Abstract
Background:
We hypothesized that echocardiographic indices of right ventricular to pulmonary artery (RV-PA) coupling were comparable to cardiac magnetic resonance imaging (CMRI)-derived RV volumetric indices in predicting disease severity in chronic pulmonary regurgitation (PR).
Methods:
Patients with ≥moderate PR (2003–2015) with and without prior CMRI scans were enrolled into the study cohort and validation cohort, respectively. Endpoint was to determine the association between noninvasive RV-PA coupling indices (tricuspid annular plane systolic excursion/right ventricular systolic pressure [TAPSE/RVSP] and fractional area change [FAC]/RVSP ratio) and markers of disease severity, and compared this association to that of CMRI-derived RV volumetric indices and markers of disease severity (peak oxygen consumption [VO2], NT-proBNP and atrial and/or ventricular arrhythmias).
Results:
Of the 256 patients in the study cohort (age 33±6 years), 187 (73%) had tetralogy of Fallot (TOF) while 69 (27%) had valvular pulmonic stenosis (VPS). TAPSE/RVSP (r=0.73, p<0.001) and FAC/RVSP (r=0.78, p<0.001) correlated with peak VO2. Among the CMRI-derived RV volumetric indices analyzed, only right ventricular end-systolic volume index correlated with peak VO2 (r=−0.54, p<001) and NT-proBNP (r=0.51, p<0.001). These RV-PA coupling indices were tested in the validation cohort of 218 patients (age 37±9 years). Similar to the study cohort, TAPSE/RVSP (r=0.59, p<0.001) and FAC/RVSP (r=0.70, p<0.001) correlated with peak VO2. TAPSE/RVSP (but not FAC/RVSP) was also associated with arrhythmia occurrence in both the study cohort and validation cohorts.
Conclusion:
Noninvasive RV-PA coupling may provide complementary prognostic data in the management of chronic PR. Further studies are required to explore this clinical tool.
Keywords: Right ventricular to pulmonary arterial coupling, pulmonary regurgitation, Tetralogy of Fallot, Pulmonic stenosis, Exercise capacity
INTRODUCTION
Chronic pulmonary regurgitation (PR), which is one of the most common reasons for reintervention in adults with congenital heart disease, particularly in patients with tetralogy of Fallot (TOF) and valvular pulmonic stenosis (VPS) with prior right ventricular (RV) outflow tract interventions.1, 2 Chronic PR causes RV volume overload resulting in increased RV preload, as well as volume overload of the proximal central pulmonary artery (PA) and vascular remodeling ultimately resulting in increased RV afterload.3–5 The goal of pulmonary valve replacement is to prevent progressive RV dysfunction by normalizing these abnormal loading conditions.6 The timing of pulmonary valve replacement is critical in order to avoid the cumulative multiple reinterventions that can occur if pulmonary valve replacement is performed too early or the irreversible RV dysfunction that can result if pulmonary valve replacement is performed too late.7, 8
Quantitative RV volumetric assessment by echocardiography is challenging because of its complex geometry, and as a result cardiac magnetic resonance imaging (CMRI) has become the most commonly used modality for RV volumetric assessment.6 Right ventricular to pulmonary arterial (RV-PA) coupling is a load independent measure of RV performance, which incorporates both RV systolic function and PA vascular function.9 Although RV-PA coupling is typically measured by invasive cardiac catheterization, several studies have demonstrated the feasibility and prognostic value of noninvasively measured RV-PA coupling in patients with heart failure due to acquired heart disease.10–13 This concept has not been studied in adults with congenital heart disease. We hypothesized that echocardiographic indices of RV-PA coupling were comparable to CMRI-derived RV volumetric indices in predicting disease severity in patients with chronic PR.
METHODS
Patient Selection
The MACHD (Mayo Adult Congenital Heart Disease) database was queried for patients with native PR following RV outflow tract and/or pulmonary valve intervention from January 1, 2003 through December 31, 2015. The inclusion criteria were: age >18 years, at least one CMRI scan, ≥moderate PR (defined as regurgitant fraction >25% by CMRI and/or qualitative assessment by Doppler echocardiography), and echocardiographic images of sufficient quality to measure fractional area change (FAC) or tricuspid annular plane systolic excursion (TAPSE). The patients with concomitant pulmonic stenosis (defined as pulmonary valve peak velocity >2 m/s) and the patients with prior pulmonary valve replacements or RV to PA conduit placement were excluded. The Mayo Clinic institutional review board approved this study and waived informed consent for patients that provided research authorization.
A total of 256 patients were identified based on the above inclusion criteria and this comprised the study cohort. A validation cohort of 218 patients with native PR and no prior CMRI was also selected from the MACHD database using the following search criteria: age >18 years, no CMRI, ≥moderate PR by qualitative assessment Doppler echocardiography, and echocardiographic images of sufficient quality to measure FAC or TAPSE. Supplementary Figure 1 shows flowchart of cohort selection.
Data collection
The following electronic health records were reviewed in details: transthoracic echocardiograms, CMRI reports, cardiopulmonary exercise test, clinical notes, and surgical records. The clinical data obtained within 12 months from the time of CMRI were analyzed as the baseline characteristics of the study cohort, and we analyzed only the first CMRI in patients with multiple CMRI scans. For the validation cohort, we analyzed the first transthoracic echocardiogram with adequate image quality for TAPSE or FAC, and we used the clinical data obtained within 12 months from the time of the index echocardiogram as the baseline characteristics. For the purpose of this study, the different types of RV outflow tract interventions were grouped into 3 categories: balloon pulmonary valvuloplasty, transannular patch repair, and non-transannular patch repair (surgical valvotomy, valvectomy or commissurotomy).
The digital echocardiographic images of both the study cohort and the validation cohort were reviewed and offline measurements performed (R.P) and these measurements were verified in randomly selected sample (25% of the cohort) by one of the investigators (A.C.E). All 2D and Doppler variables relevant to the study were collected and analyzed. The severity of tricuspid regurgitation, PR, RV enlargement, and RV systolic dysfunction were graded as none/trivial, mild, mild-moderate, moderate, moderate-severe, and severe based on standard assessment by comprehensive echocardiogram.14 The protocol for volumetric assessment using CMRI at this institution has been previously described.15 RV stroke volume and ejection fraction were calculated from end-diastolic and end-systolic volumes. PR fraction was calculated as: (RV stroke volume – left ventricular stroke volume)/RV stroke volume. All volumetric data were indexed to the body surface area. Plasma volume at the time of echocardiography was estimated by: (1−hematocrit) (a+[b x weight in kg]), where a=1530 in men and 864 in women, and b=41 in men and 47.9 in women.16
Study Endpoints
The primary endpoint was to determine the association between echocardiographic indices of RV-PA coupling and markers of disease severity, and compare this association to that of CMRI-derived RV volumetric indices and markers of disease severity. The secondary endpoint was to assess the predictive value of these echocardiographic indices of RV-PA coupling in the validation cohort. We performed separate analyses for the patients with tetralogy of Fallot (TOF) and those with valvular pulmonic stenosis (VPS), because of differences in the pathophysiology of both diseases.17, 18
RV-PA coupling was assessed by the ratio of fractional area change/right ventricular systolic pressure (FAC/RVSP) and tricuspid annular plane systolic excursion/right ventricular systolic pressure (TAPSE/RVSP). FAC/RVSP and TAPSE/RVSP ratio have been studied as noninvasive indices of RV-PA coupling and provide a measure of in-vivo RV length-forced relationship, and validated as prognostic indices in patients with heart failure due to acquired cardiovascular diseases. 10, 12, 13 The CMRI-derived RV volumetric induces that were analyzed include RV end-diastolic volume index (RVEDVI), RV end-systolic volume index (RVESVI), RV stroke volume index, and RV ejection fraction.
The following indices of disease severity were used for this study: exercise capacity (peak oxygen consumption [VO2]), biomarkers of neurohormonal activation (N-terminal pro b-type natriuretic peptide [NT-proBNP]), and atrial and/or ventricular arrhythmia documented on electrocardiogram, Holter monitor or telemetry. These indices have been shown to be prognostic for heart failure related mortality in patients with congenital and acquired heart diseases.10–13 Only peak VO2 derive from symptom-limited treadmill tests with maximum effort defined as respiratory exchange ratio > 1.1 were used for the analysis.
Statistical Analysis
We reported categorical variables as percentages, and continuous variables as mean ± standard deviation or median (interquartile range) for skewed data. We compared categorical variables with χ2 test or Fisher exact test, and continuous variables with a 2-sided unpaired t test or Mann Whitney test, as appropriate. Linear regression analyses were used to assess the relationships between continuous variables. Univariable logistic regression analyses were used to assess the association between RV indices and arrhythmia occurrence, and the degree of association was compared using the area under the curve. The inter-observer variability between the indices (FAC, TAPSE and RVSP) measured by R.P and A.C.E were assessed by intraclass correlation coefficient. We performed all statistical analyses with JMP software (version 13.0; SAS Institute Inc, Cary NC), a p<0.05 was considered statistically significant.
RESULTS
Baseline clinical and hemodynamic data of study cohort
The study cohort comprised of 256 patients, mean age was 33±6 years, and 132 (52%) were males. Among these 256 patients, 187 (73%) had TOF while 69 (27%) had VPS (Table 1). Of the 187 patients with TOF, 43 (23%) had prior palliative shunt procedures, the age of initial RV outflow tract intervention was 4±2 years, and the types of RV outflow tract interventions were transannular patch repair 126 (67%) and non-transannular patch repair 61 (33%). Of the 69 patients with VPS, the age of initial RV outflow tract intervention was 5±3 years, and the types of RV outflow tract interventions were transannular patch repair 6 (9%) and non-transannular patch repair 47 (68%), and balloon pulmonary valvuloplasty 16 (23%). Table 2 shows baseline hemodynamic data of the study cohort. The RV-PA coupling indices are baseline were: TAPSE/RVSP 0.49±0.08 and FAC/RVSP 0.98±0.17. Of the 256 patients, 122 underwent pulmonary valve replacement, and among these patients, TAPSE/RVSP increased from 0.46±0.09 to 0.51±0.08 (p=0.041) and FAC/RVSP increased from 0.94±0.14 to 0.99±0.14 (p=0.1.93).
Table 1:
Baseline Characteristics
| n=256 | |
|---|---|
| Age, years | 33±6 |
| Male | 132 (52%) |
| Tetralogy of Fallot | 187 (73%) |
| Valvular pulmonic stenosis | 69 (27%) |
| Body surface area, m2 | 29±6 |
| Body mass index, kg/m2 | 2.0±0.3 |
| Systemic arterial saturation, % | 94±4 |
| Comorbidities | |
| Hypertension | 54 (21%) |
| Hyperlipidemia | 59 (23%) |
| Coronary artery disease | 14 (6%) |
| Current or prior smoker | 31 (12%) |
| Diabetes mellitus | 29 (11%) |
| Sleep apnea | 35 (14%) |
| Prior stroke | 14 (6%) |
| NYHA III/IV | 54 (29%)[n=187] |
| Laboratory tests | |
| Hemoglobin, g/dl | 14.6±1.2 |
| Platelet, x109/l | 267±41 |
| Creatinine, mg/dl | 1.2±0.3 |
| Albumin, g/dl | 4.4±0.4 |
| Aspartate aminotransferase, U/l | 32±4 |
| Alanine aminotransferase, U/l | 43±5 |
| Alkaline phosphatase, U/l | 82±8 |
| NT-proBNP, pg/ml | 259±108 [n=173] |
| Estimated plasma volume, ml | 3121± 614 |
| Medications | |
| Diuretics | 57 (22%) |
| Beta and/or calcium channel blockers | 52 (20%) |
| RAAS antagonist | 49 (19%) |
| Class I/III antiarrhythmic drug | 24 (9%) |
| Warfarin | 16 (6%) |
| Direct oral anticoagulants | 7 (3%) |
| Aspirin | 87 (34%) |
NYHA: New York Heart Association; RAAS: renin angiotensin aldosterone system; U/l: unit per liter; pg/ml: picogram per milliliter; NT-proBNP: N-terminal pro b-type natriuretic peptide
Table 2:
Hemodynamic Data
| Echocardiography | n=256 |
|---|---|
| ≥Moderate RV enlargement* | 233 (91%) |
| ≥Moderate RV systolic dysfunction* | 61 (24%) |
| ≥Moderate tricuspid regurgitation* | 69 (27%) |
| Severe Pulmonary regurgitation* | 251 (98%) |
| Tricuspid regurgitation velocity, m/s | 3.3±0.6 |
| RVSP, mmHg | 42±5 |
| Pulmonary valve peak velocity, m/s | 1.4±0.4 |
| TAPSE, cm | 20±6 |
| FAC, % | 39±8 |
| RV S’, cm/s | 11±4 |
| TAPSE/RVSP ratio | 0.49±0.08 |
| FAC/RVSP ratio | 0.98±0.17 |
| PA acceleration time, m/s | 109±14 |
| RV stroke volume index, ml/m2 | 61±11 [n=219] |
| Lateral E/e’ | 9±5 |
| LV ejection fraction, % | 51±6 |
| LV cardiac index, l/min/m2 | 2.9±0.5 |
| Heart rate, bpm | 74±6 |
| Magnetic Resonance Imaging | n=256 |
| RVEDV index, ml/m2 | 136±39 |
| RVESV index, ml/m2 | 69±11 |
| RV stroke volume index, ml/m2 | 69±8 |
| PR volume index, ml/m2 | 28±7 |
| RV ejection fraction, % | 48±11 |
| LV ejection fraction, % | 53±9 |
| LV stroke volume index, ml/m2 | 41±9 |
| Catheterization | n=94 |
| Heart rate, BPM | 66±4 |
| Right atrial pressure, mmHg | 9(6–13) |
| RV EDP, mmHg | 13(11–17) |
| RVSP, mmHg | 46±6 |
| Mean PA pressure, mmHg | 21(17–27) |
| PA wedge pressure, mmHg | 11(9–16) |
| Mean arterial pressure, mmHg | 93±11 |
| Mixed venous O2 saturation, % | 68±7 |
| Systemic O2 saturation, % | 94±4 |
| Cardiac index, l/min*m2 | 2.8±0.6 |
| Qp:Qs | 0.98±0.02 |
| PVRI (WU*m2) | 3.3(2.5–4.7) |
| SVRI (WU*m2) | 29.6±2.3 |
| Cardiopulmonary exercise test | (n=164) |
| Peak VO2, ml/kg/min | 24±5 |
| Peak VO2, % predicted | 68±11 |
| VE/VCO2 nadir | 29±5 |
RV: right ventricle; RVSP: right ventricular systolic pressure; TAPSE: tricuspid annular plane systolic excursion; LV: left ventricle; FAC: fractional area change; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; PR: Pulmonary regurgitation; PA: pulmonary artery; EDP: end-diastolic pressure; Qp:Qs: ratio of pulmonary to systemic blood flow; PVRI: pulmonary vascular resistance index; SVRI: systemic vascular resistance index; WU*m2; Wood units x meter squared;
: Quantitative assessment; O2: oxygen; VO2: oxygen consumption; VE/VCO2: ventilatory equivalent for carbon dioxide
Indices of disease severity
Of the 187 TOF patients, peak VO2 and NT-proBNP data were available in 122 (65%) and 134 (72%) patients respectively. The peak VO2 was 23±5 ml/kg/min (67±12% of predicted) and NT-proBNP was 268±112 pg/ml. The following arrhythmias were present in the TOF patients: atrial flutter (n=38, 20%), atrial fibrillation (n=19, 10%), atrial tachycardia (n=17, 9%), non-sustained ventricular tachycardia (n=24, 13%), and sustained ventricular tachycardia (n=14, 8%). A history of atrial and/or ventricular arrhythmia was present 71 (38%) patients, with several patients had more than one type of arrhythmia.
Of the 69 VPS patients, peak VO2 and NT-proBNP data were available in 42 (61%) and 39 (57%) respectively. The peak VO2 was 25±3 ml/kg/min (70±8% of predicted) and NT-proBNP was 241±68 pg/ml. The following arrhythmias were present in the VPS patients: atrial flutter (n=11, 16%), atrial fibrillation (n=4, 6%), atrial tachycardia (n=6, 9%), non-sustained ventricular tachycardia (n=7, 10%), and sustained ventricular tachycardia (n=1, 2%). Similar to the TOF group, several patients had more than one type of arrhythmia, and a history of atrial and/or ventricular arrhythmia was present 19 (28%) patients.
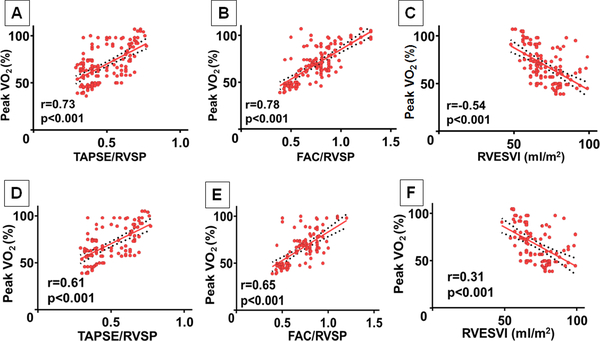
Relationship between echocardiographic and CMRI Indices and disease severity, There was a modest correlation between TAPSE/RVSP and peak VO2 (r=0.73, p<0.001), and between FAC/RVSP and peak VO2 (r=0.78, p<0.001) in TOF patients. Among the CMRI-derived RV volumetric indices analyzed, there was a negative correlation between RVESVI and peak VO2 (r=−0.54, p<0.001), (Figure 1 A-C). Peak VO2 was independent of RVEDVI, RV stroke volume index, and RV ejection fraction in TOF patients. Similar analyses performed in VPS patients also showed similar correlations between TAPSE/RVSP and peak VO2, FAC/RVSP and peak VO2, and RVESVI and peak VO2, (Figure 1 D-F).
Figure 1:
Linear correlation of percent-predicted peak oxygen consumption (peak VO2) and echocardiographic and cardiac magnetic resonance imaging derived indices in the study cohort.
(A) Peak VO2 and tricuspid annular plane systolic excursion/ right ventricular systolic pressure (TAPSE/RVSP) ratio in TOF patients; (B) Peak VO2 and fractional area change/right ventricular systolic pressure (FAC/RVSP) ratio in TOF patients; (C) Peak VO2 and right ventricular end-systolic volume index (RVESVI) in TOF patients; (D) Peak VO2 and TAPSE/RVSP in VPS patients; (E) Peak VO2 and FAC/RVSP in VPS patients; (F) Peak VO2 and RVESVI in VPS patients.
Analyses were performed to determine the associations between CMRI-derived indices and neurohormonal activation, and this showed a correlation between RVESVI and NT-proBNP both in TOF patients (r=0.51, p<0.001) and VPS patients (r=0.46, p=0.003) (figure not shown). There was no association between NT-proBNP and the other CMRI-derived indices and echocardiographic indices.
Analyses for arrhythmia history showed that TAPSE/RVSP was associated with arrhythmia history (AUC 0.697; TAPSE/RVSP <0.45: odds ratio 2.73, 95% confidence interval 1.26–4.11, p=0.031), and RVESVI was also associated with arrhythmia history (AUC 0.778; RVESVI >90 ml/m2: odds ratio 2.94, 95% confidence interval 2.06–4.39, p<0.001) in TOF patients but not in VPS patients. Arrhythmia history was independent of the other CMRI-derived indices and echocardiographic indices.
Validation cohort
A validation cohort of 218 patients was selected based on the criteria described in the Methods section. Supplementary Tables 1 and 2 show a comparison of the clinical and hemodynamic characteristics of the study cohort and validation cohort. The patients in the validation cohort were older (33±6 vs years 37±9 years, p=0.031), and were more likely to be on diuretics, warfarin, and class I/III antiarrhythmic drugs. The other clinical and hemodynamic variables were similar between the study and validation cohorts (Supplementary Tables 1 and 2).
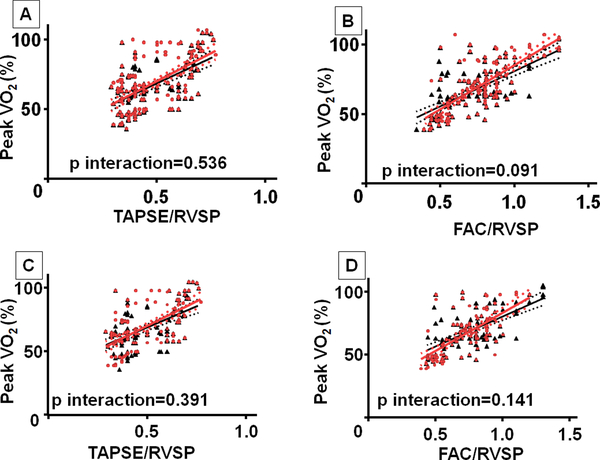
Similar to the observation in the study cohort, there was a modest association between TAPSE/RVSP and peak VO2 (r=0.59, p<0.001), and between FAC/RVSP and peak VO2 (r=0.70, p<0.001) in TOF patients (Figure 2). Similarly, peak VO2 was also associated with TAPSE/RVSP (r=0.50, p<0.001) and FAC/RVSP (r=0.64, p<0.001) in VPS patients. The strength of these associations was similar in the study cohort and validation cohort for all analyses (p interaction > 0.05) (Figure 2). Similar to the observation in the study cohort, TAPSE/RVSP was associated with arrhythmia history (AUC 0.646; TAPSE/RVSP <0.45: odds ratio 1.82, 95% confidence interval 1.17–6.25, p=0.043) in TOF patients but not in VPS patients. Again NT-proBNP was independent of all echocardiographic indices analyzed for both TOF and VPS patients.
Figure 2:
Comparison of linear correlations between study cohort and validation cohort. (A) Peak VO2 and TAPSE/RVSP in TOF patients; (B) Peak VO2 and FAC/RVSP in TOF patients; (C) Peak VO2 and TAPSE/RVSP in VPS patients; (D) Peak VO2 and FAC/RVSP in VPS patients
DISCUSSION
Chronic PR results in abnormal increase in RV preload and afterload, and over time leads to irreversible RV dysfunction and cardiovascular morbidities.3–5 The current study showed that echocardiographic indices of RV-PA coupling were comparable to CMRI derived RV volumetric indices in predicting exercise capacity and arrhythmia in patients with chronic PR.
Chronic PR and exercise capacity
Peak VO2 is a known prognostic marker in congenital and acquired heart diseases,19–21and impaired peak VO2 is a marker of disease severity and risk factor for mortality in the TOF population.22The current study showed modest correlations between peak VO2 and noninvasively measured RV-PA coupling indices (TAPSE/RVSP and FAC/RVSP) in patients with chronic PR (Figure 1).
Latus and colleagues demonstrated that patients with TOF and chronic PR had impaired RV-PA coupling both at rest and with dobutamine stress based on invasive hemodynamic assessment in 24 patients.9 Although the RV end-systolic elastance increased with dobutamine stress, there was a disproportionate increase in PA elastance resulting in a decrease in RV-PA coupling (RV-PA uncoupling) in TOF patients.9 A very important observation in that study was that the patients that had more impaired RV-PA coupling at rest tended to have more RV-PA ‘uncoupling’ with dobutamine stress because of disproportionate increase in PA elastance.9 Although the physiologic effect of exercise differ from that of dobutamine stress, the above study highlights the importance of abnormal PA vascular function and its potential impact on RV pulsatile afterload in the setting of increased pulmonary blood flow. The importance of RV afterload on peak VO2 is also demonstrated in a different study of 42 TOF patients with chronic PR, and in that study the patients with higher RV afterload (due to RV outflow tract obstruction) had significantly lower peak VO2 compared to the rest of the cohort.23 This shows that increase RV afterload, either due to RV outflow tract obstruction or pulmonary vascular dysfunction, has a negative impact on exercise capacity.
The ability of noninvasively measure RV-PA coupling indices (TAPSE/RVSP and FAC/RVSP) to predict peak VO2 in the current study is most likely because it takes into account the effect of abnormal RV afterload due to chronic PR. Even though the pathophysiology and natural history of TOF differs from VPS, 17, 18 a similar relationship between TAPSE/RVSP, FAC/RVSP and peak VO2 was observed in the VPS group. Furthermore there was no difference in the ability of non-invasive RV-PA coupling indices (TAPSE/RVSP and FAC/RVSP) to predict peak VO2 when it was tested in the validation cohort (Figure 2). All these highlight the importance of noninvasive RV-PA coupling indices as a potential supplementary tool in the assessment of patients with chronic PR. The echocardiographic indices used for the assessment of RV-PA coupling this study are part of data collection during routine comprehensive echocardiography and hence do not require any additional resource utilization.
In contrast to noninvasive RV-PA coupling indices, only RVESVI correlated with peak VO2 out of all the CMRI-derived volumetric indices analyzed in this study. CMRI-derived volumetric assessment is currently the gold standard for deciding the timing of pulmonary valve replacement based on studies stipulating RV volume thresholds beyond which complete RV reverse remodeling was unlikely to occur even after pulmonary valve replacement.8, 24 Although CMRI-derived RV volumes can predict RV remodeling after pulmonary valve replacement, it has not been shown to predict improvement in exercise capacity after pulmonary valve replacement.25 Similarly there are mixed results about the association between RV volumes and peak VO2 in chronic PR. We speculated that the discordance in the association between CMRI volumetric indices and peak VO2 may be because CMRI-derived indices only incorporates RV end-diastolic volume (preload) and ejection fraction (load dependent measure of systolic function) but does not take into account the effect of abnormal RV afterload which is present in chronic PR.6
Chronic PR and arrhythmia
Atrial and ventricular arrhythmias are common after TOF repair, and are associated with heart failure and sudden cardiac death in this population.26, 27 Several factors such as left and right ventricular ejection fraction, right atrial enlargement, multiple surgical scars and age at the time of initial repair have been proposed as risk factors for atrial and ventricular arrhythmia after TOF repair.26, 27 In this study we showed that similar to RVESVI, TAPSE/RVSP ratio was associate with arrhythmia occurrence, and there was a 2-fold increase in arrhythmia burden in patients with TAPSE/RVSP <0.45 both in the original study cohort and validation cohort. It is unclear why a similar relationship was not seen with FAC/RVSP ratio. The association between abnormal RV-PA coupling and arrhythmia occurrence in this study further highlights the impact of abnormal hemodynamics (loading conditions) in the pathogenesis of atrial and ventricular arrhythmia in TOF patients. Similarly pulmonary valve replacement as a stand-alone procedure (without concomitant anti-arrhythmia surgery), which normalizes loading conditions, is effective in reducing arrhythmia burden.28
Clinical implications and future directions
RV-PA coupling is a measure of RV performance adjusted for abnormal RV afterload. Since RV afterload is not taken into account in CMRI-derived RV volumetric assessment, perhaps noninvasive RV-PA coupling indices may be complementary to CMRI in the management of chronic PR. RV-PA coupling indices may be most beneficial in the assessment of patients with chronic PR and unexplained exertional dyspnea, and in patients with contraindications for CMRI.
This is the first study about the role of non-invasive RV-PA coupling in adults with congenital heart disease. Although the study has several limitations, it provides preliminary data for future studies such as: (1) Simultaneous invasive and noninvasive assessment of RV-PA coupling in chronic PR; (2) Simultaneous invasive and noninvasive assessment of RV-PA coupling at rest and with exercise in chronic PR patients with unexplained exertional dyspnea; (3) Comparison of noninvasive RV-PA coupling before and after pulmonary valve replacement to determine if RV performance improves after the normalization of loading conditions.
Limitations
The major limitation of this study is the lack of simultaneously acquired invasive hemodynamic data that is necessary for correlation and validation of the noninvasively measured RV-PA coupling indices reported in the current study. The other important limitation is that it is a retrospective single center study from a tertiary center is therefore prone to selection bias, which limits the generalizability of the results.
Conclusions
This study demonstrates that noninvasively measured RV-PA coupling in chronic PR was associated with exercise capacity and arrhythmia occurrence. The ability of these indices to predict peak VO2 and arrhythmia burden was reproducible when applied to a validation cohort. Noninvasive RV-PA coupling indices are not routinely used in the congenital heart disease population currently but may potentially provide complementary prognostic data in the management of chronic PR. Further studies are required to explore other potential applications of this clinical tool.
Supplementary Material
(A) Flowchart showing cohort selection. CMRI: Cardiac magnetic resonance imaging; PR: pulmonary regurgitation
Acknowledgement:
Rae Parker (R.P)
Funding: Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL141448-01
ABBREVIATIONS:
- RV
Right ventricle
- PA
Pulmonary artery
- RV-PA
Right ventricular to pulmonary arterial
- PR
Pulmonary regurgitation
- TOF
Tetralogy of Fallot
- VPS
Valvular pulmonic stenosis
- CMRI
Cardiac magnetic resonance imaging
- FAC
Fractional area change
- TAPSE
tricuspid annular plane systolic excursion
- RVSP
Right ventricular systolic pressure
- VO2
Peak oxygen consumption
Footnotes
Alexander C. Egbe, William R. Miranda, Patricia A. Pellikka,Sorin V. Pislaru, MD:Study design, data collection, data analysis, manuscript drafting, critical revision, final review/approval
Barry A. Borlaug; Srikanth Kothapalli; Sindhura Ananthaneni; Harigopal Sandhyavenu; Maria Najam, MD, Mohamed Farouk Abdelsamid,; Heidi M. Connolly: manuscript drafting, critical revision, final review/approval
REFERENCES
- 1.Karamlou T, Diggs BS, Person T, Ungerleider RM and Welke KF. National practice patterns for management of adult congenital heart disease: operation by pediatric heart surgeons decreases in-hospital death. Circulation. 2008;118:2345–52. [DOI] [PubMed] [Google Scholar]
- 2.Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, McGoon DC, Kirklin JW and Danielson GK. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med 1993;329:593–9. [DOI] [PubMed] [Google Scholar]
- 3.Kilner PJ, Balossino R, Dubini G, Babu-Narayan SV, Taylor AM, Pennati G and Migliavacca F. Pulmonary regurgitation: the effects of varying pulmonary artery compliance, and of increased resistance proximal or distal to the compliance. Int J Cardiol 2009;133:157–66. [DOI] [PubMed] [Google Scholar]
- 4.Rommel JJ, Yadav PK and Stouffer GA. Causes and hemodynamic findings in chronic severe pulmonary regurgitation. Catheter and Cardiovasc Interv 2015;23:1002–06 [DOI] [PubMed] [Google Scholar]
- 5.Bossers GPL, Hagdorn QAJ, Ploegstra MJ, Borgdorff MAJ, Sillje HHW, Berger RMF and Bartelds B. Volume load-induced right ventricular dysfunction in animal models: insights in a translational gap in congenital heart disease. Eur J Heart Fail 2017. [DOI] [PubMed] [Google Scholar]
- 6.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Mag Res 2011;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oosterhof T, Vliegen HW, Meijboom FJ, Zwinderman AH, Bouma B and Mulder BJ. Long-term effect of pulmonary valve replacement on QRS duration in patients with corrected tetralogy of Fallot. Heart. 2007;93:506–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokma JP, Winter MM, Oosterhof T, Vliegen HW, van Dijk AP, Hazekamp MG, Koolbergen DR, Groenink M, Mulder BJ and Bouma BJ. Preoperative thresholds for mid-to-late haemodynamic and clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Eur Heart J. 2016;37:829–35. [DOI] [PubMed] [Google Scholar]
- 9.Latus H, Binder W, Kerst G, Hofbeck M, Sieverding L and Apitz C. Right ventricular-pulmonary arterial coupling in patients after repair of tetralogy of Fallot. JTCVS. 2013;146:1366–72. [DOI] [PubMed] [Google Scholar]
- 10.Melenovsky V, Hwang SJ, Lin G, Redfield MM and Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur H J. 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, Mathier MA, Lacomis J, Champion HC and Simon MA. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL and Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013;305:H1373–81. [DOI] [PubMed] [Google Scholar]
- 13.Hussain I, Mohammed SF, Forfia PR, Lewis GD, Borlaug BA, Gallup DS and Redfield MM. Impaired Right Ventricular-Pulmonary Arterial Coupling and Effect of Sildenafil in Heart Failure With Preserved Ejection Fraction: An Ancillary Analysis From the Phosphodiesterase-5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure (RELAX) Trial. Circ Heart Fail 2016;9:e002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD and Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 15.Sabate Rotes A, Bonnichsen CR, Reece CL, Connolly HM, Burkhart HM, Dearani JA and Eidem BW. Long-term follow-up in repaired tetralogy of fallot: can deformation imaging help identify optimal timing of pulmonary valve replacement? J Am Soc Echocardiogr 2014;27:1305–10. [DOI] [PubMed] [Google Scholar]
- 16.Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, Mendonca M, Rashid M, Kang S, Papalia F, Weissert S, Coats CJ, Thomas M, Kuskowski M, Cohn JN, Woldman S, Anand IS and Okonko DO. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015;17:35–43. [DOI] [PubMed] [Google Scholar]
- 17.Joynt MR, Yu S, Dorfman AL, Mahani Ghadimi M, Agarwal PP and Lu JC. Differential Impact of Pulmonary Regurgitation on Patients With Surgically Repaired Pulmonary Stenosis Versus Tetralogy of Fallot. Am J Cardiol 2016;117:289–94. [DOI] [PubMed] [Google Scholar]
- 18.Bokma JP, Winter MM, Oosterhof T, Vliegen HW, van Dijk AP, Pieper PG, Meijboom FJ, Groenink M, Mulder BJ and Bouma BJ. Pulmonary Valve Replacement After Repair of Pulmonary Stenosis Compared With Tetralogy of Fallot. J Am Coll Cardiol 2016;67:1123–4. [DOI] [PubMed] [Google Scholar]
- 19.Chaix MA, Marcotte F, Dore A, Mongeon FP, Mondesert B, Mercier LA and Khairy P. Risks and Benefits of Exercise Training in Adults With Congenital Heart Disease. Can J Cardiol 2016;32:459–66. [DOI] [PubMed] [Google Scholar]
- 20.Egbe AC, Driscoll DJ, Khan AR, Said SS, Akintoye E, Berganza FM and Connolly HM. Cardiopulmonary exercise test in adults with prior Fontan operation: The prognostic value of serial testing. Int J Card 2017;235:6–10. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL and Investigators H-A. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babu-Narayan SV, Diller GP, Gheta RR, Bastin AJ, Karonis T, Li W, Pennell DJ, Uemura H, Sethia B, Gatzoulis MA and Shore DF. Clinical outcomes of surgical pulmonary valve replacement after repair of tetralogy of Fallot and potential prognostic value of preoperative cardiopulmonary exercise testing. Circulation. 2014;129:18–27. [DOI] [PubMed] [Google Scholar]
- 23.Freling HG, Willems TP, van Melle JP, van Slooten YJ, Bartelds B, Berger RM, van Veldhuisen DJ and Pieper PG. Effect of right ventricular outflow tract obstruction on right ventricular volumes and exercise capacity in patients with repaired tetralogy of fallot. Am J Cardiol 2014;113:719–23. [DOI] [PubMed] [Google Scholar]
- 24.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A and Mulder BJ. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–51. [DOI] [PubMed] [Google Scholar]
- 25.Sabate Rotes A, Johnson JN, Burkhart HM, Eidem BW, Allison TG and Driscoll DJ. Cardiorespiratory Response to Exercise before and after Pulmonary Valve Replacement in Patients with Repaired Tetralogy of Fallot: A Retrospective Study and Systematic Review of the Literature. Cong Heart Dis 2015;10:263–70. [DOI] [PubMed] [Google Scholar]
- 26.Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, Valente AM, Earing MG, Lui G, Gersony DR, Cook S, Ting JG, Nickolaus MJ, Webb G, Landzberg MJ, Broberg CS and Alliance for Adult Research in Congenital C. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868–75. [DOI] [PubMed] [Google Scholar]
- 27.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD and Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 2000;356:975–81. [DOI] [PubMed] [Google Scholar]
- 28.Therrien J, Siu SC, Harris L, Dore A, Niwa K, Janousek J, Williams WG, Webb G and Gatzoulis MA. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001;103:2489–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Flowchart showing cohort selection. CMRI: Cardiac magnetic resonance imaging; PR: pulmonary regurgitation