Abstract
Context
The study of gonadal hormone effects on adolescent wellbeing has been limited by logistical challenges. Urine hormone profiling offers new opportunities to understand the health and behavioral implications of puberty hormones.
Objective
To characterize pubertal change in urinary testosterone and estradiol among male and female adolescents, respectively.
Design
Three-year prospective cohort study.
Setting
Australian regional community.
Participants
282 (163 male) normally developing adolescents aged 11.8 ± 1.0 years at baseline.
Main outcome measure
Quarterly urine measurements of testosterone and estradiol (mass spectrometry); annual anthropometric assessment and Tanner stage (TS) self-report.
Results
Two-class sigmoidal and quadratic growth mixture models (centered on age at TS3) were identified as best-fit for describing testosterone (male) and estradiol (female) change. Classes 1 (male: 63%; female: 82%) and 2 (male: 37%; female: 18%) were respectively named the “stable” and “unstable” trajectories, characterized by different standard deviation of quarterly hormone change and magnitude of hormone peaks and troughs (all P < 0.001). Compared with class 1 (stable), class 2 males were taller at baseline (154 vs 151 cm), reported earlier and faster TS progression (P < 0.01), and showed higher serum testosterone levels at baseline and 3 years (P ≤ 0.01). Class 2 females exhibited smaller height and weight gains over the 3 years and had higher baseline serum estradiol (249 vs 98 pmol/L; P = 0.002) than class 1.
Conclusions
Adolescents showed 2 distinct urinary gonadal hormone trajectories, characterized by stability of change over time, which were not associated with consistent anthropometric differences. Results provide a methodology for studying gonadal hormone impacts on other aspects of biopsychosocial wellbeing. Identification of potential “at-risk” hormone groups would be important for planning supportive interventions.
Keywords: puberty, adolescent, urine, testosterone, estradiol, longitudinal
Introduction
Puberty, the transitional period between childhood and adulthood, is a remarkable physical event orchestrated by dramatic changes in gonadal and pituitary hormones. The 2 primary hormones of puberty, testosterone in males and estradiol in females, operate synergistically with growth hormone to produce an increase in body size and a transformation to the body to become physically and behaviorally capable of reproduction [1]. Other characteristic changes during this period include mood alterations, disengagement from education, and oppositional and other health risk behaviors, some of which are intermittent/transitory and some more permanent [1–3]. However, to date, the effect that gonadal hormones have on these other aspects of adolescent wellbeing and behavior remains unclear. Situations where gonadal hormones change rapidly, such as the menstrual cycle [4], pregnancy, and the postpartum period [5, 6], as well as in androgen abuse, are all linked to significant mood and behavior disturbances [7]. Thus, it is perhaps unsurprising that clinicians, parents, and the community commonly attribute adolescent mood and behavior change to puberty hormones, along with other influencing factors, such as neurocognitive development [8] and relationships with peers and family [9].
Understanding the true impact of gonadal hormones on adolescent health and wellbeing is important for 3 reasons. First, there is a large body of evidence demonstrating that off-timed pubertal onset (earlier or later) predicts long-term health including depression and metabolic disease risk [10, 11], indicating a potential hormonal mechanism. Tempo or the speed of change of puberty hormones might also be associated with mood or behavior alterations [12], although this research area is in its infancy and existing studies generally define tempo using proxies rather than direct gonadal hormone measurement [13, 14]. Second, the widespread distribution of gonadal hormone receptors, including in the brain [15, 16], makes the possibility of impacts outside the reproductive system very likely. Third, erroneous attribution of puberty hormone effects may draw attention away from other modifiable risk factors and result in missed opportunities for effective therapy/intervention. For example, the speculation that testosterone heightens antisocial or aggressive behaviors in adolescence is not supported by currently available data [17]. Such behaviors may instead be related to lack of learned impulse control or parental modeling, factors that are directly modifiable.
Studies seeking to examine the health and behavioral implications of puberty hormone change should directly measure relevant hormones, although such studies are challenging. Circulating testosterone rises in males from childhood levels by 20- to 30-fold over 2 to 5 years, while serum estradiol in females increase by 4- to 9-fold [18, 19]. Marked inter-individual variation in these hormones are observed across the physical stages of maturation [20–22]. These observations indicate that a high frequency of biological sampling is required to profile individual patterns/trajectories of testosterone and estradiol change, which is constrained by participant acceptability and resource limitations. Common practice has been to use infrequent blood sampling or proxies such as Tanner stage (TS), somatic growth parameters such as peak height velocity (PHV) and pubertal milestones, such as menarche and voice-break [10, 23-25]. On these measures, it is well recognized that significant inter-individual variation exists in timing and duration of puberty [26, 27]. More recently, the measurement of gonadal hormones in urine has been explored [28, 29]. Such research indicates advantages of urinalysis as a less invasive alternative to blood sampling and as a more detailed and temporally-integrated description of individual hormone trajectory than annual/biannual measurement of blood hormones [28].
The aim of this study was to examine pubertal change in urinary testosterone and estradiol in a sample of healthy adolescent males and females, respectively, followed over 3 years. The hypothesis was that individuals will show varying gonadal hormone trajectories, in particular, differences in the rate at which these hormones change. A secondary aim was to present novel insights and knowledge on longitudinal urinary gonadal hormone change in both sexes, given that we were unable to identify a previous study with such intensive and lengthy biological sampling.
1. Materials and Methods
A. Study Setting and Participants
The protocol and baseline characteristics of the Adolescent Rural Cohort study of Hormones, Health, Environments, Education and Relationships (ARCHER) are published [30, 31]. In brief, the ARCHER study was designed to investigate the impact of puberty hormones on adolescent health, wellbeing, and behavior. Participants were recruited through schools and community groups from 2 regional cities and their vicinities in the state of New South Wales, Australia [32]. A total of 400 participants, allowing for 30% attrition, was needed for the study to be adequately powered. This was achieved with enrollment of 342 adolescents and an 82% retention rate at the end of 3 years. Given the intensity of the study protocol, 3 years was the maximum duration considered possible, based on feedback from initial feasibility studies and prior research experience in adolescents [33, 34]. A 3-year study duration would allow adolescents to reach at least mid-puberty (commonly described by TS3), which is when a noticeable upswing in gonadal hormone levels is evident [19, 35, 36], and when typical adolescent mood and behaviors become more prevalent [1, 30, 37]. Inclusion criteria were school grades fifth to seventh, capturing mostly ages 10 to 12 years at enrollment, and English proficiency for the completion of questionnaires on personal, family, and environmental information. These data, together with measured anthropometry and self-reported Tanner stage were collected annually as described elsewhere [30, 38]. Adolescents were asked to rate their pubertal status against standardized line drawings of genital and breast development for males and females, respectively (with no descriptive text) [26, 27]. Females were asked every quarter if/when they had reached menarche.
B. Ethics
The ARCHER study was approved by the Human Research Ethics Committee of The University of Sydney (HREC 2010/13094 and 2015/199). Written informed consent was obtained from a parent/guardian prior to study commencement, together with adolescent assent.
C. Specimen Collection and Biochemical Analyses
Fasting, first-morning urine samples (cycle days 7-10 in postmenarcheal females) were collected every 3 months over 3 years for the measurement of testosterone and estradiol. Fasting, morning serum samples were collected annually and contemporaneously with the corresponding urine sample over the 3 years, with the same menstrual cycle considerations. All biological samples were stored at −80 °C (−112 °F) across multiple aliquots. Stability of samples under such storage conditions has been demonstrated previously [39, 40]. Liquid chromatography tandem mass spectrometry was used to measure urine and serum testosterone and estradiol concentration. The methodology was modified from a protocol for analyzing serum [41] and adapted for urine specimens after enzymatic deconjugation with full assay performance characteristics published [28]. Urine specific gravity (SG) was estimated via reagent strip (ChoiceLine 10, Roche Diagnostics), with dipstick color changes compared visually against a SG color chart. All urine hormone concentrations were adjusted to a standard SG of 1.020 using the equation: hormone concentrationsample × (1.020−1) ÷ (SGsample−1) [29, 42]. Hydration status was not found to significantly influence hormone results in this study [29]. Urine testosterone (r = 0.77; P < 0.001) and estradiol (r = 0.72; P < 0.001) were correlated with but higher than serum levels [28]. As such, both urine hormones are expressed in nmol/L from here on, whereas serum hormone levels are presented in SI units. Conversion from SI to conventional units: testosterone nmol/L ÷ 3.467 = ng/mL; estradiol pmol/L ÷ 3.671 = pg/mL.
D. Statistical Analysis
Growth mixture models (GMM) were employed to identify varying trajectories of urinary hormone change in each sex. Briefly, GMM is a data-driven analytic approach that has the capacity to handle complex and variable change (in both positive and negative directions) in longitudinal data, which enabled us to quantifiably describe urinary hormone patterns over time in individuals [43]. GMMs allow identification of (latent) classes that are defined by their patterns of change, with no predetermination of class number, type, or pattern. The advantage of GMMs over conventional analyses of change using random effects models is that GMMs do not rely on an assumption that all participants of the cohort come from the same underlying distribution. Hence, GMMs are more flexible and robust than conventional approaches to analyzing heterogeneous longitudinal data such as the urine hormones in this study. Specifically, GMMs test the hypothesis that there are subgroups of individuals who share a common hormone trajectory, but the hormone trajectories differ between groups. The general pattern of hormone change identified, by preliminary plots, was an increase over time to a level higher than baseline in both sexes. However, sex-specific plots of individual trajectories did not show a clear polynomial shape/trend over time. Thus, within the GMM framework various polynomial, semiparametric, and nonparametric shapes were tested. To determine the optimal number of hormone classes, a series of sex-specific GMMs with 1 to 3 classes were constructed for each functional form. The following criteria were used to determine the optimal number of classes: (i) convergence; (ii) Bayesian Information Criterion (BIC); (iii) adjusted BIC (aBIC); (iv) Akaike’s Information Criterion (AIC); (v) entropy; (vi) class size; and (vii) interpretability [44]. Each individual within the cohort is allocated to the class for which they had the highest probability of membership. Our input variables for GMM were individual urine testosterone or estradiol concentrations (for males and females, respectively) and centered age (treated as the time exposure variable). Age alone is not a consistent marker of puberty. Hence, the chronological age corresponding to each urine sample was centered on the participant’s age at TS3, so as to standardize individual hormone trajectories to a common level of physical maturity. Age at TS3 was selected as the centering value common to both sexes, representing both the midpoint of puberty and the stage identified in previous studies as marking the acceleration of gonadal hormone increase [18, 45].
For completeness, age at menarche in females and age at PHV in both sexes were tested as alternate centering values. These alternate centering strategies had limitations, namely the study was powered on the assumption that the majority of participants would reach at least Tanner stage 3, and height was assessed annually with PHV assigned to the midpoint age between 2 annual height measures. Given the 3-year duration of the study, PHV was missed (male: n = 21; female: n = 52) or not yet reached (male: n = 35; female: n = 7) in some participants. Similarly, not all girls had reached menarche. Full details of our analytic strategy, including decisions for participant inclusion and model selection, can be found in the online supplementary material [46]. Mplus version 7.3 was used for growth mixture modeling [47].
Independent sample t-tests were used to assess differences in age, anthropometry, timing and tempo of TS progression, male serum testosterone (log transformed) and female serum estradiol (log transformed) between included and excluded participants and between classes obtained from GMM. The same tests were applied to compare urine hormone trajectory characteristics between GMM classes. Timing and tempo of TS progression were derived mathematically for each individual using published methods involving linear and logistic mixed modeling of annual self-reported TS [48, 49]. Results are provided as mean ± standard deviation (SD) or median (interquartile range), with statistical significance set at P < 0.05. SAS Version 9.4 (SAS Institute, Cary, NC) was used for this set of analyses.
2. Results
Of the total 342 participants recruited to the ARCHER study, 282 (163 males, 119 females) were included in the GMM analyses (Fig. 1). These participants provided an average of 9.7 urine hormone measures (out of a maximum 13) over the 3 years. Baseline age was 11.9 ± 1.0 years for males and 11.7 ± 0.9 years for females. Comparison of participants included and excluded from GMM analyses showed no age, anthropometric, baseline serum estradiol, or TS differences among females (Table S1) [46]. Excluded males were identified as the later developers of the cohort, exhibiting lower TS (baseline: 2.1 vs 2.7; 3 years: 2.3 vs 4.5; both P < 0.01) and serum testosterone levels (baseline: 0.04 vs 1.1 nmol/L; 3 years: 1.7 vs 10.3 nmol/L; both P < 0.01) (Table S1) [46]. Height change over the study duration was also smaller among excluded males (14.1 vs 19.6 cm; P = 0.018). A PHV year was evident in 110 males and 58 females.
Figure 1.
Flow chart outlining the ARCHER study recruitment process and exclusion of participants from growth mixture modeling. *Submission of an EOI was not essential for recruitment into the study. (EOIs, expressions of interest)
Comparison of model-fit statistics between various GMMs showed that, regardless of centering value, urinary testosterone and estradiol change in males and females were best described by sigmoidal/logistic and quadratic 2-class models, respectively (Table 1). Among the 2 classes identified by the TS3-centered GMMs, class 1 contained 63% (n = 103) of males and 82% (n = 98) of females, and was arbitrarily named the “stable class” for a more consistent and steady rise in urine hormone over 3 years. Class 2, containing 37% (n = 60) of males and 18% (n = 21) of females, was named the “unstable class” due to a pattern of urinary hormone rise characterized by greater fluctuation and higher overall levels across the study duration. These patterns were apparent upon observing the male (Fig. 2) and female (Fig. 3) GMM plots and were subsequently quantified through calculation of hormone trajectory characteristics (Table 2). Centering on the late pubertal event of menarche resulted in 77% (82/106) of females being allocated to class 1 (Table S2; Fig. S1) [46]. Alternatively, centering on age at PHV yielded a class 1 size of 94% (103/110) among males and 86% (50/58) among females (Table S3; Fig. S2) [46]. A complete set of model-fit statistics (Tables S4-S8), including considerations for 3-class models and plots for several alternative curve shapes (Figs S3-S4), are available elsewhere [46].
Table 1.
Comparison of Model-Fit Statistics for Various 2-Class Growth Mixture Models Used to Describe Urine Testosterone and Estradiol Change in Males and Females, Respectively
| Model Description | Parameters | AIC | BIC | aBIC | Entropya | Class 1 n (%) | Class 2 n (%) |
|---|---|---|---|---|---|---|---|
| Urine testosterone, centered on age at TS3 | |||||||
| Sigmoidal/logistic (2-class; best fit) | 36 | 15777 | 15888 | 15774 | 0.760 | 103 (63.2) | 60 (36.8) |
| Gompertz (2 class) | 36 | 15840 | 15951 | 15837 | 0.773 | 113 (69.3) | 50 (30.7) |
| Cubic (2-class) | 30 | 16242 | 16335 | 16240 | 0.927 | 109 (66.9) | 54 (33.1) |
| Quadratic (2-class) | 28 | 16329 | 16415 | 16327 | 0.931 | 101 (61.9) | 62 (38.0) |
| Urine testosterone, centered on age at PHV | |||||||
| Sigmoidal/logistic (2-class; best fit) | 36 | 11759 | 11856 | 11742 | 0.970 | 103 (93.6) | 7 (6.3) |
| Gompertz (2 class) | 36 | 11764 | 11862 | 11748 | 0.914 | 105 (95.5) | 5 (4.5) |
| Cubic (2-class) | 30 | 12346 | 12427 | 12332 | 0.990 | 103 (93.6) | 7 (6.3) |
| Quadratic (2-class) | 28 | 12402 | 12478 | 12390 | 0.986 | 103 (93.6) | 7 (6.3) |
| Urine estradiol, centered on age at TS3 | |||||||
| Quadratic (2-class; best fit) | 28 | 8031 | 8109 | 8020 | 0.851 | 98 (82.4) | 21 (17.6) |
| Cubic (2-class) | 30 | 8032 | 8115 | 8020 | 0.862 | 99 (83.2) | 20 (16.8) |
| Linear (2-class) | 26 | 8029 | 8102 | 8020 | 0.845 | 100 (84.0) | 19 (16.0) |
| Urine estradiol, centered on age at menarche | |||||||
| Quadratic (2-class; best fit) | 32 | 7250 | 7335 | 7234 | 0.798 | 82 (77.4) | 24 (22.6) |
| Cubic (2-class) | 34 | 7251 | 7341 | 7234 | 0.802 | 81 (76.4) | 25 (23.6) |
| Linear (2-class) | 30 | 7278 | 7358 | 7263 | 0.801 | 86 (81.1) | 20 (18.9) |
| Urine estradiol, centered on age at PHV | |||||||
| Quadratic (2-class; best fit) | 24 | 3958 | 4007 | 3932 | 0.954 | 50 (86.2) | 8 (13.8) |
| Cubic (2-class) | 26 | 3944 | 3997 | 3915 | 0.954 | 50 (86.2) | 8 (13.8) |
| Linear (2-class) | 22 | 3967 | 4013 | 3944 | 0.936 | 50 (86.2) | 8 (13.8) |
Abbreviations: AIC, Akaike’s Information Criterion; aBIC, sample size adjusted Bayesian Information Criterion; BIC, Bayesian Information Criterion; PHV, peak height velocity; TS, Tanner stage.
aEntropy quantifies the uncertainty with which participants were categorized into latent classes, where 0 = random and 1 = perfect fit.
Figure 2.
Final 2-class sigmoidal/logistic model, centered on age at Tanner Stage 3, which show a (a) stable and (b) unstable pattern of urinary testosterone change in males. Colored lines denote the average trajectory among participants in that class.
Figure 3.
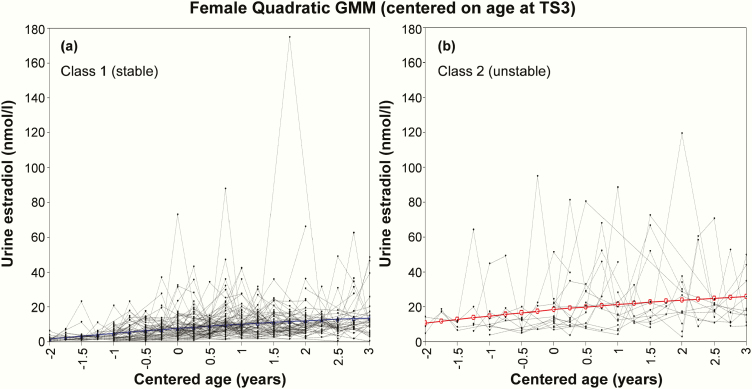
Final 2-class quadratic model, centered on age at Tanner Stage 3, which show a (a) stable and (b) unstable pattern of urinary estradiol change in females. Colored lines denote the average trajectory among participants in that class.
Table 2.
Comparison of Hormone Trajectory Characteristics Between The 2 Urine Hormone Classes Identified by the TS3-Centered Growth Mixture Models
| Male Urine Testosterone Classes (centered on age at TS3) | Female Urine Estradiol Classes (centered on age at TS3) | |||||
|---|---|---|---|---|---|---|
| Hormone Trajectory Characteristics | Class 1 (stable) | Class 2 (unstable) | P value | Class 1 (stable) | Class 2 (unstable) | P value |
| N | 103 | 60 | 98 | 21 | ||
| Study participation duration, y | 2.9 (0.4) | 2.9 (0.4) | 0.839 | 2.8 (0.5) | 2.6 (0.7) | 0.297 |
| Number of hormone measures | 9.9 (2.1) | 9.5 (2.1) | 0.191 | 9.9 (2.8) | 8.0 (2.7) | 0.010 |
| Absolute hormone concentration, nmol/L | ||||||
| At baseline | 17.0 (32.2) | 77.7 (111.6) | <0.001 | 4.4 (4.8) | 11.7 (7.3) | 0.001 |
| At 3 years | 134.2 (141.8) | 330.1 (200.4) | <0.001 | 12.5 (11.0) | 21.7 (12.1) | 0.003 |
| Median over 3 years | 52.4 (64.5) | 167.5 (109.6) | <0.001 | 7.0 (3.7) | 15.7 (5.1) | <0.001 |
| Lowest during study | 13.9 (26.3) | 51.7(55.1) | <0.001 | 2.6 (2.6) | 6.6 (3.3) | <0.001 |
| Highest during study | 163.3 (176.1) | 451.4 (289.8) | <0.001 | 24.6 (21.3) | 60.9 (24.2) | <0.001 |
| Hormone change (any direction), nmol/L | ||||||
| Change from baseline to 3 years | 116.8 (135.6) | 252.4 (233.0) | <0.001 | 7.7 (11.0) | 9.5 (12.5) | 0.546 |
| Change per year | 42.3 (48.5) | 88.4 (80.1) | <0.001 | 2.9 (4.4) | 4.8 (6.6) | 0.241 |
| Mean quarterly change | 13.9 (16.3) | 32.6 (38.1) | <0.001 | 1.1 (1.5) | 1.8 (2.2) | 0.154 |
| Median quarterly change | 9.0 (15.3) | 25.3 (55.1) | <0.001 | 0.4 (1.8) | 0.7 (4.0) | 0.744 |
| SD of quarterly change | 44.0 (61.7) | 151.5 (126.2) | <0.001 | 9.5 (11.0) | 29.7 (15.8) | <0.001 |
| Max. and min. quarterly hormone change, nmol/L | ||||||
| Max. quarterly change (any direction) | 93.6 (115.8) | 300.2 (223.6) | <0.001 | 18.7 (20.9) | 50.3 (24.2) | <0.001 |
| Min. quarterly change (any direction) | 3.5 (6.9) | 12.5 (23.6) | 0.005 | 0.7 (1.1) | 2.6 (2.9) | 0.014 |
| Max. quarterly peak | 90.5 (109.9) | 285.0 (211.8) | <0.001 | 17.6 (20.9) | 46.6 (25.7) | <0.001 |
| Min. quarterly peak | 4.9 (8.7) | 24.6 (46.1) | 0.002 | 1.5 (2.2) | 8.8 (16.9) | 0.058 |
| Max. quarterly trough | −51.7 (96.7) | −211.1 (232.3) | <0.001 | −13.6 (19.8) | −44.1 (22.4) | <0.001 |
| Min. quarterly trough | −12.1 (26.0) | −33.3 (50.3) | 0.004 | −2.2 (2.9) | −7.3 (12.8) | 0.086 |
Data are presented as mean (SD). All P values below 0.05 are shown in bold text. Abbreviations: Max, maximum; Min, minimum; TS, Tanner stage.
Compared with class 1 (stable), adolescents allocated to class 2 (unstable) in the TS3-centered GMMs showed significantly greater SD of quarterly testosterone/estradiol change (male: 151.5 vs 44.0; female: 21.3 vs 7.0 nmol/L; both P < 0.001) (Table 2). Class 2 adolescents also exhibited significantly larger maximum quarterly hormone peaks (male: 285.0 vs 90.5; female: 32.3 vs 13.2 nmol/L; both P < 0.001) and troughs (male: −211.1 vs −51.7; female: −26.8 vs −9.9 nmol/L; both P < 0.001) over the study duration. Median hormone level across 3 years was 2- to 3-fold higher in class 2 compared with class 1 adolescents (male: 167.5 vs 52.4; female: 13.2 vs 6.6 nmol/L; both P < 0.001). Absolute change in urine hormone level from baseline to 3 years was significantly greater among class 2 males (252.4 vs 116.8 nmol/L; P < 0.001) but not females (Table 2). Similar hormone trajectory differences were evident between the 2 menarche-centered GMM classes (Table S2) but not the PHV-centered classes (Table S3) [46]. Notably, the menarche-centered plots showed some premenarcheal spikes in urinary estradiol concentration that were similar in magnitude to those observed postmenarche (Fig. S1). This observation was supported statistically by the absence of a significant difference in premenarcheal (11.7 ± 14.7 nmol/L) versus postmenarchal (15.1 ± 15.8 nmol/L) maximum quarterly estradiol peaks (P = 0.35), irrespective of class.
A comparison of physical growth and maturation characteristics between the TS3-centered hormone trajectory classes is presented in Table 3. Class 2 (unstable) males showed significantly taller baseline height (P = 0.048) and earlier age at maximal height change (P = 0.005). Serum levels of testosterone among class 2 males were also significantly higher at baseline (P < 0.001) and at 3 years (P = 0.010). Class 2 males reported a significantly lower TS at baseline (2.5 vs 2.9; P = 0.033), despite TS3 centering, but not at 3 years (P = 0.079). This difference translated into significantly later timing and faster tempo of puberty (estimated from linear and logistic modeling of TS progression) among class 2 males (all P < 0.01).
Table 3.
Descriptive Characteristics of Participants in the 2 Urine Hormone Classes Identified By the TS3-Centered Growth Mixture Models
| Male Urine Testosterone Classes (centered on age at TS3) | Female Urine Estradiol Classes (centered on age at TS3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Class 1 (stable) | Class 2 (unstable) | Class 1 (stable) | Class 2 (unstable) | |||||||
| Characteristic | n | Mean (SD) | n | Mean (SD) | P value | n | Mean (SD) | n | Mean (SD) | P value |
| Age, y | ||||||||||
| At baseline | 103 | 11.8 (1.0) | 60 | 12.0 (1.0) | 0.125 | 98 | 11.6 (0.9) | 21 | 11.8 (1.1) | 0.351 |
| At 3 yearsa | 97 | 14.7 (1.0) | 56 | 15.0 (1.0) | 0.095 | 90 | 14.7 (0.9) | 19 | 14.9 (1.1) | 0.276 |
| Serum hormone concentrationb,c | ||||||||||
| At baseline | 93 | 0.6 (0.1, 4.9) | 51 | 3.4 (0.8, 13.0) | <0.001 | 89 | 97.6 (55.1, 175.5) | 19 | 248.6 (139.1, 287.8) | 0.002 |
| At 3 years | 75 | 9.8 (6.0, 12.6) | 42 | 11.2 (9.3, 13.3) | 0.010 | 54 | 114.2 (77.1, 161.5) | 13 | 186.9 (82.2, 230.2) | 0.102 |
| Age at menarche, y | - | - | - | - | - | 86 | 12.9 (1.5) | 18 | 12.5 (1.1) | 0.334 |
| Tanner stage | ||||||||||
| At baseline | 103 | 2.9 (1.0) | 59 | 2.5 (1.2) | 0.033 | 96 | 2.3 (1.0) | 21 | 2.7 (1.1) | 0.194 |
| At 3 years | 96 | 4.4 (0.9) | 56 | 4.7 (0.6) | 0.079 | 90 | 4.2 (0.8) | 19 | 4.2 (0.8) | 0.786 |
| Maximum TS | 103 | 4.5 (0.7) | 60 | 4.7 (0.6) | 0.047 | 98 | 4.3 (0.8) | 21 | 4.2 (0.7) | 0.925 |
| Linear timing (age at TS3)d | 103 | 11.9 (1.1) | 60 | 12.7 (1.0) | <0.001 | 98 | 12.3 (1.1) | 21 | 12.4 (1.1) | 0.802 |
| Linear tempo (average ∆TS/y)d | 103 | 0.5 (0.3) | 60 | 0.7 (0.4) | 0.004 | 98 | 0.7 (0.4) | 21 | 0.5 (0.4) | 0.133 |
| Logistic timing (age at TS3)e | 103 | 12.3 (0.8) | 60 | 12.6 (0.7) | 0.004 | 98 | 12.6 (0.8) | 21 | 12.5 (0.9) | 0.646 |
| Logistic tempo (slope at TS3; TS/y)e | 103 | 1.0 (0.4) | 60 | 1.2 (0.4) | 0.002 | 98 | 0.9 (0.2) | 21 | 0.8 (0.2) | 0.188 |
| Height, cm | ||||||||||
| At baseline | 103 | 151.1 (10.2) | 60 | 154.4 (10.0) | 0.048 | 98 | 150.1 (8.9) | 21 | 153.7 (8.0) | 0.093 |
| At 3 years | 97 | 170.2 (9.6) | 55 | 173.7 (6.8) | 0.992 | 89 | 163.9 (6.6) | 17 | 163.6 (4.8) | 0.856 |
| ∆ over 3 years | 97 | 19.4 (5.0) | 55 | 20.0 (5.5) | 0.494 | 89 | 14.0 (5.5) | 17 | 10.3 (4.5) | 0.015 |
| Maximum ∆ height, cm/y | 103 | 8.8 (1.9) | 60 | 9.2 (2.2) | 0.176 | 98 | 6.7 (2.6) | 21 | 5.7 (2.5) | 0.114 |
| Age at maximum ∆ height, y | 66 | 13.7 (1.0) | 44 | 13.1 (0.9) | 0.005 | 49 | 12.2 (0.8) | 6 | 11.5 (0.6) | 0.048 |
| Weight (kg) | ||||||||||
| At baseline | 103 | 44.0 (12.7) | 60 | 46.4 (11.3) | 0.243 | 98 | 44.8 (12.1) | 21 | 48.5 (12.4) | 0.218 |
| At 3 years | 97 | 62.0 (16.6) | 56 | 66.3 (13.8) | 0.107 | 89 | 60.3 (13.1) | 17 | 58.5 (11.4) | 0.578 |
| ∆ over 3 years | 97 | 18.3 (6.3) | 56 | 20.0 (7.2) | 0.134 | 89 | 16.1 (7.1) | 17 | 11.2 (5.9) | 0.012 |
P values below 0.05 are shown in bold text. Abbreviations: ∆, change; SD, standard deviation; TS, Tanner stage.
aReduced sample size for measures at 3 years are due to study attrition and adolescent refusal to provide a blood sample.
bData presented as median (interquartile range).
cSerum testosterone in males expressed in nmol/L; serum estradiol in females expressed in pmol/L.
dTiming and tempo derived from linear models of Tanner stage change (Beltz et al. Dev Psychol 2014;50:2715–26).
eTiming and tempo derived from logistic models of Tanner stage change (Marceau et al. Dev Psychol 2011;47:1389–409).
Based on the TS3-centered GMM (Table 3), females in class 2 (unstable) exhibited significantly smaller height (10.3 vs 14.0 cm; P = 0.015) and weight (11.2 vs 16.1 kg; P = 0.012) gain over 3 years. However, absolute measures of height and weight were similar to class 1 at baseline and 3 years. Class 2 females had higher serum estradiol levels at baseline (248.6.0 vs 97.6 pmol/L; P = 0.002), with no differences at 3 years (P = 0.102). Age at menarche, together with the timing and tempo of TS progression were similar between the TS3-centered classes. Comparison of participants allocated to the 2 menarche- and PHV-centered classes showed no physical maturation or serum estradiol differences (Tables S9-S10) [46].
3. Discussion
This study used more frequent biological sampling than many other longitudinal studies of puberty which address gonadal hormone change. A research protocol of such intensity was necessary to capture the rapidly changing hormone levels at this life stage and provides a strong foundation for studying the true impact of gonadal hormones on adolescent health, wellbeing, and behavior. It is important to quantify the health and behavioral implications of puberty hormones so as to avoid falsely over- or under-attributing characteristic adolescent mood and behavioral disturbances to “their hormones”; especially as these disturbances have potential lifelong consequences.
A similar study using serum samples would unlikely have been achievable, given the demonstrable challenges of recruiting a large community cohort of this age group [32] and considering the burden that repeated blood collections would place on young participants. Saliva as an alternate biological medium is prone to blood contamination; an issue that is not resolvable by mass spectrometry and is problematic especially in the early pubertal stage when detectable hormone levels are in the low ranges [50]. Our method for urinalysis has been developed and published [28, 29]. The urine values, higher than those in serum and thus easier to measure, correlate strongly with serum values [28]. Ultimately, the time-integrated nature of overnight urine collection is advantageous and more representative when compared with blood sampling, which captures hormone level at 1 instant in time. This is especially true of early puberty, during which gonadotropin activity is greatest at night [51]. The urine collection was also timed to the follicular phase in postmenarchal females.
A key and novel finding was the observation of 2 distinct urine hormone classes/trajectories in both sexes. These could be summarized in terms of stability of quarterly hormone change, together with a trend towards higher levels in those adolescents with the more unstable pattern. We have been unable to identify a study of similar sampling intensity in the literature. The majority of previous studies addressing this question have used less frequent and often randomly collected gonadal hormone samples in blood or saliva, or proxy markers such as anthropometry or Tanner stage. The latter physical measures are easy to perform but have recognized limitations [22].
Our analyses consistently identified 2-class GMMs as best fitting compared with 1- or 3-class models, regardless of centering value. Tanner stage 3 was chosen as a marker where rapid hormone upswings occur and when characteristic adolescent behaviors emerge. Substantial statistical power was lost with age at PHV centering as this was not a study of puberty onset. Centering on menarche is limited by its lateness in puberty and being a single-sex event. Thus, we believe that our data provide robust findings, given the analyses focused on relating hormone patterns to Tanner stage which reflects primarily gonadal hormone change, despite limitations around self-reporting. PHV is a mixed hormonal event occurring at clearly different Tanner stages and degrees of gonadal hormone rise in males and females [52]. Chronological age is also an inadequate indicator of pubertal progression given the wide range in onset [26, 27]. There were no easily identifiable anthropometric markers to delineate these 2 urinary hormone classes.
A second and intriguing finding was that peak urine estradiol levels in premenarcheal females may be as high as levels seen in menarchal females. Estradiol peaks prior to menarche indicate variably active follicular function in the ovaries from late childhood to early- to mid-puberty, supporting prior evidence from ultrasound and inhibin studies [53, 54]. Our data on menarchal onset are highly reliable, either provided by mothers in the small number of girls who had already reached menarche at recruitment, or recorded with the 3-monthly urine sample collections. One explanation for the premenarcheal estradiol peaks may be an intrinsic sex difference in the GnRH pulse regulator. These peaks may make hormone trajectories/patterns less clear in females. An example of this was our inability to replicate differences in TS progression observed between class 1 and 2 males.
In line with recent recommendations to expand scientific research on puberty [55], our findings afford the unique opportunity to test hypotheses concerning the relationship between gonadal hormone change and many commonly observed mood and behavioral changes in adolescence including mood lability, proneness to anxiety and depressive symptoms, disengagement with education, and increased health risk behaviors. It could be further hypothesized that the 2 identified trajectories of urine hormone change represent differences in gonadotropin-releasing hormone (GnRH) production patterns, although this cannot be confirmed on the available data.
The ARCHER study was never intended as a study of pubertal timing or onset, which has been well addressed by the impressive mixed cross-sectional/longitudinal Copenhagen Puberty Study [56], which used a lower biological sampling frequency. Rather, the intent of ARCHER was the detailed description of gonadal hormone change, over a sufficiently long period and in enough participants, to capture such change until the completion or near-completion of puberty. A limitation was the inability to conduct clinician staging of puberty as determined by our institutional Ethics Review Committee. In addition, urine collection over a longer duration would have enabled us to ascertain whether the 2 hormone trajectory classes remain with increasing gonadal maturity. We had neither resources nor adolescent consent or willingness to extend the intensive urine collection beyond 3 years.
In conclusion, a foundational study is presented which allows the exploration as to why some adolescents may be more affected by their puberty hormones than others. Other factors that need to be taken into account with such an exploration are the known neurocognitive development during adolescence [8, 57], when hyper-emotionality, risk-taking and reward-seeking are slowly modulated by ongoing maturation of the prefrontal cortex, as well as by exogenous factors such as family and peer influences. Our study has collected much data relevant to these considerations.
Acknowledgments
The authors thank the ARCHER study participants and their families, and research assistants Lisa Riley and Janet Symons. Acknowledgments to the schools and communities of Central Western NSW which were key to the recruitment of this cohort.
Acknowledgments are made to the ARCHER study investigators who contributed to the design, running of the study with its successful completion: Adrian Bauman, David Bennett, Robert Booy, Chin Moi Chow, Robert G. Cumming, Gregory Fulcher, Catherine Hawke, Philip Hazell, Rebecca Ivers, Patrick Kelly, Andrew J. Martin, Geoffrey Morgan, Karen Paxton, Margot Rawsthorne, S. Rachel Skinner and Jean Starling. Additional acknowledgements to Catherine Hawke, Philip Hazell, Rebecca Ivers and S. Rachel Skinner for their contributions to data interpretation and the critical review of earlier manuscript drafts.
Glossary
Abbreviations
- ARCHER
Adolescent Rural Cohort study of Hormones, Health, Education, Environments and Relationships
- GMM
growth mixture model
- GnRH
gonadotropin-releasing hormone
- PHV
peak height velocity
- SD
standard deviation
- TS
Tanner stage
Financial support: The ARCHER study was funded by a grant from National Health and Medical Research Council of Australia (1003312). Additional support was received from the Thyne Reid Foundation, The Balnaves Foundation, Country Women’s Association of New South Wales and Sydney Medical School. Australian Rotary Health and the Sydney Medical School Foundation supported the initial feasibility studies.
Clinical Trial Information: Australian New Zealand Clinical Trials Registry Number: ACTRN12617000964314
Author contributions: KSS and DJH designed research; KSS, HLC, GML and DJH conducted research; DJH provided essential laboratory advice and materials; FLG, HLC, and KSS analyzed data; all authors interpreted data; KSS and FLG wrote paper; KSS had primary responsibility for final content; all authors read and approved the final manuscript.
Additional Information
Disclosure summary: DJH has received institutional (but no personal) funding for investigator-initiated testosterone pharmacology studies and has provided expert testimony to anti-doping and professional standards tribunals as well as testosterone litigation. All other authors have nothing to disclose.
References and Notes
- 1. Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130–1139. [DOI] [PubMed] [Google Scholar]
- 2. Copeland WE, Angold A, Shanahan L, Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2014;53(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dimler LM, Natsuaki MN. The effects of pubertal timing on externalizing behaviors in adolescence and early adulthood: a meta-analytic review. J Adolesc. 2015;45:160–170. [DOI] [PubMed] [Google Scholar]
- 4. Laessle RG, Tuschl RJ, Schweiger U, Pirke KM. Mood changes and physical complaints during the normal menstrual cycle in healthy young women. Psychoneuroendocrinology. 1990;15(2):131–138. [DOI] [PubMed] [Google Scholar]
- 5. Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015;20(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckwalter JG, Stanczyk FZ, McCleary CA, et al. Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology. 1999;24(1):69–84. [DOI] [PubMed] [Google Scholar]
- 7. Oberlander JG, Henderson LP. The Sturm und Drang of anabolic steroid use: angst, anxiety, and aggression. Trends Neurosci. 2012;35(6):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beam MR, Gil-Rivas V, Greenberger E, Chen C. Adolescent problem behavior and depressed mood: risk and protection within and across social contexts. J Youth Adolesc. 2002;31(5):343–357 [Google Scholar]
- 10. Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;5:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee Y, Styne D. Influences on the onset and tempo of puberty in human beings and implications for adolescent psychological development. Horm Behav. 2013;64(2):250–261. [DOI] [PubMed] [Google Scholar]
- 12. Mendle J. Beyond pubertal timing: new directions for studying individual differences in development. Curr Dir Psychol Sci. 2014;23(3):215–219 [Google Scholar]
- 13. Mendle J, Harden KP, Brooks-Gunn J, Graber JA. Development’s tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Dev Psychol. 2010;46(5):1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castellanos-Ryan N, Parent S, Vitaro F, Tremblay RE, Séguin JR. Pubertal development, personality, and substance use: a 10-year longitudinal study from childhood to adolescence. J Abnorm Psychol. 2013;122(3):782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95(1-2):24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duke SA, Balzer BW, Steinbeck KS. Testosterone and its effects on human male adolescent mood and behavior: a systematic review. J Adolesc Health. 2014;55(3):315–322. [DOI] [PubMed] [Google Scholar]
- 18. Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sizonenko PC. Endocrinology in preadolescents and adolescents. I. Hormonal changes during normal puberty. Am J Dis Child. 1978;132(7):704–712. [DOI] [PubMed] [Google Scholar]
- 20. Kushnir MM, Rockwood AL, Bergquist J, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129(4):530–539. [DOI] [PubMed] [Google Scholar]
- 21. Kushnir MM, Blamires T, Rockwood AL, et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56(7):1138–1147. [DOI] [PubMed] [Google Scholar]
- 22. Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl Dev Sci. 2006;10(1):30–56. [Google Scholar]
- 23. Wang H, Lin SL, Leung GM, Schooling CM. Age at onset of puberty and adolescent depression: “Children of 1997” birth cohort. Pediatrics. 2016;137(6):e20153231. [DOI] [PubMed] [Google Scholar]
- 24. Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond). 2013;37(8):1036–1043. [DOI] [PubMed] [Google Scholar]
- 25. Galvao TF, Silva MT, Zimmermann IR, Souza KM, Martins SS, Pereira MG. Pubertal timing in girls and depression: a systematic review. J Affect Disord. 2014;155:13–19. [DOI] [PubMed] [Google Scholar]
- 26. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh GK, Balzer BW, Kelly PJ, et al. Urinary sex steroids and anthropometric markers of puberty - a novel approach to characterising within-person changes of puberty hormones. Plos One. 2015;10(11):e0143555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh GK, Balzer BW, Desai R, Jimenez M, Steinbeck KS, Handelsman DJ. Requirement for specific gravity and creatinine adjustments for urinary steroids and luteinizing hormone concentrations in adolescents. Ann Clin Biochem. 2015;52(Pt 6):665–671. [DOI] [PubMed] [Google Scholar]
- 30. Steinbeck K, Hazell P, Cumming RG, et al. The study design and methodology for the ARCHER study–adolescent rural cohort study of hormones, health, education, environments and relationships. BMC Pediatr. 2012;12:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luscombe GM, Cheng HL, Balzer BWR, et al. 2017 The ARCHER study of health and wellbeing in young rural Australians. 14th National Rural Health Conference; 2017; Cairns, Australia http://www.ruralhealth.org.au/14nrhc/sites/default/files/Luscombe%2C%20Georgina_E6_0.pdf. Accessed January 10, 2020. [Google Scholar]
- 32. Amon K, Paxton K, Klineberg E, et al. Recruiting a young adolescent rural cohort: costs and lessons learnt. Adv Pediatr Res. 2016;3(5):1–9 [Google Scholar]
- 33. Cooper Robbins SC, Rawsthorne M, Paxton K, Hawke C, Skinner SR, Steinbeck K. “You can help people”: adolescents’ views on engaging young people in longitudinal research. J Res Adolesc. 2012;22(1):8–13 [Google Scholar]
- 34. Chow CM, Wong SN, Shin M, et al. Defining the rest interval associated with the main sleep period in actigraph scoring. Nat Sci Sleep. 2016;8:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. August GP, Grumbach MM, Kaplan SL. Hormonal changes in puberty. 3. Correlation of plasma testosterone, LH, FSH, testicular size, and bone age with male pubertal development. J Clin Endocrinol Metab. 1972;34(2):319–326. [DOI] [PubMed] [Google Scholar]
- 36. Apter D. Serum steroids and pituitary hormones in female puberty: a partly longitudinal study. Clin Endocrinol (Oxf). 1980;12(2):107–120. [DOI] [PubMed] [Google Scholar]
- 37. Hemphill SA, Kotevski A, Herrenkohl TI, et al. Pubertal stage and the prevalence of violence and social/relational aggression. Pediatrics. 2010;126(2):e298–e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng HL, Sainsbury A, Garden F, et al. Ghrelin and peptide YY change during puberty: relationships with adolescent growth, development, and obesity. J Clin Endocrinol Metab. 2018;103(8):2851–2860. [DOI] [PubMed] [Google Scholar]
- 39. Jiménez C, de la Torre R, Segura J, Ventura R. Stability studies of testosterone and epitestosterone glucuronides in urine. Rapid Commun Mass Spectrom. 2006;20(5):858–864. [DOI] [PubMed] [Google Scholar]
- 40. Fuhrman BJ, Xu X, Falk RT, et al. Stability of 15 estrogens and estrogen metabolites in urine samples under processing and storage conditions typically used in epidemiologic studies. Int J Biol Markers. 2010;25(4):185–194. [PMC free article] [PubMed] [Google Scholar]
- 41. Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta. 2009;409(1-2):78–84. [DOI] [PubMed] [Google Scholar]
- 42. WADA. Athlete Biological Passport Operating Guidelines (Version 7.0). Montreal: World Anti-Doping Agency; 2019. [Google Scholar]
- 43. Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008;2:302–317 [Google Scholar]
- 44. Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569 [Google Scholar]
- 45. Huang B, Hillman J, Biro FM, Ding L, Dorn LD, Susman EJ. Correspondence between gonadal steroid hormone concentrations and secondary sexual characteristics assessed by clinicians, adolescents, and parents. J Res Adolesc. 2012;22(2):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steinbeck KS, Garden FL, Cheng HL, Luscombe GM, Handelsman DJ. Online supplementary material - Bumpy and smoother pathways of puberty hormone change: A novel way to define gonadal hormone trajectories in adolescents. 2019. doi: 10.25910/5dc1eb49c6556. URL: . [DOI] [PMC free article] [PubMed]
- 47. Muthén LK, Muthén BO.. Mplus User’s Guide. 6th ed. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- 48. Beltz AM, Corley RP, Bricker JB, Wadsworth SJ, Berenbaum SA. Modeling pubertal timing and tempo and examining links to behavior problems. Dev Psychol. 2014;50(12):2715–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol. 2011;47(5):1389–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amatoury M, Lee JW, Maguire AM, Ambler GR, Steinbeck KS. Utility of salivary enzyme immunoassays for measuring estradiol and testosterone in adolescents: a pilot study. Int J Adolesc Med Health. 2016;30(1). [DOI] [PubMed] [Google Scholar]
- 51. Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: distinguishing unique physiologic roles. Gynecol Endocrinol. 2014;30(3):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Granados A, Gebremariam A, Lee JM. Relationship between timing of peak height velocity and pubertal staging in boys and girls. J Clin Res Pediatr Endocrinol. 2015;7(3):235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cacciatore B, Apter D, Alfthan H, Stenman U-H. Ultrasonic characteristics of the uterus and ovaries in relation to pubertal development and serum LH, FSH, and estradiol concentrations. Adolesc Pediatr Gynecol. 1991;4(1):15–20 [Google Scholar]
- 54. Crofton PM, Evans AE, Groome NP, Taylor MR, Holland CV, Kelnar CJ. Dimeric inhibins in girls from birth to adulthood: relationship with age, pubertal stage, FSH and oestradiol. Clin Endocrinol (Oxf). 2002;56(2):223–230. [DOI] [PubMed] [Google Scholar]
- 55. Dorn LD, Susman EJ. Fostering the developmental science of puberty: tackling deficits and enhancing dissemination. J Adolesc Health. 2019;64(5):561–562. [DOI] [PubMed] [Google Scholar]
- 56. Mouritsen A, Aksglaede L, Soerensen K, Hagen CP, Petersen JH, Main KM, Juul A. The pubertal transition in 179 healthy Danish children: associations between pubarche, adrenarche, gonadarche, and body composition. Eur J Endocrinol. 2013;168(2):129–136. [DOI] [PubMed] [Google Scholar]
- 57. Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]