Abstract
The conserved Ypt/Rab GTPases regulate the different steps of all intracellular trafficking pathways. Ypt/Rabs are activated by their specific nucleotide exchangers termed GEFs, and when GTP-bound they recruit their downstream effectors, which mediate vesicular transport sub-steps. In the yeast exocytic pathway, Ypt1 and Ypt31/32 regulate traffic through the Golgi and the conserved modular TRAPP complex acts a GEF for both Ypt1 and Ypt31/32. However, the precise localization and function of these Ypts have been under debate, as is the identity of their corresponding GEFs. We have established that Ypt1 and Ypt31 reside on the two sides of the Golgi, early and late, respectively, and regulate Golgi cisternal progression. We and others have shown that whereas a single TRAPP complex, TRAPP II, activates Ypt31, three TRAPP complexes can activate Ypt1: TRAPPs I, III, and IV. We propose that TRAPP I and II activate Ypt1 and Ypt31, respectively, at the Golgi, whereas TRAPP III and IV activate Ypt1 in autophagy. Resolving these issues is important because both Rabs and TRAPPs are implicated in multiple human diseases, ranging from cancer to neurodegenerative diseases.
Keywords: Golgi, Ypt/Rab GTPases, Ypt1, Ypt31, TRAPP complexes, Golgi cisternal progression, cisternal maturation
Introduction
Ypt/Rab GTPases are key regulators of all membrane trafficking pathways in eukaryotic cells [1]. Their cycling between the cytoplasmic GDP-bound form and the membrane-bound GTP-bound form is controlled by upstream regulators. In the cytoplasm, they are in complex with the GDP-inhibiting factor, GDI, which protects their lipid tail from the hydrophilic environment of the cytoplasm. Guanine-nucleotide exchange factors, GEFs, activate Ypt/Rabs by stimulating their switch to the GTP-bound form in which they are attached to membranes through their lipid tail. GTPase-activating proteins, GAPs, negatively regulate Ypt/Rabs because the resultant GDP-bound form can be extracted from membranes by GDI for multiple rounds of activation (Figure 1). Whereas GEFs and GAPs are specific for individual Ypt/Rabs, GDI is a generic chaperone for multiple Ypt/Rabs [2].
Figure 1: Nucleotide and membrane cycling of Ypt/Rab GTPases.

Ypt/Rab GTPases cycle between the GDP- and GTP-bound forms. To be active, they have to be in the GTP-bound form and be attached to membranes through their lipid tail. This activation is stimulated by guanine-nucleotide exchange factors, GEFs. When activated, Ypt/Rabs recruit their downstream effectors to assemble membrane micro-domains that can mediate a specific vesicular transport step. Inactivation of Ypt/Rabs is done by GTPase-activating proteins, GAPs, that stimulate their switch to the GDP-bound form. In that form, Ypt/Rabs can interact with the GDP-inhibiting protein, GDI, a chaperone that extracts GDP-bound Ypt/Rabs from membranes and keeps them in the cytoplasm for multiple rounds of activity.
Membrane-attached GTP-bound Ypt/Rabs interact with their downstream effectors (Figure 1). Collectively, Ypt/Rab effectors include all the components of vesicular transport machinery required for vesicle formation, motility, tethering and fusion. Importantly, an individual activated Ypt/Rab can interact with multiple effectors [2]. This ability allows an individual Ypt/Rab to recruit multiple effectors to a specific location and organize membrane micro-domains that contain diverse machinery components required for completion of a single vesicular transport step [3]. Moreover, individual Ypt/Rabs can regulate more than one vesicular transport step, and in fact in more than one intra-cellular trafficking pathway. This plasticity probably requires different GEFs in addition to different effectors. We have proposed that Ypt/Rabs function in GEF-GTPase-effector modules, which are specific to individual transport steps and/or pathways [1].
Historically, Ypt/Rabs were shown to regulate the different steps of the exocytic and endocytic pathways. More recently their involvement in autophagy was also established [4]. In yeast, nine Ypts fall into six groups that have mammalian Rab orthologs and for which functions have been assigned (Figure 2). Four exocytic Ypts, Ypt1, the Ypt31/Ypt32 functional pair and Sec4, are required for viability and regulate the beginning, intermediate and late steps of the exocytic pathway, respectively. Their closest mammalian orthologs are Rab1, Rab11 and Rab8, respectively. The other five Ypts fall into three groups and are not required for viability: Vps21 (Ypt51), Ypt52 and Ypt53 are Rab5 homologs and regulate early steps of the endocytic pathway, Ypt7 is a Rab7 homolog and regulates late endocytic steps, and Ypt6, a Rab6 homolog, regulates recycling steps form endosomes to the Golgi and within the Golgi [5]. Roles for Ypt1, Vps21, and Ypt7 have been ascribed also in autophagy [6–8]. Human cells have ~70 Rabs and together with their upstream regulators and downstream effectors they constitute ~1% of the human proteome. Rabs, like Ypts, were shown to regulate intra-cellular trafficking and autophagy pathways [5, 9]. Here, we will discuss the Golgi Ypt/Rabs including Ypt6/Rab6 (Table 1).
Figure 2: The yeast Ypts in intra-cellular trafficking.
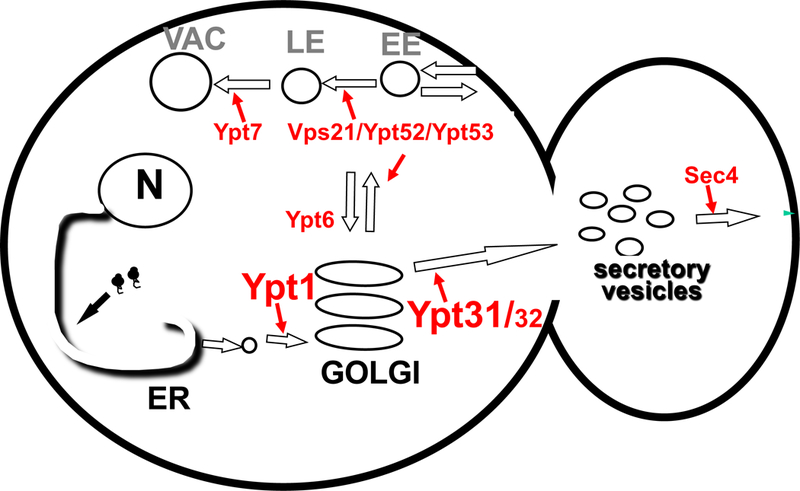
Current view of the Ypts and the transport step they regulate. In the exocytic pathway, Ypt1, Ypt31/32 and Sec4 are required for early, middle and late transport steps, with Ypt1 and Ypt31/32 in Golgi entry and exit, and Ypt31/32 and Sec4 in polarized secretion of trans-Golgi vesicles to the growing bud [1]. In the endocytic pathway, Vps21, Ypt52 and Ypt53 regulate early steps of the pathway and Ypt7 the end [5]. Ypt6 has been implicated in cross talk between the exocytic and endocytic pathways and intra-Golgi recycling [30]. ER, endoplasmic reticulum; N, nucleus; EE, early endosomes, LE, late endosomes, VAC, vacuole (the yeast lysosome).
Table 1:
The Golgi Ypt/Rabs
| A. | |||
|---|---|---|---|
| Ypt1 | h-RAB1A | h-RAB1B | |
| Sc-Ypt1 | x | 70 | 68 |
| h-Rab1A | x | 92 | |
| h-Rab1B | x | ||
| B. | |||||
|---|---|---|---|---|---|
| Sc-Ypt31 | Sc-Ypt32 | h-RAB11A | h-RAB11B | h-RAB25 | |
| Sc-Ypt31 | x | 81 | 57 | 58 | 49 |
| Sc-Ypt32 | x | 58 | 57 | 48 | |
| h-Rab11A | x | 90 | 58 | ||
| h-Rab11B | x | 57 | |||
| h-Rab25 | x | ||||
| C. | ||||
|---|---|---|---|---|
| Sc-Ypt6 | h-RAB6A | h-RAB6B | h-RAB6C | |
| Sc- Ypt6 | x | 56 | 57 | 44 |
| h- Rab6A | x | 91 | 74 | |
| h-Rab6B | x | 67 | ||
| h-Rab6C | x | |||
Numbers show percent amino-acid identity shared by the two indicated proteins (Align, UniProt).
Fonts: Bold, paralogs (same organism); red, orthologs (different organisms); gray, distant homologs. Sc, Saccharomyces cerevisiae; h-human.
Another family of conserved small GTPases, Arfs, mediates the vesicle formation sub-step of vesicular transport. Arfs are activated by their Sec7-domain containing GEFs, which are also conserved. In yeast, Arf1/2 and their GEFs, Gea1/2 and Sec7, regulate transport in early and late Golgi, respectively [10]. Based on genetic interactions, we proposed that a GTPase cascade of Ypts and Arf GEFs functions at the Golgi [11]. We will discuss these interactions in the context of the Golgi Ypt effectors.
A major question in the Ypt/Rab field has been whether individual GTPases are specific to a compartment and/or a trafficking step. Based on mutant phenotypes, we have proposed that two Ypts regulate entry into and exit from the Golgi apparatus, Ypt1 and Ypt31/32, respectively [12]. However, this view was challenged when Ypt1 was reported to localize to and function in the late Golgi [13].
TRAPP complexes were identified as GEFs for Ypt1 and Ypt31 [14, 15]. There are at least four TRAPP complexes, I-IV, which contain the four-subunit core TRAPP, while the different complexes contain additional subunits. In yeast, seven of the ten TRAPP subunits are essential for viability and three are not (Table 2 and Figure 3). The structure and/or architecture of TRAPP complexes is known [16]. However, assignment of TRAPP complexes to intra-cellular transport steps or pathways and their specific Ypt substrates is still controversial [17].
Table 2:
TRAPP complexes subunits
| TRAPP Complex |
Yeast Subunit |
Yeast Viability |
Human Subunit |
Human Viability a |
Identity (%) b |
|---|---|---|---|---|---|
| Core / I | Bet3 | Essential | TRAPPC3 | Essential | 50 |
| Bet5 | Essential | TRAPPC1 | Essential | 27 | |
| Trs23 | Essential | TRAPPC4 | Essential | 26 | |
| Trs31 | Essential | TRAPPC5 | Essential | 19 | |
|
Adaptor
(II & III) |
Trs20 | Essential | TRAPPC2 | Non-essential c | 32 |
| II | Trs120 | Essential | TRAPPC9 | Non-essential | 14 |
| Trs130 | Essential | TRAPPC10 | Non-essential | 13 | |
| Trs65 | Non-essential | TRAPPC13 | Non-essential | 12 | |
| III | Trs85 | Non-essential | TRAPPC8 | Essential | 8–18 d |
| IV | Trs33 | Non-essential | TRAPPC6A/B | Non-essential e | 16/19 |
| TRAPPC11 | Essential | NA | |||
| TRAPPC12 | Non-essential | NA |
Essentiality for human cell viability [16]
Identity in amino acids sequence between yeast and human subunits (Align, UniProt)
TRAPPC2 has a paralog, TRAPPC2L (20% identity)
18% identity considering that Trs85 has 698 amino acids compared to 1435 of TRAPPC8
TRAPPC6 A and B share 56% identity; their combined essentiality was not reported
Figure 3: Yeast TRAPP complexes in secretion and autophagy. Whereas the structure of TRAPP complexes is known, their functions are still controversial.

Core TRAPP, or TRAPP I, contains four different subunits: Bet3 (two copies, 3a and 3b), Bet5, Trs23, and Trs31. These subunits are common to all other TRAPP complexes. Trs20 (orange) is an adaptor required for assembly of two large TRAPP complexes: TRAPP II, contains three TRAPP II-specific subunits (grey), Trs120, Trs130 and Trs65 or Trs33; TRAPP III contains one TRAPP III-specific subunit, Trs85 (green). TRAPP IV contains Trs33. We propose that while TRAPP I and II regulate early and late steps in the secretory pathway, TRAPP III and IV regulate autophagy [16]. However, the uncertainties concerning the roles of TRAPP I and TRAPP III in secretion and of TRAPP II in PM recycling are indicated by question marks (?). Subunit diagram depicts relative size of the subunits and is modeled based on complexes structure or architecture (TrsX, X is roughly the size of the subunit in Kd). In the shown orientation, Ypt1 interacts with Bet5 and Trs23 on the bottom surface [53].
In summary, assignment of both Ypts and TRAPP complexes to specific Golgi cisterna has been contentious. This is true in yeast, where the interactome of Ypts and their accessory factors is minimal compared to human cells. The problems with addressing such questions stem from difficulty in distinguishing between direct and indirect effects of mutations on multiple-step interconnected pathways and in determining precise localization of dynamic proteins.
Both Ypts and TRAPP subunits are conserved from yeast to human cells, and in human cells they were implicated in diseases ranging from cancer to neurodegeneration [18, 19]. Therefore, clarifying the functions of Ypts and TRAPPs in the yeast Golgi is important as a springboard for establishing the function of their human orthologs. Here, we summarize our current understanding of the functions that Ypt/Rab GTPases and their TRAPP GEFs play at the different cisterna of the yeast Golgi and highlight questions that are still open about their functions.
I. The Golgi Ypt/RABs
In early studies, we used temperature-sensitive Ypt1 and Ypt31/32 mutant cells to determine their loss-of-function phenotypes. These mutations, ypt1-A136D and ypt32-A141D (the latter in the background of ypt31Δ), change an amino acid conserved in Ypt/Rabs. They render a rapid block of secretion (<1 minute), and thereby a fair chance for following direct phenotypes. These studies implicated Ypt1 and Ypt31/32 in entry into and exit from the Golgi [20, 21]. Later studies from multiple groups suggested that Ypt1 and Ypt31/32 function in other steps of intra-cellular trafficking pathways and in autophagy [8, 13]. Localization studies added confusion to the field [13, 22]. To clarify these issues, we performed careful mutational and localization analyses for Ypt1 and Ypt31 that supported our original proposal. In addition, these experiments provided the first evidence for regulation of Golgi cisternal progression.
Ia. Functions of the Golgi Ypts.
In addition to their defects in Golgi entry and exit, mutations in both Ypt1 and Ypt31 affect other steps of the exocytic pathway and/or autophagy. The challenge has been to distinguish between direct and indirect effects. To establish functions in different steps, we employed Ypt alleles or growth conditions specific for each process and identified process-specific effectors (Figure 4).
Figure 4: GTPase modules of Ypt1 and Ypt31.

For each process, a GTPase module contains a GEF, the GTPase and an effector (middle, red). Ypt1 functions in two different modules: Golgi entry (left) and autophagy (right): The Golgi-specific GEF and effector are TRAPP I and Uso1; the autophagy specific GEF and effector are TRAPP III and Atg11. Ypt31 functions in two modules: Golgi exit (left), and PM recycling (right). The only known GEF for Ypt31 is TRAPP II, and it activates it both in Golgi exit and in PM recycling to the Golgi [67]. Two known Ypt31/32 effectors are Myo2 for Golgi exit and Rcy1 for PM recycling. Other Ypt1 and Ypt31/32 effector were reported.
Ypt1 in Golgi entry and in autophagy: We have shown that Ypt1 is required for normal growth and autophagy early on [23]. To establish that these roles are separate, we used two different mutations: the first is a temperature-sensitive mutation that affects the exocytic pathway within a minute after the temperature shift, while the other has no effect on secretion or cell growth and only inhibits autophagy [21, 24]. As for process-specific effectors: Two Ypt1 effectors were proposed to function in vesicle formation and tethering at the early Golgi. First, Sec7, a GEF for Arf GTPase required for vesicle formation [25]. Second, the Uso1 tether was suggested as an effector for Ypt1 in ER-to-Golgi transport [26]. We identified Atg11, a bona fide autophagy-specific protein, as an effector of Ypt1 in autophagy. Our mutant analysis showed that Ypt1 and its Atg11 effector play a role in the onset of autophagy: formation of the pre-autophagosomal structure, PAS [8].
Ypt31 in Golgi exit and plasma membrane (PM) recycling: To distinguish between the roles of Ypt31/32 in exit from the Golgi and in PM recycling, we used different growth conditions for the same mutation. While the effect on the exocytic pathway occurs only at the restrictive temperature, PM recycling is blocked at the permissive temperature. As for process-specific effectors: Three Ypt31/32 effectors were proposed to function in the Golgi-to-PM transport step, from vesicle formation and motility to handover to Sec4 GTPase. First, Sec7, a GEF for Arf GTPase that mediates vesicle formation [25]. Second, we identified Myo2, a myosin V molecular motor, as an effector required for the function of Ypt31 in exit from the Golgi [27]. Third, Sec2, a GEF for Sec4 GTPase, which mediates late Golgi vesicle tethering and fusion [28]. For PM-recycling, we identified Rcy1, a F-box containing ubiquitin ligase, as a Ypt31/32 effector required specifically for this transport step [29].
Ypt6 is not essential to cell viability, but its deletion renders a temperature-sensitive phenotype. It plays a role in Golgi organization and was implicated in three retrograde transport steps that involve the Golgi: endosome-to-Golgi, Golgi-to-ER and intra Golgi [30].
Ib. Ypts localization at the Golgi.
Intra-cellular localization of Ypt1 and Ypt31/32 has been problematic and did not match their assigned function [13]. We decided to determine their localization using a number of rigorous criteria. First, when using a tagged Ypt, it should be tagged at its N-terminus. Localization of Ypt/Rabs tagged at their C-terminus (which is easier in yeast when tagging endogenous genes) is meaningless because the tag prevents the lipidation needed for their membrane attachment. Second, we made sure that the tagged Ypt is fully functional and can function as the only Ypt in the cell. For tagged Ypt1 and Ypt31, we confirmed that they support cell viability. Third, the Ypt should be expressed in its normal level either from its endogenous locus or, if expressed exogenously, under its own promoter and terminator. This is especially important when the Ypt localizes to more than one compartment. Lastly, we used two independent methods to localize the Ypts, live-cell microscopy of fluorescently-tagged Ypts and immuno-fluorescence (IF) microscopy using specific antibodies. For localization on specific Golgi cisterna, we used a set of well-documented cisternal markers, at least two for each cisterna. Co-localization of the early and late Golgi markers themselves showed that these markers partially co-localize (~15%) on an intermediate compartment we termed “transitional”.
When the Ypts were co-localized with those Golgi cisternal markers, live-cell and IF approaches gave the same answer: Ypt1 and Ypt31 reside on the opposite sides of the Golgi apparatus, with Ypt1 mostly on the early Golgi and Ypt31 on late Golgi. In addition, approximately 20% of Ypt1 and Ypt31 also localize to the transitional cisterna, where they co-localize with early and late Golgi markers and with each other [31]. Thus, functional and rigorous localization analyses agree that Ypt1 resides and functions in the early Golgi whereas Ypt31 functions and localizes at the late Golgi.
Using live-cell microscopy of N-terminally tagged Ypt6 and Golgi cisternal markers, Ypt6 was localized mostly to the early Golgi [32]. Dynamics studies of Ypt1, Ypt6, and Ypt31/32 showed that they temporarily localize to the Golgi in this order. Interestingly, deletion of Gyp1, a Ypt1 GAP, or Gyp6, the GAP for Ypt6, delayed the removal of Ypt1 or Ypt6, respectively, from the Golgi. These results combined with interaction of Gyp1 and Gyp6 with Ypt32 suggest that Rab-GAP cascades coordinate sequential localization of Ypt1, Ypt6 and Ypt32 on the Golgi [22, 33].
Ic. The Golgi Ypts and Golgi cisternal progression.
Whereas transport between membrane-bound compartments is mediated by vesicles, it has been suggested that transport within sorting compartments, i.e., Golgi and endosomes, occurs via cisternal maturation or progression [34]. In yeast, the idea that transport through the Golgi occurs via cisternal progression was supported by time-lapse microscopy analysis. Such analysis was possible due to the fact that yeast Golgi cisterna are not stacked like mammalian Golgi cisterna, but are separated. Therefore, maturation from early to late Golgi can be observed by following discrete cisterna and observing the switch of early and late Golgi markers on them [35, 36]. However, it took a decade to show that this process can be separated to two sequential steps and that these steps are regulated by Ypt1 and Ypt31. For this analysis, we used the early and late Golgi cisternal markers we established for the Ypt localization studies, in combination with Ypt mutant proteins with altered activity. We used two different approaches to determine the effects of these mutant proteins: effect on co-localization of Golgi cisternal markers using steady-state live-cell microscopy, and effect on the dynamics of Golgi cisternal markers using time-lapse fluorescence microscopy.
Using Ypt1 mutant protein trapped in the “activated” GTP-bund form and titrating its level, we showed that the switch from early to transitional cisterna was accelerated by up to 2-fold when compared to wild-type Ypt1; from 20 seconds gap between the early and transitional markers (reaching 50% fluorescence) to 8 seconds. In addition, the level of the transitional compartment was increased by 3-fold under these conditions. Inactivation of Ypt1 resulted in opposite effects. On the other hand, titration of activated Ypt31 caused up to 2.5-fold increase in the rate of maturation of transitional to late cisterna (from 10 to 4 seconds), and a five-fold increase in the accumulation of the trans-Golgi vesicles compared to those of the wild type, while inactivation of Ypt31 had the opposite effect. These effects were specific for each Ypt [31]. These results implicate Ypt1 in regulation of early to transitional and Ypt31 in transitional to late Golgi progression. In summary, in addition to providing a regulatory mechanism for Golgi cisternal progression, we also showed that it can be separated to two steps (Figure 5).
Figure 5: Assignment of Ypts and TRAPPs at the yeast Golgi.

Our data supports a model in which Ypt1 and TRAPP I reside and function at the early Golgi whereas Ypt31/32 and TRAPP II do the same at the late Golgi. In addition, we have shown that Ypt1 and Ypt31/32 regulate two steps of Golgi cisternal progression, early to transitional and transitional to late, respectively.
Id. Golgi Rabs.
We have shown the conservation of Ypt1 and its intra-cellular localization between yeast and mammalian cells early on [37]. The closest human homologs of Ypt1 are Rab1A and Rab1B with about 70% identity at the amino acid level. The two closest homologs of Ypt31/32 are Rab11A and Rab11B, with ~60% identity. Rab25 also belongs to the Rab11 sub-family, with~60% and ~50% shared identity to Rab11A/B and Ypt31/32, respectively. The closest Ypt6 homologs are Rab6A and Rab6B with ~57% shared identity, and a more distant homolog, Rab6C, with ~44% shared identity (Table 1). Remarkedly, the closest Ypt1 human homolog Rab1A can replace Ypt1 in yeast [38], namely Rab1A is a functional homolog of the yeast Ypt1. This implies that not only the GTPase itself is conserved, but also its interactions that are important for cell viability are conserved.
Each Golgi Rab pair, Rab1A-B, Rab11A-B, and Rab6A-B, share ~90% identity. None of the Golgi Rabs is individually required for cell viability. Only for the Rab1A and Rab1B it was shown that at least one of them is required for cell viability [39]. In contrast, double knockout of Rab11A and Rab11B or Rab6A and Rab6B showed that neither pair is required for cell viability [40].
Functional analyses of the Golgi Rabs were done mostly using dominant interfering mutations and gene knockdown and localization analyses were usually done with tagged overexpressed Rabs. These analyses agreed with the overall findings regarding their yeast Ypt homologs [30]. However, more rigorous localization and functional studies are needed for assigning the Golgi Rabs to specific cisterna and testing their possible roles in Golgi cisternal progression.
Ie. Golgi Rabs in disease.
Mutations in both Rab1A/B and Rab11A/B were implicated in neural disorders and cancer [41, 42]. Mutations in Rab25, a close relative of Rab11, are associated with a neural disease, Binocular Vision Disease, while elevated expression of Rab25 is linked to poor prognosis and aggressiveness of a number of cancers [43].
II. TRAPP GEFs at the Golgi
The structure and/or architecture of different TRAPP complexes have been determined to different extents. The crystal structure of core TRAPP with Trs33 and Trs20 has been resolved by X-ray crystallography, as was that of core TRAPP in complex with Ypt1 [44, 45]. The architecture of the larger complex TRAPP III was determined by cryo-EM and interaction analyses [46, 47]. The largest complex, TRAPP II, was suggested to exist as a dimer in cell lysates [48, 49] and the structure of this dimer was characterized by single-particle EM [49]. However, the in vivo occurrence or importance of TRAPP II dimerization is not clear because TRAPP II dimers were not observed in lysates of trs65Δ mutant cells [48], which exhibit no growth or autophagy phenotypes. We showed that one essential subunit, Trs20, while not required for assembly of core TRAPP or its Ypt1 GEF activity, can assemble with core-TRAPP and acts as an adaptor for the assembly of both TRAPP II and TRAPP III [47, 50].
While most TRAPP subunits are essential for cell viability, three subunits are not: Trs33, Tr65, and Trs85. There are two possible explanations, which are not mutually exclusive, for the non-essentiality of these subunits: first, they are involved in a process not essential for cell viability, e.g., autophagy. Alternatively, their functions overlap and together they are essential. As discussed below, we think that both options are in accordance for the non-essential TRAPP subunits: While the function of Trs85 and Trs33, alone or together, in autophagy is not essential for viability, the functions of Trs33 or Trs65 in assembly of TRAPP II overlap and at least one of them is essential for cell viability [51, 52].
Our model that GTPases function in process-specific modules assumes the exitance of module-specific GEFs (Figure 4). Early work on the TRAPP complexes suggested function and localization of core TRAPP subunits to early Golgi and of TRAPP II-specific subunits to late Golgi [53]. We have shown that in vitro TRAPP I and TRAPP II act as GEFs for Ypt1 and Ypt31, respectively. In addition, genetic interactions and effect of TRAPP mutations on Ypt localization supported the in vitro data [15].
Together with assigning Ypt1 and Ypt31 to early and late Golgi cisterna and GEF specificity of the TRAPP complexes, we proposed the combined model for Ypts and TRAPP at the Golgi. In this model, TRAPP I activates Ypt1 in the early Golgi and TRAPP II activates Ypt31 in late Golgi (Figure 5). TRAPP III is another Ypt1 GEF, and its specific subunit, Trs85, is not required for cell viability [54] but was implicated in autophagy [55, 56]. However, more recent studies have put into question the GEF specificity of TRAPPs to Ypts [44], and suggested that TRAPP I does not exist and that TRAPP III functions as the GEF for Ypt1 in early Golgi [57].
IIa. TRAPP I or Core TRAPP.
This is the smallest TRAPP complex and its GEF activity for Ypt1 is clear. This pentameric complex contains four small subunits (each <30 KDa), with two copies of Bet3. Convincingly, recombinant core TRAPP can act as a GEF for recombinant Ypt1 with a minimum of the four core TRAPP subunits [45]. However, because it is the core for all other TRAPP complexes and due to its low level in yeast and mammalian cell lysates [58], currently, TRAPP I is the most controversial TRAPP complex. In fact, its mere existence in vivo has been questioned [57]. However, we argue that because TRAPP complexes tend to form dimers and aggregates [48, 58], the low level of TRAPP I in cell lysates might not reflect the in vivo situation. Namely, while TRAPP I might exist in vivo, in cell lysates it associates with the larger TRAPP complexes. Thus, the jury is still out on the existence of TRAPP I, its localization, and its in vivo function.
IIb. TRAPP II and Trs65/Trs33.
This is the largest TRAPP complex. In addition to core TRAPP, TRAPP II contains two large subunits specific to this complex, Trs120 and Trs130. The assembly of this largest complex requires the adaptor Trs20 [50] and one of the two non-essential subunits, Trs65 or Trs33 [51, 52]. We showed that TRAPP II can act as a Ypt31 GEF in vitro and that TRAPP II-specific mutations affect Ypt31 localization to the Golgi in vivo [15]. We also showed that the TRAPP II-specific subunits Trs120 and Trs130 co-localize with a late Golgi marker [50], where Ypt31 resides [31], and this was corroborated for Trs130 by others [59]. However, the GEF substrate of TRAPP II has been controversial.
TRAPP II assembly:
The idea that either Trs65 or Trs33 is required for TRAPP II assembly is based on the findings that Trs33 and Trs65 interact with the TRAPP II-specific subunits Trs120 and Trs130, respectively, and deletion of either results in lower level of TRAPP II and in lower Golgi localization of Ypt31/32. We proposed that the essential function of Trs33 and Trs65 for cell viability is in TRAPP II assembly. The rationale for this idea is that while the two TRAPP II-specific subunits, Trs120 and Trs130, are each essential for viability, only double deletion of trs65Δ and trs33Δ is lethal. Thus, the essential function of Trs33 or Trs65 can be fulfilled by either subunit [51, 52].
GEF substrate of TRAPP II:
Does TRAPP II act as a Ypt1 or Ypt31 GEF? This dispute stems from biochemical assays of components purified from cell lysates that depend on the conditions used. While we showed that TRAPP I acts as a GEF for Ypt1 and TRAPP II as a GEF for Ypt31 [15], others suggested that they both act as GEFs for Ypt1 [17, 44]. Later studies from different labs supported the idea that TRAPP II acts as a GEF for Ypt31 and not Ypt1. First, in the fungus Aspergillus, TRAPP II was shown to activate RabE, the Ypt31 homolog, and not RabO, the Ypt1 homolog, in vivo [60]. Second, in vitro recombinant TRAPP II acts as a potent GEF for prenylated Ypt31 in the presence of membranes [59]. Thus, current notion assigns TRAPP II as a GEF for Ypt31/32.
IIc. TRAPP III and Trs85.
TRAPP III contains core TRAPP, the Trs20 adaptor and the TRAPP III-specific subunit, Trs85 [16]. While plenty of evidence supports the idea that TRAPP III functions as Ypt1 GEF in autophagy [8, 55, 56], recent functional and localization studies implicate it also as a Ypt1 GEF in the exocytic pathway [57]. If true, the latter observation means that TRAPP III can activate Ypt1 in both ER-to-Golgi transport and autophagy. This would imply that there is no GEF specificity in the two Ypt1 GTPase modules that act in the exocytic and autophagic pathways.
We reason that while the role of Ypt1 is essential for cell viability, Trs85 is not. Thus, even if TRAPP III can activate Ypt1 in ER to Golgi transport, there must be another way to activate Ypt1 in this step. We propose that the best candidate is TRAPP I, or core TRAPP, because early studies implicated core TRAPP subunits in ER-to-Golgi transport [61], which matches the localization and function of Ypt1. However, currently the identity of the Ypt1 TRAPP GEF in the Golgi is under debate.
IId. TRAPP IV and Trs33:
While the Trs33 subunit can assemble with core TRAPP, it is not essential for TRAPP complex assembly, nor for the Ypt1 GEF activity of this complex [45]. In terms of structure, Trs33 and Trs85, via its association with Trs20, are positioned in the two opposite ends of core TRAPP [45, 46].
The existence of TRAPP IV and its role in autophagy was suggested by phenotypic analysis of double deletion of trs85Δ and trs33Δ. Whereas trs85Δ mutant cells exhibit severe autophagy phenotypes, trs33Δ show milder autophagy defects. Importantly, mutant cells with a double deletion of trs85Δ and trs33Δ exhibit more severe autophagy defects than those of the single deletions trs85Δ or trs33Δ. In addition, Trs33 localizes to PAS and the localization of Trs33 and Trs85 to PAS is independent of each other [62].
We also showed that Trs33 functions in autophagy in the context of the TRAPP complex, because, like the trs85Δ, trs33Δ affects the localization of a core TRAPP subunit to PAS; in the double deletion this localization is even lower. Thus, functional and localization analyses support the existence of a second TRAPP complex in autophagy, TRAPP IV, which contains core TRAPP and the specific subunit, Trs33 [62].
The idea that TRAPPs III and IV act in autophagy through Ypt1 is supported by the effect of the single trs85Δ and trs33Δ deletions, and even more the double deletion, on the localization of Ypt1 to PAS. Furthermore, while over-expression of Ypt1 can suppress autophagy phenotypes of the single trs85Δ or trs33Δ deletions, it does not suppress the autophagy phenotypes of the double deletion mutant. This shows that the Ypt1 GEF activity of either TRAPP III or TRAPP IV is required for autophagy and that in the absence of Trs85, Trs33 can supplement this function [62].
Importantly for the Golgi TRAPPs: While Trs33 plays a role in autophagy in the context of TRAPP IV, it is also relevant for the assembly of TRAPP II at the Golgi. In addition, because historically Trs33 was assigned as a TRAPP I subunit, it is still considered an early Golgi TRAPP subunit [17].
IIe. Mammalian TRAPPs.
All the yeast TRAPP subunits have mammalian homologs and there are two mammalian-specific subunits. The similarity at the level of amino-acid sequence between the yeast and human orthologs varies and is not as striking as that of the Ypt/Rabs (Table 2). However, the structure of the mammalian and yeast core TRAPP is similar, with the exception of insertions of a PDZ-like domain in TRAPPC4 and four amino acid in TRAPPC5 [53]. Like the yeast core subunits, their four human orthologs are essential for viability [16]. The conservation from yeast to human for the other TRAPP subunits is very limited and they also differ in their essentiality for viability (Table 2). Therefore, it is not immediately clear that the function of the large TRAPP complexes is conserved.
Like in yeast, only two TRAPP complexes were observed in mammalian cell lysates: TRAPP II and TRAPP III (not TRAPP I), with the latter containing also the two mammalian-specific subunits [63]. In Drosophila, these complexes were shown to activate Rab11 (Ypt31 homolog) and Rab1 (Ypt1 homolog), respectively [64], which is in agreement with the GEF specificity of the yeast complexes. Interestingly, the fact that TRAPP II-specific subunits are essential in yeast and not in human cells (Table 2) can be explained by the existence of an additional metazoan-specific GEF for Rab11, SH3BP5 [65].
Roles for TRAPP II and III complexes in secretion and for TRAPP III in autophagy were suggested [66]. However, as in yeast, we question whether the fact that TRAPP I complex was not observed in mammalian cell lysates is due to the tendency of TRAPP complexes to aggregate.
IIf. TRAPP subunits and disease.
Mutations in five of the ten conserved TRAPP subunits were associated with a wide variety of human diseases. To date, the only core TRAPP subunit associated with disease is TRAPPC3, which was associated with cilliopathies. TRAPPC2 was associated with a skeletal disorder, and mutations in TRAPPC6B and the TRASPP II-specific subunits TRAPPC9 and TRAPPC10 were associated with different neurological and brain development disorders (Gene Cards, June 2016). Mutations in the two metazoan-specific subunits, TRAPPC11 and TRAPPC12, were also associated with muscular disorders and microcephaly, respectively [66]. While it is expected that more TRAPP subunits would be associated with human disease, the current spread of TRAPP-related diseases suggests that the different TRAPP complexes play roles in multiple cell processes.
Conclusions and Perspectives
Our current view about Ypt GTPases and TRAPP complexes at the yeast Golgi is simple: Ypt1 is activated by TRAPP I at the early Golgi whereas the Ypt31/32 functional pair is activated by TRAPP II at the late Golgi. In addition to their respective roles in ER-to Golgi and exit from the trans Golgi, we suggest that Ypt1 and Ypt31 affect transport through the Golgi by regulating two steps of Golgi cisternal progression, early to transitional and transitional to late (Figure 5). However, although plenty is known about the Golgi Ypt/Rabs and their TRAPP GEFs, including their detailed structural features and interactions, there are still multiple open questions about their function even in the relatively simple yeast model system. Answering such questions is relevant both to basic cell biology and human disease.
The Golgi Ypt/Rabs:
Our recent work shows localization of Ypt1 and Ypt31 to the two opposite ends of the Golgi [31]. This localization supports our earlier functional analysis of these Ypts [20, 21] and their newly identified role in Golgi cisternal maturation [31]. For achieving a comprehensive view of the Golgi Ypts, consideration should be given to the relationship between Ypt1 and Ypt31/32 with Ypt6. Dynamic studies showed that Ypt6 localizes to the Golgi after Ypt1 and before Ypt31, and a GAP cascade was proposed for this orderly localization [22]. However, it is not clear what is the role of this Ypt1-Ypt6-Ypt31 GTPase cascade in the Golgi.
Much less is known about the Golgi Rabs. The closest human homologs of Ypt1, Ypt31/32 and Ypt6, Rab1A/B Rab11A/B, and Rab6A/B, respectively, were implicated in similar processes. The roles of Rabs has been mostly determined using dominant mutations or knockdown analyses. Recent knockout studies have addressed the essentiality of the Golgi Rabs, individually and in pairs [39, 40]. Like the yeast Ypt1 and Ypt6, the Rab1A/B pair is required for viability and Rab6A/B is not. In contrast, while the Ypt31/32 pair is essential for yeast cell viability, Rab11A/B is not. The exact localization and function of the Golgi Rabs, including their possible role in the regulation of Golgi cisternal progression, are still obscure and would require rigorous studies similar to those that were done in yeast.
The Golgi TRAPPs:
Our current view is that in yeast two TRAPP complexes function in transport through the Golgi, TRAPP I and TRAPP II, while the other two complexes function in autophagy. As for the three non-essential TRAPP subunits, we propose that Trs85 in the context of TRAPP III fulfills a major role in autophagy and Trs65 fulfills a major role in the assembly of TRAPP II. Trs33 acts as a backup for both: in the context of TRAPP IV it can substitute TRAPP III in autophagy, while in the absence of Trs65 it can help in the assembly of TRAPP II (Figure 3). However, this view is currently controversial, specifically implicating TRAPP III at the early Golgi instead of TRAPP I. Addressing this and related questions about the Ypt GEF specificity of the Golgi TRAPPs and whether TRAPPs also regulate Golgi cisternal progression, would probably require in vivo analyses.
Mammalian TRAPP complexes are even less understood then yeast TRAPPs. Compared to the impressive similarity of their GTPase substrates (60–70% amino-acid identity; Table 1), the conservation of TRAPP subunits between yeast and human cells varies between twelve (TRAPPC13) to fifty (TRAPPC3) percent of amino-acid identity (Table 2). Such low similarity might point to TRAPP subunit evolution, but not necessarily to their functional conservation. The simplistic view about the existence of only two mammalian TRAPPs, which is based on isolation of complexes from cell lysates, also needs to be re-evaluated.
Another open question about yeast and mammalian TRAPP complexes is why they are distinct from other Ypt/Rab GEFs in size and complex architecture. One possible explanation for this distinction is that TRAPP complexes act as GEFs for more than one Ypt/Rab, Ypt1/Rab1 and Ypt31/Rab11, and in more than one pathway, exocytosis and autophagy. Another idea is that TRAPP complexes have functions other than acting as Ypt/Rab GEFs, especially as tethering complexes [66]. The idea that TRAPP complexes can act as tethers is supported by their size and tendency to form multimers [16]. For example, dimerization of TRAPP II complexes that reside on opposite membranes could bridge between these membranes. However, there is currently no good evidence that TRAPPs actually function as such in vivo. Moreover, while the fact that TRAPP II can form dimers makes it a candidate for tethering membranes, it seems that this dimerization is not essential for its function.
Ypt/Rab-TRAPP Modules:
One basic question in the Ypt/Rab field is how a single GTPase can regulate completely different transport steps and pathways, e.g., Ypt1 in exocytosis and autophagy. The ability of individual Ypt/Rabs to recruit different effectors provides only a partial answer to this question. We have proposed that the specificity of Ypt/Rabs modules that regulate different cellular processes rely on specific GEFs (Figure 4). For the Ypt1 modules in the exocytic and autophagic pathway, this view is currently under debate and requires additional research. Future studies should address this question and related questions regarding mechanisms of localization of specific TRAPP complexes to specific cellular compartments and how the upstream regulators and downstream effectors assemble to specific modules.
Rabs and TRAPPs in human disease:
The intracellular trafficking pathways regulated by Rabs and TRAPPs, e.g., secretion and autophagy, are key to the wellbeing of cells and organisms. Indeed, mutations in the Golgi Rabs and at least seven of the twelve human TRAPP subunits, were implicated in human disease to date. In addition, changes in levels of these proteins were associated with other diseases. Interestingly, not only individual Rabs and TRAPP subunits were implicated in diseases ranging from cancer to neurodegeneration, e.g., Rab1, the diseases associated with different TRAPP subunits do not overlap with each other or with those associated with their Rab substrates. Future studies should tell whether this current non-overlap holds, and whether it reflects yet unknown complexities of human TRAPP complexes composition and function. In summary, better understanding of how Ypt/Rabs and TRAPPs function in yeast and human cells would allow developing Rab- and TRAPP-related clinical markers or therapeutic targets.
Acknowledgments
Our research is supported by grant GM-45444 from NIH to N. Segev.
References:
- 1.Lipatova Z, Hain AU, Nazarko VY & Segev N (2015) Ypt/Rab GTPases: principles learned from yeast, Crit Rev Biochem Mol Biol. 50, 203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segev N (2001) Ypt and Rab GTPases: insight into functions through novel interactions, Curr Opin Cell Biol. 13, 500–11. [DOI] [PubMed] [Google Scholar]
- 3.Zerial M & McBride H (2001) Rab proteins as membrane organizers, Nat Rev Mol Cell Biol. 2, 107–17. [DOI] [PubMed] [Google Scholar]
- 4.Lipatova Z & Segev N (2014) Ypt/Rab GTPases regulate two intersections of the secretory and the endosomal/lysosomal pathways, Cell Logist. 4, e954870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wandinger-Ness A & Zerial M (2014) Rab proteins and the compartmentalization of the endosomal system, Cold Spring Harb Perspect Biol. 6, a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Zhou F, Zou S, Yu S, Li S, Li D, Song J, Li H, He Z, Hu B, Bjorn LO, Lipatova Z, Liang Y, Xie Z & Segev N (2014) A Vps21 endocytic module regulates autophagy, Mol Biol Cell. 25, 3166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, Langemeyer L, Kummel D, Reggiori F & Ungermann C (2018) Molecular mechanism to target the endosomal Mon1-Ccz1 GEF complex to the pre-autophagosomal structure, Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipatova Z, Belogortseva N, Zhang XQ, Kim J, Taussig D & Segev N (2012) Regulation of selective autophagy onset by a Ypt/Rab GTPase module, Proc Natl Acad Sci U S A. 109, 6981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ao X, Zou L & Wu Y (2014) Regulation of autophagy by the Rab GTPase network, Cell Death Differ. 21, 348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sztul E, Chen PW, Casanova JE, Cherfils J, Dacks JB, Lambright DG, Lee FS, Randazzo PA, Santy LC, Schurmann A, Wilhelmi I, Yohe ME & Kahn RA (2019) ARF GTPases and their GEFs and GAPs: concepts and challenges, Mol Biol Cell. 30, 1249–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones S, Jedd G, Kahn RA, Franzusoff A, Bartolini F & Segev N (1999) Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers, Genetics. 152, 1543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segev N (2001) Ypt/rab gtpases: regulators of protein trafficking, Sci STKE. 2001, re11. [DOI] [PubMed] [Google Scholar]
- 13.Sclafani A, Chen S, Rivera-Molina F, Reinisch K, Novick P & Ferro-Novick S (2010) Establishing a role for the GTPase Ypt1p at the late Golgi, Traffic. 11, 520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S, Newman C, Liu F & Segev N (2000) The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32, Mol Biol Cell. 11, 4403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD & Segev N (2006) TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF, Nat Cell Biol. 8, 1263–9. [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Lipatova Z & Segev N (2016) TRAPP Complexes in Secretion and Autophagy, Front Cell Dev Biol. 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrowman J, Bhandari D, Reinisch K & Ferro-Novick S (2010) TRAPP complexes in membrane traffic: convergence through a common Rab, Nat Rev Mol Cell Biol. 11, 759–63. [DOI] [PubMed] [Google Scholar]
- 18.Brunet S & Sacher M (2014) In sickness and in health: the role of TRAPP and associated proteins in disease, Traffic. 15, 803–18. [DOI] [PubMed] [Google Scholar]
- 19.Mitra S, Cheng KW & Mills GB (2011) Rab GTPases implicated in inherited and acquired disorders, Semin Cell Dev Biol. 22, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jedd G, Mulholland J & Segev N (1997) Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment, J Cell Biol. 137, 563–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jedd G, Richardson C, Litt R & Segev N (1995) The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway, J Cell Biol. 131, 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suda Y, Kurokawa K, Hirata R & Nakano A (2013) Rab GAP cascade regulates dynamics of Ypt6 in the Golgi traffic, Proc Natl Acad Sci U S A. 110, 18976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segev N & Botstein D (1987) The ras-like yeast YPT1 gene is itself essential for growth, sporulation, and starvation response, Mol Cell Biol. 7, 2367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipatova Z, Shah AH, Kim JJ, Mulholland JW & Segev N (2013) Regulation of ER-phagy by a Ypt/Rab GTPase module, Mol Biol Cell. 24, 3133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonold CM & Fromme JC (2014) Four GTPases differentially regulate the Sec7 Arf-GEF to direct traffic at the trans-golgi network, Dev Cell. 30, 759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao X, Ballew N & Barlowe C (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins, EMBO J. 17, 2156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS & Segev N (2008) Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion, Mol Biol Cell. 19, 4177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz D, Medkova M, Walch-Solimena C & Novick P (2002) Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast, J Cell Biol. 157, 1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SH, Chen S, Tokarev AA, Liu F, Jedd G & Segev N (2005) Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling, Mol Biol Cell. 16, 178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S & Storrie B (2012) Are Rab proteins the link between Golgi organization and membrane trafficking?, Cell Mol Life Sci. 69, 4093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JJ, Lipatova Z, Majumdar U & Segev N (2016) Regulation of Golgi Cisternal Progression by Ypt/Rab GTPases, Dev Cell. 36, 440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura S, Nagano M, Toshima JY & Toshima J (2014) Analysis of subcellular localization and function of the yeast Rab6 homologue, Ypt6p, using a novel amino-terminal tagging strategy, Biochem Biophys Res Commun. 450, 519–25. [DOI] [PubMed] [Google Scholar]
- 33.Rivera-Molina FE & Novick PJ (2009) A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway, Proc Natl Acad Sci U S A. 106, 14408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glick BS & Luini A (2011) Models for Golgi traffic: a critical assessment, Cold Spring Harb Perspect Biol. 3, a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ & Glick BS (2006) Golgi maturation visualized in living yeast, Nature. 441, 1002–6. [DOI] [PubMed] [Google Scholar]
- 36.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K & Nakano A (2006) Live imaging of yeast Golgi cisternal maturation, Nature. 441, 1007–10. [DOI] [PubMed] [Google Scholar]
- 37.Segev N, Mulholland J & Botstein D (1988) The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery, Cell. 52, 915–24. [DOI] [PubMed] [Google Scholar]
- 38.Haubruck H, Prange R, Vorgias C & Gallwitz D (1989) The ras-related mouse ypt1 protein can functionally replace the YPT1 gene product in yeast, EMBO J. 8, 1427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blomen VA, Majek P, Jae LT, Bigenzahn JW, Nieuwenhuis J, Staring J, Sacco R, van Diemen FR, Olk N, Stukalov A, Marceau C, Janssen H, Carette JE, Bennett KL, Colinge J, Superti-Furga G & Brummelkamp TR (2015) Gene essentiality and synthetic lethality in haploid human cells, Science. 350, 1092–6. [DOI] [PubMed] [Google Scholar]
- 40.Homma Y, Kinoshita R, Kuchitsu Y, Wawro PS, Marubashi S, Oguchi ME, Ishida M, Fujita N & Fukuda M (2019) Comprehensive knockout analysis of the Rab family GTPases in epithelial cells, J Cell Biol. 218, 2035–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly EE, Horgan CP & McCaffrey MW (2012) Rab11 proteins in health and disease, Biochem Soc Trans. 40, 1360–7. [DOI] [PubMed] [Google Scholar]
- 42.Yang XZ, Li XX, Zhang YJ, Rodriguez-Rodriguez L, Xiang MQ, Wang HY & Zheng XF (2016) Rab1 in cell signaling, cancer and other diseases, Oncogene. 35, 5699–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Hu C, Wu F & He S (2017) Rab25 GTPase: Functional roles in cancer, Oncotarget. 8, 64591–64599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz EM, Ferro-Novick S & Reinisch KM (2008) The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes, Cell. 133, 1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YG, Raunser S, Munger C, Wagner J, Song YL, Cygler M, Walz T, Oh BH & Sacher M (2006) The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering, Cell. 127, 817–30. [DOI] [PubMed] [Google Scholar]
- 46.Tan D, Cai Y, Wang J, Zhang J, Menon S, Chou HT, Ferro-Novick S, Reinisch KM & Walz T (2013) The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway, Proc Natl Acad Sci U S A. 110, 19432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taussig D, Lipatova Z & Segev N (2014) Trs20 is required for TRAPP III complex assembly at the PAS and its function in autophagy, Traffic. 15, 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi C, Davey M, Schluter C, Pandher P, Fang Y, Foster LJ & Conibear E (2011) Organization and assembly of the TRAPPII complex, Traffic. 12, 715–25. [DOI] [PubMed] [Google Scholar]
- 49.Yip CK, Berscheminski J & Walz T (2010) Molecular architecture of the TRAPPII complex and implications for vesicle tethering, Nat Struct Mol Biol. 17, 1298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taussig D, Lipatova Z, Kim JJ, Zhang X & Segev N (2013) Trs20 is required for TRAPP II assembly, Traffic. 14, 678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y, Morozova N, Tokarev AA, Mulholland JW & Segev N (2007) The role of Trs65 in the Ypt/Rab guanine nucleotide exchange factor function of the TRAPP II complex, Mol Biol Cell. 18, 2533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tokarev AA, Taussig D, Sundaram G, Lipatova Z, Liang Y, Mulholland JW & Segev N (2009) TRAPP II complex assembly requires Trs33 or Trs65, Traffic. 10, 1831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sacher M, Kim YG, Lavie A, Oh BH & Segev N (2008) The TRAPP complex: insights into its architecture and function, Traffic. 9, 2032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sacher M, Barrowman J, Schieltz D, Yates JR 3rd & Ferro-Novick S (2000) Identification and characterization of five new subunits of TRAPP, Eur J Cell Biol. 79, 71–80. [DOI] [PubMed] [Google Scholar]
- 55.Meiling-Wesse K, Epple UD, Krick R, Barth H, Appelles A, Voss C, Eskelinen EL & Thumm M (2005) Trs85 (Gsg1), a component of the TRAPP complexes, is required for the organization of the preautophagosomal structure during selective autophagy via the Cvt pathway, J Biol Chem. 280, 33669–78. [DOI] [PubMed] [Google Scholar]
- 56.Nazarko TY, Huang J, Nicaud JM, Klionsky DJ & Sibirny AA (2005) Trs85 is required for macroautophagy, pexophagy and cytoplasm to vacuole targeting in Yarrowia lipolytica and Saccharomyces cerevisiae, Autophagy. 1, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas LL, Joiner AMN & Fromme JC (2018) The TRAPPIII complex activates the GTPase Ypt1 (Rab1) in the secretory pathway, J Cell Biol. 217, 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brunet S, Noueihed B, Shahrzad N, Saint-Dic D, Hasaj B, Guan TL, Moores A, Barlowe C & Sacher M (2012) The SMS domain of Trs23p is responsible for the in vitro appearance of the TRAPP I complex in Saccharomyces cerevisiae, Cell Logist. 2, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas LL & Fromme JC (2016) GTPase cross talk regulates TRAPPII activation of Rab11 homologues during vesicle biogenesis, J Cell Biol. 215, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinar M, Arst HN Jr., Pantazopoulou A, Tagua VG, de los Rios V, Rodriguez-Salarichs J, Diaz JF & Penalva MA (2015) TRAPPII regulates exocytic Golgi exit by mediating nucleotide exchange on the Ypt31 ortholog RabERAB11, Proc Natl Acad Sci U S A. 112, 4346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M & Ferro-Novick S (2001) TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport, Mol Cell. 7, 433–42. [DOI] [PubMed] [Google Scholar]
- 62.Lipatova Z, Majumdar U & Segev N (2016) Trs33-Containing TRAPP IV: A Novel Autophagy-Specific Ypt1 GEF, Genetics. 204, 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bassik MC, Kampmann M, Lebbink RJ, Wang S, Hein MY, Poser I, Weibezahn J, Horlbeck MA, Chen S, Mann M, Hyman AA, Leproust EM, McManus MT & Weissman JS (2013) A systematic mammalian genetic interaction map reveals pathways underlying ricin susceptibility, Cell. 152, 909–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riedel F, Galindo A, Muschalik N & Munro S (2018) The two TRAPP complexes of metazoans have distinct roles and act on different Rab GTPases, J Cell Biol. 217, 601–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenkins ML, Margaria JP, Stariha JTB, Hoffmann RM, McPhail JA, Hamelin DJ, Boulanger MJ, Hirsch E & Burke JE (2018) Structural determinants of Rab11 activation by the guanine nucleotide exchange factor SH3BP5, Nat Commun. 9, 3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sacher M, Shahrzad N, Kamel H & Milev MP (2019) TRAPPopathies: An emerging set of disorders linked to variations in the genes encoding transport protein particle (TRAPP)-associated proteins, Traffic. 20, 5–26. [DOI] [PubMed] [Google Scholar]
- 67.Cai H, Zhang Y, Pypaert M, Walker L & Ferro-Novick S (2005) Mutants in trs120 disrupt traffic from the early endosome to the late Golgi, J Cell Biol. 171, 823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


