS version 9.3 (Cary, NC). A value of p < 0.05 was considered significant.
Ethical considerations
This study was approved by the Institution ethics board, and authorization was obtained from the Commision d’accès à l’information du Québec to collect data through Logivac Registry.
Funding Statement
This study was funded with the public health subsidy program of the Eastern Townships public health department. The authors have no financial relationships relevant to this article to disclose.
Abbreviations
- CHUS
Centre hospitalier universitaire de Sherbrooke
- CI
confidence intervals
- GEE
generalized estimating equations
- ITT
intention-to-treat
- MI
motivational interview
- PP
per-protocol
- RR
relative risk
- VC
vaccine coverage
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed.
Acknowledgments
The authors would like to thank all mothers who took part in this study during their post-partum stay. Then, we would like to thank all the nurses as well as health professionals of the CHUS maternity ward. We also would like to thank Pierrot Richard and Arianne Grégoire from the Eastern Townships public health department for their precious assistance in the collect of vaccination data.
Contributor’s Statements
Thomas Lemaitre acquired, analyzed and interpreted the data, drafted the article, and approved the final version of the manuscript to be submitted.
Nathalie Carrier performed data analysis and data interpretation, and reviewed and edited the final manuscript.
Anne Farrands participated in the mothers’ recruitment, performed MI sessions, collected data, and reviewed and edited the final manuscript.
Virginie Gosselin performed data interpretation, and reviewed and edited the final manuscript.
Geneviève Petit participated in the conception and design of the study, performed data interpretation, and reviewed and edited the final manuscript.
Arnaud Gagneur developed the strategy, designed the study, performed data analysis and data interpretation, wrote, reviewed and edited the final manuscript.
References
- 1.Centers for Disease Control and Prevention Ten great public health achievements: United States, 1900-1999. Morb Mortal Wkly Rep. 1999;48(12):241–243. [PubMed] [Google Scholar]
- 2.Ministère de la Santé et des Services Sociaux Programme national de santé publique 2003-2012. ministère de la santé et des services sociaux, Québec; 2008. p. 103. [Google Scholar]
- 3.Boulianne N, Audet D, Ouakki M, Guay M, Duval B, De Serres G.. Enquête sur la couverture vaccinale des enfants québécois en 2006. Québec: Institut national de santé publique du Québec; 2007. p. 104 p. [Google Scholar]
- 4.Guay M, Gallagher F, Petit G, Ménard S, Clement P, Boyer G. Pourquoi les couvertures vaccinales chez les nourrissons de l’Estrie sont-elles sous optimales? de Sherbrooke: CSSS-IUG, Québec, Canada; 2009. p. 77 p. [Google Scholar]
- 5.Boulianne N, Audet D, Ouakki M, Dubé È, De Serres G, Duay M. 2015. Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2014. Institut national de santé publique du Quebec, Canada. [Google Scholar]
- 6.Dubé E, Sauvageau C, Boulianne N, Guay M, Petit G. Plan québécois de promotion de la vaccination. Institut national de santé publique du Québec; 2010. p. 78 p. [Google Scholar]
- 7.Dietz VJ, Stevenson J, Zell ER, Cochi S, Hadler S, Eddins D. Potential impact on vaccination coverage levels by administering vaccines simultaneously and reducing dropout rates. Arch Pediatr Adolesc Med. 1994;148(9):943–949. doi: 10.1001/archpedi.1994.02170090057008. [DOI] [PubMed] [Google Scholar]
- 8.Valiquette L, Allard R, Guay M. Enquête sur la couverture vaccinale des enfants de 24 à 36 mois de Montréal Centre. Montréal: Direction de la santé publique, Québec, Canada; 1998. p. 1–53. [Google Scholar]
- 9.Boulianne N, Deceuninck G, Duval B. Pourquoi certains enfants sont incomplètement vaccinés à l’âge de 2 ans? Revue canadienne de santé publique. 2003;94(3):218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strine TW, Luman ET, Okoro CA, McCauley MM, Barker LE. Predictors of age-appropriate receipt of DTaP dose 4. Am J Prev Med. 2003;25(1):45–49. [DOI] [PubMed] [Google Scholar]
- 11.Dombkowski KJ, Lantz PM, Freed GL. Risk factors for delay in age-appropriate vaccination. Public Health Rep. 2004;119(2):144–155. doi: 10.1177/003335490411900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dannetun E, Tegnell A, Hermansson G, Törner A, Giesecke J. Timeliness of MMR vaccination–influence on vaccination coverage. Vaccine. 2004;22(31–32):4228–4232. doi: 10.1016/j.vaccine.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States: days undervaccinated and number of vaccines delayed. JAMA. 2005;293(10):1204–1211. doi: 10.1001/jama.293.10.1204. [DOI] [PubMed] [Google Scholar]
- 14.Sadaf A, Richards JL, Glanz J, Salmon DA, Omer SB. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:.4293–4304. doi: 10.1016/j.vaccine.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Dubé E, Gagnon D, MacDonald NE. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:.4191–4203. doi: 10.1016/j.vaccine.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernier RR, Carande-Kulis VG, Yusuf HR, Ndiaye SM, Williams SM. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The task force on community preventive services. Am J Prev Med. 2000;18:97–140. doi: 10.1016/S0749-3797(99)00118-X. [DOI] [PubMed] [Google Scholar]
- 17.Ritvo P, Wilson K, Willms D, Upshur R, Goldman A, Kelvin D, Rosenthal KL, Rinfret A, Kaul R, Krahn M. Vaccines in the public eye. Nat Med. 2005;11:.S20–4. doi: 10.1038/nm1220. [DOI] [PubMed] [Google Scholar]
- 18.Lagarde F. Summary of Public Opinion on Immunization in Canada. Ottawa: Public Health Agency of Canada; 2005. Ontario, Canada p. 19. [Google Scholar]
- 19.Sauvageau C, Duval B, Gilca V, Lavoie F, Ouakki M. Human papilloma virus vaccine and cervical cancer screening acceptability among adults in Quebec, Canada. BMC Public Health. 2007;7:.304. doi: 10.1186/1471-2458-7-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A, Yarwood J, Salisbury DM. Tracking mothers’ attitudes to MMR immunisation 1996-2006. Vaccine. 2007;25:.3996–4002. doi: 10.1016/j.vaccine.2007.02.071. [DOI] [PubMed] [Google Scholar]
- 21.Zimet GD, Liddon N, Rosenthal SL, Lazcano-Ponce E, Allen B. Chapter 24: psychosocial aspects of vaccine acceptability. Vaccine. 2006;24(Suppl 3):S3/201–9. doi: 10.1016/j.vaccine.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Fabry P, Gagneur A, Pasquier J-C. Determinants of A (H1N1) vaccination: cross-sectional study in a population of pregnant women in Quebec. Vaccine. 2011;29:.1824–1829. doi: 10.1016/j.vaccine.2010.12.109. [DOI] [PubMed] [Google Scholar]
- 23.Appiah-Brempong E, Okyere P, Owusu-Addo E, Cross R. Motivational interviewing interventions and alcohol abuse among college students: a systematic review. Am J Health Promot. 2014;29:.e32–42. doi: 10.4278/ajhp.130502-LIT-222. [DOI] [PubMed] [Google Scholar]
- 24.Rollnick S, Miller WR, Butler CC. 2008. Motivation interviewing in health care: helping patients change behavior. New York, NY: The Guilford Press. [Google Scholar]
- 25.Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:.91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 26.Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 55;2005:305–312. [PMC free article] [PubMed] [Google Scholar]
- 27.Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:.147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 28.Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd New York, NY: The Guilford Press 2013. [Google Scholar]
- 29.Gagneur A, Petit G, Valiquette L, De Wals P. An innovative promotion of vaccination in maternity ward can improve childhood vaccination coverage. Report of the PromoVac study in the Eastern townships. [in French] Library and National Archives of Canada; 2013. ISBN: 978-2-9813830-0-6 (print version), 978-2-9813830-1-3 (pdf version), Sherbrooke. p. 112. [Google Scholar]
- 30.Gagneur A, Lemaître T, Gosselin V, Farrands A, Carrier N, Petit G, Valiquette L, De Wals P. A postpartum vaccination promotion intervention using motivational interviewing techniques improves short-term vaccine coverage: PromoVac study. BMC Public Health. 2018;18(1):811. doi: 10.1186/s12889-018-5724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Des Jarlais DC, Lyles C, Crepaz N,and TREND Group . Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. [DOI] [PubMed] [Google Scholar]
- 33.Ministère de la Santé et des Services sociaux Protocole d’immunisation du Québec. Ministère de la Santé et des Services sociaux, Québec; 2014. p. 485 p. [Google Scholar]
- 34.Boulianne N, Hemon YA, Mawhinney T, Strong D, Gemmill I, Dobson S, Sartison E, Sargent M, Naus M, Tuchscherer R, et al. National eligible, due, and overdue guidelines for immunization registries: draft recommendations from the Canadian immunization registry network, data standards task group. Can Commun Dis Rep. 2004;30:53–59. [PubMed] [Google Scholar]
- 35.Briss PA, Zaza S, Pappaioanou M, Fielding J, Wright-De Agüero L, Truman BI, Hopkins DP, Dolan Mullen P, Thompson RS, Woolf SH, et al. Developing an evidence-based guide to community preventive services–methods. The Task Force on Community Preventive Services Am J Prev Med. 2000;18(1 Suppl):35–43. doi: 10.1016/S0749-3797(99)00119-1. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman J, Synnot A, Ryan R, Hill S, Horey D, Willis N, Lin V, Robinson P. Face to face interventions for informing or educating parents about early childhood vaccination. Cochrane Database Syst Rev. 2013;5:CD010038. [DOI] [PubMed] [Google Scholar]
- 37.Dubé È, MacDonald NE. Managing the risks of vaccine hesitancy and refusals. Lancet Infect Dis. 2016;16(5):518–519. doi: 10.1016/S1473-3099(16)00028-1. [DOI] [PubMed] [Google Scholar]
- 38.Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4):1–8. doi: 10.1542/peds.2013-2365. [DOI] [PubMed] [Google Scholar]
- 39.Dandekar P, Goel A, Lee DT. Biased assimilation, homophily, and the dynamics of polarization. Proc Natl Acad Sci USA. 2013;110(15):5791–5796. doi: 10.1073/pnas.1217220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahan D, Braman D, Cohen G, Gastil J, Slovic P. Who fears the HPV vaccine, who doesn’t, and why? An experimental study of the mechanisms of cultural cognition. Law Hum Behav. 2010;34(6):501–516. doi: 10.1007/s10979-009-9201-0. [DOI] [PubMed] [Google Scholar]
- 41.Paulussen TG, Hoekstra F, Lanting CI, Buijs GB, Hirasing RA. Determinants of Dutch parents’ decisions to vaccinate their child. Vaccine. 2006;24(5):644–651. doi: 10.1016/j.vaccine.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 42.Appiah-Brempong E, Okyere P, Owusu-Addo E, Cross R. Motivational interviewing interventions and alcohol abuse among college students: a systematic review. Am J Health Promot. 2014;29(1):e32–42. doi: 10.4278/ajhp.130502-LIT-222. [DOI] [PubMed] [Google Scholar]
- 43.Nyamathi A, Sinha K, Greengold B, Cohen A, Marfisee M. Predictors of HAV/HBV vaccination completion among methadone maintenance clients. Res Nurs Health. 2010;33(2):120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]


