Abstract
Electrochemical measurements of neurotransmitters provide insight into the dynamics of neurotransmission. In this review, we describe the development of electrochemical measurements of neurotransmitters and how they started with extrasynaptic measurements, but now are pushing to synaptic measurements. Traditionally, behavioral measurements with biosensors or fast-scan cyclic voltammetry have monitored extrasynaptic levels of neurotransmitters, such as dopamine, serotonin, adenosine, glutamate, and acetylcholine. Amperometry and electrochemical cytometry techniques have revealed mechanisms of exocytosis, suggesting partial release. Advances in nanoelectrodes now allow spatially resolved, electrochemical measurements in a synapse, which is only 20–100 nm wide. Synaptic measurements of dopamine and acetylcholine have been made. In this article, electrochemical measurements are also compared to optical imaging and mass spectrometry measurements, and while these other techniques provide enhanced spatial or chemical information, electrochemistry is best at monitoring real-time neurotransmission. Future challenges include combining electrochemistry with these other techniques, in order to facilitate multisite and multianalyte monitoring.
Keywords: voltammetry, amperometry, dopamine, microelectrode, nanoelectrodes, glutamate
1. INTRODUCTION
The brain is the most complex organ in the body, with the average human brain having 11.5 trillion neurons. These neurons communicate with each other with chemical neurotransmitters released into a synaptic cleft between two neurons. Synapses are intercellular junctions that connect the presynaptic terminals of axons with the postsynaptic dendrites of another neuron (Figure 1a) (1). Neurotransmitters are released into the synaptic cleft after an action potential causes the influx of Ca2+ ions through voltage-gated calcium ion channels and activates vesicular exocytosis. For direct transmission, the released neurotransmitters bind to corresponding receptors on the postsynaptic terminal to elicit a response. Receptors are also located on the presynaptic cell membrane that autoregulate the amount of neurotransmitter released. Neurotransmitters are cleared by uptake via transporters or diffusion (2). Given that the average neuron has 29,800 synapses (3), it is vital to understand chemical neurotransmission and to develop techniques that allow the study of these neurotransmitters.
Figure 1.
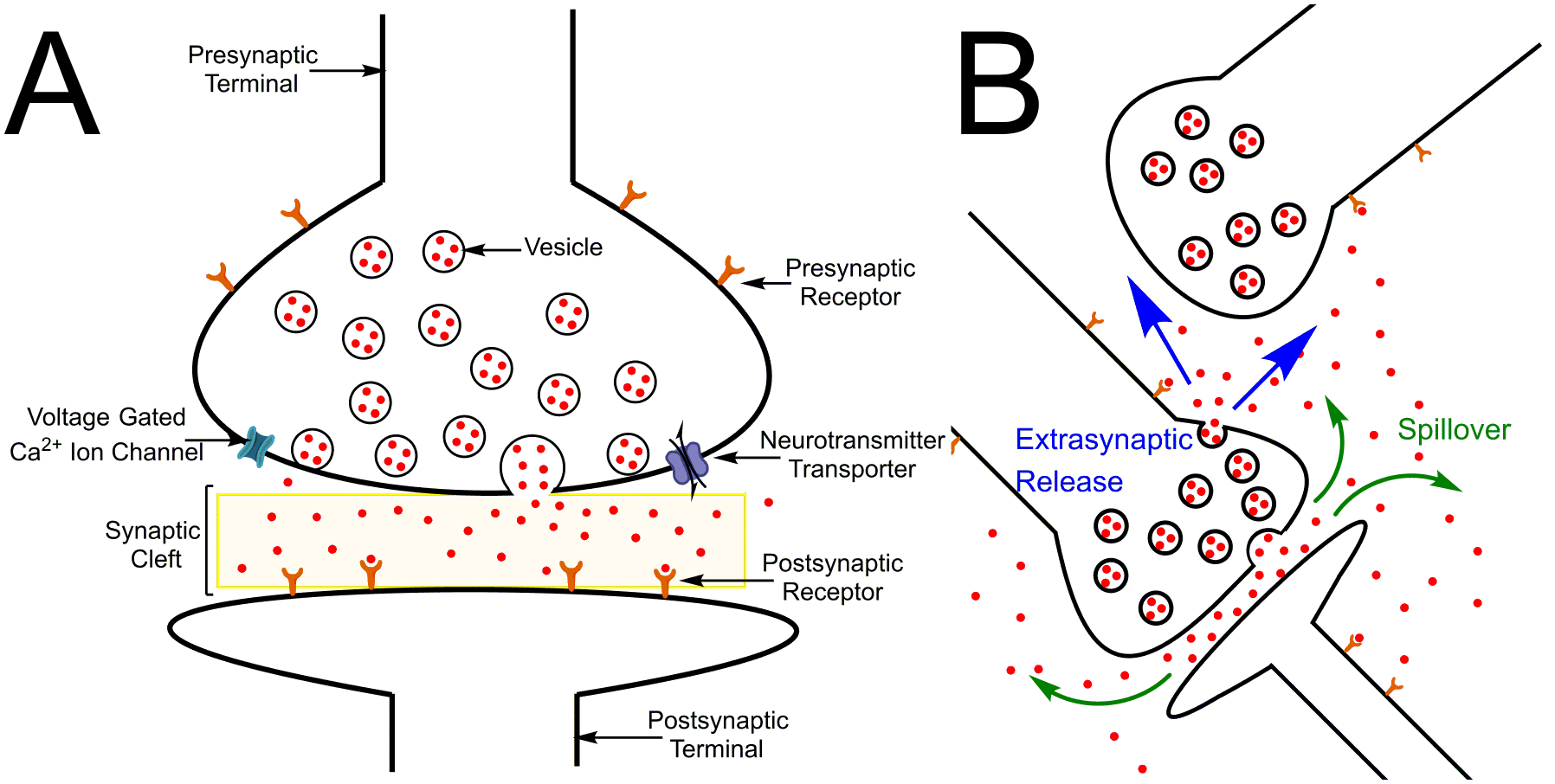
Schematics of neurotransmission. (a) Direct synaptic transmission. The neurotransmitter is released from the vesicle into the synaptic cleft and interacts with the receptor on the postsynaptic terminal. (b) Volume transmission. Two mechanisms are pictured: synaptic spillover (green) and extrasynaptic exocytosis (blue). Here, neurotransmitters diffuse and act at a more distant neuron not forming a synapse.
Direct transmission, however, is not the only method of chemical communication. In volume transmission, neurotransmitters can diffuse in the extracellular space and act at more distant neurons. There are two types of volume transmission: spillover, in which neurotransmitters escape the synaptic cleft/active zone, and extrasynaptic exocytosis, where the vesicle is released outside the synaptic active zone (Figure 1b) (4–6). Volume transmission is of particular importance with neurotransmitters such as dopamine or serotonin, and they are sometimes called neuromodulators because of their range of signaling.
In order to study chemical neurotransmission, a variety of tools and techniques have been developed. Microdialysis is a versatile, in vivo sampling technique that is useful for monitoring changes in neurochemical basal concentrations, but it is not small enough (typically with a probe diameter or 200 μm or more) to localize in a synapse (7). Microelectrodes are smaller, typically 7 μm, but the synaptic cleft is 20–100 nm, so they also mostly measure in the extracellular space. Electrochemistry does have the advantage that it is fast and can be used to monitor changes in real time. Recently, advances in nanoelectrode fabrication have facilitated manufacturing of smaller electrodes that are small enough to measure in a single synapse. These electrodes have been used to measure in artificial synapses (8) and a single synapse in neuron cultures (9).
In this review, we trace the development of electrochemical methods to measure both extrasynaptic and synaptic neurotransmission. Electrochemical measurements of extracellular levels are discussed, with a focus on how they correlate with behavior. Mechanisms and modes of exocytosis as well as how they provide insight into quantal release are also discussed. The first attempts at measuring neurotransmitters in a single synapse are highlighted, as this is an emerging field. Finally, we compare electrochemical measurements at the synapse to information gained from other techniques and discuss future challenges for developing more robust electrochemical measurements at the synapse.
2. EXTRASYNAPTIC NEUROTRANSMITTER MEASUREMENTS
Electrochemical measurements of neurotransmitters have traditionally measured extrasynaptic volume transmission, providing valuable information linking neuronal communication to behavior and pathology. Fast-scan cyclic voltammetry (FSCV) and amperometry are popular approaches to monitor real-time fluctuations of neurotransmitters because they quantitate electroactive species (10). With amperometry, a constant voltage is applied, but there is limited chemical information. FSCV is a rapid voltage scanning technique that provides a cyclic voltammogram to identify the detected analytes. Amperometry is also used in biosensors, which rely on enzyme cascades that produce an electroactive product such as hydrogen peroxide. Biosensors are useful because they can detect nonelectroactive analytes. For direct electrochemistry, carbon-fiber microelectrodes (CFMEs) are used because of their small size, biocompatibility, and good electrochemical properties, especially for catecholamines. This section outlines some of the important findings of electrochemical sensors in measuring extracellular neurotransmitters and how those measurements contribute to a new understanding of chemical signaling underlying behavior and disease.
2.1. Dopamine Measurements
Dopamine plays an important role in various aspects of neurological functions governing reward, motivation, reinforcement, and locomotion. Dopamine is the most extensively studied neurotransmitter with FSCV, partly because of its biological importance and partly because of its excellent electrochemistry. At CFMEs, dopamine adsorbs to oxide groups due to the positively charged amine group, which enhances sensitivity (11). Many studies focused on waveform improvements for dopamine, either extending the anodic limit (12) or increasing the scan rate (13) to improve the signal sensitivity. Other studies focused on improving the electrode, by adding carbon nanomaterials, polymer coatings, or making arrays (10, 14, 15). This section focuses on biological findings from measuring extrasynaptic dopamine.
In vivo FSCV studies have elucidated the fundamental mechanisms of dopamine regulation. For example, regional heterogeneity of dopamine release and uptake in the brain has been explored, finding differences in release and uptake within the compartments of striatum, nucleus accumbens (NAc), and olfactory tubercle (16, 17). An interesting recent study implanted two CFMEs to demonstrate that dopamine transients are perfectly synchronized in the NAc on both sides of the brain (18). Optogenetic studies used viral vectors to express light-activated channels in discrete neuronal populations and monitor their importance in dopamine regulation (19).
In vivo electrochemistry has also been critical to reveal the function of drugs of abuse to modify dopamine signaling. Psychostimulants such as cocaine and amphetamine impaired dopamine neurotransmission by altering dopamine transporters and vesicular transporters (20), increasing neuronal firing rates (21), or mobilizing the dopamine reserve pool of synaptic vesicles (22). Many behavioral experiments used self-administration to allow animals to push a lever and receive an injection of a drug of abuse. Self-administration of cocaine for five days caused tolerance, and evoked dopamine was lower in these cocaine-treated animals than in naïve animals (23). However, after one month of abstinence from cocaine, evoked dopamine release increased with cocaine (24). Amphetamine also induced dopamine transients in the NAc, and amphetamine-induced transients were abolished by inhibiting the cannabinoid type 1 receptor (21). With the current opioid crisis, attention is now shifting to the effects of opioids on dopamine dynamics. Oxycodone increased the frequency and concentration of dopamine release as high as 500 nM in the NAc shell (25). A single exposure to morphine, followed by naloxone-induced withdrawal, significantly decreased the frequency and concentration of spontaneous dopamine transients in the NAc, whereas no difference was observed in evoked release (26). These experiments provide an understanding of the adaptation of dopamine signaling to drugs of abuse, which is vital to developing effective treatments.
Dopamine has also been measured in other behaviors, such as intracranial self-stimulation (ICSS), where an animal presses a lever to electrically stimulate its dopamine neurons (27). Early studies using the traditional dopamine waveform found that phasic dopamine release disappeared during the continuous ICSS. Newer studies with an improved waveform coupled with principal component regression reevaluated the original finding and showed that while phasic dopamine release is not required to maintain ICSS behavior, small amounts of dopamine were still released (28). Numerous studies measured cue-evoked dopamine release in response to natural rewards or alcohol, using an audio or visual cue to predict the reward. Dopamine release upon food reward was observed in the NAc (29) and dorsolateral and dorsomedial striatum (30), and the magnitude and duration of cue-evoked transients in the NAc shell were larger than those in the NAc core (17). Animals were trained to choose either a larger reward with longer delay interval to the reward or a small reward with a shorter delay; dopamine was larger when the reward was greater (31). In alcohol studies, animals with a history of chronic alcohol exposure increased dopamine release after a reward-predictive cue (32). The Phillips group (33) has introduced chronic CFMEs encased in fused-silica capillaries (Figure 2a) that measured evoked dopamine release for up to four months. Dopamine increased upon food reward delivery (Figure 2b). Behaviorally evoked dopamine transients measured throughout Pavlovian conditioning showed elevated dopamine during cue- and reward-evoked transients during the early sessions (34).
Figure 2.
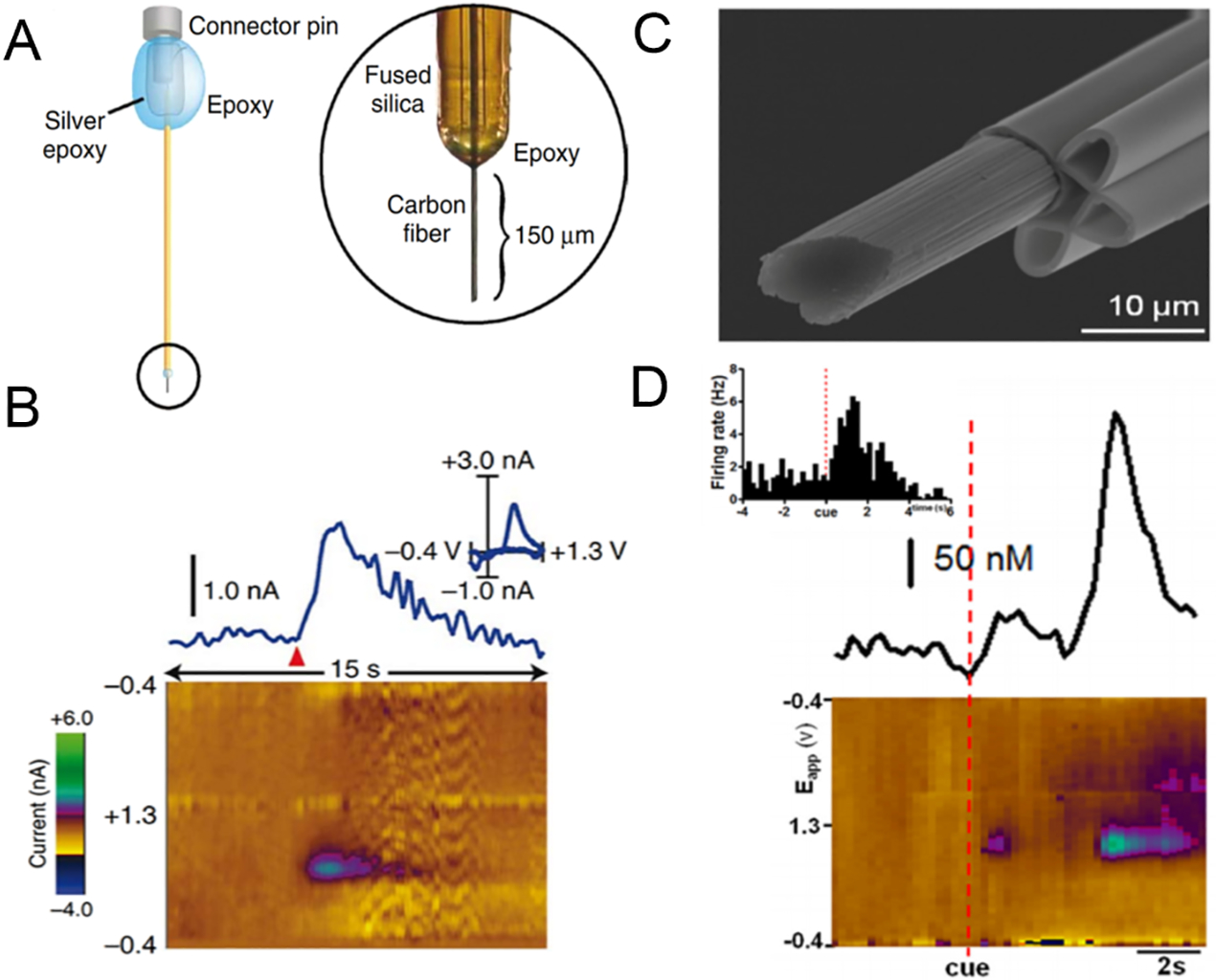
Chronic and multimodal carbon-fiber microelectrodes. A schematic of (a) the chronic microelectrode, which is made of a carbon fiber encased in a polyimide-fused silica. (b) FSCV data of food-evoked dopamine release measured at the chronic microelectrode. Panels a, b adapted with permission from Reference 33. Copyright 2010, Springer Nature. (c) Multimodal microelectrode with iontophoresis barrels. (d) FSCV data and histogram of firing rate (insert) of cue-evoked dopamine transient during ICSS. Panels c, d adapted with permission from Reference 37. Copyright 2016, CCC Republication. Abbreviations: FSCV, fast-scan cyclic voltammetry; ICSS, intracranial self-stimulation.
Electrochemical measurements of dopamine in vivo were further expanded by introducing multimodal recording. Combining FSCV and electrophysiology permitted simultaneous measurements of dopamine release and neuronal firing rates, allowing the correlation of dopamine release and subsequent firing (35). Additionally, an iontophoresis barrel was incorporated to precisely deliver drugs to the brain and simultaneously measure neuronal firing (Figure 2c,d) (36, 37). Dopamine receptor antagonists were delivered prior to the cue. The cue-mediated dopamine response was due to the activation of D2 receptors, whereas after lever press, dopamine was modulated by both D2 and D1 receptors (37).
2.2. Norepinephrine Measurements
Norepinephrine is another important neurotransmitter modulating stress, sleep, and learning (38). However, norepinephrine measurements in vivo are challenging because its cyclic voltammogram is nearly identical to dopamine (39), norepinephrine projections are widely distributed but not particularly dense (40), and the predominant region for norepinephrine measurements, the ventral bed nucleus of the stria terminalis (vBNST), is only a couple hundred microns across (41). The signal must be validated using pharmacology and histology to confirm it is norepinephrine. Compared to dopamine, the release and uptake of norepinephrine is much slower (41). A noxious stimulation, obtained by pinching the animal’s tail, increased norepinephrine release in the vBSNT and inhibited dopamine release in NAc shell (42). Morphine increased dopamine release in the NAc but had no effect on norepinephrine release in the vBSNT (26). In contrast, a significant decrease in dopamine release was observed during morphine withdrawal, while norepinephrine release increased, proving that norepinephrine is associated with drug exposure and withdrawal (26).
2.3. Serotonin Measurements
Serotonin is a neurotransmitter implicated in sleep, cognition, and mood. FSCV measurements of serotonin are challenging because of the formation of oxidative byproducts that foul the CFME surface, both for serotonin and its metabolite 5-HIAA (43). Therefore, a modified waveform and Nafion coating are used to repel interfering species and to stop fouling (44). For in vivo FSCV experiments, serotonin is measured in the substantia nigra reticulata (SNR) because it has dense serotonin terminals and few other electroactive neurotransmitters. The concentration of stimulated serotonin in the SNR is relatively small (~100 nM), although serotonin tissue content is similar to dopamine (45). The low stimulated release is due to negative feedback of autoreceptors (46) and corelease of inhibitory neurotransmitters (47). Moreover, serotonin regulation is more tightly controlled by uptake and metabolism (45). The serotonin transporter exhibits a high affinity for serotonin, and serotonin is also cleared by other transporters, such as dopamine, norepinephrine, and organic cation transporters (46). Serotonin release in vivo was not stimulation frequency dependent like other neurotransmitters (48). Hashemi’s group (49) simultaneously measured histamine and serotonin and showed that a rapid inhibition of serotonin occurs upon histamine release in the hypothalamus.
FSCV is a differential technique because it is background subtracted and thus measures fast concentration changes. To measure ambient neurotransmitter levels, Heien and Hashemi’s group (50, 51) developed fast-scan controlled-adsorption voltammetry (FSCAV), which employs an analyte accumulation time at a negative potential, followed by a scan at fast rates to measure the concentration that adsorbed to the electrode. This approach was first used to estimate ambient levels of dopamine at 90 nM (51) and has recently been extended to show that the ambient levels of serotonin in the hippocampus are 65 nM (52). FSCAV and FSCV combined would allow the correlation of ambient and fast changes in serotonin, which would provide valuable information about serotonin regulation.
2.4. Adenosine Measurements
Adenosine, a product of ATP degradation, is a purine signaling molecule that modulates cerebral blood flow and other neurotransmitters in the brain. The initial adenosine FSCV measurements were made in slices of the lamina II of the mouse spinal cord (53), and in vivo measurements of adenosine have been pioneered by the Venton group. Adenosine FSCV uses a triangular waveform with an extended anodic limit to oxidize adenosine (54), and two characteristic oxidation peaks are detected (55). A modified waveform with an anodic scan can be applied for the simultaneous measurement of transient adenosine release and oxygen (56). ATP is a potential interferent, but the negative holding potential makes the electrode more sensitive to adenosine (55); alternatively, Nafion can be used to exclude ATP (57). Hydrogen peroxide has an oxidation potential similar to adenosine but has no secondary oxidation peak so it can be differentiated (58).
Early in vivo studies characterized adenosine release evoked by electrical (59) and mechanical stimulations (60). However, spontaneous, unstimulated adenosine transients were discovered that occur every 2–3 min in the brain (54). These transient adenosine release events lasted only a few seconds, and their frequency was modulated by A1 receptors. Adenosine was also controlled by NMDA and GABAB receptors (61). The clearance was regulated by the equilibrative nucleoside transporters and multiple adenosine metabolic enzymes (62). Transient adenosine release modulated phasic dopamine release, showing that it can quickly modulate neurotransmission (63). Approximately one-third of adenosine transients were followed by a short change in oxygen (56), suggesting rapid adenosine modulated blood flow on a rapid time scale. Spontaneous adenosine transients increased during ischemia and reperfusion; thus, they may be a rapid, local neuroprotective signal during brain injury (64).
2.5. Glutamate Measurements
Biosensors have been made for some nonelectroactive neurotransmitters to measure extrasynaptic levels. Glutamate biosensors have been fabricated mostly from Pt electrodes that have a hydrogel or polymer that encases glutamate oxidase, which reacts with glutamate to form hydrogen peroxide (65). Adding ceria and titania nanoparticles to the sensor facilitates measurements of glutamate under low oxygen conditions (66). Silicon wafer–based arrays have also been made and used to measure cortically evoked glutamate in the striatum (67). Glutamate was measured during Pavlovian fear conditioning and was transiently elevated around reward seeking in the basolateral amygdala (68). Glutamate also rose during exposure to repeated cigarette smoke and was highly correlated with psychomotor symptoms (69). Commercial glutamate biosensors are available, but they are not as popular as CFMEs for dopamine, so there are many future opportunities to explore glutamate dynamics.
2.6. Acetylcholine Measurements
Biosensors are also commonly used to measure acetylcholine in the extrasynaptic space. Acetylcholine is the main neurotransmitter at the neuromuscular junction and is also an important biomarker in neurological disease, such as Alzheimer’s. Acetylcholine is rapidly degraded to choline in the brain, and many biosensors rely on detection of choline using choline oxidase. Alternatively, acetylcholinesterase and choline oxidase can be coimmobilized. Although most biosensors detect hydrogen peroxide with amperometry, a recent biosensor has been developed to use FSCV detection; this biosensor was demonstrated to detect stimulated acetylcholine release in striatal brain slices (70). In addition, a self-powered biosensor, containing a fuel cell, was used to measure acetylcholine in plasma (71). Acetylcholine biosensors have determined that a deletion in the choline transporter gene affects acetylcholine release and attention, which could be a cause of attention deficit hyperactivity disorder (72). Using chronically implanted acetylcholine biosensors, acetylcholine signaling was measured over several time domains, and tonic levels were elevated during training for a memory task and rapid eye movement (REM) sleep (73). In contrast, phasic bursts of acetylcholine occurred during the memory task in reward delivery areas.
2.7. Conclusions
Extrasynaptic measurements of neurotransmitters have provided information on how neurotransmitters are regulated during behavior. Although the electrodes are typically larger than the synapse, they are smaller than cell bodies and cause minimal tissue damage (7). Electrodes can be chronically implanted for long-term measurements, over weeks to months, providing longitudinal data on how neurotransmitters change with age or treatments. However, behavioral experiments with electrochemistry are not on the nanoscale and thus do not report on synaptic neurotransmission.
3. SINGLE-CELL MEASUREMENTS
Vesicular exocytosis involves docking, activation, and fusion of vesicles at the active zone of the presynapse. Vesicles are normally small, with a diameter of 50–800 nm and contain a limited amount of neurotransmitters (74). Single-vesicle exocytotic events are fast, occurring on the millisecond timescale. Therefore, measurements require high sensitivity, spatial resolution, and temporal resolution. Amperometry with CFMEs facilitates quantitation of neurotransmitter release at single cells and single vesicles. Chemical cytometry reveals the neurotransmitter content of vesicles. These techniques are pushing electrochemical analysis to the single-cell and -neuron level.
3.1. Amperometry to Measure Modes of Exocytosis
Amperometry has high sensitivity, allowing quantitation of neurotransmitters released from individual vesicles, and high temporal resolution, providing information of the release kinetics of exocytosis. Wightman’s group (75) introduced single-cell amperometry in the 1990s, measuring exocytosis from chromaffin cells. A CFME is placed in close proximity to a cell, a constant potential is applied to oxidize electroactive neurotransmitters, and neurotransmitters are quantitated from the current spikes using Faraday’s law. The current spike rise time corresponds to the opening of the fusion pore and half width of spike represents the duration of the exocytotic event. Pre-spike feet, where most current spikes are preceded by a small foot signal, were observed, indicating a slow leakage of neurotransmitter after formation of the fusion pore (76).
Single-cell amperometry has been used to identify different modes of exocytosis. The mechanism of exocytosis has been traditionally considered an all-or-nothing process, otherwise known as full release. The vesicular membrane would fully distend into the plasma membrane, resulting in an irreversible pore opening and full release. However, modes of partial release have been identified using amperometry. In the kiss-and run-mechanism, a small fusion pore is transiently formed, which partially releases its contents and closes (77). The vesicle reloads and is used again. Using modeling and statistical analysis, researchers estimated the maximum aperture angle of the fusion pore to be less than ten degrees during exocytosis; therefore, the full fusion of a vesicle is highly unlikely (78). The fusion pore can open and close multiple times, which is known as flickering (79). Flickering fusion pores caused complex amperometric spikes with multiple rising and falling phases as well as longer durations. Each flicker of the fusion pore corresponded to an approximately 25–30% release of dopamine in small synaptic vesicles. A third mechanism of exocytosis was recently proposed by the Ewing group: open and closed (77); a detailed review of this was recently published (74). Open and closed is different from kiss-and-run because the fusion pore is wider and the duration of pore opening is longer. Thus, a large fraction is released, but the fusion pore closes again before the vesicle fully empties its content (77). Open and closed mechanism spikes had a post-spike feet feature, where a stable plateau formed during the decay of amperometric spikes from exocytosis, indicating that the vesicle closed after partial release of its content (77). This partial release mechanism may be the primary mechanism during regular exocytosis.
3.2. Quantification of Neurotransmitter Content in Single Vesicles
To understand if the amount of release measured during amperometry is full release, information is needed about the quantity of neurotransmitters stored in a vesicle. Electrochemical cytometry measures the neurotransmitter content of a single vesicle by integration of capillary electrophoresis, microfluidics, and electrochemistry (80). Individual vesicles were isolated, and their membranes lysed and content directly detected by cylindrical carbon-fiber microelectrodes. On average, there are 33,000 dopamine molecules per vesicle (81), meaning that exocytosis releases only about 40% of neurotransmitter content, which supports the theories of partial release (82).
Vesicle impact electrochemical cytometry (VIEC) was later developed as a simplified method to quantify the content in a single vesicle (83). Vesicles were adsorbed to a disk-shaped carbon electrode and ruptured by electroporation, resulting in a pore opening on the vesicle membrane and subsequent release of neurotransmitter. The vesicle opening is slowed by proteins on the vesicle surface (84) and is also temperature and size dependent (85). Fluorescent dyes useful for visualizing the pore opening also increase the number of amperometric events by producing reactive oxygen species during excitation (86). Intracellular vesicle impact electrochemical cytometry (IVIEC) was recently introduced to quantify the neurotransmitter content of vesicles intracellularly (87). A conical carbon-fiber microelectrode was flame etched to obtain a sharp tip with 50–100 nm diameter, which penetrated the cell membrane into the cytoplasm of live cells with minimum damage. Similar to the VIEC method, the intracellular vesicles adsorbed on the electrode surface and ruptured by electroporation to release their neurotransmitter content. In IVIEC, the collection efficiencies of nanotip conical electrodes was dependent on the position of vesicle release pore, but 75–100% of content was captured (88). Although electrochemical cytometry determines vesicle content, the method cannot detect endocytic processes, where vesicles are retrieved after partial release.
3.3. Factors Affecting Exocytosis
Analysis of exocytotic processes by single-cell amperometry has revealed several key proteins that participate in the regulation of exocytosis. For example, dynamin, a GTPase that regulates membrane remodeling during endocytosis, controls the duration and kinetics of exocytotic release (89). Blocking the activity of dynamin by dynasore inhibited exocytosis in a dose-dependent manner, leading to smaller and shorter amperometric spikes. Glycocalyx, a biopolymer that covers the cell outer membrane, decreased the release kinetics of neurotransmitters during exocytosis (90). Actin also affected the fraction of neurotransmitters released during open and closed exocytosis by regulating the closing dynamics of the pore (91). In addition, cholesterol modulated the rigidity of the plasma membrane, as the addition of cholesterol on a giant unilamellar liposome decelerated the opening of the fusion pore (92).
Pharmacological treatment and changes in ions also affect exocytosis. For example, curcuminoids affect learning and memory of aged animals or animals with Alzheimer’s disease. Single-cell amperometry showed that long-term treatment with curcumin accelerated the event dynamics, and another curcuminoid demethoxycurcumin decreased the amount of release from a single event (93). Barbiturates decrease the fraction of neurotransmitter release during exocytosis in PC12 cells (94). Although IVIEC showed that the vesicular content was unaffected by barbiturates, unstable fusion pores were observed that shortened the duration of amperometric pre-spike feet. Furthermore, treatment with a local anesthetic, lidocaine, led to a longer duration of pore opening at high dose, but less release than controls (95). In contrast to lidocaine, treatment with 100 μM of zinc did not change the amount of catecholamine release during exocytosis but decreased the vesicular content (96).
3.4. Development of Electrodes for Single-Cell Measurements
Whereas all the previous studies were performed with CFMEs, new methods for detection are expanding the analytes that can be detected with single-cell amperometry. For example, the Huang group (97) developed an enzymatic biosensor to monitor glutamate release during exocytosis from single hippocampal varicosities. A platinized disk CFME was fabricated by immobilizing glutamate oxidase, and hydrogen peroxide was detected. This study was performed on varicosities in cultured hippocampal cells. A novel core–shell nanowire electrode was designed for detection of reactive oxygen species and reactive nitrogen species in living cells (98). The electrode consisted of an SiC nanowire covered with a thin carbon layer and a nanopipette filled with liquid metal. The electrode was super flexible and easily inserted into living phagolysosomes of macrophages with minimal damage. Recently, the Chen group (99) used a nanocapillary with an electrochemical detector to quantify the activity of β-glucosidase, which is related to Gaucher’s disease, in individual lysosomes of single living cells. The nanocapillary with a thin Pt layer was used to sort fluorescently labeled liposomes from living cells into the capillary by electrophoresis, where an enzymatic reaction generated hydrogen peroxide that was proportional to the activity of β-glucosidase. This method revealed that the activity of β-glucosidase was homogeneous in the same cell but heterogeneous between cells.
3.5. Conclusions
Single-cell amperometry and electrochemical cytometry provide an overview of the mechanism of catecholamine release during exocytosis and the amount of catecholamine stored in individual vesicles. The combination of these two methods provides fundamental information regarding the extent of full release and different factors that influence the neurotransmitter storage and release. This knowledge could be applied to development of selective and safe drugs that target exocytosis. However, these techniques are limited in providing chemical information on species identity and, generally, only electroactive molecules are detected. In addition, most studies of exocytotic release of neurotransmitters are performed in cultured cells and not in synapses, so the feedback regulation between pre- and postsynaptic active site zones is not present. However, these methods are useful for extension to single synapses.
4. ELECTROCHEMICAL MEASUREMENTS AT THE SYNAPSE
Electrochemical measurements at a single synapse are challenging because of the small synaptic cleft (20–100 nm). Early attempts to circumvent the size problem involved making measurements at artificial synapses using liposomes (8). These models are good for examining the molecular machinery needed for exocytosis and the modes of exocytosis (100). Recent advances in the development of nanoelectrodes have produced electrodes that have tip diameters less than 100 nm. These nanoelectrodes are inserted into the synaptic cleft to measure exocytosis of neurotransmitter release. In addition, important nonelectroactive neurotransmitters, such as acetylcholine, are also detected. Thus, electrochemistry is now pushing toward the synaptic length scale.
4.1. Measurements of Electroactive Neurotransmitters in a Synapse
The Huang and Amatore groups (101) introduced carbon-fiber nanoelectrodes to directly measure chemical neurotransmission inside individual synapses in real time. The CFME was flame etched to produce a nanotip with a 50–200-nm tip diameter, then etched by a microforge to form a needle-shaped electrode with a radius less than 100 nm and shaft length less than 1 μm (Figure 3a). Single events and complex events were detected by amperometric monitoring of exocytosis inside the synapse of a neuromuscular junction, demonstrating that different types of vesicle fusion coexist inside a single synapse. Simultaneous detection of exocytosis inside a synapse and on top of the corresponding synapse revealed a nonuniform distribution of active release zones at the synapses, as the presynaptic membrane that faced the synaptic cleft had more active hot spots and produced larger numbers of complex events. In addition, they modeled assembled groups of oriented neural networks of superior cervical ganglion neurons (SCGs) and their effector smooth muscle cells (SMCs) in a microfluidic device (Figure 3b–d) (101). This model allows amperometric detection of vesicular release inside SCG–SMC synapses with carbon-fiber nanoelectrodes and measurement of postsynaptic membrane potential with glass nanopipette electrodes.
Figure 3.
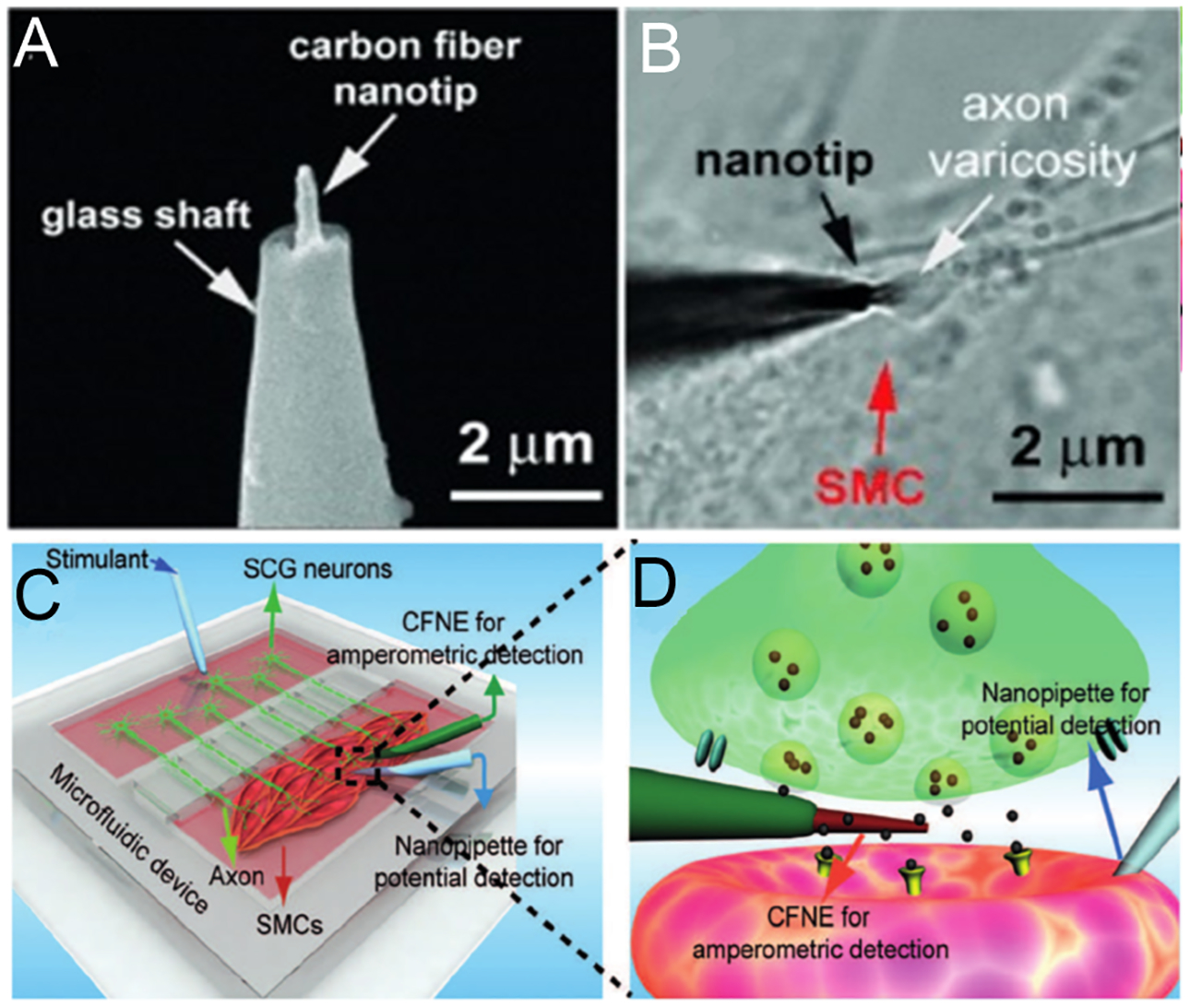
Nanoelectrode amperometric monitoring of neurotransmitter release inside a SCG–SMC synapse. (a) SEM of a carbon-fiber nanoelectrode. (b) Brightfield image of the nanoelectrode inserted inside a synapse between a varicosity of an SCG neuron and an SMC. (c) Schematics of in vivo-like neuromuscular junction in a microfluidic device. (d) Schematics of a CFNE inside a synapse and a glass nanopipette inside an SMC. Figure adapted with permission from Reference 101. Copyright 2015, John Wiley and Sons. Abbreviations: CFNE, carbon-fiber nanoelectrode; SCG, superior cervical ganglion; SEM, scanning electron microelectrode; SMC, smooth muscle cell.
4.2. ITIES Electrodes for Nonelectroactive Species
The Shen group (102) developed a new type of nanopipette electrode to directly detect nonelectroactive neurotransmitters without electrode modification. The pipette electrode is based on ion transfer across an interface between two immiscible electrolyte solutions (ITIES). A laser-pulled pipette with nanometer tip size was filled with an organic solution and immersed into biological media, where detection of neurotransmitters relied on potential driven ion transfer across the liquid–liquid interface. Ionophores are often added to achieve selective detection of specific analytes. Nonelectroactive acetylcholine and GABA as well as electroactive tryptamine and serotonin were successfully detected in vitro (102, 103).
Recently, acetylcholine exocytosis from a single Aplysia californica neuronal soma was studied by combining nanoITIES electrodes and scanning electrochemical microscopy (SECM) (104). SECM offers nanometer resolution by facilitating positioning of ITIES electrodes close to the release site on the soma. Acetylcholine release during exocytosis at a single synaptic cleft of Aplysia pedal ganglion neurons was studied by nanoITIES electrodes with a diameter of 30 nm (105). The concentration of acetylcholine around the synapse was up to 2.4 mM. Singlet, doublet, and multiplet amperometric spikes were observed, where multiple spikes may mean multiple vesicles released or a flickering of the fusion pore.
4.3. Conclusions
Carbon-fiber nanoelectrodes and nanoITIES electrodes facilitate the detection of neurotransmission inside the synapse. The study of exocytosis in a single synapse using carbon-fiber nanoelectrodes reveals heterogeneity of release mechanism. The application of nanoITIES enables the detection of electroactive and nonelectroactive molecules, broadening the range of analytes. Future research will continue to expand the type of molecules that can be detected using nanoITIES and refine the use of SECM to position the electrode in a synapse. In addition, simultaneous monitoring of multiple analytes at multiple sites would provide more information about the neurotransmitter dynamics in a synapse.
5. ELECTROCHEMISTRY IN COMPARISON TO OTHER TECHNIQUES
5.1. Optical Imaging Techniques
Imaging techniques have always been at the forefront of neuroscience, but they have traditionally imaged labeled cells or proteins on cells and not neurotransmitters. Recent advances in imaging are pushing it into the mode of measuring actual neurotransmitters, and high-resolution imaging can reach the synaptic spatial scale. Early efforts to image neurotransmission involved fluorescent false neurotransmitters (FFNs) released from presynaptic terminals (106). With FFNs that mimic dopamine, heterogeneity in release was observed that was controlled in part by D2 receptors. Recently, FFNs were developed that can measure norepinephrine distinct from dopamine (107). Methods to measure actual neurotransmitters are also being developed. The Glass lab (108) developed small organic molecules that can diffuse into synapses and are turn-on fluorescent in response to neurotransmitters, such as serotonin. The Landry group (109) recently developed near infrared (IR) catecholamine sensors (nIRCats) based on single-walled carbon nanotubes that have DNA attached to bind catecholamines and turn-on fluorescence. These nIRCats also diffuse into the synapse, and dopamine has been detected from active release sites that are approximately 2 μm wide. The molecular probes are good for studies in brain slices, because many require the probe to be added to the extracellular space, but in vivo imaging is more difficult.
A new approach to imaging molecules in the brain is to use viral vectors to express genetically encoded G protein–coupled receptors (GPCRs) that have been engineered to become fluorescent when bound to the molecule of interest (Figure 4). Tian’s group (110) developed genetically encoded sensors for dopamine, and these GPCRs on the cell surface lit up when dopamine was present. They used these sensors not only in brain slices, but also in vivo, with fiber photometry imaging in behavioral experiments. A similar strategy was used by Li’s group (111), where genetically encoded dopamine sensors were applied to measurements in Drosophila and zebrafish. Dopamine release measured with these genetically encoded sensors is approximately 10–30 μM at the release site. The concentration is larger than measured with electrochemical sensors in the extrasynaptic space, but the overall time course is similar. The strategy of modifying receptors to make them fluorescent is general and can be applied to a variety of neurotransmitters, including acetylcholine (112). Future work to integrate electrochemical and imaging techniques would be useful to understand the interplay between synaptic levels of neurotransmitters and the extrasynaptic levels of volume transmission.
Figure 4.
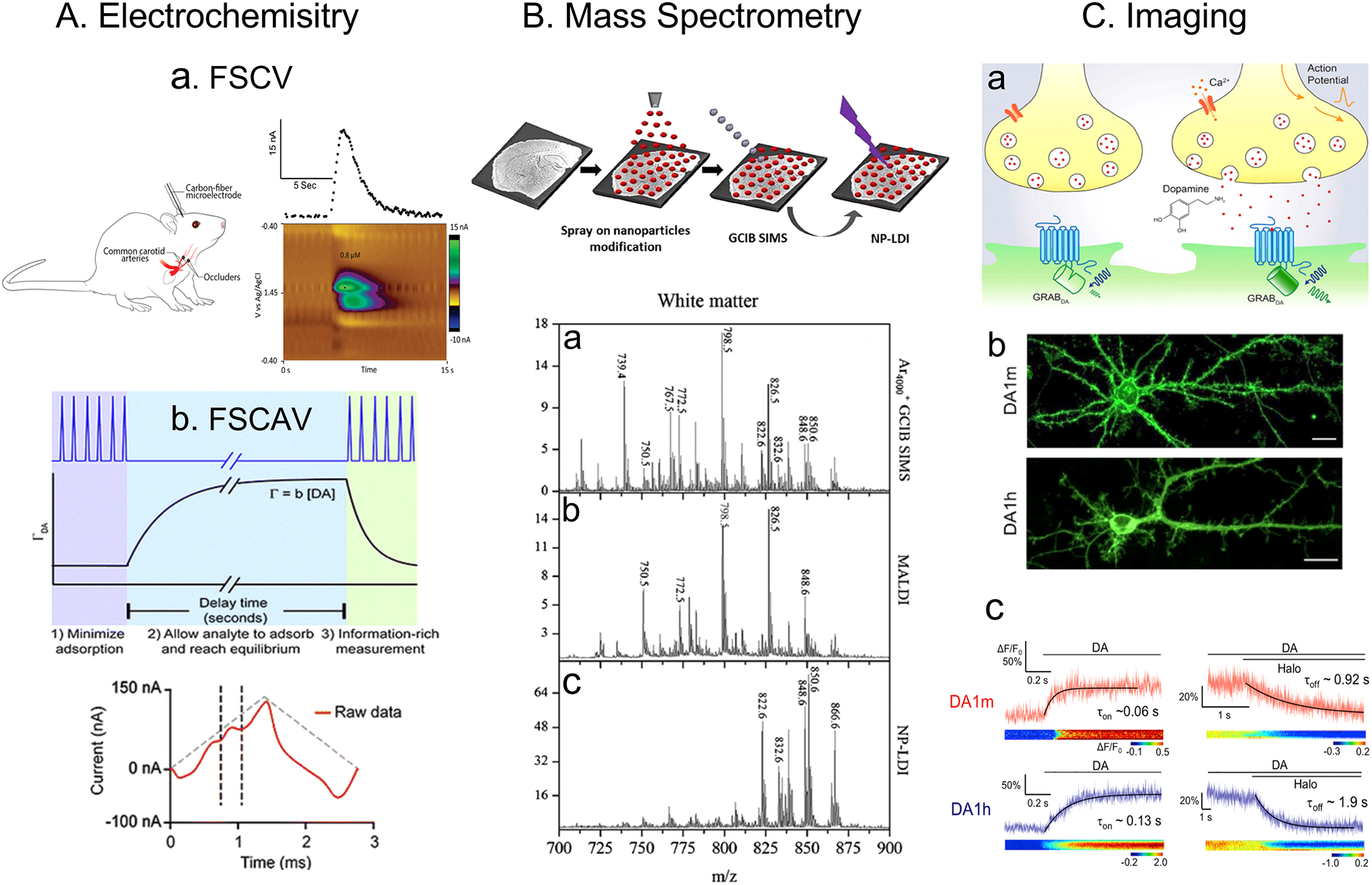
Comparisons of electrochemistry to other techniques. A. Electrochemistry. a. FSCV is typically used in vivo to measure neurochemical dynamics, such as measurements here of adenosine during ischemia. This method has 100 millisecond response times and the color plots allow different molecules to be distinguished. Figure adapted with permission from Reference 64. Copyright 2018, the authors (Venton group). b. FSCAV is used for basal measurements, and the waveform is turned off for 10 s to allow DA to adsorb to the electrode. The CV of the first few scans after the waveform is resumed give information about the ambient levels. This method has ~10 s time resolution and can distinguish between molecules with the voltammogram. Figure adapted with permission from Reference 51. Copyright 2016. Royal Society of Chemistry. B. Mass spectrometry. Mass spectrometry experiments are typically performed in brain slices, not in vivo, and require surface preparation, such as nanoparticles. Here, three types of mass spectrometry are compared a. gas cluster ion beam secondary ion mass spectrometry (GCIB SIMS), b. matrix-assisted laser desorption ionization (MALDI) and c. nanoparticle laser desorption ionization (NP-LDI) and they give different lipid profiles. Mass spectrometry data is rich in chemical information but there is no data for changes over time. Figure adapted with permission from Reference 118. Copyright 2016, Springer Nature. C. Imaging. a. GPCR-based sensors are expressed in different neurons and b. fluoresce when the target molecule is present. Here, DA1m fluoresces in the red and DA1h fluoresces blue, although the camera is not in color, so all fluorescence appears green. c. The time response of these sensors is fast, on the order of 100 ms for the rise time and 1–2 s for the decay time. These sensors are specific to the target molecule but pharmacology experiments are more difficult than with electrochemistry. Reference adapted with permission from Reference 111. Copyright 2018, Elsevier.
5.2. Mass Spectrometry Techniques
Mass spectrometry techniques identify molecules by their molecular mass, which is a unique advantage over other techniques. Modern mass spectrometry is mainly focused on proteomics, identifying the proteins and posttranslational modifications, and proteomics is now being taken to the synapse level. Heintz’s group (113) used genetically engineered mice, affinity purification, and mass spectrometry to profile 60 postsynaptic proteins at a specific type of synapse. Mass spectrometry was also useful for understanding differences in presynaptic proteins that regulate exocytosis and how they lead to diseases, such as Alzheimer’s (114). Quantitative proteomics is tackling questions about how dynamic changes in proteins at the synapse (115) change learning and memory (116).
The previous proteomic studies investigated harvested, processed tissue, but another focus of mass spectrometry is tissue imaging, which can be used for proteins, lipids, fatty acids, and small molecules. Ewing’s group combined nanoscale secondary ion mass spectrometry (nanoSIMS) with transmission electron microscopy to map the spatial distribution of dopamine in vesicles (117). These results had nanometer-scale resolution to pinpoint where dopamine was synthesized and how it was loaded into vesicles. Lipid imaging is also very significant, as changes in lipid membranes affect exocytosis and lead to different diseases, including Alzheimer’s, Parkinson, and multiple sclerosis. Ewing’s group (118) developed different mass spectrometry techniques to measure lipids in tissue, including nanoparticle-assisted laser desorption ionization mass spectrometry, matrix-assisted laser desorption ionization (MALDI), and gas cluster ion beam secondary ion mass spectrometry (Figure 4). Each ionization technique gave a unique lipid profile and the combination of these techniques provided rich information about the lipid profile of different areas. Recent work has shown that an anticancer drug, cisplatin, affects exocytosis by changing brain lipids (119). Sweedler’s group (120) also developed MALDI methods to deposit matrix on brain slices and examine fatty acid levels.
5.3. Summary of Comparisons
Electrochemistry has distinct advantages compared to optical imaging and mass spectrometry. Imaging requires an optical window or an implanted optrode to measure in vivo, which either limits the depth of tissue that can be measured or creates more tissue damage than a small electrode. On the other hand, imaging can be used to look across tissue at many sites at once, which is more difficult to do electrochemically. Mass spectrometry has not been amenable to in vivo measurement and has been primarily used for tissue studies. Spatial resolution is better for electrochemistry than most of the mass spectrometry imaging studies. However, mass spectrometry provides the mass for chemical identification and can distinguish many chemicals in a mixture. Overall, each technique has unique applications where it is best, and different techniques provide complementary chemical information about the synapse. Electrochemistry is the best at providing real-time chemical measurements in vivo.
6. FUTURE DIRECTIONS: CHALLENGES FOR ELECTROCHEMISTRY AT THE SYNAPSE
6.1. Making Smaller Electrodes
Synaptic measurements inherently require small electrodes, and advances in nanoelectrodes have facilitated recent research of electrochemistry at the synapse. Simple methods, like electrochemical (9, 121) and flame etching of carbon fibers (122), have been used but are often more art than science to produce the correct size and shaped tips. Thus, new strategies for nanoelectrodes are necessary to reproducibly fabricate robust, implantable electrodes with tiny tips. One strategy is to use nanopipette-based electrodes. Carbon can be deposited on nanopipettes to make a small conical tip for voltammetric measurement (123, 124). Small, 5–200-nm tipped carbon nanoelectrodes have been used for intracellular chemical measurements and presumably could be used for synaptic measurements as well (125). However, sensitivity scales with area for these electrodes, so smaller tips mean less current. Thus, a strategy to create recessed tips that trap dopamine, used in scanning electrochemical microscopy (126, 127), could amplify the signals. Nanopipettes are also used for ITIES measurements in the synapse and detect analytes that are not electroactive (103). While carbon is often the material of choice for direct, voltammetric detection of neurotransmitters, biosensors often use Pt electrodes. Indeed, carbon electrodes can be platinized (125), or small, 100-nm Pt tips can be etched that might be suitable for biosensor fabrication (128, 129). A different, recent strategy is to make field-effect transistor sensors, where aptamers recognize the neurotransmitters (130). Nanotechnology is a highly active research field, so new discoveries in the fabrication of nanoelectrodes should push development of nanoelectrodes that are useful for synaptic measurements.
6.2. Multianalyte Monitoring
Currently, electrochemistry at the synapse is focused on detecting a single analyte, and traditional dogma stated that just one neurotransmitter was released per synapse. However, costorage and release of neurochemicals are now widely described at a single synapse and thus multianalyte monitoring is needed to understand cotransmission. For example, ATP, which is rapidly metabolized to adenosine, is coreleased with neuropeptides and norepinephrine (131), all of which could be detected electrochemically. In addition, investigators are researching how serotonin neurons might function to release dopamine during Parkinson disease (132). Computational techniques are currently being developed to disambiguate chemical signals in FSCV, with principal components regression being used to distinguish different analytes and pH shifts (133). Newer, neural network based computational approaches have been used to pick out dopamine and serotonin in humans (134, 135). Thus, further computational methods to do multianalyte monitoring in a single synapse would provide a better understanding of the complex chemical dynamics during exocytosis.
6.3. Multisite Monitoring
Monitoring multiple synapses simultaneously would provide information into differences in chemical transmission at different synapses. Although imaging techniques are also very good at multisite monitoring, electrochemical arrays also have the potential to solve this problem. Arrays have been made to allow monitoring at different sites for single-cell amperometry experiments (136). Chemical lithography techniques are available that can make thousands of carbon nanorods in a highly organized manner (137). Carbon nanotubes can also be fabricated into microelectrode arrays, and functionalized to promote neurotransmitter detection (138, 139). Microelectrode arrays have also been used to measure dopamine and cell firing response such as action potentials (140, 141). To truly measure at different sites, you need individually addressable arrays, where each nanoelectrode can be measured independently. Microfabrication techniques have created arrays with multiple pads for voltammetric or biosensor detection (142, 143), but these are currently much larger than a synapse. Thus, future work is needed to make individually addressable nanoarrays necessary for multisynapse monitoring.
6.4. Localization in the Synapse
One of the biggest challenges for making electrochemical measurements in the synapse is the need to precisely position the electrode. It is challenging to position a nanometer-sized probe in a synapse by optical microscopy alone. Growing cells on an array of nanopillars is a strategy for approaching the synapse in both fluorescence imaging (144) and electrophysiology (145). Carbon nanotube or nanorod arrays could be used as the substrate, and electrochemical measurements could be made of synaptic and extrasynaptic neurotransmission, but this strategy is not ideal for measurements in intact tissue. Shen’s group (104) has used SECM feedback mechanisms to position electrodes in the synapse. Using a noninvasive, electroactive mediator, they can make an approach curve and use modeling to tell how far the electrode is from the soma. This strategy is best for cultured neurons or tissue preps, where the synapses are already established. Multitechnique measurements, such as cell imaging by scanning ion conductance microscopy and electrochemical measurements using SECM (146), may also be useful for localization and imaging of the synapse while measuring synaptic release.
6.5. Multitechnique Measurements
Electrochemistry is very amenable to multiplexing and can be combined with other techniques to provide additional information about the brain. For example, electrochemistry and electrophysiology can be measured at the same electrode to provide information about chemical release and cell firing (35). An alternative is to measure electrochemistry at a carbon-fiber microelectrode and electrophysiology with a traditional high-density array of microwires (141). Newer methods are integrating carbon electrodes for electrochemistry and electrophysiology with optrodes to deliver light stimulations for optogenetic experiments with light-activated channels (147). Electrochemistry is also easily integrated with optical imaging experiments. For example, genetically encoded calcium sensors have been integrated with FSCV recordings of dopamine in brain slices (148). The newest genetically encoded neurotransmitter sensors could also be integrated with voltammetric measurements. In particular, the optical measurements would give an idea of neurotransmitters at the surface of the cell and directly in the synapse, while CFMEs would provide information about the extrasynaptic spillover of neurotransmitters. Imaging and electrochemistry are both limited in the number of molecules they can measure simultaneously, but measuring different molecules with diverse techniques would provide a better way to understand neurotransmitter interactions. Thus, the quest for a true understanding the synapse may lie at the intersection of many techniques and not electrochemical information alone.
7. CONCLUSIONS: THE POTENTIAL OF ELECTROCHEMISTRY AT THE SYNAPSE TO ADVANCE NEUROSCIENCE
Electrochemical monitoring of neurotransmitters in the brain has significantly advanced our knowledge of how chemical changes affect behavior and disease. Experiments in behaving animals have discovered how dopamine acts in reward behavior and how that signaling contributes to addiction. Electrochemistry has also been used to explore dopamine changes during Parkinson disease and serotonin changes in response to antidepressant drugs. Amperometric measurements of quantal release have revealed a new understanding of the mechanism of exocytosis and how it is regulated, challenging long-standing views of full release as the main mechanism of exocytosis. Techniques continue to improve, with advances in traditional voltammetry, as well as in biosensors and ITIES, expanding the types of neurotransmitters that can be monitored. Nanotechnology advances are producing smaller, functional electrodes, and these have led to recent studies exploring electrochemical measurements directly in the synapse, a holy grail for neuroscience. In the future, the flexibility to combine electrochemistry with electrophysiology, optogenetics, and imaging will allow multimodal platforms for monitoring cell firing and multiple neurochemicals simultaneously. Electrochemistry at the synapse is important for answering many questions related to disease. For example, changes in synapse structure or number have been observed from Alzheimer’s to epilepsy to autism spectrum disorders (149), but no studies have identified how those changes affect chemical neurotransmission. The question of how synaptic architecture affects neurotransmission would be interesting to study with nanoelectrodes in the synapse. Electrochemistry will continue to provide real-time information about chemical changes to provide a better fundamental understanding of the chemical synapse and how that relates to behavior and disease.
SUMMARY POINTS.
Traditional electrodes used for neurochemistry measurements are too large for the synapse but provide an understanding of real-time changes in extrasynaptic neurotransmitter levels.
Single-cell amperometry and electrochemical cytometry reveal that not all neurotransmitter is released from the vesicle during exocytosis.
Nanoelectrodes can be positioned in the synapse to make electrochemical measurements of neurotransmitters.
Concentrations in the synapse are higher than those in the extracellular space.
There are new opportunities to now measure exocytosis in a synapse and understand its regulation with a postsynaptic cell present.
FUTURE ISSUES.
New nanoelectrodes and electrode placement strategies are needed to expand the analytes that can be detected.
Electrode arrays, particularly with individually addressable electrodes, are needed to measure at multiple synapses simultaneously.
Computational and electrochemical techniques need to be developed to enable multiple analytes to be detected simultaneously to investigate cotransmission.
Electrochemistry should be integrated with other techniques, such as imaging and mass spectrometry, to provide a better picture of chemical changes in the synapse.
ACKNOWLEDGMENTS
Research in the Venton lab is funded by NIH grants R01MH085159 and R01EB026497.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Eroglu C, Barres BA. 2015. Regulation of synaptic connectivity by glia. Nature 468(7321):223–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Südhof TC. 2012. The presynaptic active zone. Neuron 75(1):11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth G, Dicke U. 2005. Evolution of the brain and intelligence. Trends Cogn. Sci 9(5):250–57 [DOI] [PubMed] [Google Scholar]
- 4.Rossi DJ, Hamann M. 1998. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron 20(4):783–95 [DOI] [PubMed] [Google Scholar]
- 5.Trueta C, Méndez B, De-Miguel FF. 2003. Somatic exocytosis of serotonin mediated by L-type calcium channels in cultured leech neurones. J. Physiol 547(2):405–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford CP, Gantz SC, Phillips PEM, Williams JT. 2010. Control of extracellular dopamine at dendrite and axon terminals. J. Neurosci 30(20):6975–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaquins-Gerstl A, Michael AC. 2015. A review of the effects of FSCV and microdialysis measurements on dopamine release in the surrounding tissue. Analyst 140(11):3696–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cans AS, Wittenberg N, Eves D, Karlsson R, Karlsson A, et al. 2003. Amperometric detection of exocytosis in an artificial synapse. Anal. Chem 75(16):4168–75 [DOI] [PubMed] [Google Scholar]
- 9.Li Y-T, Zhang S-H, Wang L, Xiao R-R, Liu W, et al. 2014. Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses. Angew. Chem. Int. Ed 53(46):12456–60 [DOI] [PubMed] [Google Scholar]
- 10.Huffman ML, Venton BJ. 2009. Carbon-fiber microelectrodes for in vivo applications. Analyst 134(1):18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. 2000. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Anal. Chem 72(24):5994–6002 [DOI] [PubMed] [Google Scholar]
- 12.Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, et al. 2010. Carbon microelectrodes with a renewable surface. Anal. Chem 82(5):2020–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keithley RB, Takmakov P, Bucher ES, Belle AM, Owesson-White CA, et al. 2011. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal. Chem 83(9):3563–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthongkham P, Yang C, Venton BJ. 2018. Carbon nanohorn-modified carbon fiber microelectrodes for dopamine detection. Electroanalysis 30(6):1073–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. 2013. A carbon-fiber electrode array for long-term neural recording. J. Neural Eng 10(4):046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wightman RM, Heien MLA V, Wassum KM, Sombers LA, Aragona BJ, et al. 2007. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur. J. Neurosci 26(7):2046–54 [DOI] [PubMed] [Google Scholar]
- 17.Cacciapaglia F, Saddoris MP, Wightman RM, Carelli RM. 2012. Differential dopamine release dynamics in the nucleus accumbens core and shell track distinct aspects of goal-directed behavior for sucrose. Neuropharmacology 62(5–6):2050–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox ME, Mikhailova MA, Bass CE, Takmakov P, Gainetdinov RR, et al. 2016. Cross-hemispheric dopamine projections have functional significance. PNAS 113(25):6985–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, et al. 2011. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci 31(30):10829–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang KH, Penmatsa A, Gouaux E. 2015. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521(7552):322–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA. 2016. Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur. J. Neurosci 43(12):1661–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, et al. 2006. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci 26(12):3206–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siciliano CA, Ferris MJ, Jones SR. 2015. Cocaine self-administration disrupts mesolimbic dopamine circuit function and attenuates dopaminergic responsiveness to cocaine. Eur. J. Neurosci 42(4):2091–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron CM, Wightman RM, Carelli RM. 2016. One month of cocaine abstinence potentiates rapid dopamine signaling in the nucleus accumbens core. Neuropharmacology 111:223–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, et al. 2014. Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur. J. Neurosci 40(7):3041–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox ME, Rodeberg NT, Wightman RM. 2017. Reciprocal catecholamine changes during opiate exposure and withdrawal. Neuropsychopharmacology 42(3):671–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. 1999. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature 398(6722):67–69 [DOI] [PubMed] [Google Scholar]
- 28.Rodeberg NT, Johnson JA, Bucher ES, Wightman RM. 2016. Dopamine dynamics during continuous intracranial self-stimulation: effect of waveform on fast-scan cyclic voltammetry data. ACS Chem. Neurosci 7(11):1508–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. 2004. Dopamine operates as a subsecond modulator of food seeking. J. Neurosci 24(6):1265–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shnitko TA, Robinson DL. 2015. Regional variation in phasic dopamine release during alcohol and sucrose self-administration in rats. ACS Chem. Neurosci 6(1):147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. 2015. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol. Psychiatry 77(10):903–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorenza AM, Shnitko TA, Sullivan KM, Vemuru SR, Gomez-A A, et al. 2018. Ethanol exposure history and alcoholic reward differentially alter dopamine release in the nucleus accumbens to a reward-predictive cue. Alcohol. Clin. Exp. Res 42(6):1051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, et al. 2010. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 7(2):126–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart AS, Clark JJ, Phillips PEM. 2015. Dynamic shaping of dopamine signals during probabilistic Pavlovian conditioning. Neurobiol. Learn. Mem 117:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takmakov P, McKinney CJ, Carelli RM, Wightman RM. 2011. Instrumentation for fast-scan cyclic voltammetry combined with electrophysiology for behavioral experiments in freely moving animals. Rev. Sci. Instrum 82(7):074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belle AM, Owesson-White C, Herr NR, Carelli RM, Wightman RM. 2013. Controlled iontophoresis coupled with fast-scan cyclic voltammetry/electrophysiology in awake, freely moving animals. ACS Chem. Neurosci 4(5):761–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owesson-White C, Belle AM, Herr NR, Peele JL, Gowrishankar P, et al. 2016. Cue-evoked dopamine release rapidly modulates D2 neurons in the nucleus accumbens during motivated behavior. J. Neurosci 36(22):6011–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onaka T, Yagi K. 1998. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res 788(1–2):287–93 [DOI] [PubMed] [Google Scholar]
- 39.Park J, Kile BM, Wightman RM. 2009. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur. J. Neurosci 30(11):2121–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson SD, Plummer NW, de Marchena J, Jensen P. 2013. Developmental origins of central norepinephrine neuron diversity. Nat. Neurosci 16(8):1016–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J, Takmakov P, Wightman RM. 2011. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J. Neurochem 119(5):932–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J, Bucher ES, Budygin EA, Wightman RM. 2015. Norepinephrine and dopamine transmission in 2 limbic regions differentially respond to acute noxious stimulation. Pain 156(2):318–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson BP, Wightman RM. 1995. Dynamics of 5-hydroxytryptamine released from dopamine neurons in the caudate putamen of the rat. Brain Res. 674(1):163–66 [DOI] [PubMed] [Google Scholar]
- 44.Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. 2009. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal. Chem 81(22):9462–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashemi P, Dankoski EC, Lama R, Wood KM, Takmakov P, Wightman RM. 2012. Brain dopamine and serotonin differ in regulation and its consequences. PNAS 109(29):11510–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood KM, Zeqja A, Nijhout HF, Reed MC, Best J, Hashemi P. 2014. Voltammetric and mathematical evidence for dual transport mediation of serotonin clearance in vivo. J. Neurochem 130(3):351–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dankoski EC, Wightman RM. 2013. Monitoring serotonin signaling on a subsecond time scale. Front. Integr. Neurosci 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dankoski EC, Carroll S, Wightman RM. 2016. Acute selective serotonin reuptake inhibitors regulate the dorsal raphe nucleus causing amplification of terminal serotonin release. J. Neurochem 136(6):1131–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samaranayake S, Abdalla A, Robke R, Nijhout HF, Reed MC, et al. 2016. A voltammetric and mathematical analysis of histaminergic modulation of serotonin in the mouse hypothalamus. J. Neurochem 138(3):374–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atcherley CW, Laude ND, Parent KL, Heien ML. 2013. Fast-scan controlled-adsorption voltammetry for the quantification of absolute concentrations and adsorption dynamics. Langmuir 29(48):14885–92 [DOI] [PubMed] [Google Scholar]
- 51.Atcherley CW, Wood KM, Parent KL, Hashemi P, Heien ML. 2015. The coaction of tonic and phasic dopamine dynamics. Chem. Commun 51(12):2235–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdalla A, Atcherley CW, Pathirathna P, Samaranayake S, Qiang B, et al. 2017. In vivo ambient serotonin measurements at carbon-fiber microelectrodes. Anal. Chem 89(18):9703–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Street SE, Walsh PL, Sowa NA, Taylor-Blake B, Guillot TS, et al. 2011. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol. Pain 7(27):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. 2014. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLOS ONE 9(1):e87165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swamy BEK, Venton BJ. 2007. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal. Chem 79(2):744–50 [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Venton BJ. 2017. Correlation of transient adenosine release and oxygen changes in the caudate-putamen. J. Neurochem 140(1):13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross AE, Venton BJ. 2012. Nafion-CNT coated carbon-fiber microelectrodes for enhanced detection of adenosine. Analyst 137(13):3045–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spanos M, Gras-Najjar J, Letchworth JM, Sanford AL, Toups JV, Sombers LA. 2013. Quantitation of hydrogen peroxide fluctuations and their modulation of dopamine dynamics in the rat dorsal striatum using fast-scan cyclic voltammetry. ACS Chem. Neurosci 4(5):782–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cechova S, Venton BJ. 2008. Transient adenosine efflux in the rat caudate-putamen. J. Neurochem 105(4):1253–63 [DOI] [PubMed] [Google Scholar]
- 60.Ross AE, Nguyen MD, Privman E, Venton BJ. 2014. Mechanical stimulation evokes rapid increases in extracellular adenosine concentration in the prefrontal cortex. J. Neurochem 130(1):50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen MD, Wang Y, Ganesana M, Venton BJ. 2017. Transient adenosine release is modulated by NMDA and GABAB receptors. ACS Chem. Neurosci 8(2):376–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen MD, Ross AE, Ryals M, Lee ST, Venton BJ. 2015. Clearance of rapid adenosine release is regulated by nucleoside transporters and metabolism. Pharmacol. Res. Perspect 3(6):e00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross AE, Venton BJ. 2015. Adenosine transiently modulates stimulated dopamine release in the caudate-putamen via A1 receptors. J. Neurochem 132(1):51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganesana M, Venton BJ. 2018. Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLOS ONE 13(5):e0196932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian F, Gourine AV, Huckstepp RTR, Dale N. 2009. A microelectrode biosensor for real time monitoring of l-glutamate release. Anal. Chim. Acta 645(1–2):86–91 [DOI] [PubMed] [Google Scholar]
- 66.Özel RE, Ispas C, Ganesana M, Leiter JC, Andreescu S. 2014. Glutamate oxidase biosensor based on mixed ceria and titania nanoparticles for the detection of glutamate in hypoxic environments. Biosens. Bioelectron 52:397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weltin A, Kieninger J, Urban GA. 2016. Microfabricated, amperometric, enzyme-based biosensors for in vivo applications. Anal. Bioanal. Chem 408(17):4503–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malvaez M, Greenfield VY, Wang AS, Yorita AM, Feng L, et al. 2015. Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions. Sci. Rep 5:12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryu IS, Kim J, Seo SY, Yang JH, Oh JH, et al. 2018. Repeated administration of cigarette smoke condensate increases glutamate levels and behavioral sensitization. Front. Behav. Neurosci 12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asri R, O’Neill B, Patel JC, Siletti KA, Rice ME. 2016. Detection of evoked acetylcholine release in mouse brain slices. Analyst 141(23):6416–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreira FTC, Sale MGF, Di Lorenzo M. 2017. Towards timely Alzheimer diagnosis: a self-powered amperometric biosensor for the neurotransmitter acetylcholine. Biosens. Bioelectron 87:607–14 [DOI] [PubMed] [Google Scholar]
- 72.Parikh V, St. Peters M, Blakely RD, Sarter M. 2013. The presynaptic choline transporter imposes limits on sustained cortical acetylcholine release and attention. J. Neurosci 33(6):2326–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teles-Grilo Ruivo LM, Baker KL, Conway MW, Kinsley PJ, Gilmour G, et al. 2017. Coordinated acetylcholine release in prefrontal cortex and hippocampus is associated with arousal and reward on distinct timescales. Cell Rep 18(4):905–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ren L, Mellander LJ, Keighron J, Cans A-S, Kurczy ME, et al. 2016. The evidence for open and closed exocytosis as the primary release mechanism. Q. Rev. Biophys 49:e12. [DOI] [PubMed] [Google Scholar]
- 75.Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Near JA, Wightman RM. 1990. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J. Biol. Chem 265(25):14736–37 [PubMed] [Google Scholar]
- 76.Chow RH, von Rüden L, Neher E. 1992. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature 356(6364):60–63 [DOI] [PubMed] [Google Scholar]
- 77.Mellander LJ, Trouillon R, Svensson MI, Ewing AG. 2012. Amperometric post spike feet reveal most exocytosis is via extended kiss-and-run fusion. Sci. Rep 2:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amatore C, Oleinick AI, Svir I. 2010. Reconstruction of aperture functions during full fusion in vesicular exocytosis of neurotransmitters. Chem. Phys. Chem 11(1):159–74 [DOI] [PubMed] [Google Scholar]
- 79.Staal RGW, Mosharov EV, Sulzer D. 2004. Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci 7:341–46 [DOI] [PubMed] [Google Scholar]
- 80.Omiatek DM, Santillo MF, Heien ML, Ewing AG. 2009. Hybrid capillary-microfluidic device for the separation, lysis, and electrochemical detection of vesicles. Anal. Chem 81(6):2294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Omiatek DM, Bressler AJ, Cans A-S, Andrews AM, Heien ML, Ewing AG. 2013. The real catecholamine content of secretory vesicles in the CNS revealed by electrochemical cytometry. Sci. Rep 3(1):1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omiatek DM, Dong Y, Heien ML, Ewing AG. 2010. Only a fraction of quantal content is released during exocytosis as revealed by electrochemical cytometry of secretory vesicles. ACS Chem. Neurosci 1(3):234–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dunevall J, Fathali H, Najafinobar N, Lovric J, Wigstrom J, et al. 2015. Characterizing the catecholamine content of single mammalian vesicles by collision-adsorption events at an electrode. J. Am. Chem. Soc 137(13):4344–46 [DOI] [PubMed] [Google Scholar]
- 84.Lovrić J, Najafinobar N, Dunevall J, Majdi S, Svir I, et al. 2016. On the mechanism of electrochemical vesicle cytometry: chromaffin cell vesicles and liposomes. Faraday Discuss 193:65–79 [DOI] [PubMed] [Google Scholar]
- 85.Li X, Dunevall J, Ren L, Ewing AG. 2017. Mechanistic aspects of vesicle opening during analysis with vesicle impact electrochemical cytometry. Anal. Chem 89(17):9416–23 [DOI] [PubMed] [Google Scholar]
- 86.Najafinobar N, Lovrić J, Majdi S, Dunevall J, Cans A, Ewing A. 2016. Excited fluorophores enhance the opening of vesicles at electrode surfaces in vesicle electrochemical cytometry. Angew. Chemie Int. Ed 55(48):15081–85 [DOI] [PubMed] [Google Scholar]
- 87.Li X, Majdi S, Dunevall J, Fathali H, Ewing AG. 2015. Quantitative measurement of transmitters in individual vesicles in the cytoplasm of single cells with nanotip electrodes. Angew. Chemie Int. Ed 54(41):11978–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Ren L, Dunevall J, Ye D, White HS, et al. 2018. Nanopore opening at flat and nanotip conical electrodes during vesicle impact electrochemical cytometry. ACS Nano 12(3):3010–19 [DOI] [PubMed] [Google Scholar]
- 89.Trouillon R, Ewing AG. 2013. Amperometric measurements at cells support a role for dynamin in the dilation of the fusion pore during exocytosis. Chem. Phys. Chem 14:2295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trouillon R, Ewing AG. 2013. Single cell amperometry reveals glycocalyx hinders the release of neurotransmitters during exocytosis. Anal. Chem 85(9):4822–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trouillon R, Ewing AG. 2014. Actin controls the vesicular fraction of dopamine released during extended kiss and run exocytosis. ACS Chem. Biol 9(3):812–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Najafinobar N, Mellander LJ, Kurczy ME, Dunevall J, Angerer TB, et al. 2016. Cholesterol alters the dynamics of release in protein independent cell models for exocytosis. Sci. Rep 6(1):33702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, Mohammadi AS, Ewing AG. 2016. Single cell amperometry reveals curcuminoids modulate the release of neurotransmitters during exocytosis from PC12 cells. J. Electroanal. Chem 781:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye D, Ewing A. 2018. On the action of general anesthetics on cellular function: barbiturate alters the exocytosis of catecholamines in a model cell system. Chem. Phys. Chem 19(10):1173–79 [DOI] [PubMed] [Google Scholar]
- 95.Ye D, Gu C, Ewing A. 2018. Using single-cell amperometry and intracellular vesicle impact electrochemical cytometry to shed light on the biphasic effects of lidocaine on exocytosis. ACS Chem. Neurosci In press. 10.1021/acschemneuro.8b00130 [DOI] [PubMed] [Google Scholar]
- 96.Ren L, Pour MD, Majdi S, Li X, Malmberg P, Ewing AG. 2017. Zinc regulates chemical-transmitter storage in nanometer vesicles and exocytosis dynamics as measured by amperometry. Angew. Chem. Int. Ed 56(18):4970–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu Q, Zhang F, Tang Y, Zhang X, Jiang H, et al. 2018. Real‐time monitoring of exocytotic glutamate release from single neuron by amperometry at an enzymatic biosensor. Electroanalysis 30(6):1054–59 [Google Scholar]
- 98.Zhang X-W, Qiu Q-F, Jiang H, Zhang F-L, Liu Y-L, et al. 2017. Real-time intracellular measurements of ROS and RNS in living cells with single core-shell nanowire electrodes. Angew. Chem. Int. Ed 129(42):13177–80 [DOI] [PubMed] [Google Scholar]
- 99.Pan R, Xu M, Burgess JD, Jiang D, Chen H-Y. 2018. Direct electrochemical observation of glucosidase activity in isolated single lysosomes from a living cell. PNAS 115(16):4087–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mellander LJ, Kurczy ME, Najafinobar N, Dunevall J, Ewing AG, Cans A-S. 2014. Two modes of exocytosis in an artificial cell. Sci. Rep 4:3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y-T, Zhang S-H, Wang X-Y, Zhang X-W, Oleinick AI, et al. 2015. Real-time monitoring of discrete synaptic release events and excitatory potentials within self-reconstructed neuromuscular junctions. Angew. Chem. Int. Ed 54(32):9313–18 [DOI] [PubMed] [Google Scholar]
- 102.Colombo ML, Sweedler JV, Shen M. 2015. Nanopipet-based liquid-liquid interface probes for the electrochemical detection of acetylcholine, tryptamine, and serotonin via ionic transfer. Anal. Chem 87(10):5095–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwai NT, Kramaric M, Crabbe D, Wei Y, Chen R, Shen M. 2018. GABA detection with nano-ITIES pipet electrode: a new mechanism, water/DCE-octanoic acid interface. Anal. Chem 90(5):3067–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Welle TM, Alanis K, Colombo ML, Sweedler JV, Shen M. 2018. A high spatiotemporal study of somatic exocytosis with scanning electrochemical microscopy and nanoITIES electrodes. Chem. Sci 9(22):4937–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen M, Qu Z, DesLaurier J, Welle TM, Sweedler JV, Chen R. 2018. Single synaptic observation of cholinergic neurotransmission on living neurons: concentration and dynamics. J. Am. Chem. Soc 140(25):7764–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gubernator NG, Zhang H, Staal RGW, Mosharov EV, Pereira DB, et al. 2009. Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science 324(5933):1441–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dunn M, Henke A, Clark S, Kovalyova Y, Kempadoo KA, et al. 2018. Designing a norepinephrine optical tracer for imaging individual noradrenergic synapses and their activity in vivo. Nat. Commun 9(1):2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hettie KS, Glass TE. 2016. Turn-on near-infrared fluorescent sensor for selectively imaging serotonin. ACS Chem. Neurosci 7(1):21–25 [DOI] [PubMed] [Google Scholar]
- 109.Beyene AG, Delevich K, ODonnell JTDB, Piekarski DJ, Lin WC, et al. 2018. Imaging striatal dopamine release using a non-genetically encoded near-infrared fluorescent catecholamine nanosensor. bioRxiv 356543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, et al. 2018. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360(6396):eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun F, Zeng J, Jing M, Zhou J, Feng J, et al. 2018. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 174(2):481–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jing M, Zhang P, Wang G, Feng J, Mesik L, et al. 2018. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat. Biotechnol 36:726–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Selimi F, Cristea IM, Heller E, Chait BT, Heintz N. 2009. Proteomic studies of a single CNS synapse type: the parallel fiber/Purkinje cell synapse. PLOS Biol 7(4):e1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brinkmalm A, Brinkmalm G, Honer WG, Moreno JA, Jakobsson J, et al. 2014. Targeting synaptic pathology with a novel affinity mass spectrometry approach. Mol. Cell. Proteom 13(10):2584–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramos-Ortolaza DL, Bushlin I, Abul-Husn N, Annangudi SP, Sweedler J, Devi LA. 2010. Quantitative neuroproteomics of the synapse. Methods Mol. Biol 615:227–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dieterich DC, Kreutz MR. 2016. Proteomics of the synapse—a quantitative approach to neuronal plasticity. Mol. Cell. Proteom 15(2):368–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lovrić J, Dunevall J, Larsson A, Ren L, Andersson S, et al. 2017. Nano secondary ion mass spectrometry imaging of dopamine distribution across nanometer vesicles. ACS Nano 11(4):3446–55 [DOI] [PubMed] [Google Scholar]
- 118.Mohammadi AS, Phan NTN, Fletcher JS, Ewing AG. 2016. Intact lipid imaging of mouse brain samples: MALDI, nanoparticle-laser desorption ionization, and 40 keV argon cluster secondary ion mass spectrometry. Anal. Bioanal. Chem 408(24):6857–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohammadi AS, Li X, Ewing AG. 2018. Mass spectrometry imaging suggests that cisplatin affects exocytotic release by alteration of cell membrane lipids. Anal. Chem 90(14):8509–16 [DOI] [PubMed] [Google Scholar]
- 120.Wu Q, Comi TJ, Li B, Rubakhin SS, Sweedler JV. 2016. On-tissue derivatization via electrospray deposition for matrix-assisted laser desorption/ionization mass spectrometry imaging of endogenous fatty acids in rat brain tissues. Anal. Chem 88(11):5988–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strein TG, Ewing AG. 1992. Characterization of submicron-sized carbon electrodes insulated with a phenol-allylphenol copolymer. Anal. Chem 64(13):1368–73 [Google Scholar]
- 122.Strand AM, Venton BJ. 2008. Flame etching enhances the sensitivity of carbon-fiber microelectrodes. Anal. Chem 80(10):3708–15 [DOI] [PubMed] [Google Scholar]
- 123.Rees HR, Anderson SE, Privman E, Bau HH, Venton BJ. 2015. Carbon nanopipette electrodes for dopamine detection in Drosophila. Anal. Chem 87(7):3849–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schrlau MG, Dun NJ, Bau HH. 2009. Cell electrophysiology with carbon nanopipettes. ACS Nano 3(3):563–68 [DOI] [PubMed] [Google Scholar]
- 125.Actis P, Tokar S, Clausmeyer J, Babakinejad B, Mikhaleva S, et al. 2014. Electrochemical nanoprobes for single-cell analysis. ACS Nano 8(1):875–84 [DOI] [PubMed] [Google Scholar]
- 126.Yu Y, Noël J-M, Mirkin MV, Gao Y, Mashtalir O, et al. 2014. Carbon pipette-based electrochemical nanosampler. Anal. Chem 86(7):3365–72 [DOI] [PubMed] [Google Scholar]
- 127.Chen R, Hu K, Yu Y, Mirkin MV, Amemiya S. 2016. Focused-ion-beam-milled carbon nanoelectrodes for scanning electrochemical microscopy. J. Electrochem. Soc 163(4):H3032–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hao R, Zhang B. 2016. Nanopipette-based electroplated nanoelectrodes. Anal. Chem 88(1):614–20 [DOI] [PubMed] [Google Scholar]
- 129.Yang M, Qu F, Lu Y, He Y, Shen G, Yu R. 2006. Platinum nanowire nanoelectrode array for the fabrication of biosensors. Biomaterials 27(35):5944–50 [DOI] [PubMed] [Google Scholar]
- 130.Nakatsuka N, Andrews AM. 2016. Neurochips enable nanoscale devices for high-resolution in vivo neurotransmitter sensing. Neuropsychopharmacology 41(1):378–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huidobro-Toro JP, Donoso MV. 2004. Sympathetic co-transmission: the coordinated action of ATP and noradrenaline and their modulation by neuropeptide Y in human vascular neuroeffector junctions. Eur. J. Pharmacol 500:27–35 [DOI] [PubMed] [Google Scholar]
- 132.Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A. 2010. Role of serotonin neurons in the induction of levodopa- and graft-induced dyskinesias in Parkinson’s disease. Mov. Disord 25:S174–79 [DOI] [PubMed] [Google Scholar]
- 133.Keithley RB, Wightman RM. 2011. Assessing principal component regression prediction of neurochemicals detected with fast-scan cyclic voltammetry. ACS Chem. Neurosci 2(9):514–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kishida KT, Saez I, Lohrenz T, Witcher MR, Laxton AW, et al. 2016. Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. PNAS 113(1):200–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moran RJ, Kishida KT, Lohrenz T, Saez I, Laxton AW, et al. 2018. The protective action encoding of serotonin transients in the human brain. Neuropsychopharmacology 43(6):1425–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang J, Trouillon R, Lin Y, Svensson MI, Ewing AG. 2013. Individually addressable thin-film ultramicroelectrode array for spatial measurements of single vesicle release. Anal. Chem 85(11):5600–8 [DOI] [PubMed] [Google Scholar]
- 137.Demuru S, Nela L, Marchack N, Holmes SJ, Farmer DB, et al. 2018. Scalable nanostructured carbon electrode arrays for enhanced dopamine detection. ACS Sens 3(4):799–805 [DOI] [PubMed] [Google Scholar]
- 138.Li S, Guo J, Chan M, Yuan J. 2014. Multi-walled carbon nanotube coated microelectrode array for high-throughput, sensitive dopamine detection In 9th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, pp. 643–46. New York: IEEE [Google Scholar]
- 139.Clark J, Chen Y, Hinder S, Silva SRP. 2017. Highly sensitive dopamine detection using a bespoke functionalised carbon nanotube microelectrode array. Electroanalysis 29(10):2365–76 [Google Scholar]
- 140.Suzuki I, Fukuda M, Shirakawa K, Jiko H, Gotoh M. 2013. Carbon nanotube multi-electrode array chips for noninvasive real-time measurement of dopamine, action potentials, and postsynaptic potentials. Biosens. Bioelectron 49:270–75 [DOI] [PubMed] [Google Scholar]
- 141.Parent KL, Hill DF, Crown LM, Wiegand JP, Gies KF, et al. 2017. Platform to enable combined measurement of dopamine and neural activity. Anal. Chem 89(5):2790–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zachek MK, Park J, Takmakov P, Wightman RM, McCarty GS. 2010. Microfabricated FSCV-compatible microelectrode array for real-time monitoring of heterogeneous dopamine release. Analyst 135(7):1556–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Belle AM, Enright HA, Mukerjee EV, Soscia DA, Osburn JJ, et al. 2017. Measurement of glutamate in dorsal root ganglion cell culture with integrated electrochemical biosensors In 2017 IEEE International Symposium Medical Measurements and Applications, pp. 453–57. New York: IEEE [Google Scholar]
- 144.Kruss S, Salem DP, Vuković L, Lima B, Vander Ende E, et al. 2017. High-resolution imaging of cellular dopamine efflux using a fluorescent nanosensor array. PNAS 114(8):1789–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie C, Hanson L, Xie W, Lin Z, Cui B, Cui Y. 2010. Noninvasive neuron pinning with nanopillar arrays. Nano Lett. 10(10):4020–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Comstock DJ, Elam JW, Pellin MJ, Hersam MC. 2010. Integrated ultramicroelectrode-nanopipet probe for concurrent scanning electrochemical microscopy and scanning ion conductance microscopy. Anal. Chem 82(4):1270–76 [DOI] [PubMed] [Google Scholar]
- 147.Budai D, Vizvári AD, Bali ZK, Márki B, Nagy LV, et al. 2018. A novel carbon tipped single micro-optrode for combined optogenetics and electrophysiology. PLOS ONE 13(3):e0193836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sgobio C, Kupferschmidt DA, Cui G, Sun L, Li Z, et al. 2014. Optogenetic measurement of presynaptic calcium transients using conditional genetically encoded calcium indicator expression in dopaminergic neurons. PLOS ONE 9(10):e111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Spronsen M, Hoogenraad CC. 2010. Synapse pathology in psychiatric and neurologic disease. Curr. Neurol. Neurosci. Rep 10(3):207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]


