SUMMARY
SETTING:
Treatment of multidrug-resistant tuberculosis (MDR-TB) is lengthy and utilizes second-line anti-TB drugs associated with frequent adverse drug reactions (ADRs).
OBJECTIVE:
To evaluate the prevalence of and risk factors for ADRs among patients with MDR- and extensively drug-resistant TB (XDR-TB).
DESIGN:
A retrospective chart review of patients initiating treatment for M/XDR-TB in 2010–2012 in Tbilisi, Georgia.
RESULTS:
Eighty (54%) and 38 (26%) of 147 patients developed nephrotoxicity per RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) classification and ototoxicity, respectively. Twenty-five (17%) patients required permanent interruption of injectables due to an ADR. Median hospital stay, total treatment duration and number of regimen changes were higher among those with nephrotoxicity and/or ototoxicity, compared to those without (P < 0.01). Multinomial logistic regression analysis identified increasing age (per year) as a risk factor for nephrotoxicity (aOR 1.08,95%CI 1.03–1.12) and for both, nephro- and ototoxicity (aOR 1.11, 95%CI 1.05–1.17). Low baseline creatinine clearance (CrCl) was a significant risk factor for developing nephrotoxicity (aOR 1.05, 95%CI 1.02–1.07).
CONCLUSION:
Second-line injectable drug-related ADRs are common among M/XDR-TB patients. Patients with increasing age and low baseline CrCl should be monitored closely for injectable-related ADRs. Notably, our findings support WHO’s latest recommendations on introduction of injectable free anti-TB treatment regimens.
Keywords: drug resistance, second-line drugs, kanamycin, capreomycin, ADRs
RÉSUMÉ
CONTEXTE :
Le traitement de la tuberculose multirésistante (MDR-TB) est prolongé et utilise des médicaments de deuxième ligne associés à de fréquents effets secondaires (ADR).
OBJECTIF :
Evaluer la prévalence des ADR et leurs facteurs de risque parmi les patients atteints de MDR-TB et de TB ultrarésistante (XDR-TB).
SCHÉMA :
Une revue rétrospective des dossiers des patients débutant un traitement de M/XDR-TB en 2010–2012 à Tbilissi, Géorgie.
RÉSULTATS :
Sur 147 patients, 80 (54%) et 38 (26%) ont respectivement développé une néphrotoxicité selon la classification de RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) et une ototoxicité ; 25 (17%) patients ont dû arrêter définitivement les injectables à cause d’ADR. La durée médiane d’hospitalisation, la durée totale de traitement et le nombre de modifications du protocole ont été plus élevés parmi les patients ayant souffert de néphrotoxicité/d’ototoxicité, comparés aux autres (P < 0,01). Une analyse de régression logistique multinomiale a identifié l’âge croissant (par année) comme un facteur de risque de néphrotoxicité; (OR ajusté [ORa] 1,08 ; IC 95% 1,03–1,12) et à la fois de néphro- et d’ototoxicité (ORa 1,11 ; IC 95% 1,05–1,17). Une clairance de la créatinine (CrCl) basse au départ a été un facteur de risque significatif de développement d’une néphrotoxicité (ORa 1,05 ; IC 95% 1,02–1,07).
CONCLUSION :
Les ADR liés aux médicaments injectables de deuxième ligne sont fréquents parmi les patients M/XDR-TB. Les patients d’âge plus avancé et ayant une CrCl de départ basse doivent être étroitement suivis à la recherche d’ADR liés aux injectables. Nos résultats sont notamment en accord avec les dernières recommandations de l’Organisation Mondiale de la Santé relatives à l’introduction de protocoles gratuits de traitement anti-TB injectables.
RESUMEN
MARCO DE REFERENCIA:
El tratamiento de la tuberculosis multirresistente (MDR-TB) es prolongado y comporta fármacos antituberculosos de segunda línea que se asocian con reacciones adversas (ADR) frecuentes.
OBJETIVO:
Evaluar la prevalencia de reacciones adversas a los medicamentos y los factores de riesgo en los pacientes con MDR y extensivemente resistente (XDR-TB).
MÉTODO:
Fue este un estudio retrospectivo a partir del analisis de las historias clínicas de los pacientes que iniciaban tratamiento por MDR- y XDR-TB del 2010 al 2012 en Tbilisi, Georgia.
RESULTADOS:
De los 147 pacientes, 80 (54%) presentaron nefrotoxicidad definida por los criterios RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) y 38 (26%) ototoxicidad. Veinticinco pacientes (17%) necesitaron una interruptión permanente de los fármacos inyectables debido a ADR. La mediana de la estancia hospitalaria, la duratión total del tratamiento y el número de modificaciones del esquema terapéutico fue más alta en los pacientes que presentaron nefrotoxicidad u ototoxicidad, que en los pacientes sin estas reacciones adversas (P < 0,01). El análisis de regresión logística polinomial determinó que el aumento de la edad (poraño) es un factor de riesgo de nefrotoxicidad (OR ajustado [ORa] 1,08; IC 95% 1,03–1,12) y de nefrotoxicidad y ototoxicidad (ORa 1,11; IC 95% 1,05–1,17). Una depuración de creatinina (CrCl) inicial baja fue un factor de riesgo significativo solo de aparición de nefrotoxicidad (ORa 1,05; IC 95% 1,02–1,07).
CONCLUSIÓN:
Las reacciones adversas a los fármacos inyectables de segunda línea son frecuentes con los pacientes con MDR y XDR-TB. Los pacientes de mayor edad y con una CrCl inicial baja se deben vigilar de cerca a fin de detectar estas reacciones adversas. Cabe señalar que los resultados del presente estudio respaldan las recomendaciones más recientes de la Organization Mundial de la Salud sobre la introducción de esquemas de tratamiento antituberculoso sin medicamentos inyectables.
THE INCREASING PREVALENCE of multidrug-resistant tuberculosis (MDR-TB) is a major problem worldwide, threatening to limit our progress towards the goal of eliminating TB as global public health problem by 2050.1 MDR-TB is defined as Mycobacterium tuberculosis resistant to at least isoniazid (INH) and rifampicin (RMP), the two most important first-line anti-TB drugs. Extensively drug-resistant TB (XDR-TB) is additional resistance to a fluoroquinolone and at least one injectable second-line anti-TB drug (amikacin, kanamycin [KM], and/or capreomycin [CPM]).2 In 2016, an estimated 4.1% of new and 19% of previously treated TB cases globally had rifampicin-resistant (RR)/MDR-TB which equated to an estimated 600 000 incident cases and 6.2% of these cases had XDR-TB.3 The highest rate of drug resistance is found in former Soviet Union countries, including Georgia. In 2016, the incidence of RR/MDR-TB in Georgia was 20 per 100000 population and the prevalence of RR/MDR-TB was 11% among new and 31% among previously treated TB cases.3
Compared to the standard 6-month treatment for drug-susceptible TB, treatment for M/XDR-TB is more complex, less effective, toxic, costly and requires the use of second-line drugs (SLDs) for up to 24 months.4 The long duration of therapy and concurrent use of SLDs often results in frequent adverse drug reactions (ADRs), ranging from mild nausea and vomiting to life-threatening renal failure and hepatotoxicity. Depending on the severity, an ADR might require temporary or permanent discontinuation of one or more SLDs. This subsequently may lead to decreased treatment efficacy and poor patient outcomes including failure or even death.5–8
The purpose of our study was to assess the prevalence of and risk factors for ADRs among patients with M/XDR-TB in Georgia. Specifically, our study focused on injectable anti-TB drug-related (KM and CPM) ototoxicity and nephrotoxicity.9 We determined the frequency and types of injectable-related ADRs, we also assessed time to first appearance of ADRs, and determined risk factors for ototoxicity and nephrotoxicity among M/XDR-TB patients. A better understanding of frequency and occurrence of injectable-related toxicity will be beneficial for closer monitoring and management of patients at risk.
STUDY POPULATION AND METHODS
Design and setting
A retrospective chart review of patients with pulmonary M/XDR-TB initiating treatment with SLDs between 1 December 2010 and 31 December 2012 was conducted at the National Center for Tuberculosis and Lung Disease (NCTLD) in Tbilisi, Georgia. This was an exploratory study utilizing a convenience sample of all patients meeting eligibility criteria and with available medical records during the study period.
The study was approved by the NCTLD Ethics Committee (Tbilisi, Georgia) and Emory University (Atlanta, GA, USA) Institutional Review Board.
Participants
All participants had confirmed TB based on a positive sputum culture for Mycobacterium tuberculosis. Drug resistance was confirmed by the GenoType® MTBDRplus assay (Hain Lifescience, Nehren, Germany) and/or conventional drug susceptibility testing (DST), performed using the absolute concentration method on Löwenstein-Jensen (LJ) medium and/or in 7H9 broth with the BACTEC MGIT™ 960™ systems (BD, Sparks, MD, USA).10,11 DST was performed on the following first-line drugs (FLDs): streptomycin, INH, RMP, and ethambutol; and SLDs: KM, ofloxacin, ethionamide, CPM and para-aminosalicylic acid (PAS). Demographic information, socioeconomic status, medical history (comorbidities, human immunodeficiency virus [HIV] status, treatment regimens, ADRs) and laboratory data were abstracted from inpatient and outpatient medical charts as well as electronic database of the NCTLD, using a standardized data collection form.
Treatment and follow-up
Standard treatment for M/XDR-TB in Georgia consists of two phases including an initial intensive phase which includes the use of an injectable agent (KM or CPM) for the first 6–8 months of treatment and a continuation phase without an injectable, with a recommended total treatment duration between 18–24 months. The duration of the intensive phase, and total treatment depends on sputum culture conversion time, the clinical status of the patient and treatment adherence. An empirical treatment regimen (ETR) was initiated for every patient before second-line DST results were available and all regimens included pyrazinamide, fluoroquinolone, an injectable agent, PAS and additional agents chosen by the clinician. After DST results for SLDs, treatment regimens were individualized as per WHO recommendations.10 All treatment was provided using directly observed therapy (DOT).
Definitions
Time to culture conversion was defined as the time from sputum collection to the date of the first of two consecutive negative sputum cultures performed at least one month apart. Renal function was assessed by measuring serum creatinine level on a monthly basis during treatment. The RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) classification of acute kidney injury (AKI) was used to assess nephrotoxicity.12–16 The ‘risk’ category of RIFLE includes 1.5 times increase of serum creatinine compared to the baseline creatinine and decrease in glomerular filtration rate (GFR) of more than 25%; the ‘injury’ stage is defined as an increase in serum creatinine for more than twice baseline value and decrease in GFR for more than 50%; ‘failure’ stage—GFR decrease more than 75% and serum creatinine increase more than three times. Ototoxicity was assessed by patient self-report of hearing loss and/or tinnitus or by audiometry in a few cases. Abnormal potassium levels were defined as deviations from the normal ranges of blood potassium level (3.6–5.5 mmol/l).17 Treatment outcomes were classified according to the WHO definitions: successful outcome included cure and completion and unsuccessful treatment outcome comprised of failure, loss to follow-up and death.18
Statistical methods
Abstracted data were entered into a REDCap database (Vanderbilt University, Nashville, TN, USA) and data analysis was performed using SAS software (SAS Institute, Cary, NC, USA).19 Descriptive statistics were performed to examine the frequencies of sociodemographic and clinical characteristics, adverse events and treatment regimen changes. The differences in patient characteristics by ADRs was analyzed with either a X2 or Fisher exact test for categorical variables and a 2-sample t-test for continuous variables, respectively. A multinomial logistic regression was performed to determine the risk factors associated with the development of nephrotoxicity or ototoxicity alone, or both together. The model building strategy was based on purposeful selection of variables.20
RESULTS
Overall, 1452 patients were initiated for treatment with SLDs for M/XDR-TB in Georgia during the study period, including 788 patients who initiated treatment in Tbilisi and 176 patients who received their entire treatment course at the NCTLD. Of 176 patients, 147 were included in the study (Figure 1). Among those analysed, 147 patients, 121 (82%) had MDR-TB and 26 (18%) had XDR-TB. Median age was 35 years and 96 (65%) were male. Most of the patients (n = 91, 62%) were newly diagnosed TB cases and 56 (38%) were retreatment cases (33 had a history of drug-susceptible and 23 had a history of drug-resistant TB). The most common comorbidity was hepatitis C (20%), followed by diabetes mellitus (12%) and only 3% of patients had HIV. The median length of treatment was 612 days (range 28–1054), while the median length of inpatient treatment during first hospitalization was 65 days (range 11–302). Sixty-four (47%) patients had an unsuccessful treatment outcome: 51 (38%) patients were lost to follow-up, 7 (5%) died, 2(1%) failed treatment and 4 (3%) patients were transferred out of Georgia for further treatment (Table 1). The demographic and clinical characteristics of patients stratified according to either having nephrotoxicity and/or ototoxicity are summarized in Table 1.
Figure 1.
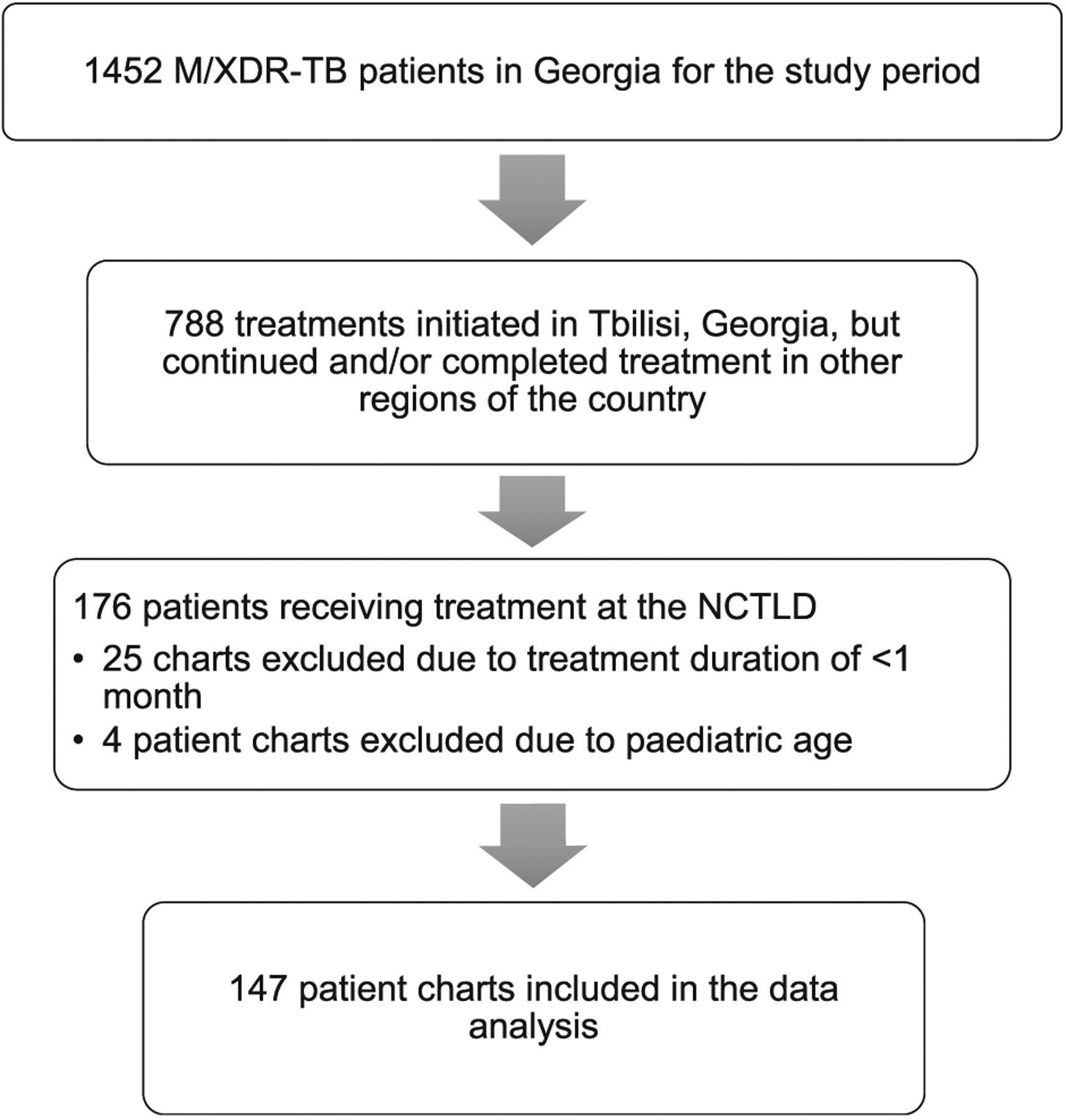
Flow diagram for patients included in Study Cohort. MDR/XDR-TB = multidrug-resistant and extensively drug-resistant tuberculosis; NCTLD = National Center for Tuberculosis and Lung Disease.
Table 1.
Characteristics of M/XDR-TB patients stratified by AKI (n = 147)
| Nephrotoxicity (AKI) | Ototoxicity | ||||
|---|---|---|---|---|---|
| Variable | Total n (%) |
Yes (n = 80, 54%) n (%) |
No (n = 67, 46%) n (%) |
Yes (n = 38, 26%) n (%) |
No (n = 109, 74%) n (%) |
| Demographics and behaviour | |||||
| Age, years, median (range) | 35.3 (17–73) | 40.2 (18–73) | 31.8 (17–72) | 50 (17–72) | 33 (17–72) |
| BMI, kg/m2, median (range) | 20.3 (14–35) | 20.5 (14–29) | 19.6 (14–35) | 21 (14–29) | 20 (14–35) |
| Male | 96 (65) | 50 (62) | 46 (69) | 28 (74) | 68 (62) |
| History of incarceration | 27 (18) | 11 (14) | 16 (24) | 3 (8) | 24 (22) |
| Tobacco user | 76 (52) | 34 (43) | 42 (63) | 20 (53) | 56 (51) |
| Alcohol user | 50 (34) | 26 (33) | 24 (36) | 13 (34) | 37 (34) |
| Injection drug user | 10 (7) | 5 (6) | 5 (7) | 1 (3) | 9 (8) |
| Comorbidities | |||||
| HIV | 4 (3) | 1 (1) | 3 (4) | 2 (5) | 2 (2) |
| Diabetes | 18 (12) | 13 (16) | 5 (7) | 5 (13) | 13 (12) |
| Hepatitis C | 29 (20) | 14 (18) | 15 (22) | 7 (18) | 22 (20) |
| Initial injectable drug | |||||
| Kanamycin | 130 (88) | 71 (89) | 59 (88) | 31 (82) | 99 (91) |
| Capreomycin | 17 (12) | 9 (11) | 8 (12) | 7 (18) | 10 (9) |
| Clinical characteristics | |||||
| History of TB | 56 (38) | 28 (35) | 28 (42) | 15 (39) | 41 (38) |
| History of MDR-TB | 23 (16) | 8 (10) | 15 (22) | 6 (16) | 17 (16) |
| Cavitary disease | 26 (18) | 12 (15) | 14 (21) | 6 (16) | 20 (18) |
| Treatment follow-up | |||||
| Number of serum Cr measurements, median (range) | 14 (2–21) | 15 (3–21) | 10 (2–21) | 15.5 (5–21) | 13 (2–21) |
| Baseline CrCl, median (range) | 95 (45–236) | 97 (46–236) | 90 (45–165) | 76 (45–127) | 101 (49–236) |
| Baseline CrCl < 60 μmol/l | 14 (9) | 6 (8) | 8 (12) | 9 (24) | 5 (5) |
| Any abnormal K follow-up | 31 (21) | 22 (28) | 9 (13) | 8 (21) | 23 (21) |
| Hyperkalemia (maximum observed K > 5.5 mmol/l | 23 (16) | 17 (21) | 6 (9) | 7 (18) | 16 (15) |
| Time to first culture conversion, days, median (range)* | 95.5 (37–505) | 82 (37–352) | 100.5 (39–505) | 103 (37–268) | 78 (38–505) |
| Time in hospital (first hospitalization), days, median (range) | 65 (11–302) | 72 (12–302) | 52 (11–207) | 81.5 (33–302) | 52 (11–275) |
| Follow-up period, median (range) | 612 (28–1054) | 640 (81–1054) | 540 (28–1028) | 669 (102–1054) | 606 (28–1039) |
| Treatment regimen change | 135 (92) | 80 (54) | 55 (37) | 37 (97) | 98 (90) |
| Number of regimen changes, median (range) | 3 (0–9) | 3 (1–9) | 2 (0–8) | 4 (0–9) | 2 (0–8) |
| Treatment outcomes | |||||
| Cured | 26 (19) | 15 (20) | 11 (18) | 9 (24) | 17 (17) |
| Completed | 46 (34) | 31 (41) | 15 (25) | 16 (43) | 30 (30) |
| Failure | 2 (1) | 1 (1) | 1 (2) | 1 (3) | 1 (1) |
| Lost to follow-up | 51 (38) | 24 (32) | 27 (44) | 10 (27) | 41 (41) |
| Died | 7 (5) | 1 (1) | 6 (10) | 1 (3) | 6 (6) |
| Transferred out† | 4 (3) | 3 (4) | 1 (2) | 0 | 4 (4) |
Excluding LTFU, died and transferred out patients.
Patients who left the country for further treatment abroad.
MDR/XDR-TB = multidrug-resistant and extensivelydrug-resistant tuberculosis; AKI = acute kidney injury (RIFLE classification); BMI = bodymass index; HIV=human immunodeficiency virus; Cr = creatinine; CrCl = creatinine clearance; K = potassium; LTFU = loss to follow-up; RIFLE = Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease.
Median days to first culture conversion were shorter among AKI patients when excluding patients who were lost to follow-up, died or transferred outside of Georgia for further treatment (median 82 days, range 37–352 vs. median 100.5 days, range 39–505), but were almost similar in case of patients with or without ototoxicity (median 103 days, 37–268 vs. median 78 days, range 38–505).
Ototoxicity developed among 38 (26%) patients. Twenty-four (16%) had tinnitus and 33 (22%) reported decreased hearing (Figure 2). Audiometry was performed for only three patients who were found to have mild, moderate, and severe hearing loss, respectively. Among those with tinnitus, 23 were receiving KM while one was taking CPM. Thirteen of the 24 (54%) patients with tinnitus required permanent interruption of an injectable agent. Among 33 patients with decreased hearing, 31 were receiving KM and 2 were receiving CPM as a part of initial treatment regimen. Among those (n = 33) with impaired hearing, 16 (48%) required permanent interruption of an injectable.
Figure 2.
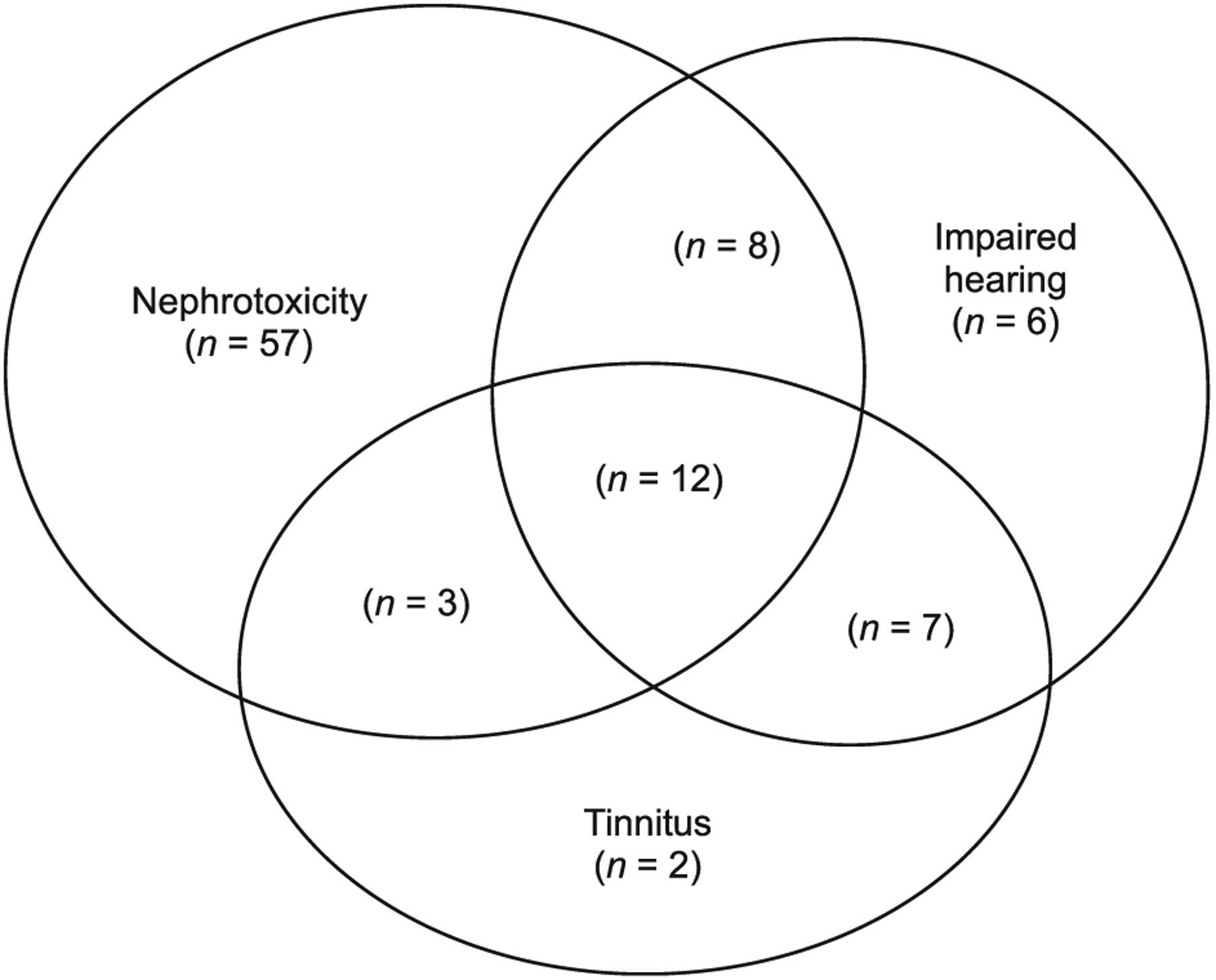
Venn diagram: overlapping of RIFLE nephrotoxicity and ototoxicity (impaired hearing and/or tinnitus).
Median age was significantly higher among those with AKI (40 vs. 32 years, P < 0.01) compared to those without AKI, as well as among those with ototoxicity (50 vs. 33 years, P < 0.01) compared to those without ototoxicity. The median hospital stay, total treatment duration and number of treatment regimen changes were higher among those with AKI and/or ototoxicity, compared to those without (P < 0.01) (Table 1).
A total of 80 (54%) patients had any AKI according to the RIFLE criteria (Table 2) with 15 (10%) of them being at the Injury stage. Any abnormal potassium levels were observed among 22 (28%) AKI patients (n = 80) and 9 (13%) patients without AKI (n = 67) (P = 0.03). Confirmed hyperkalemia (potassium level > 5.5 mmol/l) was found in 17 (21%) patients with AKI and 6 (9%) patients with no AKI (P = 0.04) (Table 1).
Table 2.
Rates of acute kidney injury according to the RIFLE classification (the two RIFLE stages are mutually exclusive)
| RIFLE stages | Total population (n = 147) n (%) |
Kanamycin use (n = 130) n (%) |
Capreomycin use (n = 17) n (%) |
|---|---|---|---|
| Risk | |||
| 1.5× creatinine increase | 3 (2) | 2 (2) | 1 (6) |
| 25% GFR decrease | 65 (44) | 57 (44) | 8 (47) |
| Either | 65 (44) | 57 (44) | 8 (47) |
| Injury | |||
| 2× creatinine increase | 2 (1) | 2 (2) | 0 (0) |
| 50% GFR decrease | 15 (10) | 14 (11) | 1 (6) |
| Either | 15 (10) | 14 (11) | 1 (6) |
RIFLE = Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; GFR = glomerular filtration rate.
Among 130 (88%) patients initially treated with KM, 17/130 (13 %) permanently interrupted drug due to an ADR; 9 (53%) out of 17 patients switched to CPM. Among 17(12%) of 147 patients initially treated with CPM, 8/17 (47%) required permanent drug interruption due to ADR; 4 (50%) out 8 patients switched to KM. In total, 25 (17%) patients had permanent discontinuation of an injectable agent due to one or more ADR; 21 of whom had ototoxicity and/or AKI and 4 patients had an allergic reaction (e.g., skin rash; irritation of the injection site).
The association of AKI and/or ototoxicity occurrence with an unsuccessful treatment outcome was not found to be significant. Among 80 patients with nephrotoxicity, 34 (43%) patients had unsuccessful treatment outcome out of which 24 (70%) were lost to follow-up. Among 38 patients with any kind of ototoxicity 13 (34%) patients had unsuccessful treatment outcome out of which 10 (77%) patients were lost to follow-up (P = 0.17).
Multinomial logistic regression analysis confirmed age to be a significant risk factor for developing nephrotoxicity only (aOR 1.08, 95%CI 1.03–1.12) and concomitant nephrotoxicity and ototoxicity (aOR 1.11, 95%CI 1.05–1.17). Low baseline creatinine clearance was a significant risk factor for developing nephrotoxicity (aOR 1.05, 95%CI 1.02–1.07). The development of nephrotoxicity, ototoxicity, or either one was not found to be injectable drug dose-dependent (Table 3).
Table 3.
Multinomial logistic regression analysis of risk factors for nephrotoxicity and ototoxicity
| RIFLE nephrotoxicity vs. no ADR | Ototoxicity vs. no ADR | Nephrotoxicity AND ototoxicity vs. no ADR | ||||
|---|---|---|---|---|---|---|
| Variable | aOR | 95%CI | aOR | 95%CI | aOR | 95%CI |
| Age (per 1-year increase) | 1.08 | 1.03–1.12 | 1.01 | 0.94–1.07 | 1.11 | 1.05–1.17 |
| Male | 0.58 | 0.17–2.04 | 3.67 | 0.54–24.82 | 2.88 | 0.50–16.67 |
| Tobacco use | 0.24 | 0.08–0.75 | 0.26 | 0.06–1.18 | 0.20 | 0.05–0.80 |
| Baseline CrCl (per 1 unit increase) | 1.05 | 1.02–1.07 | 0.96 | 0.92–1.00 | 1.01 | 0.98–1.04 |
| Initial injectable 1000 mg vs. 750 mg | 1.27 | 0.24–6.77 | 1.30 | 0.15–11.18 | 0.29 | 0.04–1.97 |
RIFLE = Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; aOR = adjusted odds ratio; CI = confidence interval; ADR = adverse drug reaction; CrCl = creatinine clearance.
DISCUSSION
Our findings indicate that ADRs to second-line injectable anti-TB drugs are common in Georgia. Due to ADRs, patients required prolonged treatment and frequent changes in treatment regimen. In total 25 (17%) patients had a permanent withdrawal of second-line injectable agent as a result of ADRs. Permanent withdrawal of an injectable agent could potentially lead to an unsuccessful treatment outcome. We found that lower baseline CrCL (<60 ml/min) is a significant risk factor for development of nephrotoxicity, thus clinicians should carefully monitor such patients. Increasing age was also associated with the development of injectable-related ADRs.21
The prevalence of nephrotoxicity among our study cohort (80, 54%) was much higher compared to nephrotoxicity prevalence in a previous study from Latvia (4.3%) and in the meta-analysis performed by Wu et al (4%).5,6 This difference could be due to differences in the definition of renal impairment: at least one-time elevation of Cr >141 mmol/l and decrease of CrCl <50 ml/min was used in the Latvian study, and the meta-analysis defined renal impairment as any elevation of Cr or any decrease of CrCl, which differs from RIFLE stages used in our study. The prevalence of ototoxicity was similar to ours (n = 38, 26%) both in the Latvian study (19% impaired hearing, 12.1% tinnitus) and the meta-analysis (ototoxicity 14.6%).5,6 Furthermore, a number of studies reported hearing impairment, prevalence ranging from 6 to 28% and renal dysfunction, prevalence ranging from 4 to 10%, as ADRs to injectable agents.8,22–28 Similar to other studies, nephrotoxicity and ototoxicity studied in Georgia also tended to occur mainly in the first 6–8 months of treatment, during the intensive phase, when injectable agents are administered.5,6,29 Closer monitoring of ADRs is essential during the treatment of M/XDR-TB to ensure successful treatment outcome.
Our findings suggest that the introduction of injectable free anti-TB treatment regimens might be better tolerated. Furthermore, there is also a lack of RCT-based evidence showing efficacy of injectable drugs in general. Based on new WHO guidelines, replacement of injectable agents in case of resistance or intolerance with newly rolled out anti-TB medications (bedaquiline, delamanid, linezolid and imipenem/cilastatin) is highly recommended.30,31 As per the latest WHO communication, KM and CPM are no longer recommended anti-TB medications, given increased risk of treatment failure and relapse associated with their use in longer MDR-TB regimens.32
A potential limitation of our study was a reporting bias. Tinnitus and impaired hearing were patient reported, as audiometry was not a routine part of the treatment monitoring; that could have led to underdetection of asymptomatic cases. Another limitation of the study is the small cohort size, which could be a reason for not finding significant association between potential risk factors and ADRs, and ADRs and final treatment outcomes. Further detailed analysis of a bigger cohort from the whole country and a better understanding of what additional factors could lead to an increased likelihood of experiencing ADRs may help with monitoring and management of TB patients. The pharmacovigilance program launched in Georgia in 2015 is another opportunity to monitor ADRs of anti-TB drugs more closely.
CONCLUSIONS
In summary, nephrotoxicity and ototoxicity were common among patients receiving second-line anti-TB injectable agents leading to permanent discontinuation of a drug due to an ADR. Elderly patients and patients with low baseline CrCl should be monitored closely for development of nephrotoxicity and/or ototoxicity. ADRs lead to prolongation of time to culture conversion and total treatment duration, therefore could seriously affect treatment adherence and final treatment outcome. Our findings support WHO’s latest recommendations on introduction of injectable free anti-TB treatment regimens.
Acknowledgements
The authors wish to thank all the clinical and administrative staff of all the institutions involved in the study project, especially the staff of the National Center for Tuberculosis and Lung Diseases, Tbilisi, Georgia, for their contribution.
This work was supported in part by the National Institutes of Health Fogarty International Center (Bethesda, MD, USA; grant D43TW007124).
References
- 1.StopTB Partnership. The paradigm shift 2016–2020. Geneva, Switzerland: StopTB, 2015. [Google Scholar]
- 2.World Health Organization/International Union Against Tuberculosis and Lung Disease. Anti-tuberculosis drug resistance in the world. Geneva, Switzerland: WHO, 2008. [Google Scholar]
- 3.World Health Organization. Global tuberculosis report, 2017. WHO/HTM/TB/2017.23 Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 4.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 5.Bloss E, Kuksa L, Holtz TH, et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000–2004. Int J Tuberc Lung Dis 2010; 14(3): 275–281. [PubMed] [Google Scholar]
- 6.Wu S, Zhang Y, Sun F, et al. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther 2016; 23(2): e521–530. [DOI] [PubMed] [Google Scholar]
- 7.de Jager P, van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis 2002; 6(7): 622–627. [PubMed] [Google Scholar]
- 8.Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2001; 5(7): 648–655. [PubMed] [Google Scholar]
- 9.Chang CH, Chen YF, Wu VC, et al. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect Dis 2014; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipiani M, Mirtskhulava V, Tukvadze N, Magee M, Blumberg HM, Kempker RR. Significant clinical impact of a rapid molecular diagnostic test (GenoType MTBDRplus assay) to detect multidrug-resistant tuberculosis. Clin Infect Dis 2014; 59(11): 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tukvadze N, Kempker RR, Kalandadze I, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One 2012; 7(2): e31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuhaci B More data on epidemiology and outcome of acute kidney injury with AKIN criteria: benefits of standardized definitions, AKIN and RIFLE classifications. Crit Care Med 2009; 37(9): 2659–2661. [DOI] [PubMed] [Google Scholar]
- 13.Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013; 6(1): 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakula AM, Skinner RA. Acute kidney injury in the critically ill patient: a current review of the literature. J Intensive Care Med 2015; 31(5): 319–324. [DOI] [PubMed] [Google Scholar]
- 15.Fujigaki Y [Acute kidney injury: progress in diagnosis and treatments. Topics: III. Approach to diagnosis; 1. Acute kidney injury definition and staging according to risk/injury/failure/loss/end-stage (RIFLE), acute kidney injury network (AKIN), and kidney disease. Nihon Naika Gakkai Zasshi 2014; 103(5): 1061–1067. [Japanese] [DOI] [PubMed] [Google Scholar]
- 16.Pan HC, Chien YS, Jenq CC, et al. Acute kidney injury classification for critically ill cirrhotic patients: a comparison of the KDIGO, AKIN, and RIFLE classifications. Sci Rep 2016; 6: 23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoss S, Elizur Y, Luria D, Keren A, Lotan C, Gotsman I. Serum potassium levels and outcome in patients with chronic heart failure. Am J Cardiol 2016; 118(12): 1868–1874. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Definitions and reporting framework for tuberculosis. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42(2): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aung TY, Thwin MH, Zin T, Oo KN, Aye NN, Sin S. Comparative pharmacokinetics of kanamycin between multidrug-resistant tuberculosis patients and healthy volunteers. Int J Med Pharm 2014; 2: 45–59. [Google Scholar]
- 22.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. IntJTuberc Lung Dis 2004; 8(11): 1382–1384. [PubMed] [Google Scholar]
- 23.Duggal P, Sarkar M. Audiologic monitoring of multi-drug-resistant tuberculosis patients on aminoglycoside treatment with long-term follow-up. BMC Ear Nose Throat Disord 2007; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturdy A, Goodman A, Jose RJ, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother 2011; 66(8): 1815–1820. [DOI] [PubMed] [Google Scholar]
- 25.Schnippel K, Berhanu RH, Black A, et al. Severe adverse events during second-line tuberculosis treatment in the context of high HIV Co-infection in South Africa: a retrospective cohort study. BMC Infect Dis 2016; 16(1): 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathews A, Bailie GR. Clinical pharmacokinetics, toxicity and cost-effectiveness analysis of aminoglycosides and aminoglycoside dosing services. J Clin Pharm Ther 1987; 12(5): 273–291. [DOI] [PubMed] [Google Scholar]
- 27.Seddon JA, Godfrey-Faussett P, Jacobs K, Ebrahim A, Hesseling AC, Schaaf HS. Hearing loss in patients on treatment for drug-resistant tuberculosis. Eur Respir J 2012; 40(5): 1277–1286. [DOI] [PubMed] [Google Scholar]
- 28.Van der Walt M, Lancaster J, Odendaal R, Davis JG, Shean K, Farley J. Serious treatment related adverse drug reactions amongst anti-retroviral naive MDR-TB patients. PLoS One 2013; 8(4): e58817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis 2007; 11(12): 1314–1320. [PubMed] [Google Scholar]
- 30.D’Ambrosio L, Centis R, Sotgiu G, Pontali E, Spanevello A, Migliori GB. New anti-tuberculosis drugs and regimens: 2015 update. ERJ Open Res 2015; 1(1): 00010–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. WHO treatment guidleines for drug-resistant tuberculosis. WHO/HTM/TB/2016.04 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 32.World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). WHO/CDS/TB/2018.18 Geneva, Switzerland: WHO, 2018. [Google Scholar]


