Abstract
This tutorial reviews the principles of dose individualisation with an emphasis on target concentration intervention (TCI). Once a target effect is chosen then pharmacodynamics can predict the target concentration and pharmacokinetics can predict the target dose to achieve the required response. Dose individualisation can be considered at three levels: population, group and individual. Population dosing, also known as fixed dosing or “one size fits all” is often used but is poor clinical pharmacology; group dosing uses patient features such as weight, organ function and co-medication to adjust the dose for a typical patient; individual dosing uses observations of patient response to inform about pharmacokinetic and pharmacodynamics in the individual and use these individual differences to individualise dose.
Keywords: Target concentration, Target concentration intervention, Target dose, Target effect, Therapeutic drug monitoring
Objectives
1) Appreciate how a target concentration (TC) strategy is essential for rational clinical use of medicines
2) Understand when and why individual patient monitoring can be used for dose individualisation
3) Distinguish target concentration intervention (TCI) from therapeutic drug monitoring (TDM)
The target principle of dosing
Therapeutics involves a two-step decision process. First, a suitable medicine is chosen to treat the disease process. Second, the right dose of the medicine must be administered to achieve the required effect to treat the disease.
The target approach links pharmacokinetics (PK) with pharmacodynamics (PD) to predict the right dose for a patient.[1] Once a medicine has been chosen to achieve a desired clinical response, the target effect for the patient should then be established. The choice of target effect is always a balance between therapeutic benefit and toxicity and this requires clinical judgement along with comprehensive knowledge of the properties of the medicine. Once the target effect has been decided, the prediction of the right dose can then be done rationally based on knowledge of PD and PK.
All drug effects are linked to concentration and that link is defined by a PD model. The most widely used is the Emax model (Equation 1) which is described by the maximum response, Emax, and the concentration producing 50% of Emax, C50.[2]
| Equation 1 |
The Emax model can be re-arranged to predict the target concentration required to achieve the target effect (Equation 2).
| Equation 2 |
With the target concentration in hand it is then simple to predict the loading dose to reach the target concentration (Equation 3) and the maintenance dose rate to keep the effect on target (Equation 4).[3,4]
| Target Loading Dose = Target Concentration × Volume of Distribution | Equation 3 |
| Target Maintenance Dose Rate = Target Concentration × Clearance | Equation 4 |
How does this work?
How can the target concentration be calculated if there is no PD model available? Suppose the target effect for morphine is to reduce post-operative pain to an acceptably mild degree without unacceptable adverse effects. A commonly recommended dose of morphine sulfate is 5 mg repeated every 4 h according to response.[5] Because morphine sulfate is only 75% morphine this corresponds to a morphine dose of 3.76 mg/4h or 0.94 mg/h. For a 70kg adult, the plasma clearance is about 86 L/h,[6] so the steady state target concentration (using Equation 4) is 0.94/86 = 0.011 mg/L. For a 70kg adult, the steady state volume of distribution of morphine is about 350 L so the intravenous loading dose (using Equation 3) is 350 L x 0.011 mg/L which is about 3.8 mg morphine or 5.1 mg morphine sulfate. This loading dose is consistent with the usual starting dose of 5 mg morphine sulfate. This shows how the target concentration can be worked out based on doses that have already been worked out by trial and error.
For some medicines a so called therapeutic range of concentrations has been established by trial and error approaches. This range of concentrations is better thought of as an acceptable range as it does not define the target concentration. With the initial guidance of the acceptable range, a clinical trial can be designed to compare potential target concentrations. This approach was used find the target concentration for starting treatment with theophylline in patients with severe airway obstruction.[7] Patients were randomised to targets of 10 and 20 mg/L and clinicians adjusted the dose after measuring concentrations to reach the target. This trial showed 10 mg/L was better than 20 mg/L. It produced a reasonable bronchodilator effect without serious adverse effects. Subsequent analysis of the results led to development of a PD model for theophylline.[2,8]
Three ways to dose
There are 3 ways to think about choosing the dose.
The population dosing method uses the same dose for everyone. This method is also known as fixed dosing or “one size fits all”. Due to its convenience it is the most commonly used method, however, by treating everyone as though they were the same, it ignores differences between patients. This means that some patients are either under-dosed or over-dosed.
The group dosing method uses patient factors such as weight, organ function or co-medication to predict the dose suitable for a group of patients with similar factors. Dosing in children is nearly always based on weight or age. The use of weight or renal function to adjust the dose is recommended for some medicines but is probably not used as often as it should be.
Individual dosing based on patient response is widely used when the response is easily measured. For example, anti-hypertensive doses are usually adjusted based on the blood pressure response. The use of other response markers such as the international normalised ratio (INR) response to warfarin or the concentration of an antibiotic such as gentamicin are also examples.
Understanding patient variability and dose individualisation
The key properties of a medicine that determine concentrations and effects are CL, V, Emax and C50. Patient variability in response can be explained by individual differences in these properties. Some variability is predictable and some is unpredictable.[9]
Predictable variability
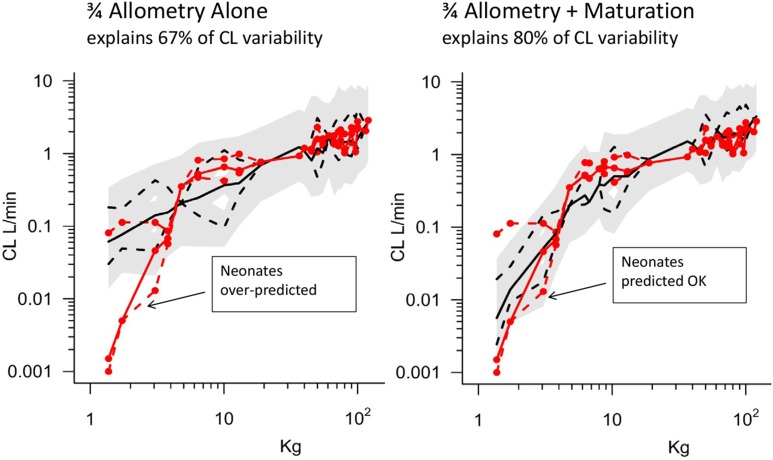
Propofol is a commonly used intravenous anaesthetic whose dose is largely predictable from weight and age (Fig. 1). This figure shows observed clearance values (red) over a wide range of weight and ages. Predictions of clearance based only in weight (allometry) or weight combined with age (maturation) show that up to 80% of variation in clearance is predictable and this can be used to work out the infusion rate of propofol. The plasma clearance of propofol in a 70 kg adult is about 2 L/min or 120 L/h and the target concentration is 5 mg/L so the required infusion rate is 600 mg/h (using Equation 4).
Figure 1. Predictable Sources of Variability in the Clearance of Propofol (data from Peeters, Allegaert et al. 2010).[10].
Unpredictable variability
Despite the consideration of covariates (e.g. weight, sex, age, disease, concomitant medications), there remains a substantial variability in clearance which cannot be predicted. This is attributable to seemingly random differences between and within patients. This between patient unpredictable variability is the reason for measuring patient responses on treatment because the response can be used to identify the individual difference. This now allows a better prediction of the dose for that individual. This principle applies not only to clearance differences but also the other 3 key drug properties of V, Emax and C50.
Therapeutic drug monitoring or target concentration intervention?
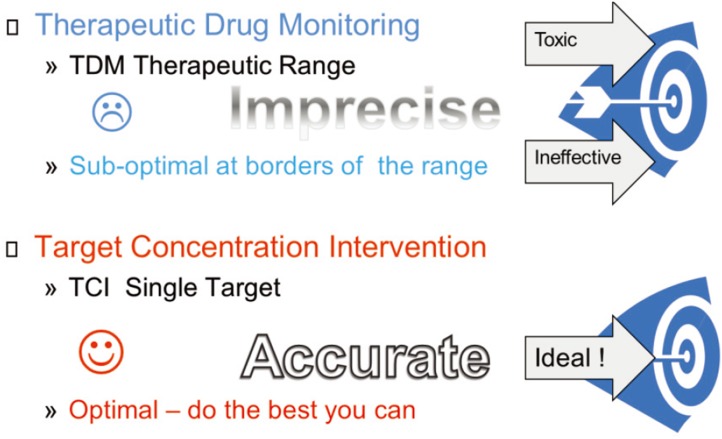
Therapeutic drug monitoring (TDM) is a traditional concept associated with empirical ‘seat of the pants’ dose adjustment determined by a measurement being outside a ‘therapeutic range’. The therapeutic range is hard to identify and is often mistakenly justified because it is based on the same concept as a normal reference range for endogenous substances (e.g. sodium, potassium). As drug concentrations are continuous, a concentration at the bottom of the range has a very different meaning (close to being ineffective) from one at the top (close to being toxic), however, TDM practitioners usually ignore this and are happy to do nothing as long as the concentration is ‘within range’. TDM is typically limited to measuring a response such as concentration without any clear understanding that the response provides feedback on how the individual patient differs from the other patients, and this needs to be used to predict the required dose and then that dose should be administered.
Target concentration intervention (TCI) is a science based method that uses PK and PD principles to identify how patients are different in terms of parameters such as CL, V, Emax and C50 and give the dose needed to reach the target. The first component of TCI is to use the individual parameters to predict the dose required to achieve the target as explained above. The second component of TCI is to administer the predicted dose in order to achieve the therapeutic target. It has been shown to improve clinical outcome as well as being a cost-effective use of health resources.
When to use TCI
The first reason for using TCI is when the effect of treatment on the desired outcome cannot be easily measured. Cardiac anti-arrhythmic drugs and anti-convulsants (brain anti-arrhythmic drugs) may be having a useful effect but often arrhythmias (heart or brain) occur only intermittently so direct observation may not provide enough feedback to know if the treatment is effective. TCI can be used to maintain drug concentration, a surrogate for anti-arrhythmic effect, at a target which is known to be effective in most people (Fig. 2).
Figure 2. The Properties of the Drug and Treatment That Point to the Need for TCI.
The second reason for using TCI is when group based dosing (e.g. using weight) does not reduce between subject variability sufficiently so it becomes safe and effective within such a group. Group based dosing removes the predictable component of between subject variability but the remaining unpredictable component may still be too large for the medicine to be used safely and effectively. This is where the use of individual patient response can further reduce variability and improve the probability of patients being within an acceptable range around the target concentration.[11] Eventually a limit of variability reduction is reached, attributable to within subject variability, that cannot be influenced by TCI.
The target concentration strategy
The target concentration strategy (Fig. 3) is an algorithm for reaching the best individual dose. It starts with choosing a target concentration.[12] A group value for volume (V) and or clearance (CL) is determined before the medicine is given. These PK parameters are then used to calculate the initial loading dose (LD) and maintenance dose rate (MDR). A response is measured reflecting how the individual is different from the group of patients with similar covariates (e.g. weight, renal function, etc). If the response is a measure of drug effect (e.g. INR), then it can be used to revise the target concentration. If the response is a concentration then it can be used to revise V and CL. Most commonly the focus will be on CL so that a new individualised maintenance dose rate can be calculated.
Figure 3. The target concentration strategy.
Target concentrations and PK parameters are known for most medicines which are helped by TCI. The PD parameters for medicines that use TCI are typically not well known. This is a reflection of the difficulty of measuring a clinical response that can be related to concentration.
When to measure the response?
A rational approach to measuring drug concentrations is based on using the measurement to predict PK parameters - most commonly clearance.
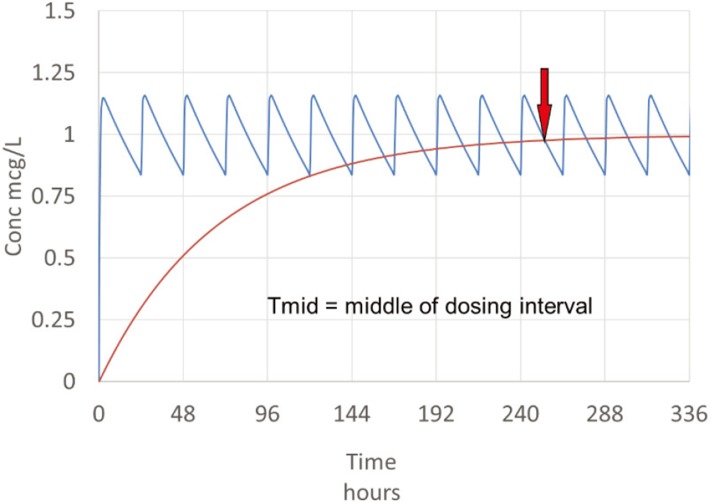
The least informative time to measure concentrations is just before the next dose (the ‘trough’ concentration) unless this is paired with another ‘peak’ concentration. This is because clearance determines the average concentration, so measuring a concentration in the middle of the dosing interval will be closer to the average and therefore more useful for predicting clearance.
A concentration in the middle of the dosing interval (Ctmid) will be closer to the average steady state concentration (Css) then either a peak or trough concentration (Fig. 4).
Figure 4. Sampling strategy for most drugs.
Clearance is easily calculated from CL=DoseRate/Css which can be approximated by CL=DoseRate/CTmid.
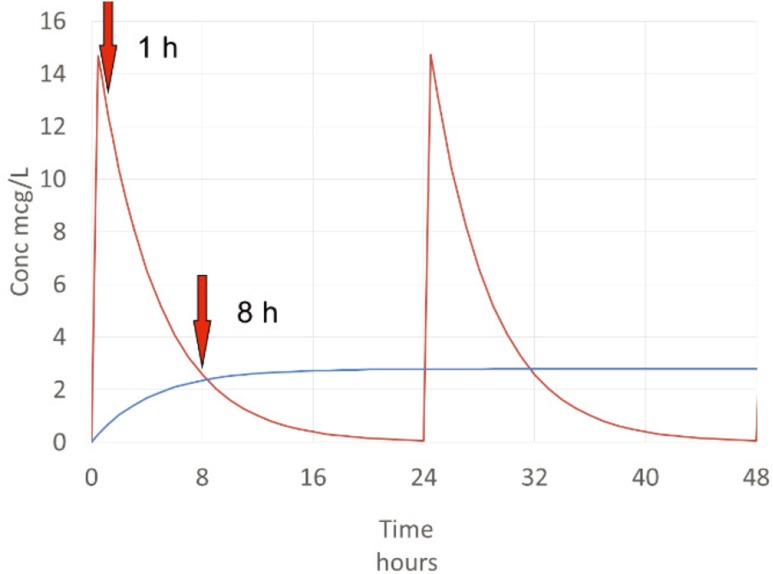
Gentamicin concentrations with once a day dosing vary considerably. The trough concentration, typically measured at 24 hours after the last dose, is often unmeasurable because it is below the limit of quantitation. Gentamicin concentrations vary widely in a dosing interval so two concentrations are needed to reliably estimate clearance. The sooner the sample is taken the sooner concentrations can be used to improve dosing. These concentrations are best measured at one and eight hours after the dose. In this example a dose of 240 mg was given every 24 hours (Fig. 5).
Figure 5. Sampling strategy for gentamicin.
The concentrations measured at these times can be used to estimate the individual V and CL parameters. V can be approximated from the first concentration (C1 ≈ 13 mg/L) assuming about 15% of the dose is eliminated in 1 hour:
Half-life can be estimated from C1 at time T1 (1 h) and the second concentration (C2 ≈ 2.5 mg/L) at time T2 (8 h):
CL can then be calculated:
The bottom line - TCI is better than TDM
Figure 6 shows the main differences between the TDM and TCI approaches. Because TDM focusses on concentration and not on changing the dose to achieve the desired outcome it is a sub-optimal method. TCI on the other hand focusses on changing the dose to achieve the target response and the desired outcome. The interpretation of the concentration may be relatively easy in some cases if the sample is likely to be an average steady state concentration. More commonly the interpretation needs to be taken note of the wider context of what is known about the PK (and PD). Methods such as Bayesian forecasting make the most out of limited sampling data. They have been in use since the early 1970s [13] and are the still the state of the art.
Figure 6. TDM and TCI.
The difference in clinical outcome between the TCI and TDM approaches has been clearly demonstrated when using mycophenolate concentrations to optimise immunosuppression after renal transplantation. There are 2 landmark TCI trials - the first [14] used a randomised concentration controlled trial approach to compare 3 potential targets. The target dose was predicted using Bayesian methods and the predicted dose was administered resulting in three groups of narrowly defined concentrations. The rate of transplantation rejection was clearly reduced. The second TCI trial [15] compared an exposure target identified from the Hale 1998 study and compared it, using Bayesian dose individualisation, to standard of care. Once again there was a dramatic reduction in transplant rejection. Two other large trials which used similar concentration sampling but left it to the clinician to calculate the dose and decide whether to adjust the dose showed no benefit in the TDM group compared with the control group.[16,17]
TDM has been common in clinical practice because it is easy to do (measure the concentration) and requires little further effort from the clinician. Trough concentrations are widely measured but this is the least informative time to learn about a key parameter such as CL. This means that the dose may be only changed if the concentration is outside the therapeutic range and when inside the range the measurement is often confused with the target.
TCI is an active process that builds upon the measurement of a concentration sampled at a time that can aid the identification of parameters such as CL in an individual. The parameters are used to predict the target dose and the dose is administered with further iterations of the target concentration strategy.
Acknowledgement
Mr Guangda Ma is thanked for his helpful suggestions.
Footnotes
Reviewer: This article was invited and reviewed by the editors of TCP.
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
References
- 1.Holford NH. Target concentration intervention: beyond Y2K. Br J Clin Pharmacol. 1999;48:9–13. doi: 10.1046/j.1365-2125.1999.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holford N. Pharmacodynamic principles and the time course of immediate drug effects. Transl Clin Pharmacol. 2017;25:157–161. doi: 10.12793/tcp.2017.25.4.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holford N, Yim DS. Clearance. Transl Clin Pharmacol. 2015;23:42–45. [Google Scholar]
- 4.Holford N, Yim DS. Volume of distribution. Transl Clin Pharmacol. 2016;24:74–77. [Google Scholar]
- 5.New Zealand Formulary. Morphine monograph. 2019. https://nzf.org.nz/nzf_2515.
- 6.Holford NH, Ma SC, Anderson BJ. Prediction of morphine dose in humans. Paediatr Anaesth. 2012;22:209–222. doi: 10.1111/j.1460-9592.2011.03782.x. [DOI] [PubMed] [Google Scholar]
- 7.Holford N, Black P, Couch R, Kennedy J, Briant R. Theophylline target concentration in severe airways obstruction - 10 or 20 mg/L? A randomised concentration-controlled trial. Clin Pharmacokinet. 1993;25:495–505. doi: 10.2165/00003088-199325060-00007. [DOI] [PubMed] [Google Scholar]
- 8.Holford N, Hashimoto Y, Sheiner LB. Time and theophylline concentration help explain the recovery of peak flow following acute airways obstruction. Population analysis of a randomised concentration controlled trial. Clin Pharmacokinet. 1993;25:506–515. doi: 10.2165/00003088-199325060-00008. [DOI] [PubMed] [Google Scholar]
- 9.Holford N. Pharmacokinetic variability due to environmental differences. Transl Clin Pharmacol. 2017;25:59–62. doi: 10.12793/tcp.2017.25.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peeters MY, Allegaert K, Blussé van Oud-Alblas HJ, Cella M, Tibboel D, Danhof M, et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed-exponent allometric model. Clin Pharmacokinet. 2010;49:269–275. doi: 10.2165/11319350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Holford NH, Buclin T. Safe and effective variability - a criterion for dose individualization. Ther Drug Monit. 2012;34:565–568. doi: 10.1097/FTD.0b013e31826aabc3. [DOI] [PubMed] [Google Scholar]
- 12.Sheiner L, Tozer T. Clinical pharmacokinetics: The use of plasma concentrations of drugs. In: Melmon K, Morelli H, editors. Clinical Pharmacology: Basic Principles of Therapeutics. New York: Macmillan; 1978. pp. 71–109. [Google Scholar]
- 13.Sheiner LB, Rosenberg B, Melmon KL. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res. 1972;5:441–459. doi: 10.1016/0010-4809(72)90051-1. [DOI] [PubMed] [Google Scholar]
- 14.Hale MD, Nicholls AJ, Bullingham RE, Hené R, Hoitsma A, Squifflet JP, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998;64:672–683. doi: 10.1016/S0009-9236(98)90058-3. [DOI] [PubMed] [Google Scholar]
- 15.Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain F, Lavaud S, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 16.van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261–266. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 17.Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, et al. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial. Am J Transplant. 2009;9:1607–1619. doi: 10.1111/j.1600-6143.2009.02668.x. [DOI] [PubMed] [Google Scholar]