Abstract
Heart failure (HF) remains a major cause of mortality, morbidity, and poor quality of life. It is an area of active research. This article is aimed to give an update on recent advances in all aspects of this syndrome. Major changes occurred in drug treatment of HF with reduced ejection fraction (HFrEF). Sacubitril/valsartan is indicated as a substitute to ACEi/ARBs after PARADIGM‐HF (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.73 to 0.87 for sacubitril/valsartan vs. enalapril for the primary endpoint and Wei, Lin and Weissfeld HR 0.79, 95% CI 0.71–0.89 for recurrent events). Its initiation was then shown as safe and potentially useful in recent studies in patients hospitalized for acute HF. More recently, dapagliflozin and prevention of adverse‐outcomes in DAPA‐HF trial showed the beneficial effects of the sodium–glucose transporter type 2 inhibitor dapaglifozin vs. placebo, added to optimal standard therapy [HR, 0.74; 95% CI, 0.65 to 0.85;0.74; 95% CI, 0.65 to 0.85 for the primary endpoint]. Trials with other SGLT 2 inhibitors and in other patients, such as those with HF with preserved ejection fraction (HFpEF) or with recent decompensation, are ongoing. Multiple studies showed the unfavourable prognostic significance of abnormalities in serum potassium levels. Potassium lowering agents may allow initiation and titration of mineralocorticoid antagonists in a larger proportion of patients. Meta‐analyses suggest better outcomes with ferric carboxymaltose in patients with iron deficiency. Drugs effective in HFrEF may be useful also in HF with mid‐range ejection fraction. Better diagnosis and phenotype characterization seem warranted in HF with preserved ejection fraction. These and other burning aspects of HF research are summarized and reviewed in this article.
Keywords: heart failure, HFrEF, HFpEF, HFmrEF, acute heart failure, treatment
Introduction
Heart failure (HF) remai006Es a major cause of mortality, morbidity, namely hospitalizations, and poor quality of life. Landmark trials, able to cause major changes in evidence based clinical practice, continue to be too few. However, new data are continuously produced, and a summary of recent new findings is provided herewith.
Epidemiology
Heart failure has a worldwide diffusion. Several studies assessed its characteristics in different regions of the world. Asian patients with HF are younger and have different risk factors, compared with Western patients.1 In an analysis of the ASIAN‐HF registry, the prevalence of diabetes ranged between 34.1% in Japanese/Koreans and 51.9% in Malaysian patients. The increase in the risk of death and HF hospitalizations associated with diabetes remained significant and independent from ethnicity at multivariable analysis (hazard ratio [HR] 1.37, 95% confidence intervals [CI] 1.19–1.57 for death or HF hospitalizations).2 Outcomes differ in Asian patients, compared with the European ones. Nagai et al. assessed the accuracy of published prediction models to predict mortality in British vs. Japanese patients. Whereas these models were moderately accurate in British patients, they consistently overestimated mortality in Japanese, consistently with their better prognosis.3
Classification and specific causes
Classification of HF remains based on left ventricular (LV) ejection fraction (EF). The main reason is that trials enrolling patients with a reduced EF (HFrEF) were successful in the identification of drugs and devices improving their outcomes.4 Thus, a reduced LVEF defines a phenotype of patients in whom neurohormonal activation and other mechanisms, such as tachycardia or LV dissynchrony, contribute to the progression of LV dysfunction and patients' outcomes. Accordingly, a network meta‐analysis showed that the combination of three neurohormonal antagonists, possibly with the addition of ivabradine, when indicated, is associated with the largest reduction in the risk of death or HF hospitalizations or of deaths alone.5
A still often overlooked cause of HF is cardiac amyloidosis. This is even more critical nowadays as a specific treatment for transthyretin amyloidosis has been identified in a randomized clinical trial. As stated in a recent consensus document by the HF Association, older patients with HF, particularly those with preserved ejection fraction (HFpEF) who are not hypertensive or who have features of hypertrophic or restrictive cardiomyopathy, degenerative aortic stenosis, and progressive HF, should be considered for screening for cardiac transthyretin amyloidosis. Hereditary transthyretin amyloidosis may have some peculiar features, such as younger age of onset and higher likelihood of a mild neurological involvement, compared with wild type transthyretin amyloidosis.6
Genetic analysis may allow personalized treatment. Specific mutations in the gene for lamin A and lamin C may be associated with different outcomes of their carriers. The amount of mutated protein in the nuclear envelope is associated with poorer outcomes of the patients with lamin A and lamin C missense mutations carriers.7 Titin mutations may also directly cause a dilated cardiomyopathy phenotype or increase heart's susceptibility to injury.8
Diagnosis and prognosis
A diagnostic algorithm for acute and chronic HF is provided by the 2016 European Society of Cardiology (ESC) guidelines.4 The use of natriuretic peptides (NPs) is recommended to rule‐out HF, but is not diagnostic, since its positive predictive value is low. The diagnostic accuracy of NPs is particularly reduced in elderly subjects with acute dyspnoea as they do not discriminate cardiac vs. respiratory origin.9 Beyond age, multiple variables influence NPs values, such as sex, obesity,10 and heart rhythm with higher levels in atrial fibrillation.11
Heart failure remains associated with poor outcomes, comparable, if not worse, to that of the most common cancers.12 Unplanned hospitalizations for HF and, in general, episodes of worsening HF are landmark events in patients' clinical course and are followed by a marked increase in mortality and rehospitalizations, compared with when the patients remain ambulatory.13, 14, 15, 16
Clinical signs
Simple clinical signs retain a main prognostic value as summarized in guidelines and position statements.4, 17 Poorer outcome are associated with a lower systolic blood pressure and higher heart rate and their decrease or lack of decrease, respectively.18, 19, 20 The role of comorbidities is outlined in the next chapters.
Biomarkers
Biomarkers maintain a major role for the prognostic assessment of HF patients. Multimarker strategies are emerging. More data compare novel biomarkers with established ones, such as serum creatinine and BNP. N‐terminal pro BNP (NT‐proBNP) and troponin outperformed other emerging biomarkers in prediction of adverse outcome in some recent studies.21, 22, 23 Multimarker models and serial measurements during the hospitalization may improve risk prediction.24, 25
Plasma renin activity has prognostic value with poorer outcomes in those patients with its greater activation. However, it did not allow to identify the patients with a better response to treatment with aliskiren in the Aliskiren Trial on Acute HF Outcomes.26 Elevated levels of growth differentiation factor‐15, were associated with diabetes, age, creatinine, high‐sensitive troponin T, NT‐proBNP, and New York Heart Association (NYHA) Class III/IV in patients with ambulatory HFrEF from the Prospective comparison of Angiotensin Receptor neprilysin inhibitor (ARNI) with Angiotensin converting enzyme inhibitor (ACEi) to Determine Impact on Global Mortality and morbidity in HF trial (PARADIGM‐HF). Growth differentiation factor‐15 was strongly related with mortality and cardiovascular (CV) outcomes but this relation was not modified by sacubitril/valsartan.27
MicroRNAs are another major area of research. Circulating levels of different microRNAs may be differentially expressed in patients with HF of different aetiologies (ischaemic and nonischaemic).28 Plasma levels may have prognostic value in acute or chronic HF and may change after treatment, although final evidence is still lacking.29, 30, 31
Risk stratification models
A multiparametric approach is often proposed for risk stratification of HF patients. The prognostic accuracy of the metabolic exercise test data combined with cardiac and kidney indexes risk score was found as superior to the HF Survival Score and Seattle HF model risk scores in a cohort of 6112 ambulatory stable HF patients.32 Predictors of mortality differ from predictors of HF hospitalization. In an analysis of the systems BIOlogy Study to TAilored Treatment in Chronic HF cohort, the five strongest predictors of mortality were more advanced age, higher blood urea nitrogen and NT‐proBNP, lower haemoglobin, and failure to prescribe a beta blocker. The five strongest predictors of HF hospitalization were more advanced age, previous HF hospitalization, presence of oedema, lower systolic blood pressure, and lower estimated glomerular filtration rate.33
Imaging techniques and invasive haemodynamics
Algorithms to estimate LV filling pressure based on echocardiography have been developed and validated.34, 35 Assessment of right ventricular (RV) function also requires a multiparametric approach. RV function has an independent value compared with pulmonary pressure both in patients with HFrEF and, interestingly, also in patients with preserved EF (HFpEF).36, 37
Right heart catheterization allows discrimination between those patients with LV dysfunction who have isolated post capillary pulmonary hypertension and those with combined postcapillary and precapillary pulmonary hypertension. These last patients are those with the worst outcomes.38 The value of the finding of a negative diastolic pulmonary pressure gradient is now challenged by its high dependency from mitral regurgitation and other variables.39
Medical treatment of HFrEF
Neurohormonal antagonists
Medical treatment of ambulatory patients with HFrEF is based on diuretics to relieve congestion and neurohormonal antagonists to improve the clinical course, to reduce HF hospitalizations, and to improve mortality. ACEi or angiotensin receptor blockers (ARB), beta blockers, and mineralocorticoid antagonists (MRA) are the pillars of current management of HFrEF.4 Ivabradine is indicated in patients whose heart rate remains >70 bpm.4 The Prospective comparison of ARNI with ACEi to PARADIGM‐HF trial showed a reduction in the combined endpoint of CV death or HF hospitalizations, (HR, 0.80; 95% CI, 0.73 to 0.87) as well as all‐cause death, CV death and HF hospitalizations alone, and an improvement in symptoms with sacubitril/valsartan vs. enalapril.40 Further, 34% of the patients who had a primary event experienced at least one other HF hospitalization and sacubitril/valsartan had a similar efficacy to reduce recurrent events (Figure 1).41 Quality of life improved and diuretic doses were slightly but significantly reduced in the patients on sacubitril/valsartan, consistent with favourable effects also on symptoms.42, 43 Based on these results,40, 44 sacubitril/valsartan is currently recommended as a replacement for ACEi/ARBs in ambulatory patients with HFrEF who remain symptomatic despite optimal medical treatment.4, 45 Based on more recent trials,44, 46 it may be considered also in patients hospitalized for acute HF, including also those with new onset HF.45
Figure 1.
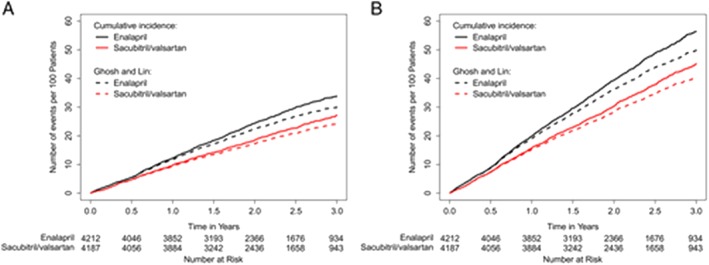
Effect of sacubitril/valsartan vs. ACE inhibitors on recurrent events. Cumulative rate of heart failure hospitalizations (A) and the primary composite endpoint (B). From reference 41.
Combined treatment with the drugs above is associated with the largest reduction in mortality and hospitalizations.5 Adherence to evidence based therapy and guidelines has an independent determinant of patients' outcomes.47, 48 Further research is therefore focused on the optimization of medical treatment in HFrEF patients.49 When the inclusion criteria of PARADIGM‐HF were applied in a large cohort of ambulatory patients with HFrEF, trial, only 12% at baseline and 21% during follow‐up met the criteria for treatment. However, this proportion rose to 60% if background treatment was not considered.50 A slow titration of sacubitril/valsartan was associated with better tolerance and persistence on maximal doses in the patients with lower systolic blood pressure in the titration study.51
Potassium lowering agents
Data from large observational studies have consistently shown a U‐shaped relationship between serum potassium levels and mortality in patients with HF as well as hypertension and myocardial infarction. Ideal serum potassium levels range between 3.5 and 4.5 mmol/L and an increase in mortality can be shown with serum potassium levels >5 mmol/L.52 Hyperkaliaemia has a major role limiting the use and titration to target doses of ACEi/ARBs and, to an even larger extent, MRAs, in patients with HFrEF, especially when associated with chronic kidney disease (CKD). 53, 54, 55, 56, 57 Novel potassium binders may be considered in patients with HF, with or without CKD, and may allow the initiation and titration of ACEi/ARB and, to an even larger extent, MRAs in more patients with HFrEF. Their efficacy in lowering serum potassium levels is proven. However, we lack of data showing the impact of these drugs on patients' outcomes.45
Treatment of iron deficiency
In addition to its role in anaemia, iron has direct effects on mitochondrial function in skeletal muscle and the heart itself. Its deficiency may be also a cause of impaired contractility.58, 59, 60 Ferric carboxymaltose improves functional capacity, symptoms, and quality of life of HF patients with iron deficiency.61, 62 A meta‐analysis of data from the four major randomized controlled trials conducted, to date, demonstrated also a reduction in recurrent CV hospitalizations (Figure 2).63 Ongoing trials are aimed to showing the impact of iron therapy on outcomes, including mortality.45, 62
Figure 2.
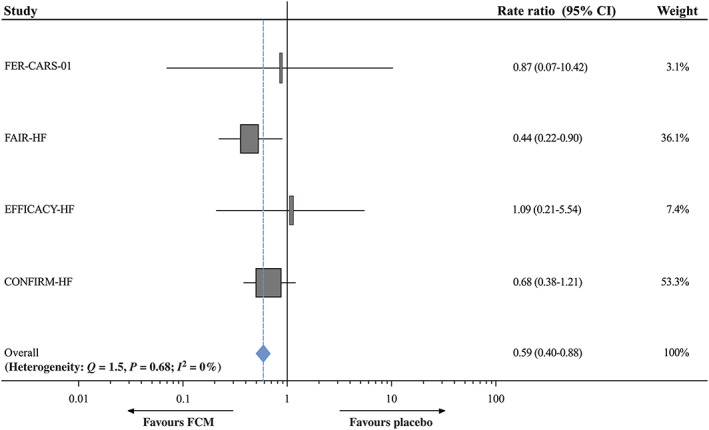
Potential pathways linking empagliflozin with heart failure outcome improvement. CV, cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; MI, myocardial infarction; REG, removal of excess glucose; SGLT2i, sodium–glucose co‐transporter 2 inhibitors. UNa, urinary sodium. From reference 63
Antidiabetic drugs
The choice of antidiabetic drugs has a pivotal role in the development and progression of HF and other CV outcomes.64, 65 Since the discovery that rosiglitazone is associated with an increased risk of myocardial infarction and CV death,66 the assessment of CV events has gained a central role in the evaluation of antidiabetic treatment. These studies has allowed the identification of antidiabetic drugs that may improve CV outcomes of diabetic patients and has then opened the pathway for a new treatment of HF, independently from the presence of diabetes.
An analysis of 24 012 patients with HF from four large randomized trials and of an administrative database of 4 million individuals, 103 857 of whom with HF, showed that insulin treatment is independently associated with increased all‐cause mortality and HF hospitalizations.67 Incretin‐based therapies, glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors, do not seem to have major effects on HF events, although an increase in HF admissions was observed with the dipeptidyl peptidase‐4 inhibitor saxagliptin.68 The glucagon‐like peptide‐1 agonist liraglutide reduced CV deaths and all‐cause deaths but had no significant effect on HF hospitalizations in a major trial.69 No effects on LV function were found in another smaller trial.70
The Empagliflozin CV Outcome Event Trial in type 2 diabetes mellitus patients at high risk of CV events was the first trial showing a favourable effect of sodium–glucose transporter type 2 (SGLT2) inhibitors on HF events. Compared with placebo, empagliflozin reduced CV mortality, all‐cause mortality and HF hospitalizations by 38%, 30%, and 35%, respectively.64, 71, 72 Further trials a reduction in HF hospitalizations in diabetic patients at risk of CV events also with the two other SGLT2 inhibitors canaglifozin and dapaglifozin.73, 74 Rates of major CV events, namely stroke and myocardial infarction, were not changed whereas renal events and CKD progression were reduced in these trials.72, 73, 74
Although the mechanisms of action are still unsettled and likely go beyond the glycosuric and natriuretic effects and involve direct effects on myocardial metabolism and function,71, 75, 76, 77 trials' results consistently showed that SGLT2 inhibitors have favourable that are specific for the development and progression of HF (Figure 3). This led to the start of randomized controlled trials in patients with HF, both with and without diabetes and the first trial has been recently accomplished. The Dapagliflozin And Prevention of Adverse‐outcomes in HF trial was an international, multicentre, parallel group, randomized, double‐blind, study in 4744 patients with chronic HFrEF. It assessed the effect of dapagliflozin 10 mg, compared with placebo, administered once daily, in addition to standard therapy, on the primary composite outcome of a worsening HF event (hospitalization or equivalent event, i.e. an urgent HF visit) or CV death.78 Median follow‐up was of 18.2 months. The primary outcome occurred in 16.3% of the patients in the dapagliflozin group vs. 21.2% of the patients on placebo (HR, 0.74; 95% CI, 0.65 to 0.85). Reductions in the worsening HF event (HR, 0.70; 95% CI, 0.59 to 0.83), CV death (HR, 0.82; 95% CI, 0.69 to 0.98), and all‐ cause death (HR, 0.83; 95% CI, 0.71 to 0.97) were also significant with dapaglifozin vs. placebo. Importantly, results were similar in patients with diabetes vs. those without diabetes with no interaction with drug efficacy.79 The frequency of adverse events did not differ between treatment groups.79 Trials with other SGLT2 inhibitors are ongoing. These results open a new pathway for HFrEF, if not HF overall, treatment with drugs having different mechanisms of action, independent from the neurohormonal pathways exploited, to date.
Figure 3.
Comparison of hard endpoints from MITRA‐FR and COAPT. COAPT, Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation; GDMT, guideline‐directed medical therapy; MITRA‐FR, Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation. From reference 71
Other options
Treatment of advanced chronic HF remains a major unmet need.17 A meta‐analysis has recently suggested that ambulatory infusions of inotropic agents may have favourable effects on symptoms with neutral effects on mortality.80 Intermittent inotropes infusions improve quality of life in these patients and do not seem to affect long‐term survival.81, 82 In recent trial, intermittent administration of intravenous levosimendan lowered NT‐proBNP levels, primary endpoint of the trial, reduced HF hospitalization and improved the quality of life in 69 outpatients with advanced chronic HF.83
Currently, routine use of ultrafiltration is not recommended and should be limited to patients who fail to respond to diuretics.4 Differently from the original intention‐to‐treat analysis a per‐protocol analysis of CARdiorenal REScue Study in acute decompensated HF showed a larger fluid removal with ultrafiltration vs. diuretic therapy.84, 85
Palliative care is still largely underused in patients with advanced HF, and its implementation should be a target of next efforts.17, 86, 87
Devices
Cardiac resynchronization therapy
Currently, the use of cardiac resynchronization therapy (CRT) is lower than what could be expected based on guidelines indications.88, 89, 90 Multiple predictors of the response to CRT have been identified. An individual patient meta‐analysis of three double‐blind, randomized trials showed that longer QRS duration and lower LVEF independently predict early clinical response to CRT.91 Both QRS duration and morphology, with a greater response in patients with left bundle branch block, were significant in other studies.92, 93 Contractile reserve after dobutamine infusion predicted the response to CRT in a meta‐analysis of nine observational studies including 767 patients.
An individual‐patient data meta‐analysis of five randomized controlled trials assessed the impact of sex, QRS duration, HF aetiology, LV end‐diastolic diameter, and height on the effects of CRT, vs. control, on deaths and the composite endpoint of deaths and HF hospitalizations.94 QRS duration was the only independent predictor of CRT benefit on mortality. For the composite outcome, height and QRS duration, but not sex, were independent predictors of CRT benefit with a continuous and independent relationship with the risk reduction with CRT vs. control. The effect of height may explain the greater benefit of CRT in women. The authors of this analysis concluded that QRS threshold for CRT indication, now fixed at 130 ms,4 may need adjustment based on patient's height.94
An analysis from the ESC CRT Survey II showed that the upgrade to CRT in carriers of permanent pacemaker or of an implantable cardioverter defibrillator (ICD) has the same success and complications rates as for patients undergoing de novo CRT implantation.95 In patients with atrial fibrillation treated with CRT, atrioventricular junction ablation was associated with reduced ICD shocks and hospitalizations compared with the use of medical therapy to slow heart.96
New pacing modalities are under study. LV‐only pacing timed with native RV activation when the AV interval is normal showed better results, compared with traditional CRT, on LVEF and strain through better apical and septal function.97 The effects of this new pacing modality is currently tested in large multicentre trial.98
Implantable cardioverter defibrillator
Current indications to ICD implantation may need some revision after publication of DANISH, a Danish randomized, controlled, multicentre study to assess the efficacy of ICD in patients with nonischaemic systolic HF on mortality.99 In his study, implantation of an ICD reduced SCD by 50% but not all‐cause mortality, (HR, 0.87; 95% CI, 0.68 to 1.12) in patients with nonischaemic cardiomyopathy. A significant treatment‐by‐subgroup interaction was found for age with a 49% reduction in mortality in the patients aged <58 years, P = 0.02, a nonsignificant 25% reduction in mortality in the patients aged 58 to 67 years and a nonsignificant 19% increase in mortality in the patients aged >68 years (P = 0.009 for interaction).99 These results were likely caused by the larger contribution of non‐HF‐related causes of deaths in older patents. Based on these data, it has been hypothesized that one may consider not to implant an ICD in patients with nonischaemic HFrEF who are aged >70 years or have advanced symptoms of HF or have life‐shortening co‐morbidity (e.g. severe lung disease or Stage IV CKD) as they are likely to die for non‐SCD related reasons.45
Consistent results regarding the limitations of ICDs in the prevention of all‐cause deaths were found in a patient‐level combined‐analysis of four major primary prevention trials in HFrEF patients. The effects of ICD on all‐cause deaths were assessed in diabetic vs. nondiabetic patients. ICDs were associated with a reduced risk of all‐cause mortality among patients without diabetes (HR, 0.56; 95% CI, 0.46–0.67) but not among patients with diabetes (HR, 0.88; 95% CI, 0.7–1.12; interaction P = 0.015).100 More generally, an increase in the comorbidity burden is associated with a reduced efficacy of ICDs for mortality reduction.101
Telemedicine
Telemedicine has often yielded disappointing results in randomized controlled trials. For instance, in a recent randomized controlled trial, remote monitoring through the CRT‐ defibrillator, compared with standard therapy, did not reduce mortality or hospitalizations, primary endpoint of the study, with, however, a reduction in in‐office visits.102 Neutral results may be caused by both the type of the intervention and patients selection. A network meta‐analysis including 53 randomized controlled trials (12.356 patients) showed that, among services that decreased all‐cause mortality and all‐cause readmissions after HF hospitalizations, nurse home visits were the most effective in both cases, compared with usual care.103 Nurse home visits had also the greatest pooled cost savings. Telephone, telemonitoring, pharmacist, and education interventions did not improve clinical outcomes.103 The HF Outpatient Monitoring Evaluation was a randomized controlled trial testing the feasibility of home BNP measurement to prevent events in HF patients. Although the trial showed the feasibility of this approach, it was terminated early because of slow enrolment, low event rates, and the need of an algorithm taking care of spontaneous BNP fluctuations.104 Telemedical Interventional Management in HF II (TIM‐HF2) was a randomized, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalizations and mortality in HF patients. Study patients were selected based on the analysis of the previous TIM‐HF trial, which was neutral. In this trial, the patients that seemed to fair better were those who had a recent hospitalization for HF and who did not present with major depression and who were from rural, rather than urban areas.105 TIM‐HF2 showed a reduction in the percentage of days lost due to unplanned CV hospitalizations and all‐cause death with telemonitoring, compared with usual care with also a reduction in all‐cause death alone. These data show the efficacy of telemonitoring when used in a well‐defined HF population.105
Percutaneous treatment of mitral regurgitation
Functional mitral regurgitation may be both an effect or a cause of HF. It results from left‐chambers remodelling and may lead to volume overload, pulmonary hypertension, and worsening of HF signs and symptoms.
Recently, two randomized controlled clinical trials have investigated the impact of percutaneous treatment of mitral regurgitation with the MitraClip device on the outcomes of HF patients: Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA‐FR) and Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT). Both trials randomized patients to MitraClip plus guideline‐directed medical therapy or guideline‐directed medical therapy alone. No reduction in the primary endpoint of all‐cause mortality or HF hospitalizations was shown in MITRA‐FR, whereas a significant reduction in HF hospitalizations (primary endpoint) as well as in mortality alone was observed in COAPT (Figure 4).106, 107 These different results may be, at least partially, explained by differences in the patients enrolled. Compared with MITRA‐FR patients, those in COAPT had more severe mitral regurgitation, less dilated left ventricles, and no signs of right ventricular failure.108 Optimistic data come from the German Transcatheter Mitral Valve Interventions registry, suggesting the safety, feasibility, and benefit of MitraClip therapy even in advanced stages of disease such as in patients with pulmonary hypertension and severe reduced LV function. 109, 110
Figure 4.
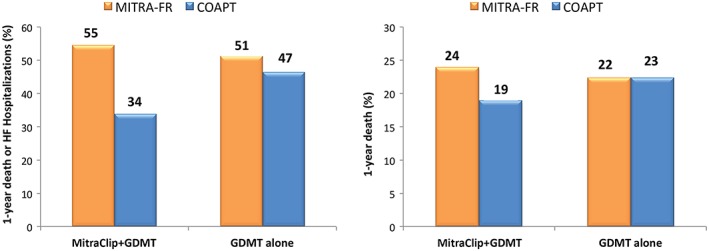
Effects of ferric carboxymaltose on cardiovascular hospitalizations and cardiovascular mortality in iron‐deficient heart failure patients. Relative risks of cardiovascular hospitalizations and cardiovascular mortality. Individual randomized controlled trials meta‐analysis. CI, confidence interval; FCM, ferric carboxymaltose. Frome reference 108
Functional tricuspid regurgitation may contribute to right heart failure and HF severity and poor prognosis. Orban et al. described the 6‐months outcomes of 50 patients undergoing transcatheter edge‐to‐edge tricuspid valve repair for severe tricuspid regurgitation. These patients had an improvement in NYHA class, 6‐min walk test distance, and quality of life after this procedure.111
Mechanical circulatory support
The role of proper physicians' and patients' education was shown by a multicentre prospective study conducted in Sweden. Patients with HFrEF, NYHA Class II–IV and on CRT and/or ICD were screened for an indication to LV assist device (LVAD) implantation. Up to 26% of the patients with low LVEF and NYHA Class II–IV had a potential indication to LVAD and only half of them accepted the procedure once offered.112
In patients with HFrEF who cannot be stabilized with medical therapy, MCS can be used as a bridge to heart transplantation or as destination therapy.4, 113 In a retrospective study conducted in Spain on 291 patients listed for urgent heart transplantation, bridging with temporary LVAD was associated with more favourable outcomes than bridging with temporary biventricular assist devices or venoarterial extracorporeal membrane oxygenation.114 LVAD implantation may be associated with some degree of recovery of LV function. Treatment with neurohormonal antagonists may help with this regard. Eighty‐one patients on LVAD support underwent paired myocardial tissue samples prior to LVAD implantation and at transplantation for histopathology. Patients had a significant improvements in cardiac structure and function over the 6 months following LVAD implantation. The degree of improvement was greater in the patients ion neurohormonal antagonists and this difference persisted after adjustment for baseline differences. Treatment with neurohormonal antagonists was associated with a reduction in fibrosis.115
Infections and thromboembolic and haemorrhagic complications remain major causes of morbidity and mortality.113 Predictors of such adverse events, such as abnormalities in platelet activity,116 are sought. Major advances have been obtained with the most recent devices. In the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3, the HeartMate 3 centrifugal‐flow LVAD was compared with the HeartMate II axial‐flow device, either as a bridge to transplantation or as destination therapy, in 1028 patients with advanced HF. Heartmate 3 was associated with less frequent need for pump replacement, mainly caused by ump thrombosis and was superior with respect to survival free of disabling stroke or reoperation to replace or remove a malfunctioning device.117
Experimental treatments
Increased adrenergic activity and reduced vagal tone play a major role in the progression of HF. Autonomic nervous system is therefore a potential target for HF treatment. Four major trials of vagal nerve stimulation did not show favourable effects in patients with HF. The ideal stimulation protocol and therapeutic dose are, however, still unsettled and may have had a major role for the results of these trials.118 Further trials, including trials of baroreceptor activation therapy, are ongoing.118 Carotid body resection was associated with a reduction in muscle sympathetic activity, reduced chemosensitivity, and better exercise tolerance in a pilot study in patients with HFrEF.119
Central sleep apnoea (CSA) is associated with increased CV morbidity and mortality in patients with HF. The treatment of sleep‐disordered breathing with predominant central sleep apnoea by adaptive SERvo VEntilation in patients with HF trial randomized 1325 patients with HFrEF and predominant CSA to adaptive servo ventilation or optimal medical treatment alone. The incidence of the composite primary endpoint was similar between the two treatment groups. However, all‐cause and CV mortality were significantly increased in the adaptive servo‐ventilation group.120 No effects on the LV remodelling and function, cardiac biomarkers, renal function, and systemic inflammation was shown by this device.121 Another trial, the effect of Adaptive servo VENTilation on survival and hospital admissions in HF, including patients with HFrEF and CSA, is ongoing.122 Phrenic nerve stimulation is another tool to treat CSA. It has improved sleep metrics and quality of life and reduced HF hospitalizations in a first study in patients with HF. 123
Regenerative therapy is still an area of active research for HFrEF treatment. Tools to increase its efficacy, such as the injection of muscle‐derived stem/progenitor cells modified with connexin‐43 gene124 or the administration of granulocyte colony stimulating factor combined with autologous bone‐marrow derived cells125 showed promising results. The Congestive Heart Failure Cardiopoietic Regenerative Therapy study evaluated the effects of intramyocardial administration of cardiopoietic stem cells in patients with HFrEF. Although results on the primary endpoint were neutral, cardiopoietic ecells administration was associated with a decrease in LV volumes, and the effect was larger in the patients who received a moderate number of injections (<20).126
HF with preserved EF
Diagnosis and prognosis: Role of exercise testing and cardiac imaging
Ezekowitz et al. prospectively tested the ESC 2007 and ESC 2016 criteria for the diagnosis of HFpEF in a community‐based cohort of 565 patients.127 Both criteria lacked sensitivity to detect HFpEF, 44.1% and 51.8%, respectively but were highly specific, 93.9% and 89%, respectively. Thus, the likelihood ratios of the existing criteria did not met the level necessary of diagnostic accuracy and further progress is needed.127
Diastolic stress testing with echocardiography and/or invasive haemodynamic monitoring are now considered the best methods to establish a diagnosis of HFpEF in patients with breathlessness.128 It is still controversial to which extent echocardiography can substitute invasive hemodynamic monitoring, above all during exercise, for the diagnosis of HFpEF.35
The mechanisms of reduced exercise tolerance are multiple in patients with HFEF. They include an impairment of the cardiac output and of the skeletal muscle diffusion of oxygen during exercise with a different contribution in each patient with HFpEF.129 Chronotropic incompetence has a major role in the limitation of the cardiac output response, in addition to the impairment of the stroke volume increase.130 Pulmonary mechanisms leading to an increase in the lung dead space have also been shown.131
Cardiac and extracardiac comorbidities have a major role in patients with HFpEF.132, 133, 134 They may influence exercise capacity and limit the value of cardiopulmonary exercise testing or the 6‐min walk test distance for the assessment of the patients with HFpEF.135, 136, 137, 138 A flattening oxygen consumption trajectory during exercise has been recently introduced as a new measurement of disease severity and a prognostic determinant and its value was independent from LVEF in a first study.139
Two‐dimensional speckle tracking echocardiography can evaluate LV global longitudinal strain, which can detect LV systolic dysfunction at an earlier stage than EF and whose impairment is associated with a worse outcome.140, 141, 142 Global longitudinal strain may be abnormal also in patients with HFpEF, showing that LV systolic dysfunction is present also in these patients, despite a normal LVEF.141, 142
Different phenotypes
Heart failure with preserved ejection fraction is a heterogeneous syndrome with different clinical presentations. Identification of specific phenotypes may be helpful. Among them, that of the patients with obesity‐related HFpEF represents a large subgroup. These patients have specific characteristics, such as an increase in epicardial fat, total heart volume, and right ventricular filling pressure.143, 144 Increased epicardial fat volume is positively correlated with markers of myocardial injury.145 Neurohormonal activation with heightened beta‐adrenergic drive and increased aldosterone and neprilysin activity leading to myocardial fibrosis and diastolic dysfunction are possible specific mechanisms in these patients.146, 147, 148 Drugs blocking these pathways might therefore be beneficial also in this HFpEF phenotype.147, 148, 149, 150
Treatment: Lack of favourable results
Heart failure with preserved ejection fraction is associated with severe profibrotic changes, among which excessive cross‐linking and deposition of collagen type I plays a major role. A phenotype of high collagen cross‐linking identifies patients resistant to the effects of spironolactone.149 Unfortunately, no drug has been identified as able to inhibit fibrosis and be effective in these patients and biomarkers to better estimate myocardial fibrosis are under investigation.151, 152
No treatment has been shown to reduce mortality and morbidity, so far. Geographical differences and enrolment of patients from Russia and Georgia may have played a major role for the neutral results on the primary outcome in the Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist (TOPCAT) trial.153, 154, 155 Analysis of TOPCAT based on the patients' LVEF has, however, fostered the hypothesis that the beneficial effects of spironolactone may be LVEF dependent.156 On the other hand, the anti‐hypertensive effects of spironolactone have no role since there was no correlation between baseline systolic blood pressure and outcomes.157
The Prospective comparison of ARNI with angiotensin‐receptor blockers Global Outcomes in HFpEF (PARAGON‐HF) is another major randomized trial in patients with HFpEF is the last major randomized trial concluded in patients with HFpEF.158 The effects of sacubitril/valsartan were compared with those of valsartan alone in 4822 patients with HF, a LVEF >45%, elevated NPs and structural heart disease. Sacubitril–valsartan failed to reduce significantly the incidence of the primary endpoint of study, a combination CV death and HF hospitalizations (HR, 0.87; 95% CI, 0.75 to 1.01; P = 0.06) as well as the incidence of CV deaths alone (HR, 0.95; 95% CI, 0.79 to 1.16). HF hospitalizations were reduced and NYHA class and quality of life were improved by the active treatment. Subgroup analysis suggested heterogeneity of the results with possible benefits with sacubitril–valsartan in patients with lower EF and in women.158
Heart rate reduction with ivabradine had no effect on diastolic function and exercise tolerance in patients with HFpEF.159 Similar sildenafil, a powerful phosphodiesterase type 5 (PDE‐5) inhibitor, useful for the treatment of primary pulmonary hypertension, has failed to improve hemodynamic measurements, exercise capacity and quality of life in patients with HFpEF in a randomized controlled trial.160, 161 The SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF study investigated the role of the soluble guanylate cyclase stimulator vericiguat on health status, finding an improvement in patient‐reported outcomes, although further research is needed.162, 163 A major Phase 3 clinical trial is ongoing.164
Physical training improved exercise capacity and quality of life in HFpEF patients, above all when used in specific phenotypes, such as the obese HFpEF patients.165, 166, 167, 168 Caloric restriction and exercise training had a synergic effect in a randomized controlled trial in obese patients with chronic stable HFpEF.169
Heart failure with midrange ejection fraction
The 2016 guidelines for HF of ESC created the new category of patients with a midrange LVEF, e.g. a LVEF between 40% and 49% (HFmrEF).4 This started a number of analyses aimed at showing the characteristics of these patients as well as their response to treatment. Compared with the patients with HFpEF, those with heart failure with mid‐range ejection fraction (HFmrEF) show a higher prevalence of coronary artery disease and, generally, have characteristics more similar to those of the patients with HFrEF so that they may be considered as having a milder form of HFrEF.170, 171, 172, 173, 174, 175, 176 With respect of outcomes, prognosis of the patients with HFmrEF is slightly better than that of the patients with HFrEF in most, but not all,177 of the studies. Progression from HFmrEF to HFrEF is an ominous prognostic sign as well as having neurohormonal activation and/or multiple echocardiographic signs of LV systolic and diastolic dysfunction.171, 172, 175, 178, 179
Retrospective analyses of clinical trials suggest that the response to treatment of the patients with HFmrEF is similar to that of those with HFrEF with favourable effects on outcomes of beta blockers, ARB, such as candesartan (Figure 5), mineralocorticoid receptor antagonists, such as spironolactone, and even digoxin.45, 156, 180, 181, 182 More recently, these results were confirmed by the Prospective comparison of ARNI with angiotensin‐receptor blockers Global Outcomes in HFpEF with the patients' subgroup with a LVEF <57%, median value, having a decrease in major events with sacubitril/valsartan.158
Figure 5.
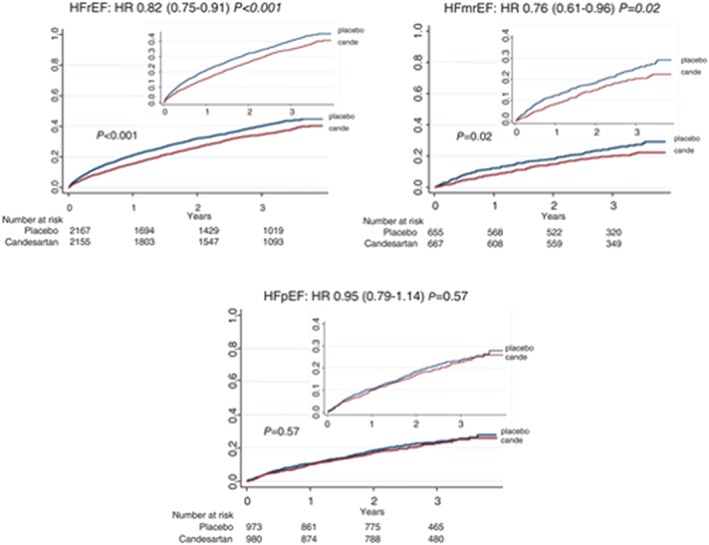
Effect of candesartan on the primary outcome by ejection fraction category. Kaplan–Meier time to event rates for candesartan vs. placebo for the primary composite outcome: time to cardiovascular death or first heart failure hospitalization in the three ejection fraction categories. Large graphs show y‐axis up to 1.0; inserted graphs show y‐axis up to 0.4. HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio. From reference 181
Amyloidosis
Cardiac amyloidosis (CA) is a cause of HF. It impairs exercise capacity and causes breathlessness through diastolic dysfunction and a reduction in the stroke volume response to exercise.183 Immunoglobulin light‐chain amyloidosis is the most frequent form of CA. It has a poor prognosis further worsened when myocardial inflammation is present.184 Transthyretin amyloidosis (ATTR) represents about 20% of CA and commonly occurs in elderly patients, including those with severe aortic stenosis.185 Different phenotypes of ATTR, including hereditary forms associated with specific mutations, have been identified.6 Bone scintigraphy is a reliable tool for its diagnosis.186 Tafamidis, preventing transthyretin tetramer dissociation, may improve clinical outcomes of ATTR patients. In the Safety and Efficacy of Tafamidis in Patients with Transthyretin Cardiomyopathy trial, 441 patients with ATTR and HF received, in a 2:1:2 ratio, 80 mg of Tafamidis, 20 mg of Tafamidis, or placebo for 30 months. Compared with placebo, Tafamidis increased survival free from of all‐cause death and cardiovascular hospitalization and improved functional capacity and quality of life.187 In a recent consensus document, it was stated that tafamidis should be considered in patients with symptomatic HF due to confirmed ATTR to improve exercise capacity and quality of life and reduce CV hospitalizations and mortality though also considering its high costs.45
Comorbidities
Comorbidities now have a major role for the clinical presentation and outcomes of the HF patients.101, 102, 188, 189 The contribution of comorbidities to patients’ outcomes may be similar in patients with HFrEF, compared with those with HFpEF,189 or be larger in those with HFpEF according to other analyses.132
The most frequent noncardiac comorbidities include chronic kidney disease,21, 32, 134, 190, 191, 192, 193, chronic obstructive pulmonary disease,194 central nervous system abnormalities,195 sleep disordered breathing,196, 197 diabetes mellitus,65, 198, 199 cancer,188, 200 and iron deficiency.60, 61, 63, 201 They were all shown to have a major impact on clinical presentation, response to treatment and outcomes. It is, however, unproven that their specific treatment may be associated with better outcomes. The only exception are SGLT2 inhibitors whose administration to diabetic patients at high risk of CV events was associated with a lower rate of the primary outcomes and HF hospitalizations.45, 65
The prevalence of sarcopenia, cachexia, and anorexia is increased in patients suffering from chronic HF. Losing muscle with or without weight loss impairs functional capacity, quality of life, and outcome to a larger extent than weight loss alone.202, 203, 204, 205, It is well‐established that frailty has a key role in HF. It is most commonly defined as meeting three out of five phenotypic criteria: low physical activity, unintentional weight loss, slow walking speed, weak grip strength, and/or exhaustion. Similar to the results in the patients with HFrEF, frailty was very common also among patients with HFpEF, and it was associated with higher risk of cardiovascular outcomes and mortality in TOPCAT.206
Adiposity is associated with increased ventricular‐arterial stiffness among the elderly and suggest a potential role in the development of HF.207 Further to a mere assessment of body mass index, abdominal fat, measured via waist‐to‐hip ratio has a tighter relationship with outcomes, especially in female patients.208
Cancer therapies are associated with side effects including up to nine categories of cardiovascular complications.200, 209 Cardiotoxicity involves direct effects of the cancer treatment on heart function and structure as well as accelerated development of CV disease, especially in the presence of traditional cardiovascular risk factors. Anthracycline‐based chemotherapy for the treatment of breast cancer is associated with an increased risk of HF.210 The incidence of cardiotoxicity for anthracyclines, anti‐HER2 agents and tyrosine kinase inhibitors were 75.8%, 69.8%, and 61.1%, respectively in a large cardio‐oncology service.211 Development of cardiac dysfunction after anthracycline therapy is associated with similar outcomes as other nonischaemic cardiomyopathies.212 High rates of cardiac therapy optimization and cancer treatment continuation were shown after the establishment of a cardio‐oncology service.211 Prospective registries are established.213
Acute heart failure
Worsening of symptoms and/or signs of HF generally caused by congestion are the main cause of unplanned emergency visits and hospitalizations of the patients with HF, independently from their LVEF and from whether worsening HF developed in an outpatient vs. an inpatient setting.214, 215 Precipitating factors may be found in most of the cases of acute HF, and they may also have a prognostic role. Poorer outcomes were described in the patients with a cardiovascular, compared with a noncardiovascular, precipitating factor, in one analysis, and in the patients with an infection, in another study.15, 216, 217, 218
Multiple factors may contribute to the poor outcomes of the patents after the hospitalization for acute HF, including socioeconomic factors, poor patient support, and poor adherence to prescribed medications.47, 57, 90, 150, 219, 220, 221 This is consistent with the relatively high proportion of patients who have an early rehospitalization for noncardiac causes.222 Other mechanisms are more directly related with congestion and possibly with myocardial, renal, and hepatic injury with persistent organ dysfunction. Such acute injury, shown by laboratory markers, has independent prognostic value and may be a cause of poor post‐discharge outcomes.24, 223, 224, 225, 226, 227 Unfortunately, strategies aimed at a better medical treatment of the acute phase, have failed to improve outcomes in major multicentre trials.228, 229 The role of sudden cardiac death (SCD) in postdischarge outcomes was investigated in an analysis from Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure trial. In this trial, about 2% of patients had SCD, resuscitated SCD, or ventricular tachycardia/ventricular fibrillation within 30 days of admission with SCD alone to account for 17% of all deaths within 30 days.230
Clinical signs have a major role for the prognostic assessment and treatment selection in the patients with acute HF. Among them, the most important is by far systolic blood pressure. A low blood pressure identifies the patient with low cardiac output and peripheral hypoperfusion, and these patients have the highest mortality during follow‐up.15, 231, 232 A decrease in blood pressure after treatment is also an ominous prognostic sign and a possible reason of the lack of efficacy of drugs with vasodilating activity when administered to patients who are not hypertensive.233 Heart rate has prognostic value also in acute HF with tachycardia and a lack of decrease in heart rate before discharge associated with poorer outcomes.18
In addition to clinical signs, imaging techniques are used to detect the severity of the haemodynamic impairment and relief from congestion.225 Echocardiography allows an estimate of LV filling pressure and pulmonary artery pressure.34, 234 The assessment of lung B‐lines by lung ultrasound allows an accurate estimate of lung congestion and has prognostic value.235, 236 Inferior vena cava diameter and jugular venous distension are indexes of venous congestion and right ventricular filling pressure.236
Among laboratory exams, the role for the diagnosis and prognostic stratification of acute HF patients is well‐established.4, 237 Hypochloraemia has emerged as a main prognostic variable with also a potential role as a cause of HF decompensation as it may cause activation of the renin‐angiotensin system.238 Its value outperforms that of hyponatremia itself.239, 240, 241 Another simple laboratory parameter, such as haemoconcentration, may be used to estimate decongestion in clinical practice.242, 243 Hypoalbuminemia as well as high blood urea nitrogen are repeatedly shown as a major independent prognostic factors likely because of their relation with the nutritional status.213, 244
Whereas treatment of acute HF has failed to have a favourable impact on outcomes, to date, a major role remains for optimization of oral treatment before discharge. This remains associated with subsequent outcomes and remains the most powerful tool in our hands to change patients' outcomes.245 Consistently, early administration of sacubitril/valsartan before discharge was shown to be safe and associated with favourable changes in biomarkers and outcomes in recent studies.44, 46
Medical therapy
The European Society of Cardiology Heart Failure Long‐Term investigated the long‐term safety of intravenous cardiovascular agents in acute HF; vasodilators did not present any association with long‐term outcome, while inotropes and/or vasopressors were associated with an increased all‐cause mortality.246
BMS‐986231, a novel second‐generation nitroxyl donor with potential inotropic, lusitropic and vasodilatory effects in patients hospitalized with decompensated heart failure and reduced ejection fraction (HFrEF), was safe and had beneficial haemodynamic effects.247 A major trial is ongoing.248 Loop diuretics represent the first option for the treatment of congestion in AHF and diuretic resistance is associated with poorer outcomes. The cause of such diuretic resistance is mostly due to some defects at the level of renal tubules, rather than reduced diuretic delivery.249 The ADVOR trial is designed to investigate if acetazolamide combined to loop diuretic therapy can improve decongestion in acute HF with volume overload.250
The use of venoarterial extracorporeal membrane oxygenation (VA‐ECMO) support is becoming more frequent in refractory cardiogenic shock but is associated with a rise in LV afterload. Different techniques of LV unloading may be used to overcome this limitation. 251 The concomitant implantation of Impella on top of VA‐ECMO reduced in‐hospital mortality (47% vs. 80%, P < 0.001) and increased successful bridging to either recovery or further therapy (68% vs. 28%, P < 0.001), compared with VA‐ECMO alone.252
Conclusions
We have summarized the most recent data regarding the epidemiology, diagnosis, and prognosis of HF as well as its main clinical phenotypes as outlines in the most recent ESC HF guidelines. Major changes are occurring for the treatment of the patients with HFrEF with respect of the implementation and, possibly, somehow larger indications to sacubitril/valsartan and the revolutionary recent data with SGLT2 inhibitors. Mitral regurgitation has a role in HF progression, but recent data from major randomized trials need further assessment and possible confirmation from ongoing trials. Treatment of comorbidities, such as hyperkalaemia and iron deficiency might further improve prognosis through the implementation of neurohormonal antagonists with potassium lowering agents and direct effects with iron replacement therapy. Whereas treatment of HFrEF is undergoing continuous improvement, treatment of HFpEF remains elusive with the only progress regarding the patients with HFmrEF whose pathogentic mechanisms and response to treatment seem similar to those of the patients with HFrEF.
Tomasoni, D. , Adamo, M. , Lombardi, C. M. , and Metra, M. (2019) Highlights in heart failure. ESC Heart Failure, 6: 1105–1127. 10.1002/ehf2.12555.
References
- 1. Lam CSP. Heart failure in Southeast Asia: facts and numbers. ESC Heart Fail 2015; 2: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooper LB, Yap J, Tay WT, Teng TK, MacDonald M, Anand IS, Sharma A, O'Connor CM, Kraus WE, Mentz RJ, Lam CS, Hf A, Investigators A‐H. Multi‐ethnic comparisons of diabetes in heart failure with reduced ejection fraction: insights from the HF‐ACTION trial and the ASIAN‐HF registry. Eur J Heart Fail 2018; 20: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 3. Nagai T, Sundaram V, Shoaib A, Shiraishi Y, Kohsaka S, Rothnie KJ, Piper S, McDonagh TA, Hardman SMC, Goda A, Mizuno A, Sawano M, Rigby AS, Quint JK, Yoshikawa T, Clark AL, Anzai T, Cleland JGF. Validation of U.S. mortality prediction models for hospitalized heart failure in the United Kingdom and Japan. Eur J Heart Fail 2018; 20: 1179–1190. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M and Document R . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 5. Komajda M, Bohm M, Borer JS, Ford I, Tavazzi L, Pannaux M, Swedberg K. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta‐analysis. Eur J Heart Fail 2018; 20: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 6. Gagliardi C, Perfetto F, Lorenzini M, Ferlini A, Salvi F, Milandri A, Quarta CC, Taborchi G, Bartolini S, Frusconi S, Martone R, Cinelli MM, Foffi S, Reggiani MLB, Fabbri G, Cataldo P, Cappelli F, Rapezzi C. Phenotypic profile of Ile68Leu transthyretin amyloidosis: an underdiagnosed cause of heart failure. Eur J Heart Fail 2018; 20: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 7. Al‐Saaidi RA, Rasmussen TB, Birkler RID, Palmfeldt J, Beqqali A, Pinto YM, Nissen PH, Baandrup U, Molgaard H, Hey TM, Eiskjaer H, Bross P, Mogensen J. The clinical outcome of LMNA missense mutations can be associated with the amount of mutated protein in the nuclear envelope. Eur J Heart Fail 2018; 20: 1404–1412. [DOI] [PubMed] [Google Scholar]
- 8. Jansweijer JA, Nieuwhof K, Russo F, Hoorntje ET, Jongbloed JD, Lekanne Deprez RH, Postma AV, Bronk M, van Rijsingen IA, de Haij S, Biagini E, van Haelst PL, van Wijngaarden J, van den Berg MP, Wilde AA, Mannens MM, de Boer RA, van Spaendonck‐Zwarts KY, van Tintelen JP, Pinto YM. Truncating titin mutations are associated with a mild and treatable form of dilated cardiomyopathy. Eur J Heart Fail 2017; 19: 512–521. [DOI] [PubMed] [Google Scholar]
- 9. Plichart M, Orvoen G, Jourdain P, Quinquis L, Coste J, Escande M, Friocourt P, Paillaud E, Chedhomme FX, Labouree F, Boully C, Benetos A, Domerego JJ, Komajda M, Hanon O. Brain natriuretic peptide usefulness in very elderly dyspnoeic patients: the BED study. Eur J Heart Fail 2017; 19: 540–548. [DOI] [PubMed] [Google Scholar]
- 10. Suthahar N, Meijers WC, Ho JE, Gansevoort RT, Voors AA, van der Meer P, Bakker SJL, Heymans S, van Empel V, Schroen B, van der Harst P, van Veldhuisen DJ, de Boer RA. Sex‐specific associations of obesity and N‐terminal pro‐B‐type natriuretic peptide levels in the general population. Eur J Heart Fail 2018; 20: 1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan MS, Fonarow GC, Khan H, Greene SJ, Anker SD, Gheorghiade M, Butler J. Renin‐angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. ESC Heart Fail 2017; 4: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P, Buchan I, Hannaford PC, Myint PK. Do patients have worse outcomes in heart failure than in cancer? A primary care‐based cohort study with 10‐year follow‐up in Scotland. Eur J Heart Fail 2017; 19: 1095–1104. [DOI] [PubMed] [Google Scholar]
- 13. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Candesartan in heart failure: assessment of reduction in M and morbidity I. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007; 116: 1482–1487. [DOI] [PubMed] [Google Scholar]
- 14. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlstrom U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A, and Heart Failure Association of the European Society of C . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 15. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, Investigators ESCHFL‐TR . Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 16. AlFaleh H, Elasfar AA, Ullah A, AlHabib KF, Hersi A, Mimish L, Almasood A, Al Ghamdi S, Ghabashi A, Malik A, Hussein GA, Al‐Murayeh M, Abuosa A, Al Habeeb W, Kashour T. Worsening heart failure in 'real‐world' clinical practice: predictors and prognostic impact. Eur J Heart Fail 2017; 19: 987–995. [DOI] [PubMed] [Google Scholar]
- 17. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hulsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 18. Kitai T, Grodin JL, Mentz RJ, Hernandez AF, Butler J, Metra M, McMurray JJ, Armstrong PW, Starling RC, O'Connor CM, Swedberg K, Tang WH. Insufficient reduction in heart rate during hospitalization despite beta‐blocker treatment in acute decompensated heart failure: insights from the ASCEND‐HF trial. Eur J Heart Fail 2017; 19: 241–249. [DOI] [PubMed] [Google Scholar]
- 19. Schmid FA, Schlager O, Keller P, Seifert B, Huang R, Frohlich GM, Luscher TF, Ruschitzka F, Enseleit F. Prognostic value of long‐term blood pressure changes in patients with chronic heart failure. Eur J Heart Fail 2017; 19: 837–842. [DOI] [PubMed] [Google Scholar]
- 20. Nikolovska Vukadinovic A, Vukadinovic D, Borer J, Cowie M, Komajda M, Lainscak M, Swedberg K, Bohm M. Heart rate and its reduction in chronic heart failure and beyond. Eur J Heart Fail 2017; 19: 1230–1241. [DOI] [PubMed] [Google Scholar]
- 21. Welsh P, Kou L, Yu C, Anand I, van Veldhuisen DJ, Maggioni AP, Desai AS, Solomon SD, Pfeffer MA, Cheng S, Gullestad L, Aukrust P, Ueland T, Swedberg K, Young JB, Kattan MW, Sattar N, McMurray JJV. Prognostic importance of emerging cardiac, inflammatory, and renal biomarkers in chronic heart failure patients with reduced ejection fraction and anaemia: RED‐HF study. Eur J Heart Fail 2018; 20: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greene SJ, Butler J, Fonarow GC, Subacius HP, Ambrosy AP, Vaduganathan M, Triggiani M, Solomon SD, Lewis EF, Maggioni AP, Bohm M, Chioncel O, Nodari S, Senni M, Zannad F, Gheorghiade M, Investigators A and Coordinators . Pre‐discharge and early post‐discharge troponin elevation among patients hospitalized for heart failure with reduced ejection fraction: findings from the ASTRONAUT trial. Eur J Heart Fail 2018; 20: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demissei BG, Postmus D, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Givertz MM, Bloomfield DM, van Veldhuisen DJ, Dittrich HC, Hillege HL, Voors AA. Plasma biomarkers to predict or rule out early post‐discharge events after hospitalization for acute heart failure. Eur J Heart Fail 2017; 19: 728–738. [DOI] [PubMed] [Google Scholar]
- 24. Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM, Wang Y, Qian M, Teerlink JR, Metra M, Davison BA, Voors AA. A multimarker multi‐time point‐based risk stratification strategy in acute heart failure: results from the RELAX‐AHF trial. Eur J Heart Fail 2017; 19: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 25. Cleland JGF, Teerlink JR, Davison BA, Shoaib A, Metra M, Senger S, Milo O, Cotter G, Bourge RC, Parker JD, Jondeau G, Krum H, O'Connor CM, Torre‐Amione G, van Veldhuisen DJ, McMurray JJV, Investigators V . Measurement of troponin and natriuretic peptides shortly after admission in patients with heart failure‐does it add useful prognostic information? An analysis of the Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Studies (VERITAS). Eur J Heart Fail 2017, 19: 739–747. [DOI] [PubMed] [Google Scholar]
- 26. Vaduganathan M, Cheema B, Cleveland E, Sankar K, Subacius H, Fonarow GC, Solomon SD, Lewis EF, Greene SJ, Maggioni AP, Bohm M, Zannad F, Butler J, Gheorghiade M. Plasma renin activity, response to aliskiren, and clinical outcomes in patients hospitalized for heart failure: the ASTRONAUT trial. Eur J Heart Fail 2018; 20: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouabdallaoui N, Claggett B, Zile MR, McMurray JJV, O'Meara E, Packer M, Prescott MF, Swedberg K, Solomon SD, Rouleau JL, Investigators P‐H, Committees . Growth differentiation factor‐15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2018; 20: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 28. De Rosa S, Eposito F, Carella C, Strangio A, Ammirati G, Sabatino J, Abbate FG, Iaconetti C, Liguori V, Pergola V, Polimeni A, Coletta S, Gareri C, Trimarco B, Stabile G, Curcio A, Indolfi C, Rapacciuolo A. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail 2018; 20: 1000–1010. [DOI] [PubMed] [Google Scholar]
- 29. Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 2016; 18: 457–468. [DOI] [PubMed] [Google Scholar]
- 30. van Boven N, Kardys I, van Vark LC, Akkerhuis KM, de Ronde MWJ, Khan MAF, Merkus D, Liu Z, Voors AA, Asselbergs FW, van den Bos EJ, Boersma E, Hillege H, Duncker DJ, Pinto YM, Postmus D. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur J Heart Fail 2018; 20: 89–96. [DOI] [PubMed] [Google Scholar]
- 31. Bayes‐Genis A, Lanfear DE, de Ronde MWJ, Lupon J, Leenders JJ, Liu Z, Zuithoff NPA, Eijkemans MJC, Zamora E, De Antonio M, Zwinderman AH, Pinto‐Sietsma SJ, Pinto YM. Prognostic value of circulating microRNAs on heart failure‐related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur J Heart Fail 2018; 20: 67–75. [DOI] [PubMed] [Google Scholar]
- 32. Agostoni P, Paolillo S, Mapelli M, Gentile P, Salvioni E, Veglia F, Bonomi A, Corra U, Lagioia R, Limongelli G, Sinagra G, Cattadori G, Scardovi AB, Metra M, Carubelli V, Scrutinio D, Raimondo R, Emdin M, Piepoli M, Magri D, Parati G, Caravita S, Re F, Cicoira M, Mina C, Correale M, Frigerio M, Bussotti M, Oliva F, Battaia E, Belardinelli R, Mezzani A, Pastormerlo L, Guazzi M, Badagliacca R, Di Lenarda A, Passino C, Sciomer S, Zambon E, Pacileo G, Ricci R, Apostolo A, Palermo P, Contini M, Clemenza F, Marchese G, Gargiulo P, Binno S, Lombardi C, Passantino A, Filardi PP. Multiparametric prognostic scores in chronic heart failure with reduced ejection fraction: a long‐term comparison. Eur J Heart Fail 2018; 20: 700–710. [DOI] [PubMed] [Google Scholar]
- 33. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, Ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 2017; 19: 627–634. [DOI] [PubMed] [Google Scholar]
- 34. Nagueh SF. Non‐invasive assessment of left ventricular filling pressure. Eur J Heart Fail 2018; 20: 38–48. [DOI] [PubMed] [Google Scholar]
- 35. Hummel YM, Liu LCY, Lam CSP, Fonseca‐Munoz DF, Damman K, Rienstra M, van der Meer P, Rosenkranz S, van Veldhuisen DJ, Voors AA, Hoendermis ES. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail 2017; 19: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 36. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL, all i . Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 2017; 19: 873–879. [DOI] [PubMed] [Google Scholar]
- 37. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail 2016; 18: 1472–1487. [DOI] [PubMed] [Google Scholar]
- 38. Palazzini M, Dardi F, Manes A, Bacchi Reggiani ML, Gotti E, Rinaldi A, Albini A, Monti E, Galie N. Pulmonary hypertension due to left heart disease: analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur J Heart Fail 2018; 20: 248–255. [DOI] [PubMed] [Google Scholar]
- 39. Nagy AI, Venkateshvaran A, Merkely B, Lund LH, Manouras A. Determinants and prognostic implications of the negative diastolic pulmonary pressure gradient in patients with pulmonary hypertension due to left heart disease. Eur J Heart Fail 2017; 19: 88–97. [DOI] [PubMed] [Google Scholar]
- 40. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P‐H and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 41. Mogensen UM, Gong J, Jhund PS, Shen L, Kober L, Desai AS, Lefkowitz MP, Packer M, Rouleau JL, Solomon SD, Claggett BL, Swedberg K, Zile MR, Mueller‐Velten G, McMurray JJV. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2018; 20: 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T, McMurray JJV, Solomon SD. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM‐HF Trial. JAMA Cardiol 2018; 3: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM‐HF trial. Eur J Heart Fail 2019; 21: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, Investigators P‐H. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 45. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]
- 46. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bohmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, Alb J, Gniot J, Mozheiko M, Lelonek M, Noe A, Schwende H, Bao W, Butylin D, Pascual‐Figal D, Investigators T . Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019; 21: 998–1007. [DOI] [PubMed] [Google Scholar]
- 47. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS, Investigators Q. Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017; 19: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 48. Howlett J, Comin‐Colet J, Dickstein K, Fuat A, Polzl G, Delaney S. Clinical practices and attitudes regarding the diagnosis and management of heart failure: findings from the CORE Needs Assessment Survey. ESC Heart Fail 2018; 5: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ambrosy AP, Mentz RJ, Fiuzat M, Cleland JGF, Greene SJ, O'Connor CM, Teerlink JR, Zannad F, Solomon SD. The role of angiotensin receptor‐neprilysin inhibitors in cardiovascular disease‐existing evidence, knowledge gaps, and future directions. Eur J Heart Fail 2018; 20: 963–972. [DOI] [PubMed] [Google Scholar]
- 50. Pellicori P, Urbinati A, Shah P, MacNamara A, Kazmi S, Dierckx R, Zhang J, Cleland JGF, Clark AL. What proportion of patients with chronic heart failure are eligible for sacubitril‐valsartan? Eur J Heart Fail 2017; 19: 768–778. [DOI] [PubMed] [Google Scholar]
- 51. Senni M, McMurray JJV, Wachter R, McIntyre HF, Anand IS, Duino V, Sarkar A, Shi V, Charney A. Impact of systolic blood pressure on the safety and tolerability of initiating and up‐titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail 2018; 20: 491–500. [DOI] [PubMed] [Google Scholar]
- 52. Butler J, Vijayakumar S, Pitt B. Need to revisit heart failure treatment guidelines for hyperkalaemia management during the use of mineralocorticoid receptor antagonists. Eur J Heart Fail 2018; 20: 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferreira JP, Mentz RJ, Pizard A, Pitt B, Zannad F. Tailoring mineralocorticoid receptor antagonist therapy in heart failure patients: are we moving towards a personalized approach? Eur J Heart Fail 2017; 19: 974–986. [DOI] [PubMed] [Google Scholar]
- 54. Ferreira JP, Rossignol P, Machu JL, Sharma A, Girerd N, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Lang CC, Ter Maaten JM, Metra M, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Voors A, Zannad F. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: findings from BIOSTAT‐CHF. Eur J Heart Fail 2017; 19: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 55. Beusekamp JC, Tromp J, van der Wal HH, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Rossignol P, Zannad F, Voors AA, van der Meer P. Potassium and the use of renin‐angiotensin‐aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT‐CHF. Eur J Heart Fail 2018; 20: 923–930. [DOI] [PubMed] [Google Scholar]
- 56. Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, Barany P, Jernberg T, Lund LH, Carrero JJ. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail 2018; 20: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlstrom U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 58. Melenovsky V, Petrak J, Mracek T, Benes J, Borlaug BA, Nuskova H, Pluhacek T, Spatenka J, Kovalcikova J, Drahota Z, Kautzner J, Pirk J, Houstek J. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail 2017; 19: 522–530. [DOI] [PubMed] [Google Scholar]
- 59. Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Limited contractile reserve contributes to poor peak exercise capacity in iron‐deficient heart failure. Eur J Heart Fail 2018; 20: 806–808. [DOI] [PubMed] [Google Scholar]
- 60. Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018; 20: 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen‐Solal A, Kindermann I, Manito N, Pfister O, Pohjantahti‐Maaroos H, Taylor J, Comin‐Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail 2019; 7: 36–46. [DOI] [PubMed] [Google Scholar]
- 63. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Luscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 64. Fitchett DH, Udell JA, Inzucchi SE. Heart failure outcomes in clinical trials of glucose‐lowering agents in patients with diabetes. Eur J Heart Fail 2017; 19: 43–53. [DOI] [PubMed] [Google Scholar]
- 65. Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschope C, Hoes AW, Seferovic JP, Logue J, McDonagh T, Riley JP, Milinkovic I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 853–872. [DOI] [PubMed] [Google Scholar]
- 66. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356: 2457–2471. [DOI] [PubMed] [Google Scholar]
- 67. Cosmi F, Shen L, Magnoli M, Abraham WT, Anand IS, Cleland JG, Cohn JN, Cosmi D, De Berardis G, Dickstein K, Franzosi MG, Gullestad L, Jhund PS, Kjekshus J, Kober L, Lepore V, Lucisano G, Maggioni AP, Masson S, McMurray JJV, Nicolucci A, Petrarolo V, Robusto F, Staszewsky L, Tavazzi L, Teli R, Tognoni G, Wikstrand J, Latini R. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail 2018; 20: 888–895. [DOI] [PubMed] [Google Scholar]
- 68. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, Committee S‐TS and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 69. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS, Investigators LT. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hanselmann A, Nilsson B, Moller JE, Hjort J, Rasmussen J, Boesgaard TW, Schou M, Videbaek L, Gustafsson I, Flyvbjerg A, Wiggers H, Tarnow L. Effect of liraglutide, a glucagon‐like peptide‐1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)‐a multicentre, double‐blind, randomised, placebo‐controlled trial. Eur J Heart Fail 2017; 19: 69–77. [DOI] [PubMed] [Google Scholar]
- 71. Butler J, Hamo CE, Filippatos G, Pocock SJ, Bernstein RA, Brueckmann M, Cheung AK, George JT, Green JB, Januzzi JL, Kaul S, Lam CSP, Lip GYH, Marx N, McCullough PA, Mehta CR, Ponikowski P, Rosenstock J, Sattar N, Salsali A, Scirica BM, Shah SJ, Tsutsui H, Verma S, Wanner C, Woerle HJ, Zannad F, Anker SD, Program ET. The potential role and rationale for treatment of heart failure with sodium‐glucose co‐transporter 2 inhibitors. Eur J Heart Fail 2017; 19: 1390–1400. [DOI] [PubMed] [Google Scholar]
- 72. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E‐RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 73. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 74. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Investigators D‐T. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 75. Pabel S, Wagner S, Bollenberg H, Bengel P, Kovacs A, Schach C, Tirilomis P, Mustroph J, Renner A, Gummert J, Fischer T, Van Linthout S, Tschope C, Streckfuss‐Bomeke K, Hasenfuss G, Maier LS, Hamdani N, Sossalla S. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail 2018; 20: 1690–1700. [DOI] [PubMed] [Google Scholar]
- 76. Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter‐2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation 2017; 136: 1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia 2018; 61: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 78. McMurray JJV, DeMets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjostrand M, Solomon SD, Committees D‐H, Investigators . A trial to evaluate the effect of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF). Eur J Heart Fail 2019; 21: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D‐HT and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 80. Nizamic T, Murad MH, Allen LA, McIlvennan CK, Wordingham SE, Matlock DD, Dunlay SM. Ambulatory inotrope infusions in advanced heart failure: a systematic review and meta‐analysis. JACC Heart Fail 2018; 6: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chernomordik F, Freimark D, Arad M, Shechter M, Matetzky S, Savir Y, Shlomo N, Peled A, Goldenberg I, Peled Y. Quality of life and long‐term mortality in patients with advanced chronic heart failure treated with intermittent low‐dose intravenous inotropes in an outpatient setting. ESC Heart Fail 2017; 4: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Silvetti S, Belletti A, Fontana A, Pollesello P. Rehospitalization after intermittent levosimendan treatment in advanced heart failure patients: a meta‐analysis of randomized trials. ESC Heart Fail 2017; 4: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Comin‐Colet J, Manito N, Segovia‐Cubero J, Delgado J, Garcia Pinilla JM, Almenar L, Crespo‐Leiro MG, Sionis A, Blasco T, Pascual‐Figal D, Gonzalez‐Vilchez F, Lambert‐Rodriguez JL, Grau M, Bruguera J, Investigators L‐HS. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018; 20: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 84. Grodin JL, Carter S, Bart BA, Goldsmith SR, Drazner MH, Tang WHW. Direct comparison of ultrafiltration to pharmacological decongestion in heart failure: a per‐protocol analysis of CARRESS‐HF. Eur J Heart Fail 2018; 20: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 85. Bart BA, Goldsmith SR, Lee KL, Redfield MM, Felker GM, O'Connor CM, Chen HH, Rouleau JL, Givertz MM, Semigran MJ, Mann D, Deswal A, Bull DA, Lewinter MM, Braunwald E. Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS‐HF, for the Heart Failure Clinical Research Network. J Card Fail 2012; 18: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Campbell RT, Petrie MC, Jackson CE, Jhund PS, Wright A, Gardner RS, Sonecki P, Pozzi A, McSkimming P, McConnachie A, Finlay F, Davidson P, Denvir MA, Johnson MJ, Hogg KJ, McMurray JJV. Which patients with heart failure should receive specialist palliative care? Eur J Heart Fail 2018; 20: 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Riley JP, Beattie JM. Palliative care in heart failure: facts and numbers. ESC Heart Fail 2017; 4: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lund LH, Svennblad B, Dahlstrom U, Stahlberg M. Effect of expanding evidence and evolving clinical guidelines on the prevalence of indication for cardiac resynchronization therapy in patients with heart failure. Eur J Heart Fail 2018; 20: 769–777. [DOI] [PubMed] [Google Scholar]
- 89. Dickstein K, Normand C, Auricchio A, Bogale N, Cleland JG, Gitt AK, Stellbrink C, Anker SD, Filippatos G, Gasparini M, Hindricks G, Blomstrom Lundqvist C, Ponikowski P, Ruschitzka F, Botto GL, Bulava A, Duray G, Israel C, Leclercq C, Margitfalvi P, Cano O, Plummer C, Sarigul NU, Sterlinski M, Linde C. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients‐who is doing what to whom and how? Eur J Heart Fail 2018; 20: 1039–1051. [DOI] [PubMed] [Google Scholar]
- 90. Lund LH, Braunschweig F, Benson L, Stahlberg M, Dahlstrom U, Linde C. Association between demographic, organizational, clinical, and socio‐economic characteristics and underutilization of cardiac resynchronization therapy: results from the Swedish Heart Failure Registry. Eur J Heart Fail 2017; 19: 1270–1279. [DOI] [PubMed] [Google Scholar]
- 91. Linde C, Abraham WT, Gold MR, Daubert JC, Tang ASL, Young JB, Sherfesee L, Hudnall JH, Fagan DH, Cleland JG. Predictors of short‐term clinical response to cardiac resynchronization therapy. Eur J Heart Fail 2017; 19: 1056–1063. [DOI] [PubMed] [Google Scholar]
- 92. van der Bijl P, Khidir M, Leung M, Mertens B, Ajmone Marsan N, Delgado V, Bax JJ. Impact of QRS complex duration and morphology on left ventricular reverse remodelling and left ventricular function improvement after cardiac resynchronization therapy. Eur J Heart Fail 2017; 19: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 93. Khidir MJ, Delgado V, Ajmone Marsan N, Schalij MJ, Bax JJ. QRS duration versus morphology and survival after cardiac resynchronization therapy. ESC Heart Fail 2017; 4: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Linde C, Cleland JGF, Gold MR, Claude Daubert J, Tang ASL, Young JB, Sherfesee L, Abraham WT. The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: an individual‐patient data meta‐analysis. Eur J Heart Fail 2018; 20: 780–791. [DOI] [PubMed] [Google Scholar]
- 95. Linde CM, Normand C, Bogale N, Auricchio A, Sterlinski M, Marinskis G, Sticherling C, Bulava A, Perez OC, Maass AH, Witte KK, Rekvava R, Abdelali S, Dickstein K. Upgrades from a previous device compared to de novo cardiac resynchronization therapy in the European Society of Cardiology CRT Survey II. Eur J Heart Fail 2018; 20: 1457–1468. [DOI] [PubMed] [Google Scholar]
- 96. Gasparini M, Kloppe A, Lunati M, Anselme F, Landolina M, Martinez‐Ferrer JB, Proclemer A, Morani G, Biffi M, Ricci R, Rordorf R, Mangoni L, Manotta L, Grammatico A, Leyva F, Boriani G. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter‐defibrillator therapies and hospitalizations. Eur J Heart Fail 2018; 20: 1472–1481. [DOI] [PubMed] [Google Scholar]
- 97. Burns KV, Gage RM, Curtin AE, Gorcsan J 3rd, Bank AJ. Left ventricular‐only pacing in heart failure patients with normal atrioventricular conduction improves global function and left ventricular regional mechanics compared with biventricular pacing: an adaptive cardiac resynchronization therapy sub‐study. Eur J Heart Fail 2017; 19: 1335–1343. [DOI] [PubMed] [Google Scholar]
- 98. Filippatos G, Birnie D, Gold MR, Gerritse B, Hersi A, Jacobs S, Kusano K, Leclercq C, Mullens W, Wilkoff BL, AdaptResponse I. Rationale and design of the AdaptResponse trial: a prospective randomized study of cardiac resynchronization therapy with preferential adaptive left ventricular‐only pacing. Eur J Heart Fail 2017; 19: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp‐Pedersen C, Pehrson S, Investigators D . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 100. Sharma A, Al‐Khatib SM, Ezekowitz JA, Cooper LB, Fordyce CB, Michael Felker G, Bardy GH, Poole JE, Thomas Bigger J, Buxton AE, Moss AJ, Friedman DJ, Lee KL, Steinman R, Dorian P, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Peterson ED, Inoue LYT, Sanders GD. Implantable cardioverter‐defibrillators in heart failure patients with reduced ejection fraction and diabetes. Eur J Heart Fail 2018; 20: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 101. Ruwald AC, Vinther M, Gislason GH, Johansen JB, Nielsen JC, Petersen HH, Riahi S, Jons C. The impact of co‐morbidity burden on appropriate implantable cardioverter defibrillator therapy and all‐cause mortality: insight from Danish nationwide clinical registers. Eur J Heart Fail 2017; 19: 377–386. [DOI] [PubMed] [Google Scholar]
- 102. Boriani G, Malavasi VL. Patient outcome after implant of a cardioverter defibrillator in the 'real world': the key role of co‐morbidities. Eur J Heart Fail 2017; 19: 387–390. [DOI] [PubMed] [Google Scholar]
- 103. Van Spall HGC, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, Coppens M, Brian Haynes R, Connolly S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta‐analysis. Eur J Heart Fail 2017; 19: 1427–1443. [DOI] [PubMed] [Google Scholar]
- 104. McDonald K, Troughton R, Dahlstrom U, Dargie H, Krum H, van der Meer P, McDonagh T, Atherton JJ, Kupfer K, San George RC, Richards M, Doughty R. Daily home BNP monitoring in heart failure for prediction of impending clinical deterioration: results from the HOME HF study. Eur J Heart Fail 2018; 20: 474–480. [DOI] [PubMed] [Google Scholar]
- 105. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Winkler S, Vettorazzi E, Polze A, Stangl K, Hartmann O, Marx A, Neuhaus P, Scherf M, Kirwan BA, Anker SD. Telemedical Interventional Management in Heart Failure II (TIM‐HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail 2018; 20: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 106. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, Investigators C. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 107. Obadia JF, Messika‐Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort‐Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N, Investigators M‐F. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 108. Senni M, Adamo M, Metra M, Alfieri O, Vahanian A. Treatment of functional mitral regurgitation in chronic heart failure: can we get a 'proof of concept' from the MITRA‐FR and COAPT trials? Eur J Heart Fail 2019; 21: 852–861. [DOI] [PubMed] [Google Scholar]
- 109. Tigges E, Blankenberg S, von Bardeleben RS, Zurn C, Bekeredjian R, Ouarrak T, Sievert H, Nickenig G, Boekstegers P, Senges J, Schillinger W, Lubos E. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2018; 20: 585–594. [DOI] [PubMed] [Google Scholar]
- 110. Geis NA, Puls M, Lubos E, Zuern CS, Franke J, Schueler R, von Bardeleben RS, Boekstegers P, Ouarrak T, Zahn R, Ince H, Senges J, Katus HA, Bekeredjian R. Safety and efficacy of MitraClip therapy in patients with severely impaired left ventricular ejection fraction: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2018; 20: 598–608. [DOI] [PubMed] [Google Scholar]
- 111. Orban M, Besler C, Braun D, Nabauer M, Zimmer M, Orban M, Noack T, Mehilli J, Hagl C, Seeburger J, Borger M, Linke A, Thiele H, Massberg S, Ender J, Lurz P, Hausleiter J. Six‐month outcome after transcatheter edge‐to‐edge repair of severe tricuspid regurgitation in patients with heart failure. Eur J Heart Fail 2018; 20: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 112. Lund LH, Trochu JN, Meyns B, Caliskan K, Shaw S, Schmitto JD, Schibilsky D, Damme L, Heatley J, Gustafsson F. Screening for heart transplantation and left ventricular assist system: results from the ScrEEning for advanced Heart Failure treatment (SEE‐HF) study. Eur J Heart Fail 2018; 20: 152–160. [DOI] [PubMed] [Google Scholar]
- 113. Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017; 19: 595–602. [DOI] [PubMed] [Google Scholar]
- 114. Barge‐Caballero E, Almenar‐Bonet L, Gonzalez‐Vilchez F, Lambert‐Rodriguez JL, Gonzalez‐Costello J, Segovia‐Cubero J, Castel‐Lavilla MA, Delgado‐Jimenez J, Garrido‐Bravo IP, Rangel‐Sousa D, Martinez‐Selles M, De la Fuente‐Galan L, Rabago‐Juan‐Aracil G, Sanz‐Julve M, Hervas‐Sotomayor D, Mirabet‐Perez S, Muniz J, Crespo‐Leiro MG. Clinical outcomes of temporary mechanical circulatory support as a direct bridge to heart transplantation: a nationwide Spanish registry. Eur J Heart Fail 2018; 20: 178–186. [DOI] [PubMed] [Google Scholar]
- 115. Catino AB, Ferrin P, Wever‐Pinzon J, Horne BD, Wever‐Pinzon O, Kfoury AG, McCreath L, Diakos NA, McKellar S, Koliopoulou A, Bonios MJ, Al‐Sarie M, Taleb I, Dranow E, Fang JC, Drakos SG. Clinical and histopathological effects of heart failure drug therapy in advanced heart failure patients on chronic mechanical circulatory support. Eur J Heart Fail 2018; 20: 164–174. [DOI] [PubMed] [Google Scholar]
- 116. Consolo F, Sferrazza G, Motolone G, Contri R, Valerio L, Lembo R, Pozzi L, Della Valle P, De Bonis M, Zangrillo A, Fiore GB, Redaelli A, Slepian MJ, Pappalardo F. Platelet activation is a preoperative risk factor for the development of thromboembolic complications in patients with continuous‐flow left ventricular assist device. Eur J Heart Fail 2018; 20: 792–800. [DOI] [PubMed] [Google Scholar]
- 117. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ, Investigators M . A fully magnetically levitated left ventricular assist device—Final report. N Engl J Med 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 118. van Bilsen M, Patel HC, Bauersachs J, Bohm M, Borggrefe M, Brutsaert D, Coats AJS, de Boer RA, de Keulenaer GW, Filippatos GS, Floras J, Grassi G, Jankowska EA, Kornet L, Lunde IG, Maack C, Mahfoud F, Pollesello P, Ponikowski P, Ruschitzka F, Sabbah HN, Schultz HD, Seferovic P, Slart R, Taggart P, Tocchetti CG, Van Laake LW, Zannad F, Heymans S, Lyon AR. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2017; 19: 1361–1378. [DOI] [PubMed] [Google Scholar]
- 119. Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, Jazwiec P, Banasiak W, Fudim M, Sobotka PA, Javaheri S, Hart EC, Paton JF, Ponikowski P. Carotid body resection for sympathetic modulation in systolic heart failure: results from first‐in‐man study. Eur J Heart Fail 2017; 19: 391–400. [DOI] [PubMed] [Google Scholar]
- 120. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cowie MR, Woehrle H, Wegscheider K, Vettorazzi E, Lezius S, Koenig W, Weidemann F, Smith G, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnoea in systolic heart failure: results of the major substudy of SERVE‐HF. Eur J Heart Fail 2018; 20: 536–544. [DOI] [PubMed] [Google Scholar]
- 122. Lyons OD, Floras JS, Logan AG, Beanlands R, Cantolla JD, Fitzpatrick M, Fleetham J, John Kimoff R, Leung RS, Lorenzi Filho G, Mayer P, Mielniczuk L, Morrison DL, Ryan CM, Series F, Tomlinson GA, Woo A, Arzt M, Parthasarathy S, Redolfi S, Kasai T, Parati G, Delgado DH, Bradley TD, Investigators A‐H . Design of the effect of adaptive servo‐ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT‐HF trial. Eur J Heart Fail 2017; 19: 579–587. [DOI] [PubMed] [Google Scholar]
- 123. Costanzo MR, Ponikowski P, Coats A, Javaheri S, Augostini R, Goldberg LR, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, McKane S, Abraham WT, remede System Pivotal Trial Study G . Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur J Heart Fail 2018; 20: 1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gwizdala A, Rozwadowska N, Kolanowski TJ, Malcher A, Cieplucha A, Perek B, Seniuk W, Straburzynska‐Migaj E, Oko‐Sarnowska Z, Cholewinski W, Michalak M, Grajek S, Kurpisz M. Safety, feasibility and effectiveness of first in‐human administration of muscle‐derived stem/progenitor cells modified with connexin‐43 gene for treatment of advanced chronic heart failure. Eur J Heart Fail 2017; 19: 148–157. [DOI] [PubMed] [Google Scholar]
- 125. Choudhury T, Mozid A, Hamshere S, Yeo C, Pellaton C, Arnous S, Saunders N, Brookman P, Jain A, Locca D, Archbold A, Knight C, Wragg A, Davies C, Mills P, Parmar M, Rothman M, Choudry F, Jones DA, Agrawal S, Martin J, Mathur A. An exploratory randomized control study of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with ischaemic cardiomyopathy: the REGENERATE‐IHD clinical trial. Eur J Heart Fail 2017; 19: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G, Investigators C. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART‐1) study. Eur J Heart Fail 2017; 19: 1520–1529. [DOI] [PubMed] [Google Scholar]
- 127. Ezekowitz JA, McAlister FA, Howlett J, Alemayehu W, Paterson I, Belenkie I, Oudit GY, Kaul P, Dyck JR, Anderson T, Alberta HI. A prospective evaluation of the established criteria for heart failure with preserved ejection fraction using the Alberta HEART cohort. ESC Heart Fail 2018; 5: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation 2017; 135: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation 2018; 137: 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dominguez E, Palau P, Nunez E, Ramon JM, Lopez L, Melero J, Bellver A, Santas E, Chorro FJ, Nunez J. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC Heart Fail 2018; 5: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail 2017; 19: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wolsk E, Claggett B, Kober L, Pocock S, Yusuf S, Swedberg K, McMurray JJV, Granger CB, Pfeffer MA, Solomon SD. Contribution of cardiac and extra‐cardiac disease burden to risk of cardiovascular outcomes varies by ejection fraction in heart failure. Eur J Heart Fail 2018; 20: 504–510. [DOI] [PubMed] [Google Scholar]
- 133. Hensen LCR, Goossens K, Delgado V, Abou R, Rotmans JI, Jukema JW, Bax JJ. Prevalence of left ventricular systolic dysfunction in pre‐dialysis and dialysis patients with preserved left ventricular ejection fraction. Eur J Heart Fail 2018; 20: 560–568. [DOI] [PubMed] [Google Scholar]
- 134. Lofman I, Szummer K, Dahlstrom U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid‐range, and reduced ejection fraction. Eur J Heart Fail 2017; 19: 1606–1614. [DOI] [PubMed] [Google Scholar]
- 135. Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, Lam CSP, Ponikowski P, Shah SJ, Gustafsson F. Resting and exercise haemodynamics in relation to six‐minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2018; 20: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Corra U, Agostoni PG, Anker SD, Coats AJS, Crespo Leiro MG, de Boer RA, Harjola VP, Hill L, Lainscak M, Lund LH, Metra M, Ponikowski P, Riley J, Seferovic PM, Piepoli MF. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 3–15. [DOI] [PubMed] [Google Scholar]
- 137. Kaye DM, Silvestry FE, Gustafsson F, Cleland JG, van Veldhuisen DJ, Ponikowski P, Komtebedde J, Nanayakkara S, Burkhoff D, Shah SJ. Impact of atrial fibrillation on rest and exercise haemodynamics in heart failure with mid‐range and preserved ejection fraction. Eur J Heart Fail 2017; 19: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 138. Cattadori G, Segurini C, Picozzi A, Padeletti L, Anza C. Exercise and heart failure: an update. ESC Heart Fail 2018; 5: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Popovic D, Arena R, Guazzi M. A flattening oxygen consumption trajectory phenotypes disease severity and poor prognosis in patients with heart failure with reduced, mid‐range, and preserved ejection fraction. Eur J Heart Fail 2018; 20: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 140. Tops LF, Delgado V, Marsan NA, Bax JJ. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail 2017; 19: 307–313. [DOI] [PubMed] [Google Scholar]
- 141. DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, Lin G, Redfield MM, Lewis G, Semigran MJ, Anstrom KJ, Hernandez AF, Velazquez EJ. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail 2017; 19: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Buggey J, Alenezi F, Yoon HJ, Phelan M, DeVore AD, Khouri MG, Schulte PJ, Velazquez EJ. Left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: outcomes following an acute heart failure hospitalization. ESC Heart Fail 2017; 4: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017; 136: 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, Becton JT, Nelson MD, Chen H, Kitzman DW. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail 2018; 6: 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail 2018; 20: 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Olivier A, Pitt B, Girerd N, Lamiral Z, Machu JL, McMurray JJV, Swedberg K, van Veldhuisen DJ, Collier TJ, Pocock SJ, Rossignol P, Zannad F, Pizard A. Effect of eplerenone in patients with heart failure and reduced ejection fraction: potential effect modification by abdominal obesity. Insight from the EMPHASIS‐HF trial. Eur J Heart Fail 2017; 19: 1186–1197. [DOI] [PubMed] [Google Scholar]
- 147. Packer M. Derangements in adrenergic‐adipokine signalling establish a neurohormonal basis for obesity‐related heart failure with a preserved ejection fraction. Eur J Heart Fail 2018; 20: 873–878. [DOI] [PubMed] [Google Scholar]
- 148. Packer M. Augmentation of glucagon‐like peptide‐1 receptor signalling by neprilysin inhibition: potential implications for patients with heart failure. Eur J Heart Fail 2018; 20: 973–977. [DOI] [PubMed] [Google Scholar]
- 149. Ravassa S, Trippel T, Bach D, Bachran D, Gonzalez A, Lopez B, Wachter R, Hasenfuss G, Delles C, Dominiczak AF, Pieske B, Diez J, Edelmann F. Biomarker‐based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo‐DHF trial. Eur J Heart Fail 2018; 20: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 150. Al‐Omary MS, Khan AA, Davies AJ, Fletcher PJ, McIvor D, Bastian B, Oldmeadow C, Sverdlov AL, Attia JR, Boyle AJ. Outcomes following heart failure hospitalization in a regional Australian setting between 2005 and 2014. ESC Heart Fail 2018; 5: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Trippel TD, Van Linthout S, Westermann D, Lindhorst R, Sandek A, Ernst S, Bobenko A, Kasner M, Spillmann F, Gonzalez A, Lopez B, Ravassa S, Pieske B, Paulus WJ, Diez J, Edelmann F, Tschope C. Investigating a biomarker‐driven approach to target collagen turnover in diabetic heart failure with preserved ejection fraction patients. Effect of torasemide versus furosemide on serum C‐terminal propeptide of procollagen type I (DROP‐PIP trial). Eur J Heart Fail 2018; 20: 460–470. [DOI] [PubMed] [Google Scholar]
- 152. Ferreira JP, Machu JL, Girerd N, Jaisser F, Thum T, Butler J, Gonzalez A, Diez J, Heymans S, McDonald K, Gyongyosi M, Firat H, Rossignol P, Pizard A, Zannad F. Rationale of the FIBROTARGETS study designed to identify novel biomarkers of myocardial fibrosis. ESC Heart Fail 2018; 5: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42. [DOI] [PubMed] [Google Scholar]
- 154. Bristow MR, Enciso JS, Gersh BJ, Grady C, Rice MM, Singh S, Sopko G, Boineau R, Rosenberg Y, Greenberg BH. Detection and management of geographic disparities in the TOPCAT trial: lessons learned and derivative recommendations. JACC Basic Transl Sci 2016; 1: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bristow MR, Sharma K, Assmann SF, Linas S, Gersh BJ, Grady C, Rice MM, Singh S, Boineau R, McKinlay SM, Greenberg BH. Data and safety monitoring board evaluation and management of a renal adverse event signal in TOPCAT. Eur J Heart Fail 2017; 19: 457–465. [DOI] [PubMed] [Google Scholar]
- 156. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, Investigators T . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, Desai AS, Lewis EF, Pitt B, Sweitzer NK, Pfeffer MA, Solomon SD. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur J Heart Fail 2018; 20: 483–490. [DOI] [PubMed] [Google Scholar]
- 158. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, Investigators P‐H and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 159. Komajda M, Isnard R, Cohen‐Solal A, Metra M, Pieske B, Ponikowski P, Voors AA, Dominjon F, Henon‐Goburdhun C, Pannaux M, Bohm M, prEserve DlvefchFwisI . Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo‐controlled trial. Eur J Heart Fail 2017; 19: 1495–1503. [DOI] [PubMed] [Google Scholar]
- 160. Liu LC, Hummel YM, van der Meer P, Berger RM, Damman K, van Veldhuisen DJ, Voors AA, Hoendermis ES. Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health‐related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. Eur J Heart Fail 2017; 19: 116–125. [DOI] [PubMed] [Google Scholar]
- 161. Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J 2015; 36: 2565–2573. [DOI] [PubMed] [Google Scholar]
- 162. Filippatos G, Maggioni AP, Lam CSP, Pieske‐Kraigher E, Butler J, Spertus J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Bamber L, Gheorghiade M, Pieske B. Patient‐reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES‐PRESERVED) study. Eur J Heart Fail 2017; 19: 782–791. [DOI] [PubMed] [Google Scholar]
- 163. Pieske B, Maggioni AP, Lam CSP, Pieske‐Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES‐PRESERVED) study. Eur Heart J 2017; 38: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, Ezekowitz J, Hernandez AF, Koglin J, O'Connor CM. A multicenter, randomized, double‐blind, placebo‐controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail 2018; 6: 96–104. [DOI] [PubMed] [Google Scholar]
- 165. Pandey A, Kitzman DW, Brubaker P, Haykowsky MJ, Morgan T, Becton JT, Berry JD. Response to endurance exercise training in older adults with heart failure with preserved or reduced ejection fraction. J Am Geriatr Soc 2017; 65: 1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta‐analysis of randomized control trials. Circ Heart Fail 2015; 8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bobenko A, Bartels I, Munch M, Trippel T, Lindhorst R, Nolte K, Herrmann‐Lingen C, Halle M, Duvinage A, Dungen HD, Gelbrich G, Tschope C, Hasenfuss G, Wachter R, Pieske B, Edelmann F. Amount or intensity? Potential targets of exercise interventions in patients with heart failure with preserved ejection fraction. ESC Heart Fail 2018; 5: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Angadi SS, Jarrett CL, Sherif M, Gaesser GA, Mookadam F. The effect of exercise training on biventricular myocardial strain in heart failure with preserved ejection fraction. ESC Heart Fail 2017; 4: 356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016; 315: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, Savarese G, Lam CSP, Lund LH. A comprehensive population‐based characterization of heart failure with mid‐range ejection fraction. Eur J Heart Fail 2017; 19: 1624–1634. [DOI] [PubMed] [Google Scholar]
- 171. Rastogi A, Novak E, Platts AE, Mann DL. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid‐range ejection fraction. Eur J Heart Fail 2017; 19: 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rickenbacher P, Kaufmann BA, Maeder MT, Bernheim A, Goetschalckx K, Pfister O, Pfisterer M, Brunner‐La Rocca HP, Investigators T‐C. Heart failure with mid‐range ejection fraction: a distinct clinical entity? Insights from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME‐CHF). Eur J Heart Fail 2017; 19: 1586–1596. [DOI] [PubMed] [Google Scholar]
- 173. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S. Shimokawa H and Investigators C‐. Characterization of heart failure patients with mid‐range left ventricular ejection fraction‐a report from the CHART‐2 Study. Eur J Heart Fail 2017; 19: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 174. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, Braun OO, Savarese G, Dahlstrom U, Lund LH. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail 2017; 10: pii: e003875. [DOI] [PubMed] [Google Scholar]
- 175. Nauta JF, Hummel YM, van Melle JP, van der Meer P, Lam CSP, Ponikowski P, Voors AA. What have we learned about heart failure with mid‐range ejection fraction one year after its introduction? Eur J Heart Fail 2017; 19: 1569–1573. [DOI] [PubMed] [Google Scholar]
- 176. Delepaul B, Robin G, Delmas C, Moine T, Blanc A, Fournier P, Roger‐Rolle A, Domain G, Delon C, Uzan C, Boudjellil R, Carrie D, Roncalli J, Galinier M, Lairez O. Who are patients classified within the new terminology of heart failure from the 2016 ESC guidelines? ESC Heart Fail 2017; 4: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bhambhani V, Kizer JR, Lima JAC, van der Harst P, Bahrami H, Nayor M, de Filippi CR, Enserro D, Blaha MJ, Cushman M, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Brouwers FP, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL Jr, Levy D, Herrington DM, van Gilst WH, Bertoni AG, Larson MG, de Boer RA, Gottdiener JS, Shah SJ, Ho JE. Predictors and outcomes of heart failure with mid‐range ejection fraction. Eur J Heart Fail 2018; 20: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Gori M, Redfield MM, Calabrese A, Canova P, Cioffi G, De Maria R, Grosu A, Fontana A, Iacovoni A, Ferrari P, Parati G, Gavazzi A, Senni M. Is mild asymptomatic left ventricular systolic dysfunction always predictive of adverse events in high‐risk populations? Insights from the DAVID‐Berg study. Eur J Heart Fail 2018; 20: 1540–1548. [DOI] [PubMed] [Google Scholar]
- 179. Lauritsen J, Gustafsson F, Abdulla J. Characteristics and long‐term prognosis of patients with heart failure and mid‐range ejection fraction compared with reduced and preserved ejection fraction: a systematic review and meta‐analysis. ESC Heart Fail 2018; 5: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Bohm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson A, Wikstrand J, Kotecha D, Beta‐blockers in Heart Failure Collaborative G . Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J 2018; 39: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018; 20: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 182. Abdul‐Rahim AH, Shen L, Rush CJ, Jhund PS, Lees KR, McMurray JJV, Collaborators VI‐HF . Effect of digoxin in patients with heart failure and mid‐range (borderline) left ventricular ejection fraction. Eur J Heart Fail 2018; 20: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 183. Clemmensen TS, Molgaard H, Sorensen J, Eiskjaer H, Andersen NF, Mellemkjaer S, Andersen MJ, Tolbod LP, Harms HJ, Poulsen SH. Inotropic myocardial reserve deficiency is the predominant feature of exercise haemodynamics in cardiac amyloidosis. Eur J Heart Fail 2017; 19: 1457–1465. [DOI] [PubMed] [Google Scholar]
- 184. Siegismund CS, Escher F, Lassner D, Kuhl U, Gross U, Fruhwald F, Wenzel P, Munzel T, Frey N, Linke RP, Schultheiss HP. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur J Heart Fail 2018; 20: 751–757. [DOI] [PubMed] [Google Scholar]
- 185. Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, George I, Kodali S, Leon MB, Hahn R, Bokhari S, Maurer MS. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017; 38: 2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 187. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, Investigators A‐AS. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 188. Ameri P, Canepa M, Anker MS, Belenkov Y, Bergler‐Klein J, Cohen‐Solal A, Farmakis D, Lopez‐Fernandez T, Lainscak M, Pudil R, Ruschitska F, Seferovic P, Filippatos G, Coats A, Suter T, Von Haehling S, Ciardiello F, de Boer RA, Lyon AR, Tocchetti CG, Heart Failure Association Cardio‐Oncology Study Group of the European Society of C . Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 2018; 20: 879–887. [DOI] [PubMed] [Google Scholar]
- 189. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, Di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, Di Lenarda A. Prevalence and prognostic impact of non‐cardiac co‐morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community‐based study. Eur J Heart Fail 2018; 20: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 190. Nayor M, Larson MG, Wang N, Santhanakrishnan R, Lee DS, Tsao CW, Cheng S, Benjamin EJ, Vasan RS, Levy D, Fox CS, Ho JE. The association of chronic kidney disease and microalbuminuria with heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2017; 19: 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sokolski M, Zymlinski R, Biegus J, Siwolowski P, Nawrocka‐Millward S, Todd J, Yerramilli MR, Estis J, Jankowska EA, Banasiak W, Ponikowski P. Urinary levels of novel kidney biomarkers and risk of true worsening renal function and mortality in patients with acute heart failure. Eur J Heart Fail 2017; 19: 760–767. [DOI] [PubMed] [Google Scholar]
- 192. Jain A, Scott C, Chen HH. The renal‐cardiac connection in subjects with preserved ejection fraction: a population based study. ESC Heart Fail 2017; 4: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jenkins R, Mandarano L, Gugathas S, Kaski JC, Anderson L, Banerjee D. Impaired renal function affects clinical outcomes and management of patients with heart failure. ESC Heart Fail 2017; 4: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Canepa M, Straburzynska‐Migaj E, Drozdz J, Fernandez‐Vivancos C, Pinilla JMG, Nyolczas N, Temporelli PL, Mebazaa A, Lainscak M, Laroche C, Maggioni AP, Piepoli MF, Coats AJS, Ferrari R, Tavazzi L, Investigators E‐HHFL‐TR. Characteristics, treatments and 1‐year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long‐Term Registry. Eur J Heart Fail 2018; 20: 100–110. [DOI] [PubMed] [Google Scholar]
- 195. Doehner W, Ural D, Celutkiene J. Heart‐brain interactions in patients with heart failure, including takotsubo syndrome: a need to monitor autonomic sympathetic activity: reply. Eur J Heart Fail 2018; 20: 1164–1165. [DOI] [PubMed] [Google Scholar]
- 196. Pearse SG, Cowie MR. Sleep‐disordered breathing in heart failure. Eur J Heart Fail 2016; 18: 353–361. [DOI] [PubMed] [Google Scholar]
- 197. Pietrock C, von Haehling S. Sleep‐disordered breathing in heart failure: facts and numbers. ESC Heart Fail 2017; 4: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sharma A, Demissei BG, Tromp J, Hillege HL, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Davison BA, Givertz MM, Bloomfield DM, Dittrich H, van Veldhuisen DJ, Cotter G, Ezekowitz JA, Khan MAF, Voors AA. A network analysis to compare biomarker profiles in patients with and without diabetes mellitus in acute heart failure. Eur J Heart Fail 2017; 19: 1310–1320. [DOI] [PubMed] [Google Scholar]
- 199. Kristensen SL, Rorth R, Jhund PS, Shen L, Lee MMY, Petrie MC, Kober L, McMurray JJV, Investigators B . Microvascular complications in diabetes patients with heart failure and reduced ejection fraction‐insights from the Beta‐blocker Evaluation of Survival Trial. Eur J Heart Fail 2018; 20: 1549–1556. [DOI] [PubMed] [Google Scholar]
- 200. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Baron‐Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol C, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GY, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Vaz Carneiro A, Windecker S, Authors/Task Force M, Guidelines ESCCfP and Document R . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017; 19: 9–42. [DOI] [PubMed] [Google Scholar]
- 201. Lam CSP, Doehner W, Comin‐Colet J, Group IC . Iron deficiency in chronic heart failure: case‐based practical guidance. ESC Heart Fail 2018; 5: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Barkhudaryan A, Scherbakov N, Springer J, Doehner W. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail 2017; 4: 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Saitoh M, Dos Santos MR, Emami A, Ishida J, Ebner N, Valentova M, Bekfani T, Sandek A, Lainscak M, Doehner W, Anker SD, von Haehling S. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). ESC Heart Fail 2017; 4: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lena A, Coats AJS, Anker MS. Metabolic disorders in heart failure and cancer. ESC Heart Fail 2018; 5: 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail 2018; 5: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018; 20: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 207. Fernandes‐Silva MM, Shah AM, Claggett B, Cheng S, Tanaka H, Silvestre OM, Nadruz W, Borlaug BA, Solomon SD. Adiposity, body composition and ventricular‐arterial stiffness in the elderly: the Atherosclerosis Risk in Communities Study. Eur J Heart Fail 2018; 20: 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Streng KW, Voors AA, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, van der Meer P, Lang CC. Waist‐to‐hip ratio and mortality in heart failure. Eur J Heart Fail 2018; 20: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 209. Anker MS, von Haehling S, Landmesser U, Coats AJS, Anker SD. Cancer and heart failure‐more than meets the eye: common risk factors and co‐morbidities. Eur J Heart Fail 2018; 20: 1382–1384. [DOI] [PubMed] [Google Scholar]
- 210. Banke A, Fosbol EL, Moller JE, Gislason GH, Andersen M, Bernsdorf M, Jensen MB, Schou M, Ejlertsen B. Long‐term effect of epirubicin on incidence of heart failure in women with breast cancer: insight from a randomized clinical trial. Eur J Heart Fail 2018; 20: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 211. Pareek N, Cevallos J, Moliner P, Shah M, Tan LL, Chambers V, Baksi AJ, Khattar RS, Sharma R, Rosen SD, Lyon AR. Activity and outcomes of a cardio‐oncology service in the United Kingdom‐a five‐year experience. Eur J Heart Fail 2018; 20: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 212. Fornaro A, Olivotto I, Rigacci L, Ciaccheri M, Tomberli B, Ferrantini C, Coppini R, Girolami F, Mazzarotto F, Chiostri M, Milli M, Marchionni N, Castelli G. Comparison of long‐term outcome in anthracycline‐related versus idiopathic dilated cardiomyopathy: a single centre experience. Eur J Heart Fail 2018; 20: 898–906. [DOI] [PubMed] [Google Scholar]
- 213. Ancion A, Allepaerts S, Oury C, Gori AS, Pierard LA, Lancellotti P. Serum albumin level and hospital mortality in acute non‐ischemic heart failure. ESC Heart Fail 2017; 4: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CSP, Cohen‐Solal A, Mebazaa A, Seronde MF. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 2018; 20: 738–747. [DOI] [PubMed] [Google Scholar]
- 215. Ferreira JP, Metra M, Mordi I, Gregson J, Ter Maaten JM, Tromp J, Anker SD, Dickstein K, Hillege HL, Ng LL, van Veldhuisen DJ, Lang CC, Voors AA, Zannad F. Heart failure in the outpatient versus inpatient setting: findings from the BIOSTAT‐CHF study. Eur J Heart Fail 2019; 21: 112–120. [DOI] [PubMed] [Google Scholar]
- 216. Platz E, Jhund PS, Claggett BL, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Solomon SD, McMurray JJ. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: recurrent hospitalizations and mortality. Eur J Heart Fail 2018; 20: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi DJ, Park JJ, Alhabib KF, Sato N, Miro O, Maggioni AP, Zhang Y, Spinar J, Cohen‐Solal A, Iwashyna TJ, Mebazaa A, Network G. Precipitating factors and 90‐day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail 2017; 19: 201–208. [DOI] [PubMed] [Google Scholar]
- 218. Arrigo M, Tolppanen H, Sadoune M, Feliot E, Teixeira A, Laribi S, Plaisance P, Nouira S, Yilmaz MB, Gayat E, Mebazaa A, Network G. Effect of precipitating factors of acute heart failure on readmission and long‐term mortality. ESC Heart Fail 2016; 3: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Laufs U, Griese‐Mammen N, Krueger K, Wachter A, Anker SD, Koehler F, Rettig‐Ewen V, Botermann L, Strauch D, Trenk D, Bohm M, Schulz M. PHARMacy‐based interdisciplinary program for patients with Chronic Heart Failure (PHARM‐CHF): rationale and design of a randomized controlled trial, and results of the pilot study. Eur J Heart Fail 2018; 20: 1350–1359. [DOI] [PubMed] [Google Scholar]
- 220. Rorth R, Fosbol EL, Mogensen UM, Kragholm K, Nume AK, Gislason GH, Jhund PS, Petrie MC, McMurray JJV, Torp‐Pedersen C, Kober L, Kristensen SL. Employment status at time of first hospitalization for heart failure is associated with a higher risk of death and rehospitalization for heart failure. Eur J Heart Fail 2018; 20: 240–247. [DOI] [PubMed] [Google Scholar]
- 221. Koudstaal S, Pujades‐Rodriguez M, Denaxas S, Gho J, Shah AD, Yu N, Patel RS, Gale CP, Hoes AW, Cleland JG, Asselbergs FW, Hemingway H. Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population‐based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail 2017; 19: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Fudim M, O'Connor CM, Dunning A, Ambrosy AP, Armstrong PW, Coles A, Ezekowitz JA, Greene SJ, Metra M, Starling RC, Voors AA, Hernandez AF, Michael Felker G, Mentz RJ. Aetiology, timing and clinical predictors of early vs. late readmission following index hospitalization for acute heart failure: insights from ASCEND‐HF. Eur J Heart Fail 2018; 20: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR, Investigators R‐A . Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX‐AHF) development program: correlation with outcomes. J Am Coll Cardiol 2013; 61: 196–206. [DOI] [PubMed] [Google Scholar]
- 224. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner‐La Rocca HP, Chioncel O, Collins SP, Doehner W, Filippatos GS, Flammer AJ, Fuhrmann V, Lainscak M, Lassus J, Legrand M, Masip J, Mueller C, Papp Z, Parissis J, Platz E, Rudiger A, Ruschitzka F, Schafer A, Seferovic PM, Skouri H, Yilmaz MB, Mebazaa A. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2017; 19: 821–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Harjola VP, Parissis J, Brunner‐La Rocca HP, Celutkiene J, Chioncel O, Collins SP, De Backer D, Filippatos GS, Gayat E, Hill L, Lainscak M, Lassus J, Masip J, Mebazaa A, Miro O, Mortara A, Mueller C, Mullens W, Nieminen MS, Rudiger A, Ruschitzka F, Seferovic PM, Sionis A, Vieillard‐Baron A, Weinstein JM, de Boer RA, Crespo‐Leiro MG, Piepoli M, Riley JP. Comprehensive in‐hospital monitoring in acute heart failure: applications for clinical practice and future directions for research. A statement from the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2018; 20: 1081–1099. [DOI] [PubMed] [Google Scholar]
- 226. Zymlinski R, Biegus J, Sokolski M, Siwolowski P, Nawrocka‐Millward S, Todd J, Jankowska EA, Banasiak W, Cotter G, Cleland JG, Ponikowski P. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur J Heart Fail 2018; 20: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 227. Zymlinski R, Sokolski M, Biegus J, Siwolowski P, Nawrocka‐Millward S, Sokolska JM, Dudkowiak M, Marciniak D, Todd J, Jankowska EA, Banasiak W, Ponikowski P. Multi‐organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur J Heart Fail 2019; 21: 744–750. [DOI] [PubMed] [Google Scholar]
- 228. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA, Adams KF, Anker SD, Arias‐Mendoza A, Avendano P, Bacal F, Bohm M, Bortman G, Cleland JGF, Cohen‐Solal A, Crespo‐Leiro MG, Dorobantu M, Echeverria LE, Ferrari R, Goland S, Goncalvesova E, Goudev A, Kober L, Lema‐Osores J, Levy PD, McDonald K, Manga P, Merkely B, Mueller C, Pieske B, Silva‐Cardoso J, Spinar J, Squire I, Stepinska J, Van Mieghem W, von Lewinski D, Wikstrom G, Yilmaz MB, Hagner N, Holbro T, Hua TA, Sabarwal SV, Severin T, Szecsody P, Gimpelewicz C, Investigators R‐A‐C . Effects of serelaxin in patients with acute heart failure. N Engl J Med 2019; 381: 716–726. [DOI] [PubMed] [Google Scholar]
- 229. Teerlink JR, Voors AA, Ponikowski P, Pang PS, Greenberg BH, Filippatos G, Felker GM, Davison BA, Cotter G, Gimpelewicz C, Boer‐Martins L, Wernsing M, Hua TA, Severin T, Metra M. Serelaxin in addition to standard therapy in acute heart failure: rationale and design of the RELAX‐AHF‐2 study. Eur J Heart Fail 2017; 19: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Pokorney SD, Al‐Khatib SM, Sun JL, Schulte P, O'Connor CM, Teerlink JR, Armstrong PW, Ezekowitz JA, Starling RC, Voors AA, Velazquez EJ, Hernandez AF, Mentz RJ. Sudden cardiac death after acute heart failure hospital admission: insights from ASCEND‐HF. Eur J Heart Fail 2018; 20: 525–532. [DOI] [PubMed] [Google Scholar]
- 231. Ferreira JP, Duarte K, Pfeffer MA, McMurray JJV, Pitt B, Dickstein K, Zannad F, Rossignol P, High‐Risk Myocardial Infarction Database I . Association between mean systolic and diastolic blood pressure throughout the follow‐up and cardiovascular events in acute myocardial infarction patients with systolic dysfunction and/or heart failure: an analysis from the High‐Risk Myocardial Infarction Database Initiative. Eur J Heart Fail 2018; 20: 323–331. [DOI] [PubMed] [Google Scholar]
- 232. Bohm M, Cammann VL, Ghadri JR, Ukena C, Gili S, Di Vece D, Kato K, Ding KJ, Szawan KA, Micek J, Jurisic S, D'Ascenzo F, Frangieh AH, Rechsteiner D, Seifert B, Ruschitzka F, Luscher T, Templin C, Inter TAKC. Interaction of systolic blood pressure and resting heart rate with clinical outcomes in takotsubo syndrome: insights from the International Takotsubo Registry. Eur J Heart Fail 2018; 20: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 233. Cotter G, Metra M, Davison BA, Jondeau G, Cleland JGF, Bourge RC, Milo O, O'Connor CM, Parker JD, Torre‐Amione G, van Veldhuisen DJ, Kobrin I, Rainisio M, Senger S, Edwards C, McMurray JJV, Teerlink JR, Investigators V. Systolic blood pressure reduction during the first 24 h in acute heart failure admission: friend or foe? Eur J Heart Fail 2018; 20: 317–322. [DOI] [PubMed] [Google Scholar]
- 234. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent LB, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 235. Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail 2017; 19: 1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Pellicori P, Shah P, Cuthbert J, Urbinati A, Zhang J, Kallvikbacka‐Bennett A, Clark AL, Cleland JGF. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail 2019; 21: 904–916. [DOI] [PubMed] [Google Scholar]
- 237. Darche FF, Baumgartner C, Biener M, Muller‐Hennessen M, Vafaie M, Koch V, Stoyanov K, Rivinius R, Katus HA, Giannitsis E. Comparative accuracy of NT‐proBNP and MR‐proANP for the diagnosis of acute heart failure in dyspnoeic patients. ESC Heart Fail 2017; 4: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kataoka H. Proposal for heart failure progression based on the 'chloride theory': worsening heart failure with increased vs. non‐increased serum chloride concentration. ESC Heart Fail 2017; 4: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Cuthbert JJ, Pellicori P, Rigby A, Pan D, Kazmi S, Shah P, Clark AL. Low serum chloride in patients with chronic heart failure: clinical associations and prognostic significance. Eur J Heart Fail 2018; 20: 1426–1435. [DOI] [PubMed] [Google Scholar]
- 240. Ter Maaten JM, Damman K, Hanberg JS, Givertz MM, Metra M, O'Connor CM, Teerlink JR, Ponikowski P, Cotter G, Davison B, Cleland JG, Bloomfield DM, Hillege HL, van Veldhuisen DJ, Voors AA, Testani JM. Hypochloremia, Diuretic Resistance, and Outcome in Patients With Acute Heart Failure. Circ Heart Fail 2016; 9; pii: e003109. [DOI] [PubMed] [Google Scholar]
- 241. Grodin JL, Testani JM, Pandey A, Sambandam K, Drazner MH, Fang JC, Tang WHW. Perturbations in serum chloride homeostasis in heart failure with preserved ejection fraction: insights from TOPCAT. Eur J Heart Fail 2018; 20: 1436–1443. [DOI] [PubMed] [Google Scholar]
- 242. Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, Rubini Gimenez M, Kozhuharov N, Sabti Z, Breitenbucher D, Wildi K, Puelacher C, Honegger U, Wagener M, Schumacher C, Hillinger P, Osswald S, Mueller C. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail 2017; 19: 226–236. [DOI] [PubMed] [Google Scholar]
- 243. Ter Maaten JM, Valente MA, Damman K, Cleland JG, Givertz MM, Metra M, O'Connor CM, Teerlink JR, Ponikowski P, Bloomfield DM, Cotter G, Davison B, Subacius H, van Veldhuisen DJ, van der Meer P, Hillege HL, Gheorghiade M, Voors AA. Combining diuretic response and hemoconcentration to predict rehospitalization after admission for acute heart failure. Circ Heart Fail 2016; 9; pii: e002845. [DOI] [PubMed] [Google Scholar]
- 244. Jujo K, Minami Y, Haruki S, Matsue Y, Shimazaki K, Kadowaki H, Ishida I, Kambayashi K, Arashi H, Sekiguchi H, Hagiwara N. Persistent high blood urea nitrogen level is associated with increased risk of cardiovascular events in patients with acute heart failure. ESC Heart Fail 2017; 4: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Gayat E, Arrigo M, Littnerova S, Sato N, Parenica J, Ishihara S, Spinar J, Muller C, Harjola VP, Lassus J, Miro O, Maggioni AP, AlHabib KF, Choi DJ, Park JJ, Zhang Y, Zhang J, Januzzi JL Jr, Kajimoto K, Cohen‐Solal A, Mebazaa A, Network G. Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity‐score matched study. Eur J Heart Fail 2018; 20: 345–354. [DOI] [PubMed] [Google Scholar]
- 246. Mebazaa A, Motiejunaite J, Gayat E, Crespo‐Leiro MG, Lund LH, Maggioni AP, Chioncel O, Akiyama E, Harjola VP, Seferovic P, Laroche C, Julve MS, Roig E, Ruschitzka F, Filippatos G, Investigators ESCHFL‐TR . Long‐term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long‐Term Registry. Eur J Heart Fail 2018; 20: 332–341. [DOI] [PubMed] [Google Scholar]
- 247. Tita C, Gilbert EM, Van Bakel AB, Grzybowski J, Haas GJ, Jarrah M, Dunlap SH, Gottlieb SS, Klapholz M, Patel PC, Pfister R, Seidler T, Shah KB, Zielinski T, Venuti RP, Cowart D, Foo SY, Vishnevsky A, Mitrovic V. A Phase 2a dose‐escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS‐986231 in hospitalized patients with heart failure with reduced ejection fraction. Eur J Heart Fail 2017; 19: 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Felker GM, Borentain M, Cleland JG, DeSouza MM, Kessler PD, O'Connor CM, Seiffert D, Teerlink JR, Voors AA, McMurray JJV. Rationale and design for the development of a novel nitroxyl donor in patients with acute heart failure. Eur J Heart Fail 2019; 21: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 249. Ter Maaten JM, Rao VS, Hanberg JS, Perry Wilson F, Bellumkonda L, Assefa M, Sam Broughton J, D'Ambrosi J, Wilson Tang WH, Damman K, Voors AA, Ellison DH, Testani JM. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail 2017; 19: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Mullens W, Verbrugge FH, Nijst P, Martens P, Tartaglia K, Theunissen E, Bruckers L, Droogne W, Troisfontaines P, Damman K, Lassus J, Mebazaa A, Filippatos G, Ruschitzka F, Dupont M. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail 2018; 20: 1591–1600. [DOI] [PubMed] [Google Scholar]
- 251. Meani P, Gelsomino S, Natour E, Johnson DM, Rocca HB, Pappalardo F, Bidar E, Makhoul M, Raffa G, Heuts S, Lozekoot P, Kats S, Sluijpers N, Schreurs R, Delnoij T, Montalti A, Sels JW, van de Poll M, Roekaerts P, Poels T, Korver E, Babar Z, Maessen J, Lorusso R. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail 2017; 19: 84–91. [DOI] [PubMed] [Google Scholar]
- 252. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Mullerleile K, Colombo A, Sydow K, De Bonis M, Wagner F, Reichenspurner H, Blankenberg S, Zangrillo A, Westermann D. Concomitant implantation of Impella((R)) on top of veno‐arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017; 19: 404–412. [DOI] [PubMed] [Google Scholar]


