Abstract
Non-melanoma skin cancer primarily affects geriatric patients as evidenced by the fact that only 20% of these cancers are diagnosed in patients under the age of 60 years. Of importance, geriatric skin responds to procarcinogenic ultraviolet B radiation (UVB) in a manner that permits the establishment of tumor cells. Recent studies have indicated that wounding of geriatric skin with fractionated resurfacing lasers and dermabrasion upregulates fibroblast production of insulin-like growth factor-1 (IGF-1) and normalizes the procarcinogenic acute UVB response consisting of basal keratinocytes proliferating while still harboring unrepaired DNA damage. The present studies tested the ability of wounding with a commercially available microneedling device to upregulate IGF-1 levels and normalize the geriatric UVB response. Geriatric volunteers were treated with a microneedling device on buttock skin and three months later the IGF-1 levels and UVB responses tested in wounded vs control skin. Wounding via microneedling upregulated IGF-1 and resulted in lower levels of basal keratinocytes proliferating with unrepaired DNA damage. The ability of microneedling to protect against the formation of UVB-damaged proliferating keratinocytes indicates the potential of this wounding modality to reduce aging-associated non-melanoma skin cancer.
Introduction
Non-melanoma skin cancer (NMSC) is commonly associated with age, with an estimated 80% of all cancers diagnosed in patients who are over 60 years of age [1]. Furthermore NMSC, particularly squamous cell carcinoma and actinic keratosis, tend to only develop in areas of the body that have received substantial sun exposure. The implementation of sunscreen and sun avoidance practices have been shown to protect against actinic neoplasia in geriatric populations, providing evidence that this is an ongoing process, not just the end result of preceding sun exposure decades earlier [2]. Our laboratories have provided considerable evidence which provides an explanation as to why geriatric patients have an increased susceptibility to NMSC [3–5]. Our studies have shown that the manner in which skin keratinocytes respond to UVB irradiation is dependent on the activation state of the keratinocyte insulin-like growth factor-1 receptor (IGF-1R) [3,5]. Ligand-bound activated IGF-1R is mandatory for keratinocytes to respond appropriately to UVB exposure. Inasmuch as human epidermal keratinocytes do not produce IGF-1, the keratinocyte IGF-1R is primarily activated by IGF-1 generated by adjacent fibroblasts in the papillary dermis.
Of importance, the expression of IGF-1 in the dermis diminishes with age due to a population shift to senescent fibroblasts [3,4]. Thus, geriatric skin is frequently deficient in IGF-1 resulting in the insufficient activation of the IGF-1R in geriatric epidermal keratinocytes [3]. Consistent with decreased numbers of active dermal fibroblasts, collagen 1 levels are also decreased in geriatric skin [6–8]. Following exposure to UVB radiation, the IGF-1 deficiency associated with aged skin results in an inappropriate proliferation of basal keratinocytes harboring DNA damage. This “abnormal” response is observed via co-expression of both proliferation marker Ki-67 and thymine dimers (TD). The occurrence of keratinocytes proliferating with unrepaired UV photoproducts following acute UVB exposure could then allow the establishment of UVB-induced mutations in geriatric skin, an initial step in the eventual progression to NMSC. According to this new paradigm, therapies that correct the aging-associated loss of IGF-1 expression should restore the appropriate UVB response in geriatric skin and thus reduce the incidence of NMSC in this susceptible population.
Previous studies by our group have determined that photorejuvenation procedures such as dermabrasion and fractionated laser resurfacing (FLR) result in a decrease in the numbers of senescent fibroblasts, increased dermal collagen I and IGF-1 levels and protect against the production of keratinocytes proliferating with unrepaired DNA lesions twenty-four hours following exposure to minimal erythema doses of UVB [3,4]. The current studies tested if controlled wounding of geriatric skin by a microneedling device, the type which is gaining popularity for photorejuvenation as well as for its ability to enhance drug absorption [8] can achieve this potentially protective effect. Thus, these pilot studies tested if wounding via a commercially-available microneedling device can upregulate dermal IGF-1 levels and normalize the acute pro-carcinogenic UVB response found in geriatric skin.
Materials and Methods
Methods
Geriatric volunteers (aged 65 years old and older) with Fitzpatrick Types I and II skin who were non-diabetic were recruited from patients treated at dermatology clinics within the Boonshoft School of Medicine at Wright State University. The studies have the approval of the Wright State University Institutional Review Board. Specific requirements for inclusion and exclusion criteria were identical to previous studies [4]. Briefly, subjects were excluded if had a history of poor wound healing or abnormal scarring, active skin diseases such as psoriasis or atopic dermatitis, or were on any photosensitizing medicines.
All subjects were thoroughly briefed on the risks and benefits of participating in the study and they signed an informed consent statement attesting to their willful participation. Geriatric volunteers who met the criteria for inclusion in this study underwent wounding of a small 5 × 5 cm area of either the upper buttocks with 10 passes both vertically and horizontally using a commercially available microneedling device (Dermaroller™ with 2.5 mm needles) after application of topical xylocaine anesthesia. Patients were given wound care instructions and asked to return at 90 days. On return, a localized 1 × 1 cm area of either microneedle-treated skin or untreated normal skin (on the opposite hip/buttock) was irradiated with dose of 350 J/m2 of UVB using our Philips F20T12/UVB lamp [4]. In Fitzpatrick Skin Types I and II, this dose of UVB is sufficient to cause a minimal erythematous reaction. Permanent marker was used to outline the areas of skin that was irradiated. Twenty-four hours following UVB exposure, photographs were taken of the skin to document the extent of the UVB reaction. The irradiated skin, as well as unirradiated adjacent skin, was removed by punch biopsy, (5 mm punch biopsies of the UVB-treated skin and 5 mm punch biopsies of unirradiated skin; 4 biopsies per individual). The epidermal response to UVB irradiation (of measuring Ki67 and thymine dimers) was assayed as previously described in the UVB-treated skin. Collagen I and IGF-1 mRNA was measured in non-UVB-treated skin by QRT-PCR as previously described [3].
Results
Results
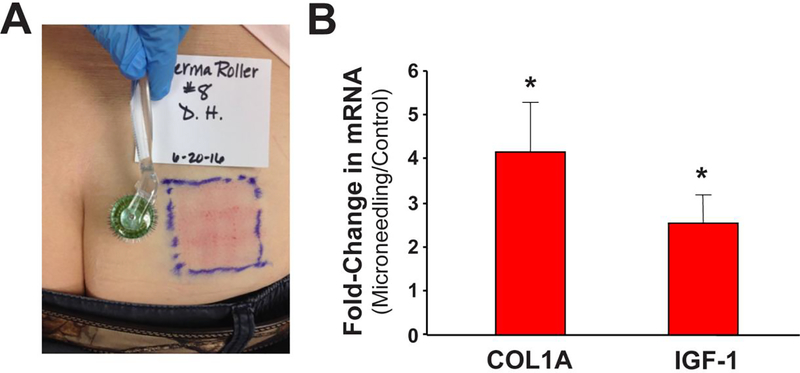
Nine geriatric subjects (7 males, 2 females; age 65 years and older) completed the studies examining UVB responses and IGF-1 expression levels at 3 months post-wounding; data from seven subjects for the UVB-responses was used as two samples were not prepared appropriately and thus were unusable. All subjects tolerated the procedure without any side effects such as wound infection, pain after 1 week, or scarring. As noted in Figure 1 A, microneedling treatment resulted in a reliable wounding response. At three months post-wounding, biopsies of wounded vs control skin were tested for both collagen 1 and IGF-1 levels. As shown in Figure 1B and Table I, mRNA levels of both collagen 1 and IGF-1 were increased in previously wounded skin. The individual changes in mRNA levels of collagen 1 and IGF-1 following wounding tended to correlate with each other. Of importance, the changes in IGF-1 mRNA levels caused by microneedling were similar to the effects caused by dermabrasion or FLR [3]. These studies indicate that the wounding response from a microneedling injury resulted in the significant improvement of geriatric dermis at three months post-treatment.
Figure 1. Microneedling increases Collagen I and IGF-1 levels in geriatric skin.
(A) Sun-protected (upper buttocks) on geriatric (≥65 years old) volunteers were treated with a microneedling device. Image shown were obtained immediately following treatment with the microneedling device. (B) Relative collagen I and IGF-1 mRNA expression levels of wounded vs control skin (N = 9) was assayed by QRT-PCR (standardized by beta-2 microglobulin expression) from biopsies obtained from skin previously wounded and healed (3 months) or untreated skin. The values are Mean relative collagen I and IGF-1 mRNA levels (Error bars indicate SEM); asterisk denotes statistical significant (p < 0.05) changes in wounded vs control skin using the Student’s t test.
Table I.
Microneedling effects on Collagen I and IGF-1 mRNA levels and numbers of dual-positive Ki67+/TD+ basal keratinocytes following UVB in geriatric skin.
| Fold Increase in mRNA (Treated/Control) | Ki67+/TD+ cells per 100 Basement Membrane | % Decrease in Ki67+/TD+ cells on wounded skin | |||
|---|---|---|---|---|---|
| Subject (M/F) | COLIA | IGF-1 | Control | Treated | |
| 1 (M) | 1.33 | 1.65 | 0.37 | 0.11 | 70.2 |
| 2 (M) | 7.47 | 4.71 | 0.17 | 0.01 | 94.1 |
| 3 (F) | 2.53 | 0.99 | 0.20 | 0.00 | 100 |
| 4 (M) | 6.20 | 5.12 | 0.29 | 0.00 | 100 |
| 5 (M) | 2.26 | 1.42 | 0.64 | 0.04 | 93.8 |
| 6 (F) | 3.98 | 0.94 | 0.16 | 0.10 | 37.5 |
| 7 (M) | 10.85 | 5.32 | 0.16 | 0.00 | 100 |
| 8 (F) | 1.08 | 0.81 | UNDET | UNDET | UNDET |
| 9 (M) | 1.70 | 1.93 | UNDET | UNDET | UNDET |
| AVG | 4.16 | 2.54 | 0.28 | 0.04 | 85.1 |
| SEM | 1.12 | 0.64 | 0.07 | 0.02 | 8.9 |
M/F; male/female; UNDET; data not available due to technical reasons.
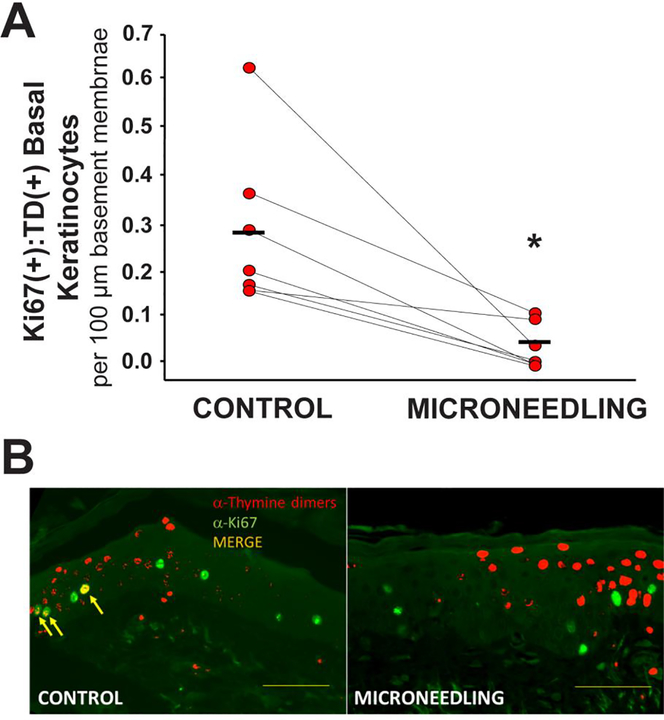
The next studies tested whether microneedling injury can protect against the formation of UVB-damaged proliferating keratinocytes in geriatric skin. At three months post-wounding, a small 1 × 1 cm area of previously wounded or normal control skin was treated with UVB and 24 h later biopsies performed of control- and UVB-treated wounded/non-wounded skin. As shown in Figure 2 and Table I, we noted a statistically significant decrease in the numbers of Ki67+/TD+ basal keratinocytes in previously wounded skin in comparison to control. It should be noted that the numbers of basal Ki67+TD+ keratinocytes following UVB treatment of microneedle-treated geriatric skin resembles the responses noted following dermabrasion and FLR wounding of geriatric skin [3,4]. Importantly, these UVB responses in previously wounded geriatric skin are similar to the “normal” responses we have previously documented in young (age < 30 years) skin [3,4].
Figure 2. Microneedling results in diminished numbers of basal keratinocytes proliferating whilst still harboring unrepaired thymine dimers following UVB in geriatric skin.
(A) The level of basal layer keratinocytes in the biopsies that co-expressed UVB-induced DNA damage (TD+) and cellular markers of proliferation (Ki67+) at 24 hours following an exposure to UVB. Error bars indicate SEM; asterisk denotes statistical significance (p < 0.006) of changes using the Student’s t test. (B) Examples of Ki67+ (green) and TD+ (red) staining of control vs previously microneedled geriatric skin revealing double-positive (yellow) Ki67+:TD+ cells.. Bar = 50 μm
Though promising, there are several important limitations to the current pilot studies. First, the short time frame of three months does not allow adequate follow up time to ascertain the longer-term effects of this wounding procedure. Second, the sample size is small, though the findings were statistically significant.
Conclusions
These pilot studies indicate that wounding of geriatric skin with a microneedling device increases dermal collagen 1 and IGF-1 levels, and normalizes the procarcinogenic UVB response. Given that these commercially available devices are less expensive and potentially more widely available than FLR, this modality could have potential use in future studies to assess the protective effect of dermal wounding on photocarcinogenesis.
ACKNOWLEDGEMENTS
We acknowledge the assistance of the Wright State Physicians Pharmacology Translational Unit for assistance in these studies. This work was supported by grants from the National Institutes of Health (R01 HL062996 to JBT; R01 AG048946 to JBT and DFS), VA Merit Award (1101CX000809 to JBT). The authors have no conflicts of interest in regard to the data presented in this manuscript.
Footnotes
Disclaimers: The authors declare no conflicts of interest with the data or ideas presented in this manuscript.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Kraemer KH. (1997) Sunlight and skin cancer: another link revealed. Proc Natl Acad Sci USA 94: 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson SC, Marks R. (1993) Reduction of solar keratoses by regular sunscreen use. N Engl J Med 329: 1147–1151. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DA, Travers JB, Somani AK, Spandau DF. (2010) The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene 29: 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spandau DF, Lewis DA, Somani AK, Travers JB. (2012) Fractionated laser resurfacing corrects the inappropriate UVB response in geriatric skin. J Invest Dermatol 132: 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp MG, Spandau DF, Travers JB. (2017) Impact of age and insulin-like growth factor-1 on DNA damage responses in UV-irradiated human skin. Molecules 22(3): E356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher GJ, Varani J, Voorhees JJ. (2008) Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol 144: 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole MA, Quan T, Voorhees JJ, Fisher GJ. (2018) Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J Cell Comm Signal 12: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mine S, et al. (2008) Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a view of skin morphogenesis and aging. PLoS One 3:e4066; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]