Abstract
Genotoxicity is a critical endpoint of toxicity to regulate environmental chemicals. Genotoxic chemicals are believed to have no thresholds for the action and impose genotoxic risk to humans even at very low doses. Therefore, genotoxic carcinogens, which induce tumors via genotoxic mechanisms, are regulated more strictly than non-genotoxic carcinogens, which induce tumors through non-genotoxic mechanisms such as hormonal effects, cell proliferation and cell toxicity. Although Ames bacterial mutagenicity assay is the gold standard to identify genotoxicity of chemicals, the genotoxicity should be further examined in rodents because Ames positive chemicals are not necessarily genotoxic in vivo. To better evaluate the genotoxicity of chemicals in a whole body system, gene mutation assays with gpt delta transgenic mice and rats have been developed. A feature of the assays is to detect point mutations and deletions by two distinct selection methods, ie, gpt and Spi− assays, respectively. The Spi− assay is unique in that it allows analyses of deletions and complex DNA rearrangements induced by double-strand breaks in DNA. Here, I describe the concept of gpt delta gene mutation assays and the application in food safety research, and discuss future perspectives of genotoxicity assays in vivo.
Key words: : gpt delta, transgenic, gene mutation, deletion, food safety
1. Introduction
Humans are continuously exposed to a variety of chemicals, some of which interact with DNA, thereby inducing mutations and cancer. The most notable example of environmental mutagens and carcinogens is cigarette smoke, which plays a major role in the etiology of lung cancer and a variety of chronic diseases1). Cigarette smoke is a mixture of 4,000 chemicals and contains more than 60 known carcinogens2,3). The International Agency for Research on Cancer (IARC) has evaluated nearly 1,000 chemicals for their potential to induce cancer in humans and concluded that about 100 chemicals are carcinogenic to humans (Group 1)4). The conclusion is supported by epidemiological evidence as well as rodent carcinogenicity assays and mechanistic studies. Group 1 chemicals including aflatoxin B1 and 4-aminobiphenyl and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (nicotine-derived nitrosaminoketone (NNK)) are all potent mutagens and carcinogens.
To identify mutagenic and carcinogenic chemicals in the environment and effectively evaluate the health effects, international organizations have set up guidelines of genotoxicity assays. The examples are guidelines by International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) for pharmaceuticals5) and those by the Organization for Economic Co-operation and Development (OECD) for industrial chemicals6). In general, (1) a bacterial reverse mutation assay (Ames test), (2) a chromosome aberration assay (CA), a micronucleus assay (MN) or a mouse lymphoma gene mutation assay in vitro (MLA) and (3) an MN assay in vivo constitute a standard battery for genotoxicity assays (Table 1). However, it has been reported that CA, MN and MLA in vitro have many false positives, ie, positive in the assays but negative in rodent two-year cancer bioassays7). In other words, these assays have low specificity to predict rodent carcinogens. Therefore, an alternative battery that skips CA, MN and MLA in vitro but includes a second in vivo assay has been proposed by ICH8). Transgenic rodent gene mutation assays, which I will describe below, are one of the candidates for the second in vivo assay. The assays detect heritable genetic changes, ie, gene mutations, and thus are suitable to examine the results of Ames test, which also detects gene mutations.
Table 1. Remodeling of the standard battery of genotoxicity assays.
| Classical battery | Alternative battery |
|---|---|
| 1. Ames test | 1. Ames test |
| 2. CA, MN or MLA in vitro | |
| 3. MN or CA in vivo | 2. MN in vivo |
| 3. The second in vivo test |
These batteries are recommended by ICH as guideline S2(R1)8), where the classical battery and the alternative battery are called option 1 and 2, respectively. In the alternative battery, it is recommended that two different tissues are examined with two in vivo assays, such as bone marrow by micronucleus test (MN) and liver by the second in vivo test. Chromosome aberration test (CA) in vivo is not recommended in the alternative battery because CA does not detect aneuploidies. In the classical battery, MN in vitro can detect aneuploidies and thus CA in vivo is included in the battery.
2. Gene Mutation Assays in Vivo
Before establishment of transgenic rodent gene mutation assays in 1980s and 1990s, in vivo mutation assays were limited to particular tissues such as melanoblasts or spleen lymphocytes. An example of the classical in vivo gene mutation assays is “mouse spot test” where developing embryo are exposed to test chemicals9). If mutations are induced in the genes that control the pigmentation of coat color of mice, the offspring will have spots of changed color in the coat. The frequency of such spots in the treated group is compared with that of spots in the control group. Although this assay surely detects various gene mutations in vivo, the target cells are restricted to melanoblasts in embryo. The assay was deleted from OECD test guidelines in 2014, because few people used it for evaluation of genotoxicity of chemicals. Another example is “mouse spleen lymphocytes assay”, where mice are treated with chemicals and then the spleen lymphocytes are cultured in vitro to select 6-thioguanine (6-TG) mutants10). If mutations occur in the hypoxanthine phosphoribosyltransferase (Hprt) locus in the spleen, the mutated lymphocytes should be resistant to 6-TG in vitro. Although this assay allows detection of mutations in adult mice, the target organ is only spleen and the mutant selection in vitro takes about 10 days. No molecular analyses of the 6-TG mutations are allowed. Thus, the assay is not popular in the field of genotoxicology. The transgenic rodent gene mutation assays are superior to the classical in vivo assays in that they allow detection of mutations in any organ of rodents including germ cells and molecular analyses of the mutants by DNA sequencing11).
Key technological innovation for the transgenic rodent gene mutation assays is rescue of lambda phage vectors from mammalian chromosomes by in vitro packaging reactions. Mutation is a very rare event, ie, 1 × 10−5 to 10−6 or 0.001 to 0.0001%. Thus, reporter genes for mutations should be rescued from mammalian chromosomes with high yield and high fidelity. The innovative idea to rescue lambda phage DNA carrying reporter genes for mutations by in vitro packaging reactions was first reported by Glazer et al12). They introduced multiple copies of a lambda phage vector containing supF in Escherichia coli, a reporter gene for mutations, into the chromosomes of mouse L-cells and irradiated the cells with ultraviolet light (UV). Then they rescued the phage with in vitro packaging extracts and introduced the rescued phages to E. coli host cells to identify mutant phages. This strategy worked well, and they identified a characteristic mutation of C to T in the reporter gene after recovery from mammalian cells irradiated with UV. The in vitro packaging extracts are extracts of E. coli having defective lambda phage, which enable excision of lambda DNA from the mammalian chromosome and promote packaging of the excised DNA into phage particles13).
The idea to rescue the lambda vector DNA carrying reporter genes for mutations from mammalian cells by the in vitro packaging reactions was applied to rescue the reporter genes from transgenic mice. Two prototypes of transgenic mice for mutation assays were established in late 1980s. Muta™ Mouse has a lambda vector carrying lacZ14) and Big Blue® Mouse has a lambda vector with lacI15). The lacZ and lacI are genes of lac operon of E. coli, where lacI encodes the repressor protein LacI, and lacZ encodes β-galactosidase, whose expression is repressed by LacI. Initially, the Muta™ Mouse assay was very time consuming because one had to find a very small number of colorless mutant plaques among millions of blue plaques. The lacZ mutants are colorless because they cannot hydrolyze X-gal, which is a substrate of β-galactosidase and produces blue color after the hydrolysis, while the wild-type phage produces blue plaques because of the intact enzyme activity. The mutant selection in Big Blue® Mouse was better than that of Muta™ Mouse because the mutant plaques were blue and the wild-type plaques were colorless. The intact lacI gene produces a repressor protein LacI, which shuts down the expression of β-galactosidase, resulting in colorless plaques. On the contrary, the lacI mutants allow the expression of β-galactosidase, which results in blue plaques. Nevertheless, both selections were time-consuming and expensive because X-gal is an expensive agent. To circumvent the problem, a positive selection for lacZ mutations was introduced. E. coli mutants deficient in galE are sensitive to galactose because they accumulate a toxic intermediate, ie, galactose-6-phosphate, generated from galactose11). The galE gene encodes galactose epimerase, which converts the toxic galactose-6-phosphate to non-toxic glucose-6-phosphate. This fact was employed for the development of the positive selection. E. coli galE cells infected with lambda phage having wild-type lacZ metabolize phenyl-β-D-galactoside (P-gal) into the toxic intermediate and result in cell death with no phage propagation. Only lacZ mutant phage can form plaques in the lawn of E. coli galE cells in the presence of P-gal, which is converted to galactose by the action of β-galactosidase. This positive selection substantially enhanced the performance of lacZ mutation assay and reduced the cost. Nevertheless, the coding size of lacZ is about 3 kilo base pairs (kb), which is not short enough for routine DNA sequencing for identification of mutations. In 1996, the cII gene of lambda phage was introduced as a novel reporter gene for mutations16). The cII gene encodes a repressor protein involved in lambda lysogenic/lytic cycle. In the hfl− E. coli, phages with active cII gene cannot enter a lytic cycle and form no plaques because of the deficit of Hfl protease. This protease normally degrades CII protein and lets the phage enter a lytic cycle. The only phages with inactive cII mutants can make plaques with the E. coli hfl− cells, which lack Hfl protease that degrades CII protein. Thus, this is a positive selection. The coding size of cII is about 300 base pairs (bp), which is about 1/10 of lacZ and 1/3 of lacI. In addition, the cII selection is applicable to both Big Blue® Mouse and Muta™ Mouse. Therefore, the cII selection is much more frequently used for Big Blue® and Muta™ Mouse assays than the original lacI or lacZ selections.
3. Establishment of Gpt Delta Mice
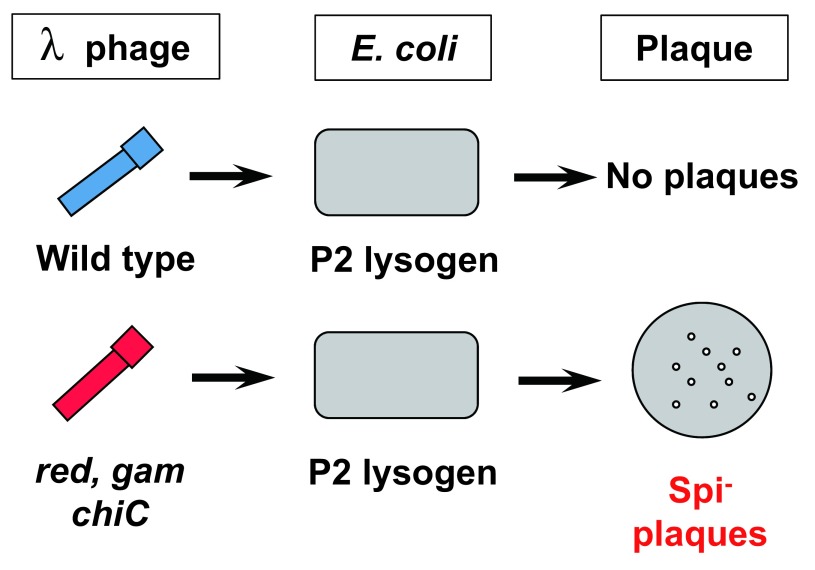
Despite the advancement of the positive selections, both assays with Muta™ Mouse and Big Blue® Mouse were insensitive to deletions, which are induced by radiation or chemical treatments with cross-linking agents such as mitomycin C (MMC)17,18). This insensitivity may be due to the high spontaneous background levels of lacZ, lacI and cII, ie, in the mid-10−5. The spontaneous mutations are mainly due to deamination of 5-methylcytosine to thymine in the CpG sites, ie, C to T transitions, since bacterial transgenes are highly methylated in mammalian cells19). Because of the high levels of spontaneous base substitutions, rare mutations such as deletions are overlooked by the selections. For example, when the level of base substitutions is 5 × 10−5 and deletion is 5 × 10−7, small number of deletion mutations will be hard to be identified. To effectively detect deletion mutations, I decided to use Spi− selection when I started to develop gpt delta transgenic mouse assay20–22) (Fig. 1). Spi− stands for Sensitive to P2 Interference. Wild-type lambda phages can lyse E. coli and make phage plaques. However, if P2 phage is already in the chromosome of E. coli (P2 lysogen), the wild-type lambda phage cannot lyse P2 lysogen. This phenomenon is called P2 interference. Interestingly, defective lambda phages, which lack the functions of the red and gam genes, can lyse P2 lysogen, thereby making phage plaques. These plaques are called Spi− plaques. The gam gene product inactivates exonuclease V, which is encoded by recBCD genes in E. coli. In P2 lysogen, there is a gene old, which kills the host E. coli when the exonuclease V is inactivated. When P2 lysogen is infected with wild-type lambda phage, the gene product of old kills the host cells, thereby preventing the propagation of incoming lambda phage. In the absence of the red and gam genes, the defective phage has a chance to propagate in P2 lysogen because the exonuclease V is not inactivated. However, the incoming lambda phage must have a Chi sequence (GCTGGTGG) to prevent the DNA from digestion by the exonuclease V. The chi sequence inactivates the recD gene product, thereby inhibiting the exonuclease. The red gene is composed of redA and redB. They are involved in recombination that resolve the replication form of phage DNA. The Spi− phage that are red− and gam− must be propagated in recA+ E. coli to allow efficient packaging DNA. No satisfactory explanation of Red in Spi− selection has been offered.
Fig. 1.
Principle of Spi− selection.
This selection takes advantage of the fact that propagation of wild-type lambda phage is restricted in P2 lysogen, which is E. coli having P2 prophage. Defective lambda phage deficient in the functions of both the gam and red genes can propagate well in P2 lysogen and display Spi− plaques as long as they carry the chiC mutation and the host strain is recA+.
Since the red and gam genes are located side-by-side in the lambda DNA and inactivation of two genes by two independent point mutations is very rare, the inactivation of both red and gam genes is most likely induced by deletions in the region. The chiC sequence makes the Spi− plaques bigger and visible. Spi− selection is unique in that it preferentially and positively selects deletion mutations. As expected, deletion mutations and complex gene rearrangements were successfully identified by Spi− selection when gpt delta mice were irradiated with heavy ion or γ-ray, or treated with MMC23,24). Molecular analyses of the Spi− mutants revealed the characteristics of junctions of the deletion mutants, ie, some deletion mutants have short overlapping sequences, ie, 1–8 bp, which is sometimes called microhomology, while others have no such sequences. Because of the size limitation of in vitro packaging, the size of deletions detectable by Spi− selection is less than 10 kb. Lambda phage should have two cos sites for the packaging at the both ends and the sites should be separated by 38–51 kb in DNA. In other words, too small or too large lambda DNA cannot be packaged to the phage particles even when they have two cos sites at the end. Therefore, deletions detectable by Spi− selection are mostly intragenic deletions. Nevertheless, gpt delta mice have tandem array of more than 50 copies of about 48 kb lambda EG10 in the chromosome 1725). Thus, they have potentially detect large deletions of approximately 2.5 mega bps (Mb).
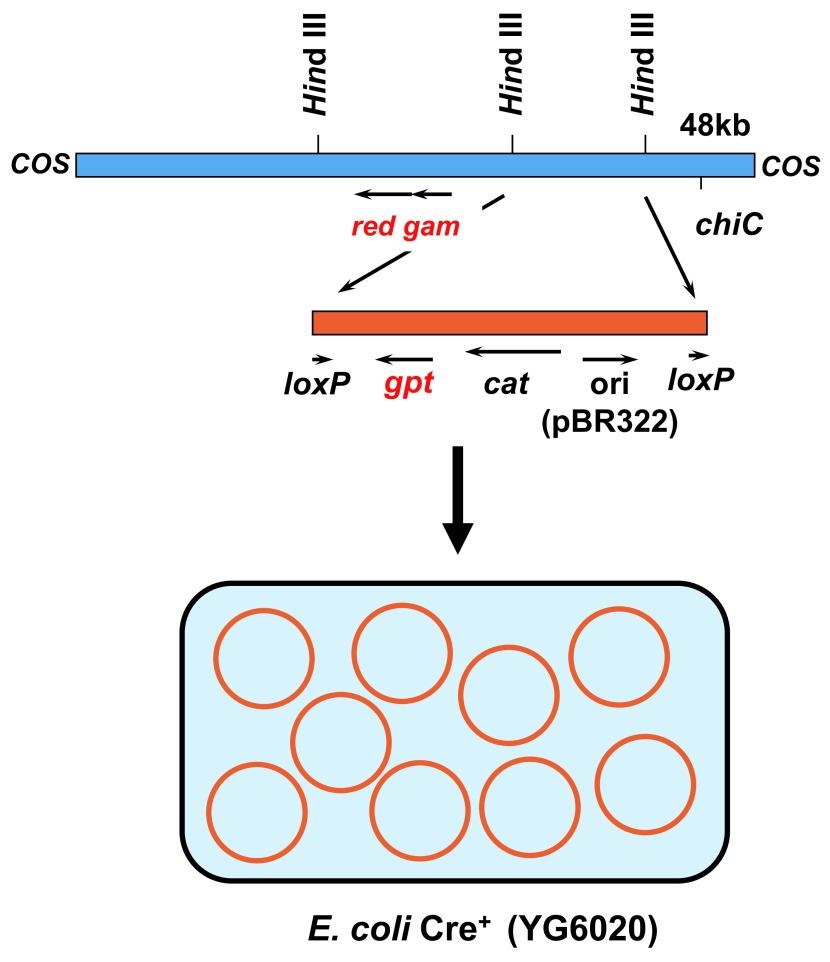
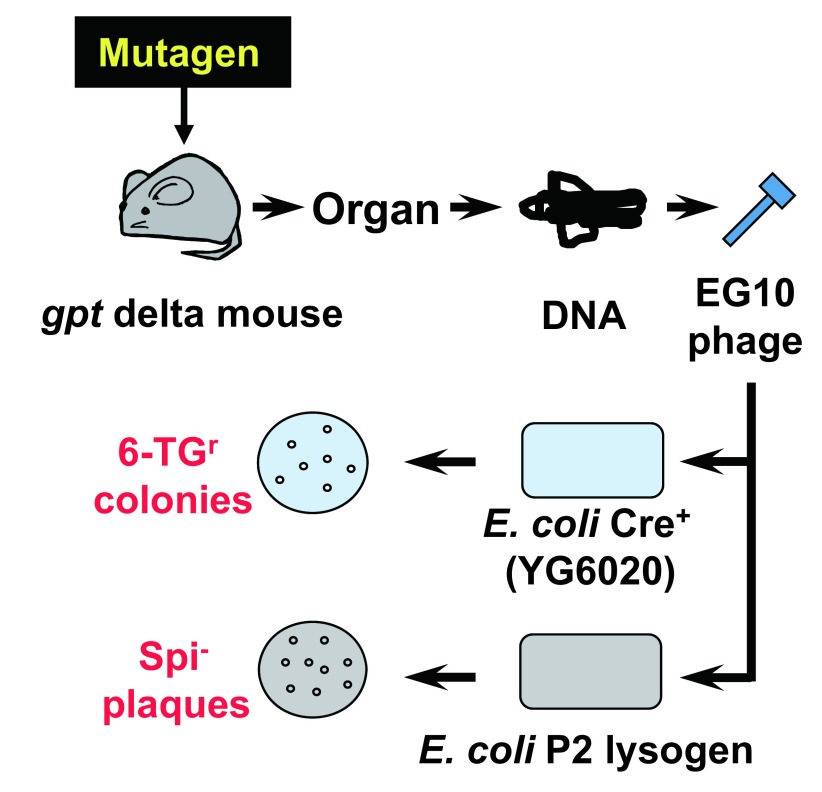
In addition to deletion mutations, point mutations such as base substitutions and frameshift should have been detected by a positive selection with a reporter gene having a convenient size for DNA sequencing. To this end, I decided to use the gpt gene of E. coli as the reporter11,20,23). The gene encodes guanine phosphoribosyltransferase, which is a bacterial counterpart of mouse Hprt. The coding size is 456 bp, which is appropriate for identification of mutations by DNA sequencing. The gpt mutants of E. coli can be positively selected with medium containing 6-TG because the wild-type enzyme metabolizes 6-TG to a toxic substance and kills the host bacterial cells. However, the gpt selection is effective only when the gene is on a multi-copy-number plasmid. Thus, the lambda phage vector carrying the gpt gene should have been converted into plasmid DNA when the phage was introduced into host bacterial cells. To solve this problem, I took an advantage of the cre-lox system. The cre gene encodes a site-specific recombinase that excises the loxP sites. If the target DNA sequence is flanked by two loxP sites, the target sequence will be excised and circulated carrying the target sequence by Cre recombinase. We made a novel lambda vector EG10 carrying a linearized plasmid DNA where the gpt gene, the chloramphenicol acetyltransferase (CAT) gene and a ColE1 replication origin are flanked by two direct repeat sequences of loxP (Fig. 2). This lambda vector also carries the red/gam genes and the chiC sequence necessary for Spi− selection. We also engineered the host E. coli strain YG6020, which originally lacks the gpt gene in the chromosome but has the cre gene20). The strain also lacks recA to suppress ex-vivo mutations after introduction of the reporter gene in the host E. coli cells. The “ex-vivo mutations” are mutations that occur in E. coli but not in mice or rats. They occur when lambda DNA having DNA lesions such as DNA adducts are propagated in E. coli. If the recA gene is activated, such unwanted “ex-vivo” mutations may occur. Therefore, the host E. coli must be recA− in the gpt selection. In the assay, rescued lambda EG10 phages are introduced into distinct E. coli host strains for Spi− and gpt selections (Fig. 3). In Spi− selection, the rescued phages are infected into E. coli strain XL1-Blue MRA (P2), which is a P2 lysogen, to select Spi− mutants. In gpt selection, the rescued phages are infected to strain YG6020, where the lambda phage is converted to a multi-copy-number plasmid carrying the gpt gene plus the drug resistance gene, and the colonies harboring plasmid carrying the mutated gpt genes are selected with plates containing 6-TG and chloramphenicol. Transgenic mice were established by microinjection of lambda EG10 DNA into fertilized eggs of BDF1 or C57BL/6J mice20). We made more than 30 transgenic lines and selected one that was originated from C57BL/6J mice, ie, #30. The mice exhibited very high rescue efficiency and low spontaneous mutation frequencies of gpt and Spi−. Because the reporter gene for point mutations is gpt and deletions can be detected by Spi− selection, we named the transgenic mice “gpt delta”.
Fig. 2.
Structure of lambda EG10 and its conversion to plasmid pYG142 in E. coli YG6020 Cre+.
The lambda EG10 is about 48 kb, which is composed of lambda 2001 and the linearized plasmid pYG144. The linearized plasmid region of the vector has been expanded for detail. The vector carries the red and gam regions and a chiC mutation involved in Spi− selection. The linearized plasmid region contains a ColE1 replication origin (ori), a chloramphenicol resistance gene (cat), the gpt gene of E. coli (gpt), and two direct-repeat sequences of loxP, which are recognition sequences of Cre recombinase. The linearized plasmid region can be excised and converted to the multi-copy-number plasmid pYG142 carrying a single loxP sequence when the lambda EG10 is introduced to E. coli YG6020 expressing Cre recombinase.
Fig. 3.
Protocols of gpt delta transgenic mouse gene mutation assay.
Two E. coli host cells are infected with the rescued lambda EG10 phages. One is E. coli YG6020 expressing Cre recombinase for 6-TG selection and the other is P2 lysogen for Spi− selection. In E. coli YG6020, lambda EG10 is converted to the multi-copy-number plasmid pYG142 carrying gpt and CAT. E. coli cells harboring plasmid carrying mutant gpt can form colonies on the plates containing chloramphenicol and 6-TG (gpt selection). Mutant lambda phage lacking the functions of red and gam can form Spi− plaques on plates containing P2 lysogen (Spi− selection).
So far, gpt delta mice have been utilized mainly in two research areas. One is radiation biology and another is chemical safety evaluation23). In the field of radiation biology, gpt delta mice were irradiated and Spi− and gpt mutations were analyzed. Interestingly, the spectra of mutations induced by irradiation are varied depending on the radiation species26). For example, Spi− mutation frequency was higher for carbon particle irradiation than γ-ray irradiation while gpt mutation frequency was higher for γ-ray than carbon particle. DNA sequence analyses revealed that carbon particle irradiation induces deletions with the size more than 1 kb, whereas gamma-ray irradiation induces smaller sized deletions and base substitutions. The results suggest that heavy-ion beam irradiation is effective at inducing deletions via double-strand breaks in DNA but ineffective at producing oxidative DNA damage by free radicals. In addition, gpt delta mice were used to study combined effects of radiation and chemical exposure, eg, low dose γ-ray irradiation plus NNK exposure27), and X-ray irradiation plus N-ethyl-N-nitrosourea (ENU) exposure28).
In the field of chemical safety evaluation, the mice are exposed to chemical carcinogens and the in vivo mutations are analyzed to examine whether genotoxicity is involved in the mechanisms of carcinogenesis. One interesting example is dicyclanil, a pyrimidine-derived insect growth regulator29). This chemical is all negative in various genotoxicity assays including Ames test while it is a hepatocarcinogen in female mice. The mutagenicity of dicyclanil was examined in the liver of male and female gpt delta mice by Umemura et al and it was found that the chemical induces gpt mutations, ie, mainly G:C to T:A, in female mice but not in male. 8-Oxo-dexoxyguanosine, an oxidative lesion in DNA, was induced in both genders, and cell proliferation and increases in liver weights were enhanced only in female. It was concluded that the female specific cell proliferation plus oxidative damage in DNA induces gender-specific gpt mutations in the liver. The results caution that decision whether the carcinogen is genotoxic or not should be made based on the results of in vivo mutagenicity assays in the target organ of carcinogenesis in rodents.
4. Establishment of Gpt Delta Rats
Although mice are predominantly used as a tester rodent in genotoxicology assays, rats are routinely used for toxicology including two-year cancer bioassays. In addition, there are a number of species-specific carcinogens, which are carcinogenic in rats but not in mice and vice versa30). An example is aflatoxin B1, a mycotoxin, whose carcinogenic potential varies among species31). Aflatoxin B1 is carcinogenic in the liver of humans and rats but mice are known to be relatively resistant. The species difference may be caused by the different metabolic activation and detoxication capacities in the species. Therefore, it was expected that transgenic rats for gene mutation assays in vivo should have been established. In fact, Big Blue® Rat had been established32,33). In 2003, lambda EG10 DNA was introduced into fertilized eggs of Sprague Dawley (SD) rats, thereby establishing gpt delta rats34). The DNA was introduced in the chromosome 4 with 5 to 10 copies per haploid. An attempt to make homozygous rats having lambda EG10 in both chromosomes was failed because the teeth development of the homozygous rats was retarded. The rats could not take the diet and survive. The established hemizygous gpt delta rats exhibited a reasonable sensitivity to mutagenicity of benzo[a]pyrene in the liver. The gpt and Spi− mutant frequencies of liver of rats treated with benzo[a]pyrene were increased in a dose dependent manner. Later, SD gpt delta rats were used to examine the relationship between mutagenicity and carcinogenicity of phenacetin, an analgesic drug, in long-term repeated dose studies35). The rats were fed diet containing 0.5% phenacetin for 26 or 52 weeks and the mutations were examined in the liver, a non-target organ of carcinogenesis, and the kidney, the target organ. The gpt and Spi− mutant frequencies were enhanced by the administration and the frequencies were higher in 52 weeks than in 26 weeks. Interestingly, both gpt and Spi− mutant frequencies were much higher in the liver than in the kidney. The results suggest that the intensity of genotoxicity does not correlate with the induction of tumor formation, and also that an organ where the highest mutation frequency was observed is not necessarily a target organ for carcinogenesis.
Since Fischer 344 (F344) rats are more frequently used for two-year cancer bioassays than SD rats, SD gpt delta rats were backcrossed with F344 rats for 15 generations, thereby establishing F344 gpt delta rats36). 2,4-Diaminotoluene, a liver carcinogen, and the non-carcinogenic structural isomer, 2,6-diaminotoluene, were administered in F344 gpt delta rats for 13 weeks. These chemicals were both mutagenic in Ames Salmonella strains in the presence of S9 activation. 2,4-Diaminotoluene enhanced gpt and Spi− mutant frequencies in the liver while 2,6-diaminotoluene did not. The results strongly support the notion that Ames positive chemicals are not necessarily positive in vivo. The non-carcinogenic 2,6-diaminotoluene was mutagenic in vitro because detoxication metabolism may be ineffective in the S9 activation system. F344 gpt delta rats were used for chemoprevention studies, too37). Male gpt delta rats were given a single injection of 1,2-dimethylhydrazine (DMH) and followed by dextran sodium sulfate (DSS) in drinking water for a week. They were fed diets containing silymarin, a natural flavonoid from the seeds of milk thistle, for 4 weeks before the DMH injection and samples were collected at 32 weeks after the DMH treatment. Silymarin suppressed the tumor formation in the colon in a dose dependent manner. In the mutation assays, DMH plus DSS enhanced the gpt mutant frequency in the colon, and the silymarin treatments reduced the mutant frequencies significantly. The results suggest that F344 gpt delta rats allow direct comparison of tumor formation and mutation induction in identical organs in identical rats. This characteristics are useful for making decisions whether the carcinogen is genotoxic or non-genotoxic. The decision is important for food safety because no acceptable daily intake (ADI) can be set for the chemical that induces mutations in vivo.
5. Gpt Delta Rodent Gene Mutations Assays in Food Safety Research
So far, about 20 chemicals have been examined with gpt delta mice or rats for food safety (Table 2). The chemicals include mycotoxins, food additives, heterocyclic amines and other compounds generated in cooking processes and others. Almost all the chemicals examined are rodent carcinogens. Therefore, the purpose of the assays appears to examine whether the genotoxicity is involved in the carcinogenesis. The experimental conditions were designed to mimic longer-term cancer bioassays. The administration period was 8 weeks, 13 weeks and in one case 78 weeks, which are longer than the standard administration period of 4 weeks38). In some cases, C57BL/6J gpt delta mice were crossed with C3H/He mice, thereby generating B6C3F1 gpt delta mice, which are the same genetic background for two-year cancer bioassays. As a result, citrinin39), flumequine40), ginkgo biloba extract41) and 3-monochloropropane-1,2-diol (3-MCPD) esters42) were turned out to be negative in the target organ(s) of carcinogenesis in gpt delta transgenic assays, and hence they could be non-genotoxic carcinogens. In contrast, estragole43), madder color44) and methyleugenol45) were positive in the transgenic assays, and therefore genotoxic mechanisms may participate in the carcinogenesis. It seems that gpt delta transgenic mice/rats gene mutation assays are effective to distinguish genotoxic and non-genotoxic carcinogens in vivo.
Table 2. Application of gpt delta rodent gene mutation assays in food safety research.
| Chemical | Species | Organ | Mutagenicity | Carcinogenicity | Administration and Dose | Note | Reference |
|---|---|---|---|---|---|---|---|
| ABAQ*1) | Male mice | Liver, kidney | + (liver) − (kidney) |
Gavage (25, 50 mg/kg/week) for 3 weeks | A product of Maillard reaction between
glucose and L-tryptophan |
63 | |
| Acrylamide | Mice | Lung | + | + (multiple organs in rats and lung in mice) |
Drinking water (100, 200, 400 ppm) for 4 weeks | A product formed in many heat-processed foods |
64 |
| 3 week- and 11 week-old male rats | Liver, testis | + (testis in young rats) − (liver in young and adult rats) |
Drinking water (20, 40, 80 ppm) for 4 weeks | 65 | |||
| Aflatoxin B1 | Male and female B6C3F1 new born mice | Liver | + Infant male and female mice exhibit similar gpt mutation frequencies |
+ (liver) |
Intraperitoneal injection on postnatal either day 4 with a single dose of 6 mg/kg or days 4, 7, and 10 with 2 mg/kg (3 × 2 mg/kg) | A mycotoxin that induces liver cancer in humans |
66 |
| APNH*2) | Male mice | Liver, colon | + | + (liver and colon in rats) |
Diet (10, 20 ppm) for 12 weeks | A product formed from nonmutagenic norharman and aniline | 67 |
| Arecoline hydrobromide | Male mice | Oral, liver | ± (oral tissues) - (liver) |
+ (oral tissue) |
Drinking water (300, 700 ppm) for 6 weeks | A major alkaloid in areca nut, chewing of which is carcinogenic to humans | 68 |
| Aristolochic acid I and II | Male mice | Kidney | + Aristolochic acid II is more mutagenic than I |
+ | Gavage (5 mg/kg) for 6 weeks | Products of Aristolochia plant species. They are associated with aristolochic acid nephropathy and Balkan endemic nephropathy. | 69 |
| Citrinin | Male rats | Kidney | - | + (kidney in rats) |
Gavage (20, 40 mg/kg/day) for 4 weeks | A food-contaminating mycotoxin | 39 |
| Dicyclanil | Male and female B6C3F1 mice | Liver | + (liver in female mice) |
+ (liver in female mice) |
Diet (0.15%) for 13 weeks | An insect growth regulator | 29 |
| Estragole | Male rats | Liver | + | + (liver in mice) |
Gavage (22, 66, 200, 600 mg/kg) for 4 weeks | A natural organic compound used as a food additive | 43 |
| Flumequine | Male and female B6C3F1 mice | Liver | - | + (liver in mice) |
Diet (0.4%) for 13 weeks | An anti-bacterial quinolone agent | 40 |
| Ginkgo biloba | Male B6C3F1 mice | Liver | - | + (liver in mice) |
Gavage (20, 200, 2000 mg/kg) for 13 weeks | A herbal supplement | 41 |
| High fat diet | Male and female mice | liver, kidney, colon | - | High fat diet for 13 or 26 weeks | 70 | ||
| Madder color and lucidin-3-O-primeveroside (LuP) | Male rats | Kidney | + | + (for madder color liver and kidney in rats) |
Diet (5.0% w/w for madder color or 0.3% w/w for LuP) for 8 weeks | Madder color is a food additive. LuP is a constituent of madder color. | 44 |
| Methyleugenol | Male and female rats | Liver | + | + (liver in rats and mice) |
Gavage (10, 30 100 mg/kg) for 13 weeks | A fragrance and flavoring agent | 45 |
| MeIQx*3) | Male mice | Liver, colon | + | + (liver and other organs in rats and mice) |
Diet (3, 30, 300 ppm) for 12 weeks | A heterocyclic amine present in cooked foods | 71 |
| MX*4) | Male and female mice | Liver, lung | - | + (rats) |
Drinking water (10, 30, 100 ppm) for 12 weeks, and 100 ppm for 78 weeks | A by-product of water chlorination | 72 |
| 3-MCPD*5) | Male rats | Kidney, testis | - | + (kidney and testis of rats) |
Gavage (40 mg/kg and the equimolar esters) for 4 weeks | 3-MCPD is a food processing contaminant. The esters are generated in foods. | 42 |
| Ochratoxin A | Male and female rats | Kidney (outer medulla) | + (outer medulla of kidney) Only Spi- mutant frequency was increased. |
+ (outer zone of renal medulla in rodents, mainly in male rats) |
Diet (5 ppm) for 4 or 13 weeks | A mycotoxin that might be associated with Balkan endemic nephropathy and urinary tract tumors in humans. | 46 |
| Male rats | Diet (5 ppm) for 4 weeks | 47 | |||||
| p53 Proficient and deficient male mice | Kidney | + Spi− mutant frequency was increased only in p53 deficient mice. |
Gavage (1, 5 mg/kg) for 4 weeks | 48 | |||
| Gavage (5 mg/kg) for 4 weeks | 49 | ||||||
| PhIP*6) | Male and female mice | Colon, spleen, liver, brain, bone marrow, testis | + (colon, spleen, liver) − (testis, brain, bone marrow) No gender difference in mutagenicity |
+ (colon and prostate of male rats and mammary glands in female rats) |
Diet (400 ppm) for 13 weeks | A heterocyclic amine in cooked food. | 73 |
| Potassium bromate | Male and female rats | Kidney | + | + (kidney of male and female rats) |
Drinking water (500 ppm) for 9 weeks | A food additive as a flour improvement for bread making | 74 |
| Safrole*7) | Male and female rats | Liver | + | + (liver of rats and mice) |
Diet (0.1, 0.5%) for 13 weeks | A natural plant constituent in the essential oils of sassafras, sweet basil, cinnamon and spices | 75 |
Rats are F344 gpt delta rats and mice are C57BL/6J gpt delta mice unless otherwise indicated.
*1 ABAQ: 5-amino-6-hydroxy-8H-benzo[6,7]azepino[5,4,3-de]quinolin-7-one
*2 APNH: aminophenylnorharman
*3 MeIQx: 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
*4 MX: 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone
*5 3-MCPD: 3-monochloropropane-1,2-diol
*6 PhIP: 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine
*7 Safrole: 4-allyl-1,2-methylenedioxybenzene
Among the chemicals examined, ochratoxin A, a mycotoxin that induces renal tumors in male rats, is interesting because it induces Spi− mutations but not gpt46,47). At the target site of carcinogenesis, ie, outer medulla of kidney, Spi− mutant frequency was statistically increased when male gpt delta rats were treated with ochratoxin A. Large deletion with the size of more than 1 kb was induced by the treatment. However, gpt mutation frequency was not increased by the treatment. Further examination with p53 deficient gpt delta mice indicates that Spi− mutant frequency, but not gpt, was increased in p53-deficient mice by ochratoxin A48,49). No increases in Spi− and gpt mutant frequencies were observed in p53-proficient mice. It seems that ochratoxin A induces double-strand breaks in DNA in the target site of kidney of rats, which resulted in large deletions. The double-strand breaks in DNA may be suppressed by p53. It is puzzling, however, why gpt mutant frequency was not increased. It is not uncommon that gpt assay and Spi−assay behave differently. However, in most cases, gpt mutant frequency was increased more effectively than Spi− mutant frequency. Only exception was heavy ion irradiation where Spi− mutant frequency was enhanced without increasing gpt mutant frequencies26). If ochratoxin A induces mutagenic DNA adducts in the target site, they will induce base substitutions (gpt) as well as deletions (Spi−). It is tempting to speculate, therefore, that ochratoxin A may interact with proteins involved in DNA replication, repair or chromosome segregation, thereby inducing double-strand breaks in DNA, which resulted in deletions without inducing point mutations. Alternatively, ochratoxin A may induce DNA adducts, but the DNA adducts are not bypassed by any DNA polymerases in outer medulla of kidney. If so, DNA replication will be completely inhibited and DNA strand breaks will be induced without induction of point mutations. Further molecular analyses may solve the interesting puzzling problem.
6. Future Perspectives
A current trend in toxicology is 3R, ie, Replacement, Reduction and Refinement, for research and testing with animals. In ICH guideline, it is recommended to integrate in vivo genotoxicity assays into repeated-dose toxicology studies8). As described above, many gpt delta gene mutation assays, in particular with rats, are already integrated into repeated-dose studies with longer period of administration. The integration enables the direct examination of participation of genotoxicity in carcinogenesis in the target organ(s) of rodents. Recently, a medium-term assay with gpt delta rats was proposed for rapid identification of renal carcinogens50). In gpt delta mice, several knockout or knockin alleles were introduced to clarify the mode of action of test chemicals. Examples include p5348,51), Atm52), Parp-153), Ogg154), Nrf255), Polk56), IL-1057) and P450 reductase-null alleles58). Mechanistic understanding of carcinogenesis may be more facilitated when knockout or knockin technologies are introduced into rats. In this regard, CRISPR/Cas9 genome editing system may play an important role in the future59).
Rapid advancing DNA sequencing technology is a novel approach to analyze mutations. Single cell whole-genome DNA sequencing has a potential to identify mutations in normal somatic cells60). In bacteria, furylfuramide-induced mutations in Salmonella typhimurium T100 are directly identified by a high-throughput DNA sequencing analysis61). Mutations in many types of cancer cells are identified by DNA sequencing and the characteristic mutations are reported62). Transition mutations of C to T at CpG site by deamination of 5-methylcytosine, another transition of C to T by UV irradiation and transversion mutations of G to T by smoking are observed in cancer cells. Therefore, mutation spectra associated with specific exposure to chemicals determined by the transgenic assays may help reveal the causes of human cancer. Although the high throughput DNA sequencing technologies are still under development and need more sophistication to reduce the error rates, we should apply new technologies as much as possible to evaluate the potential genotoxic and carcinogenic risk of chemicals to humans.
Acknowledgements
This work is partly supported by a grant-in-aid for Scientific Research (B) (JSPS KAKENHI Grant 26281029). I acknowledge Drs. Masami Yamada and Kenichi Masumura, National Institute of Health Sciences (NIHS), for valuable comments on the manuscript, and Dr. Yuji Ishii (NIHS) for helpful information.
Abbreviations:6-TG, 6-thioguanine; CA, chromosome aberration assay; CAT, chloramphenicol acetyltransferase; DMH, 1,2-dimethylhydrazine; DSS, dextran sodium sulfate; F344, Fischer 344; Hprt, hypoxanthine phosphoribosyltransferase; ICH, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; MLA, mouse lymphoma gene mutation assay; MMC, mitomycin C; MN, micronucleus assay; NNK, nicotine-derived nitrosaminoketone; OECD, Organization for Economic Co-operation and Development; P-gal, phenyl-β-D-galactoside; SD, Sprague Dawley
References
- 1.Tobacco smoking. IARC Monogr Eval Carcinog Risk Chem Hum. 1986; 38: 35–394. Lyon, France, International Agency for Research on Cancer. [PubMed] [Google Scholar]
- 2.Hecht SS. Tobacco smoke carcinogens and lung cancer. JNCI Journal of the National Cancer Institute. 1999; 91: 1194–1210. 10.1093/jnci/91.14.1194 [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature Reviews Cancer. 2003; 3: 733–744. 10.1038/nrc1190 [DOI] [PubMed] [Google Scholar]
- 4.Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. JNCI Journal of the National Cancer Institute. 2011; 103: 1827–1839. 10.1093/jnci/djr483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safety Guidelines of ICH Available at: http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html.
- 6.OECD Guidelines for Testing of Chemicals Section 4. Available at: http://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788.
- 7.Kirkland D, Aardema M, Henderson L, Müller L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2005; 584: 1–256. 10.1016/j.mrgentox.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use S2(R1) Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step4/S2R1_Step4.pdf. [PubMed]
- 9.OECD Guideline for Testing of Chemicals “Genetic Toxicology: Mouse Spot Test”. Available at: http://www.oecd.org/env/ehs/testing/E484_Genetic_Toxicology.pdf.
- 10.Jones IM, Burkhart-Schultz K, Carrano AV. A method to quantify spontaneous and in vivo induced thioguanine-resistant mouse lymphocytes. Mutation Research/Environmental Mutagenesis and Related Subjects. 1985; 147: 97–105. 10.1016/0165-1161(85)90022-6 [DOI] [PubMed] [Google Scholar]
- 11.Nohmi T, Suzuki T, Masumura K. Recent advances in the protocols of transgenic mouse mutation assays. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2000; 455: 191–215. 10.1016/S0027-5107(00)00077-4 [DOI] [PubMed] [Google Scholar]
- 12.Glazer PM, Sarkar SN, Summers WC. Detection and analysis of UV-induced mutations in mammalian cell DNA using a lambda phage shuttle vector. Proceedings of the National Academy of Sciences. 1986; 83: 1041–1044. 10.1073/pnas.83.4.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunther EJ, Murray NE, Glazer PM. High efficiency, restriction-deficient in vitro packaging extracts for bacteriophage lambda DNA using a new E.coli lysogen. Nucleic Acids Research. 1993; 21: 3903–3904. 10.1093/nar/21.16.3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gossen JA, de Leeuw WJ, Tan CH, et al. Efficient rescue of integrated shuttle vectors from transgenic mice: a model for studying mutations in vivo. Proceedings of the National Academy of Sciences. 1989; 86: 7971–7975. 10.1073/pnas.86.20.7971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohler SW, Provost GS, Kretz PL, Fieck A, Sorge JA, Short JM. The use of transgenic mice for short-term, in vivo mutagenicity testing. Gene Analysis Techniques. 1990; 7: 212–218. 10.1016/0735-0651(90)90003-X [DOI] [PubMed] [Google Scholar]
- 16.Jakubczak JL, Merlino G, French JE, et al. Analysis of genetic instability during mammary tumor progression using a novel selection-based assay for in vivo mutations in a bacteriophage lambda transgene target. Proceedings of the National Academy of Sciences. 1996; 93: 9073–9078. 10.1073/pnas.93.17.9073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T, Hayashi M, Sofuni T, Myhr BC. The concomitant detection of gene mutation and micronucleus induction by mitomycin C in vivo using lacZ transgenic mice. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1993; 285: 219–224. 10.1016/0027-5107(93)90109-S [DOI] [PubMed] [Google Scholar]
- 18.Tao KS, Urlando C, Heddle JA. Comparison of somatic mutation in a transgenic versus host locus. Proceedings of the National Academy of Sciences. 1993; 90: 10681–10685. 10.1073/pnas.90.22.10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boer de JG, Provost S, Gorelick N, Tindall K, Glickman BW. Spontaneous mutation in lacI transgenic mice: a comparison of tissues. Mutagenesis. 1998; 13: 109–114. 10.1093/mutage/13.2.109 [DOI] [PubMed] [Google Scholar]
- 20.Nohmi T, Katoh M, Suzuki H, et al. Other transgenic mutation assays: A new transgenic mouse mutagenesis test system using Spi− and 6-thioguanine selections. Environmental and Molecular Mutagenesis. 1996; 28: 465–470. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda H, Shimizu H, Ukita T, Kumagai M. A novel assay for illegitimate recombination in Escherichia coli: Stimulation of? bio transducing phage formation by ultra-violet light and its independence from RecA function. Advances in Biophysics. 1995; 31: 197–208. 10.1016/0065-227X(95)99392-3 [DOI] [PubMed] [Google Scholar]
- 22.Nohmi T, Suzuki M, Masumura K, et al. Spi? selection: An efficient method to detect? -ray-induced deletions in transgenic mice. Environmental and Molecular Mutagenesis. 1999; 34: 9–15. [DOI] [PubMed] [Google Scholar]
- 23.Nohmi T, Masumura K. Molecular nature of intrachromosomal deletions and base substitutions induced by environmental mutagens. Environmental and Molecular Mutagenesis. 2005; 45: 150–161. 10.1002/em.20110 [DOI] [PubMed] [Google Scholar]
- 24.Nohmi T, Masumura K. delta transgenic mouse: A novel approach for molecular dissection of deletion mutations. Advances in Biophysics. 2004; 38: 97–121. 10.1016/S0065-227X(04)80106-0 [DOI] [PubMed] [Google Scholar]
- 25.Masumura K, Matsui M, Katoh M, et al. Spectra ofgpt mutations in ethylnitrosourea-treated and untreated transgenic mice. Environmental and Molecular Mutagenesis. 1999; 34: 1–8. [DOI] [PubMed] [Google Scholar]
- 26.Masumura K, Kuniya K, Kurobe T, Fukuoka M, Yatagai F, Nohmi T. Heavy-ion-induced mutations in thegpt delta transgenic mouse: Comparison of mutation spectra induced by heavy-ion, X-ray, and? -ray radiation. Environmental and Molecular Mutagenesis. 2002; 40: 207–215. 10.1002/em.10108 [DOI] [PubMed] [Google Scholar]
- 27.Ikeda M, Masumura K, Sakamoto Y, et al. Combined genotoxic effects of radiation and a tobacco-specific nitrosamine in the lung of gpt delta transgenic mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007; 626: 15–25. 10.1016/j.mrgentox.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi K, Kakinuma S, Sudo S, et al. Differential effects of low- and high-dose X-rays on N-ethyl-N-nitrosourea-induced mutagenesis in thymocytes of B6C3F1 gpt-delta mice. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2008; 640: 27–37. 10.1016/j.mrfmmm.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Umemura T, Kuroiwa Y, Tasaki M, et al. Detection of oxidative DNA damage, cell proliferation and in vivo mutagenicity induced by dicyclanil, a non-genotoxic carcinogen, using gpt delta mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007; 633: 46–54. 10.1016/j.mrgentox.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Green T. Species differences in carcinogenicity: The role of metabolism in human risk evaluation. Teratogenesis, Carcinogenesis, and Mutagenesis. 1990; 10: 103–113. 10.1002/tcm.1770100206 [DOI] [PubMed] [Google Scholar]
- 31.Wogan GN, Kensler TW, Groopman JD. Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review. Food Additives & Contaminants: Part A. 2012; 29: 249–257. 10.1080/19440049.2011.563370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dycaico MJ, Scott Provost G, Kretz PL, Ransom SL, Moores JC, Short JM. The use of shuttle vectors for mutation analysis in transgenic mice and rats. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1994; 307: 461–478. 10.1016/0027-5107(94)90257-7 [DOI] [PubMed] [Google Scholar]
- 33.Dycaico MJ, Stuart GR, Tobal GM, de Boer JG, Glickman BW, Provost GS. Species-specific differences in hepatic mutant frequency and mutational spectrum among lambda/ lacl transgenic rats and mice following exposure to aflatoxin B 1. Carcinogenesis. 1996; 17: 2347–2356. 10.1093/carcin/17.11.2347 [DOI] [PubMed] [Google Scholar]
- 34.Hayashi H, Kondo H, Masumura K, Shindo Y, Nohmi T. Novel transgenic rat for in vivo genotoxicity assays using 6-thioguanine and Spi? selection. Environmental and Molecular Mutagenesis. 2003; 41: 253–259. 10.1002/em.10152 [DOI] [PubMed] [Google Scholar]
- 35.Kawamura Y, Hayashi H, Masumura K, Numazawa S, Nohmi T. Genotoxicity of phenacetin in the kidney and liver of Sprague-Dawley gpt delta transgenic rats in 26-week and 52-week repeated-dose studies. Toxicology. 2014; 324: 10–17. 10.1016/j.tox.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 36.Toyoda-Hokaiwado N, Inoue T, Masumura K, et al. Integration of in vivo genotoxicity and short-term carcinogenicity assays using F344 gpt delta transgenic rats: in vivo mutagenicity of 2,4-diaminotoluene and 2,6-diaminotoluene structural isomers. Toxicological Sciences. 2010; 114: 71–78. 10.1093/toxsci/kfp306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyoda-Hokaiwado N, Yasui Y, Muramatsu M, et al. Chemopreventive effects of silymarin against 1,2-dimethylhydrazine plus dextran sodium sulfate-induced inflammation-associated carcinogenicity and genotoxicity in the colon of gpt delta rats. Carcinogenesis. 2011; 32: 1512–1517. 10.1093/carcin/bgr130 [DOI] [PubMed] [Google Scholar]
- 38.Thybaud V, Dean S, Nohmi T, et al. In vivo transgenic mutation assays. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2003; 540: 141–151. 10.1016/j.mrgentox.2003.07.004 [DOI] [PubMed] [Google Scholar]
- 39.Kuroda K, Ishii Y, Takasu S, et al. Cell cycle progression, but not genotoxic activity, mainly contributes to citrinin-induced renal carcinogenesis. Toxicology. 2013; 311: 216–224. 10.1016/j.tox.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 40.Kuroiwa Y, Umemura T, Nishikawa A, et al. Lack of in vivo mutagenicity and oxidative DNA damage by flumequine in the livers of gpt delta mice. Archives of Toxicology. 2007; 81: 63–69. 10.1007/s00204-006-0126-9 [DOI] [PubMed] [Google Scholar]
- 41.Maeda J, Kijima A, Inoue K, et al. In vivo genotoxicity of Ginkgo biloba extract in gpt delta mice and constitutive androstane receptor knockout mice. Toxicological Sciences. 2014; 140: 298–306. 10.1093/toxsci/kfu090 [DOI] [PubMed] [Google Scholar]
- 42.Onami S, Cho Y, Toyoda T, et al. Absence of in vivo genotoxicity of 3-monochloropropane-1,2-diol and associated fatty acid esters in a 4-week comprehensive toxicity study using F344 gpt delta rats. Mutagenesis. 2014; 29: 295–302. 10.1093/mutage/geu018 [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Umemura T, Hibi D, et al. Possible involvement of genotoxic mechanisms in estragole-induced hepatocarcinogenesis in rats. Archives of Toxicology. 2012; 86: 1593–1601. 10.1007/s00204-012-0865-8 [DOI] [PubMed] [Google Scholar]
- 44.Ishii Y, Takasu S, Kuroda K, et al. Combined application of comprehensive analysis for DNA modification and reporter gene mutation assay to evaluate kidneys of gpt delta rats given madder color or its constituents. Analytical and Bioanalytical Chemistry. 2014; 406: 2467–2475. 10.1007/s00216-014-7621-2 [DOI] [PubMed] [Google Scholar]
- 45.Jin M, Kijima A, Hibi D, et al. In vivo genotoxicity of methyleugenol in gpt delta transgenic rats following medium-term exposure. Toxicological Sciences. 2013; 131: 387–394. 10.1093/toxsci/kfs294 [DOI] [PubMed] [Google Scholar]
- 46.Hibi D, Suzuki Y, Ishii Y, et al. Site-specific in vivo mutagenicity in the kidney of gpt delta rats given a carcinogenic dose of ochratoxin A. Toxicological Sciences. 2011; 122: 406–414. 10.1093/toxsci/kfr139 [DOI] [PubMed] [Google Scholar]
- 47.Kuroda K, Hibi D, Ishii Y, et al. Ochratoxin A induces DNA double-strand breaks and large deletion mutations in the carcinogenic target site of gpt delta rats. Mutagenesis. 2014; 29: 27–36. 10.1093/mutage/get054 [DOI] [PubMed] [Google Scholar]
- 48.Hibi D, Kijima A, Suzuki Y, et al. Effects of p53 knockout on ochratoxin A-induced genotoxicity in p53-deficient gpt delta mice. Toxicology. 2013; 304: 92–99. 10.1016/j.tox.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 49.Kuroda K, Hibi D, Ishii Y, et al. Role of p53 in the progression from ochratoxin A-induced DNA damage to gene mutations in the kidneys of mice. Toxicological Sciences. 2015; 144: 65–76. 10.1093/toxsci/kfu267 [DOI] [PubMed] [Google Scholar]
- 50.Matsushita K, Ishii Y, Takasu S, et al. A medium-term gpt delta rat model as an in vivo system for analysis of renal carcinogenesis and the underlying mode of action. Experimental and Toxicologic Pathology. 2015; 67: 31–39. 10.1016/j.etp.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 51.Yatagai F, Kurobe T, Nohmi T, et al. Heavy-ion-induced mutations in thegpt delta transgenic mouse: Effect ofp53 gene knockout. Environmental and Molecular Mutagenesis. 2002; 40: 216–225. 10.1002/em.10107 [DOI] [PubMed] [Google Scholar]
- 52.Furuno-Fukushi I, Masumura K, Furuse T, et al. Effect of Atm disruption on spontaneously arising and radiation-induced deletion mutations in mouse liver. Radiation Research. 2003; 160: 549–558. 10.1667/RR3073 [DOI] [PubMed] [Google Scholar]
- 53.Shibata A, Maeda D, Ogino H, et al. Role of Parp-1 in suppressing spontaneous deletion mutation in the liver and brain of mice at adolescence and advanced age. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2009; 664: 20–27. 10.1016/j.mrfmmm.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 54.Minowa O, Arai T, Hirano M, et al. Mmh/Ogg1 gene inactivation results in accumulation of 8-hydroxyguanine in mice. Proceedings of the National Academy of Sciences. 2000; 97: 4156–4161. 10.1073/pnas.050404497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoki Y, Hashimoto AH, Amanuma K, et al. Enhanced spontaneous and benzo(a)pyrene-induced mutations in the lung of Nrf2-deficient gpt delta mice. Cancer Research. 2007; 67: 5643–5648. 10.1158/0008-5472.CAN-06-3355 [DOI] [PubMed] [Google Scholar]
- 56.Takeiri A, Wada NA, Motoyama S, et al. In vivo evidence that DNA polymerase kappa is responsible for error-free bypass across DNA cross-links induced by mitomycin C. DNA Repair. 2014; 24: 113–121. 10.1016/j.dnarep.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 57.Sato Y, Takahashi S, Kinouchi Y, et al. IL-10 deficiency leads to somatic mutations in a model of IBD. Carcinogenesis. 2006; 27: 1068–1073. 10.1093/carcin/bgi327 [DOI] [PubMed] [Google Scholar]
- 58.Luan Y, Xing G, Qi X, et al. The application of hepatic P450 reductase null gpt delta mice in studying the role of hepatic P450 in genotoxic carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced mutagenesis. Archives of Toxicology. 2012; 86: 1753–1761. 10.1007/s00204-012-0891-6 [DOI] [PubMed] [Google Scholar]
- 59.Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013; 339: 819–823. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vilfan ID, Tsai YC, Clark TA, et al. Analysis of RNA base modification and structural rearrangement by single-molecule real-time detection of reverse transcription. Journal of Nanobiotechnology. 2013; 11: 8. 10.1186/1477-3155-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda T, Takamune M, Matsuda Y, Yamada M. A pilot study for the mutation assay using a high throughput DNA sequencer. Genes and Environment. 2013; 35: 53–56. 10.3123/jemsge.35.53 [DOI] [Google Scholar]
- 62.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Australian Pancreatic Cancer Genome Initiative. ICGC Breast Cancer Consortium. ICGC MMML-Seq Consortium. ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013; 500: 415–421. 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Totsuka Y, Watanabe T, Coulibaly S, et al. In vivo genotoxicity of a novel heterocyclic amine, aminobenzoazepinoquinolinone-derivative (ABAQ), produced by the Maillard reaction between glucose and l-tryptophan. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2014; 760: 48–55. 10.1016/j.mrgentox.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 64.Ishii Y, Matsushita K, Kuroda K, et al. Acrylamide induces specific DNA adduct formation and gene mutations in a carcinogenic target site, the mouse lung. Mutagenesis. 2015; 30: 227–235. 10.1093/mutage/geu062 [DOI] [PubMed] [Google Scholar]
- 65.Koyama N, Yasui M, Kimura A, et al. Acrylamide genotoxicity in young versus adult gpt delta male rats. Mutagenesis. 2011; 26: 545–549. 10.1093/mutage/ger014 [DOI] [PubMed] [Google Scholar]
- 66.Woo LL, Egner PA, Belanger CL, et al. Aflatoxin B1-DNA adduct formation and mutagenicity in livers of neonatal male and female B6C3F1 mice. Toxicological Sciences. 2011; 122: 38–44. 10.1093/toxsci/kfr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masumura K, Totsuka Y, Wakabayashi K, Nohmi T. Potent genotoxicity of aminophenylnorharman, formed from non-mutagenic norharman and aniline, in the liver of gpt delta transgenic mouse. Carcinogenesis. 2003; 24: 1985–1993. 10.1093/carcin/bgg170 [DOI] [PubMed] [Google Scholar]
- 68.Wu M, Xing G, Qi X, et al. Assessment of the mutagenic potential of arecoline in gpt delta transgenic mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2012; 748: 65–69. 10.1016/j.mrgentox.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 69.Xing G, Qi X, Chen M, et al. Comparison of the mutagenicity of aristolochic acid I and aristolochic acid II in the gpt delta transgenic mouse kidney. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2012; 743: 52–58. 10.1016/j.mrgentox.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 70.Takasu S, Ishii Y, Matsushita K, et al. No effect of high fat diet-induced obesity on spontaneous reporter gene mutations in gpt delta mice. Asian Pacific Journal of Cancer Prevention. 2014; 15: 7149–7152. 10.7314/APJCP.2014.15.17.7149 [DOI] [PubMed] [Google Scholar]
- 71.Masumura K, Horiguchi M, Nishikawa A, et al. Low dose genotoxicity of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in gpt delta transgenic mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2003; 541: 91–102. 10.1016/S1383-5718(03)00186-4 [DOI] [PubMed] [Google Scholar]
- 72.Nishikawa A, Sai K, Okazaki K, et al. MX, a by-product of water chlorination, lacks in vivo genotoxicity ingpt delta mice but inhibits gap junctional intercellular communication in rat WB cells. Environmental and Molecular Mutagenesis. 2006; 47: 48–55. 10.1002/em.20167 [DOI] [PubMed] [Google Scholar]
- 73.Masumura K, Matsui K, Yamada M, et al. Mutagenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the new gptΔ transgenic mouse. Cancer Letters. 1999; 143: 241–244. 10.1016/S0304-3835(99)00132-9 [DOI] [PubMed] [Google Scholar]
- 74.Umemura T, Tasaki M, Kijima A, et al. Possible participation of oxidative stress in causation of cell proliferation and in vivo mutagenicity in kidneys of gpt delta rats treated with potassium bromate. Toxicology. 2009; 257: 46–52. 10.1016/j.tox.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 75.Jin M, Kijima A, Suzuki Y, et al. Comprehensive toxicity study of safrole using a medium-term animal model with gpt delta rats. Toxicology. 2011; 290: 312–321. 10.1016/j.tox.2011.09.088 [DOI] [PubMed] [Google Scholar]