Abstract
The cardiovascular system is disrupted by chronic excessive alcohol use and often impaired in individuals with an alcohol use disorder (AUD). Less is known about cardiovascular recovery when an individual receives treatment for AUD. This observational study aimed to extend the growing body of evidence for cardiovascular biomarkers and intervention targets in the treatment of AUD. We examined cardiovascular function in 92 women before and after 12 weeks of cognitive behavioral therapy (CBT) for AUD. Participants were recruited exclusively from a randomized clinical trial comparing group versus individual CBT treatment strategies (parent study); no control group of untreated, but treatment seeking women was available. Demographic and drinking data were obtained from the parent study. Cardiovascular data were collected as part of this separate study, prior to and following the clinical trial. Mixed model analyses revealed multiple within-person cardiovascular changes indicative of improving health from pre- to post-treatment, including reduced heart rate and vessel stiffness as well as increased heart rate variability and baroreflex sensitivity. These significant improvements remained when extent of drinking during treatment was included in the models suggesting that active ingredients of AUD treatment may serve to benefit physical health over and above drinking reductions. Future studies should assess the time course of cardiovascular recovery during addiction treatment and the mechanisms by which evidence based AUD treatments may benefit physical as well as mental health.
Keywords: vascular tone, heart rate variability, addiction treatment, adaptability, baroreflex
Numerous cardiovascular disease states have been linked to heavy alcohol use (Corrao, Bagnardi, Zambon, & La Vecchia, 2004; Molina, Gardner, Souza-Smith, & Whitaker, 2014; Rehm et al., 2017). Cardiovascular problems also appear to be linked to diagnosis of an alcohol use disorder (AUD). Ischemic heart disease, for example, is significantly more prevalent among those seeking treatment for an AUD compared to the general population (Roerecke & Rehm, 2014a). In addition, epidemiological data have shown that individuals diagnosed with an AUD and in stable remission (>5 years) have greater risk for hypertension and myocardial infarction, and this risk is associated with duration of AUD and shorter remission (Udo, Vasquez, & Shaw, 2015).
The specific cardiovascular processes disrupted by alcohol, and their capacity for recovery with cessation of drinking, however, have not been well studied. Elevated heart rate and reduced heart rate variability (HRV), which are linked to cardiovascular disease, have been repeatedly observed in individuals with compared to without an AUD (Bar et al., 2006; DePetrillo, White, Liu, Hommer, & Goldman, 1999; Ingjaldsson, Laberg, & Thayer, 2003; Ralevski, Petrakis, & Altemus, 2019). Yet, research into whether these cardiovascular disruptions recover as the result of reduced drinking and AUD treatment is limited to two small studies that yielded contradictory results (Penzlin et al., 2017; Weise, Krell, & Brinkhoff, 1986). Moreover, cardiovascular processes other than heart rate and HRV are understudied in relation to AUD, but have a clearly established importance in overall cardiovascular health.
Understanding how the cardiovascular system, holistically, is impaired by AUD and determining whether, and under what conditions, it is capable of recovery is critical for optimizing AUD treatment strategies, and for more broadly defined conceptualizations of recovery. This is because cardiovascular functioning has been shown to influence attention capture, salience, and visceral reactions when individuals are viewing provocative cues (Garland, Carter, Ropes, & Howard, 2012; Garland, Franken, Sheetz, & Howard, 2012; Thayer & Lane, 2000). Cardiovascular dysfunction also has been implicated in the cascade of biopsychosocial events that lead to changes in affect, emotional arousal, and stress responding that can impact well-being at all stages of development (El-Sheikh, 2001; El-Sheikh & Buckhalt, 2005; Hugdahl, 1996; Porges, Doussard-Roosevelt, & Maita, 1994; Thayer & Lane, 2000). The cardiovascular system is thus an emerging transdiagnostic intervention target (Buckman, Vaschillo, Fonoberova, Mezic, & Bates, 2018). Moreover, body-based interventions that harness the inherent properties of the cardiovascular system (e.g., HRV biofeedback) have demonstrated clinical value for numerous physical and mental health conditions that co-occur with AUD (Alayan, Eller, Bates, & Carmody, 2018; Caldwell & Steffen, 2018; Goessl, Curtiss, & Hofmann, 2017; Hassett et al., 2007; Windthorst et al., 2017). The present observational study sought to characterize how the cardiovascular system changes during conventional AUD treatment to provide foundational support for the development of body-based interventions for AUD that may bolster recovery by improving both mental and physical health.
Decades of research have demonstrated that the cardiovascular system is highly flexible and capable of numerous, fine-grained, beat-to-beat shifts in heart rate, stroke volume, and vascular tone to meet internal and environmental demands. The electrocardiogram (ECG) is the most common cardiovascular assessment method from which heart rate (i.e., number of heart beats per minute) and HRV (i.e., changes in the time intervals between consecutive heart beats) can be calculated. HRV has become a focus of significant clinical research over the past decade as it is a powerful biomarker of health and resilience, and can provide important insight into both physical and mental well-being (Kemp & Quintana, 2013). In addition, the volume of the blood ejected by the heart per beat (i.e., stroke volume) and the balance of vasodilatory and vasoconstrictive forces (i.e., vascular tone) provide non-redundant information about cardiovascular functionality, and are likewise measureable as averages and variability over time. Each of these cardiovascular processes can act independently or in coordination in response to demand (Buckman et al., 2015; Liu et al., 2004; Vaschillo et al., 2018) and are component parts of the baroreflex system, a series of feedback loops that control blood pressure and integrate neural and cardiovascular signaling. As such, the health of the cardiovascular system is best measured by considering how each of these processes function.
The present study examined whether the health of the cardiovascular system, measured across multiple cardiovascular processes and using multiple indices, improved in a sample of women with AUD during a female-specific, 12-week outpatient cognitive behavior therapy (CBT) program for AUD (Epstein et al., 2018). This observational study was an add-on to a randomized clinical trial (Epstein et al., 2018) assessing group versus individual female-specific CBT for AUD. Within-person change in pre- to post-treatment cardiovascular function was measured as: (1) Mean resting heart rate, stroke volume, vascular tone, and blood pressure - calculated as estimates of systemic metabolic rate (Green, 2011); (2) variability in heart rate, stroke volume, and vascular tone - computed to assess real-time regulation and the system’s capacity to adapt to challenge (Buckman et al., 2015; Lehrer & Eddie, 2013; Vaschillo et al., 2018); and, (3) sensitivity of heart rate, stroke volume, and vascular tone to blood pressure changes - computed to gauge the functionality of the baroreflex system, a regulatory mechanism that coordinates cardiovascular processes and interconnects cardiac and neural signaling.
There is ample evidence of recovery of neural systems with cessation or reduction in drinking (Bates, Buckman, & Nguyen, 2013), but only limited direct evidence showing recovery of cardiovascular function. We hypothesized within-person improvements in cardiovascular functioning from pre- to post-treatment, such as decreased heart rate and blood pressure, and increased HRV and baroreflex sensitivity. Further, we expected these changes to be related to alcohol use during treatment, with individuals who reduced their alcohol use the most showing the greatest recovery.
Method
Participants
This study was approved by the university’s Institutional Review Board for the Protection of Human Subjects Involved in Research. Women between the ages of 20 and 68 years from a randomized clinical trial (RCT) of 12-week, female-specific individual- or group-modality CBT for AUD (Epstein et al., 2018) were recruited to be part of an uncontrolled observational study on cardiovascular changes during treatment. Eligibility criteria for the RCT included a minimum age of 18 years, DSM-IV-TR (American Psychiatric Association, 2000) diagnosis of current alcohol dependence, absence of psychotic symptoms and gross cognitive deficit in the prior six months, ability to read English at the sixth grade level, alcohol consumption within the past 60 days, and no current physiological dependence on drugs other than alcohol, nicotine, or cannabis. No additional eligibility criteria were added for the present study.
Of the 155 participants recruited into the parent RCT, 95 (61%) agreed to take part in this observational study. Sixty-three women (68%) completed both pre- and post-treatment cardiovascular evaluations. Data from two women were excluded from analysis due to arrhythmia or tremors that prevented accurate recording, and data from one woman was lost due to technical error. The final sample included 92 women. Demographic characteristics of this sample obtained from the parent RCT (Epstein et al., 2018) are presented in Table 1.
Table 1.
Sample Demographics.
| Variable | Value |
|---|---|
| Age (years; mean ± SD) | 49 ± 10 |
| Education (years; mean ± SD) | 16 ± 3 |
| Family history of AUD | 75% |
| Median household income | $70,000 |
| Race | 88% White |
| 8% Black | |
| 4% Mixed / Other | |
| Latina (any race) | 7% |
| Full-time employment | 32% |
| Married/committed relationship | 57% |
| Current Axis I diagnosis | 37% |
| Current or Past Axis II diagnosis | 15% |
| Any medication | 48% |
| Current tobacco use | 27% |
| Age of Diagnosis (years; mean ± SD)a | |
| Alcohol Abuse | 26 ± 11 |
| Alcohol Dependence | 36 ± 12 |
| Duration of AUD (years; mean ± SD)b | |
| Alcohol Abuse | 23 ± 12 |
| Alcohol Dependence | 13 ± 10 |
DSM-IV-R criteria
At start of parent RCT
Procedure and Materials
Women who were eligible for the RCT were recruited for this cardiovascular study at the end of the RCT clinical screening interview and before treatment began. Interested women provided written informed consent for this separate study and completed two identical laboratory sessions, the first approximately 2 weeks before starting the 12-week outpatient treatment program (pretreatment) and the second within 2 weeks of completing the treatment program (post-treatment). In both sessions, cardiovascular data were collected while the participant was seated comfortably in front of a television screen. Physiological data were recorded during a 5-minute low cognitive demand baseline task in which squares that changed color every 10 seconds were shown on the screen. The participant was asked to silently count the number of blue or green squares that appeared on the screen. This standardized, minimally demanding cognitive task produces better between- and within-subject stability and generalizability across sessions compared to an unstructured “resting” baseline (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992). The participants then completed a 5-minute paced breathing task as part of another aim of the study. They were compensated for their time following each cardiovascular assessment session with a $25 gift certificate to a local retail store.
Measures of Alcohol Consumption
Alcohol use was measured as part of the parent RCT. Timeline Follow-Back Interview (Sobell et al., 1996) was used to assess pretreatment drinking, which was collected for the 90 days prior to the last drink before the baseline interview and through the day before session 1 of the RCT. Drinking during treatment was measured using daily drinking logs that were completed by participants as part of the RCT within-treatment protocol (Epstein et al., 2018). Timeline Follow-Back drinking data collected at the end of the RCT were substituted where daily drinking log data were not available. From these data, percent of drinking days and mean number of drinks per drinking day were calculated during the 12 weeks before and during the treatment period. The maximum number of consecutive days abstinent during the treatment period was computed to identify duration of continuous periods of abstinence. Alcohol use characteristics of the sample before and during treatment are presented in Table 2.
Table 2.
Alcohol use characteristics of the sample.
| Drinking Variable | Value |
|---|---|
| Pre-treatment | |
| Percent Drinking Days | 67% |
| Mean Drinks per Drinking Day | 7.3 ± 4.7 |
| During treatment | |
| Percent Drinking Days | 27% |
| Mean Drinks per Drinking Day | 4.5 ± 3.8 |
| Maximum Consecutive Days of Abstinence during Treatment | 35 ± 28 |
| % reporting no alcohol use | 15% |
| % reporting reduced drinking frequency | 92% |
| % reporting reduced quantity consumed on drinking days | 90% |
Measures of Cardiovascular Functioning
A PowerLab Acquisition System (ADInstruments, Colorado Springs, CO) and Finometer MIDI (Finapres Medical Systems, Enschede, Netherlands) were used to collect ECG, respiration, and beat-to-beat blood pressure data (2000 Hz sampling rate). A standard lead II configuration was used for ECG measurement. A cuff-sensor for blood pressure measurement was attached to the second phalange of the right middle finger. A stretch belt with piezo-sensor for collection of respiratory data was set around the chest. Physiological data were analyzed using WinCPRS software (Absolute Alien Oy, Turku, Finland). Artifacts and missed or irregular beats were manually modified by interpolation prior to the analysis.
Cardiac functioning was measured from the ECG as the time interval between heart beats (R-wave to R-wave interval, RRI) to evaluate heart rate and HRV. Stroke volume was measured using the ModelFlow methodology for beat-to-beat cardiac output measurement (Bogert & van Lieshout, 2005). Pulse transit time, the time interval between the R-wave of the ECG and the apex of the finger pulse wave recorded by the Finometer MIDI, was calculated to evaluate vascular tone. Higher pulse transit time corresponds to lower vascular tone. Systolic and diastolic arterial pressures were measured from the finger pulse wave. Cubic interpolation of non-equidistant waveforms of the beat-to-beat sequences was completed; measurements were resampled at 4 Hz.
Average systolic and diastolic arterial pressure was computed across the 5-minute baseline task. Heart rate, stroke volume, and pulse transit time were measured across three levels: average functioning, variability, and baroreflex sensitivity. Average functioning was calculated as the mean across the 5-minute baseline task) and variability was calculated as total spectral power after Fourier transformation of the time series (Cooke et al., 1999; Taylor, Carr, Myers, & Eckberg, 1998). Variability was further measured as spectral power in the high frequency (HF HRV, 0.15–0.5 Hz) range of the RRI spectrum as a proxy of vagal activity (Task Force, 1996). Baroreflex sensitivity was measured as gain in the heart rate, stroke volume, and vascular tone baroreflex branches, which was calculated using cross-spectral analysis of simultaneously recorded beat-to-beat systolic arterial pressure (input) and RRI, stroke volume, pulse transit time, (outputs). Transfer functions were calculated in the low frequency range (0.05–0.15 Hz) where coherence between the input and outputs was > 0.5 (Vaschillo, Vaschillo, Buckman, Pandina, & Bates, 2012). Thus, baroreflex gain is presented as the magnitude of change in RRI (ms), stroke volume (ml), and pulse transit time (ms) in response to one unit of change in systolic blood pressure (mmHg).
Statistical Analysis
A mixed linear model (SAS 9.4) for each cardiovascular index was used to test change from pre- to post-treatment physiological assessment. All analyses focused on within-subject change as no reference group (i.e., untreated control) was available. Age was used as a covariate in all analyses except in analyses that covaried years since onset of alcohol dependence. Three drinking variables were individually tested as covariates to determine the influence of drinking during treatment on change in physiological functioning. False discovery rate (Benjamini & Hochberg, 1995) was used to mitigate alpha inflation related to multiple testing for each family of analyses (q = .05). Cohen’s d is reported to indicate effect size. Cohen’s d’s of >0.2, >0.5, and >0.8 are respectively considered small, medium and large effects (Cohen, 1988).
Based on National Heart, Lung, and Blood Institute guidelines, systolic and diastolic blood pressure at session 1 were categorized as hypotension, normotensive, or and hypertensive. Hypotensive was defined as systolic pressure ≤ 90 mmHg or diastolic pressure ≤ 60 mmHg. Hypertensive was defined as systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg. Change in blood pressure from session 1 and session 2 was computed and an analysis of variance (SAS 9.4) was used to calculate changes in blood pressure based on blood pressure at treatment entry. The Tukey Test was used for pairwise post-hoc comparisons.
Results
No pretreatment cardiovascular differences were noted between participants randomized to the group or individual CBT interventions, nor between women who completed versus did not complete the RCT study (all p> .05). In support of the hypothesis of cardiovascular improvements following a 12-week psychotherapeutic intervention, average heart rate significantly decreased, F(1,59)=13.58, p = .0005, and average pulse transit time significantly increased, F(1,59)=16.63, p = .0001, from pre- to post-treatment. Both total, F(1,59)=6.79, p = .0116, and high frequency, F(1,59)=11.66, p = .0012, HRV increased. Further, baroreflex sensitivity in the heart rate, F(1,56)=7.34, p = .0089, vascular tone, F(1,56)=8.23, p = .0057, and stroke volume, F(1,58)=5.57, p = .0217, branches also increased (Table 3). This same pattern of results was observed when intervention type (group versus individual) or current tobacco use was added to the model, and when years since alcohol dependence onset was used as a covariate in place of age.
Table 3.
Mean ± standard deviation of cardiovascular parameters pre- and post-treatment.
| Pre-treatment | Post-treatment | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Change | d | |
| Averages during 5 minute vanilla task | ||||||
| Heart rate [beats per minute] | 73.6 | 12.1 | 69.6 | 9.4 | −4.0* | −0.37 |
| Stroke volume [ml] | 97.6 | 23.2 | 95.1 | 23.0 | −2.5 | −0.11 |
| Pulse transit time [ms] | 267.2 | 27.2 | 282.6 | 31.8 | 15.4* | 0.52 |
| Systolic arterial pressure [mmHg] | 131.5 | 20.8 | 128.7 | 18.8 | −2.8 | −0.14 |
| Diastolic arterial pressure [mmHg] | 67.7 | 11.7 | 68.4 | 10.8 | 0.6 | 0.05 |
| [Log] Variability during 5 minute vanilla task | ||||||
| Total heart rate variability [ms2] | 6.3 | 1.0 | 6.6 | 0.9 | 0.3* | 0.28 |
| High frequency heart rate variability [ms2] | 4.6 | 1.4 | 5.0 | 1.2 | 0.4* | 0.31 |
| Total pulse transit time variability [ms2] | 1.5 | 0.6 | 1.7 | 0.8 | 0.2 | 0.30 |
| Total stroke volume variability [ml2] | 1.9 | 0.4 | 1.9 | 0.5 | 0.0 | 0.01 |
| [Log] Baroreflex sensitivity during 5 minute vanilla task | ||||||
| Heart rate baroreflex gain [ms/mmHg] | 1.6 | 0.6 | 1.9 | 0.6 | 0.2* | 0.36 |
| Vascular tone baroreflex gain [ms/mmHg] | −0.9 | 0.6 | −0.5 | 0.9 | 0.3* | 0.47 |
| Stroke volume baroreflex gain [ml/mmHg] | −0.4 | 0.5 | −0.2 | 0.6 | 0.2* | 0.33 |
Significant pre- to post-treatment changes based on False Discovery Rate
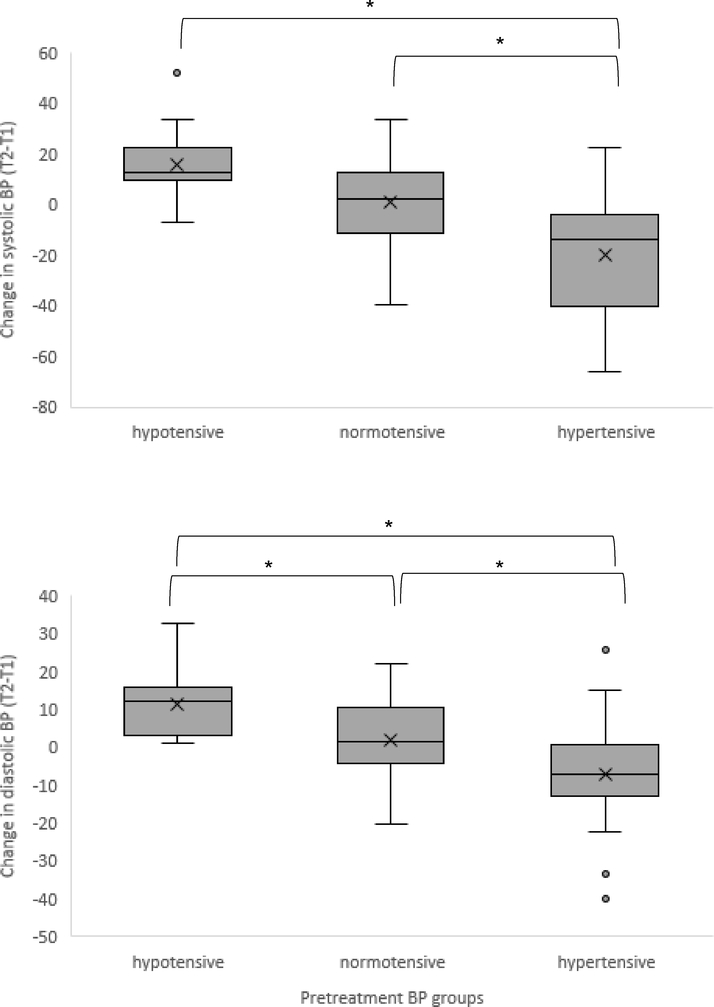
Although there was no overall change in blood pressure from pre- to post-treatment in the sample, significant differences were observed when women who entered treatment with hypotensive, normotensive, or hypertensive blood pressures were compared. Changes in systolic blood pressure across treatment in hypotensive (Δ 15.7 +/− 15.3), normotensive (Δ 0.9 +/− 17.6), or hypertensive (Δ −19.7 +/− 24.4) women were significantly different (F[2,59] = 14.43, p < .0001). Post-hoc analysis revealed that those with high blood pressure at treatment entry differed significantly from normotensive and hypotensive individuals, who did not significantly differ from one another. Changes in diastolic blood pressure across treatment in hypotensive (Δ 11.2 +/− 8.7), normotensive (Δ 1.9 +/− 10.3), or hypertensive (Δ –7.1 +/− 14.6) also were significantly different (F[2,59] = 10.79, p < .0001). Post-hoc analysis revealed that all three groups were significantly different from one another from one another.
To characterize whether the observed cardiovascular changes were related to the extent of drinking during treatment, we reassessed pre- to post-treatment changes in cardiovascular functioning while controlling for three alcohol use variables that reflected drinking during treatment (percent drinking days, maximum consecutive abstinence days, and mean drinks per drinking day). When each of these alcohol-use-during-treatment variables were added to the model, the same pattern of results was retained, except that stroke volume baroreflex sensitivity was no longer significant in any model, and vascular tone baroreflex sensitivity was no longer significant in the mean drinks per drinking day model.
Discussion
The present observational study examined within-person recovery in the cardiovascular system in women with AUD who also participated in a RCT of 12-week, outpatient, female-specific CBT for AUD (Epstein et al., 2018). We evaluated the cardiovascular system across processes that control the heart (i.e., heart rate and stroke volume) and the vasculature (i.e., pulse transit time), and considered functionality across the level of averages, variabilities, and the interrelation of the processes through the baroreflex system. Improvements in cardiovascular functioning across multiple processes and functional levels were observed from pre- to post-treatment suggesting cardiovascular system resilience.
Evidence of physiological resilience among women with an AUD has important public health implications particularly considering that women, compared to men, have shown large increases in AUD prevalence from 2001–2002 to 2012–2013 and appear to be at elevated risk for alcohol-related cardiovascular disease (Roerecke & Rehm, 2014b; Schwarzinger, Thiebaut, Baillot, Mallet, & Rehm, 2017). It is likely that physiological resilience, like psychological resilience, critically contributes to behavior change and recovery from addiction (Buckman et al., 2018). Importantly, physiological reactivity typically occurs outside of conscious awareness, and, even though an individual may not subjectively sense it, their body’s reaction to cues can provoke an arousal response and redirect attention (Kandel, Schwartz, & Jessell, 2000). Such responses often appear “automatic” in the sense that they outpace or bypass individuals’ cognitive plans and conscious intentions not to use (Robinson & Berridge, 1993; Tiffany, 1990; Wiers, Eberl, Rinck, Becker, & Lindenmeyer, 2011). This supports growing evidence that bolstering cardiovascular health to promote adaptive levels of physiological reactivity may help buffer against the everyday experiences of stress and anxiety (Goessl et al., 2017) that can serve as potent triggers for drinking (Marlatt, 1996). We speculate that evidence-based AUD treatment approaches may serve to improve cardiovascular health through currently unspecified mechanisms, possibly related to the role of the baroreflex in cognitive-emotional regulation (Critchley & Harrison, 2013).
The present study observed significant reductions in mean heart rate and arterial stiffness (i.e., significant increase in pulse transit time) after 12 weeks of CBT treatment. Average heart rate and pulse transit time reflect underlying metabolic processes that have been shown to be disrupted during acute alcohol intoxication (Buckman et al., 2015) and chronic alcohol use typical of AUD (e.g., Ingjaldsson et al., 2003 ). A reduction in heart rate is consistent with adjustments in neural innervation to the heart to reduce sympathetic and/or enhance parasympathetic activity. That both cardiac and vascular system change was observed also may suggest that these changes reflect direct improvements in organ health as well as in brain-body integration.
At the group level, changes in blood pressure were not statistically significant. This was not unexpected considering that hyper-, hypo-, and normotensive blood pressures were observed at treatment entry, and thus a healthful blood pressure response would differ based on initial values. Blood pressure has an optimal range (e.g., based on National Heart, Lung, and Blood Institute guidelines), above and below which are health conditions (i.e., hypertension and hypotension) that often require pharmacological or medical intervention. Comparison of blood pressure changes among those with hyper-, normo-, and hypotensive blood pressures at treatment entry revealed statistically significant differences consistent with a pattern of system normalization. Specifically, individuals who entered treatment with hypertensive blood pressure values showed average decreases, those with normotensive values showed minimal change, and individuals who entered treatment with evidence of hypotension showed average increases. Although an alternative explanation is that this pattern of change reflects regression to the mean, blood pressure in this study was measured throughout a 5 minute period using a research-grade device that derives blood pressure from continual beat-to-beat detection of the pulse wave. Thus, the pre- and post-treatment blood pressure values analyzed here were each based on 300 measurements of blood pressure to minimize the contribution of measurement error to the results.
The observation that physiological improvements co-occur with addiction treatment is not surprising, but nevertheless has important implications for the definition of treatment success and recovery, which are often solely defined by sustained abstinence. It is compelling that the observed changes occurred within the 12-week treatment window as it indicates that the initiation of cardiovascular recovery is possible even without a specific body-based intervention, and that inclusion of adjunctive treatments to promote heart health potentially may have a positive influence on treatment success (Alayan et al., 2018).
Unexpectedly, results of analyses that controlled for alcohol use during treatment and years of use indicated that the cardiovascular system began to recover even when stable abstinence had not yet been achieved and even after years of heavy drinking. Identification of significant change pre- to post-treatment after covarying for alcohol use suggested that most cardiovascular changes were not explained solely by alcohol use during treatment, and none were explained by years since initial diagnosis of AUD. Years of chronic, heavy alcohol use likely influences the organs as well as the neural connections of cardiovascular system. While reductions in drinking are a part of physiological recovery, it is possible that psychological and other unspecified benefits from receiving treatment also contribute to improvements in physical health (and vice versa). The present study suggests that improvements in body system function may be an important quality of life indicator of treatment outcome and argues for a more multidimensional definition of treatment gains and holistic recovery. Future studies are needed to identify biological and psychological mechanism(s) by which this recovery occurs.
Variability in heart rate also was measured in the present study as it provides insight into the adaptive capacity of heart rate in response to challenge and stress (Porges et al., 1994). A vast literature has linked low levels of resting state HRV, particularly those indices related to vagal nerve functioning (e.g., high frequency HRV), to myriad mental and physical health conditions. In the present study, we showed increases in total HRV during a low demand baseline task following a course of CBT, further supporting general physiological improvements during psychotherapeutic treatment. Moreover, high frequency HRV increased suggesting recovery of vagal functioning, which is an increasingly common biomarker and intervention target in AUD treatment and research (Price & Crowell, 2016; Ralevski et al., 2019). Changes in HRV were on the magnitude of 0.3 to 0.4 log units indicating sizeable increases within the treatment period.
Average arterial stiffness was significantly reduced. In addition, the sensitivities of the heart rate baroreflex (i.e., amount of change in heart rate in response to a 1mm/Hg change in blood pressure), vascular tone baroreflex (i.e., amount of change in pulse transit time in response to a 1mm/Hg change in blood pressure), and stroke volume baroreflex (i.e., amount of change in cardiac output in response to a 1mm/Hg change in blood pressure) were increased between 0.2 and 0.3 log units from pre- to post-treatment. This is suggestive of a generalized improvement in arterial health following AUD treatment. Improved average vascular tone across treatment is of potential clinical interest, as it is indicative of greater compliance of vessel walls to changes in blood pressure. More compliance benefits the baroreceptors, which are located in the vessel walls, by allowing them to respond in a more precise fashion to arterial stretch when blood pressure rises. This improved baroreceptor responding translates into greater sensitivity of the baroreflex, a reflexive, neurally-mediated control system best known for its role in balancing changes in blood pressure with compensatory changes in cardiovascular functions, such as heart rate, stroke volume, and vascular tone. Furthermore, a more sensitive baroreflex, via its intersection with the central nervous system, ensures better integration of cardiovascular responding within a whole body, cognitive-emotional response (Benarroch, 1997). Thus, more compliant vessels allow more sensitive regulation of blood pressure through both local and central mechanisms, which may support cognitive and emotional functioning that contribute to long-term recovery from addiction (Bates et al., 2013; Witkiewitz, Bowen, & Donovan, 2011).
One noteworthy observation was that the sensitivities of the individual baroreflex branches were differentially affected by alcohol use during treatment. The greater sensitivity of the heart rate baroreflex after treatment was not explained by drinking during treatment. The greater sensitivity of the stroke volume baroreflex, on the other hand, did appear to be driven mainly by reduced drinking during treatment. The reason for the specific influence of alcohol use on stroke volume but not heart rate baroreflex functioning is unclear. Stroke volume and heart rate measure different aspects of heart functioning; namely, the strength versus the rate of heart muscle contractions, respectively. These heart processes are mediated by separate neural pathways (i.e., neuromuscular junction and sinoatrial node innervation, respectively), which may be differentially sensitive to more general health factors such as age, fitness level, and drinking.
Based on evidence that the baroreflex supports regulation of emotion, stress, and behavior (Duschek, Werner, & Reyes Del Paso, 2013; Dworkin et al., 1994), it is possible that pre- to post-treatment changes in baroreflex sensitivity is a physiological mechanism that supports aspects of drinking- and mental health-related behavior change that are reinforced by evidence-based AUD treatment. The chronic misuse of alcohol over years, even decades, may lead to a slow degradation of cardiovascular function, yet the present results suggest that both metabolic and baroreflex processes have the capacity to rebound during early recovery. They further are consistent with the idea that improving cardiovascular health and regulation, such as with health-focused activities like exercise, HRV biofeedback, meditation, and yoga may be useful as part of the holistic recovery process due to their demonstrated beneficial influences on cardiovascular functioning (e.g.,Aggarwal et al., 2018; Chen, Sun, Wang, Lin, & Wang, 2016).
This study should be considered in light of limitations. First, cardiovascular functioning was assessed at two time points only. Prior to and following treatment, thus trajectories of change could not be determined. Future studies should assess cardiovascular function across the treatment period to capture individual cardiovascular change trajectories in relation to time varying behaviors such as alcohol use. Second, no control group was included in this study. The most appropriate control group would comprise treatment-seeking women with AUD who did not receive treatment during the 12 weeks of the study, but there were ethical concerns with delaying treatment provision in such a group, prior research has demonstrated moderate to high stability in multiple cardiovascular indicators across multiple years in a multi-site naturalistic cohort study (e.g., Hu, Lamers, Penninx, & de Geus, 2017). This study focused on within person changes, but future studies may benefit from comparisons to a nontreatment seeking AUD group or to an AUD treatment group that is also receiving an adjunctive body-based intervention. As recruitment for this study was dependent on the parent trial inclusion criteria, participants often presented with mental and physical health comorbidities that could also influence cardiovascular health. In addition, the age range of the sample was large and led to the inclusion of age as a covariate in all analyses. This sample included women only and should be replicated in a sample that includes men. As well, the sample was limited in not being racially diverse. Not all women who completed the pretreatment cardiovascular screening returned for posttreatment assessment; some dropped from the parent trial and others chose not to return to complete this separate laboratory session. To account for this, we used a mixed modeling strategy that uses all available data. Nearly half of the participants were currently taking medication, but not all medications were assessed. Future study would benefit from comprehensively tracking the influence of pharmaceutical that may affect cardiovascular function and reactivity. All participants in this study received CBT treatment for AUD. Although we did not observe differences in physiological recovery between those randomized to group or individual treatment, in the absence of testing a group that received some other psychotherapeutic intervention for AUD, these data do not speak to any specific influence of CBT, per se. Even considering these limitations, the results point to the need to better understand whether and how cardiovascular functioning serves as a physiological mechanism that supports recovery from an AUD.
Individuals entering addiction treatment are typically screened for medical conditions, and are medicated if necessary, but rarely are physical health systems considered active contributors to addiction liability. Chronic alcohol use is known to exert negative effects on the heart and blood vessels (Corrao et al., 2004; Rehm et al., 2017) and cardiovascular dysfunction is known to reduce adaptability and behavioral control (Duschek et al., 2013; Dworkin et al., 1994). Some early evidence suggests women may be more sensitive to health risks (Roerecke & Rehm, 2014b; Schwarzinger et al., 2017). Yet, the reciprocal and escalating relationship between alcohol use and cardiovascular impairment is understudied. The current study suggests that women’s cardiovascular systems may improve during treatment for AUD and that significant improvement can occur even when total abstinence is not obtained, suggesting that recovery of some cardiovascular processes may be linked to health promoting features of treatment apart from changes in drinking behavior. Adjunctive body-based interventions in AUD treatment are attractive as they expand the biobehavioral systems targeted by treatment and are well known for being simple to implement, inexpensive, and easy to disseminate. Moreover, such interventions may be particularly beneficial for AUD populations that manifest overt alcohol-related physical health problems or that are underserved, such as elderly and disabled individuals with AUD. We speculate that promoting adjunctive treatments directly aimed at improving cardiovascular functioning, such as HRV biofeedback (Lehrer & Vaschillo, 2004), may enhance treatment outcome by accelerating the rate of cardiovascular recovery and contributing to mental and physical resilience within a broad framework of AUD recovery.
Figure 1.
Changes in systolic (top) and diastolic (bottom) blood pressure differed among hypertensive, normotensive, and hypotensive women. Pre-treatment blood pressure was grouped based on published blood pressure guidelines and change in blood pressure from pre- to post-treatment was compared. * p < .05.
Acknowledgments
This work was supported in part by the National Institute on Alcoholism and Alcohol Abuse through the following grants: K02AA00325 to Jennifer F. Buckman, R21AA020367 to Evgeny G. Vaschillo, R01AA017163 to Elizabeth E. Epstein, F31AA027147 to Laura M. Lesnewich, and K24AA021778 and HHSN275201000003C to Marsha E. Bates. All authors report no conflict of interest.
Contributor Information
Jennifer F. Buckman, Rutgers University
Bronya Vaschillo, Rutgers University.
Evgeny G. Vaschillo, Rutgers University
Elizabeth E. Epstein, University of Massachusetts Medical School
Tam T. Nguyen-Louie, Johns Hopkins University
Laura M. Lesnewich, Rutgers University
David Eddie, Harvard Medical School.
Marsha E. Bates, Rutgers University
References
- Aggarwal M, Bozkurt B, Panjrath G, Aggarwal B, Ostfeld RJ, Barnard ND, . . . Litwin SE (2018). Lifestyle Modifications for Preventing and Treating Heart Failure. Journal of the American College of Cardiology, 72(19), 2391–2405. doi: 10.1016/j.jacc.2018.08.2160 [DOI] [PubMed] [Google Scholar]
- Alayan N, Eller L, Bates ME, & Carmody DP (2018). Current Evidence on Heart Rate Variability Biofeedback as a Complementary Anticraving Intervention. Journal of Alternative and Complementary Medicine, 24(11), 1039–1050. doi: 10.1089/acm.2018.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Bar KJ, Boettger MK, Neubauer R, Groteluschen M, Jochum T, Baier V, . . . Voss A (2006). Heart rate variability and sympathetic skin response in male patients suffering from acute alcohol withdrawal syndrome. Alcoholism, Clinical and Experimental Research, 30(9), 1592–1598. doi: 10.1111/j.1530-0277.2006.00191.x [DOI] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, & Nguyen TT (2013). A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychology Review, 23(1), 27–47. doi: 10.1007/s11065-013-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (1997). The central autonomic network In Low PA (Ed.), Clinical Autonomic Disorders (2nd ed., pp. 17–23). Philadelphia, PA: Lippincott-Raven. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B, 57, 289–300. [Google Scholar]
- Bogert LWJ, & van Lieshout JJ (2005). Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Experimental Physiology, 90(4), 437–446. [DOI] [PubMed] [Google Scholar]
- Buckman JF, Eddie D, Vaschillo EG, Vaschillo B, Garcia A, & Bates ME (2015). Immediate and complex cardiovascular adaptation to an acute alcohol dose. Alcoholism: Clinical and Experimental Research, 39(12), 2334–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JF, Vaschillo EG, Fonoberova M, Mezic I, & Bates ME (2018). The Translational Value of Psychophysiology Methods and Mechanisms: Multilevel, Dynamic, Personalized. Journal of Studies on Alcohol and Drugs, 79(2), 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell YT, & Steffen PR (2018). Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. International Journal of Psychophysiology 131:96–101. doi: 10.1016/j.ijpsycho.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Chen S, Sun P, Wang S, Lin G, & Wang T (2016). Effects of heart rate variability biofeedback on cardiovascular responses and autonomic sympathovagal modulation following stressor tasks in prehypertensives. Journal of Human Hypertension, 30(2), 105–111. doi: 10.1038/jhh.2015.27 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, & Eckberg DL (1999). Human responses to upright tilt: a window on central autonomic integration. Journal of Physiology, 517 (Pt 2), 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrao G, Bagnardi V, Zambon A, & La Vecchia C (2004). A meta-analysis of alcohol consumption and the risk of 15 diseases. Prevention Medicine, 38(5), 613–619. doi: 10.1016/j.ypmed.2003.11.027 [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Harrison NA (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. doi: 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- DePetrillo PB, White KV, Liu M, Hommer D, & Goldman D (1999). Effects of alcohol use and gender on the dynamics of EKG time-series data. Alcoholism: Clinical and Experimental Research, 23(4), 745–750. [PubMed] [Google Scholar]
- Duschek S, Werner NS, & Reyes Del Paso GA (2013). The behavioral impact of baroreflex function: A review. Psychophysiology 50(12):1183–1193. doi: 10.1111/psyp.12136 [DOI] [PubMed] [Google Scholar]
- Dworkin BR, Elbert T, Rau H, Birbaumer N, Pauli P, Droste C, & Brunia CH (1994). Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proceedings of the National Academy of Sciences of the United States of America, 91(14), 6329–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M (2001). Parental drinking problems and children’s adjustment: vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of Abnormal Psychology, 110(4), 499–515. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, & Buckhalt JA (2005). Vagal regulation and emotional intensity predict children’s sleep problems. Developmental Psychobiology, 46(4), 307–317. doi: 10.1002/dev.20066 [DOI] [PubMed] [Google Scholar]
- Epstein EE, McCrady BS, Hallgren KA, Cook S, Jensen NK, & Hildebrandt T (2018). A randomized trial of female-specific cognitive behavior therapy for alcohol dependent women. Psychology of Addictive Behaviors, 32(1), 1–15. doi: 10.1037/adb0000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Carter K, Ropes K, & Howard MO (2012). Thought suppression, impaired regulation of urges, and Addiction-Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. Biological Psychology, 89(1), 87–93. doi: 10.1016/j.biopsycho.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Franken IH, Sheetz JJ, & Howard MO (2012). Alcohol attentional bias is associated with autonomic indices of stress-primed alcohol cue-reactivity in alcohol-dependent patients. Experimental and Clinical Psychopharmacology, 20(3), 225–235. doi: 10.1037/a0027199 [DOI] [PubMed] [Google Scholar]
- Goessl VC, Curtiss JE, & Hofmann SG (2017). The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychological Medicine, 47(15), 2578–2586. doi: 10.1017/s0033291717001003 [DOI] [PubMed] [Google Scholar]
- Green JA (2011). The heart rate method for estimating metabolic rate: review and recommendations. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 158(3), 287–304. doi: 10.1016/j.cbpa.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Hassett AL, Radvanski DC, Vaschillo E, Vaschillo B, Sigal LH, Karavidas MK, . . . Lehrer PM (2007). A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Applied Psychophysiology and Biofeedback, 32(1), 1–10. doi: 10.1007/s10484-006-9028-0 [DOI] [PubMed] [Google Scholar]
- Hu MX, Lamers F, Penninx B, & de Geus EJC (2017). Temporal stability and drivers of change in cardiac autonomic nervous system activity. Autonomic Neuroscience, 208, 117–125. doi: 10.1016/j.autneu.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Hugdahl K (1996). Cognitive influences on human autonomic nervous system function. Current Opinions in Neurobiology, 6(2), 252–258. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, & Thayer JF (2003). Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry, 54(12), 1427–1436. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (1992). Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology, 29(742–750). [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, & Jessell TM (2000). Principles of Neural Science. New York, NY: McGraw-Hill. [Google Scholar]
- Kemp AH, & Quintana DS (2013). The relationship between mental and physical health: insights from the study of heart rate variability. International Journal of Psychophysiology, 89(3), 288–296. doi: 10.1016/j.ijpsycho.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Lehrer P, & Eddie D (2013). Dynamic processes in regulation and some implications for biofeedback and biobehavioral interventions. Applied Psychophysiology and Biofeedback, 38(2), 143–155. doi: 10.1007/s10484-013-9217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P, & Vaschillo E (2004). Heart rate variability biofeedback: A new tool for improving autonomic homeostasis and treating emotional and psychosomatic diseases. Japanese Journal of Biofeedback, 30, 7–16. [Google Scholar]
- Liu H, Yambe T, Sasada H, Nanka S, Tanaka A, Nagatomi R, & Nitta S (2004). Comparison of heart rate variability and stroke volume variability. Autonomic Neuroscience, 116(1–2), 69–75. doi: 10.1016/j.autneu.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Marlatt GA (1996). Section I. theoretical perspectives on relapse: Taxonomy of high-risk situations for alcohol relapse: Evolution and development of a cognitive-behavioral model. Addiction, 91(Supplement), S37–S49. [PubMed] [Google Scholar]
- Molina PE, Gardner JD, Souza-Smith FM, & Whitaker AM (2014). Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology, 29(3), 203–215. doi: 10.1152/physiol.00055.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzlin AI, Barlinn K, Illigens BM, Weidner K, Siepmann M, & Siepmann T (2017). Effect of short-term heart rate variability biofeedback on long-term abstinence in alcohol dependent patients - a one-year follow-up. BMC Psychiatry, 17(1), 325. doi: 10.1186/s12888-017-1480-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, & Maita AK (1994). Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development, 59(2–3), 167–186, 250–283. [PubMed] [Google Scholar]
- Price CJ, & Crowell SE (2016). Respiratory sinus arrhythmia as a potential measure in substance use treatment--outcome studies. Addiction, 111(4), 615–625. doi: 10.1111/add.13232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Petrakis I, & Altemus M (2019). Heart rate variability in alcohol use: A review. Pharmacology Biochemistry & Behavior, 176, 83–92. doi: 10.1016/j.pbb.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Rehm J, Gmel GE Sr., Gmel G, Hasan OSM, Imtiaz S, Popova S, . . . Shuper PA (2017). The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction, 112(6), 968–1001. doi: 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research: Brain Research Reviews, 18(3), 247–291. [DOI] [PubMed] [Google Scholar]
- Roerecke M, & Rehm J (2014a). Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Medicine, 12, 182. doi: 10.1186/s12916-014-0182-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, & Rehm J (2014b). Cause-specific mortality risk in alcohol use disorder treatment patients: a systematic review and meta-analysis. International Journal of Epidemiology, 43(3), 906–919. doi: 10.1093/ije/dyu018 [DOI] [PubMed] [Google Scholar]
- Schwarzinger M, Thiebaut SP, Baillot S, Mallet V, & Rehm J (2017). Alcohol use disorders and associated chronic disease - a national retrospective cohort study from France. BMC Public Health, 18(1), 43. doi: 10.1186/s12889-017-4587-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Cunningham JA, Sobell MB, Agrawal S, Gavin DR, Leo GI, & Singh KN (1996). Fostering self-change among problem drinkers: a proactive community intervention. Addictive Behaviors, 21(6), 817–833. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, & Eckberg DL (1998). Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation, 98(6), 547–555. [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. [DOI] [PubMed] [Google Scholar]
- Tiffany ST (1990). A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychology Review, 97(2), 147–168. [DOI] [PubMed] [Google Scholar]
- Udo T, Vasquez E, & Shaw BA (2015). A lifetime history of alcohol use disorder increases risk for chronic medical conditions after stable remission. Drug and Alcohol Dependence, 157, 68–74. doi: 10.1016/j.drugalcdep.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Buckman JF, Heiss S, Singh G, & Bates ME (2018). Early signs of cardiovascular dysregulation in young adult binge drinkers. Psychophysiology, 55(5), e13036. doi: 10.1111/psyp.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Buckman JF, Pandina RJ, & Bates ME (2012). Measurement of vascular tone and stroke volume baroreflex gain. Psychophysiology, 49(2), 193–197. doi: 10.1111/j.1469-8986.2011.01305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise F, Krell D, & Brinkhoff N (1986). Acute alcohol ingestion reduces heart rate variability. Drug and Alcohol Dependence, 17(1), 89–91. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, & Lindenmeyer J (2011). Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychological Science, 22(4), 490–497. [DOI] [PubMed] [Google Scholar]
- Windthorst P, Mazurak N, Kuske M, Hipp A, Giel KE, Enck P, . . . Teufel, M. (2017). Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: An exploratory pilot study. Journal of Psychosomatic Research, 93, 6–13. doi: 10.1016/j.jpsychores.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, & Donovan DM (2011). Moderating effects of a craving intervention on the relation between negative mood and heavy drinking following treatment for alcohol dependence. Journal of Consulting and Clinical Psychology, 79(1), 54–63. doi: 10.1037/a0022282 [DOI] [PMC free article] [PubMed] [Google Scholar]