Abstract
Inappropriate stoma site, improper management of stoma, and stoma complications lead to diminished quality of life of ostomates. Healthcare professionals involved in stoma creation and/or care should have the fundamental and updated knowledge of the management of stomas and their complications. This review article consists of the following major sections: principles of perioperative patient management, early complications, and late complications. In the “principles of perioperative patient management” section, the current concepts and trends in preoperative education, stoma site marking, postoperative education, and patient educational resources are discussed. In the “early complications” section, we have focused on the etiology and current management of ischemia/necrosis, fluid and electrolyte imbalances, mucocutaneous separation, and retraction. In the “late complications” section, we have focused on the etiology and current management of parastomal hernia, stoma prolapse, parastomal varices, and pyoderma gangrenosum. Pre- and postoperative patient education facilitates the patient's independence in stoma care and resumption of normal activities. Healthcare providers should have basic skills and updated knowledge on the management of stomas and complications of stomas, to act as the first crisis manager for ostomates.
Keywords: Stoma complications, perioperative management, early complications, late complications, stoma care
Introduction
Stoma creation is indicated for various clinicopathological conditions, and intestinal stomas require temporary or permanent diversion of bowel continuity. Although the purpose of stoma creation is to improve the quality of life (QoL), inappropriate stoma site, improper management of the stoma, and stoma complications can lead to diminished QoL, social isolation, and increased medical interventions and costs. Patients who undergo stoma surgery face multiple challenges and lifestyle changes[1]. Therefore, healthcare professionals involved in stoma creation and/or care should have the fundamental and updated knowledge of the management and complications of stomas[1,2].
In this study, early complications were defined as those that occurred within the first 30 days, and late complications were defined as those that occurred after the patient's physiological adjustment[3,4]. For early complications, this study focused on ischemia/necrosis, fluid and electrolyte imbalances, mucocutaneous separation, and retraction. For late complications, this study focused on parastomal hernia, stoma prolapse, parastomal varices, and pyoderma gangrenosum.
Methods
The MEDLINE database was searched for all articles in English from January 2000 to May 2019. The search terms included “stoma management,” “stoma care,” and “stoma complications.” The search was limited to humans and adults. The publication types included randomized controlled studies, prospective studies, case series, retrospective studies, and review articles. For rare disease entities and novel management, case reports were included in the references.
The institutional ethics committee determined that approval was not necessary for a review article. Also, a written consent for the publication of this article with accompanying images was obtained from the patients. The consent was written in Japanese for better understanding by the patient. The consent form will be provided to the editors of this journal on request.
Principles of Perioperative Patient Management
Preoperative education
Preoperative education enables shorter hospital stay of the patients after stoma surgery due to enhanced recovery after surgery (ERAS)[5]. Preoperative education is provided by nurse specialists, most likely the wound, ostomy, and continence (WOC) nurses. Caregivers of the patient may be invited, if necessary. The content may include the consequences of bowel resection, possible impact of stoma on relationships, sexuality, activities of daily living, anticipated clinical course after surgery, and fundamentals of stoma care and equipment[5].
Preoperative stoma site marking
The preoperative stoma site is marked to select the optimal stoma location that promotes the patient's independence in stoma care and resumption of normal activities, predicts wear times of pouching systems, and reduces postoperative complications[1,2]. The key points, procedure, and examples of stoma site marking have been detailed previously[2].
Postoperative education
Patients who undergo stoma surgery need to develop new skills in the early postoperative period, while adjusting to physical changes and new circumstances[1]. The purpose of postoperative education is to acquire skills of self-care and ability to assess stomal/peristomal conditions. However, time for patient education may be limited because of advancements in minimally invasive surgeries and shorter hospital stay with ERAS. In 2016, the WOC Nurses (WOCN) Society published the summary of their consensus conference on outcome criteria for discharging new ostomates from home healthcare[6]. There are 18 consensus statements regarding emptying the pouch, pouch change, assessment of the stoma and peristomal skin, assessment of stoma output, ostomy-related supplies and resources, nutrition and fluids, and access to a WOC nurse. This summary also provides caregivers with the information necessary for a plan of care, with outcomes, and practical advice on implementing these criteria[6].
Patient educational resources
Currently, resources for patient education are available on websites. The American College of Surgeons has developed the Ostomy Home Skills Program that offers online interactive courses and a practical kit, including broadcast video programs, booklets, sample pouches, equipment needed for pouch application, skills checklist, and an additional resource list[7]. The WOCN Society has launched patient resources online that can direct patients to the related healthcare organizations, patient care publications, products, services, and testimonials, the Peristomal Skin Assessment Guide for Consumers, and a tool to find a WOC nurse[8]. In addition, most ostomy product manufacturers provide online patient education materials, product samples, hands-on kits, and complementary support services. Regional ostomy support groups may have periodic educational meetings and teaching sessions[9].
Early Complications
Ischemia/necrosis
A newly constructed stoma appears edematous in the immediate postoperative period because of venous congestion in the mesentery, allowing adequate arterial flow but causing swelling and cyanosis in the stoma[4]. As postoperative edema decreases, the stoma shrinks. Therefore, the definitive diagnosis between venous congestion and ischemia/necrosis is crucial.
Signs of ischemia usually arise within 24 h, with bluish discoloration of the mucocutaneous junction[10]. However, delayed ischemia may occur postoperatively, as underlying medical conditions (i.e., hypoperfusion) can cause devascularization[4]. The causes of necrosis are associated with the surgical technique in stoma creation, including tension on the mesentery, ligation of the primary blood vessel, and excessive dissection of the peristomal mesentery[4,11]. The incidence of necrosis is 1.6%-11%[12-15].
The decision to proceed with stoma revision depends on the level of stoma necrosis in the abdominal wall. The extent of ischemic changes in the mucosa can be effectively assessed using endoscopy through the stoma site[4]. If the necrosis is superficial (a few millimeters), there is no need for revision[4,10,11]. If the necrosis extends above the fascia (Figure 1), an immediate revision may not be necessary. However, if the length of necrosis is more than 1 or 2 cm, early revision is recommended to prevent future stenosis[10]. If the necrosis extends below the fascia (Figure 2), an immediate surgery is required with resection of the ischemic bowel and refashioning of the new stoma[4,10,11,16,17].
Figure 1.
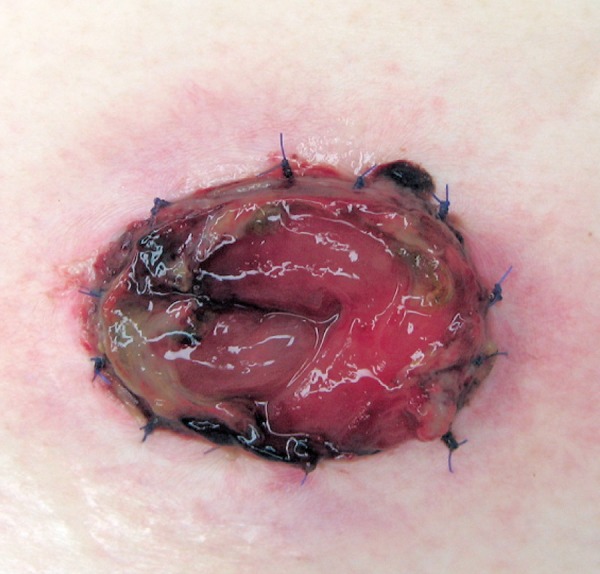
Stoma necrosis above the fascia.
Figure 2.
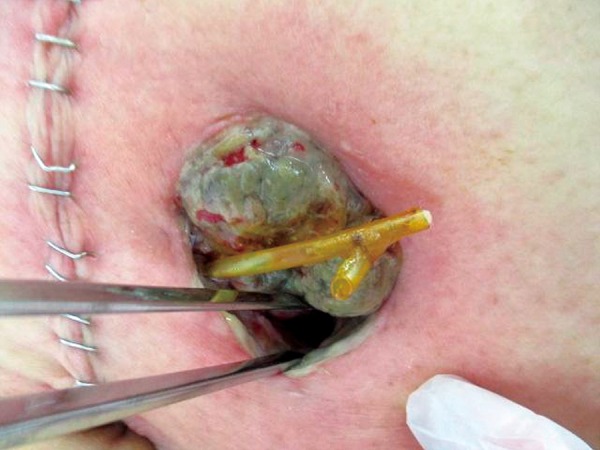
Stoma necrosis below the fascia.
Fluid and electrolyte imbalances
A newly formed ileostomy usually functions within 24 h and produces 1200 mL of watery stool per day (500-2000 mL/day)[18]. However, the amount of ileostomy output can be maintained at 300-700 mL/day, called ileostomy adaptation[11]. The underlying mechanism is a higher mean concentration of plasma aldosterone because of chronic depletion of fluid and sodium through watery stool from ileostomy[11]. Although this compensation occurs, the risk of developing severe dehydration is high in the immediate postoperative period when the oral intake of fluids is insufficient[11]. Prolonged hyperaldosteronism leads to hypokalemia and hypomagnesemia because of electrolyte imbalances. Dehydration is a major cause of readmission after stoma creation[19-21].
When the amount of fluid from the stoma exceeds 1000-2000 mL/day, it is called high output stoma (HOS), which occurs in 16% of patients with stoma creation[18,22]. It is caused by single or multiple factors, including partial bowel obstruction, intra-abdominal sepsis, prokinetic drugs (e.g., metoclopramides), sudden withdrawal of steroids or opiates, and enteritis with Clostridium difficile infection. Diuretics, coexisting diabetes mellitus, and total proctocolectomy are the risk factors for HOS[20,23], while some studies identified no specific risk factors[18,19,22].
Close monitoring of body weight, fluid balance, serum biochemistry, and electrolytes is mandatory in the immediate postoperative period. Abdominal computed tomography is helpful to identify intra-abdominal abscesses and bowel obstruction. Fluid and electrolyte resuscitation using intravenous supplements, restriction of fluid intake to 500-1000 mL/day, avoiding intake of hypotonic drinks (tea, coffee, and fruit juice), and antidiarrheal or antiperistaltic medications, such as loperamide, proton-pump inhibitors, codeine, cholestyramine, or somatostatin analogs, are recommended in the treatment protocol[18,22]. A recent pilot study showed that an intensive surveillance program comprising regular hospital visits and telephone interviews decreased both readmission rates and costs[24]; however, conflicting results were reported in a randomized study[25].
Mucocutaneous separation
Mucocutaneous separation (MCS) is characterized by partial or circumferential detachment of the mucosa from the peristomal skin. The causes of MCS include infection, diabetes mellitus, corticosteroids, malnutrition, excessive tension on the stoma, and stoma necrosis[9,10]. The incidence of MCS is 3.7%-9.7%[12,26,27].
MCS can be treated conservatively by local wound care. The separated area is irrigated with saline, and skin barrier powder is used to absorb exudates and fill the defect before applying the pouching system[1,9,28]. If the separation is deep, it may be effective to fill the separation using alginate or gelling fiber and cover it with a solid hydrocolloid or the pouch's skin barrier[1]. MCS is mostly cured with appropriate wound management; however, close observation is needed in cases of circumferential MCS because of subsequent retraction of stenosis.
Retraction
Retraction is the inversion of the mucocutaneous junction toward the abdominal wall, resulting in poor fitting of the stoma appliance and bowel leakage. Retraction is typically seen in the early postoperative period and may occur secondary to ischemia in the follow-up. The incidence of retraction is 2.9%-5.4%[26,29,30]. Retraction is more frequent in patients with high body mass index, and its suggested mechanism is difficulty in mobilizing and exteriorizing the thickened mesentery[31].
A stoma rod has traditionally been used to prevent retraction; however, a recent systematic review and meta-analysis showed that a stoma rod for loop stoma construction did not prevent stoma retraction[29]. Moreover, the incidence of peristomal dermatitis and stoma necrosis was significantly higher when a stoma rod was used[29]. In mildly symptomatic patients, a convex appliance may be useful to decrease bowel leakage. In severely symptomatic patients, local repair with partial mobilization of the proximal bowel can be attempted, although the definitive treatment is a stoma revision surgery that secures adequate bowel length and blood supply[3,10].
Late Complications
Parastomal hernia
Parastomal hernia (PSH) is an incisional hernia at the stoma site. The incidence of PSH varies from 3% to 50% because of heterogeneities in the definition of PSH, diagnostic modalities (clinical or radiological), patient population, stoma type, and follow-up duration[32-36]. The incidence of PSH increases over time and is more than 30%, 40%, and 50% by 1 year, 2 years, and longer follow-up durations, respectively[33]. Symptoms of PSH include peristomal esthetic complaints, discomfort, pain, appliance leakage, difficulty in fitting appliances, bowel obstruction, and incarceration[4,35,37]. Table 1 summarizes the risk factors for PSH.
Table 1.
| Patient factors |
| • Obesity |
| • Malnutrition |
| • Advanced age |
| • Smoking |
| • Collagen abnormalities |
| • Corticosteroid use |
| • Postoperative wound sepsis |
| • Ascites |
| • Abdominal distention |
| • Chronic constipation |
| • Obstructive uropathy |
| • Chronic obstructive lung disease |
| Surgical technical factors |
| • Inappropriate stoma site selection |
| • Oversized fascial trephine |
| • Excessive splitting and stretching of abdominal rectus muscle |
| • Epigastric nerve denervation |
| • Emergency stoma creation |
Non-invasive approaches to prevent PSH include nursing strategies for patient education in the management of body weight, exercise, and increasing the awareness of risk factors for PSH[38]. Surgical prevention includes extraperitoneal rather than transperitoneal route of construction[35] and prophylactic synthetic mesh placement[15,39] in the initial stoma creation. Management of PSH can be surgical or nonsurgical. The nonsurgical treatment includes the use of a hernia support belt and flexible pouching system and discontinuation of stoma irrigation[1]. An elective surgery may be indicated depending on the patient's comorbidities and symptoms, after a risk-benefit analysis. Emergency surgery is required for nonresolving bowel obstruction and incarcerated PSH[32,40]. In the surgery, direct suture repair or stoma relocation is not recommended because of a high recurrence rate of more than 60%. Mesh repair is considered as the standard treatment of PSH, including onlay, sublay, and open intraperitoneal mesh placement, with recurrence rates of 17%, 6.9%, and 9.2%, respectively[41]. In the laparoscopic approach, the Sugarbaker technique has a lower recurrence rate than the Keyhole technique (12% vs 35%)[41].
However, the nonoperative treatment of PSH has a lower cross-over rate (21%) than the surgical treatment, most of which is not emergency surgery[37]. Moreover, a recent nation-wide cohort study showed that the cumulative incidence of PSH causing symptoms or requiring surgery after 5 years is only 7.7%[36]. Therefore, surgical intervention for PSH should be solely indicated for symptomatic patients, and the relatively high morbidity (13%), mortality (6.3%), and recurrence (11% at 3 years) rates[40] must be informed to the patients before the treatment.
Stoma prolapse
Stoma prolapse is a full-thickness protrusion of the bowel through the stoma site, with incidence rate of 2%-3% in ileostomies and 2%-10% in colostomies[4,26,42-44]. Transverse loop colostomies are most susceptible to stoma prolapse, with an incidence rate as high as 30%[3,11]. Endostomas have lower incidence rate than loop stomas. The risk factors for stoma prolapse are advanced age, obesity, increased intra-abdominal pressure, chronic obstructive pulmonary disease, bowel redundancy, and weak fascia[4,10]. Factors associated with the surgical technique can cause stoma prolapse, including improper stoma site (outside the rectal muscle), oversized aperture, redundancy of the bowel at the stoma site, and the space between the abdominal wall and the stoma[10,45].
The main symptoms are skin irritation, difficulty fitting appliances, and mucosal ulceration with bleeding resulting from prolonged mucosal exposure[3,10]. Stoma prolapse also carries a high risk of bowel obstruction, incarceration, and strangulation[4]. Stoma prolapse can be classified into fixed and sliding types[3,4]. The fixed type is a constant prolapse, most commonly caused by improper construction of the stoma with excessive protrusion beyond the abdominal wall. The sliding type is an intermittent prolapse occurring with increased intra-abdominal pressure, more susceptible to incarceration. Typically, prolapse occurs at the efferent (distal) limb in loop stomas. For acute stoma prolapse, manual reduction can be attempted at the bed side. In cases of significant bowel edema, topical granulated sugar may be effective as an osmotic therapy[3,10,46]. Once the prolapse has reduced, adjusting the pouch size and the opening in the skin barrier may prevent trauma. A one-piece pouching system can be used[1].
The definitive treatment of stoma prolapse is surgery. If nonreducible, ischemic prolapse results in incarceration or strangulation, an emergency surgery is indicated. For reducible prolapse, surgery can be elective. If the stoma functions as fecal diversion, stoma prolapse is resolved by stoma reversal. In most cases, stoma prolapse can be managed by local surgical procedures. Intraluminal bowel fixation or resection of the prolapsed bowel segment with rematuration using modified Delorme or Altemeier procedures are the conventional techniques for local revision[4,10]. Maeda et al. first reported minimally invasive technique using a liner stapling device[47]. A curved stapling device can also be used[48], and these minimally invasive techniques can be performed with local anesthetic agents or under intravenous sedation[48-50]. Moreover, a linear stapling device allows an excision of the irreducible bowel and complete closure of the distal limb of the stoma, in patients with incarcerated loop stoma prolapse when defunction of the distal colon and rectum is not needed[51].
However, data on the long-term success rate of the local procedures are few; one study showed an acceptable recurrence of approximately 10% in the short-term follow-up[50]. In cases of failure of local surgical procedures, relocation of the stoma with laparotomy is indicated.
Parastomal varices
In patients with portal hypertension, enlarged and dilatated venous channels develop at the junction between the high-pressure portal and the low-pressure system in venous systems. The collateral portosystemic anastomoses form at the mucocutaneous junction of the stoma, resulting in engorged and pressurized subcutaneous vasculature, as the caput medusa[4,52]. It causes recurrent and torrential bleeding, affecting 5% of all ostomates[52,53]. Parastomal varices are mostly seen in patients with primary sclerosing cholangitis and liver cirrhosis, and 70% of the bleeding cases were observed in ileostomy[52]. The average time from stoma creation to the first documented episode of bleeding was 74 months (range: 1-480 months; median: 48 months)[52].
In cases of acute bleeding, direct pressure with gauze soaked in 1% epinephrine should be initially attempted. Suture ligation and/or electrocauterization may be needed for refractory bleeding. When hemostasis is achieved, refitting the appliance is considered to prevent trauma[10]. Rigid pouching products should be avoided because they may cause injury to the stoma[1]. Nonoperative local management resulted in 85% of re-bleeding rate; therefore, it is the rule rather than an exception[3,52]. Local operative management can be indicated in patients with significant systemic risks or short life expectancy. The surgical procedures include stoma revision and relocation, muco-cutaneous disconnection, or a circumferential suture technique. However, the re-bleeding rate is 81%, comparable to the nonoperative management[52]. Surgical portosystemic shunt procedures with mesocaval, portocaval, or splenorenal anastomosis can achieve reduced re-bleeding rates (38%) compared to the local management; however, these invasive procedures may be associated with increased morbidity and mortality[52]. Percutaneous embolization using a direct or transhepatic approach also has a moderate success in decreasing the re-bleeding rate (45%)[52,53]. The complications associated with this percutaneous treatment are few and limited to parastomal skin ulcers[53,54]. Transjugular intrahepatic portosystemic shunt (TIPS) with or without embolization is the most effective treatment. The re-bleeding rate is 20%, and the prevalence of TIPS for parastomal varices is increasing over time[52]. However, the complications of TIPS are hepatic encephalopathy, bleeding, biliary and hepatocellular insufficiency, and malposition or stent occlusion. The complication rate of TIPS was as high as 20% in the early 2010s, but there have been fewer procedure-related complications of TIPS in the recent studies[55,56]. Liver transplantation may be indicated for patients in whom all the above treatments fail. As prognosis of the patients with parastomal varices is associated with the underlying liver disease rather than with variceal bleeding[3], the method of management should be selected considering the patient's comorbidities and physical and social statuses.
Peristomal pyoderma gangrenosum
Pyoderma gangrenosum (PG) is a neutrophilic dermatosis associated with progressive skin ulceration, necrosis, and abscess[57,58]. Peristomal PG (PPG) is a subtype of PG that occurs in any type of intestinal stomas[57,59]. Although the etiology is unclear, inflammatory bowel diseases, autoimmune diseases, and mechanical trauma are suggested as the underlying pathophysiological factors[58]. The incidence of PPG is 0.9%-4%[58,60,61], and this rarity makes it difficult to define diagnostic and therapeutic approaches. Potential risk factors are female gender, higher body mass index, presence of autoimmune diseases, parastomal hernias, repetitive peristomal stress, irritation from bowel leakage and appliance changes, and tension or ischemia from the ostomy appliance[57,61]. Approximately 70% of the patients reported a concurrent flare of the underlying systemic disease at the time of PPG onset[57]. The reported time of PPG onset after stoma creation varies, while recent studies have reported the mean onset of 23 months[57,58]. Interestingly, these two reports also revealed the median values of onset ranging from 1.5 to 7 months[57,58].
PPG should be diagnosed based on clinical presentations that are characterized by pain, violaceous and/or undermined borders, erythema, and purulence. The differential diagnosis includes bacterial and fungal infections, chemical dermatitis, irritation from leakage, and allergic contact dermatitis. Routine biopsy and culture are not recommended because they may not provide specific findings, while aggravating the disease entity by trauma[57,59].
PPG requires long-term and multimodal treatment approaches compared to other peristomal skin ulcers. Basic skin care for PPG is to create a clean wound environment by absorbing exudates and maintaining a moist skin surface using occlusive or non-adherent dressings[57,62]. Filling the wound with an absorbent product, such as an alginate or gelling fiber, facilitates adherence of the skin barrier[1]. An example of wound management is shown in Figure 3, 4 and 5. Proper application of paste mixtures, crushed powder, and sprays is essential for an effective treatment. Mechanical trauma by pouching systems should be avoided. Mild cases without active systemic disease can be managed with topical agents as monotherapy. Corticosteroids and calcineurin inhibitors are suggested with mild recommendation[57,63]. In contrast, severe or rapidly progressive cases require systemic therapies. Corticosteroids, cyclosporine, and dapsone are suggested with mild recommendation, and the complete response rate to these agents is 50%[57]. Metronidazole, azathioprine, sulfasalazine, tacrolimus, and intravenous immunoglobulin are less commonly used but can be considered as alternatives or adjunct therapies[57]. Biologic agents, including infliximab and adalimumab, may be successful in refractory cases of PPG, regardless of the presence of inflammation[57]. Another recent review showed the effectiveness of immunomodulators, including cromolyn sodium, benzoyl peroxide, aminosalicylic acid, phenytoin, nicotine, becaplermin, and timolol[63]. Surgical intervention is the last resort for patients who do not recover with any of the aforementioned treatments, and the most effective treatment is to re-establish intestinal continuity[4,57]. Stoma revision and relocation should be avoided because of the high incidence of recurrent PPG of up to 67%[57].
Figure 3.
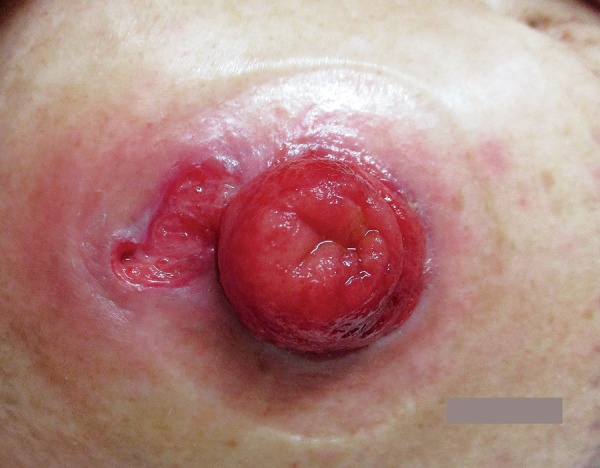
Peristomal pyoderma gangrenosum (PPG) before treatment.
Figure 4.
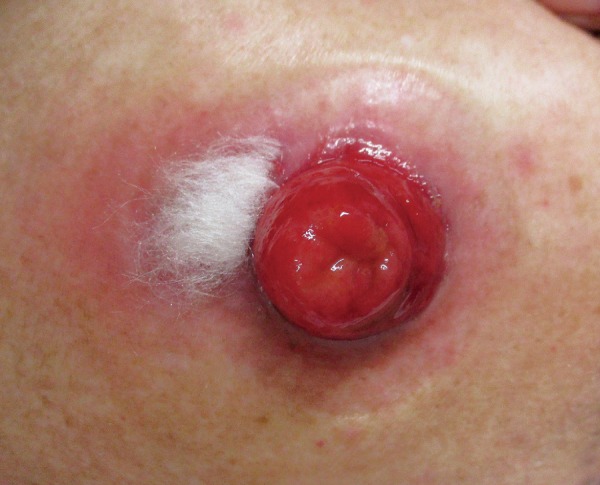
Application of alginate dressings to peristomal pyoderma gangrenosum (PPG).
Figure 5.
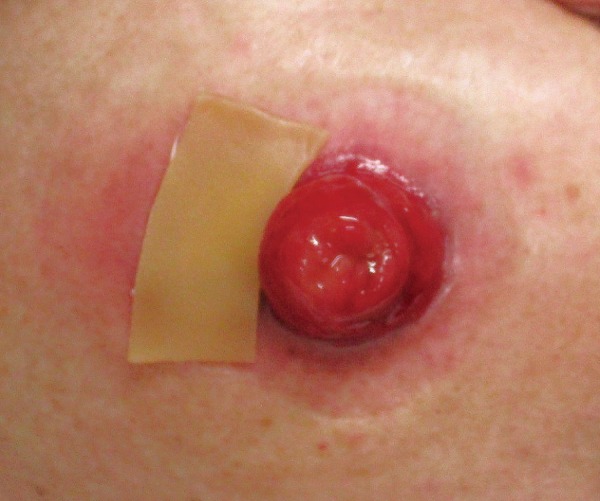
Application of wafer-type skin barrier to peristomal pyoderma gangrenosum (PPG).
Conclusions
Pre- and postoperative patient education facilitates the patient's independence in stoma care and resumption of normal activities. Healthcare providers should have basic skills and updated knowledge on the management and complications of stomas, to act as the first crisis manager for ostomates. Multidisciplinary team follow-up is crucial for optimizing the QoL of ostomates, with coordination of care and information sharing across all team members.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
Shingo Tsujinaka: Conceptualization, methodology, investigation, data curation, and wiring the original draft.
Kok-Yang Tan: Methodology, data curation, and assistance in preparing the original draft.
Yasuyuki Miyakura: Validation, revision, and editing of the manuscript.
Rieko Fukano: Conceptualization, resources (images), data curation, and visualization.
Mitsuko Oshima: Conceptualization, resources (images), data curation, and visualization.
Fumio Konishi: Critical revision and editing of the manuscript.
Toshiki Rikiyama: Critical revision and editing of the manuscript and project administration.
Approval by Institutional Review Board (IRB)
The institutional ethics committee determined that approval was not necessary for a review article.
Informed Consent
A written consent for the publication of this article with accompanying images was obtained from the patients. The consent was written in Japanese for better understanding by the patient.
References
- 1.Wound, Ostomy and Continence Nurses Society; Guideline Development Task Force. WOCN Society Clinical Guideline: Management of the Adult Patient With a Fecal or Urinary Ostomy-An Executive Summary. J Wound Ostomy Continence Nurs. 2018 Jan/Feb; 45(1): 50-8. [DOI] [PubMed] [Google Scholar]
- 2.Salvadalena G, Hendren S, McKenna L, et al. WOCN Society and ASCRS Position Statement on Preoperative Stoma Site Marking for Patients Undergoing Colostomy or Ileostomy Surgery. J Wound Ostomy Continence Nurs. 2015 May-Jun; 42(3): 249-52. [DOI] [PubMed] [Google Scholar]
- 3.Husain SG, Cataldo TE. Late stomal complications. Clin Colon Rectal Surg. 2008 Feb; 21(1): 31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnamurty DM, Blatnik J, Mutch M. Stoma Complications. Clin Colon Rectal Surg. 2017 Jul; 30(3): 193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsmo HM, Pfeffer F, Rasdal A, et al. Pre- and postoperative stoma education and guidance within an enhanced recovery after surgery (ERAS) programme reduces length of hospital stay in colorectal surgery. Int J Surg. 2016 Dec; 36(Pt A): 121-6. [DOI] [PubMed] [Google Scholar]
- 6.Colwell JC, Kupsick PT, McNichol LL. Outcome Criteria for Discharging the Patient With a New Ostomy From Home Health Care: A WOCN Society Consensus Conference. J Wound Ostomy Continence Nurs. 2016 May-Jun; 43(3): 269-73. [DOI] [PubMed] [Google Scholar]
- 7.American College of Surgeons. Ostomy Home Skills Program [https://www.facs.org/education/patient-education/skills-programs/ostomy-program]
- 8.Wound, Ostomy and Continence Nurses Society. Resources for the WOC Patient [https://www.wocn.org/page/PatientResources]
- 9.Steinhagen E, Colwell J, Cannon LM. Intestinal Stomas-Postoperative Stoma Care and Peristomal Skin Complications. Clin Colon Rectal Surg. 2017 Jul; 30(3): 184-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JT, Kumar RR. Reoperation for stoma-related complications. Clin Colon Rectal Surg. 2006 Nov; 19(4): 207-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shabbir J, Britton DC: Stoma complications: a literature overview. Colorectal Dis. 2010 Oct; 12(10): 958-64. [DOI] [PubMed] [Google Scholar]
- 12.Franklyn J, Varghese G, Mittal R, et al. A prospective randomized controlled trial comparing early postoperative complications in patients undergoing loop colostomy with and without a stoma rod. Colorectal Dis. 2017 Jul; 19(7): 675-80. [DOI] [PubMed] [Google Scholar]
- 13.Whiteley I, Russell M, Nassar N, et al. Outcomes of support rod usage in loop stoma formation. Int J Colorectal Dis. 2016 Jun; 31(6): 1189-95. [DOI] [PubMed] [Google Scholar]
- 14.Zindel J, Gygax C, Studer P, et al. A sustaining rod increases necrosis of loop ileostomies: a randomized controlled trial. Int J Colorectal Dis. 2017 Jun; 32(6): 875-81. [DOI] [PubMed] [Google Scholar]
- 15.Cross AJ, Buchwald PL, Frizelle FA, et al. Meta-analysis of prophylactic mesh to prevent parastomal hernia. Br J Surg. 2017 Feb; 104(3): 179-86. [DOI] [PubMed] [Google Scholar]
- 16.Cottam J, Richards K, Hasted A, et al. Results of a nationwide prospective audit of stoma complications within 3 weeks of surgery. Colorectal Dis. 2007 Nov; 9(9): 834-8. [DOI] [PubMed] [Google Scholar]
- 17.Robertson I, Leung E, Hughes D, et al. Prospective analysis of stoma-related complications. Colorectal Dis. 2005 May; 7(3): 279-85. [DOI] [PubMed] [Google Scholar]
- 18.Baker ML, Williams RN, Nightingale JM. Causes and management of a high-output stoma. Colorectal Dis. 2011 Feb; 13(2): 191-7. [DOI] [PubMed] [Google Scholar]
- 19.Hayden DM, Pinzon MC, Francescatti AB, et al. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: preventable or unpredictable? J Gastrointest Surg. 2013 Feb; 17(2): 298-303. [DOI] [PubMed] [Google Scholar]
- 20.Messaris E, Sehgal R, Deiling S, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012 Feb; 55(2): 175-80. [DOI] [PubMed] [Google Scholar]
- 21.Justiniano CF, Temple LK, Swanger AA, et al. Readmissions With Dehydration After Ileostomy Creation: Rethinking Risk Factors. Dis Colon Rectum. 2018 Nov; 61(11): 1297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arenas Villafranca JJ, Lopez-Rodriguez C, Abiles J, et al. Protocol for the detection and nutritional management of high-output stomas. Nutr J. 2015 May 9; 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda M, Takahashi H, Haraguchi N, et al. Factors predictive of high-output ileostomy: a retrospective single-center comparative study. Surg Today. 2019 Jun; 49(6): 482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaffer VO, Owi T, Kumarusamy MA, et al. Decreasing Hospital Readmission in Ileostomy Patients: Results of Novel Pilot Program. J Am Coll Surg. 2017 Apr; 224(4): 425-30. [DOI] [PubMed] [Google Scholar]
- 25.Grahn SW, Lowry AC, Osborne MC, et al. System-Wide Improvement for Transitions After Ileostomy Surgery: Can Intensive Monitoring of Protocol Compliance Decrease Readmissions? A Randomized Trial. Dis Colon Rectum. 2019 Mar; 62(3): 363-70. [DOI] [PubMed] [Google Scholar]
- 26.Miyo M, Takemasa I, Ikeda M, et al. The influence of specific technical maneuvers utilized in the creation of diverting loop-ileostomies on stoma-related morbidity. Surg Today. 2017 Aug; 47(8): 940-50. [DOI] [PubMed] [Google Scholar]
- 27.Sung YH, Kwon I, Jo S, et al. Factors affecting ostomy-related complications in Korea. J Wound Ostomy Continence Nurs. 2010 Mar-Apr; 37(2): 166-72. [DOI] [PubMed] [Google Scholar]
- 28.Boyd-Carson W, Thompson MJ, Trainor B, et al. Mucocutaneous separation. Nurs Stand. 2004 Jan; 18(17): 41-3. [PubMed] [Google Scholar]
- 29.Mohan HM, Pasquali A, O'Neill B, et al. Stoma rods in abdominal surgery: a systematic review and metaanalyses. Tech Coloproctol. 2019 Mar; 23(3): 201-6. [DOI] [PubMed] [Google Scholar]
- 30.Guenaga KF, Lustosa SA, Saad SS, et al.: Ileostomy or colostomy for temporary decompression of colorectal anastomosis. Cochrane Database Syst Rev. 2007 Jan; 24(1): Cd004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arumugam PJ, Bevan L, Macdonald L, et al. A prospective audit of stomas--analysis of risk factors and complications and their management. Colorectal Dis. 2003 Jan; 5(1): 49-52. [DOI] [PubMed] [Google Scholar]
- 32.ACPGBI Parastomal Hernia Group. Prevention and treatment of parastomal hernia: a position statement on behalf of the Association of Coloproctology of Great Britain and Ireland. Colorectal Dis. 2018 Jul; 20(Suppl 2): 5-19. [DOI] [PubMed] [Google Scholar]
- 33.Antoniou SA, Agresta F, Garcia Alamino JM, et al. European Hernia Society guidelines on prevention and treatment of parastomal hernias. Hernia. 2018 Feb; 22(1): 183-98. [DOI] [PubMed] [Google Scholar]
- 34.Findlay JM, Wood CPJ, Cunningham C. Prophylactic mesh reinforcement of stomas: a cost-effectiveness meta-analysis of randomised controlled trials. Tech Coloproctol. 2018 Apr; 22(4): 265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroese LF, de Smet GH, Jeekel J, et al. Systematic Review and Meta-Analysis of Extraperitoneal Versus Transperitoneal Colostomy for Preventing Parastomal Hernia. Dis Colon Rectum. 2016 Jul; 59(7): 688-95. [DOI] [PubMed] [Google Scholar]
- 36.Tivenius M, Nasvall P, Sandblom G. Parastomal hernias causing symptoms or requiring surgical repair after colorectal cancer surgery-a national population-based cohort study. Int J Colorectal Dis. 2019 Jul; 34(7): 1267-72. [DOI] [PubMed] [Google Scholar]
- 37.Kroese LF, Lambrichts DPV, Jeekel J, et al. Non-operative treatment as a strategy for patients with parastomal hernia: a multicentre, retrospective cohort study. Colorectal Dis. 2018 Jun; 20(6): 545-51. [DOI] [PubMed] [Google Scholar]
- 38.Osborne W, North J, Williams J. Using a risk assessment tool for parastomal hernia prevention. Br J Nurs. 2018 Mar 8; 27(5): 15-9. [DOI] [PubMed] [Google Scholar]
- 39.Jones HG, Rees M, Aboumarzouk OM, et al. Prosthetic mesh placement for the prevention of parastomal herniation. Cochrane Database Syst Rev. 2018 Jul 20; 7: Cd008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helgstrand F, Rosenberg J, Kehlet H, et al. Risk of morbidity, mortality, and recurrence after parastomal hernia repair: a nationwide study. Dis Colon Rectum. 2013 Nov; 56(11): 1265-72. [DOI] [PubMed] [Google Scholar]
- 41.Hansson BM, Slater NJ, van der Velden AS, et al. Surgical techniques for parastomal hernia repair: a systematic review of the literature. Ann Surg. 2012 Apr; 255(4): 685-95. [DOI] [PubMed] [Google Scholar]
- 42.Correa-Marinez A, Grenabo J, Bock D, et al. The type of stoma matters-morbidity in patients with obstructing colorectal cancer. Int J Colorectal Dis. 2018 Dec; 33(12): 1773-80. [DOI] [PubMed] [Google Scholar]
- 43.Gavriilidis P, Azoulay D, Taflampas P. Loop transverse colostomy versus loop ileostomy for defunctioning of colorectal anastomosis: a systematic review, updated conventional meta-analysis, and cumulative meta-analysis. Surg Today. 2019 Feb; 49(2): 108-17. [DOI] [PubMed] [Google Scholar]
- 44.Persson E, Berndtsson I, Carlsson E, et al. Stoma-related complications and stoma size - a 2-year follow up. Colorectal Dis. 2010 Oct; 12(10): 971-6. [DOI] [PubMed] [Google Scholar]
- 45.Maeda K, Maruta M, Utsumi T, et al. Pathophysiology and prevention of loop stomal prolapse in the transverse colon. Tech Coloproctol. 2003 Jul; 7(2): 108-11. [DOI] [PubMed] [Google Scholar]
- 46.Mohammed O, West M, Chandrasekar R. Granulated sugar to reduce an incarcerated prolapsed defunctioning ileostomy. BMJ Case Rep. 2013 Feb 28; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda K, Maruta M, Utsumi T, et al. Local correction of a transverse loop colostomy prolapse by means of a stapler device. Tech Coloproctol. 2004 Mar; 8(1): 45-6. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson HJ, Bhalerao S. Correction of end colostomy prolapse using a curved surgical stapler, performed under sedation. Tech Coloproctol. 2010 Jun; 14(2): 165-7. [DOI] [PubMed] [Google Scholar]
- 49.Monette MM, Harney RT, Morris MS, et al. Local repair of stoma prolapse: Case report of an in vivo application of linear stapler devices. Ann Med Surg (Lond). 2016 Nov; 11: 32-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masumori K, Maeda K, Hanai T, et al. Short-term outcomes of local correction of stoma prolapse with a stapler device. Tech Coloproctol. 2013 Aug; 17(4): 437-40. [DOI] [PubMed] [Google Scholar]
- 51.Masumori K, Maeda K, Koide Y, et al. Simple excision and closure of a distal limb of loop colostomy prolapse by stapler device. Tech Coloproctol. 2012 Apr; 16(2): 143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pennick MO, Artioukh DY. Management of parastomal varices: who re-bleeds and who does not? A systematic review of the literature. Tech Coloproctol. 2013 Apr; 17(2): 163-70. [DOI] [PubMed] [Google Scholar]
- 53.Setaihi R, Rousset P, Muller A, et al. Percutaneous treatment of parastomal varices: Direct or transhepatic approach? Diagn Interv Imaging. 2016 Apr; 97(4): 491-4. [DOI] [PubMed] [Google Scholar]
- 54.Minami S, Okada K, Matsuo M, et al. Treatment of bleeding stomal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol. 2007 Jan; 42(1): 91-5. [DOI] [PubMed] [Google Scholar]
- 55.Bureau C, Thabut D, Oberti F, et al. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017 Jan; 152(1): 157-63. [DOI] [PubMed] [Google Scholar]
- 56.Rabei R, Mathevosian S, Tasse J, et al. Primary constrained TIPS for treating refractory ascites or variceal bleeding secondary to hepatic cirrhosis. Br J Radiol. 2018 Feb; 91(1083): 20170409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Afifi L, Sanchez IM, Wallace MM, et al. Diagnosis and management of peristomal pyoderma gangrenosum: A systematic review. J Am Acad Dermatol. 2018 Jun; 78(6): 1195-204.e1. [DOI] [PubMed] [Google Scholar]
- 58.Toh JWT, Young CJ, Rickard M, et al. Peristomal pyoderma gangrenosum: 12-year experience in a single tertiary referral centre. ANZ J Surg. 2018 Oct; 88(10): E693-e7. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Shen B. Management of Crohn's Disease and Complications in Patients With Ostomies. Inflamm Bowel Dis. 2018 May 18; 24(6): 1167-84. [DOI] [PubMed] [Google Scholar]
- 60.Funayama Y, Kumagai E, Takahashi K, et al. Early diagnosis and early corticosteroid administration improves healing of peristomal pyoderma gangrenosum in inflammatory bowel disease. Dis Colon Rectum. 2009 Feb; 52(2): 311-4. [DOI] [PubMed] [Google Scholar]
- 61.Wu XR, Mukewar S, Kiran RP, et al. Risk factors for peristomal pyoderma gangrenosum complicating inflammatory bowel disease. Journal of Crohn's & colitis. 2013 Jun; 7(5): e171-7. [DOI] [PubMed] [Google Scholar]
- 62.Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010 Apr; 62(4): 646-54. [DOI] [PubMed] [Google Scholar]
- 63.Baltazar D, Haag C, Gupta AS, et al. A Comprehensive Review of Local Pharmacologic Therapy for Pyoderma Gangrenosum. Wounds. 2019 Jun; 31(6): 151-7. [PubMed] [Google Scholar]


