Abstract
Unpredictable and potentially dangerous aggressive behavior by youth with Autism Spectrum Disorder (ASD) can isolate them from foundational educational, social, and familial activities (Davis & Carter, 2008; Hodgetts, Nicholas, & Zwaigenbaum, 2013), thereby markedly exacerbating morbidity (Siegel & Gabriels, 2014) and costs (Croen, Najjar, Ray, Lotspeich, & Bernal, 2006) associated with ASD. This study investigates whether preceding physiological and motion data measured by a wrist-worn biosensor can predict aggression to others by youth with ASD. We recorded peripheral physiological (cardiovascular and electrodermal activity) and motion (accelerometry) signals from a biosensor worn by 20 youth with ASD (ages 6–17 years, 75% male, 85% minimally verbal) during 69 independent naturalistic observation sessions with concurrent behavioral coding in a specialized inpatient psychiatry unit. We developed prediction models based on ridge-regularized logistic regression. Our results suggest that aggression to others can be predicted 1 minute before it occurs using 3 minutes of prior biosensor data with an average area under the curve (AUC) of 0.71 for a global model and 0.84 for person-dependent models. The biosensor was well tolerated, we obtained useable data in all cases, and no users withdrew from the study. Relatively high predictive accuracy was achieved using antecedent physiological and motion data. Larger trials are needed to further establish an ideal ratio of measurement density to predictive accuracy and reliability. These findings lay the groundwork for the future development of precursor behavior analysis and just-in-time adaptive intervention systems to prevent or mitigate the emergence, occurrence, and impact of aggression in ASD.
Keywords: Aggression, Autism Spectrum Disorder, Inpatients, Autonomic Nervous System, Biosensing Techniques
LAY ABSTRACT
Unpredictable aggression can create a barrier to accessing community, therapeutic, medical, and educational services. The present study evaluated whether data from a wearable biosensor can be used to predict aggression to others by youth with Autism Spectrum Disorder (ASD). Results demonstrate that aggression to others can be predicted one minute before it occurs with high accuracy, laying the groundwork for the future development of preemptive behavioral interventions and just-in-time adaptive intervention systems to prevent or mitigate the emergence, occurrence, and impact of aggression to others in ASD.
The purpose of the present study is to evaluate whether peripheral physiological arousal and motion data measured by a wearable biosensor can be used to predict aggression to others by youth with Autism Spectrum Disorder (ASD). ASD is one of the most common childhood disorders (1 in 59) (Baio et al., 2014) and it is associated with high health care cost (Amendah, Grosse, Peacock, & Mandell, 2011). While ASD is characterized by social communication impairments and restricted, repetitive behaviors and interest (American Psychiatric Association, 2013), youth with ASD are also at increased risk for a range of co-occurring psychiatric and behavioral issues compared to the general population (Gray et al., 2012; Joshi et al., 2010; Leyfer et al., 2006; Salazar et al., 2015; Simonoff et al., 2008). Aggression is one of the most frequently observed problem behaviors in youth with ASD (Kanne & Mazurek, 2011; Matson & Cervantes, 2014), especially in the more severely affected (Bronsard, Botbol, & Tordjam, 2010; Matson & Rivet, 2008; McClintock, Hall, & Oliver, 2003; Tsiouris, Kim, Brown, & Cohen, 2011), and ranks among the most common causes for referral to behavioral healthcare services (Arnold et al., 2003). Physical aggression, including hitting, biting, scratching, and throwing objects at others, is particularly debilitating because it often occurs without warning, sometimes long after any observable trigger, creating an environment of unpredictability.
Unpredictable aggression can create a barrier to accessing community, therapeutic, medical, and educational services. For instance, families report that aggression increases their stress, isolation, and financial burden, and decreases available support options because they are understandably afraid to put their child with ASD into potentially stressful environments that might lead to aggression without warning (Davis & Carter, 2008; Hodgetts, Nicholas, & Zwaigenbaum, 2013). Frequent aggression can also have deleterious effects on professional support providers, leading to increased sick days, higher turnover rates, and compensatory payments for injury (Allen, 2000; Kiely & Pankhurst, 1998). This predicament can demoralize parents and providers, accelerate negative patient trajectories, and lead to homebound or residential living placement, collectively decreasing quality of life while increasing healthcare costs (Jewett, 2017; Price & Price, 2016). Cross-sectional and longitudinal studies suggest that even though aggression may decline in ASD over the lifespan, it can persist into adulthood and remains heightened in comparison to typically developing and intellectually impaired populations without ASD (Farmer & Aman, 2009; Farmer & Aman, 2011; Gray et al., 2012; Woodman, Mailick, & Greenberg, 2016).
Aggression is often treated with medication, which can be efficacious in some instances but can also have significant side effects and inconsistent success (Adler et al., 2015; Williamson et al., 2017; Wink, Pedapti, Horn, McDougle, & Erickson, 2017). Applied Behavior Analysis (ABA) has also been shown to be effective at reducing aggression and other problem behaviors in youth with ASD (Doehring, Reichow, Palka, Phillips, & Hagopian, 2014). Primary mechanisms utilized by ABA are identification and control of antecedents, motivating operations, and consequences that either occasion or reinforce behavior. However, identifying these factors can be challenging (particularly when they are internally mediated and the respondent cannot verbalize his or her experience; Carr & Owen-Deschryver, 2007), is time-intensive, and often requires extensive specialized expertise that is not readily accessible to most families (Xu et al., 2018).
Physiological arousal is a potentially promising objective indicator that may precede aggression toward others. Physiological arousal is consistently implicated in aggression (Lindsay & Anderson, 2000). In typically developing youth, greater ability to regulate physiological arousal is associated with fewer behavior problems (Calkins, 1997; Porges, 1996). Studies of psychiatric disorders characterized by emotional and behavioral dysregulation, such as bipolar disorder and antisocial behavior, report a strong association between physiological arousal and symptomatology (De Vries-Bouw et al., 2011; Lorber, 2004; Ortiz & Raine, 2004; Raine, 2002). Some of the earliest descriptions of ASD highlight atypical physiological arousal, including the supposition that problem behaviors are functionally related to homeostatic regulation (DesLauriers & Carlson, 1969; Hutt & Hutt, 1965; Kinsbourne, 1980; Ornitz & Ritvo, 1968; Rimland, 1964). While significant heterogeneity in individuals with ASD exists, recent reviews demonstrate that atypical autonomic reactivity is a common feature (Klusek, Roberts, & Losh, 2015; Levine, Conradt, Goodwin, Sheinkopf, & Lester, 2014; Lydon et al., 2016) and can putatively occasion maladaptive behavior when demands exceed an individual’s coping ability (Cohen, Yoo, Goodwin, & Moskowitz, 2011; Scarpa, 2015). Given the aforementioned literature, the purpose of the present study is to test the hypothesis that preceding changes in physiological arousal can be used to accurately predict aggression before it occurs.
METHODS
Participants
Twenty psychiatric inpatients with confirmed ASD were serially enrolled at one site of the Autism Inpatient Collection (AIC) study and initiated our aggression prediction protocol. The AIC is an ongoing six-site study of over 1,200 children, adolescents, and young adults admitted to specialized inpatient psychiatric units for persons with ASD and other developmental disorders. The full methods of the AIC study have been published previously (Siegel et al., 2015). Briefly, patients 4 to 20 years old with a score of ≥12 on the Social Communication Questionnaire (SCQ) (Rutter, Bailey, & Lord, 2003) or high suspicion of ASD from the inpatient clinical treatment team were eligible for enrollment. Inclusion criteria required confirmation of ASD diagnosis by research-reliable administration of the Autism Diagnostic Observation Schedule-2 (ADOS-2) (Lord et al., 2012). Exclusion criteria included not having a parent available who was proficient in English or the individual with ASD having prisoner status. Within 10 days of admission, a primary caregiver of the participant completed the Aberrant Behavior Checklist (ABC) (Aman, Singh, Stewart, & Field, 1985), Child Behavior Checklist (CBCL) (Achenbach, 1991), Vineland Adaptive Behavior Scales-2 (VABS-2) (Sparrow, Balla, & Cicchetti, 1984), and Emotion Dysregulation Inventory (EDI) (Mazefsky, Yu, White, Siegel, & Pilkonis, 2018). Participants were also administered the Leiter-3 test of non-verbal intelligence (Roid & Koch, 2017).
The 20 participants in the present aggression prediction study were 6 to 17 years old (M = 10.8, SD = 3.1) and were predominantly male (75%), white (95%), and non-Hispanic (90%) (Table 1). Most were minimally verbal (85% ADOS-2 module 1 or 2) and had intellectual disability (Leiter-3 non-verbal IQ score M = 66.1, SD = 9.0). Mean scores on the CBCL were consistently in the borderline clinical or clinical range across psychiatric diagnostic categories. Mean VABS-2 scores in the domains of daily living skills, communication, and socialization were consistently in the severely impaired range. Average ABC subscale scores were very elevated, indicating a high frequency and severity of aggression, self-injury, and tantrums (ABC-Irritability subscale M = 28.4, SD = 8.7) as well other problem behaviors. EDI Reactivity (t-score M = 58.3, SD = 8.2) and Dysphoria (t-score M = 54.9, SD = 8.2) scores were in the average range compared to the EDI’s large autism psychometric sample (Mazefsky, et al., 2018), and two and one standard deviations higher, respectively, than a general sample of 1,000 US census-matched youth (Mazefsky, Yu, White, & Pilkonis, 2019).
Table 1.
Sample Characteristics
| Demographics | |
| Age | 10.8 ± 3.1 |
| Sex, Male - N (%) | 15 (75.0) |
| Race, White - N (%) | 19 (95.0) |
| Ethnicity, Non-Hispanic - N (%) | 18 (90.0) |
| Minimally or Non-verbal - N (%) | 17 (85.0) |
| Leiter Non-verbal IQ | 66.1 ± 19.0 |
| Length of Stay (in days) | 57.8 ± 27.7 |
| Child Behavior Checklist – DSM Oriented Scales (t-scores)d | |
| Depressive Problems | 71.9 ± 4.7 |
| Oppositional Defiant Problems | 68.7 ± 7.5 |
| Conduct Problems | 66.0 ± 5.4 |
| Attention Deficit/Hyperactivity Problems | 64.5 ± 5.7 |
| Anxiety Problems | 62.3 ± 7.8 |
| Somatic Problems | 60.1 ± 7.1 |
| Vineland Adaptive Behavior Scales (standard scores)b | |
| Daily Living Skills | 55.6 ± 10.8 |
| Communication | 53.0 ± 9.0 |
| Socialization | 50.2 ± 9.6 |
| Vineland Adaptive Behavior Scales (v-scale scores)b | |
| Maladaptive Behavioral Index | 21.8 ± 1.3 |
| Internalizing | 22.0 ± 1.0 |
| Externalizing | 20.2 ± 1.4 |
| Aberrant Behavior Checklist at Admission | |
| Irritabilityb | 28.4 ± 8.7 |
| Stereotypya | 8.3 ± 5.5 |
| Lethargya | 13.7 ± 6.8 |
| Hyperactivitya | 26.1 ± 7.2 |
| Inappropriate Speecha | 3.8 ± 3.9 |
| Emotion Dysregulation Inventory at Admission (t-scores) | |
| Reactivityc | 58.3 ± 8.2 |
| Dysphoriaa | 54.9 ± 8.2 |
N=19;
N=18;
N=17;
N=15.
Both the AIC and aggression prediction protocols were approved by the IRB of the participating study site, and the guardians of all participants provided informed consent. The AIC phenotypic data is available to external investigators through SFARI Base (www.sfari.org/resource/sfari-base/), and soon will include genetic sequencing data.
Aggression Prediction Protocol
Sixty-nine naturalistic observation sessions totaling 87 hours were performed by research staff in the inpatient unit while participants wore a wireless biosensor. Biosensor data collection began on average 45 days (SD = 40) since time of admission, for an average of 10 consecutive days (SD = 10), over an average 91 days (SD = 50) of inpatient stay. Research staff conducted these observations with minimal interference to participants’ daily inpatient routines, which consisted of academic lessons, behavioral, occupational, speech and milieu therapies, meals, and free time. Research staff coded the start and stop times of each aggression episode within the observation period using a laptop computer time-synchronized to the internal clock of the biosensor worn by the participant. Aggression was operationally defined as hitting, kicking, biting, scratching, grabbing, pulling, pinching, or throwing objects at others.
Wearable Biosensor
Peripheral physiological arousal and motion activity were collected from participants using the commercially available and regulatory compliant E4 by Empatica, Inc. The E4 weighs 40 grams and is made of durable polyurethane and polycarbonate materials that make it water and shockproof. It uses photoplethysmography (PPG) (Allen, 2007) to record Blood Volume Pulse (BVP) and Inter-Beat-Interval (IBI) data at 64 Hz, from which heart rate and heart rate variability (a measure of variation in the beat-to-beat interval (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996)) can be derived. Differences in PPG as a result of skin color and external light intensity are dynamically compensated for by device firmware. Device firmware also includes motion artifact removal and IBI detection algorithms that automatically discard non-prototypical beats without smoothing IBI sequences. The E4 records electrodermal activity (EDA) at 4 Hz with a 0.01–100 microSiemen range, reflecting autonomic innervation of sweat glands and alterations in sympathetic nervous system arousal (Boucsein, 2012; Critchley, 2002). Finally, the E4 records motion-based activity up to ±8g at 32 Hz using a 3-axis accelerometer (ACCx, ACCy, ACCz). Core sensing technologies in the E4 have been validated against gold-standard laboratory-based devices during physical activity, emotional provocation, and high cognitive load in typically-developing individuals (Poh, Swenson, & Picard, 2010).
Time-Series Feature Extraction
We extracted the following E4 time-series features for BVP, IBI, EDA, ACCx, ACCy, and ACCz using successive 15-second sliding windows: first, last, maximum, minimum, mean, and median value; amount of unique values; and sum, standard deviation, and variance of values falling in a window. We also extracted the following two binary aggression labels provided by research staff coding: aggression observation flag (AOF; indicating when an aggression has occurred) and time since past aggression (TPA; indicating time elapsed since the last observation of aggression). The standard deviation of each extracted feature was also included in all prediction models.
Logistic Regression Classifier Model
Ridge-regularized logistic regression was used with extracted time-series features as input variables to make binary aggression predictions over time. Specifically, at every time point t the classifier estimated whether aggression will be observed or not, indicated by label l, in an upcoming time range (t, t +τf) using features extracted in a previous time range (t−τp, t). Samples were split into training and testing datasets using 5-fold cross-validation, repeated five times to produce confidence intervals. At each fold, the classifier was trained via maximum likelihood estimation for optimal ridge-regularized regression weights β = [β0, β1, …, βd]T, where d is the number of features. For prediction, the classifier generates probabilities for two classes l = +1 (aggression) and l = −1 (non-aggression) in the form:
where x = [1, x1, …, xd]T and corresponds to the concatenated feature vector from (t − τp, t). Receiver Operator Characteristic (ROC) curves and corresponding Area Under the Curve (AUC) values were calculated to assess decision thresholds over these probabilities.
The following five feature (signal) subsets were used as predictor variables (x) in our analyses: (1) only temporal information (AOF, TPA); (2) only motion activity (ACC); (3) only physiological activity (BVP, IBI, EDA); (4) motion and physiological activity features combined (BVP, IBI, EDA, ACC); and (5) all extracted features combined (AOF, TPA, ACC, BVP, IBI, EDA). In other words, comparing the performance of our models by iteratively enriching the feature set enabled us to determine the relative impact these various sources of information have on aggression prediction accuracy.
RESULTS
Data Collected
After a brief desensitization protocol involving increasing exposure to the wrist-worn biosensor, all 20 participants tolerated wearing the E4 and usable data were obtained in all cases (Table 2). Sixty-nine independent naturalistic observational sessions totaling 87 hours were collected (M = 4.35 hrs, SD = 4.8 hrs per participant). Within this corpus, a total of 548 aggressive episodes (M = 27, SD = 34 per participant) lasting an average duration of 28 seconds (SD = 32 sec) were observed with concurrently collected E4 data.
Table 2.
Naturalistic data collection descriptive statistics.
| Participant | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 | P19 | P20 | Group | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Sessions | 5 | 3 | 3 | 2 | 2 | 2 | 8 | 9 | 2 | 1 | 1 | 1 | 2 | 6 | 1 | 1 | 10 | 1 | 5 | 4 | 69 | 3.45 | 2.84 |
| Total Obs. Duration* | 9.33 | 3.97 | 3.77 | 3.02 | 1.25 | 0.57 | 7.02 | 8.47 | 2.72 | 1.43 | 0.22 | 1.38 | 1.6 | 8.47 | 0.52 | 1.02 | 20.48 | 0.78 | 5.1 | 5.87 | 86.99 | 4.35 | 4.80 |
| Number of Aggression Episodes | 72 | 7 | 13 | 8 | 9 | 6 | 35 | 30 | 50 | 1 | 2 | 3 | 9 | 39 | 1 | 8 | 130 | 2 | 76 | 47 | 548 | 27.40 | 33.84 |
| Mean Agg. Duration† | 9 | 102 | 77 | 11 | 19 | 9 | 19 | 18 | 50 | 3 | 1 | 15 | 19 | 6 | 51 | 7 | 103 | 4 | 7 | 22 | - | 27.6 | 31.93 |
Total observation durations are presented in hours.
Mean aggression durations are presented in seconds.
Inter-rater reliability analysis on data randomly selected from 20% of our corpus of observed aggression onsets and offsets between two research staff from the inpatient site yielded 90% agreement and a corresponding Cohen’s Kappa of 0.79. A tolerance of 2 second onset or offset difference between raters was considered an agreement. The average duration of observed aggression episodes was 28 seconds, much longer than a potential absolute 4 second difference between raters (Table 2).
Prediction Performance
Aggression prediction was performed using both global and person-dependent models, wherein data was processed every 15 seconds for decision making. In global models, a single classifier was trained over a dataset containing time-series across all sessions and all participants. In person-dependent models, individual classifiers were trained over time-series within sessions from a single participant. In both models, three minutes of prior data (τp = 180 sec) was used to make predictions in an upcoming time range (τf) since it accommodated the shortest individual observational session in our corpus.
Global prediction.
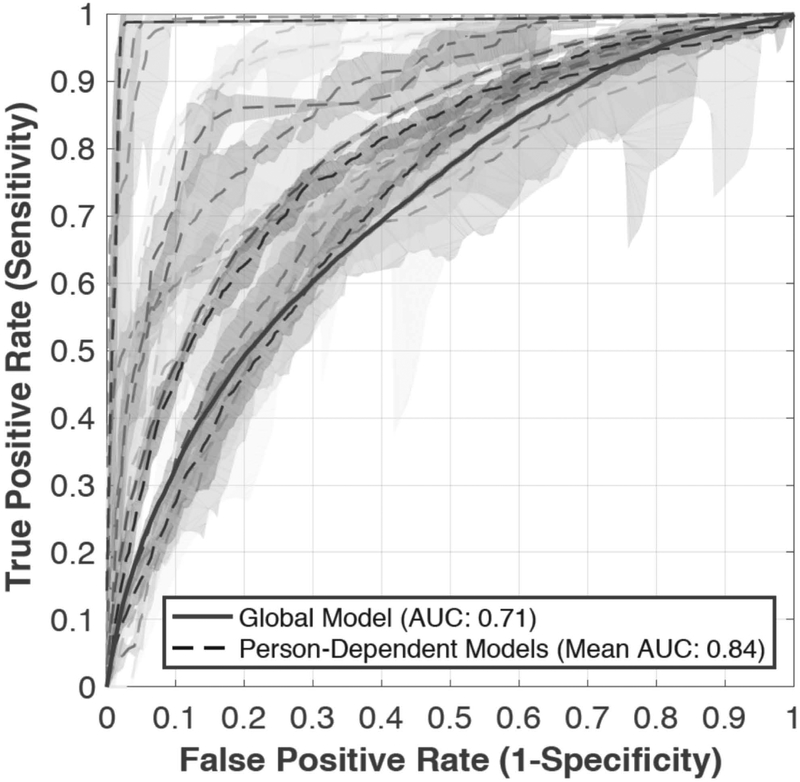
The highest accuracy achieved in the global model used all extracted features from the past three minutes to predict aggression in the upcoming one minute, with an AUC of 0.71 (represented by the solid ROC curve with 90% confidence intervals in Figure 1).
Figure 1.
ROC curves with 90% confidence intervals to predict onset of aggression in the upcoming minute, using all features from the past three minutes. The solid line represents the global model, and each curve with dashed lines represents one of the person‐dependent models.
Person-dependent prediction.
Repeating the above analysis in person-dependent models (dashed lines in Figure 1, rows in Table 3) produced an average AUC of 0.84 (min = 0.69, max = 0.99, SD = 0.10, 90% confidence intervals) predicting aggression in the upcoming one minute using all extracted features from the past three minutes.
Table 3.
Mean AUC values in person-dependent models predicting aggression onset in the next minute using accumulated data from the past three minutes.
| Features | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 | P19 | P20 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temporal | 0.67 | 0.57 | 0.58 | 0.52 | 0.83 | 0.58 | 0.62 | 0.58 | 0.83 | 0.83 | 0.82 | 0.61 | 0.64 | 0.59 | 0.92 | 0.83 | 0.65 | 0.99 | 0.53 | 0.61 | 0.69 | 0.14 |
| Motion | 0.66 | 0.84 | 0.80 | 0.49 | 0.88 | 0.73 | 0.65 | 0.79 | 0.79 | 0.98 | 0.51 | 0.85 | 0.79 | 0.64 | 0.81 | 0.73 | 0.72 | 0.99 | 0.60 | 0.75 | 0.75 | 0.13 |
| Physiological | 0.64 | 0.85 | 0.79 | 0.68 | 0.99 | 0.68 | 0.66 | 0.70 | 0.88 | 0.97 | 0.78 | 0.82 | 0.79 | 0.70 | 0.97 | 0.70 | 0.75 | 0.99 | 0.69 | 0.74 | 0.79 | 0.12 |
| Motion and Physiological | 0.71 | 0.86 | 0.81 | 0.67 | 0.98 | 0.75 | 0.68 | 0.76 | 0.88 | 0.99 | 0.83 | 0.87 | 0.85 | 0.71 | 0.99 | 0.72 | 0.78 | 0.99 | 0.70 | 0.78 | 0.82 | 0.11 |
| All Features Combined | 0.74 | 0.87 | 0.81 | 0.69 | 0.98 | 0.77 | 0.69 | 0.77 | 0.92 | 0.99 | 0.86 | 0.85 | 0.91 | 0.73 | 0.99 | 0.89 | 0.80 | 0.99 | 0.71 | 0.78 | 0.84 | 0.10 |
Comparative model performance.
Person-dependent models produced a 0.13 average increase in AUC compared to the global model. Moreover, as seen in Figure 1, person-dependent models displayed more favorable sensitivity compared to the global prediction model.
Classifier performance and feature (signal) contributions.
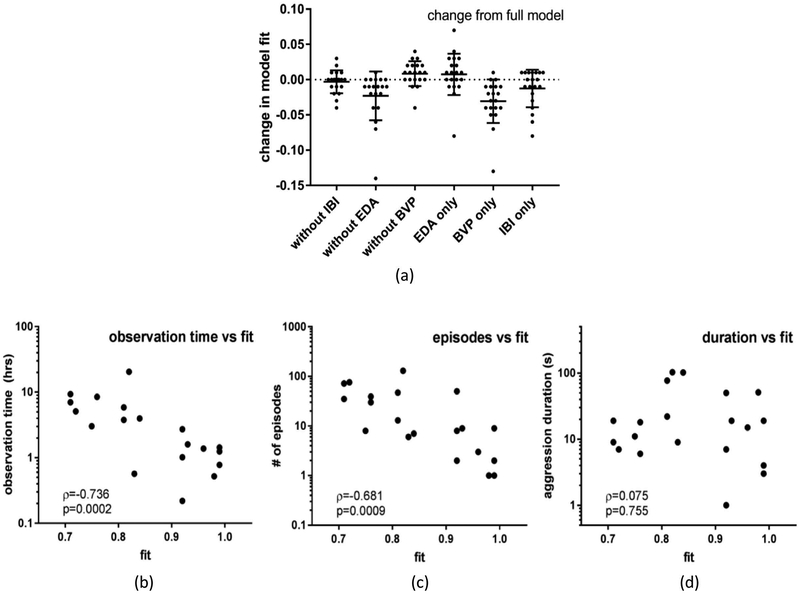
As seen in Table 3, models that included physiological and motion activity features outperformed those with only temporal features (AOF, TPA) across all participants, suggesting that biosensor data contributes unique information in the aggression prediction domain. Furthermore, Figure 2 indicates that when individual physiological features (EDA, BVP, IBI) are systematically removed, no signal alone results in significant model fit change, i.e., all individual physiological feature contributions are within the 90% confidence interval.
Figure 2.
Individual physiological feature contributions to global model fit with 90% confidence intervals (A) and relationships between global model fit and behavioral observation time (B), number of aggressive episodes observed (C), and aggression duration (D).
Classifier performance and data properties.
Correlations between model fit and total time observed, number of aggression episodes, and duration of observed aggression episodes were analyzed with Spearman’s rho since these variables were skewed due to oversampling in some participants (D’Agostino and Pearson normality test, largest p=0.0067). As seen in Figure 2, average time observed (M = 4.35 hrs, SD = 4.8 hrs) and number of observed aggressive episodes (M = 27, SD = 34) were both strong negative predictors of model fit/AUC, whereas duration of observed aggression episode (M = 28 sec, SD = 32 sec) was not. This suggests that the amount of data available to the model has a greater impact on prediction performance than duration of observed aggression episodes.
DISCUSSION
Our findings demonstrate that a wearable biosensor and the data it generates may have promise in regard to predicting aggressive behavior in children with ASD. While other researchers have incorporated physiological data into studies of problem behaviors involving youth with ASD (Barrera, Violo, & Graver, 2007; Freeman, Grzymala-Busse, Riffel, & Schroder, 2001; Freeman, Horner, & Reichle, 1999; Kushki, Khan, Brian, & Anagnostou, 2015; Lydon, Healy, & Dwyer, 2013; Nuske et al., 2018), prior work in this area has relied on artificial experimental settings and tasks, evaluated very brief time samples, or was correlational. Demonstrating that aggression can be predicted in the upcoming minute in an ecologically valid inpatient setting with 84% average accuracy is of potentially high clinical value. While we expect to increase prediction time as more data becomes available, focus groups we have conducted with parents and providers suggest that even 60 seconds of warning may be a sufficient amount of time to triage attention, rearrange the environment to make it safer, provide redirection, calm the individual directly, or promote other self-management supports to decrease the risk of progression to dangerous aggression.
Our initial focus on aggression in more severely affected youth with ASD addresses critical gaps in the literature. Despite their apparent increased vulnerability to developing serious problem behaviors (Kanne et al., 2011), those more severely affected by ASD are underrepresented in intervention research (Stedman, Taylor, Erard, Peura, & Siegel, 2018). Furthermore, it is common for this subpopulation to require high levels of intensive intervention (e.g., specialized school placements, psychopharmacology, in-home behavioral therapies), which often exceed what providers in community settings can offer (Joshi et al, 2010; Siegel et al., 2012). It can also land individuals with ASD in emergency rooms that are ill-equipped to handle them (Hoffmann, Stack, Monuteaux, Levin, & Lee, 2018) and necessitate costly psychiatric hospital care (Croen, Najjar, Ray, Lotspeich, & Bernal, 2006; Nayfack et al., 2014; Siegel & Gabriels, 2014). Our use of an inpatient psychiatric population not only allowed us to enroll youth functioning poorly across domains who are underrepresented in research, but also provided a unique opportunity to study naturally unfolding aggression in a safe environment. It is also notable that the biosensor was well tolerated and produced analyzable signal data in a severely affected and primarily minimally verbal ASD sample.
The advantages of biologically-based tools to identify processes that underlie behavioral dysregulation as it unfolds during moments of escalation are numerous and have great translational potential, especially for those unable to provide reliable self-reports on their arousal states. Our findings lay the groundwork for both expanding data collection on precursor behaviors associated with aggression (Borrero & Borrero, 2008; Hagopian, Rooker, & Yenokyan, 2018; Herscovitch, Roscoe, Libby, Bourret, Ahearn, 2009) and future development of just-in-time adaptive intervention (Nahum-Shani et al., 2018) mobile health systems (mHealth) (Kumar et al., 2013) that may enable new opportunities for intervention before distress escalates to aggression. With input from parents and providers, a mobile application could be developed that displays real-time information on the risk for imminent aggression and prompts users to initiate de-escalation or emotion regulation interventions before it occurs (e.g., Alam, Anderson, Bankole, & Lach, 2018). The effectiveness of such a system could be evaluated in its own right (i.e., directly comparing a behavioral and biosensor trial within the same study) as well as in combination with state-of-the-art pharmacological and behavioral treatment models (Conner et al., 2018; Frazier et al., 2010) to see if it further enhances aggression reduction. Uncovering physiological profiles relating to aggression in naturalistic settings may also enhance clinical trials research and precision medicine (Insel, 2014) by producing digital biomarkers (Adams et al., 2017) that better define psychiatric endophenotypes, enable recruitment of more targeted experimental groups, and help determine whether pharmaceuticals simply act as a sedative versus affect specific mechanisms that reduce physiological arousal associated with aggression. Finally, while our focus in this study was aggression to others, our methods could be applied to other prevalent problem behaviors in ASD (self-injury, property destruction, elopement, etc.), the wider outpatient ASD population, youth with other developmental disorders, as well as other populations who exhibit frequent aggression.
While we obtained good predictive performance in the current study, the design of our machine learning models reduced opportunities for cross-training along different time windows (i.e., using data from one window for another window) and may have led to overfitting (i.e., models are overparameterized). In future work, we will institute a generative non-homogeneous Poisson process model that introduces a common prior probability across windows to enable cross-learning and avoid overfitting. This approach may allow us to generate more optimal prediction performance by learning sparse linear models that identify which features correlate most strongly with aggression onsets. We also plan to address the potential issue of data quality and nonstationarity in physiological signals by extracting features that achieve immediate generalization across time. Moreover, we will explore insensitivity and modern transfer learning techniques that can be quickly adapted to changing conditions through online adaptation with limited training data in a new setting (i.e., new day for the same individual, a new individual, etc.), and assess hybrid classifiers wherein the most significant features from global and person-dependent models are combined to increase computational efficiency (i.e., start with the global features most strongly associated with high prediction accuracy and update with person-dependent features). Finally, we will work to reduce false positive and false negative predictions through feature optimization; however, as communicated to us by inpatient clinical staff, the potential benefit of avoiding or reducing a dangerous aggressive event is likely to outweigh potential harm associated with false positives in clinical practice.
Our results suggest that the person-dependent models were more accurate than the global model, and we observed individual differences in prediction accuracy (i.e., 30% difference between highest and lowest prediction performance, Table 3). However, a limitation of this study is the non-uniform frequency and duration of observed aggression across participants (evidenced in Table 2), making it difficult to determine the relative impact the amount of training data has on model prediction performance. Larger trials are needed to further establish an ideal ratio of measurement density to predictive accuracy and reliability. Also, considering the moderating influences emotion dysregulation and language ability/communication efficiency can have on aggression (Berthoz & Hill, 2005; Costa, Steffgen, & Samson, 2017; Mazefsky et al., 2013; Mazefsky & White, 2014; Samson, Hardan, Lee, Phillips, & Gross, 2015), we expect that a proportion of the person-dependent variability we observed might be explained by emotion regulation and verbal ability. In an extension of this work that we are now beginning, we will gather this information and evaluate whether it improves model prediction performance. We have also begun to explore the relationship between observable affect, internal physiology, and subsequent aggression to determine if it is concordant or discordant in individuals with ASD. Finally, we plan to include a broader sample of both verbal and minimally verbal youth with ASD in order to assess the generalizability of our results.
CONCLUSION
This study sought to define a more generalized, objective, and biological approach to understanding and predicting aggression in ASD, grounded in underlying physiological mechanisms, with a severely affected population who suffer some of the greatest morbidity but have received relatively little attention. In so doing, we seek to develop a different and potentially additive approach to traditional attempts that differentiate proactive or reactive aggression, focus on the potential function(s) of a behavior, or utilize psychopharmacology. Our results suggest that cutting-edge technology combined with multidisciplinary clinical experience has the potential to further inform what has been a largely intractable problem for a sizable segment of the ASD population, who are arguably most in need of innovative approaches. By focusing on reducing the unpredictability of aggression, we hope that the knowledge, data, and algorithms generated in this ongoing program of research could ultimately facilitate reductions in the occurrence, duration, and impact of aggression in youth with ASD, enabling them to more fully participate in their homes, schools, and communities.
ACKNOWLEDGMENTS
We are grateful to participating families and the following project administration staff and research assistants who supported the research and preparation of this manuscript: James Buckely, BA, Catalina Cumpanasoiu, BA, Yuan Guo, MS, James Heathers, PhD, Ozan Özdenizci, MS, and Peng Tian, BS of Northeastern University and Christine Peura, BS, Amy Stedman, BA, and Mary Verdi, MA of Maine Medical Center Research Institute. This work was supported by grants from the Department of Defense (12507232, 12507733), National Institute of Child Health and Human Development (R01 HD079512), National Institute on Deafness and Other Communication Disorders (P50 DC013027), National Science Foundation (SCH-1622536, IIS-1118061), Nancy Lurie Marks Family Foundation, and Simons Foundation (SFARI 296318, 618037).
Contributor Information
Matthew S. Goodwin, Northeastern University, Department of Health Sciences, Boston, MA.
Carla A. Mazefsky, University of Pittsburgh School of Medicine, Department of Psychiatry, Pittsburgh, PA.
Stratis Ioannidis, Northeastern University, Department of Electrical and Computer Engineering, Boston, MA.
Deniz Erdogmus, Northeastern University, Department of Electrical and Computer Engineering, Boston, MA.
Matthew Siegel, Maine Medical Center Research Institute, Portland, ME.
REFERENCES
- Achenbach TM (1991). Manual for the teacher’s report form and 1991 profile. Burlington: University of Vermont, Department of Psychiatry. [Google Scholar]
- Adams Z, McClure EA, Gray KM, Danielson CK, Treiber FA, & Ruggiero KJ (2017). Mobile devices for the remote acquisition of physiological and behavioral biomarkers in psychiatric clinical research. Journal of Psychiatric Research, 85, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler BA, Wink LK, Early M, Shaffer R, Minshawi N, McDougle CJ, & Erickson CA (2015). Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism, 19, 102–106. [DOI] [PubMed] [Google Scholar]
- Alam R, Anderson M, Bankole A, & Lach J (2018). Inferring physical agitation in dementia using smartwatch and sequential behavior models. In 2018 IEEE EMBS International Conference on Biomedical Health Informatics (BHI), 170–173. [Google Scholar]
- Allen D (2000). Recent research on physical aggression in persons with intellectual disability: an overview. Journal of Intellectual & Developmental Disability, 25, 41–57. [Google Scholar]
- Allen J (2007). Photoplethysmography and its application in clinical physiological measurement. Physiological Measurement, 28, R1–R39. [DOI] [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, & Field CJ (1985). The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89, 485–491. [PubMed] [Google Scholar]
- Amendah D, Grosse SD, Peacock G, & Mandell DS (2011). The economic costs of autism: a review In Amaral D, Geschwind D, & Dawson G (Eds.), Autism spectrum disorders (pp. 1347–1360). Oxford University Press. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, 5th ed: DSM 5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Arnold LE, Vitiello B, McDougle C, Scahill L, Shah B, Gonzalez NM, … Tierney E (2003). Parent-defined target symptoms respond to risperidone in RUPP autism study: customer approach to clinical trials. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 1443–1450. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries, 67, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera FJ, Violo RA, & Graver EE (2007). On the form and function of severe self-injurious behavior. Behavioral interventions, 22, 5–33. [Google Scholar]
- Berthoz S, & Hill EL (2005). The validity of using self-reports to assess emotion regulation abilities in adults with autism spectrum disorder. European Psychiatry, 20, 291–298. [DOI] [PubMed] [Google Scholar]
- Borrero CS, & Borrero JC (2008). Descriptive and experimental analyses of potential precursors to problem behavior. Journal of Applied Behavior Analysis, 41, 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein W (2012). Electrodermal activity. New York, NY: Springer Science & Business Media. [Google Scholar]
- Bronsard G, Botbol M, & Tordjman S (2010). Aggression in low functioning children and adolescents with autistic disorder. PloS One, 5, e14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD (1997). Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology, 31, 125–135. [DOI] [PubMed] [Google Scholar]
- Carr EG, & Owen-Deschryver JS (2007). Physical illness, pain, and problem behavior in minimally verbal people with developmental disabilities. Journal of Autism and Developmental Disorders, 37, 413–424. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Yoo JH, Goodwin MS, & Moskowitz L (2011). Assessing challenging behaviors in autism spectrum disorders: prevalence, rating scales, and autonomic indicators In Matson JL & Sturmey P (Eds.), International handbook of autism and pervasive developmental disorders (pp. 247–270). New York, NY: Springer New York. [Google Scholar]
- Conner CM, White SW, Beck KB, Golt J, Smith IC, & Mazefsky CA (2018). Improving emotion regulation ability in autism: the emotional awareness and skills enhancement (EASE) program. Autism. Advance online publication. doi: 10.1177/1362361318810709. [DOI] [PubMed] [Google Scholar]
- Costa AP, Steffgen G, & Samson AC (2017). Expressive incoherence and alexithymia in autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 1659–1672. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2002). Electrodermal responses: what happens in the brain. The Neuroscientist, 8, 132–142. [DOI] [PubMed] [Google Scholar]
- Croen LA, Najjar DV, Ray GT, Lotspeich L, & Bernal P (2006). A comparison of health care utilization and costs of children with and without autism spectrum disorders in a large group-model health plan. Pediatrics, 118, e1203–e1211. [DOI] [PubMed] [Google Scholar]
- Davis NO, & Carter AS (2008). Parenting stress in mothers and fathers of toddlers with autism spectrum disorders: associations with child characteristics. Journal of Autism and Developmental Disorders, 38, 1278–1291. [DOI] [PubMed] [Google Scholar]
- DesLauriers AM & Carlson CF. (1969). Your child is asleep: early infantile autism: etiology, treatment, and parental influences. Homewood, IL: Dorsey Press. [Google Scholar]
- De Vries-Bouw M, Popma A, Vermeiren R, Doreleijers TAH, Van De Ven PM, & Jansen LMC (2011). The predictive value of low heart rate and heart rate variability during stress for reoffending in delinquent male adolescents. Psychophysiology, 48, 1597–1604. [DOI] [PubMed] [Google Scholar]
- Doehring P, Reichow B, Palka T, Phillips C, & Hagopian L (2014). Behavioral approaches to managing severe problem behaviors in children with autism spectrum and related developmental disorders: a descriptive analysis. Child and Adolescent Psychiatric Clinics of North America, 23, 25–40. [DOI] [PubMed] [Google Scholar]
- Farmer CA, & Aman MG (2009). Development of the children’s scale of hostility and aggression: reactive/proactive (C-SHARP). Research in Developmental Disabilities, 30, 1155–1167. [DOI] [PubMed] [Google Scholar]
- Farmer CA, & Aman MG (2011). Aggressive behavior in a sample of children with autism spectrum disorders. Research in Autism Spectrum Disorders, 5, 317–323. [Google Scholar]
- Frazier TW, Youngstrom EA, Haycook T, Sinoff A, Dimitriou F, Knapp J, & Sinclair L (2010). Effectiveness of medication combined with intensive behavioral intervention for reducing aggression in youth with autism spectrum disorder. Journal of Child and Adolescent Psychopharmacology, 20, 167–177. [DOI] [PubMed] [Google Scholar]
- Freeman RL, Grzymala-Busse JW, Riffel LA, & Schroeder SR (2001). Analysis of self-injurious behavior by the lers data mining system In New frontiers in artificial intelligence (pp. 395–399). Springer; Berlin Heidelberg. [Google Scholar]
- Freeman RL, Horner RH, & Reichle J (1999). Relation between heart rate and problem behaviors. American Journal on Mental Retardation, 104, 330–345. [DOI] [PubMed] [Google Scholar]
- Gray K, Keating C, Taffe J, Brereton A, Einfeld S, & Tonge B (2012). Trajectory of behavior and emotional problems in autism. American Journal on Intellectual and Developmental Disabilities, 117, 121–133. [DOI] [PubMed] [Google Scholar]
- Hagopian LH, Rooker GW, & Yenokyan G (2018). Identifying predictive behavioral markers: A demonstration using automatically reinforced self‐injurious behavior. Journal of Applied Behavior Analysis, 51, 443–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch B, Roscoe EM, Libby ME, Bourret JC, & Ahearn WH (2009). A procedure for identifying precursors to problem behavior. Journal of Applied Behavior Analysis, 42, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts S, Nicholas D, & Zwaigenbaum L (2013). Home sweet home? families’ experiences with aggression in children with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 28, 166–174. [Google Scholar]
- Hoffmann JA, Stack AM, Monuteaux MC, Levin R, & Lee LK (2018). Factors associated with boarding and length of stay for pediatric mental health emergency visits. The American Journal of Emergency Medicine. Advance online publication. doi: 10.1016/j.ajem.2018.12.041. [DOI] [PubMed] [Google Scholar]
- Hutt C, & Hutt SJ (1965). Effects of environmental complexity on stereotyped behaviours of children. Animal Behaviour, 13, 1–4. [Google Scholar]
- Insel TR (2014). The NIHM research domain criteria (rdoc) project: precision medicine for psychiatry. The American Journal of Psychiatry, 171, 395–397. [DOI] [PubMed] [Google Scholar]
- Jewett C, (2017, September 26). With nowhere else to go, patients with severe autism turn to hospitals for help. The Philadelphia Inquirer. Retrieved from http://www.philly.com/philly/health/kids-families/with-nowhere-else-to-go-patients-with-severe-autism-languish-in-hospitals-across-u-s-20170926.html.
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, … Biederman J (2010). The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: a large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders, 40, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Kanne SM, & Mazurek MO (2011). Aggression in children and adolescents with ASD: prevalence and risk factors. Journal of Autism and Developmental Disorders, 41, 926–937. [DOI] [PubMed] [Google Scholar]
- Kiely J, & Pankhurst H (1998). Violence faced by staff in a learning disability service. Disability and Rehabilitation, 20, 81–89. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M (1980). Do repetitive movement patterns in children and animals serve a dearousing function? Journal of Developmental and Behavioral Pediatrics, 1, 39–42. [PubMed] [Google Scholar]
- Klusek J, Roberts JE, & Losh M (2015). Cardiac autonomic regulation in autism and fragile x syndrome: a review. Psychological Bulletin, 141, 141–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, … Swendeman D (2013). Mobile health technology evaluation: the mHealth evidence workshop. American Journal of Preventive Medicine, 45, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A, Khan A, Brian J, & Anagnostou E (2015). A kalman filtering framework for physiological detection of anxiety-related arousal in children with autism spectrum disorder. IEEE Transactions on Bio-Medical Engineering, 62, 990–1000. [DOI] [PubMed] [Google Scholar]
- Levine TP, Conradt E, Goodwin MS, Sheinkopf SJ, & Lester B (2014). Psychophysiological arousal to social stress in autism spectrum disorders In Patel VB, Preedy VR, & Martin CR (Eds.), Comprehensive guide to autism (pp. 1177–1193). New York, NY: Springer. [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, … Lainhart JE (2006). Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36, 849–861. [DOI] [PubMed] [Google Scholar]
- Lindsay JJ, & Anderson CA (2000). From antecedent conditions to violent actions: a general affective aggression model. Personality & Social Psychology Bulletin, 26, 533–547. [Google Scholar]
- Lorber MF (2004). Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychological Bulletin, 130, 531–552. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule, second edition (ados-2) manual (part i): modules 1–4. Torrance, CA: Western Psychological Services. [Google Scholar]
- Lydon S, Healy O, & Dwyer M (2013). An examination of heart rate during challenging behavior in autism spectrum disorder. Journal of Developmental and Physical Disabilities, 25, 149–170. [Google Scholar]
- Lydon S, Healy O, Reed P, Mulhern T, Hughes BM, & Goodwin MS (2016). A systematic review of physiological reactivity to stimuli in autism. Developmental Neurorehabilitation, 19, 335–355. [DOI] [PubMed] [Google Scholar]
- Matson JL, & Cervantes PE (2014). Assessing aggression in persons with autism spectrum disorders: an overview. Research in Developmental Disabilities, 35, 3269–3275. [DOI] [PubMed] [Google Scholar]
- Matson JL, & Rivet TT (2008). The effects of severity of autism and pdd-nos symptoms on challenging behaviors in adults with intellectual disabilities. Journal of Developmental and Physical Disabilities, 20, 41–51. [Google Scholar]
- Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, & White SW (2013). The role of emotion regulation in autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 52, 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, & White SW (2014). Emotion regulation: concepts & practice in autism spectrum disorder. Child and Adolescent Psychiatric Clinics of North America, 23, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky C, Yu L, White SW, Pilkonis P (2019, April). Assessment and treatment of emotion dysregulation in youth with autism spectrum disorder. In Invited Symposium: Emotion reactivity and dysregulation in at-risk youth: transdiagnostic approaches and measures. The North American Society for the Study of Personality Disorders Annual Conference, Pittsburgh, PA. [Google Scholar]
- Mazefsky CA, Yu L, White SW, Siegel M, & Pilkonis PA (2018). The emotion dysregulation inventory: Psychometric properties and item response theory calibration in an autism spectrum disorder sample. Autism Research, 11, 928–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock K, Hall S, & Oliver C (2003). Risk markers associated with challenging behaviours in people with intellectual disabilities: a meta-analytic study. Journal of Intellectual Disability Research, 47, 405–416. [DOI] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, & Murphy SA (2018). Just-in-time adaptive interventions (jitais) in mobile health: key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine, 52, 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayfack AM, Huffman LC, Feldman HM, Chan J, Saynina O, & Wise PH (2014). Hospitalizations of children with autism increased from 1999 to 2009. Journal of Autism and Developmental Disorders, 44, 1087–1094. [DOI] [PubMed] [Google Scholar]
- Nuske HJ, Finkel E, Tomczuk L, Hedley D, Parma V, Pellecchia M, Herrington J, Mandell DS, & Dissanayake C (2018). A window of opportunity: increase in heart rate prior to episodes of challenging behavior in preschoolers with autism. International Society for Autism Research (INSAR) 2018 Annual Meeting, Rotterdam, Netherlands, May 9–12. [Google Scholar]
- Ornitz EM, & Ritvo ER (1968). Perceptual inconstancy in early infantile autism. The syndrome of early infant autism and its variants including certain cases of childhood schizophrenia. Archives of General Psychiatry, 18, 76–98. [DOI] [PubMed] [Google Scholar]
- Ortiz J, & Raine A (2004). Heart rate level and antisocial behavior in children and adolescents: a meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 154–162. [DOI] [PubMed] [Google Scholar]
- Poh M-Z, Swenson NC, & Picard RW (2010). A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Transactions on Bio-Medical Engineering, 57, 1243–1252. [DOI] [PubMed] [Google Scholar]
- Porges SW (1996). Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development and Psychopathology, 8, 43–58. [Google Scholar]
- Price L & Price R, (2016, August 2). The meltdown: when the world becomes a blur of colors and deafening screams. Autism Parenting Magazine. Retrieved from https://www.autismparentingmagazine.com/how-meltdown-feels/.
- Raine A (2002). Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology, 30, 311–326. [DOI] [PubMed] [Google Scholar]
- Rimland B (1964). Infantile autism: the syndrome and its implications for a neural theory of behavior. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Roid GH, & Koch C (2017). Leiter-3: nonverbal cognitive and neuropsychological assessment In: McCallum R (eds) Handbook of nonverbal assessment. Springer, Cham. [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The social communication questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Salazar F, Baird G, Chandler S, Tseng E, O’sullivan T, Howlin P, … Simonoff E (2015). Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 2283–2294. [DOI] [PubMed] [Google Scholar]
- Samson AC, Hardan AY, Lee IA, Phillips JM, & Gross JJ (2015). Maladaptive behavior in autism spectrum disorder: the role of emotion experience and emotion regulation. Journal of Autism and Developmental Disorders, 45, 3424–3432. [DOI] [PubMed] [Google Scholar]
- Scarpa A (2015). Physiological arousal and its dysregulation in child maladjustment. Current Directions in Psychological Science, 24, 345–351. [Google Scholar]
- Siegel M, Doyle K, Chemelski B, Payne D, Ellsworth B, Harmon J, … Lubetsky M (2012). Specialized inpatient psychiatry units for children with autism and developmental disorders: a United States survey. Journal of Autism and Developmental Disorders, 42, 1863–1869. [DOI] [PubMed] [Google Scholar]
- Siegel M, & Gabriels RL (2014). Psychiatric hospital treatment of children with autism and serious behavioral disturbance. Child and Adolescent Psychiatric Clinics of North America, 23, 125–142. [DOI] [PubMed] [Google Scholar]
- Siegel M, Smith KA, Mazefsky C, Gabriels RL, Erickson C, Kaplan D, … Autism and Developmental Disorders Inpatient Research Collaborative (ADDIRC). (2015). The autism inpatient collection: methods and preliminary sample description. Molecular Autism, 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla D, & Cicchetti D (1984). Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Stedman A, Taylor B, Erard M, Peura C, & Siegel M (2018). Are children severely affected by autism spectrum disorder underrepresented in treatment studies? an analysis of the literature. Journal of Autism and Developmental Disorders. Advance online publication. doi: 10.1007/s10803-018-3844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. (1996). Heart rate variability standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043–1065. [PubMed] [Google Scholar]
- Tsiouris JA, Kim SY, Brown WT, & Cohen IL (2011). Association of aggressive behaviours with psychiatric disorders, age, sex and degree of intellectual disability: a large-scale survey. Journal of Intellectual Disability Research, 55, 636–649. [DOI] [PubMed] [Google Scholar]
- Williamson E, Sathe NA, Andrews JC, Krishnaswami S, McPheeters ML, Fonnesbeck C, … Warren Z (2017). Medical therapies for children with autism spectrum disorder—an update. Comparative Effectiveness Review No. 189. (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-2015-00003-I.) AHRQ Publication No. 17-EHC009-EF. Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Wink LK, Pedapati EV, Horn PS, McDougle CJ, & Erickson CA (2017). Multiple antipsychotic medication use in autism spectrum disorder. Journal of Child and Adolescent Psychopharmacology, 27, 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman AC, Mailick MR, & Greenberg JS (2016). Trajectories of internalizing and externalizing symptoms among adults with autism spectrum disorders. Development and Psychopathology, 28, 565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Strathearn L, Liu B, O’Brien M, Kopelman TG, Zhu J, … Bao W (2018). Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatrics. Advance online publication. doi: 10.1001/jamapediatrics.2018.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]