Abstract
Aims
Limited data are available regarding the ability of sacubitril/valsartan to provide clinically meaningful health‐related quality of life (HRQoL) improvements among individuals with heart failure (HF). Objective measurement of physical activity and sleep using actigraphy can provide insight into daily functioning and HRQoL.
Methods and results
We designed an 18 week, multicenter, randomized, double‐blind, double‐dummy, parallel‐group study to objectively assess changes in function and HRQoL directly after initiating sacubitril/valsartan vs. enalapril in participants with HF in their home environments. A total of 136 outpatient, ambulatory participants with New York Heart Association Class II or III HF with reduced ejection fraction (HFrEF) will be included in the study. Patients will undergo a 2 week baseline observational phase (continuing current HF treatment); data from the second week of this phase will be the baseline value for comparison with those of subsequent periods. Patients will then enter an 8 week blinded‐treatment phase (randomly assigned 1:1 to sacubitril/valsartan or enalapril), followed by an 8 week open‐label extension phase (treatment with only sacubitril/valsartan). The primary efficacy endpoint is the change in mean activity counts during the most active 30 min of the participant's day between baseline and the final randomized treatment phase measurement. Secondary endpoints include the change in mean sleep activity during the randomized and open‐label phases; questionnaires will also assess HRQoL measures. Rather than analysing pooled actigraphy data, the researchers are considering each participant to be acting as his or her own control.
Conclusions
This will be the first study to assess the effects of sacubitril/valsartan on objective measures of sleep and activity in individuals with HFrEF within the context of their daily lives. Wearable accelerometer devices will be used to gain insight into how the medication affects physical activity and sleep.
Keywords: Biosensor, Sacubitril/valsartan, Health‐related quality of life, Sleep, Heart failure, Physical activity
1. Introduction
Given the significant morbidity associated with heart failure (HF), researchers are focusing investigations on understanding both the symptom burden and the effect of treatments on health‐related quality of life (HRQoL). Current HRQoL assessment, including the 6 min walk test (6MWT) and questionnaires, are reliable and well validated, and provide important prognostic information in HF.1, 2 These approaches also give a validated and well‐understood basis for evaluating HRQoL in HF. Although the design and validation of assessments for HRQoL generally consider and control for factors such as patient recall and reporting bias, these approaches remain single time‐point assessments. The use of actigraphy by a wearable sensor objectively and continuously measures the frequency and intensity of activity and sleep in a patient's home environment and is not affected by recall and reporting bias.3, 4, 5 Actigraphy may also be able to provide information, such as patterns of activity, that could identify responders and nonresponders to therapy and the time course of patient improvement. Additionally, a recent scientific statement from the American Heart Association advised that ‘wearable activity monitoring devices and improved software and technology partnerships open opportunities for integrating objective physical activity data to improve the quality of care and health status of patients with cardiovascular disease risk’.6
Sacubitril/valsartan is a first‐in‐class angiotensin receptor/neprilysin inhibitor (ARNI) therapy approved for the treatment of symptomatic HF with reduced ejection fraction (HFrEF).7 In the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF; NCT01035255) trial, treatment with sacubitril/valsartan reduced the risk of the composite outcome of CV death or first hospitalization for worsening HF than did enalapril (hazard ratio 0.80; 95% confidence interval 0.73–0.87; P < 0.001).8 The cardiovascular and renal pharmacological effects of sacubitril/valsartan are attributed to the inhibition of the renin–angiotensin–aldosterone system by valsartan and neprilysin inhibition by sacubitril, which leads to increased concentrations of natriuretic peptides and subsequent natriuresis.9 The potential for sacubitril/valsartan to reduce pulmonary congestion is suggested by findings of reduced N‐terminal pro‐B‐type natriuretic peptide levels in patients hospitalized with acute decompensated HF from PIONEER‐HF.10 In theory, the decreased pulmonary congestion that results from the natriuretic effects of sacubitril/valsartan may improve sleep and reduce fatigue by improving wakeful activity. Reduction in congestion may also contribute to a reduction in sleep‐disordered breathing.11 Although sacubitril/valsartan slowed deterioration of HRQoL in PARADIGM‐HF, the timing of baseline assessments after the run‐in phase and use of subjective measures may have limited detection of clinically meaningful improvements.12 Consequently, limited clinical trial data are available to support anecdotal reports of clinically meaningful improvements in HFrEF after initiating sacubitril/valsartan.13
The Phase 4 AWAKE‐HF trial (NCT02970669) will leverage digital medicine technology to provide insight and perspective on the early clinical experience with sacubitril/valsartan in a way that approximates the real‐world setting. Continuous actigraphy will objectively assess outcomes in participants' usual home environment within 2 months of initiating therapy.
2. Methods
2.1. Design
The 18 week study will include a 2 week baseline observation phase (with participants continuing current HF drugs), followed by an 8 week blinded‐treatment phase during which participants will be randomly assigned 1:1 to sacubitril/valsartan treatment or enalapril comparator, and finally an 8 week, open‐label extension phase during which all participants will be treated with sacubitril/valsartan (Figure 1). Before start of the randomized, blinded‐treatment phase, participants previously on an angiotensin‐converting enzyme inhibitor (ACEI) will undergo a 36 h washout period. All participants will undergo another 36 h washout period before beginning the open‐label extension phase. As the PARADIGM‐HF trial demonstrated improved clinical outcomes for patients treated with sacubitril/valsartan compared with enalapril, an open‐label extension was included in the AWAKE‐HF study design to minimize exposure to sub‐optimal guideline‐directed HF therapy. The study will include eight weekly or biweekly office visits and five follow‐up visits via telephone. This trial is registered at http://ClinicalTrials.gov: NCT02970669. The study protocol was approved by the relevant institutional committees on human research review, and all participants will be required to sign written informed consent before enrolling.
Figure 1.
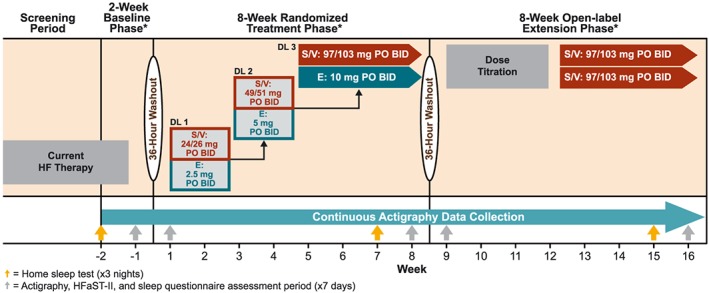
Study design. *KCCQ‐23 administered at the end of each study phase. BID, twice daily; DL, dose level; E, enalapril; HF, heart failure; HFaST‐II, Heart Failure Symptom Tracker‐II; KCCQ, Kansas City Cardiomyopathy Questionnaire‐23; PO, orally; S/V, sacubitril/valsartan.
2.2. Population
The study population will consist of outpatient men and women who are 18 to 80 years of age, with HFrEF New York Heart Association (NYHA) Class II or III (Table 1).
Table 1.
Key inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
• Written informed consent must be obtained before any assessment is performed • Men and women between 18 and 80 years of age • Participants diagnosed with NYHA Class II or III HFrEF • Participants must be a candidate for treatment with sacubitril/valsartan as per the US prescribing information • Participants must be living in a traditional residence, apartment, or noncommunal adult home where they can move freely and frequently, and they must be primarily responsible for scheduling their sleep and daily activities |
• Participants with a history of hypersensitivity to any of the study drugs, including history of hypersensitivity to drugs of similar chemical classes, or allergy to ACEIs, ARBs, or neprilysin inhibitors, as well as known or suspected contraindications to the study drugs • Participants with a history of angioedema, drug related, or otherwise • Participants with symptomatic hypotension, systolic blood pressure < 100 mmHg at screening, or <95 mmHg at randomization • Participants with any conditions in skin or upper extremities that would limit the ability to tolerate a wrist‐worn actigraphy device on the nondominant arm for 24 h/day for the duration of the study • Participants who are nonambulatory or use mobility assistive devices, such as motorized devices, wheelchairs, or walkers. The use of canes for stability while ambulating is acceptable • Participants with physical activity impairment primarily due to conditions other than HF such as: ∘ Exertional angina ∘ Inflammatory or degenerative joint disease ∘ Gout ∘ Peripheral vascular disease ∘ Neurologic disease affecting activity or mobility • Participants unwilling or unable to wear or operate study‐measurement devices for the phases required |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
2.3. Study treatment
Before randomization, a 2 week phase will occur to allow for device familiarization and baseline assessment completion. Data from the second week of this phase, the ‘baseline phase’ (Week −1), will be the baseline value for comparison with those of subsequent periods. Actigraphy data collected outside defined measurement periods will be excluded from analysis for defined study endpoints. Before randomization, site staff will evaluate the completeness of baseline actigraphy data, and participants without adequate baseline data will be asked to repeat the baseline period or will be excluded from the study.
The 36 h washout interval after randomization and before entering the open‐label extension is consistent with US Food and Drug Administration‐approved prescribing information to minimize the potential for angioedema when switching from an ACEI to an ARNI.7 To maintain blinding, the 36 h washout interval was applied to both study arms. After the washout period, participants will be randomly assigned 1:1 to sacubitril/valsartan or enalapril. Treatment begins after randomization on Day 1, at Dose Level 1 (Table S1 ).
Therapy will be up‐titrated every 2 weeks, as clinically tolerated, to target Dose Level 3. The randomized comparison phase of 8 weeks provides direct assessment of initial and early changes associated with sacubitril/valsartan treatment compared with treatment with enalapril. Study‐drug dose‐level adjustments should be mainly based on overall safety and tolerability (Table 2).
Table 2.
Safety and tolerability guidance for dose adjustments
| Parameter | Criteria |
|---|---|
| Potassium concentration | • Potassium concentration > 5.3 mEq/L |
| Kidney function |
• eGFR reduction ≥ 35% vs. baseline; or • Serum creatinine increase of ≥0.5 mg/dL with eGFR decrease of at least 25% |
| Blood pressure |
• Symptomatic hypotension • Systolic blood pressure < 90 mmHg |
| Adverse events or conditions | • Postural symptoms or any conditions that preclude continuation according to the investigator's judgement |
eGFR, estimated glomerular filtration rate.
Every attempt should be made to maintain participants on the target study‐drug dose level for as long as possible. If the investigator determines a participant is unable to tolerate the protocol‐specified target dose, attempts should be made to rectify the situation by adjusting concomitant medications before reducing the dose of the study treatment to the next lower level (Table S1 ). Participants may restart current doses of study drug following an interruption of treatment, based on investigator judgement. All participants will enter the open‐label treatment phase on sacubitril/valsartan at Dose Level 2, unless they completed the double‐blind treatment epoch on Dose Level 1, in which case they will initiate sacubitril/valsartan at that level. Therapy will then be up‐titrated in the same way as in the double‐blind treatment phase.
If a participant's condition warrants any change in concomitant HF or CV medications, changes may be made at the investigator's discretion. At their discretion, the investigator may use and adjust oral diuretics throughout the study.
2.4. Assessments
To assess the effect of sacubitril/valsartan compared with enalapril on daytime activity, sleep, and HRQoL, we will employ actigraphy to assess activity and sleep, a home sleep test, and a patient‐reported outcome assessment.
2.4.1. Activity assessments
Actigraphy has been validated and used successfully in prospective interventional studies, including studies in patients with HFrEF, to assess the impact of pharmacological treatment on physical activity during both sleep and wakefulness.14, 15 In actigraphy, wearable digital sensors called accelerometers measure the accelerations of the device in motion along reference axes. Acceleration is proportional to external force and, therefore, can reflect intensity and frequency of physical activity. In our study, individuals will wear a wrist‐worn activity monitor for 18 weeks. The primary endpoint is measured in each participant as the change from baseline (Week −1) to the end of the randomized phase (Week 8) in mean activity counts collected during the most active 30 min of the day using actigraphy. Secondary endpoints include change from baseline compared with the initiation of sacubitril/valsartan (Week 1), as well as the beginning and end of the open‐label phase (Weeks 9 and 16) in mean activity counts during the most active 30 min of the day (Table 3). Selection of the novel primary endpoint of this study was informed by previous studies. Peak activity for a 30 min duration by actigraphy is reported to discriminate between HF with or without anergia.16 Missing data will be imputed using the last‐observation‐carried‐forward method. If there is no post‐baseline value, the missing value will not be imputed, and the patient will be removed from the analysis. If data are missing or unevaluable for the week preceding randomization, data from the previous week will be used as the baseline measurement. If data are missing or unevaluable for each of these weeks, the patient will be excluded.
Table 3.
Study endpoints and statistical analysis (intent‐to‐treat full analysis set)
| Endpoint | Statistical method |
|---|---|
| Primary objective | |
| • Change in mean activity counts collected during the most active 30 min of the participant's day between baseline phase (mean of data collected each day during Week −1) and the final randomized treatment phase measurement (mean of endpoint data collected each day during Week 8) | • ANCOVA model with treatment and baseline activity as exploratory variablesa |
| Secondary objectives | |
|
Based on actigraphy data during the randomized treatment phase: • Change in mean activity during sleep between baseline phase and Week 8 • Change in mean activity during sleep between baseline phase and Week 1 • Change in mean activity counts during the most active 30 min of the participant's day between baseline phase and Week 1 |
• ANCOVA model will be used to analyse the mean change from baseline to each time point for each continuous variablea |
|
Based on actigraphy data during the open‐label extension phase: • Change in mean activity during sleep between baseline phase and Weeks 9 and 16 • Change in mean activity counts during the most active 30 min of the participant's day between baseline phase and Weeks 9 and 16 |
• Paired t‐tests will be used to analyse the change from baseline to each time point for each continuous variable o Baseline is defined as the mean of Week −1 (for the group randomized to sacubitril/valsartan) and the mean of Week 8 (for the group randomized to enalapril) |
| Exploratory objectives | |
|
During the randomized treatment phase: • Change in AHI between baseline and the randomized treatment • Actigraphy‐based endpoints [between baseline phase (mean of Week −1) and Weeks 1 and 8]: ∘ Change in WASO ∘ Proportion of participants with improvement in sleep efficiency ∘ Change in 12 h skewness and kurtosis of the participant's daytime activity patterns ∘ Change in mean activity counts collected during the most active 6 min of the participant's day • Change in HFaST‐II scores between baseline phase and Weeks 1 and 8 • Change in KCCQ‐23 clinical summary score between surveys collected at Day 1 and the end of the randomized treatment phase |
• ANCOVA model will be used to analyse the mean change from baseline to each time point for each continuous variablea • Fisher's exact test will compare the proportion of participants with improvement in sleep efficiency between treatment groups at each time point • For the HFaST‐II: ∘ ANCOVA model will be used to analyse the questions measured on a Likert scale ∘ Fisher's exact test will be used to compare between treatment groups for the yes/no questions • Sleep questionnaire data will be summarized by treatment group • Pearson's correlation coefficients will be calculated to examine the association between information from the sleep questionnaire and the actigraphy sleep data |
|
During the open‐label extension phase: • Change in AHI between baseline home sleep test and the open‐label phase home sleep test • Actigraphy‐based endpoints [between baseline phase (mean of Week −1) and Week 9 and 16]: ∘ Change in WASO ∘ Proportion of participants with improvement in sleep efficiency ∘ Change in 12 h skewness and kurtosis of the participant's daytime activity patterns ∘ Change in mean activity counts collected during the most active 6 min of the participant's day • Change in HFaST‐II scores between baseline phase and Week 9 and 16 • Change in KCCQ‐23 clinical summary score between surveys collected at Day 1 the end of the open‐label treatment phase |
• Paired t‐tests will be used to analyse the change from baseline to each time point for each continuous variable ∘ Baseline is defined as the mean of Week −1 (for the group randomized to sacubitril/valsartan) and the mean of Week 8 (for the group randomized to enalapril) • The proportion of participants with improvement in sleep efficiency will be summarized by randomized treatment group at each time point • For the HFaST‐II: ∘ ANCOVA model will be used to analyse the questions measured on a Likert scale ∘ Yes/no questions will be summarized by randomized treatment group • Sleep questionnaire data will be summarized by treatment group • Pearson's correlation coefficients will be calculated to examine the association between information from the sleep questionnaire and the actigraphy sleep data |
Analyses were conducted following the intent‐to‐treat principle for the full analysis set, defined as all patients except those who were not qualified for randomization and did not receive study drug but were inadvertently randomized into the study.
AHI, apnoea–hypopnea index; ANCOVA, analysis of covariance; CI, confidence interval; HFaST‐II, Heart Failure Symptom Tracker‐II; KCCQ‐23, Kansas City Cardiomyopathy Questionnaire‐23; WASO, wake after sleep onset.
aMissing data will be imputed using the last‐observation‐carried‐forward method. Because the Actiwatch continues to record data right up to the time of an event (i.e. serious adverse event, hospitalizations, and death), any poor activity response related to such an event would be reflected in the last observation. ANCOVA model includes least squares means of the two treatment groups, least squares mean difference between the treatment groups, 95% CI for the difference in the two treatment groups, and P value based on the fitted linear model.
The occurrence and degree of sensor motion will be captured in counts (0.000175 G‐force per activity count) that are sampled at a rate of 32 Hz and recorded in 30 s epochs. Overall activity will be assessed by the measurement of counts per minute, and patterns of peak activity will be assessed by the measurement of the most active 30 min of each day. The primary endpoint will be a previously validated16 assessment of the individual change in waking activity from baseline to the last week of randomized treatment, collected during the most active 30 min of each day. The most active periods are defined as the individual minutes of each day (not necessarily contiguous) with the highest activity counts. Change during the most active 6 min is an exploratory endpoint. Although not a formal endpoint, simple responder analysis will be performed to assess the number of subjects who achieved 5%, 10%, and 20% change in activity from baseline in each arm. Other measurements, such as total activity counts and daily minutes spent at levels of sedentary, low, moderate, and vigorous activity, will be reported. We will also be assessing the day‐to‐day intra‐variability and inter‐variability of activity for individual patients with HF. Activity will be stratified by other baseline characteristics, including age, sex, ischaemic cardiomyopathy status, ejection fraction, and other co‐morbidities. We will compare actigraphy findings with patient‐reported symptoms obtained from surveys. Finally, analyses stratified by apnoea–hypopnea index at baseline will be conducted.
Physical activity will be measured by the Philips Actiwatch Spectrum (Philips Respironics, Boston, MA; Table S2 ), a US Food and Drug Administration‐approved Class 2 medical device (registration number 983533). Participants will be trained on the use of this lightweight actigraphy device during the first study visit. The device will be worn on participants' nondominant wrist continuously for the 18 week study duration, from the time of enrolment to the end of the open‐label extension phase. The device has a battery life of ~8 months if used continuously.17 The device will be exchanged five times (at study visits) to guard against data loss and ensure adequate battery life. Data will be collected continuously, but only data collected during assessment periods will contribute to prospectively defined endpoints. Each actigraphy assessment period is seven contiguous days. This will allow for continuous actigraphy recording for each 7 day period and correlation with daily symptom assessments by e‐diary and sleep questionnaires.
2.4.2. Sleep assessments
The key secondary endpoint is the change in sleep activity from baseline to the last week of the randomized treatment phase. Sleep will be assessed using two methods: (i) employing actigraphy to measure sleep (defined as a lack of movement, <40 counts/min), including measures of sleep disruption (wake after sleep onset, defined as the number of minutes scored awake between sleep onset and end of the sleep period; Table S2 )18 and efficiency (defined as the percentage of sleep period during which subject is scored asleep) and (ii) performing a home sleep test to evaluate for sleep‐disordered breathing. Although polysomnography (PSG) is considered the gold‐standard assessment tool for the diagnosis of sleep apnoea, home sleep testing is a viable home‐based assessment tool that has been increasingly utilized.19 Portable monitors have been recommended as an alternative to PSG for assessment of sleep‐disordered breathing in patients with high pretest probability of moderate‐to‐severe sleep apnoea.19, 20 In this study, sleep‐disordered breathing will be assessed using the portable sleep monitor AccuSom III (NovaSom, Inc., Glen Burnie, MD; Table S2 ) to measure arterial oxygen saturation, pulse rate, respiratory effort, and airflow wave form. The AccuSom III device is a 501k‐registered medical device indicated for diagnostic evaluation of sleep apnoea that has been clinically validated and has been used in patients with HF. Tests will be administered on three nights of the participants' choice during the weeks preceding the first, third, and fifth actigraphy assessment periods. Data from the home sleep test will be transmitted to a central reader immediately upon completion of the test. On receipt, each test will be assessed for completeness, and participants who have fewer than 4 h of interpretable data will be asked to repeat the home sleep study for up to three nights to ensure capture of adequate data. Home sleep studies will not be taken during actigraphy assessment.
2.4.3. Participant‐reported outcome assessments
Participant‐reported outcomes will be assessed using the Kansas City Cardiomyopathy Questionnaire‐23 (KCCQ‐23), a validated, reliable, and responsive health‐status measure for congestive HF that has served as a clinically meaningful outcome measurement tool in CV clinical research, patient management, and HRQoL assessment.21 The KCCQ‐23 questionnaire will be administered at the end of each study phase (baseline, treatment, and extension) via mobile application on either the patient's or a site‐provisioned smartphone.
Two other participant‐reported questionnaires will be employed in this study: (i) the Heart Failure Symptom Tracker‐II (HFaST‐II) and (ii) a short sleep questionnaire, which will be administered digitally at the end of each day during assessment Week −1, end of Week 7, and end of Week 15, via a mobile application‐based e‐diary, using either the participant's own smartphone or a site‐provisioned smartphone. HFaST‐II is a daily questionnaire regarding the participants' HF symptom severity and functional limitations (see Supporting Information). The sleep portion will include specific sleep‐hygiene questions that are associated with measurements collected in the actigraphy portion of this study. These questions are designed to provide information that can be aligned with the sleep actigraphy data for contextual analysis.
2.4.4. Safety assessments
Safety assessments will consist of monitoring and recording of all adverse events and serious adverse events; evaluations of haematology, blood chemistry, and urine values; regular measurements of vital signs; and results of physical examinations. In addition, laboratory assessments will be routinely performed, and cases of angioedema will be reported and adjudicated by an independent committee.
2.5. Sample size
A total of 136 participants from 15 to 25 centres in the USA will be randomly assigned. The study is powered on the basis of data reported by Maurer et al., who also used a Philips activity tracker to demonstrate that participants with HF and clinical anergia recorded ~4500 fewer activity counts than other participants with HF during the most active 30 min of the day (no anergia, 25 880 ± 8285 counts vs. anergia, 21 369 ± 7418 counts; P < 0.05).16 Assuming a significance level of 0.05, a total sample size of 136 participants will provide 90% power to detect a clinically meaningful difference of 5000 mean activity counts collected during the most active 30 min from baseline between the sacubitril/valsartan treatment group and the enalapril group during Week 8, assuming a common standard deviation of 7400 activity counts,16 a 20% dropout rate, and a 10% rate of participants with nonevaluable data. Furthermore, this sample size will provide 93% power to detect a 3.5‐point difference in the change from baseline in mean activity value during the sleep (expressed as counts per minute) between the sacubitril/valsartan treatment group and the enalapril group during Week 8, assuming a common standard deviation of 4.9 points, a 20% dropout rate, and a 10% rate of participants with nonevaluable data.22
2.6. Rationale
The study design will enable assessment of changes in sequential daily physical activity, sleep efficiency, HF symptoms, and HRQoL between baseline and selected time points between patients treated with sacubitril/valsartan and patients treated with enalapril. The initial baseline week enables participants to become familiar and acclimated to the testing devices and assessment routines prior to entering the baseline phase of the study. Assessment of the primary endpoint will most likely detect the early clinical impact of initiation of sacubitril/valsartan vs. enalapril on HRQoL.
Summation of overall participant activity will be measured and reported but will not be used as the primary endpoint to distinguish between changes during resting and active periods. An actigraphy study in HF demonstrated that more symptomatic participants generally have both a reduction in activity when active and an increase in movement when resting than do healthier participants.16 If participants with HF spend most of their time at rest, treatment‐related increases in physical activity when active may be offset by a concomitant reduction in movement when at rest. As a result, this can appear as a simple decrease in overall activity if accelerometry data are compiled and averaged over long periods. Moreover, the measurement of activity during the most active 30 min of the day will facilitate the assessment of patterns of activity rather than overall activity, which may not accurately reflect symptomatic improvement. Compared with previous studies that have used pooled actigraphy data, this study will compare data at each time point vs. baseline, with each participant acting as their own control.
3. Discussion
This study aims to compare the effects of initiating sacubitril/valsartan with enalapril on objective measures, during both wakefulness and sleep, for participants with HFrEF in the context of their daily lives. While randomized controlled studies, such as the Phase 3 OUTSTEP‐HF trial (NCT02900378), have used actigraphy to evaluate physical activity in patients with HFrEF exposed to enalapril or sacubitril/valsartan, AWAKE‐HF will be the first study to objectively assess physical activity using actigraphy data collected in the real‐world setting to examine the effect of sacubitril/valsartan on HRQoL. This study will assess incremental effects on HRQoL using actigraphy and HFaST‐II questionnaire data collected at intervals within 2 months of initiating therapy. Moreover, this study will provide insight into how the effect of sacubitril/valsartan varies between participants by analysing individual patient data instead of pooled data.
Actigraphy was chosen to objectively assess activity and rest in ambulatory participants in their home environments, thereby providing reliable, valid, real‐world data regarding the effects of sacubitril/valsartan. Other studies have successfully employed actigraphy to assess similar endpoints. For example, in a prospective interventional study assessing the impact of treatment with celecoxib on daytime symptoms of participants with osteoarthritis, actigraphy assessment of the increase in participants' activity levels demonstrated a significant treatment effect, whereas questionnaire‐based assessment of pain had a high‐placebo response rate and did not meet statistical significance.15 Another study used actigraphy to evaluate treatment‐induced reduction of itch‐related sleep disruption in atopic dermatitis.14
A study of the effect of isosorbide mononitrate on activity levels in HF and preserved ejection fraction used actigraphy to assess effects on participants' movement levels over 6 weeks.23 Although no significant between‐group difference in the 6MWT or HRQoL questionnaire results (KCCQ or Minnesota Living with Heart Failure questionnaire) was observed, actigraphy data revealed a dose‐dependent trend toward lower activity levels and a statistically significant decrease in active time per day among participants in the highest‐dose treatment arm.
This study is unique in its focus on the effects of sacubitril/valsartan on sleep. Although sleep disturbance or disorder is one of the most common complaints among patients with HF, there is a dearth of evidence on the impact of interventions on sleep.24 Because treatment with sacubitril/valsartan may reduce the requirement for loop diuretic therapy in HFrEF through natriuretic and diuretic effects,25, 26 it is postulated that sacubitril/valsartan may impact fluid volume status, thereby reducing sleep apnoea severity. Thus, this study's two‐pronged approach to assess sleep using both actigraphy and at‐home sleep tests will provide objective, real‐world sleep data in the context of the participant's home life.
Although this study is novel in its objective measurement of clinical endpoints in the context of participants' everyday lives, limitations exist because of the technology used. The burden of training participants to use technology to objectively assess therapy at home is continuous and requires extensive site preparation to train the clinical research teams to adequately counsel and support participants.
Additional limitations will be related to study duration and patient characteristics. Given that changes in myocardial function and remodelling are typically associated with improved sleeping behaviours,27 the limited duration of this study assesses only the effect of treatment initiation and may not be sufficient to observe longer‐term positive impacts of treatment. Only participants with NYHA Class II–III HFrEF will be included, limiting generalizability of results. Despite exclusion of patients with co‐morbidities that limit physical activity prior to randomization, participants may develop physical limitations post‐randomization, confounding results. Similarly, variability in patients' lifestyles and home environments may impact activity levels. To control for variation between individuals, each endpoint will be measured in relation to baseline activity. Furthermore, actigraphy data may be influenced by other unforeseen factors. The endpoints chosen for this study have not yet been validated in HFrEF. The secondary endpoint and all exploratory analyses are considered supportive, and each will be tested with a type I error of 0.05. Finally, objective parameters of the severity of HFrEF, such as N‐terminal pro B‐type natriuretic peptide, were not included in this study.
This trial may also provide guidance for future studies using digital medicine techniques and offer critical early insights into the use of actigraphy in HF trials because of its unique design. The analysis of each participant as their own control may help inform advancement of personalized medicine. Moreover, the objective assessment of physical activity using actigraphy will allow for evidence‐based clinical recommendations to improve HRQoL in HF.
4. Conclusions
The increasing demand for real‐world evidence to individualize therapeutic management of patients necessitates objective assessment of rest and activity, as well as using subjective measures. Technological advancement has made it possible to move randomized controlled clinical trials into the context of participants' everyday lives at home. This study may provide valuable insights into the impact of initiating sacubitril/valsartan treatment on subjective and objective outcomes.
Conflict of interest
Raj M. Khandwalla has served as a consultant for Novartis Pharmaceuticals Corporation and has received a research grant from General Electric. Kade Birkeland has served as a consultant for Novartis Pharmaceuticals Corporation. J. Thomas Heywood has served as a consultant for Actelion Pharmaceuticals, Medtronic, and Abbott; was a speaker for Actelion Pharmaceuticals, Medtronic, Abbott, Otsuka, and Novartis Pharmaceuticals Corporation; has received grant funding from Medtronic, Abbott, and Impedimed; and has received fellowship grant support for Abbott. Robert L. Owens has received honoraria and travel reimbursements from ResMed, LLC and Itamar Medical and has served as a consultant for Novartis Pharmaceuticals Corporation. Steven Steinhubl has received financial compensation from Novartis Pharmaceuticals Corporation for serving as an adviser. Daniel Grant, Kevin McCague, Emmanuel Fombu, and Jerome B. Riebman are employees of Novartis Pharmaceuticals Corporation.
Funding
This work was supported by Novartis Pharmaceuticals Corporation. AWAKE‐HF was designed and developed by the study's steering committee in conjunction with the study sponsor, Novartis Pharmaceuticals Corporation. The sponsor provided consultant compensation to the steering committee member authors for their trial‐related activities.
Author of contributions
Raj M. Khandwalla, Kade Birkeland, and Robert L. Owens contributed to the design of the study, drafting the manuscript, and critical review of the manuscript. J. Thomas Heywood, Steven Steinhubl, and Emmanuel Fombu contributed to the design and execution of the study, and critical review of the manuscript. Daniel Grant and Jerome B. Riebman contributed to the design and execution of the study and critical review of the manuscript. Kevin McCague contributed toward the design of statistical analyses in the study and critical review of the manuscript. All authors approved the final version of this article.
Supporting information
Table S1. Dose Levels of Each Study Drug.
Table S2. Activity‐ and Sleep‐Assessment Devices.
Acknowledgements
Medical writing assistance for this manuscript was provided by Marcel Kuttab, PharmD, of Oxford PharmaGenesis Inc., Newtown, Pennsylvania, USA, and was funded by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript.
Khandwalla, R. M. , Birkeland, K. , Heywood, J. T. , Steinhubl, S. , McCague, K. , Fombu, E. , Grant, D. , Riebman, J. B. , and Owens, R. L. (2019) Activity Sensors to Evaluate the Effect of Sacubitril/Valsartan on Quality‐of‐Life in Heart Failure: rational and design of the AWAKE‐HF study. ESC Heart Failure, 6: 1313–1321. 10.1002/ehf2.12514.
AWAKE‐HF; http://ClinicalTrials.gov: NCT02970669.
References
- 1. Uszko‐Lencer N, Mesquita R, Janssen E, Werter C, Brunner‐La Rocca HP, Pitta F, Wouters EF, Spruit MA. Reliability, construct validity and determinants of 6‐minute walk test performance in patients with chronic heart failure. Int J Cardiol 2017; 240: 285–290. [DOI] [PubMed] [Google Scholar]
- 2. Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 1992; 124: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 3. Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, Guralnik JM. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2009; 64: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang MY, Hung HL, Tsai PS. The sleep log and actigraphy: congruency of measurement results for heart failure patients. J Nurs Res 2011; 19: 173–180. [DOI] [PubMed] [Google Scholar]
- 5. Sallis JF, Saelens BE. Assessment of physical activity by self‐report: status, limitations, and future directions. Res Q Exerc Sport 2000; 71: S1–S14. [PubMed] [Google Scholar]
- 6. Lobelo F, Rohm Young D, Sallis R, Garber MD, Billinger SA, Duperly J, Hutber A, Pate RR, Thomas RJ, Widlansky ME, McConnell M, Joy EA, American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Cardiovascular Surgery and Anesthesia; and Stroke Council . Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation 2018; 137: e495–e522. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Colvin MM, et al. 2017. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136:e137‐e161. [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail 2014; 16: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Entresto (sacubitril and valsartan) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 10. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2018;380: 539–548. [DOI] [PubMed] [Google Scholar]
- 11. White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol 2013; 591: 1179–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD. Health‐related quality of life outcomes in PARADIGM‐HF. Circ Heart Fail 2017; 10:pii: e003430 [DOI] [PubMed] [Google Scholar]
- 13. Bell TD, Mazer AJ, Miller PE, Strich JR, Sachdev V, Wright ME, Solomon MA. Use of sacubitril/valsartan in acute decompensated heart failure: a case report. ESC Heart Fail 2017; 5: 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, Imayama S, Kato M, Hasebe I, Taira K, Yamamoto M, Mihara R, Kabashima K, Ruzicka T, Hanifin J, Kumagai Y. The first trial of CIM331, a humanized antihuman interleukin‐31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double‐blind, placebo‐controlled study. Br J Dermatol 2016; 174: 296–304. [DOI] [PubMed] [Google Scholar]
- 15. Trudeau J, Van Inwegen R, Eaton T, Bhat G, Paillard F, Ng D, Tan K, Katz NP. Assessment of pain and activity using an electronic pain diary and actigraphy device in a randomized, placebo‐controlled crossover trial of celecoxib in osteoarthritis of the knee. Pain Pract 2015; 15: 247–255. [DOI] [PubMed] [Google Scholar]
- 16. Maurer MS, Cuddihy P, Weisenberg J, Delisle S, Strong BM, Gao Q, Kachnowski S, Howell J. The prevalence and impact of anergia (lack of energy) in subjects with heart failure and its associations with actigraphy. J Card Fail 2009; 15: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Actiwatch Spectrum Activity Monitor. https://www.usa.philips.com/healthcare/product/HC1046964/actiwatch-spectrum-activity-monitor (10 January 2019).
- 18. Shrivastava D, Jung S, Saadat M, Sirohi R, Crewson K. How to interpret the results of a sleep study. J Community Hosp Intern Med Perspect 2014; 4: 24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13: 479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3: 737–747. [PMC free article] [PubMed] [Google Scholar]
- 21. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 22. Peterson BT, Chiao P, Pickering E, Freeman J, Zammit GK, Ding Y, Badura LL. Comparison of actigraphy and polysomnography to assess effects of zolpidem in a clinical research unit. Sleep Med 2012; 13: 419–424. [DOI] [PubMed] [Google Scholar]
- 23. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter M, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty S, Velazquez EJ, Shah MR, Braunwald E, NHLBI Heart Failure Clinical Research Network . Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373: 2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Redeker NS. Sleep disturbance in people with heart failure: implications for self‐care. J Cardiovasc Nurs 2008; 23: 231–238. [DOI] [PubMed] [Google Scholar]
- 25. Vardeny O, Claggett B, Kachadourian J, Packer M, Zile M, Rouleau J, Swedberg K, Shi V, Lefkowitz M, McMurray J, Solomon SD. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: The Paradigm‐HF Study [abstract]. Circulation 2016; 134: A17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang TD, Tan RS, Lee HY, Ihm SH, Rhee MY, Tomlinson B, Pal P, Yang F, Hirschhorn E, Prescott MF, Hinder M, Langenickel TH. Effects of sacubitril/valsartan (LCZ696) on natriuresis, diuresis, blood pressures, and NT‐proBNP in salt‐sensitive hypertension. Hypertension 2017; 69: 32–41. [DOI] [PubMed] [Google Scholar]
- 27. Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando SI, Bradley TD. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348: 1233–1241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dose Levels of Each Study Drug.
Table S2. Activity‐ and Sleep‐Assessment Devices.


