CASE
A 37-year-old male with a medical history of glucose-6-phosphate dehydrogenase (G6PD) deficiency (Beaumont variant) presented at the emergency department (ED) with fatigue, malaise, generalized joint pains, and chills. On exam, he was febrile (40.2°C) and tachycardic (143 beats/min) while normotensive (124/75 mm Hg) and oxygenating well on ambient air (99% SpO2). His blood chemistries were remarkable, with elevated lactic acid at 3.27 mmol/liter (range, 0.5 to 2.20 mmol/liter) and procalcitonin at 0.35 ng/ml (range, <0.05 ng/ml). Additional lab findings showed a normal white blood cell (WBC) count (7,192 cells/μl; range, 4,000 to 11,000/μl), with 80% neutrophils, and signs of acute hemolytic anemia, with high reticulocytes at >17.97% (range, 0.59 to 2.24%), low hemoglobin (Hgb; 9.9 mg/dl; range, 12.2 to 16.4 mg/dl), hyperbilirubinemia (unconjugated bilirubin, 3 mg/dl; range, 0.1 to 1.1 mg/dl), and high ferritin level (3,240 ng/ml; range, 18 to 464 ng/ml). He was given a dose of 1 g meropenem intravenously (i.v.) and admitted for further management of suspected septicemia.
The urine and sputum cultures collected at the ED were negative, but aerobic blood cultures became positive after 18 h of incubation. The initial Gram stain showed Gram-negative rods (Fig. 1A); however, the Verigene Gram-negative blood culture nucleic acid test (BC-GN; Luminex Co., Austin, TX) did not identify any organisms. Blood bottle subcultures grew purple colonies of Gram-negative rods on both 5% sheep blood (SBA) and MacConkey (MAC) agars (Fig. 1B and C). Identification by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Vitek MS; bioMérieux, Inc., Hazelwood, MO) revealed Chromobacterium violaceum, which was confirmed by 16S rRNA sequencing (GenBank accession number MH790126) with 99.93% (1,470/1,471 nucleotides) identity to C. violaceum ATCC 12472T. Antimicrobial susceptibility testing was performed using a Sensititre Gram-negative nonfermenters MIC plate (Thermo Fisher Scientific, Waltham, MA) and an Etest (bioMérieux Inc., Hazelwood, MO) for meropenem. The MIC values were reported with no interpretation (Table 1). He was given 14 days of high dose meropenem (2 g every 8 h [q8h] i.v.) and doxycycline (100 mg twice a day oral [BID]). Due to a continuous decline in his Hgb and platelets, he was given 1 unit of packed red blood cells and platelet transfusion on hospital day (HD) 2. Subsequent blood cultures drawn 48 and 96 h postbacteremia were negative. On HD 12, he was discharged with doxycycline maintenance therapy. At follow-up visits at 2 and 4 months, examination revealed no signs of infection, and inflammatory markers were within normal ranges.
FIG 1.
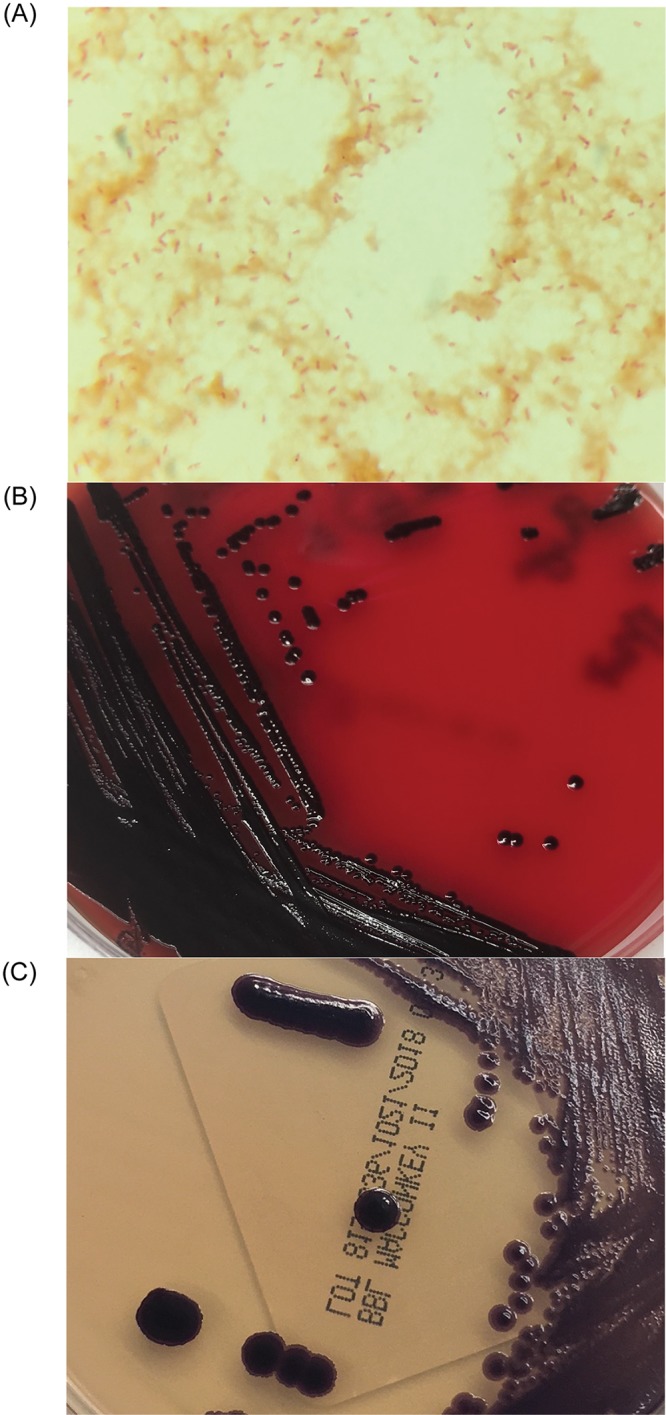
(A) Gram stain of Chromobacterium violaceum from positive aerobic blood culture bottle showing Gram-negative bacilli (×1,000). (B and C) Purple-colored colonies of C. violaceum isolated from the overnight subcultures (5% sheep blood agar [B] and MacConkey agar [C]) of positive blood culture bottle.
TABLE 1.
MIC values for Chromobacterium violaceum isolated from the wound and positive blood cultures
| Antibacterial drug | MIC (μg/ml) from: |
|
|---|---|---|
| Wound culture during previous admission | Positive blood culture during current admission | |
| Piperacillin | 8 | 32 |
| Ampicillin-sulbactam | >16/8 | >16/8 |
| Piperacillin-tazobactam | ≤8/4 | 16/4 |
| Ticarcillin-clavulanic acid | >128/2 | >128/2 |
| Ceftazidime | 16 | >16 |
| Ceftriaxone | 32 | >32 |
| Cefotaxime | >32 | >32 |
| Cefepime | 4 | 8 |
| Aztreonam | 4 | 8 |
| Imipenem | ≤1 | |
| Meropenem | 0.19a | |
| Amikacin | 16 | 8 |
| Gentamicin | 4 | 4 |
| Tobramycin | 4 | 2 |
| Tetracycline | ≤1 | ≤1 |
| Levofloxacin | ≤0.12 | ≤0.12 |
| Ciprofloxacin | ≤0.25 | ≤0.25 |
| Trimethoprim-sulfamethoxazole | ≤0.5/9.5 | ≤0.5/9.5 |
| Chloramphenicol | 4 | 4 |
Performed by Etest (bioMérieux Inc., Hazelwood, MO).
Interestingly, the patient had had a previous episode of C. violaceum bacteremia 10 months earlier secondary to a leg abscess from which the same organism was also grown. The wound developed after wading in the floodwaters of a hurricane in Texas. The isolate from his positive blood cultures was identified at another hospital; susceptibility testing was not performed. He was transferred to our hospital, and C. violaceum from his wound culture was identified in our laboratory. The susceptibility profile of the organism from the wound was similar to that of the organism isolated from the blood during the current admission (Table 1). He was given meropenem (2 g i.v.) and levofloxacin (100 mg BID) and discharged with a 6-week course of levofloxacin, which was discontinued 1 day early due to the development of arthralgias. His computed tomography (CT) scan showed hepatomegaly, splenic microinfarcts, hepatic lesions, and pulmonary nodules, all of which greatly improved at 6- and 9-week follow-up visits. Of note, his abscess was completely healed when he was admitted for the current episode, and he denied repeat exposure to stagnant water.
DISCUSSION
C. violaceum is a facultative anaerobic Gram-negative bacillus that is ubiquitously found in environments, such as soil and stagnant water, in tropical and subtropical environments worldwide (1, 2). It is catalase positive and is typically recognized for production of a violet pigment called violacein (3). As it gives variable reactions for oxidase and indole assays and its pigmentation can affect interpretation, other diagnostic measures should be taken for absolute identification (3). Colonies of C. violaceum grow well on 5% SBA and MAC and produce an almond-like smell (1). Although human infections by C. violaceum are uncommon, it has emerged as an opportunistic environmental pathogen and is associated with severe morbidity and mortality (1, 2), with a historic mortality rate as high as 65.6% (1). Certain immunodeficiencies, including chronic granulomatous disease (CGD), G6PD deficiency, and diabetes, appear to be important predisposing conditions for C. violaceum infection (1, 2, 4, 5).
C. violaceum infection usually originates from a localized contaminated wound (6). Several case reports have demonstrated C. violaceum infection in children with a history of exposure to contaminated soil and water, or soldiers with battle wounds (1, 6). The organism can rapidly disseminate through the bloodstream and cause multiorgan abscesses and fatal sepsis (1, 6). Relapses and recurrent infection are common and have been reported (1).
The ability of C. violaceum to secrete an extracellular protein collagenase and the possession of flagella (7, 8) would likely explain the rapid dissemination of C. violaceum from a localized wound into multiorgan abscesses. Fulminant septicemia caused by C. violaceum can be attributed to the production of hemolysin and other cytolytic toxins (8). Interestingly, the violacein pigment produced by C. violaceum has been a major interest in biotechnological applications due to the antimicrobial potency of this pigment (7). Although violacein has certain cytotoxic activity, it is not clear whether it is a major virulence factor for C. violaceum pathogenicity, since nonpigmented strains display similar pathogenicity to the pigmented ones (9).
The organism can lose pigmentation upon subsequent cultures from the parental strain or exposure to stressful environments, such as freeze-thaw cycles in laboratories (3). While pigmented colonies are easily identifiable, nonpigmented isolates can be mistaken for Aeromonas or Vibrio species (3). As C. violaceum can be β-hemolytic like most Aeromonas species, these organisms can be differentiated by biochemical characteristics, such as positive acid production from maltose and mannitol by Aeromonas species (3, 10). Currently, diagnostic laboratories can identify C. violaceum by automated biochemical systems (Vitek, MicroScan, and Phoenix), MALDI-TOF MS systems, or 16S rRNA sequencing (11). Of note, both pigmented and nonpigmented colonies of C. violaceum were recovered from the frozen isolates of the wound culture of our patient and were confirmed by 16S rRNA sequencing, with 99.87% (1,492/1,494 nucleotides) and 99.93% (1,471/1,472 nucleotides) identity to C. violaceum ATCC 12472T, respectively (GenBank accession numbers MG938492 and MG938493). Only pigmented colonies were observed with the bloodstream isolate during the current episode.
There are currently no recommended guidelines for interpreting antimicrobial susceptibility testing data for C. violaceum, most likely due to its rare occurrences in clinical settings. C. violaceum displays resistance to most penicillins and cephalosporins and to some β-lactam/β-lactamase inhibitor combinations (e.g., amoxicillin-clavulanate) (1, 12). It also shows various levels of resistance to other classes (1) and natural resistance to polymyxins (e.g., colistin) (13). Although β-lactam resistance appears to be increasing over time, most isolates show sensitivity to meropenem, imipenem, and piperacillin-tazobactam (1, 12). Ciprofloxacin is reported to be the most effective antibiotic among 25 antibiotics of various classes tested in vitro (1). Other antibiotics with adequate activity against C. violaceum are trimethoprim-sulfamethoxazole, tetracyclines, aminoglycosides, and chloramphenicol (1). Proposed mechanisms for antibiotic resistance include β-lactamase production and lipid A modification against β-lactams and polymyxins, respectively (12, 13).
One of the primary mechanisms of host immune responses in combating C. violaceum infection is by antimicrobial activities of neutrophils via expression of superoxide dismutase enzyme and the release of reactive oxygen species (ROS) (14). G6PD is essential in maintaining redox equilibrium of cellular NADPH that results in the production of ROS, including hydrogen peroxide (H2O2), for killing pathogens (14). Microorganisms also produce H2O2 from their metabolic processes, which the host cells can utilize to fight against infection. Catalase-positive organisms can neutralize endogenous H2O2, unlike catalase-negative microbes (14). Therefore, individuals with neutrophil deficiency or functional defects in intracellular bactericidal activity, such as CGD, G6PD deficiency, or diabetes, are especially prone to infection by catalase-positive organisms like C. violaceum (1, 2, 14). Based on the World Health Organization classification of G6PD deficiency, our patient displayed class I severe G6PD deficiency (<10% activity). Importantly, the nitroblue tetrazolium (NBT) and bacterial killing assays, which test for neutrophil superoxide production and intracellular killing of Staphylococcus aureus, respectively, conducted on the blood samples of our patient demonstrated a severe defect in bactericidal activity (5). His neutrophil G6PD activity was only 3.51% of normal (5). Besides the exposure of floodwaters to the leg wound, G6PD deficiency (Beaumont variant) with severe neutrophil defect was one of the most significant risk factors that predisposed our patient to C. violaceum infection. Interestingly, the twin brother of our patient also had G6PD deficiency and died at age 3 from septic infection with C. violaceum after playing in the mud (5). Therefore, patients with G6PD, CGD, or similar intracellular bactericidal defects should promptly seek medical treatment due to the risk of developing severe infections from the environment.
A case series in 2011 reported that reinfection with C. violaceum occurred in 7 out of 106 cases within a median of 135 days (1). Interestingly, asymptomatic colonization with C. violaceum in gastrointestinal and respiratory sites has been documented (4). The reported duration of antibiotics in successfully treated patients varies and is likely affected by host factors, source control, and the tissue penetration of selected agents. Patients without CGD typically received 6 weeks to 3 months of therapy (1). Still, significantly delayed relapses have been described (1, 2). Our patient appears to have relapsed at 10 months without any clinical risk factors for reinfection—no recent wounds nor re-exposure to standing water. It is likely that he suffered a relapse due to incomplete clearance of the previous infection caused by ineffective neutrophil killing. Possible sources of relapse may originate from the previous microabscesses in the lungs, liver, and spleen. In this situation, he was placed on lifelong suppressive therapy.
In summary, this case highlights a severe form of G6PD deficiency as a prominent risk factor in recurrent C. violaceum infection. Since C. violaceum displays several virulence factors, and the recovery of this organism from clinical specimens often indicates severe infection, the isolate should be reported, susceptibility testing performed, and an infectious disease team consulted for proper antibiotic selection. Because of the severity and increasing incidence of C. violaceum infection in clinical settings, further studies focusing on the establishment of standardized antimicrobial guidelines may be necessary in order to optimize treatment options.
Data availability.
Sequences were deposited in GenBank under accession numbers MH790126, MG938492, and MG938493.
SELF-ASSESSMENT QUESTIONS
- What antibiotic would be ineffective in treating Chromobacterium violaceum infection?
-
a.Imipenem
-
b.Colistin
-
c.Ciprofloxacin
-
d.Trimethoprim-sulfamethoxazole
-
a.
- Which condition is not shown to be a predisposition for Chromobacterium violaceum infection?
-
a.Chronic granulomatous disease
-
b.Diabetes
-
c.Factor VIII deficiency
-
d.Glucose-6-phosphate dehydrogenase deficiency
-
a.
- What activity is associated with Chromobacterium violaceum infection?
-
a.Inhaling cigarette smoke
-
b.Eating raw oysters
-
c.Drinking unpasteurized milk
-
d.Swimming in a creek
-
a.
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.00314-19 in this issue.
ACKNOWLEDGMENTS
We thank the medical technologists at the clinical microbiology laboratory of the University of Texas Medical Branch (Galveston, TX) for assistance with organism identification and antimicrobial susceptibility testing.
REFERENCES
- 1.Yang CH, Li YH. 2011. Chromobacterium violaceum infection: a clinical review of an important but neglected infection. J Chin Med Assoc 74:435–441. doi: 10.1016/j.jcma.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Macher AM, Casale TB, Fauci AS. 1982. Chronic granulomatous disease of childhood and Chromobacterium violaceum infections in the southeastern United States. Ann Intern Med 97:51–55. doi: 10.7326/0003-4819-97-1-51. [DOI] [PubMed] [Google Scholar]
- 3.Sivendra R, Lo HS. 1975. Identification of Chromobacterium violaceum: pigmented and non-pigmented strains. J Gen Microbiol 90:21–31. doi: 10.1099/00221287-90-1-21. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y, Majumdar SS, Hennessy J, Baird RW. 2016. The spectrum of Chromobacterium violaceum infections from a single geographic location. Am J Trop Med Hyg 94:710–716. doi: 10.4269/ajtmh.15-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamlok RJ, Mamlok V, Mills GC, Daeschner CW, Schmalstieg FC, Anderson DC. 1987. Glucose-6-phosphate dehydrogenase deficiency, neutrophil dysfunction and Chromobacterium violaceum sepsis. J Pediatr 111:852–854. doi: 10.1016/s0022-3476(87)80203-2. [DOI] [PubMed] [Google Scholar]
- 6.Sirinavin S, Techasaensiri C, Benjaponpitak S, Pornkul R, Vorachit M. 2005. Invasive Chromobacterium violaceum infection in children: case report and review. Pediatr Infect Dis J 24:559–561. doi: 10.1097/01.inf.0000164761.81491.3f. [DOI] [PubMed] [Google Scholar]
- 7.Batista JH, da Silva Neto JF. 2017. Chromobacterium violaceum pathogenicity: updates and insights from genome sequencing of novel Chromobacterium species. Front Microbiol 8:2213. doi: 10.3389/fmicb.2017.02213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciprandi A, da Silva WM, Santos AV, de Castro Pimenta AM, Carepo MS, Schneider MP, Azevedo V, Silva A. 2013. Chromobacterium violaceum: important insights for virulence and biotechnological potential by exoproteomic studies. Curr Microbiol 67:100–106. doi: 10.1007/s00284-013-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman SC, Ceraso D, Schugurensky A. 1986. First case report from Argentina of fatal septicemia caused by Chromobacterium violaceum. J Clin Microbiol 23:956–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott SL, Cheung WKW, Janda JM. 2003. The genus Aeromonas: biochemical characteristics, atypical reactions, and phenotypic identification schemes. J Clin Microbiol 41:2348–2357. doi: 10.1128/jcm.41.6.2348-2357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology. 2015. Manual of clinical microbiology, 11th edition ASM Press, Washington, DC. [Google Scholar]
- 12.Fantinatti-Garboggini F, Almeida R. d, Portillo V. d A, Barbosa TAP, Trevilato PB, Neto CER, Coêlho RD, Silva DW, Bartoleti LA, Hanna ES, Brocchi M, Manfio GP. 2004. Drug resistance in Chromobacterium violaceum. Genet Mol Res 3:134–147. [PubMed] [Google Scholar]
- 13.Poirel L, Jayol A, Nordmann P. 2017. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosa-Borges A, Sampaio MG, Condino-Neto A, Barreto OC, Nudelman V, Carneiro-Sampaio MM, Nogueira SA, Abreu TF, Rehder J, Costa-Carvalho BT. 2001. Glucose-6-phosphate dehydrogenase deficiency with recurrent infections: case report. J Pediatr (Rio J) 77:331–336. doi: 10.2223/JPED.243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequences were deposited in GenBank under accession numbers MH790126, MG938492, and MG938493.


