LETTER
In May and June of 2018, one of the largest documented outbreaks of Cyclospora cayetanensis occurred in the Midwestern United States (https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2018/a-062018/index.html). Of the 250 total laboratory-confirmed cases nationally, 177 (71%) were in Wisconsin. Cases were linked to consuming prepackaged mixed vegetable trays containing broccoli, cauliflower, carrots, and dill dip. Eight patients were hospitalized, and the vegetable trays were voluntarily recalled. Epidemiologic data and FDA trace-back investigation did not identify a single point source of the outbreak.
As a public health reference laboratory, the Wisconsin State Laboratory of Hygiene (WSLH) receives Cyclospora-positive specimens from clinical laboratories in Wisconsin for surveillance. The WSLH confirms Cyclospora diagnoses using autofluorescence under UV microscopy at 350 nm. The BioFire FilmArray Gastrointestinal Panel (FilmArray GI) is the only commercially available molecular multiplex panel that includes a Cyclospora target. To assess the performance of the FilmArray GI during this outbreak, specimens received at WSLH during the outbreak period were tested by autofluorescence. A total of 140 outbreak-associated Cyclospora-positive specimens were submitted by clinical laboratories, of which 99 (71%) were submitted by laboratories that used FilmArray GI. The remainder were submitted by laboratories that used either modified acid fast staining or autofluorescence. Of the FilmArray GI-positive specimens, 94/99 (95%) were confirmed as positive by autofluorescence at WSLH. This was consistent with the confirmation rate of specimens received from laboratories that used modified acid fast staining (24/26 [92%]). The high confirmation rate by autofluorescence microscopy demonstrates the reliability of FilmArray GI Cyclospora-positive results with a large number of real-world clinical specimens [the number of FilmArray GI-positive specimens in this outbreak (99 specimens) is more than 5 times higher than the number of positive specimens in the FDA 510(k) clearance data (19 specimens) (1) (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?id=K140407).
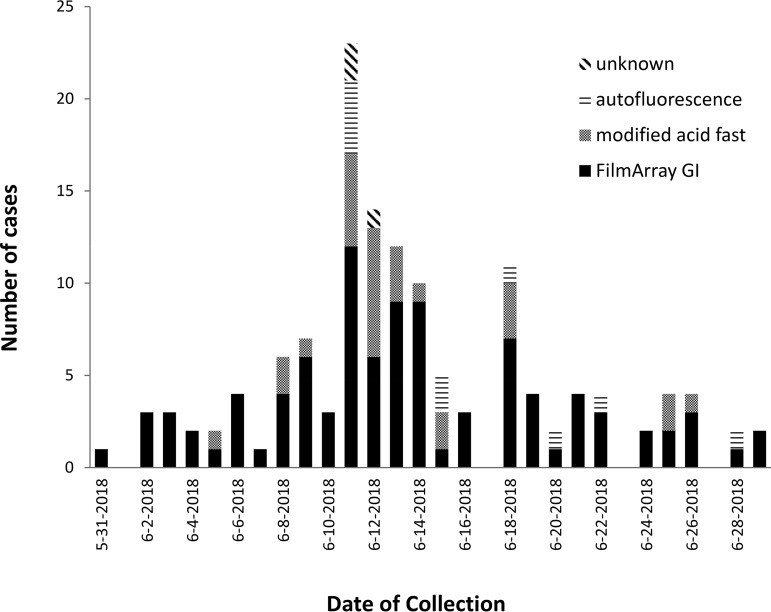
FilmArray GI results substantially contributed to the recognition of this Cyclospora outbreak, because the vast majority of positive specimens early in the outbreak were FilmArray GI-positive specimens. The Wisconsin Department of Health Services posted an alert on 8 June 2018 linking vegetable trays to Cyclospora (https://www.dhs.wisconsin.gov/news/releases/060818.htm). Of all outbreak-associated Cyclospora-positive specimens prior to this date, 15/16 (94%) were FilmArray GI-positive specimens (Fig. 1).
FIG 1.
Number of cases identified by each method at clinical laboratories, by date of collection.
Before the use of FilmArray GI in clinical laboratories, many Cyclospora infections were likely undiagnosed because clinicians infrequently order Cyclospora-specific testing and many clinical laboratories do not routinely perform Cyclospora testing (2). Therefore, the use of FilmArray GI likely increases the identification of Cyclospora infections, due to both the sensitivity of PCR and the inclusion of the Cyclospora target on the panel (3). Indeed, the number of Cyclospora infections reported nationally has risen from 2011 and 2012 (237 and 130 infections, respectively) (4) to 2018 (2,299 infections as of October 2018) (5), and it is likely that implementation of the FilmArray GI has played a role in the increase. Thus, while culture-independent diagnostic tests (CIDTs) can complicate the interpretation of long-term surveillance data and challenge real-time public health surveillance of bacterial infections where molecular subtyping currently requires an isolate, for pathogens where testing historically has been less common or available, broad molecular CIDT panels can benefit public health by identifying more cases and potentially more outbreaks.
ACKNOWLEDGMENTS
We thank Wisconsin clinical laboratories for their participation in the Wisconsin Clinical Laboratory Network and their submission of positive Cyclospora specimens to WSLH.
REFERENCES
- 1.Buss SN, Leber A, Chapin K, Fey PD, Bankowski MJ, Jones MK, Rogatcheva M, Kanack KJ, Bourzac KM. 2015. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol 53:915–925. doi: 10.1128/JCM.02674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marder EP, Cieslak PR, Cronquist AB, Dunn J, Lathrop S, Rabatsky-Ehr T, Ryan P, Smith K, Tobin-D’Angelo M, Vugia DJ, Zansky S, Holt KG, Wolpert BJ, Lynch M, Tauxe R, Geissler AL. 2017. Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance - Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2013–2016. MMWR Morb Mortal Wkly Rep 66:397–403. doi: 10.15585/mmwr.mm6615a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buss SN, Alter R, Iwen PC, Fey PD. 2013. Implications of culture-independent panel-based detection of Cyclospora cayetanensis. J Clin Microbiol 51:3909. doi: 10.1128/JCM.02238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casillas SM, Hall RL, Herwaldt BL. 2019. Cyclosporiasis surveillance – United States, 2011-2015. MMWR Surveill Summ 68:1–16. doi: 10.15585/mmwr.ss6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casillas SM, Bennett C, Straily A. 2018. Multiple cyclosporiasis outbreaks — United States, 2018. MMWR Morb Mortal Wkly Rep 67:1101–1102. doi: 10.15585/mmwr.mm6739a6. [DOI] [PMC free article] [PubMed] [Google Scholar]