Abstract
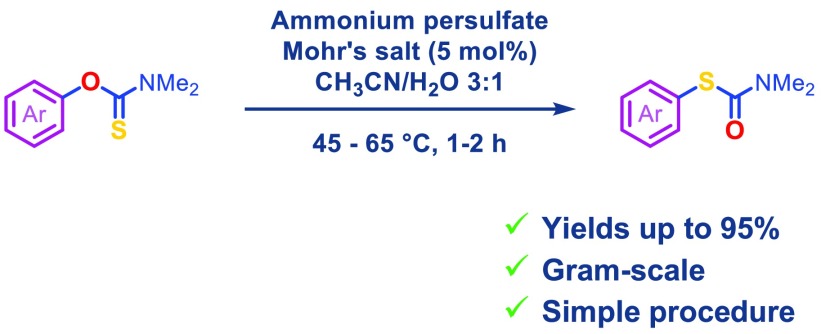
Herein, we report that iron(II)/ammonium persulfate in aqueous acetonitrile mediates the Newman–Kwart rearrangement of O-aryl carbamothioates. Electron-rich substrates react rapidly under moderate heating to afford the rearranged products in excellent yields. The mild conditions, rapid reaction rates, and suitability for scale up offers immediate practical benefits to access functionalized thiophenols.
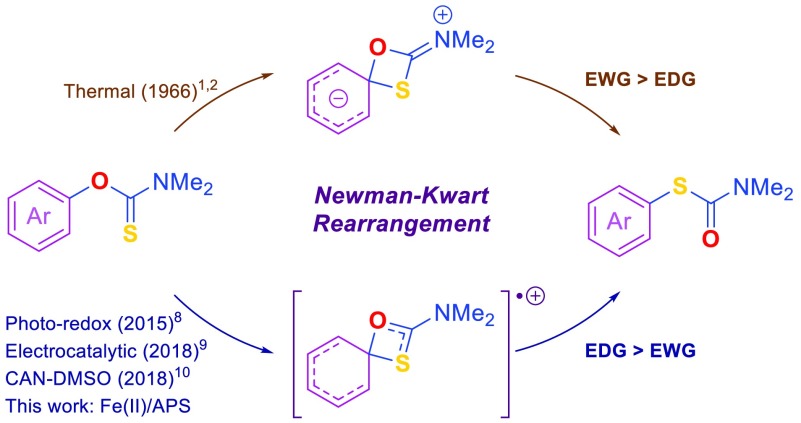
The Newman–Kwart rearrangement (NKR)—the transformation of O-aryl carbamothioates to the corresponding S-aryl carbamothioates—gives access to thiophenols from their more readily available phenol counterparts.1,2 The three-step sequence, which involves phenol protection with thiocarbamoyl chloride, NKR, and deprotection of the resulting carbamothioate, is appealing, as it avoids the need for highly reactive reagents or handling of foul-smelling chemicals. The NKR is therefore a synthetically important reaction with widespread applications.3−6 The high activation barrier (ca. 35–43 kcal·mol–1)7 of the reaction has been a long-standing limitation, as thermal activation requires temperatures of 150 °C for electron-deficient substrates to >300 °C for nonactivated arenes (Figure 1).7 At such high temperatures, compound volatility, decomposition, and charring become problematic. In practice, the thermal reaction is therefore limited to activated, thermally stable, and nonvolatile substrates. Renewed interest in the NKR has led to the discovery of several catalytic systems that favor electron-rich substrates, including a photoredox catalytic system8 and, very recently, an electrochemical method,9 as well as a chemical reaction involving single-electron oxidation of O-aryl carbamothioates with ceric ammonium nitrate (CAN) in dimethyl sulfoxide (DMSO).10 The latter method makes the NKR with electron-rich substrates widely accessible, as it overcomes the need for specialist equipment. However, the use of DMSO as the solvent, and the need for high substrate dilution, practically limits applications to small-scale reactions.
Figure 1.
Newman–Kwart Rearrangement (NKR). Abbreviation: APS, ammonium persulfate; EWG, electron-withdrawing groups; EDG, electron-donating groups.
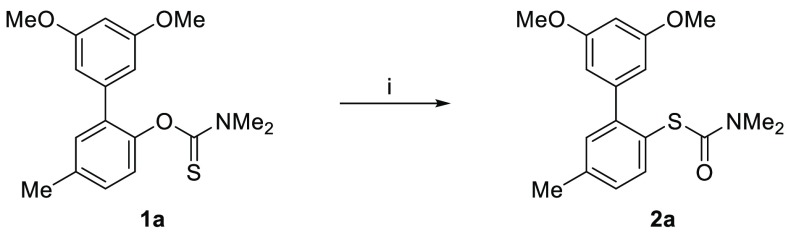
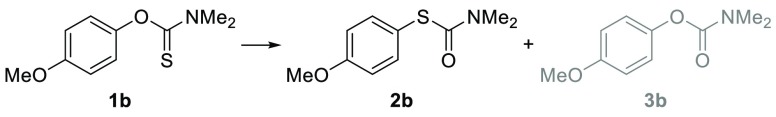
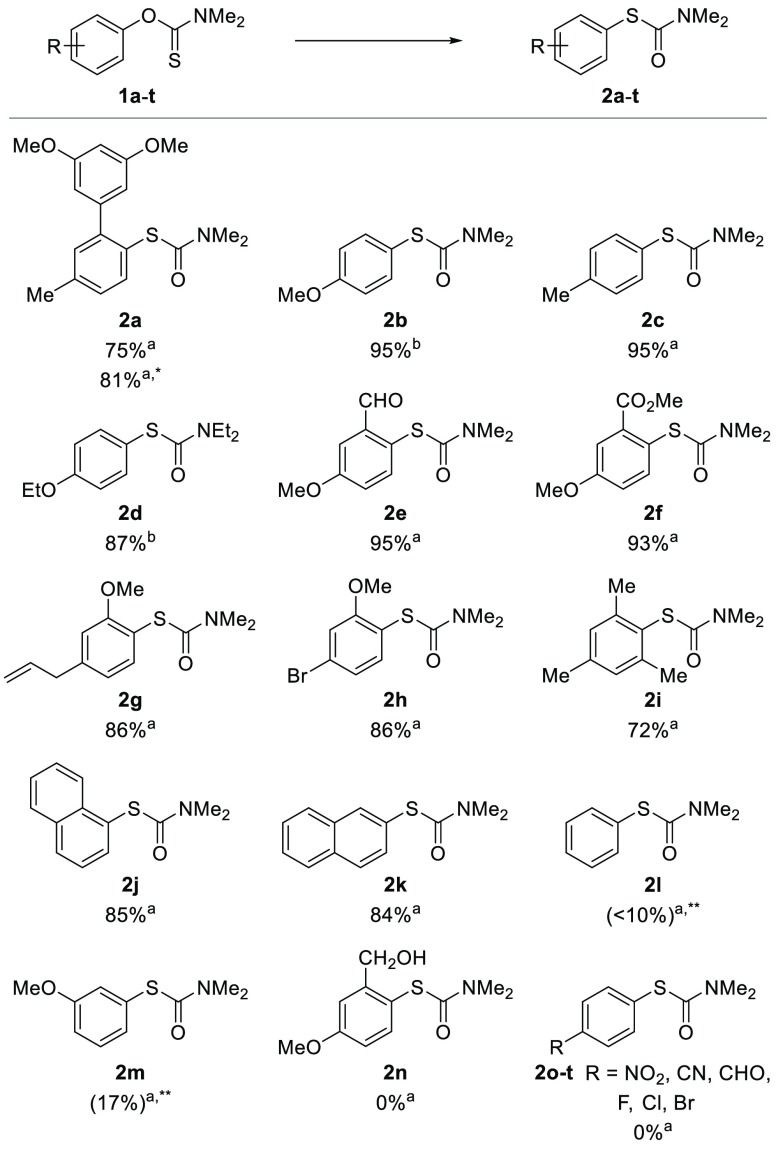
As a part of our ongoing research program, we needed a robust scalable method to access S-(3′,5′-dimethoxy-5-methyl-[1,1′-biphenyl]-2-yl) dimethylcarbamothioate 2a from the corresponding O-aryl carbamothioate 1a (Scheme 1).11 Although successful on small scale, the thermal NKR proved operationally challenging to scale to multigram quantities, as inconsistent heating resulted in variable yields. Attempted Pd-catalyzed NKR only afforded trace amounts of product 2a in agreement with the previously reported scope.12 Inspired by the work of Anderson and Kochi on radical decarboxylation of carboxylic acids,13 we attempted to use silver nitrate and ammonium persulfate (APS) as a single electron oxidant to mediate this transformation. Gratifyingly, under these conditions (35 mol % AgNO3, 1 equiv of APS, CH3CN/H2O, 85 °C) 1a rearranged to target product 2a in 78% yield (Scheme 1). In a bid to develop a practical and scalable method, we investigated the effect of the different reagents and reaction parameters using O-(4-methoxyphenyl) dimethylcarbamothioate 1b as a model compound (Table 1). When subjected to the aforementioned conditions, 1b was fully converted to 2b (Table 1, entry 1). Control experiments proved that APS is essential for the reaction to proceed (Table 1, entry 2). In the absence of silver, we observed large variations in yields depending on the source of APS. Subsequent analysis by inductively coupled plasma mass spectrometry (ICP-MS) revealed high levels of iron in the batch of APS that most effectively mediated transformation of 1b to 2b (see Supporting Information). As iron is known to accelerate the decomposition of APS in a similar manner to silver,13,14 we hypothesized that the iron impurity played a key role in the reaction. Gratifyingly, replacing silver nitrate with catalytic amounts (5 mol %) of Mohr’s salt ((NH4)2Fe(SO4)2·6H2O) afforded 2b in 95% yield (Table 1, entry 4). Lowering the reaction temperature from 85 to 45 °C still gave full conversion within 1 h and afforded 2b in 91% isolated yield (Table 1, entry 5). As described in the previously reported non-thermal NKR protocols,8−10 the reaction proved less efficient at high concentrations. At a concentration up to 0.17 M (Table 1, entry 7), the rate of transformation appeared to be unaffected; however, at 0.25 M the yield dropped to 10% under otherwise identical conditions (Table 1, entry 6). Finally, the use of water as cosolvent proved crucial for the formation of the target product. Indeed, when acetonitrile was used as the sole reaction solvent, starting material 1b was converted quantitatively to the corresponding carbamate 3b (Table 1, entry 8).
Scheme 1. Preliminary Results.
Conditions: silver nitrate (35 mol %), ammonium persulfate (1.3 equiv), CH3CN/H2O 3:1, 85 °C, 90 min, 78% yield.
Table 1. Optimization of the Novel NKR Protocol.
| no. | equiv of APS | metal (mol %) | solvents | temp (°C) | concn (M) | conv (%)a |
|---|---|---|---|---|---|---|
| 1 | 1.3 | Ag (35)b | CH3CN/H2O 3:1 | 85 | 0.083 | >95 |
| 2 | 0 | Ag (35)b | CH3CN/H2O 3:1 | 85 | 0.083 | <5 |
| 3 | 1 | – | CH3CN/H2O 3:1 | 85 | 0.083 | 10–95d |
| 4 | 1 | Fe (5)c | CH3CN/H2O 3:1 | 85 | 0.083 | >95 |
| 5 | 1 | Fe (5)c | CH3CN/H2O 3:1 | 45 | 0.083 | >95 (91) |
| 6 | 1 | Fe (5)c | CH3CN/H2O 3:1 | 45 | 0.25 | 10 |
| 7 | 1 | Fe (5)c | CH3CN/H2O 3:1 | 45 | 0.17 | >95 |
| 8 | 1 | Fe (5)c | CH3CN | 65 | 0.083 | >95e (3b 84%) |
As determined by 1H NMR, isolated yields are given in brackets.
As silver nitrate.
As Mohr’s salt.
Depending on the source of APS.
Reaction was heated for 4 h, conversion to 3b.
With optimized conditions in hand (5 mol % Mohr’s salt, 1 equiv of APS, CH3CN/H2O 3:1), we explored the scope of this novel NKR reaction (Figure 2). Substrates substituted with electron-donating groups (EDG) in the para-position afforded rearranged products 2a–f in nearly quantitative yields. Additional electron-withdrawing groups (EWG) were well tolerated, as exemplified with the formation of aldehyde and ester substituted products 2e and 2f in 95% and 93% yields, respectively. Steric hindrance had little-to-no influence on the rearrangement, as ortho-substituted products 2a and 2f–i were obtained in good-to-excellent yields. The reaction displayed good functional group tolerance, as aldehyde, ester, allyl, and bromo substituents in products 2e–h remained intact through the procedure; notably, oxidation of aldehyde 2e was not observed. However, rearrangement of benzylic alcohol 2n was problematic, as oxidation of the alcohol resulted in the formation of a complex mixture of products. S-(Naphthalene-1-yl) dimethyl-carbamothioate 2j and its 2-regioisomer 2k were obtained in 85% and 84% isolated yields, respectively. This result is of note, as the CAN10 and photoredox8 methods allow access to the 1-napthalene but not the 2-napthalene derivative. Formation of electron-neutral 2l and moderately electron-deficient meta-methoxy substituted 2m was observed, albeit in moderate conversions (<10% and 17%, respectively). Attempted reactions with electron-deficient substrates proved troublesome; nitro- 1o, nitrile- 1p, aldehyde- 1q, and halide- 1r–t substituted O-aryl carbamothioate failed to rearrange. In most cases, NMR analysis of the reaction mixture showed that the starting materials were transformed to the corresponding O-aryl carbamates instead of the expected S-aryl carbamothioate (see Supporting Information, Figures S5 and S6). Formation of carbamates has previously been reported for the CAN-DMSO mediated NKR reaction.10
Figure 2.
Scope study. Conditions: a1 (1 mmol), Mohr’s salt (5 mol %), APS (1 equiv), CH3CN/H2O 3:1, 65 °C, 2 h; b1 (1 mmol), Mohr’s salt (5 mol %), APS (1 equiv), CH3CN/H2O 3:1, 45 °C, 1 h. *Scale-up to 10 mmol. **Conversion determined by 1H NMR.
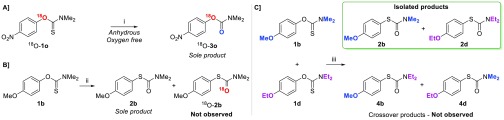
To gain a better understanding of this side reaction, isotopically labeled [18O]O-aryl carbamothioate 18O-2o was subjected to the reaction conditions with strict exclusion of water and oxygen (Scheme 2A). Carbamate 18O-3j was isolated in 60% yield. Tandem mass spectrometry (MS/MS) confirmed the position of the [18O]oxygen on the molecule as shown on Scheme 2A (see Supporting Information). In the absence of any other source of oxygen, this demonstrates that the extra oxygen added on the carbamate is likely to come from the persulfate. Furthermore, subjecting 1b to the standard reaction conditions while replacing H2O with [18O]H2O did not lead to any isotopic exchange on the rearranged product, thus suggesting that water is not actively participating in the reaction (Scheme 2B). Overall, the results of this scope study are in line with the work previously published on oxidative NKR: the reaction proceeded rapidly with electron-rich ring systems and nonactivated systems reacted more sluggishly, while electron-deficient substrates failed to react, or underwent a side-reaction to give the corresponding O-carbamates.
Scheme 2. Isotopic Labeling Experiments (A, B) and Crossover Experiment (C).
Conditions: (i) Mohr’s salt (5 mol %), APS (2 equiv), anhydrous degassed CH3CN, 65 °C, 3 h; (ii) Mohr’s salt (5 mol %), APS (1 equiv), CH3CN/[18O]H2O 3:1, 45 °C, 1 h; (iii) Mohr’s salt (5 mol %), APS (1 equiv), CH3CN/H2O 3:1, 45 °C, 1 h.
To elucidate the rearrangement mechanism itself, we first focused our attention on the reaction kinetics. 1H NMR reaction monitoring of the para-methoxy derivative 1b led to a sigmoidal kinetic profile (see Supporting Information, Figure S8). After an induction period of about 35 min, 1b was quantitatively rearranged to product 2b within 20 min on a 0.5 mmol scale (zero-order linear approximation k ≈ 5 mmol·L–1·min–1). Although not uncommon, sigmoidal kinetic profiles are difficult to interpret; unravelling which mechanisms are responsible for the induction period and then for reaction lift-off is challenging and outside of the scope of the present study. We subsequently investigated whether the reaction was inter- or intramolecular through a crossover experiment between the para-methoxy derivative 1b and its ethyl analogue 1d (Scheme 2C). Should the reaction be intermolecular, an interchange of substituents would occur, giving rise to crossover rearranged products 4b and 4d. NMR analysis of the crude reaction mixture showed exclusive formation of the two noncrossover rearranged products 2b and 2d, in equal amounts. The absence of crossover products confirms that the reaction proceeds through an intramolecular mechanism.
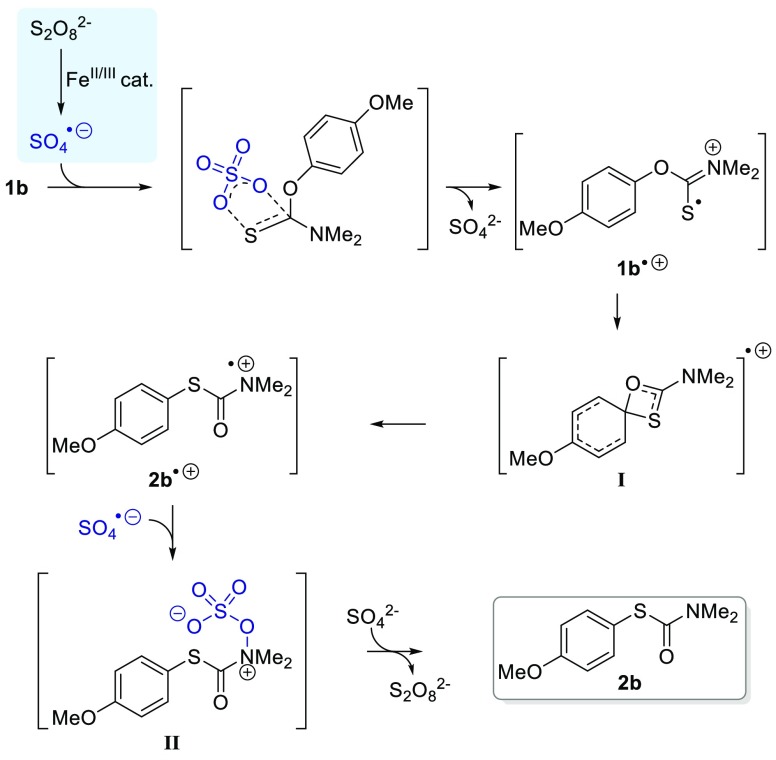
The observed reactivity points to a radical-cation transition state as reported for other nonthermal NKR.8−10 Indeed, iron(II)/(III) salts and oxides are known to decompose aqueous APS to sulfate radical anions SO4–• in a Fenton-like process.15 Consistent with this, a dark orange-brown residue was observed in the product mixtures. Blocking of the reaction with 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO), an established radical trap, provided further evidence of a radical reaction mechanism. On the basis of these observations, we propose the mechanism depicted in Scheme 3. A Fenton-like process generates sulfate radical anion SO4–• (Scheme 3, blue box), which, in turn, reacts by abstracting an electron from the sulfur atom in 1b, forming radical cation 1b+•. Subsequent intramolecular (vide supra) rearrangement of 1b+• leads to the formation of a putative four-center intermediate I, as previously described.16 Heterolytic cleavage of the OAryl bond gives radical cation 2b+•, which after single-electron reduction affords product 2b. The exact nature of the reduction step is unclear; 2b+• could potentially abstract an electron from 1b. However, experimental observations suggest that 1b alone cannot sustain a radical chain reaction. It is therefore more likely that single electron reduction is mediated by the persulfate system, possibly by combination of the sulfate radical anion SO4–• with 2b+• to give intermediate II. Nucleophilic attack by sulfate would then liberate the product 2b and regenerate the peroxide.17 The high reactivity of the APS/Fe(II) system may reflect the ability of sulfate to stabilize single electron transfer through cyclic transition states.18
Scheme 3. Proposed Mechanism.
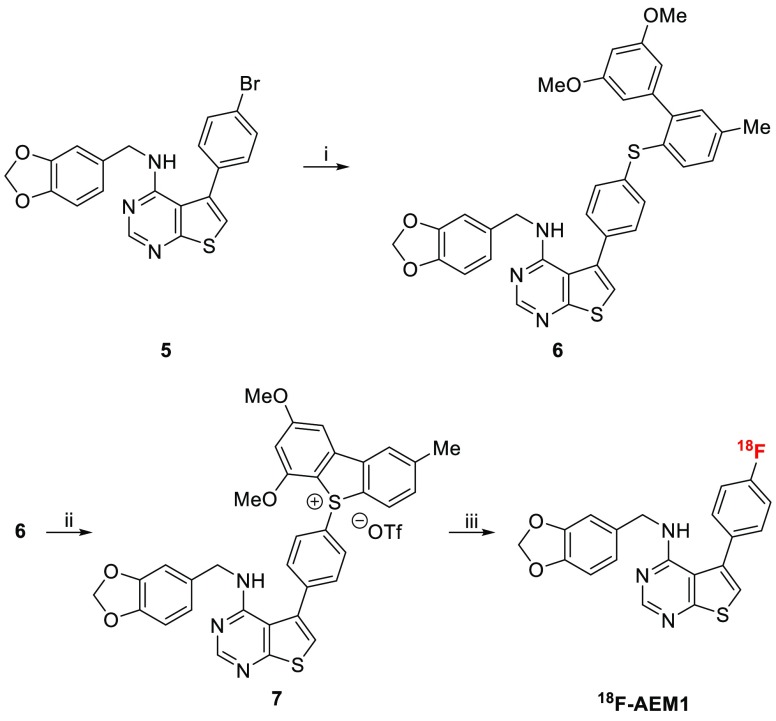
Finally, we employed the novel strategy for the synthesis of 18F-AEM1,19 a putative radiotracer for imaging of cancer drug resistance with positron emission tomography (Scheme 4). On a 3 g scale (10 mmol), 1a rearranged to give biaryl building block 2a in 81% yield. Coupling with the aryl bromide 5 gave the corresponding biaryl thioether 6 in 56% yield, which upon treatment with aqueous calcium hypochlorite11 afforded the dibenzothiophene sulfonium salt 7 in 72% yield. Labeling with [18F]fluoride (2.5 mg, DMSO, 125 °C, 25 min) under nonoptimized conditions afforded 18F-AEM1 in 15 ± 4% (n = 4) decay-corrected radiochemical yield (d.c. RCY).
Scheme 4. Application to the Labeling of 18F-AEM1.
Conditions: (i) 2a, tBuOK, Pd2(dba)3, DPEPhos, toluene, reflux, 56% yield; (ii) Ca(OCl)2, acetate buffer pH 4, acetonitrile, 3 °C, 15 min, 72% yield; (iii) 18F–, K222/KHCO3, DMSO, 125 °C, 25 min, 15 ± 4% d.c. RCY (n = 4).
Very recently, Ritter and Alcarazo independently reported late-stage, site-selective aromatic C–H insertion of aryl dibenzothiophenium salts.20,21 Although synthetically more demanding, the ring-closing route exemplified with 2a above is highly complementary in that it gives access to complex heteroatom-rich molecules such as 18F-AEM1 and allows the point of functionalization to be chosen at will.
In conclusion, we report that catalytic amounts of Fe(II) in the presence of APS mediates conversion of electron-rich and electron-neutral O-aryl carbamothioates to the corresponding S-aryl carbamothioates under mild conditions. The reaction has a similar scope to the previously reported methods for cation-radical mediated NKR, but offers clear practical advantages in that it circumvents the need for specialist equipment and proceeds with shorter reaction times and at higher substrate concentration, and the use of a volatile solvent makes it well suited for scale up. The practicability of the APS/Fe(II) system may prove beneficial for radical-driven reactions beyond the NKR.
Acknowledgments
We thank Dr Laure Benhamou (Department of Chemistry, University College London) and Dr. Julie Charpentier (Givaudan) for helpful discussions. We thank the mass spectrometry facility at King’s College London for assistance with the HRMS, ICP-MS and MS/MS experiments. The research leading to these results was funded by the European Union’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No. 602102 (EPITARGET) (E.A.), a Wellcome Trust and Royal Society Sir Henry Dale Fellowship (107610/Z/15/Z) to (T.H.W.), CRUK & EPSRC Comprehensive Cancer Imaging Centre at KCL, UCL & Imperial jointly funded by Cancer Research UK and the Engineering and Physical Sciences Research Council (EPSRC; C1519/A16463; C2536/A10337) (T.G.), and the UCL MSci in Chemistry (H.Y.A.). This work was undertaken at UCLH/UCL, which is funded in part by the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.9b04280.
Experimental procedures, spectral and analytical data (PDF)
Author Contributions
⊥ T.G. and R.P. contributed equally.
Author Contributions
*T.W. and E.A. jointly supervised this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Kwart H.; Evans E. R. The Vapor Phase Rearrangement of Thioncarbonates and Thioncarbamates. J. Org. Chem. 1966, 31 (2), 410–413. 10.1021/jo01340a015. [DOI] [Google Scholar]
- Newman M. S.; Karnes H. A. The Conversion of Phenols to Thiophenols via Dialkylthiocarbamates. J. Org. Chem. 1966, 31 (12), 3980–3984. 10.1021/jo01350a023. [DOI] [Google Scholar]
- Porter D. W.; Bradley M.; Brown Z.; Canova R.; Charlton S.; Cox B.; Hunt P.; Kolarik D.; Lewis S.; O’Connor D.; et al. The Discovery of Potent, Orally Bioavailable Pyrazolo and Triazolopyrimidine CXCR2 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2014, 24 (1), 72–76. 10.1016/j.bmcl.2013.11.074. [DOI] [PubMed] [Google Scholar]
- Sørensen A.; Rasmussen B.; Agarwal S.; Schau-Magnussen M.; Sølling T. I.; Pittelkow M. Conversion of Phenols into Selenophenols: Seleno Newman-Kwart Rearrangement. Angew. Chem., Int. Ed. 2013, 52 (47), 12346–12349. 10.1002/anie.201303773. [DOI] [PubMed] [Google Scholar]
- Otto S. Selection and Amplification of Hosts From Dynamic Combinatorial Libraries of Macrocyclic Disulfides. Science 2002, 297 (5581), 590–593. 10.1126/science.1072361. [DOI] [PubMed] [Google Scholar]
- Błaszczyk A.; Chadim M.; von Hänisch C.; Mayor M. Synthesis of Macrocyclic Molecular Rods as Potential Electronic Devices. Eur. J. Org. Chem. 2006, 2006, 3809–3825. 10.1002/ejoc.200600336. [DOI] [Google Scholar]
- Lloyd-Jones G.; Moseley J.; Renny J. Mechanism and Application of the Newman-Kwart O→S Rearrangement of O-Aryl Thiocarbamates. Synthesis 2008, 2008 (5), 661–689. 10.1055/s-2008-1032179. [DOI] [Google Scholar]
- Perkowski A. J.; Cruz C. L.; Nicewicz D. A. Ambient-Temperature Newman–Kwart Rearrangement Mediated by Organic Photoredox Catalysis. J. Am. Chem. Soc. 2015, 137 (50), 15684–15687. 10.1021/jacs.5b11800. [DOI] [PubMed] [Google Scholar]
- Broese T.; Roesel A. F.; Prudlik A.; Francke R. An Electrocatalytic Newman–Kwart-Type Rearrangement. Org. Lett. 2018, 20 (23), 7483–7487. 10.1021/acs.orglett.8b03257. [DOI] [PubMed] [Google Scholar]
- Pedersen S. K.; Ulfkjær A.; Newman M. N.; Yogarasa S.; Petersen A. U.; Sølling T. I.; Pittelkow M. Inverting the Selectivity of the Newman–Kwart Rearrangement via One Electron Oxidation at Room Temperature. J. Org. Chem. 2018, 83 (19), 12000–12006. 10.1021/acs.joc.8b01800. [DOI] [PubMed] [Google Scholar]
- Gendron T.; Sander K.; Cybulska K.; Benhamou L.; Sin P. K. B.; Khan A.; Wood M.; Porter M. J.; Årstad E. Ring-Closing Synthesis of Dibenzothiophene Sulfonium Salts and Their Use as Leaving Groups for Aromatic 18 F-Fluorination. J. Am. Chem. Soc. 2018, 140 (35), 11125–11132. 10.1021/jacs.8b06730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. N.; Jover J.; Lloyd-Jones G. C.; Moseley J. D.; Murray P.; Renny J. S. The Newman-Kwart Rearrangement of O-Aryl Thiocarbamates: Substantial Reduction in Reaction Temperatures through Palladium Catalysis. Angew. Chem., Int. Ed. 2009, 48 (41), 7612–7615. 10.1002/anie.200903908. [DOI] [PubMed] [Google Scholar]
- Anderson J. M.; Kochi J. K. Silver(I)-Catalyzed Oxidative Decarboxylation of Acids by Peroxydisulfate. Role of Silver(II). J. Am. Chem. Soc. 1970, 92 (6), 1651–1659. 10.1021/ja00709a039. [DOI] [Google Scholar]
- House D. A. Kinetics and Mechanism of Oxidations by Peroxydisulfate. Chem. Rev. 1962, 62 (3), 185–203. 10.1021/cr60217a001. [DOI] [Google Scholar]
- Wacławek S.; Lutze H. V.; Grübel K.; Padil V. V. T.; Černík M.; Dionysiou; Dionysios D. Chemistry of Persulfates in Water and Wastewater Treatment: A Review. Chem. Eng. J. 2017, 330, 44–62. 10.1016/j.cej.2017.07.132. [DOI] [Google Scholar]
- Cruz C. L.; Nicewicz D. A. Mechanistic Investigations into the Cation Radical Newman–Kwart Rearrangement. ACS Catal. 2019, 9 (5), 3926–3935. 10.1021/acscatal.9b00465. [DOI] [Google Scholar]
- The proposed mechanism implies that substoichiometric amounts of APS should mediate the NKR; However, APS is not truly catalytic in this reaction as 1 equiv is required for optimal yields. The stoichiometry of the reaction suggests that the iron catalyst only generates one sulfate radical anion per persulfate that is cleaved. We can only speculate that the second sulfate radical is consumed in a nonproductive redox cycle of iron(II/III).
- Tanwar L.; Börgel J.; Ritter T. Synthesis of Benzylic Alcohols by C–H Oxidation. J. Am. Chem. Soc. 2019, 141 (45), 17983–17988. 10.1021/jacs.9b09496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollong M. J.; Yun H.; Sherwood L.; Woods A. K.; Lairson L. L.; Schultz P. G. A Small Molecule Inhibits Deregulated NRF2 Transcriptional Activity in Cancer. ACS Chem. Biol. 2015, 10 (10), 2193–2198. 10.1021/acschembio.5b00448. [DOI] [PubMed] [Google Scholar]
- Ritter T.; Xu P.; Zhao D.; Berger F.; Hamad A.; Rickmeier J.; Petzold R.; Kondratiuk M.; Bohdan K. Site-Selective Late-Stage Aromatic 18F-Fluorination via Aryl Sulfonium Salts. Angew. Chem., Int. Ed. 2019, 10.1002/anie.201912567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafuta K.; Korzun A.; Böhm M.; Golz C.; Alcarazo M. Synthesis, Structure and Reactivity of 5-(Aryl)Dibenzothiophenium Triflates. Angew. Chem., Int. Ed. 2019, 10.1002/anie.201912383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.