Abstract
Shiga toxin (verotoxin)-producing Escherichia coli (STEC) is an important cause of foodborne disease. Since outcomes of the infections with STEC have a broad range of manifestation from asymptomatic infection or mild intestinal discomfort, to bloody diarrhea, hemolytic uremic syndrome (HUS), end-stage renal disease (ESRD), and death, the disease is a serious burden in public health and classified as a notifiable infectious disease in many countries. Cattle and other ruminants are considered to be the major reservoirs of STEC though isolation of STEC from other animals have been reported. Hence, the source of contamination extends to a wide range of foods, not only beef products but also fresh produce, water, and environment contaminated by excretes from the animals, mainly cattle. A low- infectious dose of STEC makes the disease relatively contagious, and causes outbreaks with unknown contamination sources and, therefore, as a preventive measure against STEC infection, it is important to obtain characteristics of prevailing STEC isolates in the region through robust surveillance. Analysis of the isolates by pulsed-field gel electrophoresis (PFGE) and multiple-locus variable-number tandem repeat analysis (MLVA) could help finding unrecognized foodborne outbreaks due to consumption of respective contaminated sources. However, though the results of molecular analysis of the isolates could indicate linkage of sporadic cases of STEC infection, it is hardly concluded that the cases are related via contaminated food source if it were not for epidemiological information. Therefore, it is essential to combine the results of strain analysis and epidemiological investigation rapidly to detect rapidly foodborne outbreaks caused by bacteria. This article reviews STEC infection as foodborne disease and further discusses key characteristics of STEC including pathogenesis, clinical manifestation, prevention and control of STEC infection. We also present the recent situation of the disease in Japan based on the surveillance of STEC infection.
Keywords: Shiga toxin-producing E. coli, foodborne disease, infection, HUS, Japan
1. General introduction
As an important cause of foodborne disease, it is estimated by searching references published between January 1, 1990 and April 30, 2012 that Shiga toxin (verotoxin)-producing Escherichia coli (STEC) causes 2,801,000 acute illnesses annually, and leads to 3,890 cases of hemolytic uremic syndrome (HUS), 270 cases of ESRD, and 230 deaths globally1). Similar estimation for STEC global burden, that is 2.5 million illnesses and 1.2 million foodborne illnesses annually, has been given by WHO, although Norovirus was the leading cause of foodborne illness, causing 125 million cases and Campylobacter spp. caused 96 million foodborne illnesses2,3).
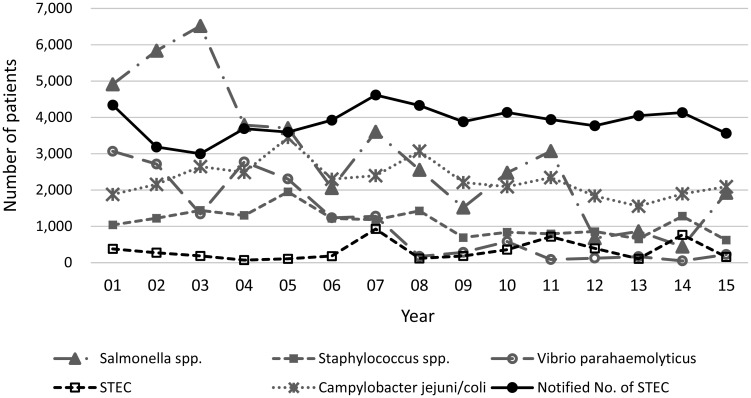
According to food poisoning statistics by the Ministry of Health, Labour and Welfare (MHLW) in Japan, the average number of food poisoning incidents and cases for the decade from 1981 to 1990 and from 2005 to 2014 were 967 and 35,618 for the former and 1,207 and 25,852 for the latter, respectively4). However, approximately 28% of reduction was observed in the number of the cases as a whole and the number of cases per incidents decreased from 36.8 to 21.4 between the two decades. On the other hand, as the number of incidents in the latter grew 1.25 times of that of the former, large foodborne outbreaks have been decreasing in number and small outbreaks and/or sporadic cases have been increasing. There are two different reporting systems for surveillance of STEC infections in Japan. One is based on the Law Concerning the Prevention of Infectious Diseases and Medical Care for Patients of Infections (the Infectious Diseases Control Law) and its purpose is to collect and compile reports of nationally notifiable infectious diseases, including STEC infections regardless of the route of infection. Since STEC infection is defined by isolation of STEC from the person except in the case of HUS, when serodiagnosis was possible, asymptomatic patients are also found in this system. The other is based on the Food Sanitation Law that collects reports of foodborne illness from municipal public health agencies and the system focuses on collecting symptomatic patients of food poisoning. Though the major cause of STEC infections is considered to be foodborne, there is a quite big difference between the numbers of STEC infection cases and the numbers of STEC food poisoning reported through each surveillance system (Fig. 1). The number of patients in food poisoning due to Salmonella spps. or Vibrio parahaemolyticus has gradually decreased since 2001 but that of patients due to Campylobacter jejuni/coli has remained relatively high compared to that of Staphylococcus spps. and STEC. The average number of the cases of STEC infection in the 11-year period from 2005 to 2015 was 3,995 but that of food poisoning due to STEC was only 364 for the same period. Although the number of cases of STEC infection includes 34% of asymptomatic patients during the period of 2009 to 20155–10), number of patients with STEC food poisoning is by far too small to compare to that of the cases of STEC infection. In this article we focus on STEC and STEC infection as foodborne illness discussing potential preventive measures against STEC infection.
Fig. 1.
The number of patients of food poisoning by various causal agents in Japan from 2001 to 2015 and the number of notified STEC infection during the same period. Note that the number of patients of food poisoning by STEC is strikingly smaller than that of notified STEC infection. The number of patients with The number of patients of food poisoning by various causal agents in Japan from 2001 to 2015 and the number of notified STEC infection during the same period. Note that the number of patients of food poisoning by STEC is strikingly smaller than that of notified STEC infection. The number of patients with Salmonella species and Vibrio parahaemolyticus are in decline.
2. Shiga toxin (verotoxin)-producing E. coli (STEC) and STEC infection
2-1. Introduction
Diarrheagenic E. coli that are capable of causing disease in healthy individuals can be categorized into six well-described categories: enteropathogenic E. coli (EPEC), Shiga toxin-producing E. coli (STEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC) and diffusely adherent E. coli (DAEC)11). The categories of diarrheagenic E. coli are differentiated on the basis of pathogenic features reflecting profile of virulence factors of the isolate. We will use the term STEC to denote strains possessing Shiga toxin independent of accompanying virulence factors.
STEC was first recognized as a human pathogen in 1982, when E.coli O157:H7 caused two outbreaks of hemorrhagic colitis associated with consumption of undercooked ground beef12,13). Since then, a number of foodborne outbreaks of hemorrhagic colitis and HUS due to not only STEC O157:H7 but also other serotypes of STEC have been reported worldwide. Since STEC resides in the gastrointestinal tract of cattle and other ruminants, contamination of meat with STEC during slaughter is a principal route by which these pathogens enter the food supply. However, a variety of foods have been identified as vehicles of STEC-associated illnesses; these include ground beef14), roast beef15), salami16), raw milk17,18), cheese19,20), ice-cream21), yogurt22), Romaine lettuce23), lettuce24), unpasteurized apple cider or juice25–28), cantaloupe29), spinach30), radish sprouts31,32), and alfalfa sprouts33). STEC O157:H7 have been predominant serotype of STEC associated with human illness and the serotype had been the major target of most detection methods. But as Shiga toxin and/or genes for Shiga toxin have become the main target of most detection methods in STEC surveillance, more cases of non-O157 STEC are reported. STEC infection remains a leading cause of gastroenteritis among notifiable disease in Japan and is sometimes followed by a severe life-threatening complication such as HUS.
2-2. Pathogenesis
2-2-1 Shiga toxins
Shiga toxins (Stxs) are key virulence factors produced by Shigella dysenteriae serotype 1 and STEC. Stxs have been shown to be responsible for exacerbating intestinal damage, and cause systemic complications involving the kidneys and central nervous system (CNS). The toxin is named after Dr. Kiyoshi Shiga, who identified the causative agent of dysentery, Shiga’s bacillus, in an outbreak of dysentery in Japan in 189734). In 1977, Konowalchuk et al. showed that some strains of E. coli produced a cytotoxin capable of killing Vero cell, and the cytotoxin was referred to as Vero cytotoxin or Verotoxin35). In addition to the findings with the purification of Shiga toxin from S. dysenteriae serotype 1 in 198036,37), it was reported that a Shiga-like toxin was produced by E. coli O157:H7 strain that had caused an outbreak of hemorrhagic colitis in the United States38) and that this toxin was the same as the verotoxin shown to be produced by E. coli O157:H739). Now, the terms Shiga toxins or Verotoxins are used to describe the same toxin40).
There are two types of Stxs produced by STEC, Shiga toxin type 1 (Stx1) and Shiga toxin type 2 (Stx2), based on their antigenic characteristics compared to the prototypical Shiga toxin produced by S. dysenteriae serotype 141–43). It has been shown that purified Stx2 has 400-fold lower median lethal dose (LD50) in mice44) and is 1,000 times more toxic to human renal endothelial cells than Stx145). Epidemiological evidence also shows that Stx2-producing strains of STEC O157:H7 are more frequently associated with HUS than are strains producing Stx146,47).
These Shiga toxin family members have a monomeric A and pentameric B molecular configuration, as revealed by X-ray crystallography48,49). A catalytic A subunit is non-covalently associated with a pentamer of identical B fragments that form the B subunit, which is responsible for binding to cell surface receptors, glycosphingolipid globotriaosylceramide (Gb3; also known as CD77 or the Pk blood group antigen)50–52). After binding to Gb3 receptors, the toxins are internalized and undergo retrograde intracellular transport to the endoplasmic reticulum (ER). During transport to the ER, catalytic A subunits dissociate from B subunits by proteolysis and disulfide bond reduction53–56). Since catalytic A subunit has a specific RNA N-glycosidase activity that cleaves an adenine base at position 4,324 of 28S ribosomal RNA of eukaryotic ribosomes57,58), it inhibits elongation factor-dependent amino-acyl tRNA binding and subsequent chain elongation59). However, delivery of the toxins to the ER and following retrotranslocation of the catalytic A subunits into the cytoplasm result in not only host cell protein synthesis inhibition, but activation of the ribotoxic stress and ER stress response and, in some cases, the induction of apoptosis, cytokines and chemokines60).
The stx genes in STEC strains are encoded by the genomes of prophages of the lambdoid family and are located downstream of the phage late gene promoter61). While stx1 is under control of the iron-regulated authentic promoter, which will result in induction of Stx1 expression at low concentration of iron62), the expression of stx2 depends primarily on the late promoter63,64). DNA-damaging agents, such as mitomycin C have been shown to increase Stx production through prophage induction65,66). Stx production in STEC strains, therefore, is intimately correlated with the Stx-encoding phages.
2-2-2 Adhesins Locus of enterocyte effacement
The ability of STEC to induce attaching and effacing (A/E) lesions of intestinal epithelia is shared by EPEC, Escherichia albertii (previously classified as Hafnia alvei), and Citrobacter rodentium67). The A/E lesions were typical histopathological observations in intestinal biopsy specimens from patients and infected animals originally reported with EPEC infection, which are characterized by effacement of microvilli and intimate adherence between the bacterium and the surface of epithelial cells68,69).
The bacterial genes involved in formation of A/E lesion were shown to be located on a 35-kb locus of the chromosome of EPEC and STEC isolates67). This locus, called locus of enterocyte effacement (LEE) is not present in non-pathogenic strains of E. coli but is found in EPEC and STEC strains capable of producing the A/E lesion. LEE-positive STEC serotypes have been referred to as enterohemorrhagic E. coli (EHEC)70) and LEE-positive STEC serotypes (such as O157:H7, O26:H11, O103:H2, O111:NM, O121:H19, and O145:NM) are much more commonly associated with HUS and with epidemic diseases than are LEE-negative serotypes11,70,71).The LEE consists of five major operons, which encode a type III secretion system, multiple secreted proteins, a bacterial adhesin called intimin, and a translocated receptor for intimin, Tir72–75). Intimin is a 95-kDa outer membrane protein that is encoded by eae gene (E. coli attaching and effacing) and necessary for the formation of A/E lesions69,76). The delivery of Tir into the host cell through type III secretion system is followed by binding of intimin to Tir that was recruited to the surface of the host cell membrane, which initiates formation of A/E lesions77). Although intimin is the primary adhesin in STEC, there are other adhesins contributing to the adhesive capabilities of STEC, including fimbrial adhesin proteins such as long-polar fimbriae78), autotransporters, flagella, and other adhesin proteins reviewed in reference79).
2-2-3 Acid tolerance
The infectious dose of STEC is estimated to be as low as or less than 100 organism80,81), which is attributed to its acid-resistant nature82–85). In addition to increasing the possibility of survival of the bacterium under gastric acid environment, the acid tolerance has enabled the pathogen to survive in various acidic food; apple cider (pH 3.7 to 4.0)86), buttermilk (pH 4.1)87), yogurt (pH4.17 to 4.39)87,88), and sour cream (pH 4.3)87). Acid resistance mechanism in E. coli includes a glucose-repressed system, glutamate- and arginine-dependent systems89). While rpoS (encoding sigma factor) is essential for expression of glucose-repressed system, glutamate- and arginine decarboxylase are required in amino acid-dependent systems. The two decarboxylase systems are believed to consume protons during the decarboxylation of glutamate or arginine, thereby preventing internal pH of the cell from decreasing to lethal levels90).
2-3. Animal Reservoir of STEC
Cattle and other ruminants91,92) are considered to be the major reservoirs of STEC, though STEC has also been isolated from other animals, such as dogs, cats, swine93), and horses94). Animal reservoirs for STEC O157:H7 including amphibians and fish, as well as invertebrates, such as insects and mollusks, were reviewed elsewhere95,96). Aquatic species such as finfish and shellfish as dead-end hosts96) could transmit the organism to other animals, when they are consumed97–99).
In a survey performed on rectal content samples from 250 beef cattle on 25 beef farms and 250 dairy cows on 25 dairy farms during summer in 2011 in Japan, STEC O157 was isolated from 16 (6.4%) beef cattle on 7 (28%) beef farms, but not obtained from any dairy cows tested100), and the previous investigation performed by the same authors four years apart showed very similar prevalence of STEC O157 (8.9%)101). In another study, prevalence of STEC strains in 932 healthy dairy cows from 123 farms was 12%, and 31 different O-serogroups, including O26 but not O157, were identified102). Using stx-PCRs for screening, the same study also found that the prevalence of the stx gene positive samples among the dairy cows was 30.4%. Hussein103) reviewed published reports and summarized that the prevalence rates of E. coli O157 ranged from 0.3 to 19.7% in feedlots and from 0.7 to 27.3% on pasture with regard to beef cattle and that corresponding prevalence rates of non-O157 STEC were 4.6 to 55.9% and 4.7 to 44.8%, respectively.
2-4. Sources of Human Infection
2-4-1. Undercooked Contaminated Beef Products
Since the most common source of STEC infection in human is consumption of contaminated foods, consumption of raw or undercooked foods of bovine origin has been the most common means of transmitting STEC infection. Ground beef is an especially efficient transmission vehicle of STEC and a multistate outbreak was traced to hamburgers distributed by a restaurant chain in 1993104), and undercooked hamburgers were implicated in a number of other outbreaks105–108). Hamburgers prepared at home were also implicated109,110). Needless to say, raw or undercooked beef products have a higher risk of transmitting contaminated bacteria to human and, in fact, the STEC O157 infections due to consumption of raw beef liver in 20106), a large STEC O111 outbreak due to consumption of Yukhoe, a Korean dish of raw beef and egg yolk7), and a diffuse outbreak from a restaurant chain due to cubically assembled meat111) were reported in Japan.
2-4-2. Contaminated Fresh Produce
Because STEC can attach to raw or processed fruits and vegetables, produce has also been a vehicle for transmission of the bacteria. Major outbreaks were linked to lettuce112), including a multistate outbreak; sprouts32) and spinach113) are implicated in numerous HUS cases, and the large outbreak of O104:H4 in 2011 in the European Union (EU) also implicated the consumption of sprouts114). Produce-associated outbreak surveillance data from the Centers for Disease Control and Prevention (CDC, U.S.A.) for the period from 2000 to 2009 showed that, among produce commodities, leafy greens were the most frequently linked to outbreaks115). Typically, produce-mediated outbreaks were linked to foliage contaminated by irrigation/spray water116); STEC O157:H7 were shown to adhere to and penetrate roots117).
Fermentation of food products can reduce the viability of STEC. STEC O157:H7 declined up to 3.5 logs in soudjouk sausage118,119), but fermented products may provide a vehicle for infection if curing conditions are inadequate; an outbreak in Sweden was traced to improperly processed sausage120). Pickled vegetables7) and lightly salted vegetables8,121,122), which were eaten fresh, as salad, have also been implicated in the outbreaks. Salmon roe that was lightly salted as a topping of sushi (ikura-sushi) was contaminated with STEC O157:H7 and caused an outbreak in Japan123).
2-4-3. Environment-mediated Transmission
Manure is a good vehicle of STEC and some outbreaks have been associated with public events held on grazing areas, presumably strewn with manure. A scouting event held in Scotland on a muddy field grazed by sheep resulted in an outbreak with Pulsedfield gel electrophoresis (PFGE) indistinguishable isolates from patients, the field, and sheep feces124); culturable bacteria were isolated for 15 weeks from the field soil after the outbreak125). In a sporadic case of STEC O157 infection in Minnesota, the isolates in garden soil linked to the case could survive on manure-amended soil for more than two months126).
Agricultural fairs exhibiting livestock are often implicated in human STEC outbreaks127–129). An investigation of a fair-associated STEC O157 outbreak suggested that infections can be caused by widespread contamination of a building, since there was no evidence implicating specific food or beverage sources but STEC O157 was recovered from the rafters of the building130). In an outbreak of STEC O157:H7 infection associated with attendance at multiple rodeos that had used bulls from the same cattle supplier, isolates from all 14 patients showed indistinguishable PFGE pattern and isolates from nine patients had identical multiple-locus variable-number tandem repeat analysis (MLVA) patterns and five had minor differences, and an isolate of STEC O157 identified from a dirt sample collected from the bullpens of one of the attended rodeos was indistinguishable by PFGE and MLVA form the main outbreak strain131). STEC O157:H7 was recovered from 3.5% of leafy green samples of the plot at 60 m away from a cattle feedlot, whereas that was recovered from 1.8% of the samples at 180 m away from the same feedlot, suggesting airborne contamination with STEC O157:H7 from cattle production area132).
Waterborne STEC infection has been implicated in a number of sporadic cases and outbreaks133,134). STEC outbreaks were associated with swimming in lakes135,136) and pools137), and consumption of water from a private water supply138–140).
2-4-4. Direct Contact Transmission
Direct human-animal contact can transmit STEC, and the most important prevention step to reduce transmission resulting from human-animal contact is hand-washing141). STEC outbreaks have often been associated with animals in public settings. Children are at most risk, as highlighted by HUS cases in petting zoo142) or farm143,144) visit and in participation in lamb feeding event145).
STEC can be transmitted to humans through person-to-person transmission. Through National Outbreak Reporting system in USA, estimated 40 foodborne outbreaks of STEC were reported in 2009, and five additional STEC outbreaks were reported as transmitted by person-to-person contact146). Person-to-person transmission of STEC is a recognized cause of outbreaks in childcare settings147–150), which may be related to close contact of children with immature immune systems and underdeveloped personal hygiene skills.
2-5. Epidemiology
2-5-1. Clinical course
STEC infection has a broad spectrum of clinical manifestation; asymptomatic infection, nonbloody diarrhea, bloody diarrhea (hemorrhagic colitis), and HUS. Typical clinical course of STEC O157:H7 infection begins with ingestion of the organisms, followed by a 3-to-4-day incubation period before the first loose stool. Illness then begins with nonbloody diarrhea and abdominal cramps. Most persons who seek medical attention develop bloody diarrhea, a typical feature of STEC O157:H7 infection, in the second or third day of illness151). Symptoms of infection with STEC O157:H7 usually subside in about a week, with no obvious sequelae. However, about 6 percent of patients develop HUS152) and it is usually diagnosed two to 14 days after the onset of diarrhea153). HUS is most likely to occur in young children and elderly152). In addition to age, risk factors for development of HUS include bloody diarrhea, fever, an elevated leukocyte count, antibiotic administration, and use of antimotility agents154–156). Although an association between non-O157 STEC and milder clinical symptoms have been reported157–159), further investigation is needed on whether features of the clinical illness vary among serotypes and how difference in virulence factors might result in differences in clinical outcomes. Although limited data are available on dose response, some findings indicate that the infectious doses of STEC are relatively low. For example, from an outbreak of O111 STEC in beef sausage in Australia, investigators extrapolated a dose range of 1 to 10 organisms, given as few as 1 cell per 10 g of sausage80). Using the concentrations of STEC O145 in contaminated ice cream in an outbreak in Belgium, the estimated infective dose was 400 colony forming unit (CFU)21). This is comparable to illness from STEC O157:H7, which can result from infection with as few as 10 cells81).
2-5-2. Surveillance
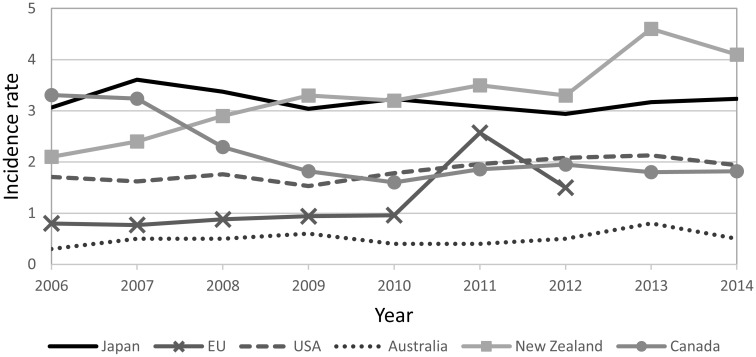
In the United States, all STEC infections that cause human illness are notifiable to the Nationally Notifiable Diseases Surveillance System. Incidence rate for STEC are shown in Fig. 2, and the mean incidence rate for STEC in the period from 2010 to 2014 was 1.98 in the United States160). On the other hand, in 2014, the Centers for Disease Control and Prevention (CDC) Emerging Infections Program analyzed the data gathered from the Foodborne Diseases Active Surveillance Network (FoodNet). A total of 697 laboratory-confirmed cases of STEC non-O157 and 444 of STEC O157 infections were identified, with incidence rates of 1.43 and 0.91 per 100,000 persons, respectively161). An examination of STEC cases from Michigan demonstrated an increase in non-O157 STEC162) and similar increases in non-O157 STEC were reported in other studies163–165). In both the Ontario and British Columbia sentinel sites in Canada, a total of 61 cases of STEC infections were reported between 2011 and 2012, representing an incidence rate of 3.1 cases/100,000 person-years166). In comparison, the annual combined incidence rate for STEC infection as notifiable disease in Canada for both years was 1.9 cases/100,000 person-years (Fig. 2). While a slight decrease in the incidence rate has been observed in Canada, the apparent increasing trend of the incidence rate was reported from New Zealand167). Except for the sudden increase of the incidence rate in the EU in 2011--probably due to a large outbreak of STEC O104 in Germany--it remained less than 1.5 cases/100, 000 in the EU, though the data was available only up to 2012168), and the incidence rate in Australia has been about 0.5 since 2007169–173).
Fig. 2.
Annual incidence rate per 100,000 population of STEC infection in Japan, EU, United States, Australia, New Zealand, and Canada, 2006 to 2014, except for the EU, for which data was available up to 2012.
In Japan, STEC infection is a category III notifiable infectious disease, along with other bacterial infections caused by Vibrio cholerae O1 or O139, Shigella species, Salmonella enterica serovar Typhi, and Salmonella enterica serovar Paratyphi A in the National Epidemiological Surveillance of Infectious Diseases (NESID) under the Infectious Diseases Control Law enacted in April 1999. Despite control measures instituted since 1996, including designating STEC infection as a notifiable disease, and the disease being monitored effectively through nationwide surveillance, the annual incidence rate remains around 3.0 per 100,000 population (Fig. 2). Under a surveillance system for food poisoning based on the Food Sanitation Law, an STEC infection is reported as food poisoning by physicians or judged as such by the director of the health center and reported as such by the local government to the MHLW. During the investigation of the outbreaks, family members and colleagues of the patients were encouraged to have stool examination and it was revealed that approximately 35% of STEC infections were asymptomatic and that the proportion of asymptomatic infection was high among the middle-aged group whereas symptomatic cases were more frequent in young and old age groups174).
Apart from NESID, results of characterization (serotypes, Stx types, etc.) of STEC isolates at prefectural and municipal PHIs are reported to the Infectious Disease Surveillance Center (IDSC) at the National Institute of Infectious Diseases (NIID). The summary showed that STEC O157 serogroup is the predominant one, followed by O26, O111, O103, O121, O145, and others. However, as seen in the United States163) and continental Europe175,176), the percentage of non-STEC O157 serogroups among all STEC isolates from human infection has been increasing slightly; the rate of isolation frequency of STEC O157 has declined from approximately 70% of all STEC isolates in 2000 to about 60% in 2015177,178). As a collaborating laboratory surveillance system between prefectural and municipal PHIs and NIID, PulseNet Japan, a national laboratory network that connects foodborne illness cases to detect outbreaks by the use of DNA fingerprinting of the isolates, has been established179). It constitutes a part of PulseNet International180), and has contributed to investigations of domestic181) and international STEC O157 outbreaks182).
2-5-3. Outbreaks in Japan
In the outbreaks with more than 10 culture-positive patients reported to the IDSC from 2000 to 2012, the main mode of transmission of the infection was person to person (41%), food borne (29%), and water borne (3%,), and in about one-third of the outbreaks, the mode of transmission of the infection remains unknown174). One major setting of these outbreaks was nursery schools, which may account for the high proportion of person-to-person transmissions of the infection in the outbreaks. The most prevalent serogroup of STEC in the outbreaks in nursery schools was O26 (52%), followed by O157 (27%), O111 (9%), O103 (4%), O121 (4%), O145 (3%), and OUT174), which may account for relatively mild clinical manifestations, including asymptomatic cases and, consequently, frequent person-to-person transmission observed in these outbreaks. Increased STEC outbreaks in childcare facilities due to non-O157 serogroups, particularly O26 and O111 during 2010 to 2013, were also reported by another group in Japan150). There were 13 STEC outbreaks that had more than 100 culture positive cases between 2000 and 2015(Table 1)183,184). All 13 outbreaks appear to have resulted from consumption of contaminated foods, and, in some of these outbreaks, microbiological testing confirmed the implicated foods. These included beef products121); lightly salted cucumber122); Koumi-ae consisting of boiled spinach and steamed chicken meat seasoned with welsh onion, ginger, and soy sauce122); boxed meals185); lettuce5); school lunches6); Yukhoe (raw beef)7), and Japanese rice cakes7).
Table 1. Foodborne Outbreaks Caused by STEC in Japan.
| Year | Prefecture/City | Setting (reference) | Serotype | Stx type | Symptomatic cases | Culture positives |
Likely mode of
transmission |
|---|---|---|---|---|---|---|---|
| 2001 | Chiba P. | Patient’s home (113) | O157:H7 | Stx1&2 | 195 | 257 | beef products (a) |
| 2002 | Fukuoka C. | Nursery school (114) | O157:H- | Stx2 | 74 | 112 | lightly salted cucumber (a) |
| 2002 | Utsunomiya C. | Hospital and home for the elderly (114) |
O157:H7 | Stx1&2 | 123 | 111 | Koumi-ae (a) |
| 2003 | Yokohama C. | Kindergarten (183) | O26:H11 | Stx1 | 141 | 449 | Foodborne |
| 2004 | Ishikawa P. | High school (184) | O111:H- | Stx1&2 | 110 | 103 | Foodborne |
| 2007 | Tokyo M. | School refectory (173) | O157:H7 | Stx2 | 467 | 204 | Foodborne |
| 2007 | Miyagi P., Sendai C. & Akita C. |
Restaurant (173) | O157:H7 | Stx1&2 | 314 | 173 | boxed meals (a) |
| 2009 | Saga P. | Nursery school (5) | O26:H11 | Stx1 | N.D. | 133 | lettuce (a) |
| 2010 | Mie P. | High school (6) | O157:H7 | Stx2 | 138 | 164 | school lunch (a) |
| 2011 | Toyama P. | Chain restaurants (7) | O111:H8 | Stx2, Stx- | 181 | 102 | Yukhoe (raw beef) (a) |
| O157:H7 | Stx1, Stx2,Stx1&2 | 38 | |||||
| 2011 | Yamagata P. | Festival (7) | O157:H7 | Stx1&2 | 287 | 189 | Japanese rice cakes (a) |
| 2012 | Osaka C. | Nursery school (8) | O26:H- | Stx1 | 68 | 115 | Foodborne |
| 2014 | Shizuoka C. | Street stall (10) | O157:H7 | Stx1&2 | 510 | 193 | Foodborne |
(a) Confirmed microbiologically; ND, no data.
Low infectious doses of STEC--possibly fewer than 10 organisms80,81)-- is a critical factor in the transmission of the STEC, when people consume raw or lightly cooked foods such as sushi and vegetables. Because sushi and raw or lightly cooked meat are popular foods in Japan, there have been outbreaks associated with consumption of salmon roe sushi in 1998123) and “rare” roast beef contaminated with STEC O157 in 2001121). In an STEC O111 outbreak associated with consumption of Yukhoe at Yakiniku chain restaurants, STEC O111:H8 was isolated from 85 of 181 patients (median age 20 years); in 34 of those patients HUS developed; encephalopathy developed in 21 patients; and 5 patients died7). HUS occurred most frequently in individuals aged 5–9 years, and this age group was significantly associated with acute encephalopathy186). STEC O111:H8 was also isolated from the conserved part of the original meat preparations distributed to the chain restaurants.
Some of the outbreaks were associated with consumption of vegetables. In addition to two large outbreaks in 2011 (Table 1), there were four outbreaks associated with consumption of vegetables7); cabbage was identified as a vehicle of STEC O26:H11 in an outbreak and STEC O157:H7 was isolated from pickled eggplant and green perilla, green perilla served with grated radish, and cucumber in three outbreaks, respectively. A large outbreak of STEC O157:H7 infection traced to a brand of lightly salted vegetables occurred in Sapporo, Hokkaido in 20128). STEC O157:H7 was isolated from the implicated product. Since the products were widely distributed, 169 patients were reported from five facilities for the elderly, hotels, restaurants, and families in Hokkaido and included four cases in different prefectures from which STEC O157:H7--with indistinguishable PFGE patterns and identical MLVA type--was isolated. STEC O157:H7 was isolated from 73 of 169 patients, eight of whom, mostly elderly, died. In August 2014, there was STEC O157 food poisoning of 510 cases, who consumed contaminated lightly pickled cucumbers sold at food stands during a fireworks display in Shizuoka Prefecture10).
2-6. HUS
HUS is a life-threatening illness characterized by hemolytic anemia, thrombocytopenia, and renal failure, and it is the most common cause of acute renal failure among children in the United States187). Foodborne Diseases Active Surveillance Network reported that among 3464 STEC O157 infections in the period of 2000-2006, 218 persons (6.3%) developed HUS and the highest proportion of HUS cases (15.3%) occurred among children aged less than 5 years and that death occurred in 0.6% of all patients with STEC O157 infection and in 4.6% of those with HUS188). In Italy, an average of 33 sporadic cases of HUS per year were observed between 1988 and 2012, with a mean annual incidence of 0.4 cases per 100,000 residents aged 0–15 years176). In 15 EU countries, A total of 382 (6.6%) confirmed STEC cases (n=5746) developed HUS in 2012 and 59 per cent of HUS cases (n=226) were reported in 0–4 year-old children with O157 and O26 as dominant serogroups followed by 5–14 year old children with O157 as a dominant serogroup (74%)168). In Argentina, postdiarrheal HUS is endemic and approximately 400 HUS cases were reported annually between 2002 and 2011. The incidence ranged from 10 to 17 cases per 100,000 children less than 5 years of age, and lethality was between 1 and 4%189).
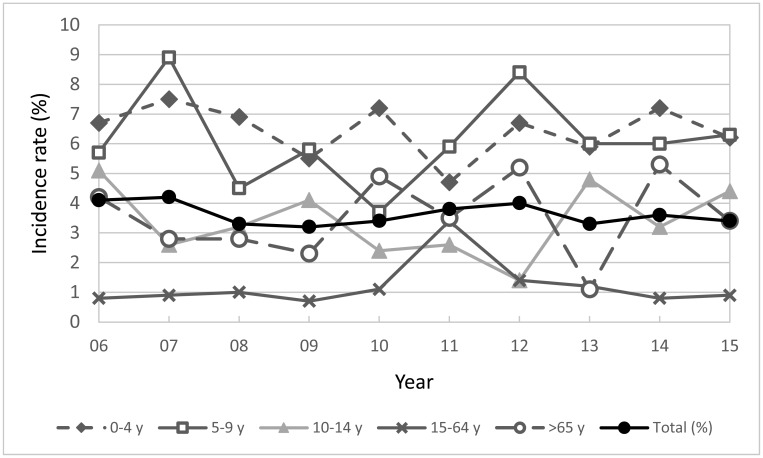
In Japan, due to an amendment of the case definition of STEC in the Infectious Diseases Control Law in 2006, HUS patient should be reported as having STEC infection if Stx was detected in feces, or O-antigen agglutinating antibody or anti-Stx antibody was detected in the serum of the patient. From 2006 to 2015, the average annual number of HUS cases (including serodiagnosed cases) was 99 and the incidence rate of HUS (HUS cases/symptomatic cases) was 3.6% (Fig. 3)111,190–197). As reported in previous studies, increased rates of HUS in children less than 10 years old and the elderly198,199) are shown in Fig. 3.
Fig. 3.
Incidence rate of HUS in STEC infection by age groups in Japan, 2006 to 2015. Incidence rate (%) was calculated as (number of patients with HUS) ÷ (number of symptomatic cases) × 100%.
From 2006 to 2015, 65% of the 985 HUS cases were culture-confirmed by laboratory testing, and the rest of the cases were diagnosed by detecting antilipopolysaccharide antibody of E. coli in the serum of the patients or Stx in the stool samples of the patients.
STEC O157 was the predominant serogroup, occupying 85% of all isolates in culture-confirmed HUS cases, followed by O111 (4.4%), O26 (2.7%), O121 (2.3%), O165 (1.2%), O145 (0.6%), and the rest of the O serogroups, including O55, O74, O76, O115, O174, O183, and unknown serogroup samples. Although non-O157 serogroup strains were isolated in the culture-confirmed HUS cases, 94% of all STEC isolates in the culture-confirmed HUS cases were either Stx2 or both Stx1 and Stx2 producers, which is consistent with epidemiological evidence that Stx2-containing STEC O157:H7 strains are more frequently associated with HUS than the strains containing Stx146,47,71).
2-7. Infection Control
Although it is rare to be able to find the source of infection in sporadic cases of STEC infection, many outbreaks of STEC infection have been associated with foods that become contaminated through direct or indirect environmental exposure to waste products of cattle. Therefore, implementing effective measures to reduce or eliminate STEC from all stages of the food chain, starting from production to consumption would lead to reducing STEC infection in humans. All steps ranging from reducing carriage of STEC by cattle used in food production to proper food preparation to kill STEC before consumption should be included. Certain farm management practices, especially those related to prevention of contamination and multiplication of STEC in feed and water, may provide practical means to reduce the prevalence of STEC in cattle on farms and in slaughter plants200). Good hygienic practices during food production are essential to keep microbiological contamination to a minimum. The most effective method of eliminating STEC from foods is to introduce a bactericidal treatment, such as heating201,202) or irradiation203–206). Since person-to-person contact is an important mode of transmission through the oral-fecal route in STEC infection, good hygiene practice is especially important in settings such as child-care facilities, where persons at high risk for STEC infection spend considerable time together. In addition, as the median duration of shedding reported from previous outbreaks in childcare facilities has been found to be between 20 and 50 days147,207–209), exclusion of ill persons from the facilities until the diarrhea has resolved should be considered. In general, routine handwashing before eating and after diaper changes and toileting is the best way to prevent the spread of infection in child-care facilities.
3. Control Measures Against Foodborne Disease Due to STEC
Among preharvest food safety interventions to reduce the prevalence and shedding level of STEC O157 by cattle, vaccines have been the most effective interventions documented to date.
Currently, only two commercially available vaccines against E. coli O157:H7 in cattle exist: a type III secreted proteins (Bioniche Life Sciences Inc., Belleville, Ontario, Canada), and a siderophore receptor and porin protein (Epitopix, LLC, Wilmar, MN, USA). Several systematic reviews and meta-analyses suggested that both vaccines effectively reduce the probability of feedlot cattle to shed STEC O157 in feces210–213).
Some of the lessons learned from STEC outbreaks have resulted in long-term improvements in food safety. In 1994, the U.S. Department of Agriculture (USDA), Food Safety and Inspection Service (FSIS) declared STEC O157:H7 an adulterant in ground beef in response to the multistate outbreak caused in 1993 by undercooked hamburgers contaminated with STEC O157214). Following these outbreaks, the U.S. Food and Drug Administration (FDA) issued more stringent guidelines for the internal temperature of cooked hamburgers215). The pronouncement from FSIS was extended in 1999 to all nonintact raw beef products216). Since the number of infections and outbreaks due to STEC non-O157 has increased, FSIS documented risk profile for pathogenic non-O157 STEC and concluded that STEC O157 was not the only STEC representing a hazard217). Furthermore, FSIS decided on the implementation of sampling and testing manufacturing trim and other raw ground beef product components for STEC non-O157 in 2012218). STEC strains of serogroups O26, O111, O103, O145, O121, and O45 were declared as adulterant in these food commodities and included in the sampling plans in addition to STEC O157.
In the European Union (EU), although the limitations exist in categorizing the level of danger associated with STEC from nonhuman sources, the seropathotypes A and B of Karmali’s scheme219) formed a large consensus in the scientific community and were endorsed by the European Food Safety Authority (EFSA). The scheme was based on the evaluation of the virulence and serological features of the strains combined with their association with severe disease and epidemic outbreaks. STEC strains belonging to serogroups O157, O26, O111, O103, and O145 were included in the seropathotypes A and B of the scheme. EFSA recommended focusing food testing for STEC on the seropathotype A and B groups220,221). However, the massive outbreak caused by an enteroaggregative STEC O104:H4 strain in 2011 in Germany and other 12 European countries forced the European Commission (EC) to take measures against the possibility of other STEC crises in the EU. EFSA was asked to assess the public health risk caused by STEC and other pathogenic bacteria that may contaminate both seeds and sprouted seeds intended for direct human consumption222,223). Finally, the European Commission issued Regulation (EU) 209/2013, containing the microbiological criteria for STEC in sprouts and amending Regulation (EC) 2073/2005, which introduced for the first time in EU legislation a specific criterion for STEC regarding the presence in sprouts of the five STEC serogroups, i.e. O157, O26, O111, O103, and O145 plus STEC O104:H4224).
In Japan, STEC infections have been routinely notifiable since 1996. They are also reported as food poisoning by physicians or judged as such by the director of the health center under a surveillance system for food poisoning. Furthermore, the Abattoir Law Enforcement Regulation (Ministry of Health and Welfare Ordinance No. 44, September 28, 1953) was amended by the MHLW so that the measures for prevention and reduction of STEC contamination at every step of the processes such as tying the rectum before evisceration at slaughter houses could be fulfilled. The MHLW has also formulated standards of a hygienic control manual for large cooking facilities based on the HACCP (Hazard Analysis and Critical Control Point) to prevent food poisoning due to food provided by these facilities. Although the official detection methods for STEC O26, O111, and O157225) was in use, a newer detection method for STEC O26, O103, O111, O121, O145, and O157 in food226) has been established and validated,227) reflecting a steady increase in non-O157 STEC infections. The method involved a combination of various chromogenic agars, targeting particular STEC serogroups, and molecular approaches such as real-time PCR based on the methods used by the USDA228) and EFSA221). In response to persistent food poisonings caused by raw beef, the MHLW revised the standards of beef product quality for raw-eating and put them into operation in October 2011. Further, after STEC O157 was detected in the inner part of cattle livers, the MHLW banned the marketing of cattle liver intended to be eaten raw. Probably as a consequence of these preventive measures, the incidence of STEC O157 cases related to consumption of raw meat decreased by almost half in one year, from 2011 to 20128).
4. Conclusions
STEC infections continue to occur due to a variety of foods contaminated by this bacterium. Although foods of bovine origin contaminated with this pathogen have been a major source for infection, evidence of disease linked to other sources, including contaminated produce, water, and other environmental exposures, indicates a necessity for a comprehensive approach to STEC reduction or elimination at all levels of food production. However, despite various control measures taken today, reported incidence of non-O157 STEC has been gradually increasing, partially due to recent improved detection methods, and massive outbreak due to a rare category of STEC that belonged to enteroaggregative E. coli occurred in Europe, indicating the complexity of securing food safety when dealing with STEC.
Surveillance of STEC infections, especially laboratory investigation of the implicated isolates based on molecular analytical methods, have revealed that complex ecology and genetics of this pathogen existed and indicated that combining the results of isolate analysis with epidemiological information were important for accurate determination of the infectious source. Therefore, cooperative interplay of relevant authorities referring to public health, food, and veterinary science are indispensable for establishment of effective control measures and prevention against STEC infection.
Acknowledgements
We thank all investigators of prefectural and municipal public health institutes for providing us samples and information, and the IDSC staff for data collection and analysis. We also thank the Food Safety Committee of Japan for its support in writing the manuscript.
Abbreviations: A/E: attaching and effacing, CDC: Centers for Disease Control and Prevention, CFU: colony forming unit, CNS: central nervous system, DAEC: diffusely adherent E. coli, EAEC: enteroaggregative E. coli, EC: European Commission EFSA: European Food Safety Authority, EHEC: enterohemorrhagic E. coli, EIEC: enteroinvasive E. coli, EPEC: enteropathogenic E. coli, ER: endoplasmic reticulum, ESRD: end-stage renal disease, ETEC: enterotoxigenic E. coli, EU: European Union, FDA: U.S. Food and Drug Administration, FoodNet: Foodborne Diseases Active Surveillance Network, FSIS: Food Safety and Inspection Service, Gb3: glycosphingolipid globotriaosylceramide, HUS: hemolytic uremic syndrome, IDSC: Infectious Disease Surveillance Center, LD50: median lethal dose, LEE: locus of enterocyte effacement, MHLW: Ministry of Health, Labour and Welfare, MLVA: Multiple-locus variable-number tandem repeat analysis, NESID: National Epidemiological Surveillance of Infectious Diseases, NIID: National Institute of Infectious Diseases, PFGE: Pulsedfield gel electrophoresis, STEC: Shiga toxin-producing E. coli, Stx: Shiga toxin, Stx1: Shiga toxin type 1, Stx2: Shiga toxin type 2, USDA: U.S. Department of Agriculture
Footnotes
Conflict of interest statement: The authors had no conflicts of interest to declare in this article.
References
- 1.Majowicz SE, Scallan E, Jones-Bitton A, et al. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathogens and Disease. 2014; 11: 447–455. 10.1089/fpd.2013.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirk MD, Pires SM, Black RE, et al. Correction: World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLOS Medicine. 2015; 12: e1001940. 10.1371/journal.pmed.1001940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk MD, Pires SM, Black RE, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLOS Medicine. 2015; 12: e1001921. 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Ministry of Health Labour and Welfare Food Poisoning Statistics Available at : http://www.mhlw.go.jp/file/06-Seisakujouhou-11130500-Shokuhinanzenbu/nenji_3.xls. Food Poisoning Statistics. 2015.
- 5.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of May 2010. Infec Agen Surv Rep. 2010; 31: 152–153. [Google Scholar]
- 6.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2011. Infec Agen Surv Rep. 2011; 32: 125–126. [Google Scholar]
- 7.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2012. Infec Agen Surv Rep. 2012; 33: 115–116. [Google Scholar]
- 8.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2013. Infec Agen Surv Rep. 2013; 34: 123–124. [Google Scholar]
- 9.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2014. Infec Agen Surv Rep. 2014; 35: 117–118. [Google Scholar]
- 10.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2015. Infec Agen Surv Rep. 2015; 36: 73–74. [Google Scholar]
- 11.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998; 11: 142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley LW, Remis RS, Helgerson SD, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. New England Journal of Medicine. 1983; 308: 681–685. 10.1056/NEJM198303243081203 [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Isolation of E. coli O157:H7 from sporadic cases of hemorrhagic colitis—United States. MMWR Morb Mortal Wkly Rep. 1982; 31: 585–588. [PubMed] [Google Scholar]
- 14.Erickson MC, Doyle MP. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. Journal of Food Protection. 2007; 70: 2426–2449. 10.4315/0362-028X-70.10.2426 [DOI] [PubMed] [Google Scholar]
- 15.Rodrigue DC, Mast EE, Greene KD, et al. A university outbreak of Escherichia coli O157:H7 infections associated with roast beef and an unusually benign clinical course. Journal of Infectious Diseases. 1995; 172: 1122–1125. 10.1093/infdis/172.4.1122 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami--Washington and California, 1994. MMWR Morb Mortal Wkly Rep. 1995; 44: 157–160. [PubMed] [Google Scholar]
- 17.Guh A, Phan Q, Nelson R, et al. Outbreak of Escherichia coli O157 associated with raw milk, Connecticut, 2008. Clinical Infectious Diseases. 2010; 51: 1411–1417. 10.1086/657304 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Escherichia coli 0157:H7 infections in children associated with raw milk and raw colostrum from cows--California, 2006. MMWR Morb Mortal Wkly Rep. 2008; 57: 625–628. [PubMed] [Google Scholar]
- 19.Gaulin C, Levac E, Ramsay D, et al. Escherichia coli O157:H7 outbreak linked to raw milk cheese in Quebec, Canada: use of exact probability calculation and casecase study approaches to foodborne outbreak investigation. Journal of Food Protection. 2012; 75: 812–818. 10.4315/0362-028X.JFP-11-385 [DOI] [PubMed] [Google Scholar]
- 20.Gill A, Oudit D. Enumeration of Escherichia coli O157 in Outbreak-Associated Gouda Cheese Made with Raw Milk. Journal of Food Protection. 2015; 78: 1733–1737. 10.4315/0362-028X.JFP-15-036 [DOI] [PubMed] [Google Scholar]
- 21.Buvens G, Possé B, De Schrijver K, De Zutter L, Lauwers S, Piérard D. Virulence profiling and quantification of verocytotoxin-producing Escherichia coli O145:H28 and O26:H11 isolated during an ice cream-related hemolytic uremic syndrome outbreak. Foodborne Pathogens and Disease. 2011; 8: 421–426. 10.1089/fpd.2010.0693 [DOI] [PubMed] [Google Scholar]
- 22.Morgan D, Newman CP, Hutchinson DN, Walker AM, Rowe B, Majid F. Verotoxin producing Escherichia coli O 157 infections associated with the consumption of yoghurt. Epidemiology and Infection. 1993; 111: 181–188. 10.1017/S0950268800056880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor EV, Nguyen TA, MacHesky KD, et al. Multistate outbreak of Escherichia coli O145 infections associated with romaine lettuce consumption, 2010. Journal of Food Protection. 2013; 76: 939–944. 10.4315/0362-028X.JFP-12-503 [DOI] [PubMed] [Google Scholar]
- 24.Doyle MP, Erickson MC. Summer meeting 2007 the problems with fresh produce: an overview. Journal of Applied Microbiology. 2008; 105: 317–330. 10.1111/j.1365-2672.2008.03746.x [DOI] [PubMed] [Google Scholar]
- 25.Besser RE, Lett SM, Weber JT, et al. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA: The Journal of the American Medical Association. 1993; 269: 2217–2220. 10.1001/jama.1993.03500170047032 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider--Connecticut and New York, October 1996. MMWR Morb Mortal Wkly Rep. 1997; 46: 4–8. [PubMed] [Google Scholar]
- 27.Tamblyn S, deGrosbois J, Taylor D, Stratton J. An outbreak of Escherichia coli O157:H7 infection associated with unpasteurized non-commercial, custom-pressed apple cider--Ontario, 1998. Can Commun Dis Rep. 1999; 25: 113–117, discussion 117–120. [PubMed] [Google Scholar]
- 28.Hilborn ED, Mshar PA, Fiorentino TR, et al. An outbreak of Escherichia coli O157∶H7 infections and haemolytic uraemic syndrome associated with consumption of unpasteurized apple cider. Epidemiology and Infection. 2000; 124: 31–36. 10.1017/S0950268899003258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beuchat LR. Pathogenic Microorganisms Associated with Fresh Produce. Journal of Food Protection. 1996; 59: 204–216. 10.4315/0362-028X-59.2.204 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach--United States, September 2006. MMWR Morb Mortal Wkly Rep. 2006; 55: 1045–1046. [PubMed] [Google Scholar]
- 31.Watanabe Y, Ozasa K, Mermin JH, et al. Factory outbreak of Escherichia coli O157:H7 infection in Japan. Emerging Infectious Diseases. 1999; 5: 424–428. 10.3201/eid0503.990313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michino H, Araki K, Minami S, et al. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. American Journal of Epidemiology. 1999; 150: 787–796. 10.1093/oxfordjournals.aje.a010082 [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention Outbreaks of Escherichia coli O157:H7 infection associated with eating alfalfa sprouts-Michigan and Virginia, June-July 1997. MMWR Morb Mortal Wkly Rep. 1997; 46: 741–744. [PubMed] [Google Scholar]
- 34.Trofa AF, Ueno-Olsen H, Oiwa R, Yoshikawa M. Dr. Kiyoshi Shiga: discoverer of the dysentery bacillus. Clinical Infectious Diseases. 1999; 29: 1303–1306. 10.1086/313437 [DOI] [PubMed] [Google Scholar]
- 35.Konowalchuk J, Speirs JI, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977; 18: 775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien AD, LaVeck GD, Griffin DE, Thompson MR. Characterization of Shigella dysenteriae 1 (Shiga) toxin purified by anti-Shiga toxin affinity chromatography. Infect Immun. 1980; 30: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsnes S, Eiklid K. Isolation and characterization of Shigella shigae cytotoxin. J Biol Chem. 1980; 255: 284–289. [PubMed] [Google Scholar]
- 38.Obrien A, Lively TA, Chen ME, Rothman SW, Formal SB. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. The Lancet. 1983; 321: 702. 10.1016/S0140-6736(83)91987-6 [DOI] [PubMed] [Google Scholar]
- 39.Johnson W. M., Lior H., Bezanson G. S. Cytotoxic Escherichia coli O157:H7 associated with haemorrhagic colitis in Canada. Lancet. 1983; 1(8314-5): 76. [DOI] [PubMed]
- 40.Kaper JB, O’Brien AD. Overview and Historical Perspectives. Microbiol Spectr. 2014; 2: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheutz F, Teel LD, Beutin L, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. Journal of Clinical Microbiology. 2012; 50: 2951–2963. 10.1128/JCM.00860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strockbine NA, Marques LR, Newland JW, Smith HW, Holmes RK, O’Brien AD. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986; 53: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheutz F. Taxonomy Meets Public Health: The Case of Shiga Toxin-Producing Escherichia coli. Microbiology Spectrum. 2014; 2. 10.1128/microbiolspec.EHEC-0019-2013 [DOI] [PubMed] [Google Scholar]
- 44.Tesh VL, Burris JA, Owens JW, et al. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect Immun. 1993; 61: 3392–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louise CB, Obrig TG. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. Journal of Infectious Diseases. 1995; 172: 1397–1401. 10.1093/infdis/172.5.1397 [DOI] [PubMed] [Google Scholar]
- 46.Ostroff SM, Tarr PI, Neill MA, Lewis JH, Hargrett-Bean N, Kobayashi JM. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. Journal of Infectious Diseases. 1989; 160: 994–998. 10.1093/infdis/160.6.994 [DOI] [PubMed] [Google Scholar]
- 47.Scotland SM, Willshaw GA, Smith HR, Rowe B. Properties of strains of Escherichia coli belonging to serogroup O 157 with special reference to production of Vero cytotoxins VTl and VT2. Epidemiology and Infection. 1987; 99: 613–624. 10.1017/S0950268800066462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraser ME, Chernaia MM, Kozlov YV, James MNG. Crystal structure of the holotoxino from Shigella dysenteriae at 2.5 Å resolution. Nature Structural & Molecular Biology. 1994; 1: 59–64. 10.1038/nsb0194-59 [DOI] [PubMed] [Google Scholar]
- 49.Stein PE, Boodhoo A, Tyrrell GJ, Brunton JL, Read RJ. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature. 1992; 355: 748–750. 10.1038/355748a0 [DOI] [PubMed] [Google Scholar]
- 50.Jacewicz M, Clausen H, Nudelman E, Donohue-Rolfe A, Keusch GT. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin- binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. Journal of Experimental Medicine. 1986; 163: 1391–1404. 10.1084/jem.163.6.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindberg AA, Brown JE, Strömberg N, Westling-Ryd M, Schultz JE, Karlsson KA. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987; 262: 1779–1785. [PubMed] [Google Scholar]
- 52.Waddell T, Cohen A, Lingwood CA. Induction of verotoxin sensitivity in receptor-deficient cell lines using the receptor glycolipid globotriosylceramide. Proceedings of the National Academy of Sciences. 1990; 87: 7898–7901. 10.1073/pnas.87.20.7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garred O, van Deurs B, Sandvig K. Furin-induced cleavage and activation of Shiga toxin. Journal of Biological Chemistry. 1995; 270: 10817–10821. 10.1074/jbc.270.18.10817 [DOI] [PubMed] [Google Scholar]
- 54.Garred Ø, Dubinina E, Polesskaya A, Olsnes S, Kozlov J, Sandvig K. Role of the disulfide bond in Shiga toxin A-chain for toxin entry into cells. Journal of Biological Chemistry. 1997; 272: 11414–11419. 10.1074/jbc.272.17.11414 [DOI] [PubMed] [Google Scholar]
- 55.Garred O, Dubinina E, Holm PK, et al. Role of processing and intracellular transport for optimal toxicity of Shiga toxin and toxin mutants. Experimental Cell Research. 1995; 218: 39–49. 10.1006/excr.1995.1128 [DOI] [PubMed] [Google Scholar]
- 56.LaPointe P, Wei X, Gariépy J. A role for the protease-sensitive loop region of Shiga-like toxin 1 in the retrotranslocation of its A1 domain from the endoplasmic reticulum lumen. Journal of Biological Chemistry. 2005; 280: 23310–23318. 10.1074/jbc.M414193200 [DOI] [PubMed] [Google Scholar]
- 57.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. European Journal of Biochemistry. 1988; 171: 45–50. 10.1111/j.1432-1033.1988.tb13756.x [DOI] [PubMed] [Google Scholar]
- 58.Saxena SK, O’Brien AD, Ackerman EJ. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28 S RNA when microinjected into Xenopus oocytes. J Biol Chem. 1989; 264: 596–601. [PubMed] [Google Scholar]
- 59.Hale TL, Formal SB. Cytotoxicity of Shigella dysenteriae 1 for cultured mammalian cells. The American Journal of Clinical Nutrition. 1980; 33(Suppl): 2485–2490. 10.1093/ajcn/33.11.2485 [DOI] [PubMed] [Google Scholar]
- 60.Johannes L, Römer W. Shiga toxins — from cell biology to biomedical applications. Nature Reviews Microbiology. 2010; 8: 105–116. 10.1038/nrmicro2279 [DOI] [PubMed] [Google Scholar]
- 61.Waldor MK, Friedman DI. Phage regulatory circuits and virulence gene expression. Current Opinion in Microbiology. 2005; 8: 459–465. 10.1016/j.mib.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 62.Calderwood SB, Mekalanos JJ. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. Journal of Bacteriology. 1987; 169: 4759–4764. 10.1128/jb.169.10.4759-4764.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner PL, Waldor MK. Bacteriophage control of bacterial virulence. Infection and Immunity. 2002; 70: 3985–3993. 10.1128/IAI.70.8.3985-3993.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyler JS, Mills MJ, Friedman DI. The operator and early promoter region of the Shiga toxin type 2-encoding bacteriophage 933W and control of toxin expression. Journal of Bacteriology. 2004; 186: 7670–7679. 10.1128/JB.186.22.7670-7679.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Jumaili I, Burke DA, Scotland SM, Al-Mardini H, Record CO. A method of enhancing verocytotoxin production by Escherichia coli. FEMS Microbiology Letters. 1992; 93: 121–125. 10.1111/j.1574-6968.1992.tb05077.x [DOI] [PubMed] [Google Scholar]
- 66.Wagner PL, Neely MN, Zhang X, Acheson DWK, Waldor MK, Friedman DI. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. Journal of Bacteriology. 2001; 183: 2081–2085. 10.1128/JB.183.6.2081-2085.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proceedings of the National Academy of Sciences. 1995; 92: 1664–1668. 10.1073/pnas.92.5.1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983; 41: 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proceedings of the National Academy of Sciences. 1990; 87: 7839–7843. 10.1073/pnas.87.20.7839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaper JB, Mellies JL, Nataro J. Pathogenicity islands and other mobile genetic elements of diarrheagenic Escherichia coli. Washington, D.C.: ASM Press; 1999. [Google Scholar]
- 71.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol. 1999; 37: 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elliott SJ, Wainwright LA, McDaniel TK, et al. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Molecular Microbiology. 1998; 28: 1–4. 10.1046/j.1365-2958.1998.00783.x [DOI] [PubMed] [Google Scholar]
- 73.Perna NT, Mayhew GF, Pósfai G, et al. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1998; 66: 3810–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997; 91: 511–520. 10.1016/S0092-8674(00)80437-7 [DOI] [PubMed] [Google Scholar]
- 75.Stevens M. P., Frankel G. M. The Locus of Enterocyte Effacement and Associated Virulence Factors of Enterohemorrhagic Escherichia coli. Microbiol Spectr. 2014;2(4):EHEC-0007-2013. [DOI] [PubMed]
- 76.Yu J, Kaper JB. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Molecular Microbiology. 1992; 6: 411–417. 10.1111/j.1365-2958.1992.tb01484.x [DOI] [PubMed] [Google Scholar]
- 77.DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay BB. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect Immun. 1999; 67: 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross BN, Rojas-Lopez M, Cieza RJ, McWilliams BD, Torres AG. The Role of Long Polar Fimbriae in Escherichia coli O104:H4 Adhesion and Colonization. PLOS ONE. 2015; 10: e0141845. 10.1371/journal.pone.0141845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McWilliams BD, Torres AG. Enterohemorrhagic Escherichia coli Adhesins. Microbiology Spectrum. 2014; 2. 10.1128/microbiolspec.EHEC-0003-2013 [DOI] [PubMed] [Google Scholar]
- 80.Paton AW, Ratcliff RM, Doyle RM, et al. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J Clin Microbiol. 1996; 34: 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tilden J, Jr, Young W, McNamara AM, et al. A new route of transmission for Escherichia coli: infection from dry fermented salami. American Journal of Public Health. 1996; 86: 1142–1145. 10.2105/AJPH.86.8_Pt_1.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arnold KW, Kaspar CW. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1995; 61: 2037–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benjamin MM, Datta AR. Acid tolerance of enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1995; 61: 1669–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Conner DE, Kotrola JS. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl Environ Microbiol. 1995; 61: 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheville AM, Arnold KW, Buchrieser C, Cheng CM, Kaspar CW. rpoS regulation of acid, heat, and salt tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1996; 62: 1822–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller LG, Kaspar CW. Escherichia coli O157: H7 Acid Tolerance and Survival in Apple Cider. Journal of Food Protection. 1994; 57: 460–464. 10.4315/0362-028X-57.6.460 [DOI] [PubMed] [Google Scholar]
- 87.Dineen SS, Takeuchi K, Soudah J, Boor KJ. Persistence of Escherichia coli O157:H7 in dairy fermentation systems. Journal of Food Protection. 1998; 61: 1602–1608. 10.4315/0362-028X-61.12.1602 [DOI] [PubMed] [Google Scholar]
- 88.Hudson LM, Chen J, Hill AR, Griffiths MW. Bioluminescence: a rapid indicator of Escherichia coli O157:H7 in selected yogurt and cheese varieties. Journal of Food Protection. 1997; 60: 891–897. 10.4315/0362-028X-60.8.891 [DOI] [PubMed] [Google Scholar]
- 89.Foster JW. Escherichia coli acid resistance: tales of an amateur acidophile. Nature Reviews Microbiology. 2004; 2: 898–907. 10.1038/nrmicro1021 [DOI] [PubMed] [Google Scholar]
- 90.Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW. Control of acid resistance in Escherichia coli. J Bacteriol. 1999; 181: 3525–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gyles CL. Shiga toxin-producing Escherichia coli: An overview1. Journal of Animal Science. 2007; 85 (suppl_13): E45–E62. 10.2527/jas.2006-508 [DOI] [PubMed] [Google Scholar]
- 92.La Ragione RM, Best A, Woodward MJ, Wales AD. Escherichia coli O157:H7 colonization in small domestic ruminants. FEMS Microbiology Reviews. 2009; 33: 394–410. 10.1111/j.1574-6976.2008.00138.x [DOI] [PubMed] [Google Scholar]
- 93.Beutin L, Geier D, Steinrück H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993; 31: 2483–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trevena WB, Hooper RS, Wray C, Willshaw GA, Cheasty T, Domingue G. Vero cytotoxin-producing Escherichia coli O157 associated with companion animals. Vet Rec. 1996; 138: 400. [PubMed] [Google Scholar]
- 95.Ferens WA, Hovde CJ. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathogens and Disease. 2011; 8: 465–487. 10.1089/fpd.2010.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Persad A. K., LeJeune J. T. Animal Reservoirs of Shiga Toxin-Producing Escherichia coli. Microbiol Spectr. 2014;2(4):EHEC-0027-2014. [DOI] [PubMed]
- 97.Gourmelon M, Montet MP, Lozach S, et al. First isolation of Shiga toxin 1d producing Escherichia coli variant strains in shellfish from coastal areas in France. Journal of Applied Microbiology. 2006; 100: 85–97. 10.1111/j.1365-2672.2005.02753.x [DOI] [PubMed] [Google Scholar]
- 98.Manna SK, Das R, Manna C. Microbiological quality of finfish and shellfish with special reference to shiga toxin-producing Escherichia coli O157. Journal of Food Science. 2008; 73: M283–M286. 10.1111/j.1750-3841.2008.00815.x [DOI] [PubMed] [Google Scholar]
- 99.Bennani M, Badri S, Baibai T, et al. First detection of Shiga toxin-producing Escherichia coli in shellfish and coastal environments of Morocco. Applied Biochemistry and Biotechnology. 2011; 165: 290–299. 10.1007/s12010-011-9251-x [DOI] [PubMed] [Google Scholar]
- 100.Sasaki Y, Murakami M, Maruyama N, et al. Comparison of the prevalence of shiga toxin-producing Escherichia coli strains O157 and O26 between beef and dairy cattle in Japan. Journal of Veterinary Medical Science. 2013; 75: 1219–1221. 10.1292/jvms.12-0514 [DOI] [PubMed] [Google Scholar]
- 101.Sasaki Y, Tsujiyama Y, Kusukawa M, Katayama MMS, Yamada Y, Yamada Y. Prevalence and characterization of Shiga toxin-producing Escherichia coli O157 and O26 in beef farms. Veterinary Microbiology. 2011; 150: 140–145. 10.1016/j.vetmic.2010.12.024 [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi H, Kanazaki M, Ogawa T, Iyoda S, Hara-Kudo Y. Changing prevalence of O-serogroups and antimicrobial susceptibility among STEC strains isolated from healthy dairy cows over a decade in Japan between 1998 and 2007. Journal of Veterinary Medical Science. 2009; 71: 363–366. 10.1292/jvms.71.363 [DOI] [PubMed] [Google Scholar]
- 103.Hussein HS. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products1,2. Journal of Animal Science. 2007; 85 (suppl_13): E63–E72. 10.2527/jas.2006-421 [DOI] [PubMed] [Google Scholar]
- 104.O’Brien AD, Melton AR, Schmitt CK, McKee ML, Batts ML, Griffin DE. Profile of Escherichia coli O157:H7 pathogen responsible for hamburger-borne outbreak of hemorrhagic colitis and hemolytic uremic syndrome in Washington. J Clin Microbiol. 1993; 31: 2799–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberts CL, Mshar PA, Cartter ML, et al. The role of heightened surveillance in an outbreak of Escherichia coli O157. H7. Epidemiology and Infection. 1995; 115: 447–454. 10.1017/S095026880005860X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shefer AM, Koo D, Werner SB, et al. A cluster of Escherichia coli O157:H7 infections with the hemolytic-uremic syndrome and death in California. A mandate for improved surveillance. West J Med. 1996; 165: 15–19. [PMC free article] [PubMed] [Google Scholar]
- 107.Cieslak PR, Noble SJ, Maxson DJ, et al. Hamburger-associated Escherichia coli O157:H7 infection in Las Vegas: a hidden epidemic. American Journal of Public Health. 1997; 87: 176–180. 10.2105/AJPH.87.2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torso LM, Voorhees R, Forest SA, et al. Escherichia coli O157:H7 Outbreak Associated with Restaurant Beef Grinding. Journal of Food Protection. 2015; 78: 1272–1279. 10.4315/0362-028X.JFP-14-545 [DOI] [PubMed] [Google Scholar]
- 109.Mead PS, Finelli L, Lambert-Fair MA, et al. Risk factors for sporadic infection with Escherichia coli O157:H7. Archives of Internal Medicine. 1997; 157: 204–208. 10.1001/archinte.1997.00440230076009 [DOI] [PubMed] [Google Scholar]
- 110.Rivas M, Caletti MG, Chinen I, et al. Home-prepared hamburger and sporadic hemolytic uremic syndrome, Argentina. Emerging Infectious Diseases. 2003; 9: 1184–1186. 10.3201/eid0909.020563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection 2010. (in Japanese) Infec Dis Weekly Rep. 2011; 13: 11–19. [Google Scholar]
- 112.Ackers ML, Mahon BE, Leahy E, et al. An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. The Journal of Infectious Diseases. 1998; 177: 1588–1593. 10.1086/515323 [DOI] [PubMed] [Google Scholar]
- 113.Grant J, Wendelboe AM, Wendel A, et al. Spinach-associated Escherichia coli O157:H7 outbreak, Utah and New Mexico, 2006. Emerging Infectious Diseases. 2008; 14: 1633–1636. 10.3201/eid1410.071341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beutin L, Martin A. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. Journal of Food Protection. 2012; 75: 408–418. 10.4315/0362-028X.JFP-11-452 [DOI] [PubMed] [Google Scholar]
- 115.Erickson MC, Doyle MP. Plant food safety issues: linking production agriculture with One Health, In Improving food safety through a One Health approach—workshop summary. Institute of Medicine, National Academies Press, Washington, DC. 2012:140–175. [Google Scholar]
- 116.Solomon EB, Pang HJ, Matthews KR. Persistence of Escherichia coli O157:H7 on lettuce plants following spray irrigation with contaminated water. Journal of Food Protection. 2003; 66: 2198–2202. 10.4315/0362-028X-66.12.2198 [DOI] [PubMed] [Google Scholar]
- 117.Wachtel MR, Whitehand LC, Mandrell R. Association of Escherichia coli O157:H7 with preharvest leaf lettuce upon exposure to contaminated irrigation water. Journal of Food Protection. 2002; 65: 18–25. 10.4315/0362-028X-65.1.18 [DOI] [PubMed] [Google Scholar]
- 118.Portofett A, Hwang C, Call J, et al. Viability of multi-strain mixtures of Listeria monocytogenes, Salmonella typhimurium, or Escherichia coli O157:H7 inoculated into the batter or onto the surface of a soudjouk-style fermented semi-dry sausage. Food Microbiology. 2008; 25: 793–801. 10.1016/j.fm.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 119.Hwang CA, Porto-Fett ACS, Juneja VK, Ingham SC, Ingham BH, Luchansky JB. Modeling the survival of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella Typhimurium during fermentation, drying, and storage of soudjouk-style fermented sausage. International Journal of Food Microbiology. 2009; 129: 244–252. 10.1016/j.ijfoodmicro.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 120.Sartz L, De JONG B, Hjertqvist M, et al. An outbreak of Escherichia coli O157:H7 infection in southern Sweden associated with consumption of fermented sausage; aspects of sausage production that increase the risk of contamination. Epidemiology and Infection. 2008; 136: 370–380. 10.1017/S0950268807008473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection as of April 2002. Infec Agen Surv Rep. 2002; 23: 137–138. [Google Scholar]
- 122.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection as of May 2003. Infec Agen Surv Rep. 2003; 24: 129–130. [Google Scholar]
- 123.Terajima J, Izumiya H, Iyoda S, Tamura K, Watanabe H. Detection of a multi-prefectural E coli O157:H7 outbreak caused by contaminated Ikura-Sushi ingestion. Jpn J Infect Dis. 1999; 52: 52–53. [PubMed] [Google Scholar]
- 124.Strachan NJC, Fenlon DR, Ogden ID. Modelling the vector pathway and infection of humans in an environmental outbreak of Escherichia coli O157. FEMS Microbiology Letters. 2001; 203: 69–73. 10.1111/j.1574-6968.2001.tb10822.x [DOI] [PubMed] [Google Scholar]
- 125.Ogden ID, Hepburn NF, MacRae M, et al. Long-term survival of Escherichia coli O157 on pasture following an outbreak associated with sheep at a scout camp. Letters in Applied Microbiology. 2002; 34: 100–104. 10.1046/j.1472-765x.2002.01052.x [DOI] [PubMed] [Google Scholar]
- 126.Mukherjee A, Cho S, Scheftel J, Jawahir S, Smith K, Diez-Gonzalez F. Soil survival of Escherichia coli O157:H7 acquired by a child from garden soil recently fertilized with cattle manure. Journal of Applied Microbiology. 2006; 101: 429–436. 10.1111/j.1365-2672.2006.02913.x [DOI] [PubMed] [Google Scholar]
- 127.Keen JE, Wittum TE, Dunn JR, Bono JL, Durso LM. Shiga-toxigenic Escherichia coli O157 in agricultural fair livestock, United States. Emerging Infectious Diseases. 2006; 12: 780–786. 10.3201/eid1205.050984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Keen JE, Durso LM, Meehan TP. Isolation of Salmonella enterica and Shiga-toxigenic Escherichia coli O157 from feces of animals in public contact areas of United States zoological parks. Applied and Environmental Microbiology. 2007; 73: 362–365. 10.1128/AEM.01563-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Centers for Disease Control and Prevention (CDC) Notes from the field: Escherichia coli O157:H7 gastroenteritis associated with a State Fair - North Carolina, 2011. MMWR Morb Mortal Wkly Rep. 2012; 60: 1745–1746. [PubMed] [Google Scholar]
- 130.Varma JK, Greene KD, Reller ME, et al. An outbreak of Escherichia coli O157 infection following exposure to a contaminated building. JAMA. 2003; 290: 2709–2712. 10.1001/jama.290.20.2709 [DOI] [PubMed] [Google Scholar]
- 131.Lanier WA, Hall JM, Herlihy RK, et al. Outbreak of Shiga-toxigenic Escherichia coli O157:H7 infections associated with rodeo attendance, Utah and Idaho, 2009. Foodborne Pathogens and Disease. 2011; 8: 1131–1133. 10.1089/fpd.2011.0884 [DOI] [PubMed] [Google Scholar]
- 132.Berry ED, Wells JE, Bono JL, et al. Effect of proximity to a cattle feedlot on Escherichia coli O157:H7 contamination of leafy greens and evaluation of the potential for airborne transmission. Applied and Environmental Microbiology. 2015; 81: 1101–1110. 10.1128/AEM.02998-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chalmers RM, Aird H, Bolton FJ. Waterborne Escherichia coli O157. Journal of Applied Microbiology. 2000; 88 (S1): 124S–132S. 10.1111/j.1365-2672.2000.tb05340.x [DOI] [PubMed] [Google Scholar]
- 134.Muniesa M, Jofre J, García-Aljaro C, Blanch AR. Occurrence of Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environmental Science & Technology. 2006; 40: 7141–7149. 10.1021/es060927k [DOI] [PubMed] [Google Scholar]
- 135.Friedman MS, Roels T, Koehler JE, Feldman L, Bibb WF, Blake P. Escherichia coli O157:H7 outbreak associated with an improperly chlorinated swimming pool. Clinical Infectious Diseases. 1999; 29: 298–303. 10.1086/520204 [DOI] [PubMed] [Google Scholar]
- 136.Samadpour M., Stewart J., Steingart K., Addy C., Louderback J., McGinn M., et al. Laboratory investigation of an E. coli O157:H7 outbreak associated with swimming in Battle Ground Lake, Vancouver, Washington. Journal of environmental health. 2002; 64: 16–20, 26, 25. [PubMed]
- 137.Verma A, Bolton FJ, Fiefield D, et al. An outbreak of E. coli O157 associated with a swimming pool: an unusual vehicle of transmission. Epidemiology and Infection. 2007; 135: 989–992. 10.1017/S0950268807007947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olsen SJ, Miller G, Breuer T, et al. A waterborne outbreak of Escherichia coli O157:H7 infections and hemolytic uremic syndrome: implications for rural water systems. Emerging Infectious Diseases. 2002; 8: 370–375. 10.3201/eid0804.000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bopp DJ, Sauders BD, Waring AL, et al. Detection, isolation, and molecular subtyping of Escherichia coli O157:H7 and Campylobacter jejuni associated with a large waterborne outbreak. Journal of Clinical Microbiology. 2003; 41: 174–180. 10.1128/JCM.41.1.174-180.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hrudey SE, Payment P, Huck PM, Gillham RW, Hrudey EJ. A fatal waterborne disease epidemic in Walkerton, Ontario: comparison with other waterborne outbreaks in the developed world. Water Science and Technology. 2003; 47: 7–14. 10.2166/wst.2003.0146 [DOI] [PubMed] [Google Scholar]
- 141.National Association of State Public Health Veterinarians, Inc. (NASPHV) Centers for Disease Control and Prevention (CDC) Compendium of measures to prevent disease associated with animals in public settings, 2011: National Association of State Public Health Veterinarians, Inc. MMWR Recomm Rep. 2011; 60 (RR-04): 1–24. [PubMed] [Google Scholar]
- 142.Goode B, O’Reilly C, Dunn J, et al. Outbreak of Escherichia coli O157. Archives of Pediatrics & Adolescent Medicine. 2009; 163: 42–48. 10.1001/archpediatrics.2008.525 [DOI] [PubMed] [Google Scholar]
- 143.Waguri A, Sakuraba M, Sawada Y, et al. Enterohemorrhagic Escherichia coli O157 infection presumably caused by contact with infected cows, Aomori Prefecture, Japan. Jpn J Infect Dis. 2007; 60: 321–322. [PubMed] [Google Scholar]
- 144.Ihekweazu C, Carroll K, Adak B, et al. Large outbreak of verocytotoxin-producing Escherichia coli O157 infection in visitors to a petting farm in South East England, 2009. Epidemiology and Infection. 2012; 140: 1400–1413. 10.1017/S0950268811002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rowell S, King C, Jenkins C, et al. An outbreak of Shiga toxin-producing Escherichia coli serogroup O157 linked to a lamb-feeding event. Epidemiology and Infection. 2016; 144: 2494–2500. 10.1017/S0950268816001229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wikswo ME, Hall AJ, Centers for Disease Control and Prevention Outbreaks of acute gastroenteritis transmitted by person-to-person contact--United States, 2009-2010. MMWR Surveill Summ. 2012; 61: 1–12. [PubMed] [Google Scholar]
- 147.Brown JA, Hite DS, Gillim-Ross LA, et al. Outbreak of shiga toxin-producing Escherichia coli serotype O26: H11 infection at a child care center in Colorado. The Pediatric Infectious Disease Journal. 2012; 31: 384–388. 10.1097/INF.0b013e3182457122 [DOI] [PubMed] [Google Scholar]
- 148.Gallagher L, Soyemi K, Conover C, et al. Outbreak of Escherichia coli O157:H7 in a child care center in Cook County, Illinois, with prolonged shedding and household transmission. American Journal of Infection Control. 2013; 41: 936–938. 10.1016/j.ajic.2013.03.312 [DOI] [PubMed] [Google Scholar]
- 149.MacDonald E, Dalane PK, Aavitsland P, Brandal LT, Wester AL, Vold L. Implications of screening and childcare exclusion policies for children with Shiga-toxin producing Escherichia coli infections: lessons learned from an outbreak in a daycare centre, Norway, 2012. BMC Infectious Diseases. 2014; 14: 673. 10.1186/s12879-014-0673-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kanayama A, Yahata Y, Arima Y, et al. Enterohemorrhagic Escherichia coli outbreaks related to childcare facilities in Japan, 2010–2013. BMC Infectious Diseases. 2015; 15: 539. 10.1186/s12879-015-1259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ostroff SM, Kobayashi JM, Lewis JH. Infections with Escherichia coli O157:H7 in Washington State. The first year of statewide disease surveillance. JAMA. 1989; 262: 355–359. 10.1001/jama.1989.03430030043031 [DOI] [PubMed] [Google Scholar]
- 152.Griffin PM. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser MJ SP, Ravdin JI, Greenberg HB, Guerrant RL, editor. Infections of the gastrointestinal tract. New York: Raven Press; 1995. p. 739-761. [Google Scholar]
- 153.Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. Journal of Infectious Diseases. 1985; 151: 775–782. 10.1093/infdis/151.5.775 [DOI] [PubMed] [Google Scholar]
- 154.Pavia AT, Nichols CR, Green DP, et al. Hemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: Clinical and epidemiologic observations. The Journal of Pediatrics. 1990; 116: 544–551. 10.1016/S0022-3476(05)81600-2 [DOI] [PubMed] [Google Scholar]
- 155.Bell BP, Griffin PM, Lozano P, Christie DL, Kobayashi JM, Tarr PI. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. PEDIATRICS. 1997; 100: e12. 10.1542/peds.100.1.e12 [DOI] [PubMed] [Google Scholar]
- 156.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. New England Journal of Medicine. 2000; 342: 1930–1936. 10.1056/NEJM200006293422601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pai CH, Ahmed N, Lior H, Johnson WM, Sims HV, Woods DE. Epidemiology of sporadic diarrhea due to verocytotoxin-producing Escherichia coli: a two-year prospective study. Journal of Infectious Diseases. 1988; 157: 1054–1057. 10.1093/infdis/157.5.1054 [DOI] [PubMed] [Google Scholar]
- 158.Huppertz HI, Busch D, Schmidt H, Aleksic S, Karch H. Diarrhea in young children associated with Escherichia coli non-O157 organisms that produce Shiga-like toxin. The Journal of Pediatrics. 1996; 128: 341–346. 10.1016/S0022-3476(96)70278-0 [DOI] [PubMed] [Google Scholar]
- 159.Hedican EB, Medus C, Besser JM, et al. Characteristics of O157 versus non-O157 Shiga toxin-producing Escherichia coli infections in Minnesota, 2000-2006. Clinical Infectious Diseases. 2009; 49: 358–364. 10.1086/600302 [DOI] [PubMed] [Google Scholar]
- 160.Adams DA, Thomas KR, Jajosky RA, et al. Nationally Notifiable Infectious Conditions Group Summary of Notifiable Infectious Diseases and Conditions — United States, 2014. MMWR. Morbidity and Mortality Weekly Report. 2016; 63: 1–152. 10.15585/mmwr.mm6354a1 [DOI] [PubMed] [Google Scholar]
- 161.Centers for Disease Control and Prevention Foodborne Diseases Active Surveillance Network (FoodNet) FoodNet Surveillance Report for 2014 (Final Report). Atlanta, Georgia: U.S. Department of Health and Human Services, CDC; 2014.
- 162.Tseng M, Sha Q, Rudrik JT, et al. Increasing incidence of non-O157 Shiga toxin-producing Escherichia coli (STEC) in Michigan and association with clinical illness. Epidemiology and Infection. 2016; 144: 1394–1405. 10.1017/S0950268815002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gould LH, Mody RK, Ong KL, et al. Emerging Infections Program Foodnet Working Group Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog Dis. 2013; 10: 453–460. 10.1089/fpd.2012.1401 [DOI] [PubMed] [Google Scholar]
- 164.Manning SD, Madera RT, Schneider W, et al. Surveillance for Shiga toxin-producing Escherichia coli, Michigan, 2001-2005. Emerging Infectious Diseases. 2007; 13: 318–321. 10.3201/eid1302.060813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stigi KA, MacDonald JK, Tellez-Marfin AA, Lofy KH. Laboratory practices and incidence of non-O157 shiga toxin-producing Escherichia coli infections. Emerging Infectious Diseases. 2012; 18: 477–479. 10.3201/eid1803.111358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Government of Canada Canadian National Enteric Pathogen Surveillance System (FoodNet Canada) 2015. Guelph, ON: Public Health Agency of Canada; 2015. [Google Scholar]
- 167.Research Institute of Environmental Science and Notifiable disease N002 - Trend rates by year since 1997 2016 [cited 2016; Available at: http://www.nzpho.org.nz/NotifiableDisease.aspx.
- 168.European Centre for Disease Prevention and Control Annual epidemiological report 2014 –food- and waterborne diseases and zoonoses. Stockholm: ECDC; 2014. [Google Scholar]
- 169.Vally H, Hall G, Dyda A, et al. Epidemiology of Shiga toxin producing Escherichia coli in Australia, 2000-2010. BMC Public Health. 2012; 12: 63. 10.1186/1471-2458-12-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.NNDSS Annual Report Writing Group Australia’s notifiable disease status, 2011: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep. 2013; 37: E313–E393. [DOI] [PubMed] [Google Scholar]
- 171.NNDSS Annual Report Writing Group Australia’s notifiable disease status, 2013: Annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep. 2015; 39: E387–E478. [DOI] [PubMed] [Google Scholar]
- 172.NNDSS Annual Report Writing Group Australia’s notifiable disease status, 2012: Annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep. 2015; 39: E46–E136. [DOI] [PubMed] [Google Scholar]
- 173.NNDSS Annual Report Working Group Australia’s notifiable disease status, 2014: Annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep. 2016; 40: E48–E145. [DOI] [PubMed] [Google Scholar]
- 174.Terajima J, Iyoda S, Ohnishi M, Watanabe H. Shiga Toxin (Verotoxin)-Producing Escherichia coli in Japan. Microbiology Spectrum. 2014; 2. 10.1128/microbiolspec.EHEC-0011-2013 [DOI] [PubMed] [Google Scholar]
- 175.Bielaszewska M, Mellmann A, Bletz S, et al. Enterohemorrhagic Escherichia coli O26:H11/H-: a new virulent clone emerges in Europe. Clinical Infectious Diseases. 2013; 56: 1373–1381. 10.1093/cid/cit055 [DOI] [PubMed] [Google Scholar]
- 176.Germinario C, Caprioli A, Giordano M, et al. Community-wide outbreak of haemolytic uraemic syndrome associated with Shiga toxin 2-producing Escherichia coli O26:H11 in southern Italy, summer 2013. Eurosurveillance. 2016; 21: 30343 10.2807/1560-7917.ES.2016.21.38.30343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2001. Infec Agen Surv Rep. 2001; 22: 135–136. [Google Scholar]
- 178.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2016. Infec Agen Surv Rep. 2016; 37: 85–86. [Google Scholar]
- 179.Watanabe H, Terajima J, Izumiya H, Iyoda S, Tamura K. [PulseNet Japan: surveillance system for the early detection of diffuse outbreak based on the molecular epidemiological method]. Journal of the Japanese Association for Infectious Diseases. 2002; 76: 842–848. 10.11150/kansenshogakuzasshi1970.76.842 [DOI] [PubMed] [Google Scholar]
- 180.Swaminathan B, Gerner-Smidt P, Ng LK, et al. Building PulseNet International: an interconnected system of laboratory networks to facilitate timely public health recognition and response to foodborne disease outbreaks and emerging foodborne diseases. Foodborne Pathogens and Disease. 2006; 3: 36–50. 10.1089/fpd.2006.3.36 [DOI] [PubMed] [Google Scholar]
- 181.Terajima J, Izumiya H, Iyoda S, Mitobe J, Miura M, Watanabe H. Effectiveness of pulsed-field gel electrophoresis for the early detection of diffuse outbreaks due to Shiga toxin-producing Escherichia coli in Japan. Foodborne Pathogens and Disease. 2006; 3: 68–73. 10.1089/fpd.2006.3.68 [DOI] [PubMed] [Google Scholar]
- 182.Centers for Disease Control and Prevention (CDC) Escherichia coli O157:H7 infections associated with ground beef from a U.S. military installation--Okinawa, Japan, February 2004. MMWR Morb Mortal Wkly Rep. 2005; 54: 40–42. [PubMed] [Google Scholar]
- 183.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection as of May 2004, Japan. Infec Agen Surv Rep. 2004; 25: 138–139. [Google Scholar]
- 184.Kato K, Shimoura R, Nashimura K, et al. Outbreak of enterohemorrhagic Escherichia coli O111 among high school participants in excursion to Korea. Jpn J Infect Dis. 2005; 58: 332–333. [PubMed] [Google Scholar]
- 185.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection in Japan as of April 2008. Infec Agen Surv Rep. 2008; 29: 117–118. [Google Scholar]
- 186.Yahata Y, Misaki T, Ishida Y, et al. E. coli O111 Outbreak Investigation Team Epidemiological analysis of a large enterohaemorrhagic Escherichia coli O111 outbreak in Japan associated with haemolytic uraemic syndrome and acute encephalopathy. Epidemiology and Infection. 2015; 143: 2721–2732. 10.1017/S0950268814003641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Siegler RL. The hemolytic uremic syndrome. Pediatric Clinics of North America. 1995; 42: 1505–1529. 10.1016/S0031-3955(16)40096-9 [DOI] [PubMed] [Google Scholar]
- 188.Gould LH, Demma L, Jones TF, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000-2006. Clinical Infectious Diseases. 2009; 49: 1480–1485. 10.1086/644621 [DOI] [PubMed] [Google Scholar]
- 189.Rivas M, Chinen I, Miliwebsky E, Masana M. Risk Factors for Shiga Toxin-Producing Escherichia coli-Associated Human Diseases. Microbiology Spectrum. 2014; 2. 10.1128/microbiolspec.EHEC-0002-2013 [DOI] [PubMed] [Google Scholar]
- 190.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection 2006, 2007. Infec Dis Weekly Rep. 2009; 11: 16–22. Japanese. [Google Scholar]
- 191.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Reported cases of hemorrhagic uremic syndrome associated with EHEC infection in 2008—NESID. Infec Dis Weekly Rep. 2009; 30: 122–123. Japanese. [Google Scholar]
- 192.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection 2009. Infec Dis Weekly Rep. 2010; 12: 11–19. Japanese. [Google Scholar]
- 193.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Enterohemorrhagic Escherichia coli infection 2011. Infec Dis Weekly Rep. 2012; 14: 9–19. Japanese. [Google Scholar]
- 194.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Hemolytic uremic syndrome that appeared in NESID: trends in 2012. Infec Agen Surv Rep. 2013; 34: 140–141. [Google Scholar]
- 195.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Hemolytic uremic syndrome among EHEC patients in Japan, 2013. Infec Agen Surv Rep. 2014; 35: 130–132. Japanese. [Google Scholar]
- 196.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Hemolytic uremic syndrome among EHEC patients in Japan, 2014. Infec Agen Surv Rep. 2015; 36: 84–86. Japanese. [Google Scholar]
- 197.National Institute of Infectious Diseases and Tuberculosis and Infectious Diseases Control Division Ministry of Health, Labour and Welfare Hemolytic uremic syndrome among EHEC patients in Japan, 2015. Infec Agen Surv Rep. 2016; 37: 97–98. Japanese. [Google Scholar]
- 198.Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. New England Journal of Medicine. 1995; 333: 364–368. 10.1056/NEJM199508103330608 [DOI] [PubMed] [Google Scholar]
- 199.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005; 365: 1073–1086. [DOI] [PubMed] [Google Scholar]
- 200.Hancock DD, Besser TE, Rice DH. Ecology of Escherichia coli O157:H7 in cattle and impact of management practices. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E coli strains. Washington, DC: ASM Press; 1998. p. 85-91. [Google Scholar]
- 201.U.S. Department of Agriculture Food Safety and Inspection Service Compliance guideline for validating cooking instructions for mechanically tenderized beef products. In: Agriculture Do, editor.; 2013.
- 202.Gill CO, Devos J, Badoni M, Yang X. Inactivation of Escherichia coli O157:H7 in Beef Roasts Cooked in Conventional or Convection Ovens or in a Slow Cooker under Selected Conditions. Journal of Food Protection. 2016; 79: 205–212. 10.4315/0362-028X.JFP-15-116 [DOI] [PubMed] [Google Scholar]
- 203.Thayer DW, Boyd G. Elimination of Escherichia coli O157:H7 in meats by gamma irradiation. Appl Environ Microbiol. 1993; 59: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thayer DW, Rajkowski KT, Boyd G, Cooke PH, Soroka DS. Inactivation of Escherichia coli O157:H7 and Salmonella by gamma irradiation of alfalfa seed intended for production of food sprouts. Journal of Food Protection. 2003; 66: 175–181. 10.4315/0362-028X-66.2.175 [DOI] [PubMed] [Google Scholar]
- 205.Rezende ACB, Igarashi MC, Destro MT, Franco BDGM, Landgraf M. Effect of gamma radiation on the reduction of Salmonella strains, Listeria monocytogenes, and Shiga toxin-producing Escherichia coli and sensory evaluation of minimally processed spinach (Tetragonia expansa). Journal of Food Protection. 2014; 77: 1768–1772. 10.4315/0362-028X.JFP-14-108 [DOI] [PubMed] [Google Scholar]
- 206.Sommers C, Rajkowski KT, Scullen OJ, Cassidy J, Fratamico P, Sheen S. Inactivation of Shiga Toxin-Producing Escherichia coli in lean ground beef by gamma irradiation. Food Microbiology. 2015; 49: 231–234. 10.1016/j.fm.2015.02.013 [DOI] [PubMed] [Google Scholar]
- 207.Shah S, Hoffman R, Shillam P, Wilson B. Prolonged fecal shedding of Escherichia coli O157:H7 during an outbreak at a day care center. Clinical Infectious Diseases. 1996; 23: 835–836. 10.1093/clinids/23.4.835 [DOI] [PubMed] [Google Scholar]
- 208.Wahl E, Vold L, Lindstedt BA, Bruheim T, Afset JE. Investigation of an Escherichia coli O145 outbreak in a child day-care centre - extensive sampling and characterization of eae- and stx 1-positive E. coli yields epidemiological and socioeconomic insight. BMC Infectious Diseases. 2011; 11: 238. 10.1186/1471-2334-11-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dabke G, Le Menach A, Black A, et al. Duration of shedding of Verocytotoxin-producing Escherichia coli in children and risk of transmission in childcare facilities in England. Epidemiology and Infection. 2014; 142: 327–334. 10.1017/S095026881300109X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Snedeker KG, Campbell M, Sargeant JM. A systematic review of vaccinations to reduce the shedding of Escherichia coli O157 in the faeces of domestic ruminants. Zoonoses and Public Health. 2012; 59: 126–138. 10.1111/j.1863-2378.2011.01426.x [DOI] [PubMed] [Google Scholar]
- 211.Varela NP, Dick P, Wilson J. Assessing the existing information on the efficacy of bovine vaccination against Escherichia coli O157:H7--a systematic review and meta-analysis. Zoonoses and Public Health. 2013; 60: 253–268. 10.1111/j.1863-2378.2012.01523.x [DOI] [PubMed] [Google Scholar]
- 212.Vogstad AR, Moxley RA, Erickson GE, Klopfenstein TJ, Smith DR. Assessment of heterogeneity of efficacy of a three-dose regimen of a type III secreted protein vaccine for reducing STEC O157 in feces of feedlot cattle. Foodborne Pathogens and Disease. 2013; 10: 678–683. 10.1089/fpd.2012.1374 [DOI] [PubMed] [Google Scholar]
- 213.Vogstad AR, Moxley RA, Erickson GE, Klopfenstein TJ, Smith DR. Stochastic simulation model comparing distributions of STEC O157 faecal shedding prevalence between cattle vaccinated with type III secreted protein vaccines and non-vaccinated cattle. Zoonoses and Public Health. 2014; 61: 283–289. 10.1111/zph.12069 [DOI] [PubMed] [Google Scholar]
- 214.Barrett TJ, Lior H, Green JH, et al. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J Clin Microbiol. 1994; 32: 3013–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.U.S. Department of Health and Human Services. Food Code 1997. 1997;section 3-401.11.
- 216.U.S. Department of Agriculture Food Safety and Inspection Service. FSIS Policy on Nonintact Raw Beef Products Contaminated with E. coli O157:H7. 1999.
- 217.U.S. Department of Agriculture Food Safety and Inspection Service Risk Profile for Pathogenic Non-O157 Shiga Toxin-Producing Escherichia coli (non-O157 STEC). In: Agriculture Do, editor.; 2012. [Google Scholar]
- 218.U.S. Department of Agriculture Food Safety and Inspection Service Shiga toxin-producing Escherichia coli in certain raw beef products. Federal Register. 2011; 76: 58157–58165. [Google Scholar]
- 219.Karmali MA, Mascarenhas M, Shen S, et al. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. Journal of Clinical Microbiology. 2003; 41: 4930–4940. 10.1128/JCM.41.11.4930-4940.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.European Food Safety Authority Panel on Biological Hazards (BIOHAZ) Scientific Opinion of the Panel on Biological Hazards on a request from EFSA on monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human pathogenic VTEC types. EFSA Journal. 2007; 579: 1–61. [Google Scholar]
- 221.Technical specifications for the monitoring and reporting of verotoxigenic Escherichia coli (VTEC) on animals and food (VTEC surveys on animals and food). EFSA Journal. 2009; 7: 1366–1408. 10.2903/j.efsa.2009.1366 [DOI] [Google Scholar]
- 222.Scientific Opinion on VTEC-seropathotype and scientific criteria regarding pathogenicity assessment. EFSA Journal. 2013; 11: 3138–3243. 10.2903/j.efsa.2013.3138 [DOI] [Google Scholar]
- 223.European Food Safety Authority Panel on Biological Hazards (BIOHAZ) Scientific Opinion on the risk posed by Shiga toxinproducing Escherichia coli (STEC) and other pathogenic bacteria in seeds and sprouted seeds. EFSA Journal. 2011; 9: 2424–2524. 10.2903/j.efsa.2011.2424 [DOI] [Google Scholar]
- 224.THE EUROPEAN COMMISSION. COMMISSION REGULATION (EU) No 209/2013 of 11 March 2013. Official Journal of the European Union. 2013;12.3:L 68/19-23.
- 225.Ministry of Health Labour and Welfare. Notification No. 1217 (17 December 2012). 2012.
- 226.Ministry of Health Labour and Welfare. Notification No. 1120 (20 November 2014). 2014.
- 227.Hara-Kudo Y, Konishi N, Ohtsuka K, et al. An interlaboratory study on efficient detection of Shiga toxin-producing Escherichia coli O26, O103, O111, O121, O145, and O157 in food using real-time PCR assay and chromogenic agar. International Journal of Food Microbiology. 2016; 230: 81–88. 10.1016/j.ijfoodmicro.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 228.U.S. Department of Agriculture. Laboratory guidebook MLG 5B appendix1.01. Primer and probe sequences and reagent concentrations for non-O157 Shiga toxin-producing Escherichia coli (STEC) real-time PCR assay. 2012.