Abstract
A major corn-related mycotoxin, fumonisin B1 (FB1), continues to attract attention of researchers as well as risk-assessors due to the diverse toxicological characteristics, including distinct target tissues in different animal species and opposite susceptibility in males and females in mice and rats. More than thirty years passed since the structure identification as a sphingoid-like chemical, but the causal mechanism of the toxicity remains obscure in spites of extensive studies. Considerable amounts of knowledge have been accumulated on the biochemical/toxicological actions of FB1, but the influence on lipid dynamics and mobilization in the body has not been focused well in relation to the FB1-mediated toxicity. Considerable influences of this toxin on mobilization of sphingolipids and phospholipids and also on adaptive changes in their compositions in tissues are implicated from recent studies on FB1-interacting ceramide synthases. Accumulated patho-physiological data also suggest a possible role of hepatic phospholipid on FB1-mediated toxicity. Thus, a mechanism of FB1-mediated toxicity is discussed in relation to the mobilization of phospholipids and sphingolipids in the body in this context.
Key words: : mycotoxin in foods, phospholipid mobilization, ceramide, sphingomyelin, species difference in target tissues, sex-related difference in susceptibility
1. Introduction
Fumonisins are isolated as mycotoxins from cultures on corn of Fusarium verticillioides (moniliforme)1–3). Fumonisin B14) (FB1*, Fig. 1) has an IUPAC name of (2R,2’R)-2,2’-[[(5R,6R,7S,9S,11R,16R,18S,19S)-19-amino-11,16,18-trihydroxy-5,9-dimethyl-6,7-icosanediyl]bis[oxy(2-oxo-2,1-ethanediyl)]]disuccinic acid (C34H59NO15, Mw 721.83). This chemical is soluble in water and stable at room temperature and to light. FB1 has values of LogP is 2.20 and LogD at pH 7.4 is −3.88. The acid part, also called as carballylic acid, has a chelating capability with metals of divalent cations5,6). This chemical is known to inhibit sphinganine/sphingosine N-acyltransferase (ceramide synthase/CerS), causing an accumulation of sphingoid bases (IC50 ~75 nM)7), and also inhibits protein phosphatases (PP); IC50 values are 80, 300, 400, 500 and 3000 μM for PP5, PP2Cα, PP2A, PP1γ2 and PP2B, respectively8). FB1 is also reported to interact with arginosuccinate synthase, a urea cycle enzyme9).
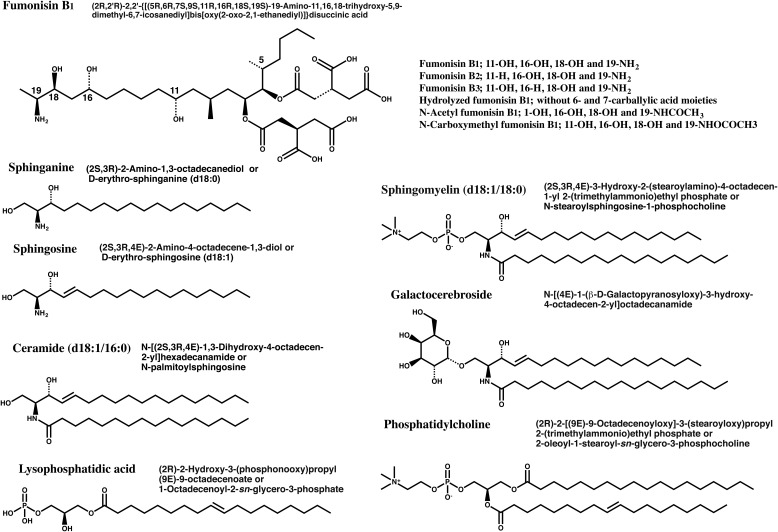
Fig. 1.
Structures of typical fumonisin derivatives, sphingoids and phospholipids Sphingoids, ceramides and phospholipids are shown with their IUPAC names and synonyms.
Only a typical fatty acid conjugate is shown for ceramide, sphingomyelin, galactocerebroside, lysophosphatidic acid and phosphatidylcholine.
D18:1, dihydroxysphingoid base with 18 carbons and one double bond18,19).
Sn (stereospecific numbering): Secondary hydroxyl group is shown to the left of C-2 in a Fischer projection. The carbon atom above C-2 is called C-1, the one below C-3; the use of this “stereospecific numbering” is indicated by the prefix “sn” before the stem-name of the compound17).
Wide ranges of food contamination and distinct toxicity targets in different animal species of this toxicant continue to attract attentions of regulatory authorities as well as academic researchers10–12). Several reports are available on the toxicity of FB1 and the related chemicals12–16). These studies suggested interactions of ceramides/sphingoids on FB1-mediated toxicity. Ceramides, sphingoids17–19) and their metabolites have been discussed as rheostats of cellular signals20–22). In fact, several reports are available on FB1-mediated alterations in cellular signals accompanying the changes in ceramide and sphingoid levels23–25). In spite of extensive elaborative researches, causal mechanisms of FB1 toxicity remain obscure in vivo in animal species.
There are couples of unanswered queries on the mechanisms of FB1-mediated toxicity. The major queries include 1) species differences in target organs, 2) sex-related difference in liver and kidney toxicities in rodents, and 3) whether sphingosine is an etiological factor or a surrogate marker. These queries are linked profoundly to a safety assessment of fumonisin intakes from foods. Previous studies on FB1 toxicity mostly dealt with the influence on biological/pharmacodynamic actions of sphingolipids and their metabolites, and poorly focused on the influence on mobilization of individual lipids. Liver is the common target of FB1-mediated toxicity throughout experimental and domestic animals, and cholestatic signs are invariably detected in the exposed animals as described in details below. These data suggested a possible role of hepatic phospholipid supply for the bile excretion on FB1-mediated toxicity. Therefore, the possible mechanism of FB1-mediated toxicity is discussed focusing on the mobilization of phospholipids and sphingolipids in FB1-exposed animals.
*FB1 preparations isolated from cultured materials are used in the biochemical and toxicological studies. The preparation is often noted to contain FB2 and/or other derivatives. Here, FB1 refers to preparations of more than 90% pure.
2. Profiles of FB1 pharmacokinetics in Typical Animal Species
2.1. Pharmacokinetics in Rats
Early studies on FB1 using fluorescent derivatization for the detection showed the rapid disappearance from blood circulation and the minimal elimination to urine. Plasma half-life of FB1 quantified as the fluorescent was shown as 3.15 hr after the p.o. administration of 10 mg/kg b.w. to male Wistar rats, whereas it was calculated 1.03 hr after the i.v. administration of 2 mg/kg b.w.26). Plasma half-lives were calculated as 1.164 and 1.69 hrs after the subcutaneous injection of FB1 0.8 and 8 mg/kg b.w., respectively, in new born male Sprague-Dawley (SD) rats27). In the oral study, FB1 stayed for longer periods in liver (t1/2, 4.07 hr) and kidney (t1/2, 7.07 hr) than in the plasma. Plasma half life of FB1 is reported to be 18 min after the i.p. administration in male BD IX rats28). Experimental data described above showed the discordance on the disappearance rates of blood FB1 between the oral and parenteral administrations26). Due to the invariability of the elimination kinetics of an identical chemical among different routes of the administration, the terminal portion of the curves observed in the oral experiments is rather likely to represent the absorption rate (in the so-called a flip-flop model). Continued absorption of FB1 is thus expected after the oral administration, although the extent of the absorption is limited in rats.
Studies using 5,9-dimethyl-14C-enriched FB129) confirm the major role of bile excretion and the minimal role of urinary excretion. The biliary excretion recovered in 24 hr shares 67% of the radioactivity given i.p. to male Wistar rats at a dose of 7.5 mg/kg b.w. of 14C-FB130).
Soon after the intravenous injection of 14C-FB1 (4.5 µg/head), the blood level dropped rapidly and the levels in liver and kidney exceed the blood level between 0.5 hr and 96 hr after the administration31). These data suggest the sequestration of FB1 in tissues including kidney and liver. In addition, a high level of 14C-FB1 is also continuously detected in the gastrointestinal tract.
The blood level of 14C-FB1 was increased rapidly after the intragastric administration (1 mg/head after addition of unlabeled FB1) and then the low but stable 14C-FB1 level was maintained until 96 hr. In the three consecutive p.o. administrations of 14C-FB1 (1 mg), levels of 14C-FB1 radioactivity in liver and kidney are somewhat higher at 24 hr after the third administration than at the point just after the third administration31). These data suggest the temporal stationary state of 14C-FB1 in both tissues during the repeated administration.
Cumulative urinary excretion of unchanged FB1 was 2.3, 0.8 and 0.4%, respectively, until four days after gavage administrations of 0.69, 6.93 or 69.3 μmol/kg FB1 (corresponding to 0.5, 5 or 50 mg/kg b.w.) to male F344 rats. After hydrolysis of the urine, 7.4, 1.2 and 0.5% of the respective doses were recovered32). Thus, no dose-dependent increases were observed in urinary excretion of FB1 in rats.
2.2. Pharmacokinetics in Pigs
After an intravenous administration of 14C-FB1 (0.4 mg/kg b.w.), the plasma disappearance rate was calculated 10.5 min in pigs33). Total 21.2% and 58.3% of the radioactivity are recovered in urine and feces for three days, respectively. High levels of the radioactivity remain in the kidney and liver among the tissue examined.
Following the intragastric administration of 14C-FB1 (0.5 mg/kg b.w.), the radioactivity appeared in the blood after 30 min and most was eliminated in the feces (87 to 95% of the dose) within 72 hr33). Less than 1% of the dose was excreted in urine. The oral bioavailability was estimated at around 4% in pigs.
In an oral study using bile duct-cannulated animals, most of the recovered radioactivity was found in the feces (86 to 94% of the dose) after 72 hr34). Only small amounts of the radioactivity were recovered from the bile (1.7% of dose) and urine (1.2% of dose).
Fusarium-cultured materials 26 g containing 125 mg of FB1 (corresponding to 5 mg/kg) was given to male piglets (5 mg/kg b.w.) orally, and plasma FB1 levels are monitored by high-performance liquid-chromatography35). Highest plasma concentration (Cmax) of FB1 appears at 2 hr (Tmax) after the administration. The sharp decline after Tmax suggested the rapid equilibrium with tissue/organ compartment and the half-life (t1/2 of β-phase) was calculated from the plasma profile as around 12 hr. The value is likely to represent the half-life of the absorption in similar to the case with rat pharmacokinetics described above. FB1 was excreted in urine (0.93%) and feces (76.5%) until 84 hr.
Absorption of FB1 was estimated 4% in pigs fed the 45 mg/kg diet for 10 days, and the total urinary excretion was 1.5% using liquid-chromatography mass-spectrometry (LC/MS)36). Mono-carballylic ester hydrolyzed (24%) and di-hydrolyzed metabolites (aminopentol, 16%) were detected together with FB1 (ca 65%) in the urine. FB1 was detected in liver, kidney, lung and spleen (noted as the decreasing order) even in 10 days after the withdrawal of the FB1-contaminated feed.
After feeding of 100 mg/kg diet of FB1 for 5 to 11 days, tissue FB1 levels of pigs euthanized or died were determined on serum, bile, lung, liver, kidney, brain, spleen, pancreas, heart, muscle, eye, and fat by LC/MS37). High levels of FB1 were detected in kidney, liver, lung, spleen and pancreas.
2.3. Pharmacokinetics in Velvet Monkey
Plasma disappearance rate of FB1 was calculated 40 min after the i.v. administration of 1.6 mg/kg b.w. in velvet monkey. Significant amounts (5–20%) were eliminated into the urine, although fecal excretion was the major route of the elimination38). More than 70% of the radioactivity was recovered within three days after an oral 14C-FB1 administration (8 mg/kg b.w.) in velvet monkeys. Fecal excretion also shared the main route of the elimination. Considerable amounts of radioactivity were detected in the intestines, while the urinary excretion ratios were slightly less in the oral study than in the parenteral study.
2.4. Urinary Excretion of FB1 in Human
Daily urine output of volunteers consuming 206 g/day of tortillas and biscuits prepared from masa and maize flour were determined for FB1 and the derivatives39). Only intact FB1 was detected in urine and the amount was estimated as 0.5% of the intake. Within five days after the cessation of consumption, urinary FB1 disappeared and thus suggests an apparent half-life of less than 48 hr in humans.
2.5. Metabolism and Transport
No enzymatic formation of FB1 metabolite was detected in rat hepatocyte and microsomal systems, suggesting FB1 as a poor substrate of cytochrome P450 forms40). Although the urinary excretion of hydrolyzed FB1 without carballylic acid side chain was detected in urine, hydrolysis of ester bonds at C6 or C7 was not observed in hepatocyte system. Thus, no substantial data is available on the enzyme responsible for FB1 hydrolysis.
Experiments with male Mdr1a/1b double knockout mice indicate that the cellular or brain transport of FB1 appears to be independent of the P-glycoprotein-mediated transporting system41). Using cell culture systems with or without verapamil or probenecid (P-gp and Mrp inhibitors respectively), involvement of P-gp in the influx/efflux mechanisms of aminopentol (hydrolyzed FB1), but not FB1, was suggested in the intestinal cells42).
To pregnant rats, 14C-FB1 (0.101 mg) was given i.v. on gestational day (GD) 15 and the distribution to fetus was examined 1 hr after the injection43). No apparent radioactivity was detected in the fetus. The sphinganine level was also not altered, suggesting the scarce distribution of FB1 in rat fetus. A report, however, noted the presence of radioactive FB1 in both placental and embryonic tissues after the intraperitoneal injection at GD 10.5 to pregnant mouse dams of LM/Bc strain, which accompanied the increase in their sphinganine levels44).
Although no clear mechanism was available on the selective retention of FB1 in liver and kidney, there are possible contributions of transporters. Interactions of FB1 with organic anion transporter of rat (rOAT1)45) and of human (hOAT3)46) were reported in the inhibition study using typical substrates. These data suggest the possibility of efficient uptake in liver and reuptake in kidney of FB1 through OAT family of transporters.
Prolonged retention of 14C-FB1 radioactivity in intestine and limited excretion in bile of pigs may imply at least partly the contribution for intestinal excretion of FB1 through transporters such as ABCG2 (BCRP) known to express in jejunum and liver47,48), although no substantial data is yet available on FB1.
Information on the tissue distribution of FB1 other than liver and kidney is limited in the pharmacokinetic studies described above, but the distributions of FB1 to thymus, adrenal, pancreas are suggested from acute or sub-acute toxicity studies in pigs and other species. These data suggest the sustained absorption at intestines and continued sequestration in susceptible tissues after the oral administration of FB1 in experimental animals.
3. Profiles of Species- and Sex-related Differences in Toxicity Target Tissues
3.1. Toxicity in Mouse
Liver apoptosis and hepatocellular hypertrophy are detected in female B6C3F1 mice fed 52 mg/kg diet or more of FB1 (corresponding to 11.5 mg/kg b.w./day or more) for 28 days10). Pathological changes and altered serum parameters indicative of hepatotoxicity are found in female B6C3F1 mice fed 81 mg/kg diet (corresponding to 28.9 mg/kg b.w./day) of FB1 for 90 days49). No apparent changes in clinical parameters are detected in male B6C3F1 mice after the FB1 administration.
In another FB1 feeding study for 28 days50), increases in blood alanine aminotransferase (ALT), alkaline phosphatase (ALP) cholesterol and total bile acids are detected in B6C3F1 female mice fed 163 kg/diet (corresponding to 41 mg/kg b.w./day) or higher doses of FB1. Bile duct hyperplasia as well as apoptosis is found in female mice fed 234 mg/kg or 484 mg/kg diets (corresponding to 62 or 105 mg/kg b.w./day). Changes in hepatotoxic parameters such as ALT, ALP, aspartate aminotransferase (AST), cholesterol and total bile acids are also found in male B6C3F1 mice fed FB1 at a high dose (484 mg/kg diet corresponding to 93 mg/kg b.w./day). These feeding studies are consistently indicative of higher susceptibility of female mice than the males to FB1. Similar differences were observed in mice fed FB1 by the gavage administration for 14 days51). Liver necrosis was detected in female B6C3F1 mice at 15 mg/kg b.w./day, whereas it was detected in the males at 35 mg/kg b.w. or higher dose. Both the sexes showed the increases of hepatotoxic parameters such as serum cholesterol and ALT.
Although no obvious damage in kidney was reported in FB1 feeding study, slight increases in the incidence of single epithelial cell necrosis in cortical and medullary renal tubules with vacuolation of the cytoplasm in epithelial cells in the outer medulla, were found in females after the gavage administration at 15 to 75 mg/kg b.w. of FB1. These effects were not detected in the male mice. In addition, adrenal cortical cell vacuolation was observed at the 15 mg/kg b.w./day in the females and at the 35 mg/kg b.w./day in the males.
Observed higher susceptibilities of female, rather than male, B6C3F1 mice to FB1 in the dysfunctions of kidney and adrenal as well as liver suggest the systemic distribution of a mutual etiological factor.
Female B6C3F1 mice were fed diets containing FB2, FB3, hydrolyzed-FB1, N-acetyl-FB1, or N-carboxymethyl-FB1 (approximately 0, 14, 70, and 140 μmol/kg diet, Fig. 1) for 28 days. None of these diets caused a decrease in body weight gain over the 28 days10).
3.2. Toxicity in Rat
Hepatocellular apoptosis was detected in female F344 rats treated with 99 mg/kg diet of FB1 or the higher doses (corresponding to 12−56 mg/kg b.w./day) for 28 days52). Bile duct hyperplasia was also detected in the females fed 234 or 484 mg/kg diets (corresponding to 28 or 56 mg/kg b.w./day). Hepatocellular apoptosis and bile duct hyperplasia were also observed in the male animals, but at the higher doses, 234 and 484 mg/kg diets, respectively. Kidney apoptosis was observed in both sexes of the animals treated with 99 mg/kg diet of FB1 or the higher doses.
In a different feeding study of FB1 for 28 days50), liver apoptosis was detected in female F344 rats treated with the 163 mg/kg diet (corresponding to 20 mg/kg b.w./day). Increases of serum cholesterol and total bile acid levels were found after feedings of the 234 mg/kg diet (corresponding to 28 mg/kg b.w./day) or the higher dose in the female rats. Liver apoptosis was also observed in the male rats treated with the 234 mg/kg diet, and increases of serum cholesterol, total bile acid and ALP are detected at the 484 mg/kg diet (corresponding to 56 mg/kg b.w./day). Kidney apoptosis was detected in the female at the 163 mg/kg diet group and in the male at the 99 mg/kg diet group. Kidney weight loss was found at the 234 mg/kg diet in both sexes of rats.
Nephrosis involving the outer medulla was found in male F344 rats fed diet containing 9 mg /kg diet of FB1 (corresponding to 0.21 mg/kg b.w./day) or the higher dose for 13 weeks, and, to a lesser degree, in the females fed FB1 containing the 81 mg/kg diet49). Renal weight decreases were found in both sexes of rats fed at dietary levels of the 9 mg/kg diet (corresponding to 5.66–6.35 mg/kg b.w./day) or the higher levels. In this study, hepatotoxicity was not found in rats fed at dietary levels of up to the 81 mg/kg diet for 90 days.
In a two-year NTP study50), renal tubule apoptosis was observed in male F344 rats fed 15 mg/kg diet of FB1 (corresponding to 0.76 mg/kg b.w./day) from six-week evaluation, and renal tubule epithelial hyperplasia is detected in the groups fed the 50 and 150 mg/kg diet (corresponding to 2.5 and 7.5 mg/kg b.w./day) at the final evaluation. No significant increases in the incidence of neoplasms and non-neoplastic lesions were detected in the females fed 5 to 100 mg/kg diet of FB1.
These diet studies using F344 rats indicate the following points; 1) kidney is the most susceptible target of FB1, 2) liver damages are also occurred at the higher doses, and 3) female F344 rats are rather resistant than the male rats to FB1.
Experiments using both sexes of SD rats also indicate hepatic lesions after the feeding of 150 mg/kg diet of FB1 (corresponding to 13 or 13.6 mg/kg b.w./day) for four weeks53). Cortical nephrosis appeared in the males fed 15 mg/kg diet of FB1 (corresponding to 1.4 mg/kg b.w./day), and in the females fed the 50 mg/kg diet (4.1 mg/kg b.w./day).
Susceptibility to FB1-mediated nephropathy was also higher in male SD rats than the females in FB1 feeding study43). In a diet study using male SD strain of rats, liver and kidney apoptosis were detected in the groups fed 53 and 6.9 mg/kg diet of total FBs (corresponding to 5.3 and 0.69 mg/kg b.w./day), respectively, for three weeks54). Increases in serum ALP and urea nitrogen were also found in male SD rats exposed to 53 mg/kg diet of total FB. Increases in apoptosis score in liver was detected at the 88.6 mg/kg diet (8.86 mg/kg b.w./day) for 10 days and in kidney at the 13.5 mg/kg diet (corresponding to 1.35 mg/kg b.w./day)55).
Significantly depressed body weight and food consumption were observed in female SD rats treated with gavage administration of FB1 at 35 and 75 mg/kg b.w./day for 11 days56). Reduced liver weight, elevated serum ALT and histopathological changes suggest hepatic dysfunction at doses of the 15 mg/kg b.w./day and the higher. Altered renal morphology, together with changes in urine osmolality and urine enzyme levels, was observed at the 5 mg/kg/day and the higher doses. In addition, transient increase in cellular vacuolation was detected in bone marrow at a dose of 5mg/kg b.w./day. Increased cytoplasmic vacuolation of adrenal cortex cells occurred in rats treated with the 15 mg/kg b.w./day. Elevated serum cholesterol was observed at 5 mg/kg b.w./day of FB1 and the higher.
In male SD rats treated with gavage administration of FB1, glomerular and tubular damages were suggested at 5 mg/kg b.w./day from the alteration of clinical parameters57). Single cell necrosis is increasingly numerous in the liver from 15–75 mg FB1/kg b.w./day. Thus, clear sex-related difference exists on FB-induced renal apoptosis, but faint in the hepatic apoptosis, in SD rats. Consistent with F344 rats, Female SD rats are more resistant than the males on FB1-induced kidney dysfunctions.
The doses yielding liver and kidney damages are equivalent between the diet study for four weeks and gavage studies for 11 days using both sexes of SD rats. These results among sub-chronic studies are consistent with the view that sustained absorption in intestine of FB1 is a key factor in oral toxicity studies.
Reductions in body weight, food consumption and feces production with polyuria without a compensatory increase in water consumption were observed in male SD rats treated in parenteral (i.p.) with 7.5 or 10 mg/kg b.w./day for four consecutive days58). In addition to dehydration, decreased absolute organ weights of liver, kidney, spleen and thymus were detected. Elevated levels of serum blood urea nitrogen (BUN) and altered kidney morphology indicate nephrotoxicity. Serum levels of ALP, ALT, AST, cholesterol, total bilirubin, and calcium were elevated, and of glucose and amylase are reduced in these animals. These data suggest the dysfunctions of pancreas as well as kidney and liver.
Damages of lung and intestine, in addition to liver and kidney, are reported in male BDIX rats treated p.o. with 4.7 mg/kg of FB1 for 12 days and 7 mg/kg for the successive 9 days of FB11).
3.3. Toxicity in Rabbit
Single or multiple doses of FB1 (less than 0.5 mg/kg i.v.) caused renal proximal tubular damage in rabbits. Serum levels of urea nitrogen and creatinine were increased and urinary glucose and protein concentrations were also altered59). These findings suggest that FB1 targets the proximal tubular epithelia, especially at the cortico-medullary junction, in rabbit kidney. The increased levels of protein in the urine may be indicative of the extensive tubular epithelial necrosis.
3.4. Toxicity in Swine
Swine showed pulmonary edema and hydrothorax after the feeding of fumonisin-contaminated corn60) or intravenous injection of FB1 (0.174 to 0.4 mg/kg b.w.) daily for 4 to 7 days61). Pancreatic lesions were present in all pigs developed pulmonary edema/hydrothorax. Liver changes were observed in all pigs fed FB1 diet for the feeding study61). The animal shows typical lesions including interlobular edema of lung and pancreatic lesions62).
Feeding of 20 mg FB1/kg b.w./day for four days resulted in the pulmonary edema and hepatotoxicity in pigs63). Pigs developed pulmonary edema beginning on day 3; none survived beyond day 4. Progressive elevations in hepatic parameters, including serum enzymes, bile acids, total bilirubin, and histologic changes, began on day 2. Early histological changes in the lung consisted of perivascular edema followed by interlobular and peribronchial edema without the sign of inflammation. Ultrastructurally, alveolar endothelial cells contained unique accumulations of membranous material within pulmonary intravascular macrophages beginning on day 2. Marked elevations in sphinganine, sphingosine, and their ratio (sphinganine/sphingosine Sa/So) began on day 1 for all tissues, irrespective of being morphologically (lung, liver) or not (kidney, pancreas)63). Thus, FB induces early elevations in sphingolipids and hepatic damage, followed by alveolar endothelial damage in pigs63,64). In an experiment using pulmonary endothelial cells, increased albumin permeability was observed in the presence of 30 to 50 mM FB165). Neither toxicity nor ultrastructural changes were, however, detected after the exposure at 5–20 µg/ml (corresponding to 7–28 μM), relevant to in vivo exposure levels, for seven days65). These data may favor the idea that pulmonary dysfunction is an indirect consequence of the damage on primary target tissue(s).
3.5. Toxicity in Horse
Leukoencephalomalacia is a typical toxicity of FB1 in horses66,67). Gavage administration of cultured materials containing FB1 (2.5 g cultured material/kg b.w./day for seven days) to a horse caused severe hepatotoxicity and mild edema of the brain68). In a similar experiment with the cultured material of 1.25 g/kg b.w./day, another horse developed mild hepatic disorder and moderate edema of the brain. In both animals the brain edema was particularly noticeable in the medulla oblongata.
A horse given intravenously 7 times from day 0 to day 9 with 0.125 mg of FB1/kg b.w./day showed clinical signs of neurotoxicity, which appeared on day 8. Euthanasia was performed on the horse on day 10 while the animal was in a tetanic convulsion. The principal lesions were severe edema of the brain and early, bilaterally symmetrical, focal necrosis in the medulla oblongata68).
In horses treated intravenously with 0.05–0.20 mg/kg of FB1 for up to 28 days, hindlimb ataxia, delayed forelimb placing, and decreased tone and movement of tongue are observed69). These results indicate that FB1 induced dose-dependent changes in the cerebrospinal fluid and nerve system.
3.6. Developmental Toxicity
Pregnant CD1 mice were treated orally with semi-purified extracts of F. moniliforme containing FB1 at a dose of 0, 12.5, 25, 50 or 100 mg/kg b.w./day between GD 7 and 15. At GD 18, litters were examined for gross abnormalities and for skeletal or visceral examinations70). Significant maternal mortality was observed in mice treated with the 50 and 100 mg/kg b.w./day. Maternal body weight gains, live-offspring number per litter, and mean body weight of the offspring were decreased at FB1 doses of 25 mg/kg b.w./day or the higher. The frequency of resorption of implants increased at all the doses in a dose-dependent manner. The incidence of ossification deficits also increased from the 25 mg/kg b.w./day dose. Cleft palate was observed at the highest FB1 dose group. Maternal intoxication manifested as ascites associated mainly with increased histopathological scores reflecting hepatocellular damage. Concomitant increases in serum ALT on GD 12 was also observed at all doses above 12.5 mg/kg b.w./day of FB1. Authors70) suggest based on these results that FB1 is developmentally toxic in mice, and that this toxicity may be mediated by maternal hepatotoxicity.
In addition, extracts from Fusarium cultures and purified FB1 have been shown to be embryotoxic to hamsters71,72), mice70,73), rats74,75), chicken76,77), and rabbits78). With exception of studies with hamsters and chicken, embryo toxicities in vivo appeared in association with signs of maternal toxicity73–75,78).
FB1 was toxic, especially to pre-somite stage, in a whole embryo system of rat. Embryo growth were retarded, and the development was inhibited without specific abnormalities. Addition of ceramide, N-acetylsphingosine or raft ganglioside Gm1 did not reverse FB1-mediated toxicity, suggesting that inhibition of sphingolipid synthesis was not the cause of the toxicity. Further, the main metabolite of FB1, aminopental (AP-1), inhibited embryonic growth, but was 100-fold less toxic than FB179).
Due to the possible association of FB1-contaminated foods with an outbreak of neural tube defects (NTD) occurred in 1991 in south Texas county bordering Mexico80,81), attentions were focused on risks of FB1 exposure to NTD.
Both in vitro82) and in vivo studies treated FB1 parenterally44) showed the association of FB1 exposure with NTD in mice.
4. Currently Known Biochemical Alterations in FB1-exposed Animals
Considerable amounts of data have been accumulated for the biochemical changes in FB1-mediated toxicities. These may be summarized and subdivided into bile acids, sphingolipids and phospholipids from the point of endobiotics involved.
4.1. Overflow of Bile Acids in Systemic Circulation
As described above, liver is the common target of FB1-mediated toxicity throughout the experimental and domestic animals examined, and cholestatic signs, including increased levels of serum bile acid, are consistently detected in the exposed animals.
The predominant bile acids in control mouse liver are reported to be taurocholic acid (86.1 ± 18.5 nmol/g liver) and cholic acid (4.7 ± 0.9), tauro-β-muricholic acid (34.2 ± 8.8) and β-muricholic acid (35.1 ± 5.0), and tauro-ω-muricholic acid (18.1 ± 4.5) and ω-muricholic acid (11.8 ± 2.1)83). These are excreted in bile, but in situations of the restricted bile excretion, bile acids are excreted in urine through kidney. The hepatic levels of major bile sulfates, taurocholic acid 7-sulfate, cholic acid 7-sulfate, and taurochenodeoxycholic acid 7-sulfate were higher in male mice than in female mice83). This indicates the male-dominant sulfation of cholic acid and chenodeoxycholic acid, and thus in turn suggests that hepatic elimination of hydrophobic bile acids is delayed in female mice. The observed sex-related difference in bile acid excretion is consistent with higher susceptibility of female mice to FB1.
Bile acid exists within enterohepatic circulation (>96%), and clearance through the plasma-renal system occurs selectively at the over-limiting capacity of liver to send bile acids into bile as described above. Obstruction of the common bile duct leads to cholestatic liver. Cholestasis results in the accumulation of hydrophobic bile acids in liver. As a possible cause of liver injury, direct action of bile acids has been proposed from apoptosis of hepatocyte cell line84), which is prevented by a pan-caspase inhibitor, z-VAD-FMK85).
Unlike in mice, urinary excretion of taurocholate is higher in female rats than in male rats due to the higher renal expression of a specific Oatp1 transporter86). An activity of MRP3 transporter mediating bile acid effluence to plasma is higher in female rats than the males87–89). Further, hepatic sulfating activity of bile acids, which is mediated by hydroxysteroid sulfotransferase, is higher in female rats than the males90,91). These sex-related differences on bile acids are accorded with the higher susceptibility of kidney of male rats to FB1.
Thus, data on plasma bile acids are in good agreement with the idea of bile acid-involvement on FB1-mediated kidney toxicity. No morphological changes with apoptosis or activation of caspases are, however, evident in vivo in bile duct ligated rats and mice92–94). In addition, serum pro-apoptotic bile acid levels are approximately 1,000-fold less than needed to stimulate apoptosis in vitro95). Instead, neutrophil-induced liver injury was proposed in bile-duct ligated animals96,97). Another study also implies that a hydrophobic lithocholic acid leads to cholestasis through the disruption of phospholipid and sphingolipid homeostasis in mice98).
Certain amounts of bile acids are also excreted in urine of pig. Hydrophilic γ-muricholic acid, but not cholic acid, is excreted in urine and bile in this species99,100).
Therefore, the role of bile acid effluence into systemic circulation for FB1-mediated kidney toxicities is yet unclear, although the chronic exposure to bile acids is possible to exacerbate functions of liver in mice and kidney in rats.
4.2. Alteration of Sphingoid and Ceramide Levels
FB1 is known to influence several distinct signaling pathways of sphingoids including ceramide synthesis, sphingosine-1-phosphate receptor and glycosylphosphatidylinositol anchoring19,101–103).
4.2.1. Sphingoid
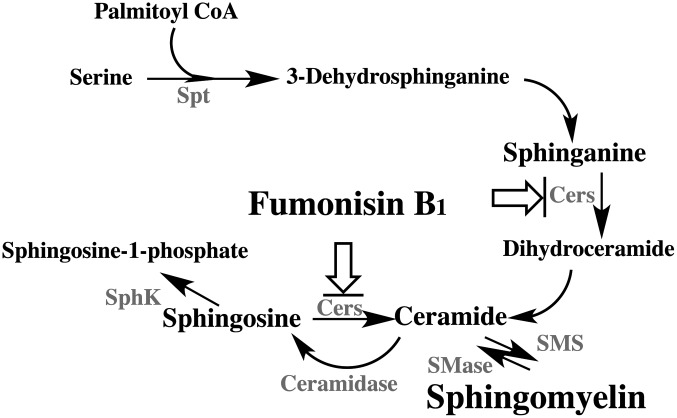
FB1 inhibits ceramide synthase, a key enzyme in the de novo biosynthesis of sphingolipids (Fig. 2). The increase of free sphinganine (and also the ratio with sphingosine) in tissue after the administration of FB1 is used as a biomarker for fumonisin exposure. A single subcutaneous injection of FB1 caused an increase in free sphingoid bases in male BALB/c mice. A significant time-dependent increase in sphingoid bases occurred in the intestine and liver peaking at 4–8 hr and declining to control levels by 24 hr. The level of free sphinganine remained high in the kidney104). This persistence in renal cells was rapidly reversed in the presence of the inhibitor (ISP-1/myriocin)105) of serine palmitoyltransferase, which catalyzes sphinganine biosynthesis.
Fig. 2.
Biosynthesis of sphingomyelin and interaction of fumonisin B1
Interaction points of fumonisin B1 are shown as arrows with bar. Enzymes are shown in gray color. CerS; ceramide synthase (sphinganine/sphingosine N-acyltransferase), SMS; sphingomyelin synthase, SMase; sphingomyelinase, SphK; sphingosine kinase, and Spt; serine palmitoyltransferase.
The associations of sphingoid levels with FB1 toxicities were also detected in kidney and liver of rats55) and in lung of pigs63). However, no clear mechanistic clues have been provided between FB1-mediated changes in sphinganine levels and organ toxicities in vivo in these animal species.
Calmodulins (CaM1-4) are small, ubiquitous adaptor proteins that amplify calcium cation’s diminutive size to the scale of proteins106). Sphingosine is a potent inhibitor of several calmodulin-dependent enzymes including multifunctional Ca2+/calmodulin-dependent protein kinase, Ca2+/calmodulin-dependent phosphodiesterase, and smooth muscle myosin light chain kinase107). Inhibition of each of the enzymes is competitive with calmodulin, suggesting that sphingosine may be a calmodulin antagonist.
The activity of acidic sphingomyelinase was enhanced and liver sphingomyelin content was decreased following five daily treatments with FB1 in mice. The expression and activities of serine palmitoyltransferase and sphingosine kinase were increased significantly in liver following FB1 treatment. These data suggest that FB1-mediated inhibition of ceramide synthase stimulates sphingolipid-metabolizing systems to maintain a balance of cellular sphingolipids23).
4.2.2. Ceramide and ceramide synthase
Ceramide is a lipid-signaling molecule that plays critical roles in regulating cell behavior108). Six distinct forms of mammalian ceramide synthases (CerS) mediate syntheses of ceramides with restricted acyl chain lengths109–111). CerS1, CerS2, CerS3, CerS4 and CerS5/6 mediate mainly the formation of C18, C22, C26, C18 and C16 dihydroceramides, respectively112).
The sphingolipid contents of lung and liver were compared in normal and FB1-exposed piglets (gavaged with 1.5 mg FB1/kg b.w. daily for 9 days)14). The effect of the toxin on each CerS form was deduced from an analysis of its effects on individual ceramide and sphingomyelin species. As expected, the total ceramide content decreased by half in the lungs of FB1-exposed piglets, Total ceramide increased 3.5-fold in the livers of FB1-exposed animals, possibly due to the degradation of sphingomyelin. These data are consistent with the view that FB1 is more prone to bind to CerS4 and CerS2 to deplete lung d18:1/C20:0 and d18:1/C22:0 ceramides113). These views are consistent with the idea that differences in CerS4, CerS2, and CerS1 expressions in targets are involved in the toxicity of FB1.
CerS2-deficient mice, carrying an insertion (gene trap) in the CerS2 gene to generate transgenic mice that lack CerS2 mRNA, lacked ceramide synthase activity toward C24:1 in the brain as well as the liver, and showed only very low activity toward C18:0-C22:0 in liver and also reduced activity toward C22:0 residues in the brain114). These mice also exhibit strongly reduced levels of ceramide components with very long fatty acid residues (>or=C22) in the liver, kidney and brain. In addition, histochemical staining of myelin is progressively lost from the early adulthood, followed by about 50% loss of compacted myelin and 80% loss of myelin basic protein. Starting around 9 months, both the medullary tree and the internal granular layer of the cerebellum showed significant signs of degeneration associated with the formation of microcysts. Beyond seven months, the CerS2-deficient mice developed hepatocarcinomas with local destruction of tissue architecture and discrete gaps in renal parenchyma.
In another CerS2-deficient mouse model115), liver ceramide and downstream sphingolipids were devoid of very long (C22-C24) acyl chains, consistent with the substrate specificity of CerS2 toward acyl-CoAs. Unexpectedly C16-ceramide levels were elevated in vivo, although C16-ceramide synthesis in vitro was not increased. Levels of sphinganine were also significantly elevated, by up to 50-fold in the liver.
With the exceptions of glucosylceramide synthase and neutral sphingomyelinase 2, none of the other enzymes tested in either the sphingolipid biosynthetic or metabolic pathways were significantly changed in the liver. Total glycerophospholipid and cholesterol levels were unaltered, although there was a marked elevation in C18:1 and C18:2 fatty acids in phosphatidylethanolamine (PE), concomitant with a reduction in C18:0 and C20:4 fatty acids. These results indicate that CerS2 activity supports different biological functions: maintenance of myelin, stabilization of the cerebellar as well as renal histological architecture, and protection against hepatocarcinomas possibly through crosstalk and regulation between the various branches of lipid metabolic pathways115). These data are consistent with the association of ceramides and sphingomyelin on FB1-mediated liver and brain toxicities.
4.2.3. Sphingolipid signals
On the interaction of sphingolipids with cellular signal molecules, hypoxia-reoxygenation of rat renal tubular epithelial NRK-52E cells resulted in a significant increase in ceramide synthase activity without any significant change in acid or neutral sphingomyelinase. The hypoxia-reoxygenation of NRK-52E cells was also associated with the release of endonuclease G from the mitochondria to the cytoplasm. It further led to the fragmentation of DNA and cell death. Addition of 50 μM FB1 suppressed hypoxia-reoxygenation–induced ceramide generation and provided protection against the release of hypoxia-reoxygenation–induced endonuclease G, DNA fragmentation, and cell death116). FB1 has also been shown to inhibit various inducer mediated-cell deaths in cell lines110).
In contrast, sphingolipid metabolites such as ceramide, glycosphingolipids, sphingosine 1-phosphate and gangliosides are involved in β-cell signaling pathways and processes implicated in β-cell diabetic disease such as apoptosis, β-cell cytokine secretion, endoplasmic reticulum-to-Golgi vesicular trafficking, islet autoimmunity and insulin gene expression117). Further, FB1-mediated activation of NF-κB (nuclear factor-kappa B) and the sequential induction of TNFα (tumor necrosis factor α) expression stimulated in the subsequent increase in caspase 3 activity in porcine renal proximal cell-derived LLC-PK1118). Thus influences of sphingoids on cellular signal molecules may depend on the cell type and experimental conditions to exert the protecting or exacerbating effects. The role of signal molecules derived from sphingoids remain obscure in vivo on FB1-mediated toxicity119).
4.3. Supply Circle of Phospholipid
4.3.1. Supply through the intestinal absorption
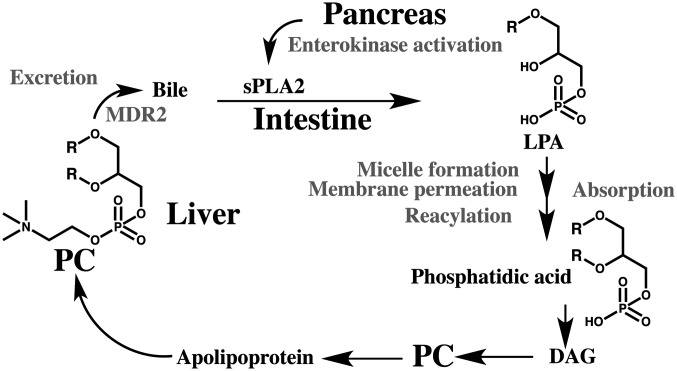
Similar to xenobiotics, various endobiotics are excreted through bile. Non-esterified cholesterol, bile acids, phosphatidylcholine (PC) and glutathione are the main components of bile. Specific transporters expressed in canalicular membrane are involved in their transfers from liver to bile duct (Fig. 3). ATP-binding cassette transporters, ABCG5/8, ABCB10 (BSEP), ABCB4 (MDR2) and ABCC2 (MRP2), mediate the transfer of cholesterol, cholates, PC and glutathione in livers, respectively. Cholesterol, bile acids and PC are forming micelles, holding certain ranges of their ratios in the bile after the membrane permeation to maintain the bile fluidity120,121), and descend to gall bladder and intestine. Insufficient supply of PC, thus, affects the excretion of cholesterol and bile acids. Bile flow is driven mainly by the levels of bile acids122) and glutathione123) and the deficient supply of PC is able to reduce the rate of bile flow. Prolonged irregular ratios of bile components would thus promote formation of cholestatic conditions.
Fig. 3.
Enterohepatic circulation of phosphatidylcholine
R; fatty acids, DAG; diacylglycerol, LPA; lysophosphatidic acid, MDR2; ABCB4 phosphatidylcholine transporter, PC; phosphatidylcholine, sPLA2; secretory phospholipase A2 (IB form). Specific process and enzymes are shown in gray color.
Dysfunctions in canalicular components are known to associate with enhanced levels of ALP and bilirubin in the body. Observed changes in biochemical parameters, excesses of cholesterol and bile acids compared to triglycerides in FB1-treated mice and rats are consistent with the notion of shortage of phospholipids, particularly PC, to excrete in bile. Liver PC is maintained by supplies from two sources124). One is a conversion from triglycerides within hepatocytes. The other is intestinal absorption of phospholipids from bile and diet. Bile supplies the majority (up to 90%) of intestinal phospholipids. Intestinal phospholipids are hydrolyzed to lysophosphatidic acid (LPA) in the presence of secretory phospholipase A2 (sPLA2) released from the pancreas. LPA forms micelles with monoacylglycerol, fatty acids, bile acids and cholesterol, and then passes through the brush border membrane. Within enterocytes, LPA is acylated to phosphatidic acid and then to PC and PE. These phospholipids are delivered as particles like chylomicron and apolipoprotein B to tissues.
4.3.2. Supply through the liver synthesis
Phospholipids are biosynthesized from diacylglycerols in tissues like liver. Liver has two routes of PC biosynthesis. The main route is a sequential pathway starting from choline to phosphocholine to CDP choline and then to PC. Another route is three-consecutive methylations of PE to form PC. Methionine is the donor of the methyl group. The latter route is found only in liver.
CTP:phosphocholine cytidylyltransferase α (CCTα) is essential for production of PC of animal cell membranes and is proteolytically sensitive125). Calmodulin stabilized CCTα from the calpain-mediated proteolysis. Mapping and site-directed mutagenesis of CCTα uncovered a motif (LQERVDKVK) harboring a vital recognition site, Gln(243), whereby calmodulin directly binds to the enzyme. Thus, calmodulin, through antagonizing calpain, serves as a novel binding partner for CCTα that stabilizes the enzyme under proinflammatory stress126). The CDP-choline pathway contributes PC supply for bile secretion. The function of CCTα may be impaired in the presence of an antagonist of calmodulin, sphingosine as described in the section of 4.2.1 (Sphingoid), and also through the direct interaction of CCTα with sphingosine127), and indirectly through the repression of CCT gene transcription128). Gene knockout of either CCTα (Pcyt1a) or PE N-methyltransferase (Pemt), however, had no strike effect on bile secretion or composition, suggesting the existence of effective metabolic compensation mechanism129,130).
PC synthesis is reduced in the liver of Pemt-null mice. Thus, treatment of Pemt null mice with choline-deficient diets results in abrupt death (within five days)131). Double knockout mice of Pemt and Abcb4 (Mdr2) are somewhat resistant to choline-deficient diets132), through knockout of the PC-transporter Abcb4 (Mdr2) to relieve PC loss. The Mdr2(−/−)/Pemt(−/−) mice also adapt to severe choline deprivation through several mechanisms, including choline recycling by induction of PLA2, by the activity of choline kinase, and CCTα activities and also by a strikingly decreased expression of choline oxidase132). These pathological models raise a possibility of uncompensated loss of PC in bile as a main etiological factor in FB1-treated animals, in addition to FB1-mediated inhibition of ceramide synthase102,133).
4.4. Possible Trigger Point of PC-supply Dysfunction
4.4.1. Lipase function
PC is produced from diacylglycerol and CDP-choline. Decreased supply of triacylgycerol from intestine may affect the PC supply in liver. Orlistat is a potent, specific and irreversible inhibitor of gastric and pancreatic lipases134). This drug forms a covalent bond with the active serine of both lipases in the lumen of the gastrointestinal tract, which prevents the hydrolysis of dietary fat into absorbable monoacylglycerol. In patients taking orlistat, levels of both cholesterol and LDL are markedly reduced135). However, orlistat does not severely affect the plasma triacylglycerol level. The reduction of plasma cholesterol level observed are rather inconsistent with the no clear changes in FB1-mediated animals. These data are rather discordant with the idea that secreted-lipase dysfunction is a main causal mechanism of FB1-mediated liver toxicity.
4.4.2. Pancreatic phospholipase function
Levels of total cholesterol in sera and livers were significantly increased in the rats fed FB1 (250 mg/kg diet) for 21 days, while the liver sphingomyelin and PE were significantly decreased and increased, respectively. In a long-term study, only PE was significantly increased in all the FB1-treated animals136). In mice treated with FB1 for more than one week, changes in biochemical parameters become apparent. Increases in plasma values of ALT, ALP, bilirubin, cholesterol and bile acids are detected. FB1 also caused significant cholestasis and liver necrosis in pigs62). These data suggest the commonality of hepato-biliary dysfunction, particularly cholestasis, in FB1-treated animals.
As described in the section of 4.3.2 (Supply through liver synthesis), insufficient supply of PC in bile secretion is likely to participate in the FB1-mediated cholestasis. Enhanced synthesis of PC through the CDP-choline pathway and from PE and methionine is necessary to compensate the loss of PC in the bile.
Studies with an experimental animal model have shown that choline and methionine deficiency causes dysfunctions of liver and pancreas137,138). Young female CD1 mice fed a choline-deficient, ethionine-supplemented (CDE) diet for 24 hr develop hemorrhagic pancreatic necrosis with a 5-day mortality rate of approximately 50%139). At the end of the diet administration, the in vivo discharge of digestive enzymes is blocked, disappearance of exocytosis took place, and zymogen granules accumulate within acinar cells. Thus, the CDE diet does not affect the general phenomenon of membrane fusion-fission but specifically inhibits the membrane fusion-fission associated with exocytosis. Twenty-four hours after withdrawal of the CDE diet, discharge of zymogen granules into lysosomes (crinophagy) can be observed, and, 24 hr later, autophagocytosis is noted.
In fact, FB1-mediated pancreatic dysfunction is observed in rats58) and pigs60,64). The pancreatic disorders may be associated with methionine deficiency as discussed in later.
Young female mice, fed a diet deficient in choline and supplemented with ethionine, develop acute necrotizing hemorrhagic pancreatitis, and the incidence is greater in young and female mice than in adult and male mice140,141). In contrast, female rats are less susceptible than the males to the acute effects of choline deficiency, such as fatty liver and impaired secretion of triglycerides into the blood plasma142). In addition, protection conferred by ethinylestradiol in the livers of rats fed a methionine- and choline-deficient diet is consistent with the relative insensitivity of female rats to the hepatotoxicity of dietary methyl donor insufficiency143). Observed sex-related differences in CDE diet-mediated liver toxicity are consistent with those in FB1-mediated liver toxicities in mice and rats. These data suggest the possible association of hepato-pancreatic changes on FB1-mediated toxicity.
For pancreatic zymogens, two steps of their activations are required with the exception of trypsinogen. In the first stage, active trypsin is generated from its zymogen by enterokinase, and in the second stage trypsin activates the remaining zymogens giving rise to the well-known activation cascade of pancreatic enzymes144). Enterokinase is synthesized in the enterocytes of the proximal small intestine145).
DL-Ethionine has a destructive effect on the acinar tissue of the pancreas of rats137). The destructive effects of ethionine on liver and pancreas can be completely prevented by the simultaneous administration of equal molar amounts of methionine. Ethionine affects protein synthesis through inhibiting the incorporation of methionine and glycine into proteins in the liver146).
As described above, treatment of Pemt-null mice with choline-deficient diets results in severe liver dysfunction and abrupt death within five days. PC deficiency is replenished by a synthesis from PE in livers in the presence of Pemt. Methionine is utilized for the methyl donor.
In a state of reduced supply of zymogen from pancreas, decreased digestion capacity of protein at intestine results in the diminished amounts of methionine availability. Methionine supplied through portal vein is consumed extensively within liver for the PC and protein syntheses147). These situations would link to methionine-deficient conditions in other tissues. Methionine deficiency in the pancreas may promote dysfunctions of pancreas in similar to those in rodents treated with CDE diet. Consistent with this idea, FB1 treatment resulted in the up-regulation of the intestinal brush border transporter B0AT and the basolateral transporter y+LAT1 in newborn chickens, suggesting the demand of an increased intracellular uptake of neutral amino acids such as methionine148).
In fetal rat pancreas cells, methionine is required for the growth of the exocrine pancreas, and the higher levels are required to achieve basal or maximal differentiation of acinar cells149). The basal requirement was determined to be 30 mg/L methionine, and a level of 80 mg/L supported maximal differentiation.
4.4.3. Secretory phospholipase
Secretory phospholipase A2s (sPLA2s) are small secreted proteins (14–18 kDa), proteolytically activated after their release from the pancreas. sPLA2s require submillimolar levels of Ca2+ to liberate LPA. An sPLA2-deficient mutant showed no clear alteration of phospholipid absorption possibly by the feedback regulation of secretins and partly as a result of compensation by other PLA2 forms150). Pancreatic PLA2 also has intrinsic secretin-releasing activity151,152). Secretin regulates exocrine secretion as well as gastric motility. A pancreas injured by FB1 may not function well to compensate the secretion. In addition, FB1 is a chelating agent of divalent cations like calcium ion. Considerable retention of FB1 in intestinal cells is expected from the increase of sphingoids even after the s.c. administration in mice104), suggesting the possible contact of the FB1 with secretory phospholipase A2. These situations would exacerbate the absorption capability for phospholipids at intestine.
In addition to its enzymatic function, sPLA2 can exert various biological responses through the binding to specific M-receptors. Physiologically, sPLA2s play important roles on the neurotransmission in the central nervous system and the neuritogenesis in the peripheral nervous system. sPLA2 are implicated to link to the neurodegenerative diseases like Alzheimer’s disease and cerebrovascular diseases like stroke153). Release of sPLA2 in the circulation during pancreatic dysfunction may evoke the signal through M-receptor.
M-type phospholipase A2 receptor (PLA2R) is expressed in podocytes in human glomeruli. One of the ligands, group IB secretory phospholipase A2 (sPLA2 IB), is expressed higher in chronic renal failure patients than in controls. After binding to human podocyte PLA2R in vitro, sPLA2 IB form induced podocyte apoptosis in a time- and concentration-dependent manner154). sPLA2 IB upregulated PLA2R and increased ERK1/2 and cPLA2α phosphorylation in podocytes, which resulted in enhanced apoptosis. In contrast, PLA2R-silenced human podocytes displayed attenuated apoptosis. These data indicate that sPLA2 IB has the potential to induce human podocyte apoptosis via binding to the PLA2R. No PLA2R1 is expressed in mouse kidney podocytes155) and thus the role of sPLA2 on FB1-mediated kidney toxicity in mice remains unclear.
4.4.4. Intestinal membrane alteration and zinc-deficiency
Feeding FB-containing diet (FB1 + FB2 25.4 mg/kg feed) for 15 days to chickens resulted in altered glycosylation of mucin and in a decreased level of mucin 2 mRNA in the duodenum148). In addition, specific mRNA level of basolateral zinc transporter (ZnT1) was reduced in jejunum of FB-treated chickens148), which may contribute to maintain the zinc ion in brush border.
Marginal zinc-deficiency is known to associate with the decreased absorption of triglyceride in rats156). Plural mechanisms including apolipoprotein B mRNA editing157) are known to participate in this phenomenon. FB1 includes two moles of carballylic acid in the molecule, which are chelating agents of divalent cations158). FB1 is thus possible to contribute intestinal zinc deficiency as a zinc-chelating agent. The primary defect in lipid absorptive processes in zinc-deficient rats occurred in the formation of chylomicrons159).
5. Interaction of Phospholipids in Tissue Membranes
5.1. Lipid Raft Anchoring and Folate
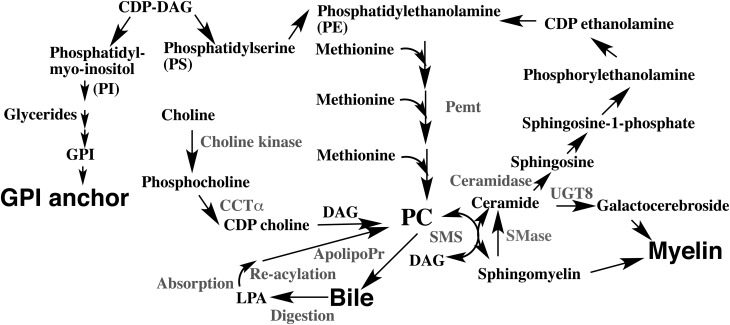
Glycosylphosphatidylinositol (GPI) functions in the integration of GPI-anchored proteins into lipid microdomains. GPI anchor is biosynthesized from phosphatidylinositol-containing unsaturated fatty acyl chains at the sn-2 position, and the lipid moieties of the GPI anchor are exchanged after GPI attachment to proteins in mammalian cells (Fig. 4). This lipid remodeling process of GPI-anchored proteins is essential for their association with the lipid microdomains160).
Fig. 4.
Interrelationship of phospholipids and sphingolipids on their biosynthesis and recycling
Enzymes and the associated biological processes are shown in gray color.
ApolipoPr; apolipoprotein, CCTα; CTP:phosphocholine cytidylyltransferase α, CDP-choline; cytidine diphosphocholine, DAG; diacylglycerol, GPI; glycosylphosphatidylinositol, LPA; lysophosphatidic acid, PC; phosphatidylcholine, PE; phosphatidylethanolamine, PI; phosphatidylinositol, PS; phosphatidylserine, SMase; sphingomyelinase and UGT8; UDP-galactose ceramide galactosyltransferase.
Granule formation and apical sorting of digestive enzymes in pancreatic acinar cells involves the selective aggregation of a mixture of regulated secretory proteins and the association of these aggregates with specific membrane domains of the trans-Golgi network, which then pinch off as condensing vacuoles.
Isolated membranes of zymogen granules from pancreatic acinar cells are rich in cholesterol and sphingomyelin and formed detergent-insoluble complexes. These complexes contained the GPI-anchored glycoprotein GP-2, the lectin ZG16p, and sulfated matrix proteoglycans. Formation of zymogen granules as well as the formation of detergent-insoluble complexes was reduced after treatment of rat pancreatic lobules with FB1103). These results suggested the possible role of GPI-anchor in zymogen processing in the pancreas. GP-2-null mice, however, displayed no gross signs of nutrient malabsorption such as weight loss, growth retardation, or diarrhea161). Zymogen granules in the GP-2 null mice appeared normal on electron microscopy and contained the normal complement of proteins excluding GP-2161). Therefore, the role of GPI anchoring on the FB1-mediated pancreatic toxicity remains obscure.
The folate receptor, like many glycosylphosphatidylinositol-anchored proteins, is found associated with membrane domains that are insoluble in Triton X-100 at low temperature and that are enriched in cholesterol and sphingolipids. The folate receptor-mediated transport of 5-methyltetrahydrofolate was almost completely blocked in cells in which sphingolipids had been reduced by approximately 40% in Caco-2 cells after FB1-treatment. FB1 had no influence on the receptor-mediated transport or facilitative transport of the folate162). FB1-treatment was shown to alter recycling of folate receptor in cell systems163).
Maternal FB1 administration (20 mg/kg of b.w./day, i.p.) to pregnant LM/Bc mice during early gestation resulted in 79% NTDs in exposed fetuses44). Sphingolipid profiles were significantly altered in maternal and embryonic tissues following the exposure, and 3H-folate levels and immunohistochemical expression of GPI-anchored folate receptor were reduced. Maternal folate supplementation partially rescued the NTD phenotype, whereas intraperitoneal injections of a lipid raft component, GM1, significantly restored folate concentrations and afforded almost complete protection against FB1-induced NTDs. Maternal FB1 exposure is thus proposed to alter folate concentrations in LM/Bc mice, resulting in a dose-dependent increase in NTDs.
Treatment with the same regimen of inbred mouse SWV strain, however, yielded different results. Approximately 15% of the implants were resorbed, and only one exencephalic fetus was observed in the 10 SWV litters examined after the intraperitoneal administration of 20 mg /kg b.w./day on GD 7.5 and GD 8.5. A similar strain difference was also observed between CD1 and LM/Bc mice101,164). CD1 mice exhibit an increased number of early embryonic resorptions and/or late fetal death following FB1 exposure, whereas LM/Bc embryos survive, but fail to complete neurulation.
5.2. Interaction of Folate, Cobalamin and Methionine
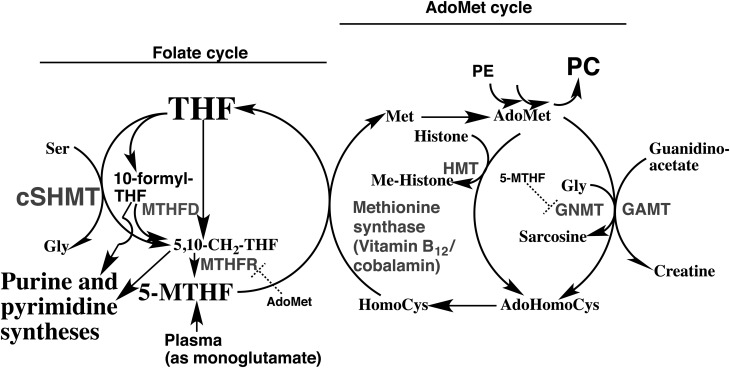
Folate, S-adenosylmethionine and cobalamin (vitamin B12) are key components of the one carbon unit (C1-unit) supply chain for nucleotide biosynthesis165) (Fig. 5). Interruption of this supply chain is known to lead to embryo and developmental toxicities166,167).
Fig. 5.
Folate, cobalamin and methionine and their interactions
Cys; cysteine, Gly; glycine, Ser; serine, 5-MTHF; 5-methyltetrahydrofolate pentaglutamate, GNMT; glycine N-methyltransferase, HMT; histone methyltransferase, MTHFD; tetrahydrofolate dehydrogenase, MTHFR; methylenetetrahydrofolate reductase, cSHMT; cytosolic serine hydroxymethyl transferase, THF; tetrahydrofolate pentaglutamate, adoMet; S-adenosylmethionine. Dotted halt-signs indicate the feedback inhibition. Enzymes are shown in gray color.
Female LM/Bc mice fed folate-sufficient (control) or folate-deficient diet were treated intraperitoneally with 0 (vehicle), 2.5 or 10 mg/kg FB1 on embryonic days 7 (E7) and E8, and their fetuses examined on E16. Dose-dependent NTD induction was found in groups fed the control diet: 3 of 13 low-FB1 dose and 10 of 11 high-FB1 dose litters were affected. Among groups fed folate-deficient diet, NTDs were found only in 4 of 11 high-FB1dose litters. In another trial, consumption of folate-deficient diet also resulted in fewer NTDs at a dose of 10 mg/kg of FB1 and reduced maternal red blood cell folate levels by 80%. In utero death did not fully account for the differences in NTD rates. Thus, folate deficiency does not exacerbate FB1-mediated NTD formation in LM/Bc mice168).
Cobalamin can bind two carrier proteins in the digestive tract, haptocorrin (R protein) and intrinsic factor, but only the binding to intrinsic factor allows its absorption. Cobalamin is bound almost exclusively to haptocorrin in the acid milieu of the stomach, rather than to intrinsic factor. Cobalamin remains bound to haptocorrin in the slightly alkaline environment of the intestine until pancreatic proteases partially degrade haptocorrin and enable cobalamin to become bound exclusively to intrinsic factor169).
In the aspirates from patients with exocrine pancreatic dysfunction, cobalamin was found to be coupled >60% to a protein identical to R proteins in terms of molecular mass (125,000), ionic nature (mean pI, 3.51), and also of the reactivity with anti-R protein and anti-intrinsic factor sera. These findings suggest that the formation of unabsorbable cobalamin complexes may be a reason for the impaired cobalamin absorption in exocrine pancreatic insufficiency. Decreased enzyme activity and nondegradation of R proteins may also be due to malactivation of pancreatic zymogens in an acidic pH of the intestinal juice170). In another study of patients showing malabsorption of cobalamin with exocrine pancreatic insufficiency, a failure to degrade haptocorrin is proposed to prevent the binding of cobalamin to intrinsic factor171). Thus currently plural types of C1-unit dysfunctions are possible causes of developmental toxicity after FB1 exposure101).
5.3. Myelin
Long-term deficiency of cobalamin or folate causes a demyelinating disease of the brain and spinal cord172). A reduced supply of methyl groups has been implicated as the mechanism of demyelination from human studies of inborn errors. One child had abnormal methylfolate metabolism, one had abnormal methylcobalamin metabolism, and one had hypermethioninaemia probably caused by methionine adenosyltransferase deficiency. Each patient had abnormal myelination before treatment, suggesting demyelination with low cerebrospinal concentration of S-adenosylmethionine. Treatment with L-methionine or betaine led to substantial clinical improvement, apparent remyelination, and increases in cerebrospinal S-adenosylmethionine concentration into the normal range172). Thus, expected decrease in circulating methionine level in FB1-exposed animals could be linked to the brain dysfunction.
Myelin is a fatty white substance surrounding the axon of nerve cells173,174). The primary lipid of myelin is glycolipid called galactocerebroside. UGT8 enzymes175) (UDP-galactose ceramide galactosyltransferases) catalyze transfer of galactose to ceramide to form galactocerebrosides (Fig. 4). Amounts of compacted myelin as well as myelin binding protein and myelin specific sphingolipids are decreased in CerS2-deficient mice carrying an insertion (gene trap) in the CerS2 gene114). CerS2 is localized to white matter of the brain and capable of synthesizing myelin sphingolipids176) These results suggest the possible involvement of FB1-mediated suppression of brain CerS2 in myelin dysfunction. Sphingomyelin is also contained in the membranous myelin sheath surrounding nerve cell axons, where it serves as an electrically insulating layer.
Myelination starts at birth in the spinal cord in the mouse, and is achieved in almost all regions of the brain around 45–60 days postnatally173). In humans, the peak of myelin formation occurs during the first year postnatally177), although it starts during the second half of fetal life in the spinal cord. These results may suggest the high susceptibility to FB1 during developing ages. Decreased production of ceramides would lead to reduced levels of galactocerebroside and PC/sphingomyelin, which may be associated with equine leukoencephalomalacia and swine pulmonary edema, respectively.
6. Roles of PC Mobilization and of Methionine-deficiency in FB1-mediated Toxicity
FB1 shows multiple organ toxicities in experimental and domestic animals. Toxicities like leukoencephalomalacia and pulmonary edema are unique and species-specific, but nephrotoxicity occurs in many species and hepatotoxicity appears in all species examined. Profiles of biochemical parameters suggest the suppressed bile excretory function possibly due to the deficiency of PC supply as the initial event of FB1-mediated liver toxicity as described above.
Typical pathological models of liver toxicity are related to phospholipid dysfunctions in rodents. One model is the Pemt-null mouse fed a choline-deficient diet131), and the second is rat and mouse fed choline-deficient diet with ethionine supplementation140–142). The third model is mouse fed a diet deficient of both choline and methionine. The first model showed severe liver disorders to lead to death within one week. The second model is characterized with both liver and pancreas damages. The third is a typical NASH (nonalcoholic steatohepatitis) model98,178). These models yield consistently deficient conditions of both CDP-choline and methyl donor for PE methylation.
Total phospholipids account for roughly 2–3% wet weight of liver of mouse and the half is PC179). Thus, total hepatic amount of PC would be around 20–30 mg in 1–2 g of liver of 20 g body weight of mouse. Apparently, the mouse liver secretes the equivalent of its entire hepatic pool of PC into bile daily. Most of the PC is reabsorbed through the intestine as LPA and is returned to the liver and other tissues after the reacylation. Administration of methionine was unable to prevent FB1-mediated liver toxicity in rats180), although methionine partly prevented an increase of sphingosine, but not sphinganine. Therefore, decreased recycling of PC, excreted in the intestine through the bile, is likely the main mechanism for the FB1-mediated deficiency of hepatic PC. No clear information is available at present on the initial disorder to lead to malabsorption of phospholipids in FB1-treated animals. Plural candidates are discussed in this context. 1) Altered functions of sPLA2 are possible to occur. Functional sPLA2 requires submillimolar concentration of calcium ion for the maximal catalysis, but the chelating of carballylic acid part of FB1 with calcium ion may lower the efficiency of the catalysis as a direct interaction of FB1. 2) Similar to rodents fed CDE diet, FB1 causes lesions of the pancreas as well as liver in rats and pigs. If discharge of digestive enzymes is blocked and zymogen granules accumulated within acinar cells of pancreas in FB1-treated animals, reabsorption of PC would be decreased even considering the compensation through other PLA2 forms. The supply of ethionine is critical in pancreas lesion in the CDE model. Auxiliary synthesis of PC from PE in livers of FB1-treated animals would consume methionine entering through portal vein and thus diminish the amount available through the systemic circulation. This may produce a situation similar to animals treated with CDE diet or diet deficient both choline and methionine as described above.
In addition to the three in vivo models described above, administration of thioacetamide is reported to elicit the hepatotoxicity181) and encephalopathy182) in rats. Brain levels of sphingomyelin and phosphatidylinositol are decreased in thioacetamide-treated rats. Similar to the CDE diet model, the hepatotoxicity was ameliorated by the coadministration of S-adenosylmethionine intraperitoneally in thioacetamide-exposed animals183). Thioacetamide is reported to reduce levels of liver-specific methionine adenyltransferase and DNA methylation184). These data also support the idea that methionine and phospholipid are associated with liver and brain toxicities.
On the association of methionine deficiency with pancreatic disorders, S-adenosylmethionine was shown to suppress autophagy through the action of methyltransferase Ppm1p in yeast185). The enzyme modifies the catalytic subunit of PP2A. Lysosomal/autophagic dysfunction was proposed to be a key-initiating event in pancreatitis and cancer186,187). CerS2 down-regulation is also reported to lead to autophagy in cell culture systems188). Autophagosome half-life in mammalian cells is within 10 min, although impaired autophagic flux prolongs the half-life189). Thus, careful examination may be necessary to detect an alteration of pancreas in FB1-treated animals.
FB1 interacts with ceramide synthases (sphingosine N-acyltransfeases) to inhibit N-acylations of sphinganine to form dihydroceramides, and of sphingosine to form ceramides (Fig. 2). Sphingomyelin synthase (SMS) mediates the biosynthesis of sphingomyelin from ceramides and PC. Human SMS1 and SMS2, rather than functioning strictly as sphingomyelin synthases, are capable of using sphingomyelin and PC as phosphocholine donors to produce PC or sphingomyelin, dependent on the relative concentrations of diacylglycerides (DAG) and ceramide as phosphocholine acceptors, respectively190). Both SMS1 and SMS2 are ubiquitously expressed190). SMS1 is associated with the Golgi apparatus, while SMS2 is primarily concentrated at the plasma membrane. The reverse transfer of phosphocholine is detected in various mammalian cells191–193), and syntheses of PC and sphingomyelin are physiologically linked194). SMS-mediated transfer of phosphocholyl group thus may be an additional pathway for supplying PC in tissues like the liver. Decreased levels of sphingomyelin and increased levels of ceramide or sphingosine are detected in livers of FB1-treated mice23), rats136) and pigs14), which may be indicative of the adaptive changes to lead to their liver disorders.
PC is a component of high-density lipoprotein195). Exchanges of PC thus occur through serum lipoproteins among tissues. PC is the predominant phospholipid of alveolar surfactant. Decreased availability of PC as a cellular membrane component, and also as a phosphocholine-donor for sphingomyelin synthesis may be linked to disorders in peripheral tissues such as the kidney and lung in FB1-treated animals. In addition, formation of PC from sphingomyelin would produce ceramides as byproducts. Excess ceramides are metabolized to sphingosine as well as galactocerebroside. Sphingosine and sphinganine are degraded to form ethanolamine phosphate and fatty aldehydes like palmitoic aldehyde. The former is used for the synthesis of PE, whereas the latter may be involved in the oxidative damage that occurs in target tissues. The high demands for PC supply in liver force the mobilization of PC from tissues through plasma components like high-density lipoprotein (HDL), and also the exchange of phosphocholine moiety between sphingomyelin and PC in tissues. Prolonged shifts in phospholipid flow may trigger the signal for FB1-mediated toxicological events.
Myelin is a fatty white substance surrounding the axons of nerve cells173,174). The primary lipid of myelin is a glycolipid called galactocerebroside. UGT8 enzymes175) (UDP-galactose ceramide galactosyltransferases) catalyze transfer of galactose to ceramide to form galactocerebrosides. Production of galactocerebroside is necessary for myelination and FB1-mediated changes in the composition of myelin is possibly associated with brain disorders. In fact, the amounts of compacted myelin as well as myelin binding protein and myelin specific sphingolipids are decreased in CerS2-deficient mice carrying an insertion (gene trap) in the CerS2 gene114). CerS2 is localized to white matter of the brain and capable of synthesizing myelin sphingolipids176) These results suggest the possible involvement of FB1-mediated suppression of brain CerS2 in equine leukoencephalomalacia. In contrast, no embryonic or prenatal abnormalities have been detected in CerS2 null mice115,196).
Sphinganine, sphingosine and the ratio are clearly increased in CerS2-deficient mice in both the liver and kidney, but only the liver toxicity, without kidney pathology, is observed196). These data may be indicative of sphinganine and sphingosine as surrogate marker of the tissue exposure, but accumulation of both substances themselves is not directly linked to the toxicological events at least in the kidney. Alternatively, subsequent metabolism such as formation of sphingosine-1-phosphate may evoke cellular signals. CerS2, having sphingosine-1-phosphate receptor-like motifs in its sequence111), may be regulated in vivo by sphingosine-1-phosphate110). Therefore altered signaling through sphingolipids, and the irregular metabolisms may be associated with chronic toxicities of FB1 as the studies of CerS2-deficient mice suggested114,115). Progress in quantitative analyses of biochemical consequence of phospholipid flows and signals would help us to clarify the detailed mechanisms of FB1-mediated toxicity.
Abbreviations:ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; C1-unit, one carbon unit; CCTα, CTP:phosphocholine cytidylyltransferase α; CDE, choline-deficient; ethionine-supplemented; CerS, ceramide synthase; DAG, diacylglycerol; ED, embryonic day; FB1, fumonisin B1; GD, gestational day; GPI, glycosylphosphatidylinositol; HDL, high-density lipoprotein; LC/MS, liquid-chromatography mass-spectrometry; LPA, lysophosphatidic acid; Mrp, multidrug resistance- associated protein/ABCC transporter; NTD, neural tube defect; PC, phosphatidylcholine; PE, phosphatidylethanolamine; Pemt, phosphatidylethanolamine N-methyltransferase; P-gp, p-glycoprotein/MDR1/ABCB1 transporter; PLA2R, phospholipase A2 receptor; PP, protein phosphatase; SD, Sprague-Dawley; sPLA2, secretory phospholipase A2; SMS, sphingomyelin synthase
Footnotes
Conflict of interest statement: The authors had no conflicts of interest to declare in this article.
Reference
- 1.Gelderblom WC, Jaskiewicz K, Marasas WF, et al. Fumonisins--novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol. 1988; 54: 1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriek NP, Kellerman TS, Marasas WF. A comparative study of the toxicity of Fusarium verticillioides (= F. moniliforme) to horses, primates, pigs, sheep and rats. Onderstepoort J Vet Res. 1981; 48: 129–131. [PubMed] [Google Scholar]
- 3.Kriek NPJ, Marasas WFO, Thiel PG. Hepato- and cardiotoxicity of Fusarium verticillioides (F. moniliforme) isolates from Southern African maize. Food and Cosmetics Toxicology. 1981; 19: 447–456. 10.1016/0015-6264(81)90449-1 [DOI] [PubMed] [Google Scholar]
- 4.Bezuidenhout SC, Gelderblom WCA, Gorst-Allman CP, et al. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. Chem Commun. 1988; 743–745. [Google Scholar]
- 5.Ito Y, Tsurufuji S, Ishibashi S, Ishidate M, Tamura Z, Takita H. Detoxication and excretion of radioactive strontium. III. Effect of tricarballylic and lactic acids. CHEMICAL & PHARMACEUTICAL BULLETIN. 1958; 6: 34–36. 10.1248/cpb.6.34 [DOI] [PubMed] [Google Scholar]
- 6.Beier RC, Elissalde MH, Stanker LH. Calculated three dimensional structures of the fumonisin B1?4 mycotoxins. Bulletin of Environmental Contamination and Toxicology. 1995; 54: 479–487. 10.1007/BF00192588 [DOI] [PubMed] [Google Scholar]
- 7.Merrill AH, Jr, van Echten G, Wang E, Sandhoff K. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J Biol Chem. 1993; 268: 27299–27306. [PubMed] [Google Scholar]
- 8.Fukuda H, Shima H, Vesonder RF, et al. Inhibition of protein serine/threonine phosphatases by fumonisin B1, a mycotoxin. Biochemical and Biophysical Research Communications. 1996; 220: 160–165. 10.1006/bbrc.1996.0374 [DOI] [PubMed] [Google Scholar]
- 9.Jenkins GR, Tolleson WH, Newkirk DK, et al. Identification of fumonisin B1 as an inhibitor of argininosuccinate synthetase using fumonisin affinity chromatography and in vitro kinetic studies. Journal of Biochemical and Molecular Toxicology. 2000; 14: 320–328. [DOI] [PubMed] [Google Scholar]
- 10.Howard P, Couch LH, Patton RE, et al. Comparison of the toxicity of several fumonisin derivatives in a 28-day feeding study with female B6C3F(1) mice. Toxicology and Applied Pharmacology. 2002; 185: 153–165. 10.1006/taap.2002.9529 [DOI] [PubMed] [Google Scholar]
- 11.Bulder AS, Arcella D, Bolger M, et al. Fumonisins (addendum). In: WHO, ed. Safety evaluation of certain food additives and contaminants. Vol 65. Geneva 2012: 325–794. [Google Scholar]
- 12.Marasas WFO. Discovery and occurrence of the fumonisins: a historical perspective. Environmental Health Perspectives. 2001; 109(Suppl 2): 239–243. 10.1289/ehp.01109s2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss KA, Riley RT. Fumonisin Toxicity and Mechanism of Action: Overview and Current Perspectives. Food Safety. 2013; 1: 2013006 10.14252/foodsafetyfscj.2013006 [DOI] [Google Scholar]
- 14.Loiseau N, Polizzi A, Dupuy A, et al. New insights into the organ-specific adverse effects of fumonisin B1: comparison between lung and liver. Archives of Toxicology. 2015; 89: 1619–1629. 10.1007/s00204-014-1323-6 [DOI] [PubMed] [Google Scholar]
- 15.Hahn I, Nagl V, Schwartz-Zimmermann HE, et al. Effects of orally administered fumonisin B1 (FB1), partially hydrolysed FB1, hydrolysed FB1 and N-(1-deoxy-D-fructos-1-yl) FB1 on the sphingolipid metabolism in rats. Food and Chemical Toxicology. 2015; 76: 11–18. 10.1016/j.fct.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 16.Voss KA, Riley RT, Snook ME, Waes JG. Reproductive and sphingolipid metabolic effects of fumonisin B(1) and its alkaline hydrolysis product in LM/Bc mice: hydrolyzed fumonisin B(1) did not cause neural tube defects. Toxicological Sciences. 2009; 112: 459–467. 10.1093/toxsci/kfp215 [DOI] [PubMed] [Google Scholar]
- 17.The nomenclature of lipids. J Lipid Res. 1967; 8: 523–528. [PubMed] [Google Scholar]
- 18.IUPAC-IUB Commission on Biochemical Nomenclature (CBN). European Journal of Biochemistry. 1967; 2: 127–131. 10.1111/j.1432-1033.1967.tb00116.x [DOI] [PubMed] [Google Scholar]
- 19.Merrill AH, Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chemical Reviews. 2011; 111: 6387–6422. 10.1021/cr2002917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature Reviews Molecular Cell Biology. 2003; 4: 397–407. 10.1038/nrm1103 [DOI] [PubMed] [Google Scholar]
- 21.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature Reviews Cancer. 2010; 10: 489–503. 10.1038/nrc2875 [DOI] [PubMed] [Google Scholar]
- 22.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2008; 1781: 424–434. 10.1016/j.bbalip.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Q, Suzuki H, Sharma N, Sharma RP. Ceramide synthase inhibition by fumonisin B1 treatment activates sphingolipid-metabolizing systems in mouse liver. Toxicological Sciences. 2006; 94: 388–397. 10.1093/toxsci/kfl102 [DOI] [PubMed] [Google Scholar]
- 24.Sharma R, He Q, Riley R. Lupus-prone NZBWF1/J mice, defective in cytokine signaling, are resistant to fumonisin hepatotoxicity despite accumulation of liver sphinganine. Toxicology. 2005; 216: 59–71. 10.1016/j.tox.2005.07.024 [DOI] [PubMed] [Google Scholar]
- 25.Merrill AH, Jr, Sullards MC, Wang E, Voss KA, Riley RT. Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environmental Health Perspectives. 2001; 109(Suppl 2): 283–289. 10.1289/ehp.01109s2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Larranaga MR, Anadon A, Diaz MJ, et al. Toxicokinetics and oral bioavailability of fumonisin B1. Vet Hum Toxicol. 1999; 41: 357–362. [PubMed] [Google Scholar]
- 27.Kwon OS, Sandberg JA, Slikker W, Jr. Effects of fumonisin B 1 treatment on blood-brain barrier transfer in developing rats. Neurotoxicology and Teratology. 1997; 19: 151–155. 10.1016/S0892-0362(96)00217-6 [DOI] [PubMed] [Google Scholar]
- 28.Shephard GS, Thiel PG, Sydenham EW. Initial studies on the toxicokinetics of fumonisin B1 in rats. Food and Chemical Toxicology. 1992; 30: 277–279. 10.1016/0278-6915(92)90004-5 [DOI] [PubMed] [Google Scholar]
- 29.Alberts JF, Gelderblom WC, Vleggaar R, Marasas WF, Rheeder JP. Production of [14C]fumonisin B1 by Fusarium moniliforme MRC 826 in corn cultures. Appl Environ Microbiol. 1993; 59: 2673–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shephard GS, Thiel PG, Sydenham EW, Alberts JF. Biliary excretion of the mycotoxin fumonisin B1 in rats. Food and Chemical Toxicology. 1994; 32: 489–491. 10.1016/0278-6915(94)90047-7 [DOI] [PubMed] [Google Scholar]
- 31.Norred WP, Plattner RD, Chamberlain WJ. Distribution and excretion of [14C] fumonisin B1 in male sprague-dawley rats. Natural Toxins. 1993; 1: 341–346. 10.1002/nt.2620010604 [DOI] [PubMed] [Google Scholar]
- 32.Hopmans EC, Hauck CC, Hendrich S, Murphy PA. Excretion of fumonisin B1, hydrolyzed fumonisin B1, and the fumonisin B1-fructose adduct in rats. Journal of Agricultural and Food Chemistry. 1997; 45: 2618–2625. 10.1021/jf960886j [DOI] [Google Scholar]
- 33.Prelusky DB, Trenholm HL, Savard ME. Pharmacokinetic fate of14C-Labelled fumonisin B1 in Swine. Natural Toxins. 1994; 2: 73–80. 10.1002/nt.2620020205 [DOI] [PubMed] [Google Scholar]
- 34.Voss KA, Smith GW, Haschek WM. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Animal Feed Science and Technology. 2007; 137: 299–325. 10.1016/j.anifeedsci.2007.06.007 [DOI] [Google Scholar]
- 35.Dilkin P, Direito G, Simas MMS, Mallmann CA, Corrêa B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticillioides culture material in weaned piglets. Chemico-Biological Interactions. 2010; 185: 157–162. 10.1016/j.cbi.2010.03.025 [DOI] [PubMed] [Google Scholar]
- 36.Fodor J, Balogh K, Weber M, et al. Absorption, distribution and elimination of fumonisin B 1 metabolites in weaned piglets. Food Additives & Contaminants: Part A. 2008; 25: 88–96. 10.1080/02652030701546180 [DOI] [PubMed] [Google Scholar]
- 37.Meyer K, Mohr K, Bauer J, Horn P, Kovács M. Residue formation of fumonisin B1 in porcine tissues. Food Additives and Contaminants. 2003; 20: 639–647. 10.1080/0265203031000119043 [DOI] [PubMed] [Google Scholar]
- 38.Shephard GS, Thiel PG, Sydenham EW, Alberts JF, Cawood ME. Distribution and excretion of a single dose of the mycotoxin fumonisin B1 in a non-human primate. Toxicon. 1994; 32: 735–741. 10.1016/0041-0101(94)90342-5 [DOI] [PubMed] [Google Scholar]
- 39.Riley RT, Torres O, Showker JL, et al. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Molecular Nutrition & Food Research. 2012; 56: 1445–1455. 10.1002/mnfr.201200166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cawood ME, Gelderblom WCA, Alberts JF, Snyman SD. Interaction of 14C-labelled fumonisin B mycotoxins with primary rat hepatocyte cultures. Food and Chemical Toxicology. 1994; 32: 627–632. 10.1016/0278-6915(94)90006-X [DOI] [PubMed] [Google Scholar]
- 41.Sharma RP, Bhandari N, Tsunoda M, Riley RT, Voss KA, Meredith FI. Fumonisin toxicity in a transgenic mouse model lacking the mdr1a/1b P-glycoprotein genes. Environmental Toxicology and Pharmacology. 2000; 8: 173–182. 10.1016/S1382-6689(00)00038-7 [DOI] [PubMed] [Google Scholar]
- 42.De Angelis I, Friggè G, Raimondi F, Stammati A, Zucco F, Caloni F. Absorption of Fumonisin B1 and aminopentol on an in vitro model of intestinal epithelium; the role of P-glycoprotein. Toxicon. 2005; 45: 285–291. 10.1016/j.toxicon.2004.10.015 [DOI] [PubMed] [Google Scholar]
- 43.Voss KA, Bacon CW, Norred WP, et al. Studies on the reproductive effects of fusarium moniliforme culture material in rats and the biodistribution of [14c]fumonisin B1 in pregnant rats. Natural Toxins. 1996; 4: 24–33. 10.1002/19960401NT4 [DOI] [PubMed] [Google Scholar]
- 44.Gelineau-van Waes J, Starr L, Maddox J, et al. Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an in vivo mouse model. Birth Defects Research Part A: Clinical and Molecular Teratology. 2005; 73: 487–497. 10.1002/bdra.20148 [DOI] [PubMed] [Google Scholar]
- 45.Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Pflügers Archiv - European Journal of Physiology. 2000; 440: 337–350. 10.1007/s004240000297 [DOI] [PubMed] [Google Scholar]
- 46.Tachampa K, Takeda M, Khamdang S, et al. Interactions of organic anion transporters and organic cation transporters with mycotoxins. Journal of Pharmacological Sciences. 2008; 106: 435–443. 10.1254/jphs.FP0070911 [DOI] [PubMed] [Google Scholar]
- 47.Agarwal S, Uchida Y, Mittapalli RK, Sane R, Terasaki T, Elmquist WF. Quantitative proteomics of transporter expression in brain capillary endothelial cells isolated from P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and P-gp/Bcrp knockout mice. Drug Metabolism and Disposition. 2012; 40: 1164–1169. 10.1124/dmd.112.044719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochemical Pharmacology. 2005; 70: 695–699. 10.1016/j.bcp.2005.05.031 [DOI] [PubMed] [Google Scholar]
- 49.Voss K, Chamberlain WJ, Bacon CW, Herbert RA, Walters DB, Norred WP. Subchronic feeding study of the mycotoxin fumonisin B1 in B6C3F1 mice and Fischer 344 rats. Fundamental and Applied Toxicology. 1995; 24: 102–110. 10.1006/faat.1995.1012 [DOI] [PubMed] [Google Scholar]
- 50.NTP. NTP technical report on the toxicology and carcinogenesis studies of fumonisin B1 (cas no. 116355–83-0) in F344/N rats and B6C3F1 mice (feed studies). Department of health and human services Public health service National institutes of health; 2001:1–355. [PubMed]
- 51.Bondy GS, Suzuki CAM, Fernie SM, et al. Toxicity of fumonisin B1 to B6C3F1 mice: A 14-day gavage study. Food and Chemical Toxicology. 1997; 35: 981–989. 10.1016/S0278-6915(97)87267-5 [DOI] [PubMed] [Google Scholar]
- 52.Tolleson WH, Dooley KL, Sheldon WG, Thurman JD, Bucci TJ, Howard PC. The mycotoxin fumonisin induces apoptosis in cultured human cells and in livers and kidneys of rats. Advances in Experimental medicine and Biology. 1996; 392: 237–250. 10.1007/978-1-4899-1379-1_21 [DOI] [PubMed] [Google Scholar]
- 53.Voss KA, Chamberlain WJ, Bacon CW, Norred WP. A preliminary investigation on renal and hepatic toxicity in rats fed purified fumonisin B1. Natural Toxins. 1993; 1: 222–228. 10.1002/nt.2620010404 [DOI] [PubMed] [Google Scholar]
- 54.Voss KA, Plattner RD, Riley RT, Meredith FI, Norred WP. In vivo effects of fumonisin B1-producing and fumonisin B1-nonproducing Fusarium moniliforme isolates are similar: fumonisins B2 and B3 cause hepato- and nephrotoxicity in rats. Mycopathologia. 1998; 141: 45–57. 10.1023/A:1006810916344 [DOI] [PubMed] [Google Scholar]
- 55.Riley RT, Voss KA. Differential sensitivity of rat kidney and liver to fumonisin toxicity: organ-specific differences in toxin accumulation and sphingoid base metabolism. Toxicological Sciences. 2006; 92: 335–345. 10.1093/toxsci/kfj198 [DOI] [PubMed] [Google Scholar]
- 56.Bondy GS, Suzuki CA, Mueller RW, et al. G. S. Bondy C. A. M. Suzuki R. W. M Gavage administration of the fungal toxin fumonisin B1 to female Sprague-Dawley rats. Journal of Toxicology and Environmental Health, Part A. 1998; 53: 135–151. 10.1080/009841098159411 [DOI] [PubMed] [Google Scholar]
- 57.Bondy G, Barker M, Mueller R, et al. Fumonisin B1 toxicity in male Sprague-Dawley rats. Advances in Experimental medicine and Biology. 1996; 392: 251–264. 10.1007/978-1-4899-1379-1_22 [DOI] [PubMed] [Google Scholar]
- 58.Bondy G, Suzuki C, Barker M, et al. Toxicity of fumonisin B1 administered intraperitoneally to male Sprague—Dawley rats. Food and Chemical Toxicology. 1995; 33: 653–665. 10.1016/0278-6915(95)00031-V [DOI] [PubMed] [Google Scholar]
- 59.Gumprecht LA, Marcucci A, Weigel RM, et al. Effects of intravenous fumonisin B1 in rabbits: Nephrotoxicityxs and sphingolipid alterations. Natural Toxins. 1995; 3: 395–403. 10.1002/nt.2620030512 [DOI] [PubMed] [Google Scholar]
- 60.Harrison LR, Colvin BM, Greene JT, Newman LE, Cole JR, Jr. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. Journal of Veterinary Diagnostic Investigation. 1990; 2: 217–221. 10.1177/104063879000200312 [DOI] [PubMed] [Google Scholar]
- 61.Colvin BM, Harrison LR. Fumonisin-induced pulmonary edema and hydrothorax in swine. Mycopathologia. 1992; 117: 79–82. 10.1007/BF00497282 [DOI] [PubMed] [Google Scholar]
- 62.Colvin BM, Cooley AJ, Beaver RW. Fumonisin toxicosis in swine: clinical and pathologic findings. Journal of Veterinary Diagnostic Investigation. 1993; 5: 232–241. 10.1177/104063879300500215 [DOI] [PubMed] [Google Scholar]
- 63.Gumprecht LA, Beasley VR, Weigel RM, et al. Development of fumonisin-induced hepatotoxicity and pulmonary edema in orally dosed swine: morphological and biochemical alterations. Toxicologic Pathology. 1998; 26: 777–788. 10.1177/019262339802600610 [DOI] [PubMed] [Google Scholar]
- 64.Haschek WM, Motelin G, Ness DK, et al. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia. 1992; 117: 83–96. 10.1007/BF00497283 [DOI] [PubMed] [Google Scholar]
- 65.Haschek WM, Gumprecht LA, Smith G, Tumbleson ME, Constable PD. Fumonisin toxicosis in swine: an overview of porcine pulmonary edema and current perspectives. Environmental Health Perspectives. 2001; 109(Suppl 2): 251–257. 10.1289/ehp.01109s2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kellerman TS, Marasas WF, Pienaar JG, Naudé TW. A mycotoxicosis of equidae caused by Fusarium moniliforme sheldon. A preliminary communication. Onderstepoort J Vet Res. 1972; 39: 205–208. [PubMed] [Google Scholar]
- 67.Marasas WF, Kellerman TS, Pienaar JG, Naudé TW. Leukoencephalomalacia: a mycotoxicosis of Equidae caused by Fusarium moniliforme Sheldon. Onderstepoort J Vet Res. 1976; 43: 113–122. [PubMed] [Google Scholar]
- 68.Marasas WF, Kellerman TS, Gelderblom WC, Coetzer JA, Thiel PG, van der Lugt JJ. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res. 1988; 55: 197–203. [PubMed] [Google Scholar]
- 69.Foreman JH, Constable PD, Waggoner AL, et al. Neurologic abnormalities and cerebrospinal fluid changes in horses administered fumonisin B1 intravenously. Journal of Veterinary Internal Medicine. 2004; 18: 223–230. 10.1111/j.1939-1676.2004.tb00165.x [DOI] [PubMed] [Google Scholar]
- 70.Gross SM, Reddy RV, Rottinghaus GE, Johnson G, Reddy CS. Developmental effects of fumonisin B1-containingFusarium moniliforme culture extract in CD1 mice. Mycopathologia. 1994; 128: 111–118. 10.1007/BF01103018 [DOI] [PubMed] [Google Scholar]
- 71.Floss JL, Casteel SW, Johnson GC, Rottinghaus GE, Krause GF. Developmental toxicity of fumonisin in Syrian hamsters. Mycopathologia. 1994; 128: 33–38. 10.1007/BF01104276 [DOI] [PubMed] [Google Scholar]
- 72.Penner JD, Casteel SW, Pittman L, Jr, Rottinghaus GE, Wyatt RD. Developmental toxicity of purified fumonisin B1 in pregnant Syrian hamsters. Journal of Applied Toxicology. 1998; 18: 197–203. [DOI] [PubMed] [Google Scholar]
- 73.Reddy RV, Johnson G, Rottinghaus GE, Casteel SW, Reddy CS. Developmental effects of fumonisin B1 in mice. Mycopathologia. 1996; 134: 161–166. 10.1007/BF00436724 [DOI] [PubMed] [Google Scholar]
- 74.Collins TFX, Shackelford ME, Sprando RL, et al. Effects of fumonisin B1 in pregnant rats. Food and Chemical Toxicology. 1998; 36: 397–408. 10.1016/S0278-6915(97)00170-1 [DOI] [PubMed] [Google Scholar]
- 75.Collins TFX, Sprando RL, Black TN, et al. Effects of fumonisin B1 in pregnant rats. Part 2. Food and Chemical Toxicology. 1998; 36: 673–685. 10.1016/S0278-6915(98)00036-2 [DOI] [PubMed] [Google Scholar]
- 76.Javed T, Bennett GA, Richard JL, Dombrink-Kurtzman MA, C�t� LM, Buck WB. Mortality in broiler chicks on feed amended withFusarium proliferatum culture material or with purified fumonisin B1 and moniliformin. Mycopathologia. 1993; 123: 171–184. 10.1007/BF01111269 [DOI] [PubMed] [Google Scholar]
- 77.Zacharias C, Van Echten-Deckert G, Wang E, Merrill AH, Jr, Sandhoff K. The effect of fumonisin B1 on developing chick embryos: correlation between de novo sphingolipid biosynthesis and gross morphological changes. Glycoconjugate Journal. 1996; 13: 167–175. 10.1007/BF00731491 [DOI] [PubMed] [Google Scholar]
- 78.LaBorde J, Terry KK, Howard PC, et al. Lack of embryotoxicity of fumonisin B1 in New Zealand white rabbits. Fundamental and Applied Toxicology. 1997; 40: 120–128. 10.1006/faat.1997.2380 [DOI] [PubMed] [Google Scholar]
- 79.Flynn TJ, Stack ME, Troy AL, Chirtel SJ. Assessment of the embryotoxic potential of the total hydrolysis product of fumonisin B1 using cultured organogenesis-staged rat embryos. Food and Chemical Toxicology. 1997; 35: 1135–1141. 10.1016/S0278-6915(97)85466-X [DOI] [PubMed] [Google Scholar]
- 80.Missmer SA, Suarez L, Felkner M, et al. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environmental Health Perspectives. 2006; 114: 237–241. 10.1289/ehp.8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hendricks K. Fumonisins and neural tube defects in South Texas. Epidemiology. 1999; 10: 198–200. [DOI] [PubMed] [Google Scholar]
- 82.Sadler TW, Merrill AH, Stevens VL, Sullards MC, Wang E, Wang P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology. 2002; 66: 169–176. 10.1002/tera.10089 [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. Journal of Lipid Research. 2010; 51: 3230–3242. 10.1194/jlr.M007641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faubion WA, Guicciardi ME, Miyoshi H, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. Journal of Clinical Investigation. 1999; 103: 137–145. 10.1172/JCI4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones B, Roberts PJ, Faubion WA, Kominami E, Gores GJ. Cystatin A expression reduces bile salt-induced apoptosis in a rat hepatoma cell line. Am J Physiol. 1998; 275: G723–G730. [DOI] [PubMed] [Google Scholar]
- 86.Kato Y, Kuge K, Kusuhara H, Meier PJ, Sugiyama Y. Gender difference in the urinary excretion of organic anions in rats. Journal of Pharmacology and Experimental Therapeutics. 2002; 302: 483–489. 10.1124/jpet.102.033878 [DOI] [PubMed] [Google Scholar]
- 87.Lu H, Klaassen C. Gender differences in mRNA expression of ATP-binding cassette efflux and bile acid transporters in kidney, liver, and intestine of 5/6 nephrectomized rats. Drug Metabolism and Disposition. 2007; 36: 16–23. 10.1124/dmd.107.014845 [DOI] [PubMed] [Google Scholar]
- 88.Zelcer N, Saeki T, Bot I, Kuil A, Borst P. Transport of bile acids in multidrug-resistance-protein 3-overexpressing cells co-transfected with the ileal Na+-dependent bile-acid transporter. Biochemical Journal. 2003; 369: 23–30. 10.1042/bj20021081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rost D, Kopplow K, Gehrke S, et al. Gender-specific expression of liver organic anion transporters in rat. European Journal of Clinical Investigation. 2005; 35: 635–643. 10.1111/j.1365-2362.2005.01556.x [DOI] [PubMed] [Google Scholar]
- 90.Yamazoe Y, Gong D, Murayama N, Abu-Zeid M, Kato R. Regulation of hepatic cortisol sulfotransferase in rats by pituitary growth hormone. Mol Pharmacol. 1989; 35: 707–712. [PubMed] [Google Scholar]
- 91.Ogura K, Kajita J, Narihata H, et al. Cloning and sequence analysis of a rat liver cDNA encoding hydroxysteroid sulfotransferase. Biochemical and Biophysical Research Communications. 1989; 165: 168–174. 10.1016/0006-291X(89)91050-4 [DOI] [PubMed] [Google Scholar]
- 92.Schoemaker MH, Gommans WM, de la Rosa LC, et al. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. Journal of Hepatology. 2003; 39: 153–161. 10.1016/S0168-8278(03)00214-9 [DOI] [PubMed] [Google Scholar]
- 93.Fickert P, Trauner M, Fuchsbichler A, et al. Oncosis represents the main type of cell death in mouse models of cholestasis. Journal of Hepatology. 2005; 42: 378–385. 10.1016/j.jhep.2004.10.016 [DOI] [PubMed] [Google Scholar]
- 94.Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004; 40: 998–1007. 10.1002/hep.1840400431 [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver International. 2012; 32: 58–69. 10.1111/j.1478-3231.2011.02662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004; 286: G499–G507. 10.1152/ajpgi.00318.2003 [DOI] [PubMed] [Google Scholar]
- 97.Gujral J, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003; 38: 355–363. 10.1053/jhep.2003.50341 [DOI] [PubMed] [Google Scholar]
- 98.Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012; 56: 118–129. 10.1002/hep.25630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang N, Yu S, Adams SH, Ronis MJJ, Badger TM. Profiling of urinary bile acids in piglets by a combination of enzymatic deconjugation and targeted LC-MRM-MS. Journal of Lipid Research. 2016; 57: 1917–1933. 10.1194/jlr.D069831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergström S, Danielsson H, Göransson Å, Sömme R, Stenhagen E, Palmstierna H. On the bile acid metabolism in the pig Bile acids and steroids 81. Acta Chemica Scandinavica. 1959; 13: 776–783. 10.3891/acta.chem.scand.13-0776 [DOI] [Google Scholar]
- 101.Gelineau-van Waes J, Voss KA, Stevens VL, Speer MC, Riley RT. Maternal fumonisin exposure as a risk factor for neural tube defects. Advances in Food and Nutrition Research. 2009; 56: 145–181. 10.1016/S1043-4526(08)00605-0 [DOI] [PubMed] [Google Scholar]
- 102.Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH, Jr. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991; 266: 14486–14490. [PubMed] [Google Scholar]
- 103.Schmidt K, Schrader M, Kern HF, Kleene R. Regulated apical secretion of zymogens in rat pancreas. Involvement of the glycosylphosphatidylinositol-anchored glycoprotein GP-2, the lectin ZG16p, and cholesterol-glycosphingolipid-enriched microdomains. Journal of Biological Chemistry. 2001; 276: 14315–14323. 10.1074/jbc.M006221200 [DOI] [PubMed] [Google Scholar]
- 104.Enongene EN, Sharma RP, Bhandari N, Voss KA, Riley RT. Disruption of sphingolipid metabolism in small intestines, liver and kidney of mice dosed subcutaneously with fumonisin B1. Food and Chemical Toxicology. 2000; 38: 793–799. 10.1016/S0278-6915(00)00065-X [DOI] [PubMed] [Google Scholar]
- 105.Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochemical and Biophysical Research Communications. 1995; 211: 396–403. 10.1006/bbrc.1995.1827 [DOI] [PubMed] [Google Scholar]
- 106.Clapham DE. Calcium Signaling. Cell. 2007; 131: 1047–1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 107.Jefferson AB, Schulman H. Sphingosine inhibits calmodulin-dependent enzymes. J Biol Chem. 1988; 263: 15241–15244. [PubMed] [Google Scholar]
- 108.Zheng W, Kollmeyer J, Symolon H, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2006; 1758: 1864–1884. 10.1016/j.bbamem.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 109.Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010; 62: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochemical Journal. 2012; 441: 789–802. 10.1042/BJ20111626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laviad EL, Albee L, Pankova-Kholmyansky I, et al. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. Journal of Biological Chemistry. 2008; 283: 5677–5684. 10.1074/jbc.M707386200 [DOI] [PubMed] [Google Scholar]
- 112.Cingolani F, Futerman AH, Casas J. Ceramide synthases in biomedical research. Chemistry and Physics of Lipids. 2016; 197: 25–32. 10.1016/j.chemphyslip.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 113.Riebeling C, Allegood JC, Wang E, Merrill AH, Jr, Futerman AH. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. Journal of Biological Chemistry. 2003; 278: 43452–43459. 10.1074/jbc.M307104200 [DOI] [PubMed] [Google Scholar]
- 114.Imgrund S, Hartmann D, Farwanah H, et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. Journal of Biological Chemistry. 2009; 284: 33549–33560. 10.1074/jbc.M109.031971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pewzner-Jung Y, Park H, Laviad EL, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. Journal of Biological Chemistry. 2010; 285: 10902–10910. 10.1074/jbc.M109.077594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Basnakian AG, Ueda N, Hong X, Galitovsky VE, Yin X, Shah SV. Ceramide synthase is essential for endonuclease-mediated death of renal tubular epithelial cells induced by hypoxia-reoxygenation. American Journal of Physiology-Renal Physiology. 2005; 288: F308–F314. 10.1152/ajprenal.00204.2004 [DOI] [PubMed] [Google Scholar]
- 117.Boslem E, Meikle PJ, Biden TJ. Roles of ceramide and sphingolipids in pancreatic β-cell function and dysfunction. Islets. 2012; 4: 177–187. 10.4161/isl.20102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gopee NV, Sharma RP. The mycotoxin fumonisin B 1 transiently activates nuclear factor-κB, tumor necrosis factor α and caspase 3 via protein kinase Cα-dependent pathway in porcine renal epithelial cells. Cell Biology and Toxicology. 2004; 20: 197–212. 10.1023/B:CBTO.0000038458.39516.40 [DOI] [PubMed] [Google Scholar]
- 119.Seefelder W, Humpf HU, Schwerdt G, Freudinger R, Gekle M. Induction of apoptosis in cultured human proximal tubule cells by fumonisins and fumonisin metabolites. Toxicology and Applied Pharmacology. 2003; 192: 146–153. 10.1016/S0041-008X(03)00262-X [DOI] [PubMed] [Google Scholar]
- 120.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996; 37: 606–630. [PubMed] [Google Scholar]
- 121.Bourgès M, Small DM, Dervichian DG. Biophysics of lipid associations. 3. The quaternary systems lecithin-bile salt-cholesterol-water. Biochim Biophys Acta. 1967; 144: 189–201. [PubMed] [Google Scholar]
- 122.Javitt NB, Emerman S. Effect of sodium taurolithocholate on bile flow and bile acid excretion. Journal of Clinical Investigation. 1968; 47: 1002–1014. 10.1172/JCI105790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson DR, Habeebu SS, Klaassen CD. Increase in bile flow and biliary excretion of glutathione-derived sulfhydryls in rats by drug-metabolizing enzyme inducers is mediated by multidrug resistance protein 2. Toxicological Sciences. 2002; 66: 16–26. 10.1093/toxsci/66.1.16 [DOI] [PubMed] [Google Scholar]
- 124.Shi Y, Burn P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nature Reviews Drug Discovery. 2004; 3: 695–710. 10.1038/nrd1469 [DOI] [PubMed] [Google Scholar]
- 125.Cornell RB, Ridgway ND. CTP:phosphocholine cytidylyltransferase: Function, regulation, and structure of an amphitropic enzyme required for membrane biogenesis. Progress in Lipid Research. 2015; 59: 147–171. 10.1016/j.plipres.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 126.Chen BB, Mallampalli RK. Calmodulin binds and stabilizes the regulatory enzyme, CTP: phosphocholine cytidylyltransferase. Journal of Biological Chemistry. 2007; 282: 33494–33506. 10.1074/jbc.M706472200 [DOI] [PubMed] [Google Scholar]
- 127.Sohal PS, Cornell RB. Sphingosine inhibits the activity of rat liver CTP:phosphocholine cytidylyltransferase. J Biol Chem. 1990; 265: 11746–11750. [PubMed] [Google Scholar]
- 128.Ryan AJ, Fisher K, Thomas CP, Mallampalli RK. Transcriptional repression of the CTP:phosphocholine cytidylyltransferase gene by sphingosine. Biochemical Journal. 2004; 382: 741–750. 10.1042/BJ20040105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacobs RL, Devlin C, Tabas I, Vance DE. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase alpha in mice decreases plasma high density and very low density lipoproteins. Journal of Biological Chemistry. 2004; 279: 47402–47410. 10.1074/jbc.M404027200 [DOI] [PubMed] [Google Scholar]
- 130.Verkade HJ, Havinga R, Shields DJ, et al. The phosphatidylethanolamine N -methyltransferase pathway is quantitatively not essential for biliary phosphatidylcholine secretion. Journal of Lipid Research. 2007; 48: 2058–2064. 10.1194/jlr.M700278-JLR200 [DOI] [PubMed] [Google Scholar]
- 131.Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. Journal of Biological Chemistry. 1998; 273: 27043–27046. 10.1074/jbc.273.42.27043 [DOI] [PubMed] [Google Scholar]
- 132.Li Z, Agellon LB, Vance DE. Phosphatidylcholine homeostasis and liver failure. Journal of Biological Chemistry. 2005; 280: 37798–37802. 10.1074/jbc.M508575200 [DOI] [PubMed] [Google Scholar]
- 133.Badiani K, Byers DM, Cook HW, Ridgway ND. Effect of fumonisin B1 on phosphatidylethanolamine biosynthesis in Chinese hamster ovary cells. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1996; 1304: 190–196. 10.1016/S0005-2760(96)00119-1 [DOI] [PubMed] [Google Scholar]
- 134.Zhi J, Mulligan TE, Hauptman JB. Long-term systemic exposure of orlistat, a lipase inhibitor, and its metabolites in obese patients. The Journal of Clinical Pharmacology. 1999; 39: 41–46. 10.1177/00912709922007543 [DOI] [PubMed] [Google Scholar]
- 135.Lucas CP, Boldrin MN, Reaven GM. Effect of orlistat added to diet (30% of calories from fat) on plasma lipids, glucose, and insulin in obese patients with hypercholesterolemia. The American Journal of Cardiology. 2003; 91: 961–964. 10.1016/S0002-9149(03)00112-7 [DOI] [PubMed] [Google Scholar]
- 136.Gelderblom WCA, Smuts CM, Abel S, et al. Effect of fumonisin B1 on the levels and fatty acid composition of selected lipids in rat liver in vivo. Food and Chemical Toxicology. 1997; 35: 647–656. 10.1016/S0278-6915(97)00036-7 [DOI] [PubMed] [Google Scholar]
- 137.Bollag W, Gallico E. The effect of dl-ethionine on the content of some enzymes in pancreas and liver. Biochimica et Biophysica Acta. 1952; 9: 193–198. 10.1016/0006-3002(52)90146-7 [DOI] [PubMed] [Google Scholar]
- 138.Farber E, Corban MS. Sex difference in ethionine inhibition of hepatic protein synthesis. J Biol Chem. 1958; 233: 625–630. [PubMed] [Google Scholar]
- 139.Koike H, Steer ML, Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol. 1982; 242: G297–G307. [DOI] [PubMed] [Google Scholar]
- 140.Lombardi B, Rao NK. Acute hemorrhagic pancreatic necrosis in mice. Influence of the age and sex of the animals and of dietary ethionine, choline, methionine, and adenine sulfate. Am J Pathol. 1975; 81: 87–100. [PMC free article] [PubMed] [Google Scholar]
- 141.Gilliland L, Steer ML. Effects of ethionine on digestive enzyme synthesis and discharge by mouse pancreas. Am J Physiol. 1980; 239: G418–G426. [DOI] [PubMed] [Google Scholar]
- 142.Tessitore L, Sesca E, Greco M, Pani P, Dianzani MU. Sexually differentiated response to choline in choline deficiency and ethionine intoxication. Int J Exp Pathol. 1995; 76: 125–129. [PMC free article] [PubMed] [Google Scholar]
- 143.Fullerton FR, Greenman DL, Blaydes BS, Poirier LA. Ethynylestradiol protection against methyl insufficiency in castrated male Wistar/Furth rats fed a methionine-choline-deficient diet. Carcinogenesis. 1993; 14: 1237–1240. 10.1093/carcin/14.6.1237 [DOI] [PubMed] [Google Scholar]
- 144.Scheele G, Jacoby R. Proteolytic processing of presecretory proteins is required for development of biological activities in pancreatic exocrine proteins. J Biol Chem. 1983; 258: 2005–2009. [PubMed] [Google Scholar]
- 145.Rinderknecht H. Activation of pancreatic zymogens. Digestive Diseases and Sciences. 1986; 31: 314–321. 10.1007/BF01318124 [DOI] [PubMed] [Google Scholar]
- 146.Simpson MV, Farber E, Tarver H. Studies on ethionine 1. inhibition of protein synthesis in intact animals. J Biol Chem. 1950; 182: 81–90. [Google Scholar]
- 147.Mato JM, Martínez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annual Review of Nutrition. 2008; 28: 273–293. 10.1146/annurev.nutr.28.061807.155438 [DOI] [PubMed] [Google Scholar]
- 148.Antonissen G, Van Immerseel F, Pasmans F, et al. The mycotoxins deoxynivalenol and fumonisins alter the extrinsic component of intestinal barrier in broiler chickens. Journal of Agricultural and Food Chemistry. 2015; 63: 10846–10855. 10.1021/acs.jafc.5b04119 [DOI] [PubMed] [Google Scholar]
- 149.Longnecker DS. Abnormal methyl metabolism in pancreatic toxicity and diabetes. The Journal of Nutrition. 2002; 132(Suppl): 2373S–2376S. 10.1093/jn/132.8.2373S [DOI] [PubMed] [Google Scholar]
- 150.Richmond BL, Boileau AC, Zheng S, et al. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A2–Deficient mice. Gastroenterology. 2001; 120: 1193–1202. 10.1053/gast.2001.23254 [DOI] [PubMed] [Google Scholar]
- 151.Li JP, Chang TM, Wagner D, Chey WY. Pancreatic phospholipase A 2 from the small intestine is a secretin-releasing factor in rats. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001; 281: G526–G532. 10.1152/ajpgi.2001.281.2.G526 [DOI] [PubMed] [Google Scholar]
- 152.Chang T, Chang CH, Wagner DR, Chey WY. Porcine pancreatic phospholipase A2 stimulates secretin release from secretin-producing cells. Journal of Biological Chemistry. 1999; 274: 10758–10764. 10.1074/jbc.274.16.10758 [DOI] [PubMed] [Google Scholar]
- 153.Yagami T, Yamamoto Y, Koma H. The role of secretory phospholipase A2 in the central nervous system and neurological diseases. Molecular Neurobiology. 2014; 49: 863–876. 10.1007/s12035-013-8565-9 [DOI] [PubMed] [Google Scholar]
- 154.Pan Y, Wan J, Liu Y, et al. sPLA2 IB induces human podocyte apoptosis via the M-type phospholipase A2 receptor. Scientific Reports. 2015; 4: 6660. 10.1038/srep06660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Murakami M, Taketomi Y, Miki Y, Sato H, Yamamoto K, Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: the 3rd edition. Biochimie. 2014; 107 Pt A: 105–113. [DOI] [PubMed] [Google Scholar]
- 156.Koo SI, Henderson DA, Algilani K, Norvell JE. Effect of marginal zinc deficiency on the morphological characteristics of intestinal nascent chylomicrons and distribution of soluble apoproteins of lymph chylomicrons. The American Journal of Clinical Nutrition. 1985; 42: 671–680. 10.1093/ajcn/42.4.671 [DOI] [PubMed] [Google Scholar]
- 157.Nassir F, Blanchard RK, Mazur A, Cousins RJ, Davidson NO. Apolipoprotein B mRNA editing is preserved in the intestine and liver of zinc-deficient rats. The Journal of Nutrition. 1996; 126: 860–864. 10.1093/jn/126.4.860 [DOI] [PubMed] [Google Scholar]
- 158.Schwartz R, Topley M, Russell JB. Effect of tricarballylic acid, a nonmetabolizable rumen fermentation product of trans-aconitic acid, on Mg, Ca and Zn utilization of rats. The Journal of Nutrition. 1988; 118: 183–188. 10.1093/jn/118.2.183 [DOI] [PubMed] [Google Scholar]
- 159.Koo SI, Turk DE. Effect of zinc deficiency on intestinal transport triglyceride in the rat. The Journal of Nutrition. 1977; 107: 909–919. 10.1093/jn/107.5.909 [DOI] [PubMed] [Google Scholar]
- 160.Fujita M, Jigami Y. Lipid remodeling of GPI-anchored proteins and its function. Biochimica et Biophysica Acta (BBA) - General Subjects. 2008; 1780: 410–420. 10.1016/j.bbagen.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 161.Yu S, Michie SA, Lowe AW. Absence of the major zymogen granule membrane protein, GP2, does not affect pancreatic morphology or secretion. Journal of Biological Chemistry. 2004; 279: 50274–50279. 10.1074/jbc.M410599200 [DOI] [PubMed] [Google Scholar]
- 162.Stevens VL, Tang J. Fumonisin B1-induced sphingolipid depletion inhibits vitamin uptake via the glycosylphosphatidylinositol-anchored folate receptor. Journal of Biological Chemistry. 1997; 272: 18020–18025. 10.1074/jbc.272.29.18020 [DOI] [PubMed] [Google Scholar]
- 163.Chatterjee S, Smith ER, Hanada K, Stevens VL, Mayor S. GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment. The EMBO Journal. 2001; 20: 1583–1592. 10.1093/emboj/20.7.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Voss KA, Riley RT, Waes JG. Trends in fumonisin research: Recent studies on the developmental effects of fumonisins and Fusarium verticillioides. Mycotoxins. 2005; 55: 91–100. 10.2520/myco.55.91 [DOI] [Google Scholar]
- 165.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. Journal of Inherited Metabolic Disease. 2011; 34: 75–81. 10.1007/s10545-010-9177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yamazoe Y, Yamada T, Mitsumori K. Embryo- and testicular-toxicities of methoxyacetate and the related: a review on possible roles of one-carbon transfer and histone modification. Food Safety. 2015; 3: 92–107. 10.14252/foodsafetyfscj.2015013 [DOI] [Google Scholar]
- 167.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature Reviews Cancer. 2013; 13: 572–583. 10.1038/nrc3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Voss KA, Riley RT, Gelineau-van Waes J. Fumonisin B 1 induced neural tube defects were not increased in LM/Bc mice fed folate-deficient diet. Molecular Nutrition & Food Research. 2014; 58: 1190–1198. 10.1002/mnfr.201300720 [DOI] [PubMed] [Google Scholar]
- 169.Allen RH, Seetharam B, Podell E, Alpers DH. Effect of proteolytic enzymes on the binding of cobalamin to R protein and intrinsic factor. In vitro evidence that a failure to partially degrade R protein is responsible for cobalamin malabsorption in pancreatic insufficiency. J Clin Invest. 1978; 61: 47–54. PMID: 22556 doi: 10.1172/JCI108924. [DOI] [PMC free article] [PubMed]
- 170.Marcoullis G, Parmentier Y, Nicolas JP, Jimenez M, Gerard P. Cobalamin malabsorption due to nondegradation of R proteins in the human intestine. Inhibited cobalamin absorption in exocrine pancreatic dysfunction. Journal of Clinical Investigation. 1980; 66: 430–440. 10.1172/JCI109873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guéant JL, Champigneulle B, Gaucher P, Nicolas JP. Malabsorption of vitamin B12 in pancreatic insufficiency of the adult and of the child. Pancreas. 1990; 5: 559–567. 10.1097/00006676-199009000-00011 [DOI] [PubMed] [Google Scholar]
- 172.Surtees R, Leonard J, Austin S. Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. The Lancet. 1991; 338: 1550–1554. 10.1016/0140-6736(91)92373-A [DOI] [PubMed] [Google Scholar]
- 173.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews. 2001; 81: 871–927. 10.1152/physrev.2001.81.2.871 [DOI] [PubMed] [Google Scholar]
- 174.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nature Reviews Neuroscience. 2005; 6: 683–690. 10.1038/nrn1743 [DOI] [PubMed] [Google Scholar]
- 175.Mackenzie PI, Walter Bock K, Burchell B, et al. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenetics and Genomics. 2005; 15: 677–685. 10.1097/01.fpc.0000173483.13689.56 [DOI] [PubMed] [Google Scholar]
- 176.Becker I, Wang-Eckhardt L, Yaghootfam A, Gieselmann V, Eckhardt M. Differential expression of (dihydro)ceramide synthases in mouse brain: oligodendrocyte-specific expression of CerS2/Lass2. Histochemistry and Cell Biology. 2008; 129: 233–241. 10.1007/s00418-007-0344-0 [DOI] [PubMed] [Google Scholar]
- 177.Carmody DP, Dunn SM, Boddie-Willis AS, DeMarco JK, Lewis M. A quantitative measure of myelination development in infants, using MR images. Neuroradiology. 2004; 46: 781–786. 10.1007/s00234-004-1241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Caballero F, Fernández A, Matías N, et al. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. Journal of Biological Chemistry. 2010; 285: 18528–18536. 10.1074/jbc.M109.099333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nelson GJ. The lipid composition of normal mouse liver. J Lipid Res. 1962; 3: 256–262. [Google Scholar]
- 180.He Q, Suzuki H, Sharma RP. S-adenosylmethionine or 5′-methylthioadenosine are unable to prevent fumonisin B1 hepatotoxicity in mice despite increased oxidation in liver. Journal of Applied Toxicology. 2006; 26: 509–516. 10.1002/jat.1170 [DOI] [PubMed] [Google Scholar]
- 181.Fitzhugh OG, Nelson AA. Liver tumors in rats fed thiourea or thioacetamide. Science. 1948; 108: 626–628. 10.1126/science.108.2814.626 [DOI] [PubMed] [Google Scholar]
- 182.Swapna I, Kumar KVSS, Reddy PVB, Murthy CRK, Reddanna P, Senthilkumaran B. Phospholipid and cholesterol alterations accompany structural disarray in myelin membrane of rats with hepatic encephalopathy induced by thioacetamide. Neurochemistry International. 2006; 49: 238–244. 10.1016/j.neuint.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 183.Osada J, Aylagas H, Sanchez-Vegazo I, Gea T, Millan I, Palacios-Alaiz E. Effect of s-adenosyl-l-methionine on thioacetamide-induced liver damage in rats. Toxicology Letters. 1986; 32: 97–106. 10.1016/0378-4274(86)90054-8 [DOI] [PubMed] [Google Scholar]
- 184.Huang ZZ, Mato JM, Kanel G, Lu SC. Differential effect of thioacetamide on hepatic methionine adenosyltransferase expression in the rat. Hepatology. 1999; 29: 1471–1478. 10.1002/hep.510290525 [DOI] [PubMed] [Google Scholar]
- 185.Sutter BM, Wu X, Laxman S, Tu BP. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell. 2013; 154: 403–415. 10.1016/j.cell.2013.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012; 303: G993–G1003. 10.1152/ajpgi.00122.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perera RM, Stoykova S, Nicolay BN, et al. Transcriptional control of autophagy–lysosome function drives pancreatic cancer metabolism. Nature. 2015; 524: 361–365. 10.1038/nature14587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Spassieva SD, Mullen TD, Townsend DM, Obeid LM. Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochemical Journal. 2009; 424: 273–283. 10.1042/BJ20090699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schworer CM, Shiffer KA, Mortimore GE. Quantitative relationship between autophagy and proteolysis during graded amino acid deprivation in perfused rat liver. J Biol Chem. 1981; 256: 7652–7658. [PubMed] [Google Scholar]
- 190.Huitema K, van den Dikkenberg J, Brouwers JFHM, Holthuis JCM. Identification of a family of animal sphingomyelin synthases. The EMBO Journal. 2004; 23: 33–44. 10.1038/sj.emboj.7600034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.van Helvoort A, van’t Hof W, Ritsema T, Sandra A, van Meer G. Conversion of diacylglycerol to phosphatidylcholine on the basolateral surface of epithelial (Madin-Darby canine kidney) cells. Evidence for the reverse action of a sphingomyelin synthase. J Biol Chem. 1994; 269: 1763–1769. [PubMed] [Google Scholar]
- 192.Ternes P, Brouwers JFHM, van den Dikkenberg J, Holthuis JCM. Sphingomyelin synthase SMS2 displays dual activity as ceramide phosphoethanolamine synthase. Journal of Lipid Research. 2009; 50: 2270–2277. 10.1194/jlr.M900230-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tafesse FG, Ternes P, Holthuis JCM. The multigenic sphingomyelin synthase family. Journal of Biological Chemistry. 2006; 281: 29421–29425. 10.1074/jbc.R600021200 [DOI] [PubMed] [Google Scholar]
- 194.Xu Z, Zhou J, McCoy DM, Mallampalli RK. LASS5 is the predominant ceramide synthase isoform involved in de novo sphingolipid synthesis in lung epithelia. Journal of Lipid Research. 2005; 46: 1229–1238. 10.1194/jlr.M500001-JLR200 [DOI] [PubMed] [Google Scholar]
- 195.van der Veen JN, Lingrell S, Vance DE. The membrane lipid phosphatidylcholine is an unexpected source of triacylglycerol in the liver. Journal of Biological Chemistry. 2012; 287: 23418–23426. 10.1074/jbc.M112.381723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pewzner-Jung Y, Brenner O, Braun S, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. Journal of Biological Chemistry. 2010; 285: 10911–10923. 10.1074/jbc.M109.077610 [DOI] [PMC free article] [PubMed] [Google Scholar]