Abstract
Background
Nosocomial infections continue to be a significant cause of morbidity and mortality among preterm and/or low birth weight (LBW) infants. Preterm infants are deficient in immunoglobulin G (IgG); therefore, administration of intravenous immunoglobulin (IVIG) may have the potential of preventing or altering the course of nosocomial infections.
Objectives
To use systematic review/meta‐analytical techniques to determine whether IVIG administration (compared with placebo or no intervention) to preterm (< 37 weeks' postmenstrual age (PMA) at birth) or LBW (< 2500 g birth weight) infants or both is effective/safe in preventing nosocomial infection.
Search methods
For this update, MEDLINE, EMBASE, CINAHL, The Cochrane Library, Controlled Trials, ClinicalTrials.gov and PAS Abstracts2view were searched in May 2013.
Selection criteria
We selected randomised controlled trials (RCTs) in which a group of participants to whom IVIG was given was compared with a control group that received a placebo or no intervention for preterm (< 37 weeks' gestational age) and/or LBW (< 2500 g) infants. Studies that were primarily designed to assess the effect of IVIG on humoral immune markers were excluded, as were studies in which the follow‐up period was one week or less.
Data collection and analysis
Data collection and analysis was performed in accordance with the methods of the Cochrane Neonatal Review Group.
Main results
Nineteen studies enrolling approximately 5000 preterm and/or LBW infants met inclusion criteria. No new trials were identified in May 2013.
When all studies were combined, a significant reduction in sepsis was noted (typical risk ratio (RR) 0.85, 95% confidence interval (CI) 0.74 to 0.98; typical risk difference (RD) ‐0.03, 95% CI 0.00 to ‐0.05; number needed to treat for an additional beneficial outcome (NNTB) 33, 95% CI 20 to infinity), and moderate between‐study heterogeneity was reported (I2 54% for RR, 55% for RD). A significant reduction of one or more episodes was found for any serious infection when all studies were combined (typical RR 0.82, 95% CI 0.74 to 0.92; typical RD ‐0.04, 95% CI ‐0.02 to ‐0.06; NNTB 25, 95% CI 17 to 50), and moderate between‐study heterogeneity was observed (I2 50% for RR, 62% for RD). No statistically significant differences in mortality from all causes were noted (typical RR 0.89, 95% CI 0.75 to 1.05; typical RD ‐0.01, 95% CI ‐0.03 to 0.01), and no heterogeneity for RR (I2 = 21%) or low heterogeneity for RD was documented (I2 = 28%). No statistically significant difference was seen in mortality from infection; in incidence of necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD) or intraventricular haemorrhage (IVH) or in length of hospital stay. No major adverse effects of IVIG were reported in any of these studies.
Authors' conclusions
IVIG administration results in a 3% reduction in sepsis and a 4% reduction in one or more episodes of any serious infection but is not associated with reductions in other clinically important outcomes, including mortality. Prophylactic use of IVIG is not associated with any short‐term serious side effects.
The decision to use prophylactic IVIG will depend on the costs and the values assigned to the clinical outcomes. There is no justification for conducting additional RCTs to test the efficacy of previously studied IVIG preparations in reducing nosocomial infections in preterm and/or LBW infants.
Plain language summary
Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants
Infants may acquire infections while in the womb or in the hospital after birth, especially if they require intensive care. Such infections may cause serious illness or death. Transport of immunoglobulin (substance in the blood that can fight infection) from the mother to the fetus mainly occurs after 32 weeks' gestation, and infants do not begin to produce immunoglobulin until several months after birth. Theoretically, the adverse effects of infection could be reduced by the preventive administration of intravenous immunoglobulin. To date, approximately 5000 infants have been enrolled in studies conducted to evaluate the effects of prophylactic use of intravenous immunoglobulin on neonatal outcomes. Intravenous administration of immunoglobulin results in a 3% reduction in blood‐borne infection and a 4% reduction in serious infection. Intravenous administration of immunoglobulin is not associated with reductions in other important neonatal outcomes or in length of hospital stay. Most important, intravenous immunoglobulin administration does not have any important effect on mortality. Prophylactic use of IVIG is not associated with any short‐term serious side effects. From a clinical perspective, a 3% to 4% reduction in nosocomial infection without a reduction in mortality or other important clinical outcomes is of marginal importance.
Background
Description of the condition
Although survival has improved for preterm and/or low birth weight (LBW) infants, nosocomial infection continues to be a significant cause of morbidity and mortality in this population. A 25% incidence of late‐onset infection has been reported in a cohort of 6911 very LBW infants who were admitted to 12 US centres and who survived beyond three days (Stoll 1996). Neonates in whom late‐onset sepsis developed were significantly more likely to die than those who were not infected (17% vs 7%; P < 0.0001) (Stoll 1996).
Description of the intervention
Intravenous immunoglobulin (IVIG) contains pooled immunoglobulin G (IgG) extracted from the plasma of more than 1000 blood donors.
IVIG is frequently given to immunodeficient patients who have decreased antibody production capabilities. In immunodeficient patients, IVIG is administered to maintain adequate antibody levels to prevent infection and to confer passive immunity.
How the intervention might work
Maternal transport of immunoglobulins to the fetus mainly occurs after 32 weeks' gestation, and endogenous synthesis does not begin until about 24 weeks after birth, so the preterm infant is especially vulnerable to infectious sources in the neonatal intensive care unit (Baker 1990a). Mean serum levels of IgG are 400 mg/dL in infants at less than 32 weeks' gestational age (GA) compared with 1000 mg/dL in term infants (Hobbs 1967; Stiehm 1966). The idea of preventing nosocomial infection with IVIG is attractive, as administration of IVIG provides IgG that can bind to cell surface receptors, provide opsonic activity, activate complement, promote antibody‐dependent cytotoxicity and improve neutrophilic chemo‐luminescence (Baley 1988).
Why it is important to do this review
Administration of IVIG to LBW infants has been studied extensively. Numerous descriptive review articles, commentaries and editorials on the use of IVIG in neonates have been published, often by the same researchers. These papers have included several randomised controlled trials (RCTs), the authors' personal experience with IVIG and/or information about the preparation or dosing regimen of IVIG (Weisman 1986; Bortolussi 1986a; Bortolussi 1986b; Fischer 1986; Stiehm 1986; Baley 1988; Fischer 1988; Gonzalez 1989; Kyllonen 1989; Stabile 1989; Noya 1989; Johnston 1990; Fischer 1990a; Fischer 1990b; Fischer 1990c; Baker 1990a; Baker 1990b; Bussel 1990b; Hammarstrom 1990; Kliegman 1990; Stiehm 1990; Whitelaw 1990; Berger 1991; Hill 1991a; Hill 1991b; Irani 1991; Kliegman 1991; Magny 1991a; Rondini 1991; Haque 1992; Siber 1992; Weisman 1992; Hill 1993; Weisman 1993; Weisman 1994b; Wolach 1997). Salzer (Salzer 1991) presented (in abstract form only) the results of a meta‐analysis of seven studies and concluded that no significant reduction was seen in the incidence of sepsis in the treated group. In "Effective Care of the Newborn Infant", Baley and Fanaroff (Baley 1992) present overviews of RCTs that studied the administration of IVIG to neonates. They reviewed seven studies of the prophylactic use of IVIG that reported an outcome of sepsis and concluded, "The preliminary data generated in trials of IVIG are promising, but use of this treatment modality still needs to be considered experimental and [it] should only, as yet, be used under study conditions." Lacy and Ohlsson (Lacy 1995) included additional trials and concluded that routine administration of IVIG to preterm infants to prevent infection is not recommended. Jenson and Pollock (Jenson 1997) used slightly different inclusion criteria and, like Lacy and Ohlsson (Lacy 1995), noted heterogeneity among studies. They concluded, "this heterogeneity probably belies the minimal benefit, at most, of prophylactic IVIG". The results of a Canadian multidisciplinary consensus‐building initiative (Consensus 1997) have been published, and the use of IVIG for prophylaxis of neonatal nosocomial infection was considered inappropriate. This review provides an update of our previous review, which was last updated with no changes in 2010 (Ohlsson 2001; Ohlsson 2007) and was first published in 1998 (Ohlsson 1998).
Objectives
To use systematic review/meta‐analytical techniques to determine whether IVIG administration (compared with placebo or no intervention) to preterm (< 37 weeks' gestational age (GA) at birth)or LBW (< 2500 g birth weight) infants or both is effective/safe in preventing nosocomial infection.
Methods
Criteria for considering studies for this review
Types of studies
Studies in which preterm and/or LBW neonates were randomly assigned to receive IVIG or placebo or no intervention.
Types of participants
Preterm and/or LBW neonates.
Types of interventions
IVIG for the prevention of bacterial or fungal infection. Studies that were designed to evaluate the effects of IVIG on humoral immune markers were excluded, as were studies in which the follow‐up period was one week or less. Studies that assessed the effectiveness of IVIG for treatment of suspected or confirmed infection were excluded.
Types of outcome measures
Primary outcomes
Sepsis, one or more episodes (clinical signs and symptoms of sepsis and positive blood culture for bacteria or fungi).
Secondary outcomes
Any serious infection (clinical signs and symptoms in conjunction with positive cultures (bacteria or fungi) from normally sterile body fluids (blood, cerebrospinal fluid, urine obtained by catheterization or suprapubic tap) or from tissue at autopsy). As per this definition, cases of sepsis if reported separately were also included in any serious infection.
Necrotizing enterocolitis (NEC) diagnosed according to Bell's criteria (Bell 1978). For repeated episodes of sepsis, any serious infection and NEC, only one occurrence per infant was counted as an outcome.
Death from all causes.
Death from infection (including death from NEC).
Length of hospital stay.
Incidence of bronchopulmonary dysplasia (BPD), defined as an additional oxygen requirement (above room air) at 28 days of age or a requirement for assisted ventilation for reasons other than apnoea of prematurity.
Incidence of intraventricular haemorrhage (IVH), any grade, classified according to Papile (Papile 1983).
Incidence of IVH, grade 3 or 4, classified according to Papile (Papile 1983).
Reports on possible side effects as described by the authors.
Search methods for identification of studies
The search strategy used to identify studies adhered to the guidelines of the Cochrane Neonatal Review Group.
Electronic searches
MEDLINE was searched from 1966 to July 2007. EMBASE (Excerpta Medica online) was searched from 1980 to July 2007. The Cochrane Library, Issue 2, 2007, was searched. No language restrictions were applied. Ms Elizabeth Uleryk developed and applied an extensive search strategy (available upon request) for MEDLINE and EMBASE in February 2001 and September 2003. The same strategy was used in 2007.
In December 2009, we updated the search as follows: MEDLINE (search via PubMed), CINAHL, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library) were searched from 2003 to December 2009. Search term: immunoglobulin. Limits: human, newborn infant and clinical trial. No language restrictions were applied.
Searches on May 28, 2013, were conducted by Ms Coleen M. Ovelman, Trials Search Co‐ordinator, and Yolanda R. Brosseau, Managing Editor, the Cochrane Neonatal Review Group. Abstracts of the Pediatric Academic Societies (PAS) Annual Meetings from 2000 to 2013 were searched on the same day by one of the review authors (AO).
Searching other resources
The search was initiated by review of personal files and published meta‐analyses. The reference list of identified studies and subsequently retrieved articles was scanned for additional references.
Data collection and analysis
Data collection and analysis were done in accordance with the methods of the Cochrane Neonatal Review Group.
Selection of studies
The criteria used to select studies for inclusion in this overview were:
design: RCT in which treatment with IVIG was compared with a placebo or no intervention provided to a control group;
population: inclusion of preterm (< 37 weeks' gestational age) and/or LBW (< 2500 g) infants;
intervention: administration of IVIG for the prevention of bacterial/fungal infection during initial hospital stay (8 days or longer). (Studies that were primarily designed to assess the effects of IVIG on humoral immune markers were excluded, as were studies in which the follow‐up period was one week or less. Studies designed to assess the effectiveness of treatment with IVIG for suspected/established infection were excluded.); and
reporting: included at least one of the following outcomes: sepsis, any serious infection, death from all causes, death from infection, length of hospital stay, IVH, NEC or BPD;and descriptions of side effects.
The titles (and abstracts when available) in MEDLINE, EMBASE and The Cochrane Library printouts were reviewed by the two review authors. Any article that the review authors believed might meet the inclusion criteria noted above or should have its reference list searched was retrieved. Informal attempts were made to locate unpublished studies, and attempts were made to request additional information from authors of published studies. Additional information was obtained on one published study (Sandberg 2000).
All identified trials (excluding those that used IVIG for treatment) are listed in the tables of Included studies and Excluded studies.
Data extraction and management
Data abstraction forms were developed and were pilot‐tested to verify definitions of terms. The two review authors independently abstracted information on each study, and one review author (AO) checked for any discrepancies and pooled the results. Data abstraction included whether the study involved prophylaxis or treatment, the number of participants enrolled, the number of participants enrolled but later excluded, the time period and geographical location of the study, baseline characteristics of participants, inclusion/exclusion criteria, the preparation and dosing regimen of IVIG and placebo and length of follow‐up.
Information on outcomes and on the numbers of affected infants was abstracted. The total number of infants with sepsis (clinical signs and symptoms plus positive blood culture (bacteria or fungi)) and any serious infection (clinical signs and symptoms in conjunction with positive cultures (bacteria or fungi) from normally sterile body fluids) was abstracted, as was information on NEC, death from all causes and death from infection. Information on length of hospital stay and on incidence of BPD and IVH was collected. Information on probable infection was not collected, as the definitions used by different investigators were too variable.
Assessment of risk of bias in included studies
Assessment of the quality of included studies (excluding abstracts) was performed independently by JBL and AO, using criteria developed by the Cochrane Neonatal Review Group. These criteria included blinding of randomisation, blinding of the intervention, complete follow‐up and blinding of outcome measurement. For each criterion, three possibilities were identified: yes, can't tell and no. The assignment was not done with the assessors blinded to author, institution, journal of publication or results, as both assessors were familiar with most of the studies and with the typographical layout of the journals and would have knowledge of these even when blinded. In addition, the results sections of articles often include methodological information. After independent evaluation was performed, the two assessors discussed the results of each study, and any discrepancies were resolved.
For the update in 2009, the following issues were evaluated and entered into the risk of bias table:
Sequence generation: Was the allocation sequence adequately generated?;
Allocation concealment: Was allocation adequately concealed?;
Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?;
Incomplete outcome data: Were incomplete outcome data adequately addressed?;
Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?; and
Other sources of bias: Was the study apparently free of other problems that could put it at high risk of bias?
Measures of treatment effect
The statistical package (RevMan 5.2) provided by the Cochrane Collaboration was used. Typical relative risk (RR) and typical risk difference (RD) with 95% confidence intervals (CIs) using the fixed‐effect model are reported. If a statistically significant reduction in RD was noted, the number needed to treat to benefit (NNTB) or harm (NNTH) was calculated.
Assessment of heterogeneity
Statistically significant between‐study heterogeneity was reported when identified, and the test for inconsistency (I2 statistic) was applied when statistically significant heterogeneity was noted (Higgins 2003). For this update, the following cut‐offs were used:
<25%: no heterogeneity;
25% to 49%: low heterogeneity;
50% to 74%: moderate heterogeneity; and
> 75%: high heterogeneity.
Data synthesis
Meta‐analysis was performed using Review Manager software (RevMan 5.2) as supplied by The Cochrane Collaboration. For estimates of typical RR and RD, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. All meta‐analyses were done using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
Future updates will consider post hoc subgroup analyses to explore any heterogeneity noted in the primary analysis.
Results
Description of studies
Included Studies Details of the included studies are provided in the Table of Included Studies.
Nineteen studies, including approximately 5000 preterm and/or LBW infants, met inclusion criteria. These studies were performed in many countries (US, Italy, UK, Saudi Arabia, France, Thailand, Belgium, Turkey, Sweden and Austria). The amount of IVIG per dose varied from 120 mg/kg (Haque 1986) to 1 g/kg (Bussel 1990a). The number of doses varied from a single dose (Atici 1996, Haque 1986, Christensen 1989, Ratrisawadi 1991, Weisman 1994a) to seven doses (Stabile 1988).
Different IVIG preparations were used: Gammagard (Baker 1992); Sandoglobulin (Atici 1996, Bussel 1990a, Chirico 1987, Clapp 1989, Fanaroff 1994, Tanzer 1997, Van Overmeire 1993, Weisman 1994a); Gamimmune (Christensen 1989); Intraglobin (Conway 1990, Haque 1986, Ratrisawadi 1991); IgVena (Didato 1988); Biotransfusion (Magny 1991b); unnamed product (Spady 1994; Sandberg 2000 (study supported by Baxter AG, Austria)); Venogamma (Stabile 1988); Gammumine‐N (Chou 1998).
Excluded Studies Six studies were excluded, as they included infants who were heavier or more mature at birth than was permitted by the inclusion criteria (Kinney 1991, Adhikari 1996); lacked information on outcomes (Kacet 1991; Malik 1990); lacked a randomised control group (Acunas 1994) or provided immunoglobulin intramuscularly (Monintja 1989).
Risk of bias in included studies
The assessment of individual studies is presented in the Characteristics of included studies table.
The methodological quality of the studies varied. Five studies were of high quality (Baker 1992, Christensen 1989, Clapp 1989, Fanaroff‐I 1994, Weisman 1994a) (i.e. complete follow‐up, blinding of randomisation, intervention and outcome measurement could be ascertained from the published reports). In the remaining 15 studies, elements of bias could not be excluded. The lack of a placebo in 10 studies (Atici 1996, Chirico 1987, Conway 1990, Didato 1988, Fanaroff 1994 (phase II), Haque 1986, Ratrisawadi 1991, Stabile 1988, Tanzer 1997, Van Overmeire 1993) precluded blinding of the caregivers. One study (Fanaroff 1994) included two phases, with phase I providing a placebo but not phase II. In several studies, blinding of randomisation was not clearly described (Chirico 1987, Magny 1991b, Ratrisawadi 1991, Stabile 1988). In the study by Sandberg (Sandberg 2000), an intention‐to‐treat analysis was not applied. One study (Spady 1994) has been published in abstract form only, and the quality therefore could not be fully assessed. The study by Bussel (Bussel 1990a) represents an interim analysis, with data lacking from a large proportion of the infants randomly assigned.
Effects of interventions
Nineteen studies met inclusion criteria. These included a total of approximately 5000 preterm and/or LBW infants and reported on at least one of the outcomes of interest for this systematic review. No new trial was identified in the literature search conducted in May 2013. One additional trial was identified in July 2007 (Lelik 2004). However, this trial enrolled infants at greater than 38 weeks' gestational age and with birth weight greater than 2500 g. The study was therefore excluded. No new studies were identified in the literature search conducted in September 2003.
For details of results, see Data and analyses. It should be noted that for most outcomes, the large study by Fanaroff (Fanaroff 1994) greatly influenced the summary statistics, with an assigned weight ranging from 42.7% for the outcome of any serious infection to 88.3% for the outcome of IVH grade 3 or 4.
IVIG VERSUS PLACEBO OR NO TREATMENT (COMPARISON 1)
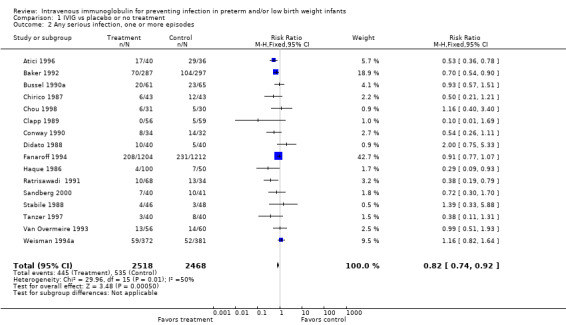
PRIMARY OUTCOME Sepsis, one or more episodes (Outcome 1.1)(Figure 1): Ten studies (including 3975 infants) reported on the outcome of one or more episodes of sepsis per infant (clinical signs and symptoms of infection and positive blood culture). Only the study by Ratrisawadi (Ratrisawadi 1991) showed a statistically significant reduction in sepsis (RR 0.38; 95% CI 0.19 to 0.79). When all studies were combined, a statistically significant (P = 0.02) reduction in sepsis was noted (typical RR 0.85, 95% CI 0.74 to 0.98); typical RD ‐0.03, 95% CI ‐0.05 to 0.00; NNT 33, 95% CI 20 to infinity). Significant between‐study heterogeneity was observed for this outcome for both RR and RD (P = 0.02; I2 = 54%; moderate for RR, 55% moderate for RD). Any serious infection, one or more episodes (Outcome 1.2)(Figure 2): Sixteen studies (including 4986 infants) reported on one or more episodes of any serious infection (sepsis, meningitis, urinary tract infection). Four studies (Atici 1996, Baker 1992, Haque 1986, Ratrisawadi 1991) showed a statistically significant reduction in any serious infection. A statistically significant reduction was found when all studies were combined (typical RR 0.82, 95% CI 0.74 to 0.92; typical RD ‐0.04, 95% CI ‐0.06 to ‐0.02; NNTB 25, 95% CI 17 to 50). Statistically significant between‐study heterogeneity was observed for this outcome (P = 0.01 and I2 = 50% (moderate) for RR; P = 0.0006 and I2 = 62% (moderate) for RD).
1.
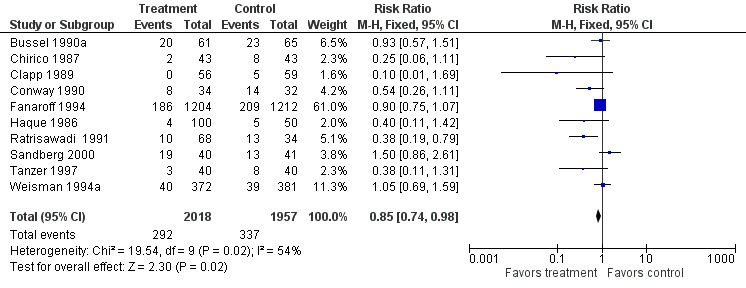
Forest plot of comparison: 1 IVIG versus placebo or no treatment, outcome: 1.1 Sepsis, one or more episodes.
2.
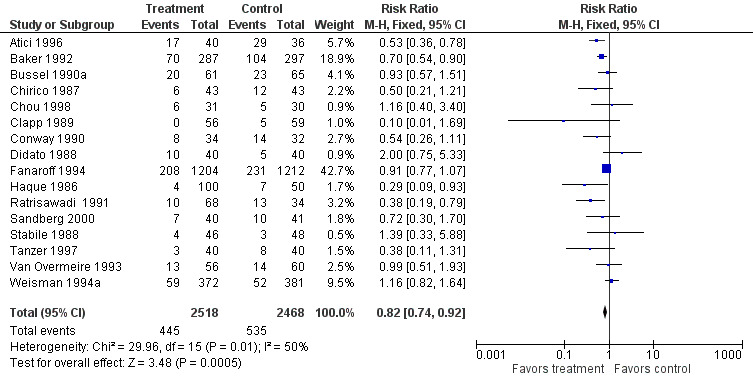
Forest plot of comparison: 1 IVIG versus placebo or no treatment, outcome: 1.2 Any serious infection, one or more episodes.
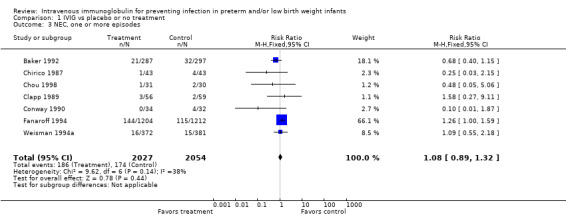
Necrotizing enterocolitis (NEC), one or more episodes (Outcome 1.3): Seven studies (including 4081 infants) reported on NEC (Bell's stage 2 or 3). One study (Fanaroff 1994) showed a borderline statistically significant increase in NEC (RR 1.26, 95% CI 1.00 to 1.59). When all studies were combined, no significant increase was evident (typical RR 1.08, 95% CI 0.89 to 1.32; typical RD 0.01, 95% CI ‐0.01 to 0.02). No statistically significant between‐study heterogeneity was observed for this outcome for RR (P = 0.14; I2 = 38% (low)), but it was observed for RD (P = 0.05, I2 = 52% (moderate)).
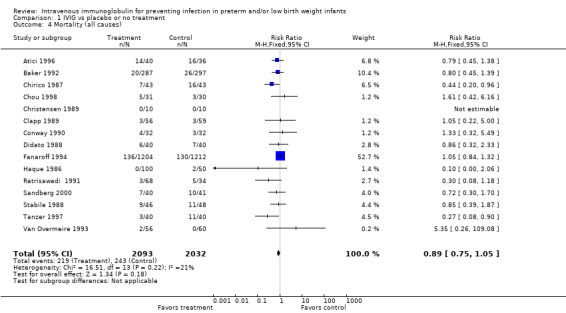
Mortality (all causes) (Outcome 1.4)(Figure 3): Fifteen studies (including 4125 infants) reported on mortality from all causes. Two studies (Chirico 1987, Tanzer 1997) showed a statistically significant reduction in this outcome. When all studies were combined, no statistically significant reduction was noted (typical RR 0.89, 95% CI 0.75 to 1.05; typical RD ‐0.01, 95% CI ‐0.03 to 0.01). No statistically significant between‐study heterogeneity was observed for this outcome for RR (P = 0.22; I2 = 21% (none)) and for RD (P = 0.15; I2 = 28% (low)).
3.
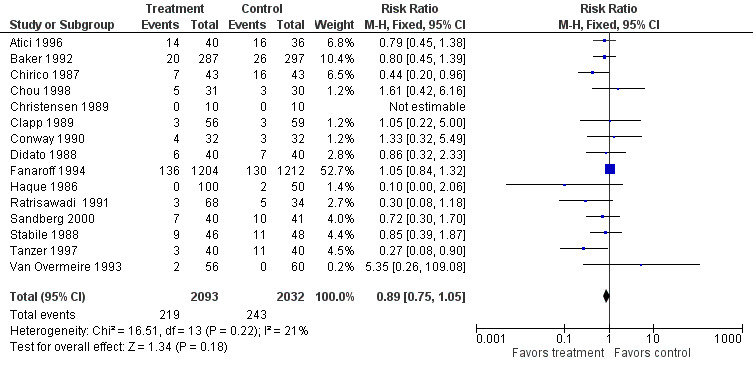
Forest plot of comparison: 1 IVIG versus placebo or no treatment, outcome: 1.4 Mortality (all causes).
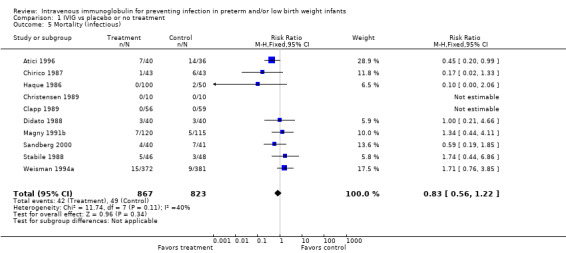
Mortality (infectious) (Outcome 1.5): Ten studies (including 1690 infants) reported on mortality from infection. One study (Atici 1996) showed a statistically significant reduction in this outcome. The overall analysis showed no significant impact of IVIG prophylaxis on this outcome (typical RR 0.83, 95% CI 0.56 to 1.22; typical RD ‐0.01, 95% CI ‐0.03 to 0.01). No statistically significant between‐study heterogeneity was observed for this outcome for RR (P = 0.11; I2 = 40% (low)) and for RD (P = 0.08; I2 = 42% (low)).
Duration of hospitalisation (Outcome 1.6): None of eight studies (including 3562 infants) reported a significant reduction in length of hospital stay after IVIG prophylaxis. The overall typical weighted mean difference was ‐2.1 days (95% CI ‐4.5 to 0.3). No statistically significant between‐study heterogeneity was observed (P = 0.67 and I2 = 0% (none) for both RR and RD).
Bronchopulmonary dysplasia (BPD) (Outcome 1.7): In only one study, the outcome of BPD was defined and data were provided. Several authors failed to define the outcome of BPD, and others defined the outcome but did not provide data. In a small study (n = 115), Clapp (Clapp 1989) showed a trend towards increased BPD (RR 1.53, 95% CI 0.78 to 3.01; RD 0.10, 95% CI ‐0.06 to 0.25). In another small study (n = 61), Chou (Chou 1998) found similar results (RR 1.61, 95% CI 0.42 to 6.16; RD 0.06, 95%CI ‐0.11 to 0.23). When combined (n = 176), the typical RR was 1.55 (95% CI 0.85 to 2.84), and the typical RD was 0.09 (95% CI ‐0.03 to 0.20). No between‐study heterogeneity was observed for this outcome for RR (P = 0.95; I2 = 0% (none)) and for RD (P = 0.74; I2 = 0% (none)).
Intraventricular haemorrhage (IVH) any grade (Outcome 1.8): Four studies (including 3176 infants) reported on IVH (any grade). Prophylactic IVIG did not have a statistically significant effect on this outcome (typical RR 1.02, 95% CI 0.88 to 1.19; typical RD 0.00, 95% CI ‐0.02 to 0.03). No statistically significant between‐study heterogeneity was observed for this outcome (RR, P = 0.39; I2 = 0.9% (none); RD, P = 0.39; I2 = 0.6% (none)).
Intraventricular haemorrhage (IVH) grade 3 or 4 (Outcome 1.9): Two studies (including 3000 infants) reported on IVH grade 3 or 4. The typical RR was 1.01 (95% CI 0.85 to 1.21), and the typical RD was 0.00 (95% CI ‐0.02 to 0.03). Statistically significant between‐study heterogeneity was observed (RR, P = 0.09, I2 = 65% (moderate); RD, P = 0.08, I2 = 68% (moderate)).
A rise in serum IgG in the treatment group was noted in all studies that measured serum levels of IgG.
No major adverse effects of IVIG were reported in any of the studies.
Results from excluded studies (see Characteristics of excluded studies) were similar to those from included studies.
Discussion
The effectiveness of IVIG in preventing nosocomial infection in neonates has been well studied. To date, more than 5000 preterm and/or LBW neonates have been enrolled in trials from many different areas of the world. No new trial was identified for this update conducted in May 2013. One additional trial was identified for the update of the review conducted in July 2007 (Lelik 2004). However, the study included infants at > 38 weeks' gestation and > 2500 g birth weight. Therefore, the study was excluded.
The methodological quality of the included trials varied. Five studies were of high quality, but elements of bias could not be excluded in the other studies, mainly because of the fact that the intervention and the assessment of outcomes were performed unblinded to group assignment, or there was lack of complete follow‐up of all randomly assigned infants. IVIG caused increased levels of IgG in serum. No major side effects were noted.
A small but statistically significant reduction in the incidence of sepsis and of any serious infection was found. Statistically significant between‐study heterogeneity was observed for these outcomes. The heterogeneity might be explained in part by variable rates of sepsis and any serious infection in the control groups; differences in preparation, dose and/or dose schedule for IVIG; differences in causative organisms for nosocomial infection; differences in attention to other preventive measures for nosocomial infection and differences in other co‐interventions by place and over time. Some asymmetry was noted when funnel plots were performed for sepsis and any serious infection. For the two main outcomes-sepsis (one or more episodes) and any serious infection (one or more episodes)-moderate inconsistency between study results was noted (I2 54% and 50%, respectively).
No statistically significant differences were noted in mortality from all causes, mortality from infection, NEC, BPD or IVH. Results for these outcomes were centred around an RR of 1.0, with very narrow CIs indicating no trends in either direction. In none of the studies that provided data on IVH was there any assurance that all neonates were subjected to ascertainment of an IVH according to a preset schedule for ultrasonographic examination. A trend towards shortened duration of hospital stay was noted with IVIG treatment [weighted mean difference (WMD) ‐2.1 days, 95% CI ‐4.5 to 0.3 days]. The outcome of hospital stay is highly dependent on the GA at birth of the neonate, the availability of institutions providing Level II care to which the neonate can be transferred and the social situation of the family.
It is possible that the IVIG preparations used in these studies did not contain the necessary antibodies to prevent infection and that the use of preparations with known specific antibodies against common pathogens in a specific neonatal intensive care unit might be more effective (Weisman 1994b).
The benefits of 3.0% and 4.0% reduction in sepsis and in any serious infection, respectively, should be weighed against the costs and the values assigned to this outcome. No serious side effects have been reported from IVIG to date, but unknown long‐term risks of administration of blood products and the pain associated with establishing an intravenous route for IVIG should be taken into account.
Units with high nosocomial infection rates may want to compare and adjust their infection control policies to those settings with low rates by using benchmarking techniques. If the rates remain high after such measures are taken, use of IVIG might be justified. Prophylactic use of IVIG should be based on a full economic evaluation and a clinical decision analysis that incorporates baseline risk for serious nosocomial infection, both clinical and economic outcomes following prophylactic IVIG and values attached to infections prevented. Such analyses have not been performed.
Although differences in inclusion criteria are seen, as well as differences in the number of studies published at the time of the reviews and the number of statistical analyses performed, the results of our systematic review are close to those of three previous meta‐analyses (Lacy 1995, Jenson 1997, Ohlsson 1998). The results of these meta‐analyses should encourage basic scientists and clinicians to pursue other avenues to enhance the immune system of preterm and/or LBW infants and to prevent nosocomial infection.
Authors' conclusions
Implications for practice.
IVIG administration results in a 3% to 4% reduction in sepsis/any serious infection but is not associated with reductions in mortality or other morbidities (NEC, IVH, length of hospital stay). Prophylactic use of IVIG is not associated with any short‐term serious side effects. The decision to use prophylactic IVIG will depend on the costs and the values assigned to the clinical outcomes.
Implications for research.
A full economic evaluation and a clinical decision analysis that incorporates baseline risk for confirmed nosocomial infection, clinical outcomes and economic outcomes after prophylactic IVIG, as well as values attached to infections prevented, is needed.
There is no justification for conducting additional RCTs to test the efficacy of previously studied IVIG preparations in reducing nosocomial infection in preterm and/or LBW infants. It is possible that the IVIG preparations used in published studies did not contain the necessary antibodies to prevent infection. The use of preparations with known specific antibodies against the common pathogens in a specific neonatal intensive care unit might be more effective, and RCTs to test the effectiveness of such preparations may be justified. The results of these meta‐analyses should encourage basic scientists and clinicians to pursue other avenues to prevent nosocomial infection.
What's new
| Date | Event | Description |
|---|---|---|
| 29 January 2020 | New citation required but conclusions have not changed | Contact author changed, and contact details updated. |
| 29 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 28 May 2013 | New search has been performed | Review updated in May, 2013. No new trials identified. Conclusions not changed. |
| 28 May 2013 | New citation required but conclusions have not changed | Updated search: May, 2013. |
| 7 April 2010 | Amended | Review updated Issue 4, 2010. Risk of Bias tables completed for this amended version. |
| 25 February 2010 | New search has been performed | This updates the existing review "Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants" published in the Cochrane Database of Systematic Reviews (Ohlsson 2007). Updated search found no new trials. No changes to conclusions. |
| 3 July 2008 | Amended | Converted to new review format. |
| 23 July 2007 | New search has been performed | This is an update of the review "Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants" published in The Cochrane Library, Issue 1, 2004 (Ohlsson 2004). One potential new trial was identified for this update conducted in July 2007. However, the infants randomized were more than 38 weeks gestation or weighed more than 2,500 g and therefore the study did not meet the inclusion criteria of preterm or low birth weight infants. Trials using species specific immunoglobulins (such as for staphylococcus aureus or epidermidis) were not included as they are reviewed separately by others within the Cochrane Collaboration. There have been two previous updates of this review (2001, 2003). In our 2001 update of this review, we identified 4 additional studies (2 single center studies from Turkey, one single center study from Taiwan and one multi‐center study conducted in four centers in Sweden and Austria). These studies reported on a total of 298 infants. We are aware of one additional unpublished study that enrolled 40 infants in Estonia. To our knowledge, this study has not been published. We were not able to identify any additional studies in our literature search in September 2003. In the 2001 update of this review, secondary analyses according to study quality were abolished as we found it exceedingly difficult to ascertain whether caregivers/researchers were blinded to the randomization process and/or the intervention or not. In 2001, the addition to our previous systematic review of outcomes from 298 randomized infants did not overturn the main results from our previous review first published in 1998. IVIG administration results in a 3‐4% reduction in sepsis and any serious infection but is not associated with reductions in mortality or other important morbidities. There is no need for further trials of currently available IVIG preparations to reduce the incidence of nosocomial infections. As we noted statistically significant heterogeneity for the two main outcomes of interest in this review (sepsis and any serious infection), we added the newly introduced "inconsistency test" (I squared). For both outcomes (sepsis and any serious infection), there was moderate "inconsistency" of 54% and 50% respectively. |
| 20 October 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Dr K. Thiringer provided us with additional information on the trial by Sandberg 2000. Ms Elizabeth Uleryk developed and executed extensive searches of MEDLINE and EMBASE in February of 2001 and September 2003. Dr Ryzhak Oleu assisted with translation of the study from Russian to English by Lelik (Lelik 2004).
Searches on May 28, 2013, were conducted by MsColleen M. Ovelman, Trials Search Co‐ordinator, and Yolanda R. Brosseau, Managing Editor, the Cochrane Neonatal Review Group.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. IVIG vs placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sepsis, one or more episodes | 10 | 3975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 2 Any serious infection, one or more episodes | 16 | 4986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.92] |
| 3 NEC, one or more episodes | 7 | 4081 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.32] |
| 4 Mortality (all causes) | 15 | 4125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.05] |
| 5 Mortality (infectious) | 10 | 1690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.56, 1.22] |
| 6 Duration of hospitalisation | 8 | 3562 | Mean Difference (IV, Fixed, 95% CI) | ‐2.12 [‐4.54, 0.30] |
| 7 Bronchopulmonary dysplasia | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.85, 2.84] |
| 8 Intraventricular haemorrhage any grade | 4 | 3176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.19] |
| 9 Intraventricular haemorrhage grade 3 or 4 | 2 | 3000 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.21] |
1.1. Analysis.
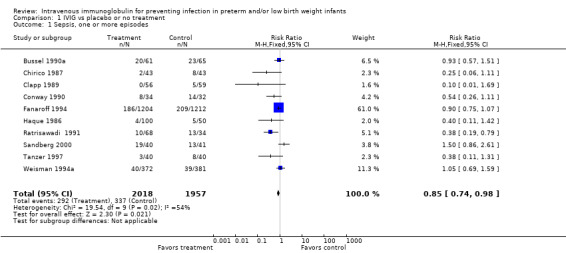
Comparison 1 IVIG vs placebo or no treatment, Outcome 1 Sepsis, one or more episodes.
1.2. Analysis.
Comparison 1 IVIG vs placebo or no treatment, Outcome 2 Any serious infection, one or more episodes.
1.3. Analysis.
Comparison 1 IVIG vs placebo or no treatment, Outcome 3 NEC, one or more episodes.
1.4. Analysis.
Comparison 1 IVIG vs placebo or no treatment, Outcome 4 Mortality (all causes).
1.5. Analysis.
Comparison 1 IVIG vs placebo or no treatment, Outcome 5 Mortality (infectious).
1.6. Analysis.
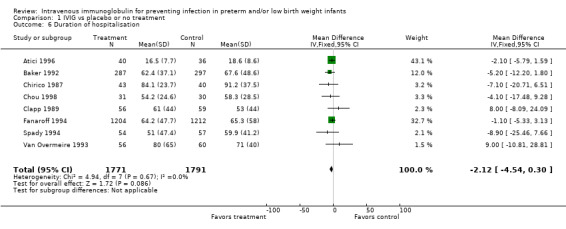
Comparison 1 IVIG vs placebo or no treatment, Outcome 6 Duration of hospitalisation.
1.7. Analysis.
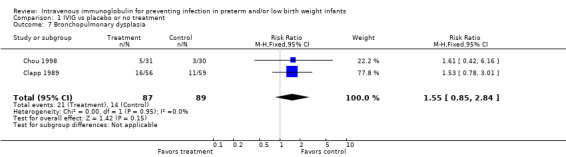
Comparison 1 IVIG vs placebo or no treatment, Outcome 7 Bronchopulmonary dysplasia.
1.8. Analysis.
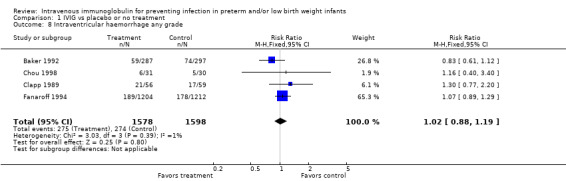
Comparison 1 IVIG vs placebo or no treatment, Outcome 8 Intraventricular haemorrhage any grade.
1.9. Analysis.
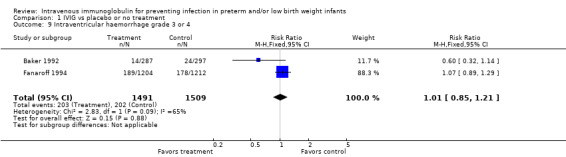
Comparison 1 IVIG vs placebo or no treatment, Outcome 9 Intraventricular haemorrhage grade 3 or 4.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Atici 1996.
| Methods | Single‐centre, randomised, controlled trial without the use of a placebo I. Blinding of randomisation-can't tell II. Blinding of intervention-no III. Complete follow‐up-yes IV. Blinding of outcome measurement(s)-no | |
| Participants | 76 infants with GA < 34 weeks Single‐centre study, Turkey May 15, 1993-June 15, 1994 | |
| Interventions | 40 infants with mean GA (SD) 31.4 ± 2.9 wk, mean BW (SD) 1623 ± 468 g received 0.5 g/kg of IVIG (Sandoglobulin, Sandoz) within 24 hours of birth 36 infants with mean GA (SD) 32.3 ± 2.4 wk, mean BW (SD) 1684 ± 519 g served as controls (no placebo was given) | |
| Outcomes | Proven infection (clinical findings and blood and/or cerebrospinal fluid culture positive for a pathogen) Total mortality, infectious mortality, days in hospital | |
| Notes | Proven infection, mortality from any cause, infectious mortality and days in hospital could be ascertained from this study No adverse effects were noted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A block randomisation method was used. No further information was provided |
| Allocation concealment (selection bias) | Unclear risk | A block randomisation method was used. No further information was provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported on all infants enrolled |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell whether there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Baker 1992.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled trial I. Blinding of randomisation-yes II. Blinding of intervention-yes III. Complete follow‐up-yes IV. Blinding of outcome measurement-yes | |
| Participants | 588 infants with a BW of 500 to 1750 g. Age 3 to 7 days Six centres in the US July 16, 1987 to December 12, 1988 | |
| Interventions | 287 infants received 500 mg/kg of IVIG (Gammagard, Baxter Healthcare, Hyland Division, Glendale, Calif) at enrolment (age 3 to 7 days), 1 week later and then every 14 days until a total of five infusions had been given or until hospital discharge, whichever came first 297 infants received an equal volume of a sterile solution of 5% albumin and 0.9% sodium chloride | |
| Outcomes | Proven infection (clinical findings of sepsis and at least one of the following: a positive blood culture (bacteria or fungi), isolation of a pathogen from a normally sterile body site (CSF, pleural, peritoneal or joint fluid; bone; soft tissue or urine obtained by suprapubic or bladder catheterization) or isolation of virus from an infant with clinical deterioration) NEC (stage II or III) IVH (grade I to IV) BPD (definition not provided) Total days in hospital Eleven neonates were excluded from the study but were included in the intention‐to‐treat analysis | |
| Notes | The following outcomes could be ascertained from this study: any serious infection (bacterial + fungal), IVH, NEC, death from all causes. Total episodes for sepsis were reported. A total of 50 episodes of sepsis were reported among 287 infants in the IVIG group and 75 episodes of sepsis among 197 infants in the placebo group. The outcome "sepsis, one or more episodes" could not be ascertained from this study. Adverse reactions were noted during 10 infusions (5 in each study group, or < 1%). Mild increases or decreases in blood pressure, heart rate or temperature that were reversed when the rate of infusion was slowed. Two infants in each group had fluid overload after an infusion and were treated with a single dose of furosemide | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation table was used |
| Allocation concealment (selection bias) | Low risk | Infants were randomly assigned by the pharmacist at the study site to receive either IVIG or albumin placebo |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Albumin placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell whether there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Bussel 1990a.
| Methods | Randomized, double‐blind, placebo‐controlled trial. I. Blinding of randomisation-yes II. Blinding of intervention-yes III. Complete follow‐up-no IV. Blinding of outcome measurement-yes | |
| Participants | 240 infants with BW < 1300 g Data for 172 participants are presented in this preliminary analysis; of these, 46 were excluded from the statistical analysis (29 because they died during the first 5 days of life, 4 because of protocol violations and 13 because of inadequate follow‐up-usually because of their return to the referring hospital. 126 infants remained) Single US centre September 1984 to October 1987 | |
| Interventions | 61 neonates (mean BW 977 g) received a dose of 1 g of a 6% solution of IVIG (Sandoglobulin, Sandoz Pharmaceuticals, East Hanover, NJ) on 4 of the first 5 days of life, and a fifth dose was administered on day 15 or as close to that day as possible (the dose could be given as late as day 21) 65 neonates (mean BW 1043 g) received an albumin placebo at equal oncotic load at the same times | |
| Outcomes | Sepsis (signs and symptoms compatible with sepsis and a positive blood or CSF culture) IVH diagnosed by ultrasonographic examination at 3 to 7 days of age 1 neonate excluded because of severe anomalies incompatible with life For additional exclusions, see "Participants" | |
| Notes | This is an interim analysis of a larger study, the results of which have not been reported to date Data are available on the outcome of sepsis but not on IVH No adverse effects were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A random number generator was used |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation-yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Albumin was used as placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data for 172 participants are presented in this preliminary analysis; of these, 46 were excluded from the statistical analysis (29 because they died during the first 5 days of life, 4 because of protocol violations and 13 because of inadequate follow‐up-usually because of their return to the referring hospital. 126 infants remained) |
| Selective reporting (reporting bias) | High risk | Noted in incomplete outcome data section above. The protocol for the study was not available to us, and we therefore cannot tell whether there were any other deviations |
| Other bias | Low risk | Appears free of other bias |
Chirico 1987.
| Methods | Randomised, controlled trial without the use of a placebo I. Blinding of randomisation-yes II. Blinding of intervention-no III. Complete follow‐up-yes IV. Blinding of outcome measurement-no | |
| Participants | In this study, a subgroup with BW </= 1500 g (n = 86) of the total population of 133 infants (BW range 550 to 3340 g; GA range 24 to 40 wk) fulfilled the inclusion criteria for this systematic review Single centre, Italy Dates not given | |
| Interventions | 43 infants received 0.5 g/kg of IVIG (Sandoglobulin) weekly for 1 month 43 infants received no placebo or other intervention | |
| Outcomes | Criteria for diagnosis of sepsis, meningitis, arthritis, pneumonia, urinary tract infection and surface infection included both a positive culture of blood, cerebrospinal fluid, tracheal aspirate, urine or pus, respectively, and the presence of clinical and non‐microbiological laboratory features. For the diagnosis of pneumonia, the appearance of a new infiltrate on a chest roentgenogram was also required NEC was diagnosed when typical clinical and radiological symptoms were present 3 infants in the control group who died within 24 hours after birth were excluded from the analysis by the authors | |
| Notes | The outcomes of sepsis, any serious infection, NEC, length of hospital stay, death from all causes and deaths from infection could be ascertained from this study. The 3 infants in the control group who died within 3 days of life are included in our analyses as per intention to treat No side effects were observed after IVIG administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 infants in the control group who died within 24 hours after birth were excluded from the analysis by the authors |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell whether there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Chou 1998.
| Methods | Randomised placebo‐controlled trial with the use of a non-identical‐looking placebo (saline) I. Blinding of randomisation-can't tell II. Blinding of intervention-no III. Complete follow‐up-yes IV. Blinding of outcome measurement(s)-no | |
| Participants | 61 infants with a BW < 1500 g were enrolled. Single‐centre study, Taiwan July 1993 to June 1994 | |
| Interventions | 31 infants, mean BW (SD) 1210 ± 340 g, mean GA (SD) 29.7 ± 2.2 wk, received Gammumine‐N (Miles Inc. Cutter Biological, USA). IVIG was infused for 30 minutes to 2 hours within the first 12 hours of birth, and every 2 weeks until the patient weighed 1800 g or was discharged. The dose of IVIG was 750 to 1000 mg/kg/dose if the infant's BW was < 1000 g and 500 to 750 mg/kg/dose if the infant's BW was between 1001 and 1500 g. 30 neonates, mean BW (SD) 1320 ± 250 g and mean GA (SD) 30.6 ± 1.7 wk received saline infusion | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly divided into 2 groups |
| Blinding (performance bias and detection bias) All outcomes | High risk | A non-identical‐looking placebo (saline) was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up-yes |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell whether there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Christensen 1989.
| Methods | Randomised, double‐blind, placebo‐controlled study I. Blinding of randomisation-yes II. Blinding of intervention-yes III. Complete follow‐up-yes IV. Blinding of outcome measurement-yes | |
| Participants | 20 preterm neonates, weight < 2000 g at entry to study and < 7 days of age Single centre, US Dates not given | |
| Interventions | 10 neonates received IVIG (Gamimmune‐N, Cutter Biologicals, Berkeley, California) 5% IgG in 10% maltose at 15 mL/kg BW as a single infusion 10 neonates received equal volume of 0.1% albumin in 10% maltose | |
| Outcomes | Nosocomial infection (not defined) Survival | |
| Notes | This study provides information on death from infections and death from all causes No differences in heart rate, respiratory rate, rectal temperature and urine output were noted before, during and after infusions of IVIG or placebo (no differences between groups) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Low risk | IVIG and placebo were dispensed from the pharmacy to study nurses in accordance with a random number sequence known only to the study pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Albumin was used as placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available to us, and we therefore cannot tell whether there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Clapp 1989.
| Methods | Randomised, double‐blind, placebo‐controlled trial I. Blinding of randomisation-yes II. Blinding of intervention-yes III. Complete follow‐up-yes IV. Blinding of outcome measurement-yes | |
| Participants | 115 infants with BW of 600 to 2000 g and < 48 hours of age Single centre, US November 1, 1986 to August 31, 1987 | |
| Interventions | 56 neonates (GA = 30 wk; mean BW (SD) 1.3 ± 0.7 kg) received IVIG (Sandoglobulin). Initial infusions of IVIG were 500 mg/kg for infants weighing > 1000 g at birth and 700 mg/kg for infants weighing < 1000 g. If serum IgG levels were < 700 mg/dL on day 2 or 6 after transfusion in the IVIG group, an additional dose of IVIG was administered at that time and subsequent doses were increased by 200 mg/kg. The objective was to maintain IgG serum levels at >700 mg/dL 59 neonates (GA 31 wk; mean BW (SD) 1.3 ± 0.4 kg) received placebo (equal volume of 6% or 10% sucrose solution). When an infant receiving IVIG required an extra dose, the paired participant in the placebo group received an additional infusion | |
| Outcomes | Sepsis (systemic clinical deterioration with a positive blood culture, cerebrospinal fluid or aspirate of another normally sterile body cavity) NEC (abdominal distension with gastric retention, abdominal erythema or bloody stools, with radiographic evidence of pneumatosis intestinalis, portal venous gas or pneumoperitoneum and staged by the modified Bell's criteria) Length of hospital stay Death from all causes Death from infection BPD (requiring O2 at 28 days for BPD) IVH (Papile classification) | |
| Notes | From the data presented, the outcomes of sepsis, any serious infection, NEC, BPD, IVH, length of hospital stay, death from all causes and death from infection could be ascertained. Three episodes of sepsis/proven infection occurred in (an) infant(s) born at 24 weeks GA and BW of 600 g. We assumed that this was only one infant and assigned only one outcome in the meta‐analyses Transient tachycardia and a decrease in blood pressure were noted in one infant who received IVIG. Transient rise in alanine aminotransferase level was noted in 1 infant who received IVIG and in 1 who received placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Low risk | All investigators and caretakers, with the exception of clinical pharmacist, were unaware of the random assignment of each infant |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | A placebo was used, but a 6% or 12% sucrose solution was used, which may not look identical and would taste differently should a caretaker have tried to taste a drop |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Conway 1990.
| Methods | Randomised controlled trial without use of a placebo I. Blinding of randomisation-yes II. Blinding of intervention-no III. Complete follow‐up-no IV. Blinding of outcome measurement-no | |
| Participants | 66 neonates of < 30 wk GA 2 centres in the UK Dates not given | |
| Interventions | 34 infants received 200 mg/kg IVIG (Intraglobin F, Biotest Pharma, FRG) within 48 hours of birth and at 3‐weekly intervals until discharge from the neonatal unit. On clinical suspicion of infection, neonates in the treatment group only were given a supplementary dose of IVIG 100 mg/kg. A further 100 mg/kg was given within the next 48 hours if infection was confirmed 32 infants received routine intensive care | |
| Outcomes | Sepsis (blood culture-proven infection) NEC (clinical findings and pneumatosis intestinalis on abdominal X‐ray or confirmed at autopsy) IVH (no definition given) BPD (no definition given) Length of stay in NICU (median and range) | |
| Notes | Outcomes of sepsis and NEC could be ascertained. We used as denominators all randomly assigned participants. We included in the outcome of mortality (all causes) the infants who were withdrawn because of early death. 1 infant with 2 episodes of NEC was counted as 1 outcome. Side effects were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eleven infants-6 in the control group and 5 in the treatment group-were withdrawn from the trial because of early death from extreme prematurity (n = 7), early return to the referring hospital (n = 3) and elective treatment with IVIG for severe congenital septicaemia (n = 1) |
| Selective reporting (reporting bias) | Unclear risk | See "Incomplete outcome data addressed?" The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Didato 1988.
| Methods | Randomised controlled trial without the use of a placebo I. Blinding of randomisation-yes II. Blinding of intervention-no III. Complete follow‐up-yes IV. Blinding of outcome measurement-no | |
| Participants | 80 infants with a BW of 2000 g or less Single centre, Italy June 1985 to December 1986 | |
| Interventions | 40 infants received 0.5 g/kg/wk of IVIG (IgVena, Sclavo; Siena, Italy) until they reached the GA of 36 weeks and during the entire period of intensive care 40 infants received no placebo or other intervention | |
| Outcomes | Sepsis defined as clinical manifestations, microbiological findings (positive blood culture or CSF culture) and non‐microbiological laboratory findings (total and differential white blood cell count, erythrocyte sedimentation rate, C‐reactive protein, platelet count, tests of haemostatic function) | |
| Notes | Any serious infection, death from all causes and death from infection could be ascertained in this study. As sepsis included neonates with positive CSF cultures, the results were included in the any serious infection category only. Data could not be separated between sepsis and meningitis No side effects or adverse reactions were observed after IVIG administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial without the use of a placebo |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all infants randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Fanaroff 1994.
| Methods | Multicentre, 2‐phase controlled trial. Phase 1 was placebo controlled and double blinded; phase 2 was not placebo controlled I. Blinding of randomisation-yes II. Blinding of intervention-yes/no* III. Complete follow‐up-yes IV. Blinding of outcome measurement-yes/no* *This study had 2 phases; in phase 1a, placebo was used, but not in phase 2 | |
| Participants | 2416 infants with BW 501 to 1500 g and randomly assigned at a mean age of 44 ± 25 hours after birth 8 centres in the US January 1, 1988 to March 31, 1991 (or through April, 1991) | |
| Interventions | In phase 1 595 infants received IVIG (Sandoglobulin, Sandoz Pharmaceuticals, East Hanover, NJ) 623 infants received placebo-equal volume of 5% albumin solution in the same vehicle prepared by the manufacturer of the immune globulin. The infants received their first dose of study drug within 24 hours of randomisation. To achieve a target level of 700 mg of immune globulin/dL, infants weighing 501 to 1000 g were given 900 mg of immune globulin per kg of body weight, and infants weighing 1001 to 1500 g were given 700 mg per kg. The infusions were repeated every 2 weeks until the infants weighed 1800 g, were transferred to another hospital, died or were sent home In phase 2 609 infants received IVIG as per above (phase 1) 589 received no intervention | |
| Outcomes | Sepsis (symptoms compatible with infection and a positive blood culture for bacteria or fungi obtained at least 96 hours after birth and before 120 days of life; for commensals, the diagnosis required 2 positive blood cultures obtained no more than 4 days apart) The diagnosis of meningitis required a positive culture of CSF. The diagnosis of urinary tract infection required a pure culture from urine obtained by catheterization or suprapubic puncture Proven infection (including septicaemia, meningitis or urinary tract infection) during the first 120 days of life NEC (Bell's modified classification) BPD (not defined) Days in hospital | |
| Notes | The following outcomes could be ascertained from this study; sepsis, any serious infection, NEC, death from all causes, death from infection, days in hospital The infusions were discontinued in < 1% of infants (10 in the IVIG group and 11 in the placebo group) because of tachycardia or acute changes in blood pressure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An unbiased coin design was used |
| Allocation concealment (selection bias) | Low risk | All lots of IVIG and placebo were marked and recorded by code |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Yes/No; this study had 2 phases; in phase 1a, placebo was used, but not in phase 2 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Fanaroff‐I 1994.
| Methods | Phase 1 of Fanaroff 1994, placebo‐controlled | |
| Participants | 1218 infants with BW 501 to 1500 g and randomly assigned at a mean age of 44 ± 25 hours after birth 8 centres in the US January 1, 1988 to March 31, 1991 (or through April 1991) | |
| Interventions | In phase 1 595 infants received IVIG (Sandoglobulin, Sandoz Pharmaceuticals, East Hanover, NJ) 623 infants received placebo-equal volume of 5% albumin solution in the same vehicle prepared by the manufacturer of the immune globulin. The infants received their first dose of study drug within 24 hours of randomisation. To achieve a target level of 700 mg of immune globulin/dL, infants weighing 501 to 1000 g were given 900 mg of immune globulin per kg of body weight, and infants weighing 1001 to 1500 g were given 700 mg per kg. The infusions were repeated every 2 weeks until the infants weighed 1800 g, were transferred to another hospital, died or were sent home | |
| Outcomes | Sepsis (symptoms compatible with infection and a positive blood culture for bacteria or fungi obtained at least 96 hours after birth and before 120 days of life; for commensals, the diagnosis required 2 positive blood cultures obtained no more than 4 days apart) The diagnosis of meningitis required a positive culture of CSF. The diagnosis of urinary tract infection required a pure culture from urine obtained by catheterization or suprapubic puncture Proven infection (including septicaemia, meningitis or urinary tract infection) during the first 120 days of life NEC (Bell's modified classification) BPD (not defined) Days in hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Multicentre, 2‐phase controlled trial. Phase 1 was placebo controlled and double blinded; phase 2 was not placebo controlled |
| Allocation concealment (selection bias) | Low risk | An unbiased coin design was used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All lots of IVIG and placebo were marked and recorded by code. A placebo was used in phase 1 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Haque 1986.
| Methods | Randomised controlled trial, without the use of a placebo I. Blinding of randomisation-yes II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement ‐ no | |
| Participants | 150 neonates of 28 to 37 weeks GA and less than 4 hours of age Single centre, Saudi Arabia Dates not given | |
| Interventions | 50 neonates received IVIG (Intraglobulin, Biotest Pharma, West Germany) 120 mg/kg within 2 to 4 hours of birth 50 neonates received IVIG (Intraglobulin) 120 mg/kg on days 1 and 8 of life 50 neonates received no intervention | |
| Outcomes | Sepsis was defined as presence of clinical features and a positive culture of blood or cerebrospinal fluid | |
| Notes | Sepsis, any serious infection, death from all causes and death from infection could be ascertained in this study. One infant developed pneumonia in the control group. Mean age at onset of infection was 46.3 hours (range 8 to 76 hours), suggesting that some infants had infection acquired in utero and were infected at the time of enrolment No adverse effect of therapy was noted during the study and at 6‐month follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Low risk | Envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Magny 1991b.
| Methods | Multicentre, randomised, controlled, double‐blind study I. Blinding of randomisation ‐ can't tell II. Blinding of intervention ‐ yes III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement ‐ yes | |
| Participants | 235 neonates of less than or equal to 32 weeks' gestation, hospitalised before 25 hours of life and having endotracheal tube and/or umbilical catheter on admission 4 centres in Paris, France 1987 to 1989 | |
| Interventions | 120 neonates received 500 mg (10 mL) of polyvalent Ig (Biotransfusion, France) on days 0, 1, 2, 3, 17 and 31 of life In the placebo group, 115 neonates received 10 mL of 0.2% albumin in the same fashion | |
| Outcomes | Death from infection Certain nosocomial infection (clinical signs of infection, positive cultures (blood, urine, cerebrospinal fluid, tracheal aspirate, stools, gastric aspirate), at least 2 biological signs of infection (abnormal number of leukocytes, immature leukocytes > 5%, thrombocytopenia < 150 000/mm3, rise in fibrinogen levels > 4.5 g/L, rise in C‐reactive protein levels > 20 mg/L) NEC was diagnosed when bloody stools were associated with radiological pneumatosis Neonatal infection and infection occurring within the first 4 days of life, potentially of maternal origin, were not counted as evaluation criteria | |
| Notes | Death from infection could be ascertained in this study. The definition of nosocomial infection did not meet our criteria for sepsis or any serious infection. The number of infants with 1 or more episodes of NEC could not be ascertained. The 46 infants for whom the protocol was broken were maintained in the statistical analyses "There were neither clinical nor biologic side effects in any of the patients after Ig infusion" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation table was prepared by the statistician for each unit |
| Allocation concealment (selection bias) | Low risk | The investigators were "blind" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In 46 infants (21 in the IVIG group; 25 in the placebo group), irregularities occurred in the protocol (1 dose forgotten or no follow‐up until 45 days of life because of transfer out of the unit). The data from these 46 infants were maintained for statistical analysis but were considered separately |
| Selective reporting (reporting bias) | Low risk | See "Incomplete outcome data addressed". The protocol for the study was not available to us, and we therefore cannot tell if there were any other deviations |
| Other bias | Low risk | Appears free of other bias |
Ratrisawadi 1991.
| Methods | Randomised controlled trial, without the use of a placebo group I. Blinding of randomisation ‐ can't tell II. Blinding of intervention ‐ no III. Complete follow‐up ‐ can't tell IV. Blinding of outcome measurement ‐ no | |
| Participants | 68 infants with a BW of 1000 to 1500 g Single centre, Bangkok, Thailand February 1988 to March 1990 | |
| Interventions | 34 neonates received 250 mg/kg of IVIG (Biotest Pharma, West Germany) within 4 hours of birth 34 neonates received 500 mg/kg of IVIG within 4 hours of birth 34 neonates received no intervention | |
| Outcomes | Sepsis (presence of clinical findings of sepsis plus positive blood cultures) | |
| Notes | The outcomes of sepsis and death from all causes could be ascertained in this study "No adverse effects were observed during the period of study" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, controlled trial, without the use of a placebo group |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation ‐ can't tell |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Infants (number not stated) who expired within 24 hours of life or required blood exchange transfusion were excluded from the study. In spite of these exclusions, the number of participants in each group is identical (n = 34) |
| Selective reporting (reporting bias) | Unclear risk | See above. The protocol for the study was not available to us, and we therefore cannot tell if there were any other deviations |
| Other bias | Low risk | Appears free of other bias |
Sandberg 2000.
| Methods | Randomised double‐blind placebo controlled trial I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ yes III. Complete follow‐up ‐ no IV. Blinding of outcome measurement(s) ‐ yes | |
| Participants | 105 infants were randomly assigned into the study. 24 infants (12 in each group) were excluded because of initial serum IgG level > 4 g/L, violation of the study protocol, withdrawal of consent or intrauterine infection | |
| Interventions | 40 infants, mean GA (SD) 27.5 ± 2.2 wk and mean BW (SD) 1.06 ± 0.39 kg, received 1 g/kg (20 mL/kg) of IVIG (Baxter) on study day 0 (< 48 hours of age) and on days 3, 7, 14 and 21. 41 infants, mean GA (SD) 27.7 ± 2.5 wk, mean BW (SD) 1.13 ± 0.38 kg, received an equal volume of placebo (human albumin 5%) | |
| Outcomes | Sepsis (symptoms and positive blood culture) Days on ventilator Total mortality from any cause. Infectious mortality | |
| Notes | Outcomes of sepsis, death from all causes and death from infection could be ascertained from this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to Dr Klara Thiringer, randomisation was achieved by a computer‐generated list for each of the 4 centres, and infants were allocated by the use of sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Infants were allocated by the use of sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 105 infants were randomly assigned into the study. 24 infants (12 in each group) were excluded because of initial serum IgG level > 4 g/L, violation of the study protocol, withdrawal of consent or intrauterine infection |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Spady 1994.
| Methods | Randomised, double‐blind trial Published in abstract form only ‐ full quality assessment not possible | |
| Participants | 111 VLBW infants | |
| Interventions | 54 infants were given 300 mg/kg of IVIG (name of product not given) as 5% solution, once between 24 and 72 hours of age and again 72 hours later 57 infants were given the same volumes as 5% dextrose | |
| Outcomes | The outcome of sepsis was not defined in this abstract, but according to the authors, sepsis occurred in 17 infants in the IVIG group and in 15 in the control group Hospital stay | |
| Notes | Length of hospital stay could be ascertained from this study, which is published in abstract form only Respiratory rate increased in the IVIG group after the first infusion. No other side effects occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Published in abstract form only ‐ full quality assessment not possible |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See above |
| Selective reporting (reporting bias) | Unclear risk | See above |
| Other bias | Unclear risk | See above |
Stabile 1988.
| Methods | Single‐centre, randomised, controlled trial without the use of a placebo group I Blinding of randomisation ‐ can't tell II Blinding of intervention ‐ no III Complete follow‐up ‐ no IV Blinding of outcome measurement ‐ no | |
| Participants | 94 neonates, GA ≤ 34 weeks' gestation or BW ≤ 1500 g Single centre, Rome, Italy May 1984 to June 1986 | |
| Interventions | 0.5 g/kg IVIG (Venogamma Polivalente, Ismunit, Pomezia, Italy) on the 1st, 2nd, 3rd, 7th, 14th, 21st and 28th days of life (treatment group) or no intervention (control group) | |
| Outcomes | Sepsis was defined as clinical signs of systemic infection and positive blood or CSF culture for a pathogen 14 neonates were excluded from the analysis. 6 neonates in the treatment group were excluded: 3 underwent exchange transfusion, 2 died from severe respiratory distress syndrome and 1 had suspected prenatal infection. 8 control neonates were excluded: 2 underwent exchange transfusion, 3 died of severe respiratory distress syndrome, 1 died of respiratory distress syndrome and IVH and 2 had suspected prenatal infection | |
| Notes | Sepsis, any serious infection, death from all causes and death from infection could be ascertained from this study. Infants who died and were excluded by the authors are included in our analysis "..no newborn infant showed any local or general reaction either during or after infusions. Three infants showed a temporary increase in IgE from 10‐18 to 28‐38 IU/mL without concurrent side effects". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Unclear risk | Infants were randomly assigned |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up ‐ no 14 neonates were excluded from the analysis. 6 neonates in the treatment group were excluded: 3 underwent exchange transfusion, 2 died from severe respiratory distress syndrome and 1 had suspected prenatal infection. 8 control neonates were excluded: 2 underwent exchange transfusion, 3 died of severe respiratory distress syndrome, 1 died of respiratory distress syndrome and IVH and 2 had suspected prenatal infection |
| Selective reporting (reporting bias) | High risk | No; see comments above. The protocol for the study was not available to us, and we therefore cannot tell whether there were any other deviations |
| Other bias | Low risk | Appears free of other bias |
Tanzer 1997.
| Methods | Single‐centre, quasi‐randomised trial without the use of a placebo I. Blinding of randomisation ‐ no II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measure(s) ‐ no | |
| Participants | 80 preterm neonates. Single centre, Turkey. Dates for the study period not provided | |
| Interventions | 40 infants with mean (SE) GA of 36.18 (0.17) weeks and mean (SE) BW of 1.85 (0.07) kg were given 500 mg/kg of IVIG (Sandoglobulin R) if they were weighing more than 1500 g and 700 mg/kg if they were weighing < 1500 g at birth on days 1, 2 and 8 of life 40 infants with mean (SE) GA of 35.58 (0.19) weeks and mean (SE) BW of 1.67 (0.07) kg got no placebo | |
| Outcomes | Blood culture ‐ proven sepsis, mortality from any cause, sepsis related mortality, days in hospital. Serum IgG levels were measured on days 1, 2, 8 and 12 | |
| Notes | Sepsis, death from all causes could be ascertained from this study. No adverse effects were noted. The outcome of days in hospital was reported only for the control group. Treatment with IVIG resulted in a statistically significant increase in serum IgG concentrations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Infants were divided into two groups on the basis of order of admission |
| Allocation concealment (selection bias) | High risk | Allocation was known based on order of admission |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes wee reported on all enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell whether there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Van Overmeire 1993.
| Methods | Randomised controlled trial without the use of a placebo group I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ no III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement ‐ no | |
| Participants | 116 neonates of < 32 wk GA and < 1500 g BW. Single centre, Antwerp, Belgium Dates not given | |
| Interventions | 56 neonates received 500 mg IVIG (Sandoglobulin) in 10 mL of saline over a period of 30 minutes within the first 12 hours of life. This infusion was repeated every 24 hours until the 7th day of life, then was administered weekly for another 3 weeks. 60 neonates received no placebo or other intervention | |
| Outcomes | The diagnosis of any serious infection was made when the clinical diagnosis, in association with suggestive laboratory data, was confirmed by a positive blood or CSF culture | |
| Notes | Any serious infection, hospital stay and death from all causes could be ascertained in this study Short‐lasting hypotension was observed shortly after IVIG administration in one patient | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Allocation was by means of envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, and we therefore cannot tell if there were any deviations |
| Other bias | Low risk | Appears free of other bias |
Weisman 1994a.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled trial I. Blinding of randomisation ‐ yes II. Blinding of intervention ‐ yes III. Complete follow‐up ‐ yes IV. Blinding of outcome measurement ‐ yes | |
| Participants | 753 neonates with a BW of 500 to 2000 g, GA ≤ 34 wk, postnatal age ≤ 12 h 9 centres in the US June 1985 to April 1989 | |
| Interventions | 372 neonates received a single intravenous infusion of 10 mL/kg of IVIG (500 mg/kg) (Sandoglobulin) 381 neonates received a single infusion of albumin 5 mg/kg | |
| Outcomes | All outcomes were recorded during the first 8 weeks (56 days) of life Sepsis was defined as clinical symptoms and signs consistent with sepsis in association with isolation of a causative organism from a blood culture specimen. For the diagnosis of sepsis caused by S epidermidis 2 positive cultures were required Infection was defined as clinical signs and symptoms and isolation of a causative organism from blood culture, CSF culture, urine culture by bladder tap or catheterization or culture of a specimen from a normally sterile site during hospitalisation or at autopsy BPD was defined as the need for continued oxygen or ventilatory support at 28 days of age for reasons other than apnoea and a chest radiograph or histopathological criteria compatible with the diagnosis. IVH was graded on a scale of 1 to 4 according to Papile. Bell's classification of stage II or greater was used to define NEC | |
| Notes | Sepsis, any serious infection, NEC and death from infection could be ascertained from this study. Although the outcomes of IVH and BPD were well defined, figures for these outcomes were not reported by group IVIG‐treated infants had a slower heart rate during the infusion than before the infusion and higher systolic blood pressure for 2 hours after the infusion than before the infusion. Both groups tolerated the infusions similarly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Other bias | Low risk | |
Abbreviations: BW = birth weight g = gram GA = gestational age IgG = immunoglobulin iv = intravenous(ly) IVIG = intravenous immunoglobulin kg = kilogram LBW = low birth weight (< 2.5 kg) mg = milligram SEM = standard error of the mean SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acunas 1994 | This is an RCT comparing the effects of fresh frozen plasma or gamma globulin on humoral immunity in neonatal sepsis. This study did not meet the inclusion criteria, as a randomised untreated control group was not included. A non‐randomised concurrent group of infants without suspicion of infection and matched for age, birth weight and gestational age served as a control group |
| Adhikari 1996 | This is a double‐blind placebo‐controlled RCT assessing the efficacy of prophylactic use of IVIG in 21 pairs of ventilated neonates weighing more than 1500 g. Mean weight in the IVIG group was 2702 g, and in the placebo group 2679 g; thus, most neonates did not fulfill the entry criterion of weight < 2500 g. In this study, IVIG did not significantly reduce the rate of infection, the duration of ventilation or the time to clinical recovery |
| Kacet 1991 | The authors do not provide enough information regarding definitions of outcomes for inclusion in this systematic review |
| Kinney 1991 | This is a double‐blind RCT designed to determine whether IVIG administration modifies the incidence of infection in high‐risk neonates. 170 infants were enrolled. The study population included neonates of > 1500 g birth weight with no upper limit stated by the authors. Thus, this study did not meet our inclusion criteria. The authors "found no evidence that the administration of IVIG affected parameters that might be related to the occurrence of systematic or localized infectious processes" |
| Lelik 2004 | This is a randomised controlled trial, but the infants enrolled were > 38 weeks' gestation and weighed > 2500 g; therefore the study population did not fulfill our inclusion criteria |
| Malik 1990 | This study has been published in abstract form only, and the authors do not provide enough information regarding definitions of outcomes for inclusion in this systematic review |
| Monintja 1989 | Immunoglobulin was given intramuscularly. It is unclear whether this is an RCT. Sepsis was not clearly defined |
Differences between protocol and review
None
Contributions of authors
Arne Ohlsson:
Literature search and identification of trials for inclusion. Evaluation of methodological quality of included trials. Abstraction of data. Verification and entry of data into RevMan. Writing of review text.
Janet Lacy:
Literature search and identification of trials for inclusion. Evaluation of methodological quality of included trials. Abstraction of data. Writing of review text.
Both reviewers contributed to this update in 2013.
Arne Ohlsson (AO) wrote the original review and updated the review in 2003 and 2007.
The February 2010 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton and Roger Soll). The 2010 update was reviewed and approved by AO.
Sources of support
Internal sources
Department of Paediatrics, Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
None
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Atici 1996 {published data only}
- Atici A, Satar M, Karabay A, Yilimaz M. Intravenous immunoglobulin for prophylaxis of nosocomial sepsis. Indian Journal of Pediatrics 1996;63:517‐21. [DOI] [PubMed] [Google Scholar]
Baker 1992 {published data only}
- Baker CJ, Melish ME, Hall RT, et al. Intravenous immune globulin for the prevention of nosocomial infection in low‐birth‐weight neonates. New England Journal of Medicine 1992;327:213‐9. [DOI] [PubMed] [Google Scholar]
Bussel 1990a {published data only}
- Bussel JB. Intravenous gammaglobulin in the prophylaxis of late sepsis in very‐low‐birth‐weight infants: preliminary results of a randomized, double‐blind, placebo‐controlled trial. Reviews of Infectious Diseases 1990;12:S457‐62. [DOI] [PubMed] [Google Scholar]
Chirico 1987 {published data only}
- Chirico G, Rondini G, Plebani A, Chiara A, Massa M, Ugazio AG. Intravenous gammaglobulin therapy for prophylaxis of infection in high‐risk neonates. Journal of Pediatrics 1987;110:437‐42. [DOI] [PubMed] [Google Scholar]
Chou 1998 {published data only}
- Chou Y‐H, Yau K‐I T. The use of prophylactic intravenous immunoglobulin therapy in very low birthweight infants. Chang Gung Medical Journal 1998;21:371‐6. [PubMed] [Google Scholar]
Christensen 1989 {published data only}
- Christensen RD, Hardman T, Thornton J, Hill HR. A randomized, double‐blind, placebo‐controlled investigation of the safety of intravenous immune globulin administration to preterm neonates. Journal of Perinatology 1989;9:126‐30. [PubMed] [Google Scholar]
Clapp 1989 {published data only}
- Clapp DW, Kliegman RM, Baley JE, et al. Use of intravenously administered immune globulin to prevent nosocomial sepsis in low birth weight infants: report of a pilot study. Journal of Pediatrics 1989;115:973‐8. [DOI] [PubMed] [Google Scholar]
Conway 1990 {published data only}
- Conway SP, Ng PC, Howel D, Maclain B, Gooi HC. Prophylactic intravenous immunoglobulin in pre‐term infants: a controlled trial. Vox Sanguinis 1990;59:6‐11. [DOI] [PubMed] [Google Scholar]
Didato 1988 {published data only}
- Didato MA, Gioeli R, Priolisi A. The use of intravenous gamma‐globulin for prevention of sepsis in pre‐term infants. Helvetica Paediatrica Acta 1988;43:283‐94. [PubMed] [Google Scholar]
Fanaroff 1994 {published data only}
- Fanaroff AA, Korones SB, Wright LL, et al. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very‐low‐birth‐weight infants. New England Journal of Medicine 1994;330:1107‐13. [DOI] [PubMed] [Google Scholar]
Fanaroff‐I 1994 {published data only}
- Fanaroff‐1. Phase 1 of Fanaroff 1994. New England Journal of Medicine 1994; Vol. 330:1107‐13.
Haque 1986 {published data only}
- Haque KN, Zaidi MH, Haque SK, Bahakim H, El‐Hazmi M, El‐Swailam M. Intravenous immunoglobulin for prevention of sepsis in preterm and low birth weight infants. Pediatric Infectious Disease 1986;5:622‐5. [DOI] [PubMed] [Google Scholar]
Magny 1991b {published data only}
- Magny JF, Bremard‐Oury C, Brault D, Menguy C, Voyer M, Landais P, et al. Intravenous immunoglobulin therapy for prevention of infection in high‐risk premature infants: report of a multicenter, double‐blind study. Pediatrics 1991;88:437‐43. [PubMed] [Google Scholar]
Ratrisawadi 1991 {published data only}
- Ratrisawadi V, Srisuwanporn T, Puapondh Y. Intravenous immunoglobulin prophylaxis for infection in very low birth‐weight infants. Journal of the Medical Association of Thailand 1991;74:14‐8. [PubMed] [Google Scholar]
Sandberg 2000 {published data only}
- Sandberg K, Fasth A, Berger A, Eibl M, Isacson K, Lisebka A, et al. Preterm infants with low immunoglobulin G levels have increased risk of neonatal sepsis but do not benefit from prophylactic immunoglobulin G. Journal of Pediatrics 2000;137:623‐8. [DOI] [PubMed] [Google Scholar]
Spady 1994 {published data only}
- Spady DW, Pabst HF, Byrnes P. Intravenous immunoglobulin (IVIG) shortens stay for low birth weight infants [abstract]. Pediatr Research 1994;35:304A. [Google Scholar]
Stabile 1988 {published data only}
- Stabile A, Sopo SM, Romanelli V, Pastore M, Pesaresi MA. Intravenous immunoglobulin for prophylaxis of neonatal sepsis in premature infants. Archives of Disease in Childhood 1988;63:441‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanzer 1997 {published data only}
- Tanzer F, Yazar N, Hakgudener Y, Kafali G. Intravenous immunoglobulin for sepsis prevention in preterm infants. Turkish Journal of Pediatrics 1997;39:341‐5. [PubMed] [Google Scholar]
Van Overmeire 1993 {published data only}
- Overmeire B, Bleyaert S, Reempts PJ, Acker KJ. The use of intravenously administered immunoglobulins in the prevention of severe infection in very low birth weight neonates. Biology of the Neonate 1993;64:110‐5. [DOI] [PubMed] [Google Scholar]
Weisman 1994a {published data only}
- Weisman LE, Stoll BJ, Kueser TJ, et al. Intravenous immune globulin prophylaxis of late‐onset sepsis in premature neonates. Journal of Pediatrics 1994;125:922‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acunas 1994 {published data only}
- Acunas BA, Peakman M, Liossis G, et al. Effect of fresh frozen plasma and gammaglobulin on humoral immunity in neonatal sepsis. Archives of Disease in Childhood 1994;70:F182‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adhikari 1996 {published data only}
- Adhikari M, Wesley AG, Fourie PB. Intravenous immunoglobulin prophylaxis in neonates on artificial ventilation. South African Medical Journal 1996;86:542‐5. [PubMed] [Google Scholar]
Kacet 1991 {published data only}
- Kacet N, Gremillet C, Zaoui C, et al. Prevention of late‐onset infections in preterm infants with intravenous gamma‐globulin: a randomized clinical trial [abstract ]. European Journal of Pediatrics 1991;150:604. [Google Scholar]
Kinney 1991 {published data only}
- Kinney J, Mundorf L, Gleason C, et al. Efficacy and pharmacokinetics of intravenous immune globulin administration to high‐risk neonates. American Journal of Diseases of Children 1991;145:1233‐8. [DOI] [PubMed] [Google Scholar]
Lelik 2004 {published data only}
- Lelik MP, Efanova EA. Prevention of nosocomial infections in newborns at artificial lung ventilation. Anesteziologiia i Reanimatologiia 2004;May‐June(3):41‐3. [PubMed] [Google Scholar]
Malik 1990 {published data only}
- Malik S, Giacoia GP, West K, Miller G. Intravenous immunoglobulin (IVIG) to prevent infections in infants with bronchopulmonary dysplasia (BPD) [abstract]. Pediatric Research 1990;27:273A. [Google Scholar]
Monintja 1989 {published data only}
- Monintja HE. Investigation on immunoglobulin fortification in preventing infections in the newborn. Paediatrica Indonesiana 1989;29:91‐6. [PubMed] [Google Scholar]
Additional references
Baker 1990a
- Baker CJ. New uses of intravenous immune globulin in newborn infants. Journal of Clinical Immunology 1990;10:47S‐55S. [DOI] [PubMed] [Google Scholar]
Baker 1990b
- Baker CJ, Rench MA, Noya FJD, Garcia‐Prats JA, and the Neonatal IVIG Study Group. Role of intravenous immunoglobulin in prevention of late‐onset infection in low‐birth‐weight neonates. Reviews of Infectious Diseases 1990;12:S463‐9. [DOI] [PubMed] [Google Scholar]
Baley 1988
- Baley JE. Neonatal sepsis: the potential for immunotherapy. Clinics in Perinatology 1988;15:755‐71. [PubMed] [Google Scholar]
Baley 1992
- Baley JE, Fanaroff AA. Neonatal infections. Part 2: Specific infectious diseases and therapies. In: Sinclair J, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:496‐506. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 1991
- Berger M. Use of intravenously administered immune globulin in newborn infants: prophylaxis, treatment, both, or neither. Journal of Pediatrics 1991;118:557‐9. [DOI] [PubMed] [Google Scholar]
Bortolussi 1986a
- Bortolussi R, Fischer GW. Opsonic and protective activity of immunoglobulin, modified immunoglobulin, and serum against neonatal Escherichia coli K1 infection. Pediatric Research 1986;20:175‐8. [DOI] [PubMed] [Google Scholar]
Bortolussi 1986b
- Bortolussi R. Potential for intravenous gamma‐globulin use in neonatal gram‐negative infection: an overview. Pediatric Infectious Diseases 1986;5:S198‐200. [PubMed] [Google Scholar]
Bussel 1990b
- Bussel JB. Neonatal uses of intravenous immunoglobulin. American Journal of Pediatric Hematol/Oncology 1990;12:505‐9. [DOI] [PubMed] [Google Scholar]
Consensus 1997
- Consensus Working Group. Present and future uses of IVIG: a Canadian multidisciplinary consensus‐building initiative. Canadian Journal of Allergy and Clinical Immunology 1997;2:176‐208. [Google Scholar]
Fischer 1986
- Fischer GW, Hemming VG, Hunter KW, et al. Intravenous immunoglobulin in the treatment of neonatal sepsis: therapeutic strategies and laboratory studies. Pediatric Infectious Disease 1986;5:S171‐5. [PubMed] [Google Scholar]
Fischer 1988
- Fischer GW. Therapeutic uses of intravenous gammaglobulin for pediatric infections. Pediatric Clinics of North America 1988;35:517‐33. [DOI] [PubMed] [Google Scholar]
Fischer 1990a
- Fischer GW, Weisman LE. Therapeutic intervention of clinical sepsis with intravenous immunoglobulin, white blood cells and antibiotics. Scandinavian Journal of Infectious Diseases 1990;73 Suppl:17‐21. [PubMed] [Google Scholar]
Fischer 1990b
- Fischer GW, Hemming VG, Gloser HP, Bachmyer H, Pilar CE, Wilson SR, et al. Polyvalent group B streptococcal immune globulin for intravenous administration: overview. Reviews of Infectious Diseases 1990;12:S483‐91. [DOI] [PubMed] [Google Scholar]
Fischer 1990c
- Fischer GW. Immunoglobulin therapy for neonatal sepsis: an overview of animal and clinical studies. Journal of Clinical Immunology 1990;10:40S‐6S. [DOI] [PubMed] [Google Scholar]
Gonzalez 1989
- Gonzalez LA, Hill HR. The current status of intravenous gamma‐globulin use in neonates. Pediatric Infectious Disease Journal 1989;8:315‐22. [PubMed] [Google Scholar]
Hammarstrom 1990
- Hammarstrom L, Smith CIE. The use of intravenous IgG as prophylaxis and for treatment of infections. Infection 1990;18:314‐24. [DOI] [PubMed] [Google Scholar]
Haque 1992
- Haque KH. Does the commercial type of IVIG used make a difference?. Pediatrics 1992;89:806‐7. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 1993
- Hill HR. Intravenous immunoglobulin use in the neonate: role in prophylaxis and therapy of infection. Pediatric Infectious Disease Journal 1993;12:549‐59. [PubMed] [Google Scholar]
Hill 1991a
- Hill RH. Is prophylaxis of neonates with intravenous immunoglobulin beneficial. American Journal of Diseases of Children 1991;145:1229‐30. [DOI] [PubMed] [Google Scholar]
Hill 1991b
- Hill HR. The role of intravenous immunoglobulin in the treatment and prevention of neonatal bacterial infection. Seminars in Perinatology 1991;15:41‐6. [PubMed] [Google Scholar]
Hobbs 1967
- Hobbs JR, Davis JA. Serum IgG‐globulin levels and gestational age in premature babies. Lancet 1967;I:757‐9. [DOI] [PubMed] [Google Scholar]
Irani 1991
- Irani SF, Wagle SU, Deshpande PG. Role of intravenous immunoglobulin in prevention and treatment of neonatal infection. Indian Pediatrics 1991;28:443‐9. [PubMed] [Google Scholar]
Jenson 1997
- Jenson HB, Pollock BH. Meta‐analyses of the effectiveness of intravenous immune globulin for prevention and treatment of neonatal sepsis. Pediatrics 1997;99:e2. [DOI] [PubMed] [Google Scholar]
Johnston 1990
- Johnston RB. Immunotherapy and immunoprophylaxis in the newborn infant: the need for definitive trials. Reviews of Infectious Diseases 1990;12:S392‐3. [DOI] [PubMed] [Google Scholar]
Kliegman 1990
- Kliegman RM, Clapp DW, Berger M. Targeted immunoglobulin therapy for the prevention of neonatal infections. Reviews of Infectious Diseases 1990;12:S443‐56. [DOI] [PubMed] [Google Scholar]
Kliegman 1991
- Kliegman RM, Clapp DW. Rational principles for immunoglobulin prophylaxis and therapy for neonatal infections. Clinics in Perinatology 1991;18:303‐324. [PubMed] [Google Scholar]
Kyllonen 1989
- Kyllonen KS, Clapp W, Kliegman RM, Baley JE, Shenker N, Fanaroff AA, et al. Dosage of intravenously administered immune globulin and dosing interval required to maintain target levels of immunoglobulin G in low birth weight infants. Journal of Pediatrics 1989;115:1013‐6. [DOI] [PubMed] [Google Scholar]
Lacy 1995
- Lacy JB, Ohlsson A. Administration of intravenous immunoglobulins for prophylaxis or treatment of infection in preterm infants: meta‐analyses. Archives of Disease in Childhood 1995;72:F151‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magny 1991a
- Magny J‐F. Les immunoglobulines sont‐elles utiles dans le traitment des infections neonatales?. La Revue du Praticien 1991;41:1368‐70. [PubMed] [Google Scholar]
Noya 1989
- Noya FJD, Baker CJ. Intravenously administered immune globulin for premature infants: a time to wait. Journal of Pediatrics 1989;115:969‐71. [DOI] [PubMed] [Google Scholar]
Papile 1983
- Papile LA, Munsick‐Bruno G, Schafer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. Journal of Pediatrics 1983;103:273‐7. [DOI] [PubMed] [Google Scholar]
Rondini 1991
- Rondini G, Chirico G, Ugazio AG. Intravenous immunoglobulin for prophylaxis of infection in preterm infants. Developmental Pharmacology and Therapeutics 1991;17:144‐9. [DOI] [PubMed] [Google Scholar]
Salzer 1991
- Salzer HR, Weninger M, Pollak A, Kolmer M. Prophylactic immunoglobulin (IG) treatment in infants less than 30 weeks gestation-a meta analysis [abstract]. European Journal of Pediatrics 1991;150:604. [Google Scholar]
Siber 1992
- Siber GR. Immune globulin to prevent nosocomial infections. New England Journal of Medicine 1992;327:269‐71. [DOI] [PubMed] [Google Scholar]
Stabile 1989
- Stabile A, Sopo SM, Pastore M, Pesaresi MA. Intravenous immune globulin doses and infection prophylaxis in very low birth weight neonates. Journal of Pediatrics 1989;114:168. [DOI] [PubMed] [Google Scholar]
Stiehm 1966
- Stiehm RE, Fudenberg HH. Serum levels of immune globulins in health and disease: a survey. Pediatrics 1966;37:715‐27. [PubMed] [Google Scholar]
Stiehm 1986
- Stiehm ER. Intravenous immunoglobulins in neonates and infants: an overview. Pediatric Infectious Disease 1986;5:S217‐9. [DOI] [PubMed] [Google Scholar]
Stiehm 1990
- Stiehm ER. Role of immunoglobulin therapy in neonatal infections: where we stand today. Reviews of Infectious Diseases 1990;12:S439‐42. [PubMed] [Google Scholar]
Stoll 1996
- Stoll BJ, Gordon T, Korones SB, et al. Late‐onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. Journal of Pediatrics 1996;129:63‐71. [DOI] [PubMed] [Google Scholar]
Weisman 1986
- Weisman LE, Fischer GW, Hemming VG, Peck CC. Pharmacokinetics of intravenous immunoglobulin (Sandoglobulin) in neonates. Pediatric Infectious Disease 1986;5:S185‐8. [PubMed] [Google Scholar]
Weisman 1992
- Weisman LE, Cruess DF, Fischer GW. Current status of intravenous immunoglobulin in preventing or treating neonatal bacterial infections. Clinical Reviews in Allergy 1992;10:13‐28. [DOI] [PubMed] [Google Scholar]
Weisman 1993
- Weisman LE, Cruess DF, Fischer GW. Standard versus hyperimmune immunoglobulin in preventing or treating neonatal bacterial infections. Clinics in Perinatology 1993;20:211‐24. [PubMed] [Google Scholar]
Weisman 1994b
- Weisman LE, Cruess DF, Fischer GW. Opsonic activity of commercially available standard intravenous immunoglobulin preparations. Pediatric Infectious Disease Journal 1994;13:1122‐5. [DOI] [PubMed] [Google Scholar]
Whitelaw 1990
- Whitelaw A. Treatment of sepsis with IgG in very low birthweight infants. Archives of Disease in Childhood 1990;65:347‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolach 1997
- Wolach B. Neonatal sepsis: pathogenesis and supportive therapy. Seminars in Perinatology 1997;21:28‐38. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ohlsson 1998
- Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000361] [DOI] [Google Scholar]
Ohlsson 2001
- Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000361] [DOI] [PubMed] [Google Scholar]
Ohlsson 2004
- Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000361.pub2] [DOI] [PubMed] [Google Scholar]
Ohlsson 2007
- Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000361] [DOI] [Google Scholar]


