Abstract
The human microbiome is known to play an essential role in influencing host health. Extracellular vesicles (EVs) have also been reported to act on a variety of signaling pathways, distally transport cellular components such as proteins, lipids, and nucleic acid, and have immunomodulatory effects. Here we shall review the current understanding of the intersectionality of the human microbiome and EVs in the emerging field of microbiota-derived EVs and their pharmacological potential. Microbes secrete several classes of EVs: outer membrane vesicles (OMVs), membrane vesicles (MVs), and apoptotic bodies. EV biogenesis is unique to each cell and regulated by sophisticated signaling pathways. EVs are primarily composed of lipids, proteins, nucleic acids, and recent evidence suggests they may also carry metabolites. These components interact with host cells and control various cellular processes by transferring their constituents. The pharmacological potential of microbiomederived EVs as vaccine candidates, biomarkers, and a smart drug delivery system is a promising area of future research. Therefore, it is necessary to elucidate in detail the mechanisms of microbiome-derived EV action in host health in a multi-disciplinary manner.
Keywords: Drug delivery system, Extracellular vesicles, Microbiome, Pharmabiotics
Introduction
Interest in extracellular vesicles (EVs) has dramatically increased over the course of three decades as their physiological importance and promising biomedical applications have been realized. Upon the initial discovery that eukaryotic cells release bi-layered vesicles into the extracellular environment in 1983, their function was not well understood and EVs were written off as the cellular equivalent of a garbage disposal.[1] However, extensive research conducted over the past several decades have revealed that EVs are expressed in prokaryotes, eukaryotes, and archaea at varying levels of environmental stress. This evidence suggests that EV secretion is an evolutionarily conserved, necessary function in cells across all domains of life.[2] While nomenclature confusion has previously been an issue in the field, the term extracellular vesicle is the general term used for all lipid-bilayer encased spherical nanoparticles that transport protein, lipid and nucleic acid cargo from the cell into the extracellular milieu. The role of EVs in intercellular communication, long-distance transport of active biomolecules, receptor-mediated host cell entry, immune modulation, disease pathogenesis, and a variety of biomedical applications including bioengineered vaccine adjuvants and diagnostic biomarkers have become a research topic of great interest in the past decade.[3,4]
The functional significance of the commensal bacteria living in and on our bodies, or microbiota, has similarly undergone a reversal of favor in the scientific community since the turn of the century. While it has been well-established that communities of bacteria naturally co-exist within our bodies, their relationship with their human host was regarded as largely commensal or potentially pathogenic. Appreciation of the essential symbiotic role microbiota plays in health has dramatically increased since 2001 when Nobel Laureate Joshua Lederberg famously coined the term microbiome as it is currently defined: the collection of genomes contributed by all microorganisms present in an ecosystem.[5] Since then, an explosion of research has revealed that the human microbiome has a multitude of essential functions in host health including immune modulation, nutrient metabolism, maintenance of the intestinal barrier, and protection from pathogen colonization.[6,7,8]
As advancements in both EV and microbiome research have dramatically increased in the past decade, the intersectionality of the microbiome and EVs is emerging as a promising research avenue. Previously, only gram-negative bacteria were thought to produce EVs. However, it was recently determined that grampositive bacteria also release ectosomes, a class of EVs that are packaged and released at the cellular membrane as cells grow.[9] Furthermore, mounting evidence suggests that single-cell bacteria as well as multicellular eukaryotic organisms undergo programmed cell death, or apoptosis.[10] This introduces another layer of complexity to bacterial EV secretion as cells that undergo apoptosis release another class of EVs, apoptotic bodies, which carry cellular cargo from cells undergoing programmed cell death into the extracellular environment. Through further exploration of the emerging field of microbe-derived extracellular vesicles, researchers can better understand the complex interplay of host-microbiota interactions and develop innovative pharmacological health solutions using microbe-derived EVs as a smart drug delivery system.
Why microbiome-derived EVs?
EVs are released from all three domains of life including our own eukaryotic human cells, so why is special interest warranted to EVs originating from our commensal bacteria? Three decades ago the foundation of human genetics was based on the assumption that the vast, complex biological functions of the human body were carried out primarily by the genetic information contained in our own cells. Thus, launched the groundbreaking Human Genome Project in 1990 which sought to sequence and map the entire human genome in order to elucidate our genetic makeup. Early estimates of the number of genes contained in the human genome ranged from 50,000 upwards to 140,000. However, at the HGP's completion in 2003 it was revealed that the human genome contained drastically fewer genes than previously estimated. At present, the human genome is estimated to encode approximately 20,500 genes, barely surpassing the genetic content of Caenorhabditis elegans, a 1 mm roundworm.[11,12]
While scientists were left scratching their heads over the unexpectedly low number of protein-encoding genes in the human genome, interest in understanding the genetic contributions of the commensal microorganisms co-existing in and on our bodies, the microbiota, began to rise. The human microbiota was generally believed to outnumber human cells by a factor of 10, so logically the next step to uncover the missing piece of the genetic puzzle making up our bodies was to launch the Human Microbiome Project (HMP) in 2007. The HMP conducted metagenomic sequencing, a culture independent methodology that selectively targets the conserved 16s ribosomal RNA region of bacteria, to identify up to the species level all the bacteria composing the microbial flora of healthy people at multiple body sites.[13] Whole genome shotgun sequencing and gene mapping further elucidated the functional characteristics of core bacterial strains in our microbiota.
The HMP and other investigations around the world have laid the foundation for an ever-increasing understanding of our microbiome. Though it was commonly believed bacterial cells outnumber our own by a factor of 10, it now estimated to be a more modest factor of 1.1. Rather than raw cell count, the critical way microbiota outnumber their human counterparts is by their genetic contributions. It is estimated that on average 1,000 different bacterial species inhabit a healthy human host and that each species encodes roughly 2,000 genes.[14] Based on these currently accepted approximations, our microbiome yields approximately 2,000,000 genes, vastly outnumbering the 20,500 genes contributed by our own human genome. Thus, the human body can be viewed as a supraorganism comprised of trillions of both human and microbial cells shaped by 99% microbial and 1% human genomic content.
The HGP and HMP have shown that the human genome comprises less than 1% of the total genetic material present in our body, highlighting the importance of understanding how the composition and activity of the other 99% contributed by our microbiota impacts human health and disease. Imbalances in commensal microbial communities have been associated with a wide variety of diseases including obesity, diabetes, cancer, depression, anxiety, asthma, atopic dermatitis, nonalcoholic fatty liver disease (NAFLD), and Irritable Bowel Syndrome (IBS).[15,16,17] As interest continues to shift from microbial composition to functionality, EVs derived from these bacterial communities have great potential as therapeutic and diagnostic tools to better understand the complex interactions between commensal microbial communities and the host.
EV biogenesis
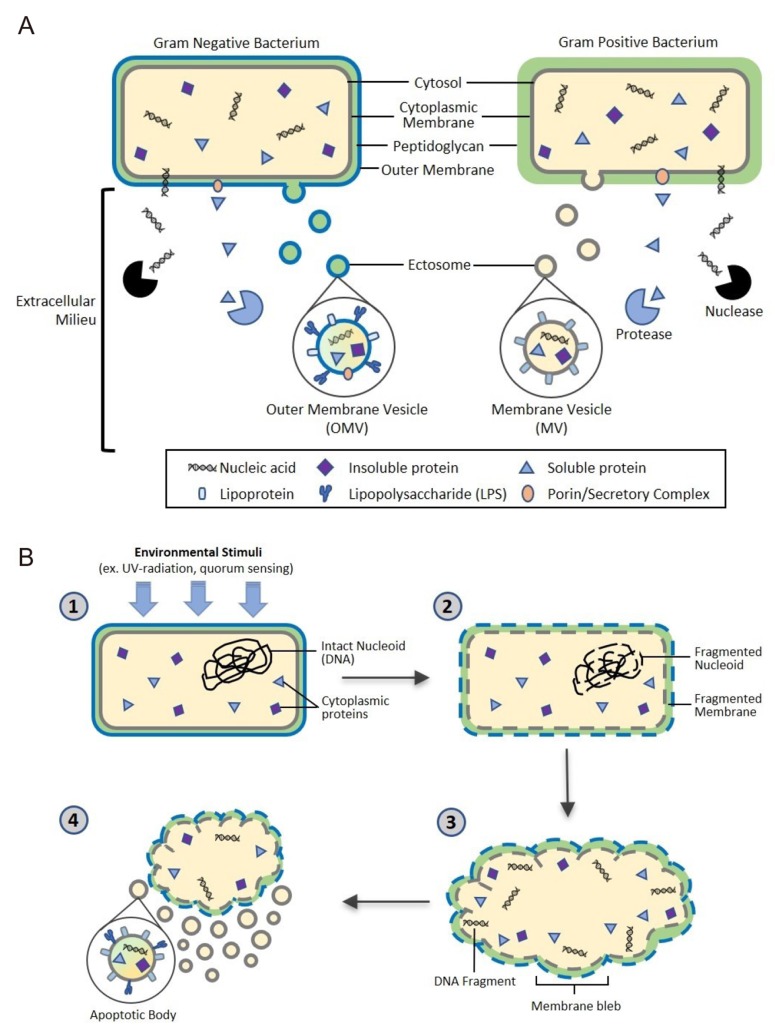
The biogenesis of EVs is a very tightly regulated process governed by multiple signaling molecules and begins with receptor activation unique for each cell type.[18] Though eukaryotic EV biogenesis is well characterized, the mechanisms of bacterial EV biogenesis have only recently begun to be elucidated.[19] Here we will describe the current understanding of the biogenesis of ectosomes, also called shedding vesicles, in gram-negative and gram-positive bacteria as well as bacterial release of apoptotic bodies as summarized in Figure 1. Additionally, we will briefly discuss the well-defined mechanisms underlying eukaryotic exosome release that can offer insights into the process of EV biogenesis.
Figure 1. Proposed bacterial extracellular vesicle secretion. Bacteria ubiquitously release extracellular vesicles (EVs) into the extracellular milieu roughly 10-300 nm in diameter. EVs are composed of a lipid bilayer containing soluble and insoluble proteins, nucleic acids, and lipids. The lipid bilayer encasing the vesicular contents confers protection from extracellular degradation by nucleases and proteases, allowing distal transfer of EV cargo. (A) Gram negative and positive bacteria secrete ectosomes, or outer membrane vesicles (OMV) and membrane vesicles (MV), respectively. OMV biogenesis through budding of the outer membrane is well characterized, however the mechanism of MV biogenesis through the thick peptidoglycan cell wall of gram positive bacteria is not yet fully understood. (B) Bacterial cells also undergo apoptosis, a conserved self-destruct mechanism instigated by environmental stimuli such as quorum sensing or UV damage. After the apoptotic pathway is initiated, nucleoid DNA is degraded and subsequently fragmented, followed by cell membrane fragmentation. Finally, blebbing of the cell membrane occurs, resulting in release of bacterial apoptotic bodies containing bacterial cellular components.
Gram-negative bacteria: Outer Membrane Vesicles (OMV)
Gram-negative bacteria produce ectosomes, known as outer membrane vesicles (OMVs), that contain periplasmic constituents including proteins, lipoproteins, phospholipids, and lipopolysaccharide (LPS).[2] The first step in OMV formation is outward bulging of the outer membrane (OM). Links between the OM and the peptidoglycan are lost, either by movement of linking protein or by breaking the connection directly in various areas. This process can result in the incorporation of peptidoglycan fragments and parts of OM-peptidoglycan bridging proteins in the OMV.[20] However, simply removing OM-peptidoglycan links cannot sufficiently explain the mechanism of OMV formation. Overexpressed and misfolded periplasmic proteins can cause budding and specific proteins are enriched/excluded in OMVs, suggesting a more sophisticated biogenesis pathway. Gathering of periplasmic proteins as well as accumulation of curvature-inducing OM proteins could induce additional budding events.[20,21]
It is often proposed that OMVs are released from the bacterial cell when the bud grows to the point at which membrane curvature forces separation. Many membrane fission processes are energy-dependent, but the gram-negative bacterial envelope does not have a direct energy source such as ATP or NADPH, so it is not clear what would drive this fission. One possibility is that energy is provided from the cytoplasm via an inner membrane conduit. Alternatively, the required energy could be stored by the folding of membrane-associated proteins and released by their conformational changes, as occurs for the membrane fusion step of many viruses.[20,22]
Gram-positive bacteria: Membrane Vesicles (MV)
Unlike gram-negative bacteria, gram-positive bacteria lack an outer membrane and have a much thicker peptidoglycan cell wall outside of the cell membrane, which led to the initial assumption that gram-positive bacteria could not release EVs. However, increasing evidence has shown that many species of gram positive bacteria release EVs, or membrane vesicles.[9,23] Currently, the specific mechanisms behind the release of EVs in gram-positive bacteria are not fully understood, but support for three exclusive hypotheses exists in scientific literature.
EVs may be forced through the cell wall by turgor pressure after release from the plasma membrane.[24] Alternatively, cell wall-modifying enzymes released with EVs may facilitate a loosening of the wall to enable EV release. Such a mechanism may be widespread, as EVs from the Gram-positive bacterium Staphylococcus aureus carry peptidoglycan-degrading enzymes, such as Sle1, that can manipulate the thick Gram-positive peptidoglycan cell wall.[9] Finally, EVs may be actively transited through channels. In other words, proteins channels or structural cables may guide EVs to the extracellular environment.[24]
Microbial apoptotic bodies
Another development in the field of microbial membrane dynamics is the recent attention given to prokaryotic programmed cell death (PCD), or apoptosis. It was originally presumed that possession of a genetically coded cellular self-destruct mechanism would confer no evolutionary advantage to single-celled organisms. However, recent evidence suggests that bacteria undergo apoptosis in an altruistic manner for the benefit of the entire colony rather than the individual cell in order to respond to environmental stress, biofilm formation, and genetic transformation.[25] Once PCD signal pathways are activated, DNA damage and fragmentation are induced, leading to blebbing at the cell membrane, which results in cell fragmentation and the release of apoptotic bodies. These apoptotic bodies contain fragments of cellular DNA, RNA, proteins, and other cellular components.[22] While in-depth research of eukaryotic apoptosis has enabled a detailed understanding of the mechanisms of eukaryotic autolysis and subsequent release of apoptotic bodies, further research and studies must be conducted to understand the role of apoptotic body release from our microbiota in immune system modulation and intercellular signaling.
Eukaryotic cell-derived exosomes
Briefly, we will discuss the formation and release of exosomes, a class of EV released in all eukaryotic cells. Exosomes, while not present in microbiota, are a well-characterized EV class that offer insight into cellular mechanisms involved in EV biogenesis that may be conserved in other EV classes, such as ectosomes.
Exosomes biogenesis begins with the characteristic formation of intraluminal vesicles (ILVs) transported in multivesicular bodies (MVBs).[26] The best-described mechanism for formation of MVBs and ILVs is carried out by the endosomal sorting complex required for transport (ESCRT). ESCRT machinery is formed at vesicle budding sites at the cell membrane through recruitment of four cytosolic protein complexes. The ESCRT-0 complex recognizes and sequesters ubiquitin-modified transmembrane proteins in the endosomal membrane. The ESCRT-I and -II complexes are believed to be responsible for membrane budding containing the sorted cargo and ESCRT-III components drive vesicle scission.[27,28] ESCRT vesicular bodies are released from the cytosolic space by a fission event involving a ring of proteins at the bud-membrane interface. This protein ring complex constricts the neck of the bud by forming increasingly smaller rings and finally pinches the budding vesicle from the membrane to be released into the extracellular milieu. [20,29] However, some evidence suggests that MVBs and ILVs can form in the absence of ESCRT.[30,31] Therefore, MVBs and their ILVs can be formed by both ESCRT-dependent and -independent mechanisms related to the cargo that is sorted within a given cell.[20]
Microbial EV components and functions
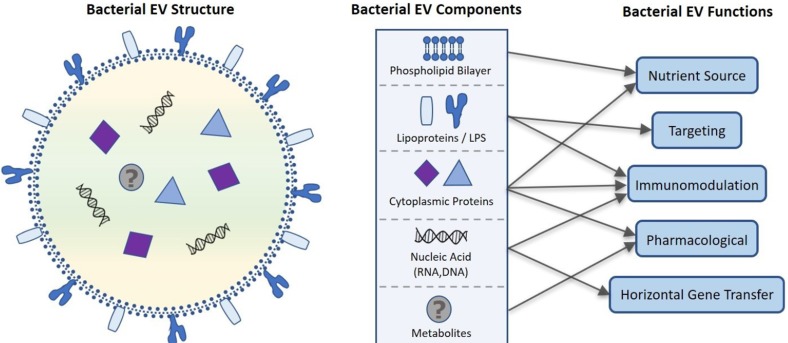
Despite their nanosized dimensions, microbial EVs contain a variety of functional components including microbe-derived lipids, luminal and membrane proteins, nucleic acid (DNA, siRNA, mRNA, tRNA, and sRNA), and possibly metabolites. [3,20,24] After these components are released extracellularly within a spherical phospholipid bilayer, they have a variety of functional roles in host health as outlined in Figure 2. Here we will discuss these functions including drug delivery, targeting, immunomodulation, and gene transfer as they relate to the pharmacological potential of microbial EVs.
Figure 2. Proposed bacterial extracellular vesicle composition and related function. Bacterial EVs are enclosed in a phospholipid bilayer originating from the bacterial membrane that along with lipo- and cytosolic proteins can be used as a nutrient source by targeted cells. Cytoplasmic proteins contained within the EV also have roles in immunomodulation and potential pharmacological applications. Lipoproteins and LPS embedded in the phospholipid bilayer moderate site-specific targeting of the EV for intra- and intercellular communication and EV constituent delivery. Nucleic acids including sRNA, mRNA, tRNA, rRNA, and DNA are transported in bacterial EVs, further contributing to immunomodulation as well as horizontal gene transfer. Recent evidence that bacterial EVs are metabolically active suggest that bacterial EV may also contain metabolites, increasing the pharmacological potential of bacterial EVs.
Role of lipids in microbial EVs
Drug delivery vehicle
The bilayered lipids of OMVs include mainly lipopolysaccharide (LPS) and outer membrane lipids.[32] A number of lipid vesicles called liposomes are incorporated in mammalian cells cultured in vitro with no observable cytotoxic effect.[33] Due to the variety of glycolipids and glycoproteins inserted into liposomal vesicles, uptake of vesicles of defined composition by cells offers a potential to modify cellular composition and to introduce biological activity of molecules into cells.[33]
EVs can be used to deliver a variety of cargo including cyclic nucleotides, enzymes, sugars, and anti-tumor drugs.[34,35,36] Recently, nano or micro particles, such as virus-like particles and liposomal vesicles, are under investigation for their use as a vaccine delivery system.[37,38] Considering this evidence, EVs have notable advantages as pharmacological vehicles. Native OMVs have the immunogenic capacity to carry a wide spectrum of endogenous antigens, and the natural self-adjuvanticity exerted by toll-like receptor (TLR) agonists, such as LPS.[39] However, to use OMVs as vehicles, safety concerns about the inclusion of endotoxic LPS must be resolved due to the potential for excessive secretion of proinflammatory cytokines instigated by LPS.[40] Many studies have explored the potential use of vesicles as a drug delivery vehicle such as Kim et. al. who successfully modified OMVs to be utilized as multifunctional vaccine delivery vehicles.[39]
Specific targeting
Regardless of the exact lipid composition of EVs, the lipid bilayer appears to be adapted to the EV's target environment. pH may differ among target tissues, and the vesicle needs to be rigid enough to maintain in these environments to function optimally.[41] In addition, carbohydrate cell membrane determinants play a significant role in intercellular recognition processes. The carbohydrate recognition systems include the receptors for glycoprotein.[42] The existence of these receptors suggests that it should be possible to control the tissue distribution and cellular uptake of phospholipid vesicles by attaching appropriate carbohydrate determinants to the vesicle surface. [43,44] The modification of the vesicle surface with specific synthetic glycolipids has been shown that the tissue distribution and stability of these vesicles are significantly affected in mice. [45,46] The surface properties of vesicles can be changed by size, surface charge, permeability, and the surface ligand groups. [47] The physical state of lipids is important in determining the pathways by which lipid vesicles are incorporated into cultured cells. Thus, the method manipulating the lipid composition of vesicles have a potential to achieve some degree of targeting of vesicles and their contents to specific regions of the cell.[33]
Role of proteins in microbial EVs
Although lipids are accepted to play important roles in EVs functions, proteins are indispensable. Such proteins can play significant roles in regulatory processes, cellular responses, host-microbe interactions and targeting.[41] According to a variety of proteomic studies, a number of proteins are detected in microbial Evs.[10,48] EV proteins are mainly located in either the membrane or luminal space and are understood to potentially function in immunity, targeting, and pathogenicity.
Membrane proteins
Outer membrane proteins mainly operate in flagellum assembly, pore formation, transport of specific substrates, and outer membrane stabilization. Additionally, various membrane proteins have been shown to be important for antibiotic resistance, proteolysis, and host-bacteria interaction.[32] For example, OprM is required for antibiotic efflux,[49] PonA is involved in β-lactam resistance,[50] and LasA is a protease virulence factor related to chronic lung infections such as cystic fibrosis.[51] In addition, EVs released by bacteria, such as Pseudomonas aeruginosa and Staphylococcus aureus, contain a variety of virulence factors that can stimulate the host immune system.[10,48]
Membrane proteins also play a critical role in targeting of the EV. The carbohydrate cell membrane recognition systems include the receptors for galactose-terminated glycoproteins in hepatocytes, 6-phosphomannosyl containing glycoproteins in fibroblasts, and mannose terminated glycoproteins in macrophages.[52,53,54] These membrane glycoprotein receptors are crucial for specific cell-targeting of bacterial EVs.
Luminal proteins
Proteins are not only localized in EV membranes, but also inside the EV lumen. For example, α-hemolysin was shown to be localized in the lumen of EVs released by Staphylococcus aureus.[55] α-hemolysin is a clinically relevant toxin which kills many types of cells including keratinocytes[56] and has been reported to be associated with atopic dermatitis severity.[57,58] In general, secreted soluble toxins are neutralized and lose their activity by engaging the host immune system.[21] EVs may offer additional protection to bacterial toxins by enveloping them in a cell membrane compared to soluble toxins. Furthermore, EV-associated α-hemolysin induces keratinocyte death more readily than soluble α-hemolysin. In addition, EV-associated α-hemolysin induces necrotic cell death by facilitating toxin entry into the cytoplasm of keratinocytes, while soluble α-hemolysin induces keratinocyte death via apoptosis. Thus, α-hemolysin delivered in the EV lumen enhances keratinocyte death and evasion of host immune defenses, illustrating the clinical significance of EVs in luminal protein delivery.[55]
Role of nucleic acid in microbial EVs
A diverse composition of genetic material is found in microbial Evs.[26] Most research has focused on the nucleic acid contents (DNA, RNA) of EVs in human and eukaryotic cell EVs. In contrast, much less is known regarding the properties and functions of the nucleic acid components of bacterial Evs.[59,60]
It has been well established that microbial EVs carry rRNA, tRNA, mRNA, and small RNA (sRNAs).[59,61] The majority of RNAs in EVs are shorter than 250 nucleotides in length and are therefore classified as sRNAs. It is interesting to note that many RNAs in EVs are noncoding, with prokaryotic RNAs in EVs frequently deriving from intergenic or nonprotein-coding regions. [59,62] In addition, a large proportion of sRNAs in EVs are from uncharacterized intergenic regions and may also modulate gene expression in target cells.[59,63] Recent evidence suggests that bacterial OMVs also carry bacterial DNA mainly at the OMV surface, though their functional role and composition are not yet fully understood.[60] The presence of bacterial DNA in secreted EVs makes it possible to target bacteria-derived EVs systemically as potential biomarkers of disease through metagenomic analysis of the conserved 16S rDNA region of bacteria.[64]
Horizontal gene transfer (HGT)
The presence of RNA in EVs raises considerable interest in the use of microbial EVs as novel mediators of horizontal gene transfer (HGT). RNAs in EVs may attenuate or enhance the expression of specific genes in the target cell.[59,65]
To affect gene expression in target cells directly, RNAs in EVs enter the cytoplasm by fusion of the EV with the target cell membrane. Once in the cytoplasm, microbial RNAs might interact with or be modified by target cell factors, such as the eukaryotic RNA-induced silencing complex (RISC) or RNases. Complementarity between noncoding microbial sRNA in EVs and target cell RNAs might lead to the attenuation or enhancement of gene expression by influencing transcription, translation, or mRNA processing or stability. The mechanisms involved are likely to vary greatly depending on the specific RNA in EV and the presence of specific target cell factors. mRNA in EV might be translated to produce microbial proteins in target cells.[59]
Immunomodulatory effects
Host immune cells and other somatic cells express a variety of pattern-recognition receptors (PRRs) and other conserved pathogen-associated molecular patterns (PAMPs).[59] Pattern recognition receptors (PRRs) include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), retinoid acid inducible gene I (RIG-I)-like receptors (RLRs), and C-type lectin-binding domain receptors. It is recognized that microbial nucleic acids, DNA and RNA, are sensed by either endosomal or cytoplasmic receptors in host cells.[66]
EVs from several bacterial species trigger PRR signaling to promote protective immunity, a property that has been harnessed for vaccine development.[59] For instance, Staphylococcus aureus EV has shown potential as a vaccine candidate by inducing innate immunity dependent on TLR2 signaling in host cells.[67] Most research regarding bacterial EVs and immune modulation as described above has focused on immune regulation by LPS and proteins, which are constituents of bacterial EVs. However, currently there is no available literature that has verified the influence of nucleic acids contained in bacterial EVs on immunomodulation. Thus, further studies are needed to determine the role of nucleic acids associated with bacterial EVs in host immune modulation.
Metabolites in microbial EVs?
Recently, it has been demonstrated that EVs are metabolically active and they present L-asparaginase activity in neural stem cells (NSCs). Although emerging evidence suggests that EVs can act as metabolic regulators, characterization of EVs in metabolic activity is a controversial issue.[68,69] In addition, little is known about the existence and function of metabolites in microbial EVs. Gut microbiota are known to produce a variety of metabolites such as N-acyl amides that can influence and regulate host physiology.[70] Thus, it is necessary to further investigate and characterize the presence and activity of metabolites in microbiota-derived EVs in order to harness their potential metabolic functions.
Conclusion and perspectives
In this review, we have elaborated on recent advancements in microbial EV research including a brief background on the intersectionality of EVs and the human microbiome, the biogenesis of bacterial EVs, the structure and function of their cargo, as well as future biomedical applications of microbiota-derived EVs. Further studies must be undertaken to thoroughly understand the mechanisms underlying the biogenesis of bacterial EVs, particularly MVs in gram-positive bacteria and bacterial apoptotic bodies. EV applications in medicine, especially as biomarkers and pharmabiotics, is an emerging field. Future biomedical applications of microbiota-derived EVs include biomarkers for disease diagnosis and prevention, drug delivery systems for beneficial proteins and miRNAs, as well as adjuvants for vaccine development. As the underlying mechanisms of bacterial EV biogenesis and subsequent physiological consequences to the human host become elucidated, their pharmacological potential can be fully realized.
Acknowledgements
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (NRF-20163A9B6901516 and NRF-2017M3A9F3047497).
Footnotes
Conflict of interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
References
- 1.H Rashed M, Bayraktar E, K Helal G, Abd-Ellah MF, Amero P, Chavez-Reyes A, et al. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017;18:E538. doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience. 2015;65:783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McConnell MJ. Extracellular vesicles and immune modulation. Immunol Cell Biol. 2018;96:681–682. doi: 10.1111/imcb.12188. [DOI] [PubMed] [Google Scholar]
- 5.Lederberg J, Mccray AT. “Ome Sweet'Omics--A Genealogical Treasury of Words”. The Scientist. 2001:8. [Google Scholar]
- 6.Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;356:j831. doi: 10.1136/bmj.j831. [DOI] [PubMed] [Google Scholar]
- 7.Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 10.Peeters SH, de Jonge MI. For the greater good: Programmed cell death in bacterial communities. Microbiol Res. 2018;207:161–169. doi: 10.1016/j.micres.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 11.National Human Genome Research Institute. An Overview of the Human Genome Project. [Accessed 03 Oct 2018]. https://www.genome.gov/12011238/an-overview-of-thehuman-genome-project/
- 12.Hillier LW, Coulson A, Murray JI, Bao Z, Sulston JE, Waterston RH. Genomics in C. elegans: so many genes, such a little worm. Genome Res. 2005;15:1651–1660. doi: 10.1101/gr.3729105. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature. 2017;550:61–66. doi: 10.1038/nature23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nature Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B, Yao M, Lv L, Ling Z, Li L. The Human Microbiota in Health and Disease. Engineering. 2017;3:71–82. [Google Scholar]
- 16.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YJ, Marsland BJ, Bunyavanich S, O'Mahony L, Leung DY, Muraro A, et al. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol. 2017;139:1099–1110. doi: 10.1016/j.jaci.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocucci E, Meldolesi J. Ectosome and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;6:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gramnegative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci U S A. 2010;107:19002–19007. doi: 10.1073/pnas.1008843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allocati N, Masulli M, Di Ilio C, De Laurenzi V. Die for the community: an overview of programmed cell death in bacteria. Cell Death Dis. 2015;6:e1609. doi: 10.1038/cddis.2014.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA cargo selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 28.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 29.Williams RL, Urbé S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 30.Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 31.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev. 2018;27:535–555. doi: 10.1002/mas.20175. [DOI] [PubMed] [Google Scholar]
- 33.Poste G, Papahadjopoulos D. Lipid vesicles as carriers for introducing materials into cultured cells: influence of vesicle lipid composition on mechanism(s) of vesicle incorporation into cells. Proc Natl Acad Sci U S A. 1976;73:1603–1607. doi: 10.1073/pnas.73.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papahadjopoulos D, Poste G, Schaeffer BE, Vail WJ. Membrane fusion and molecular segregation in phospholipid vesicles. Biochim Biophys Acta. 1974;352:10–28. doi: 10.1016/0005-2736(74)90175-8. [DOI] [PubMed] [Google Scholar]
- 35.Papahadjopoulos D, Mayhew E, Poste G, Smith S, Vail WJ. Incorporation of lipid vesicles by mammalian cells provides a potential method for modifying cell behaviour. Nature. 1974;252:163–166. doi: 10.1038/252163a0. [DOI] [PubMed] [Google Scholar]
- 36.Poste G, Papahadjopoulos D, Vail WJ. Lipid vesicles as carriers for introducing biologically active materials into cells. Methods Cell Biol. 1976;14:33–71. doi: 10.1016/s0091-679x(08)60468-9. [DOI] [PubMed] [Google Scholar]
- 37.Casal JI, Rueda P, Hurtado A. Parvovirus-like particles as vaccine vectors. Methods. 1999;19:174–186. doi: 10.1006/meth.1999.0843. [DOI] [PubMed] [Google Scholar]
- 38.Parmar MM, Edwards K, Madden TD. Incorporation of bacterial membrane proteins into liposomes: factors influencing protein reconstitution. Biochim Biophys Acta. 1999;1421:77–90. doi: 10.1016/s0005-2736(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 39.Gho YS, Kim OY, Jang SC, Yoon CM, Kim YK. Method for treating and diagnosing cancer by using cell-derived microvesicles. Biochim Biophys Acta. 2009;1788:2150–2159. [Google Scholar]
- 40.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldeschwieler JD. Phospholipid vesicle targeting using synthetic glycolipid and other determinants. Ann N Y Acad Sci. 1985;446:349–367. doi: 10.1111/j.1749-6632.1985.tb18413.x. [DOI] [PubMed] [Google Scholar]
- 43.Bussian RW, Wriston JC., Jr Influence of incorporated cerebrosides on the interaction of liposomes with HeLa cells. Biochim Biophys Acta. 1977;471:336. doi: 10.1016/0005-2736(77)90261-9. [DOI] [PubMed] [Google Scholar]
- 44.Juliano RL. Drug delivery systems: characteristics and biomedical applications. USA: Oxford University Press; 1980. pp. 189–236. [Google Scholar]
- 45.Mauk MR, Gamble RC, Baldeschwieler JD. Vesicle targeting: timed release and specificity for leukocytes in mice by subcutaneous injection. Science. 1980;207:309–311. doi: 10.1126/science.7350660. [DOI] [PubMed] [Google Scholar]
- 46.Mauk MR, Gamble RC, Baldeschwieler JD. Targeting of lipid vesicles: specificity of carbohydrate receptor analogues for leukocytes in mice. Proc Natl Acad Sci U S A. 1980;77:4430–4434. doi: 10.1073/pnas.77.8.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinstein JN. Liposomes as “targeted” drug carriers: a physical chemical perspective. Pure Appl Chem. 1981;53:2241–2254. [Google Scholar]
- 48.Choi DS, Kim DK, Choi SJ, Lee J, Choi JP, Rho S, et al. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics. 2011;11:3424–3429. doi: 10.1002/pmic.201000212. [DOI] [PubMed] [Google Scholar]
- 49.Chuanchuen R, Narasaki CT, Schweizer HP. The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J Bacteriol. 2002;184:5036–5044. doi: 10.1128/JB.184.18.5036-5044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol Rev. 2008;32:361–385. doi: 10.1111/j.1574-6976.2007.00095.x. [DOI] [PubMed] [Google Scholar]
- 51.Storey DG, Ujack EE, Rabin HR, Mitchell I. Pseudomonas aeruginosa lasR Transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashwell G, Morell AG. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41:99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- 53.Achord DT, Brot FE, Sly WS. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977;77:409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- 54.Stahl PD, Rodman JS, Miller MJ, Schlesinger PH. Evidence for receptormediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978;75:1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong SW, Choi EB, Min TK, Kim JH, Kim MH, Jeon SG, et al. An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus. PLoS One. 2014;9:e100499. doi: 10.1371/journal.pone.0100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 57.Walev I, Martin E, Jonas D, Mohamadzadeh M, Müller-Klieser W, Kunz L, et al. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wichmann K, Uter W, Weiss J, Breuer K, Heratizadeh A, Mai U, et al. Isolation of α-toxin-producing Staphylococcus aureus from the skin of highly sensitized adult patients with severe atopic dermatitis. Br J Dermatol. 2009;161:300–305. doi: 10.1111/j.1365-2133.2009.09229.x. [DOI] [PubMed] [Google Scholar]
- 59.Tsatsaronis JA, Franch-Arroyo S, Resch U, Charpentier E. Extracellular vesicle RNA: A universal mediator of microbial communication. Trends Microbiol. 2018;26:401–410. doi: 10.1016/j.tim.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, et al. Bacteria membrane vesicles transport their DNA cargo into host cells. Sci Rep. 2017;7:7072. doi: 10.1038/s41598-017-07288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambertz U, Oviedo Ovando ME, Vasconcelos EJ, Unrau PJ, Myler PJ, Reiner NE. Small RNAs derived from tRNAs and rRNAs are highly enriched in exosomes from both old and new world Leishmania providing evidence for conserved exosomal RNA Packaging. BMC Genomics. 2015;16:151. doi: 10.1186/s12864-015-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blenkiron C, Simonov D, Muthukaruppan A, Tsai P, Dauros P, Green S, et al. Uropathogenic Escherichia coli releases extracellular vesicles that are associated with RNA. PLoS One. 2016;11:e0160440. doi: 10.1371/journal.pone.0160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sjöström AE, Sandblad L, Uhlin BE, Wai SN. Membrane vesicle-mediated release of bacterial RNA. Sci Rep. 2015;5:15329. doi: 10.1038/srep15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi EB, Hong SW, Kim DK, Jeon SG, Kim KR, Cho SH, et al. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy. 2014;69:517–526. doi: 10.1111/all.12374. [DOI] [PubMed] [Google Scholar]
- 65.Kawamura Y, Yamamoto Y, Sato TA, Ochiya T. Extracellular vesicles as trans-genomic agents: Emerging roles in disease and evolution. Cancer Sci. 2017;108:824–830. doi: 10.1111/cas.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eigenbrod T, Dalpke AH. Bacterial RNA: An underestimated stimulus for innate immune responses. J Immunol. 2015;195:411–418. doi: 10.4049/jimmunol.1500530. [DOI] [PubMed] [Google Scholar]
- 67.Choi SJ, Kim MH, Jeon J, Kim OY, Choi Y, Seo J, et al. Active immunization with extracellular vesicles derived from Staphylococcus aureus effectively protects against Staphylococcal lung infections, mainly via Th1 Cell-mediated immunity. PLoS ONE. 2015;10:e0136021. doi: 10.1371/journal.pone.0136021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iraci N, Gaude E, Leonardi T, Costa ASH, Cossetti C, Peruzzotti-Jametti L, et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 2017;13:951–955. doi: 10.1038/nchembio.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397–408. doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 70.Cohen LJ, Esterhazy D, Kim SH, Lemetre C, Aguilar RR, Gordon EA, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]