Abstract
Aims
Iron deficiency is common in heart failure with reduced ejection fraction (HFrEF). In patients with cardiac resynchronization therapy (CRT), it is associated with a diminished reverse remodelling response and poor functional improvement. The latter is partially related to a loss in contractile force at higher heart rates (negative force–frequency relationship).
Methods and results
The effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy (IRON‐CRT) trial is a multicentre, prospective, randomized, double‐blind controlled trial in HFrEF patients who experienced incomplete reverse remodelling (defined as a left ventricular ejection fraction below <45%) at least 6 months after CRT. Additionally, patients need to have iron deficiency defined as a ferritin below 100 μg/L irrespective of transferrin saturation or a ferritin between 100 and 300 μg/L with a transferrin saturation <20%. Patients will be randomized to either intravenous ferric carboxymaltose (dose based according to Summary of Product Characteristics) or intravenous placebo. The primary objective is to evaluate the effect of ferric carboxymaltose on metrics of cardiac reverse remodelling and contractility, measured by the primary endpoint, change in left ventricular ejection fraction assessed by three‐dimensional (3D) echo from baseline to 3 month follow‐up and the secondary endpoints change in left ventricular end‐systolic and end‐diastolic volume. The secondary objective is to determine if ferric carboxymaltose is capable of improving cardiac contractility in vivo, by assessing the force–frequency relationship through incremental biventricular pacing. A total of 100 patients will be randomized in a 1:1 fashion.
Conclusions
The IRON‐CRT trial will determine the effect of ferric carboxymaltose on cardiac reverse remodelling and rate‐dependent cardiac contractility in HFrEF patients.
Keywords: Iron deficiency, Cardiac remodelling, Contractility, Heart failure
Introduction
Iron deficiency is common in heart failure with a reduced ejection fraction (HFrEF), affecting around 40 to 50% of patients.1, 2 Iron deficiency is associated with a reduced functional status, poor exercise performance, and increased risk for heart failure hospitalization and cardiovascular mortality.3, 4 On a molecular level, the detrimental effects of iron deficiency relate to a diminished availability of iron as a cofactor in proteins of oxidative phosphorylation and antioxidative enzymes.5, 6 Therefore, iron deficiency is involved in the process of progressive cardiac remodelling and failing cardiac energetics. In HFrEF patients receiving cardiac resynchronization therapy (CRT) specifically, iron deficiency occurs in up to 56% of patients.7 Normally, CRT induces significant cardiac reverse remodelling, not only on a macroscopic level but also on a microscopic level. On a molecular level, CRT induces the up‐regulation of proteins involved in energy metabolism, excitation–contraction coupling, and redox balance.8, 9, 10, 11, 12, 13, 14 Of these proteins, several use iron as a cofactor. Not surprisingly, the presence of iron deficiency at the time of CRT implant is associated with diminished cardiac reverse remodelling and limited functional improvement.7 Indeed, an exercise haemodynamic evaluation of HFrEF patients indicated that the presence of iron deficiency is associated with a diminished contractile reserve of the left ventricle.15 Normally, when heart rate increases, cardiac contractility increases disproportionally, a process termed ‘positive force–frequency relationship or Treppe phenomenon'.16 However, in order to attain such an increase in contractility, the myocardium requires a surge in cardiac energetics.17 Recent preclinical and animal studies have illustrated that iron deficiency results in failing cardiac energetics, potentially explaining the decrease in cardiac contractility at higher heart rates.5, 18 The effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy (IRON‐CRT) trial was specifically designed to answer two important questions: (i) if treatment with ferric carboxymaltose induces incremental reverse remodelling in CRT patients with iron deficiency and a persistently reduced left ventricular ejection fraction and (ii) if treatment with ferric carboxymaltose is capable of improving cardiac contractility (force–frequency relationship) in vivo. For the latter, a specific stepwise pacing protocol will be used to assess the impact of ferric carboxymaltose on contractility.
Methods
The IRON‐CRT trial is a double‐blind, randomized, placebo‐controlled trial conducted in two sites in Belgium (Ziekenhuis Oost‐Limburg, Genk, and Jessa Hospital, Hasselt). This trial will assess the impact of intravenous ferric carboxymaltose compared with placebo on cardiac reverse remodelling and cardiac contractility in HFrEF patients who experience incomplete reverse remodelling [defined as a left ventricular ejection fraction (LVEF) below <45%] at least 6 months after CRT. The study is conducted in accordance with the principles stated in the Declaration of Helsinki and the international conference on the harmonization of good clinical practice. The trial is registered on http://clinicaltrials.gov (identifier: NCT = 03380520). Written informed consent will be provided by all patients before any study‐related investigations will take place. A flow of the study is reflected in Figure 1 .
Figure 1.
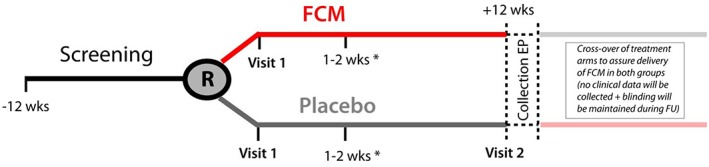
Flowchart of the IRON‐CRT study. *Additional visit for patients requiring 1.5–2.0 g ferric carboxymaltose (FCM) (visit also in placebo group). EP, endpoint; FU, follow‐up.
Study design and conduct
Eligibility
Men and women aged ≥18 years who received CRT as part of their treatment plan for HFrEF (according to a guideline indication Class IA, IB, IIa, or IIb) more than 6 months ago and have the presence of incomplete reverse remodelling, defined as an LVEF below 45% at screening, are candidate for laboratory screening. Additionally, patients need to be on maximal tolerable and stable doses of all guideline‐directed medical heart failure therapies for at least 4 weeks (with the exception of loop diuretics) and have mild functional impairment (defined as a New York Heart Association Class II or more). In addition, all patients are followed in a dedicated multidisciplinary CRT optimization clinic ensuring optimal biventricular pacing and heart failure care.19 These patients undergo a screening laboratory, and if the presence of iron deficiency is confirmed, patients are eligible for trial enrolment if none of the exclusion criteria are present. Major inclusion and exclusion criteria are listed in Table 1. Iron deficiency is defined according to the established criteria used in previous trials and recognized by heart failure guidelines, being a ferritin below 100 μg/L irrespective of transferrin saturation (TSAT) or a ferritin between 100 and 300 μg/L if TSAT was below 20%.20, 21, 22, 23
Table 1.
Major inclusion and exclusion criteria of the IRON‐CRT trial
| Inclusion criteria |
|
•Patients with chronic heart failure and implantation of cardiac resynchronization therapy more than 6 months ago and presence of iron deficiency (ferritin <100 μg/L, irrespective of TSAT or ferritine between 100 and 300 μg/L with TSAT < 20%) and presence of incomplete reverse remodelling (LVEF < 45%) •Age ≥18 years •Obtained informed consent •Stable pharmacological therapy of heart failure during the last 4 weeks (with the exception of diuretics) •At least 98% bivacing the last 6 months |
| Exclusion criteria |
|
•A TSAT > 45% •Haemoglobin >15 g/dL at inclusion •Planned cardiovascular hospitalization during study period •Known hypersensitivity to Injectafer® •Known active infection, CRP > 20 mg/L, clinically significant bleeding, and active malignancy •Chronic liver disease and/or screening ALT or AST above three times the upper limit of the normal range •Immunosuppressive therapy or renal dialysis (current or planned within the next 6 months) •History of erythropoietin, i.v. or oral iron therapy, and blood transfusion in previous 12 weeks and/or such therapy planned within the next 6 months •Unstable angina pectoris as judged by the investigator, clinically significant uncorrected valvular disease or left ventricular outflow obstruction, obstructive cardiomyopathy, poorly controlled fast atrial fibrillation or flutter, and poorly controlled symptomatic bradyarrhythmias or tachyarrhythmias •Acute myocardial infarction or acute coronary syndrome, transient ischaemic attack, or stroke within the last 3 months •Coronary artery bypass graft, percutaneous intervention (e.g. cardiac, cerebrovascular, and aortic; diagnostic catheters are allowed), or major surgery, including thoracic and cardiac surgery, within the last 3 months •Inability to fully comprehend and/or perform study procedures in the investigator's opinion •Vitamin B12 and/or serum folate deficiency according to the laboratory (re‐screening is possible after substitution therapy) •Pregnancy or lactation •Participation in another clinical trial within previous 30 days and/or anticipated participation in another trial during this study |
ALT, alanine transaminase; AST, aspartate transaminase; CRP, C‐reactive protein; LVEF, left ventricular ejection fraction; TSAT, transferrin saturation.
Randomization
If patients suffice all inclusion and exclusion criteria and provide written informed consent, patients are randomized using a web‐based randomization system (Castor EDC). Patients will be assigned in balanced blocks assuring 1:1 randomization to either intravenous placebo or intravenous ferric carboxymaltose. A block randomization strategy is used assuring an equal number of patients with a baseline LVEF < 35% and LVEF > 35% (with maximum of 45%) in both treatment arms. An LVEF of 35% was chosen to stratify into the block of lower vs. higher baseline LVEF, based on the observation that previous trials assessing the impact of ferric carboxymaltose in HFrEF patients found a mean LVEF of on average 35%, if an entry LVEF below 45% was used. We do not apply a capping method to assure a minimal of patients in the low vs. high LVEF block strata.
Drug intervention
The active treatment intervention consists of ferric carboxymaltose (Injectafer®/Ferinject®, Glattbrugg, Switzerland) diluted into 250 mL NaCl 0.9%. The placebo intervention consists of the same 250 mL NaCl 0.9% without ferric carboxymaltose. The required dose of ferric carboxymaltose will be calculated according to the regulatory‐approved dosing scheme (Table 2). Briefly, based on screening weight and screening haemoglobin, patients will require a dose of ferric carboxymaltose ranging between 500 to 2000 mg. Because the maximal allowed dose of ferric carboxymaltose during one intravenous administration is 1000 mg/week, patients who require a dose of either 1500 or 2000 mg will receive a follow‐up appointment after 1–2 weeks to receive the remaining dose (Figure 1 ). To assure maximal blinding, patients assigned to the placebo group who would also require an additional dose based on their body weight and haemoglobin levels will also receive a second dosing appointment with infusion of placebo at that time. As the collection of the endpoints in this study occur on a relative short bases, no additional maintenance doses of ferric carboxymaltose will be administered. After collection of the primary and secondary endpoints, collection of study‐specific data will be terminated [with the exception of serious adverse events (SAEs), up to 24 h after last visit]. Because at the time of enrolment of the first patients into the IRON‐CRT trial, treatment with ferric carboxymaltose had a class IIa indication in European heart failure guidelines, it was decided with the local ethical committee that all patients who were randomized to the placebo arm would receive appropriate treatment with ferric carboxymaltose after collection of the primary and secondary endpoints.22 Again, to assure optimal blinding of both patients and investigators, the administration of ferric carboxymaltose will be performed in a blinded fashion. Additionally, the patients who are assigned to ferric carboxymaltose at baseline also receive a similar appointment after study completion but receive placebo in a blinded fashion at that time. Therefore, patients will be crossed over from treatment assignment group at the end of the study, but this is performed to assure delivery of ferric carboxymaltose to all patients and is not performed to collect additional study information.
Table 2.
Dosing of ferric carboxymaltose
| Haemoglobin (g/dL) | Body weight (kg) | ||
|---|---|---|---|
| <35 | 35–70 | >70 | |
| <10 | 500 mg | 1.500 mg | 2.000 mg |
| 10–14 | 500 mg | 1.000 mg | 1.500 mg |
| >14* | 500 mg | 500 mg | 500 mg |
with a maximum up to 15 g/dL.
Blinding and study oversight
Because ferric carboxymaltose is a dark‐brown solution, additional measures will be undertaken in this trial to assure patient and investigator blinding. Both the placebo and ferric carboxymaltose solutions for infusion are covered in non‐see‐through white bag. Additionally, all infusion lines are made of non‐see‐through white plastic, avoiding the detection of the colour of the infusate (Supporting Information, Figure S1). A study member from the Clinical Trial Unit (CTU) of the Ziekenhuis Oost‐Limburg (Genk, Belgium) is assigned as part of the unblinded study team responsible for randomization and preparation and administration of the blinded study infusate. Additionally, all post‐baseline iron values are only made available by the central laboratory of Ziekenhuis Oost‐Limburg (Genk, Belgium) to the unblinded study member of the CTU. The CTU from the Ziekenhuis Oost‐Limburg (Genk, Belgium) is responsible for safety monitoring and collection of all potential SAEs and adverse events. Additionally, the CTU performs oversight of per‐protocol execution of the study. This trial is partially sponsored by an unrestricted research grant from Vifor Pharma, covering medication cost and study materials. The sponsor, Vifor Pharma, had no role in the design or the monitoring of the trial, the selection of the participating centres, recruitment of patients, or in the collection, recording, storage, retention, or analysis of data, or the writing of this methods paper.
Endpoints and ancillary data
The primary objective of this study is to determine if treatment with ferric carboxymaltose is capable of inducing cardiac reverse remodelling in iron‐deficient HFrEF patients with incomplete reverse remodelling following CRT. Therefore, the study primary endpoint is the change from baseline in LVEF measured at 3 months. Key study objectives and endpoints are reflected in Table 3. Supporting secondary endpoints of reverse remodelling are the change from baseline in left ventricular end‐systolic volume (LVESV) and end‐diastolic volume (LVEDV) both at 3 months. These volumetric measurements will be collected using 3D transthoracic echocardiography by an experienced blinded sonographer to minimize intraobserver variability.24 The second objective of this study is to determine if treatment with ferric carboxymaltose is capable of improving cardiac contractility by assessing the force–frequency relationship. The gold standard for measuring the force–frequency relationship is by plotting invasively measured contractility (Dp/Dt) against heart rate. However, previous studies have shown a good approximation using a non‐invasive cardiac contractility index (CI; for details, see the next section). This CI is the ratio between systolic blood pressure divided by LVESV index (LVESV/body surface area). Therefore, the secondary endpoint (assessing the objective of contractility) is the change in CI from baseline measured at 3 months. Further supporting secondary endpoints are endpoints of functional performance and function well‐being including (i) change in Kansas City Cardiomyopathy Questionnaire from baseline to 3 months, (ii) change in European Quality of Life 5D from baseline to 3 months, and (iii) change in cardiopulmonary exercise test (CPET) variables, being the slope of the VE/VCO2 ratio and peak VO2. Because the results from the CPET are a secondary endpoint, patients will not obligated to be able to perform a maximal CPET (respiratory exchange ratio >1.1) at screening. Because it is anticipated that a fair amount of patients would have a submaximal exercise test (respiratory exchange ratio <1.1) at baseline, both VE/VCO2 ratio and peak VO2 will be evaluated. For ancillary biomarker studies, blood samples will be collected at both baseline and follow‐up, stored directly on ice, processed within the hour, and stored for longer periods at −80 °C. Finally, safety endpoints will be collected to assure safety and tolerability of the study drug by collecting SAEs and discontinuation of ferric carboxymaltose due to any adverse event.
Table 3.
Study objectives and related endpoints
| Study objective | Related endpoint |
|---|---|
| Primary objective: to determine if treatment with ferric carboxymaltose induces cardiac reverse remodelling |
Primary endpoint: change in left ventricular ejection fraction from baseline Secondary endpoint: change in left ventricular end‐systolic volume from baseline Secondary endpoint: change in left ventricular end‐diastolic volume from baseline |
| Secondary objective: to determine if treatment with ferric carboxymaltose improves cardiac contractility (force–frequency relationship) | Secondary endpoint: change in the slope of the contractility index at incremental pacing at 70, 90, and 110 b.p.m. |
| Tertiary objective: to determine if treatment with ferric carboxymaltose improves functional status/QoL and exercise capacity | Secondary endpoint: change in the KCCQ, EQ 5D, and VO2max and slope of the VE/VCO2 ratio |
| Exploratory objective: to determine if treatment with ferric carboxymaltose changes relevant biomarkers | Secondary endpoint: change in biomarkers and peripheral metabolome |
| Safety objective: to evaluate the safety and tolerability of ferric carboxymaltose | Secondary endpoint: SAEs and adverse events leading to study drug discontinuation and occurrence of heart failure hospitalization and all‐cause mortality |
EQ 5D, European Quality of Life 5D; KCCQ, Kansas City Cardiomyopathy Questionnaire; QoL, quality of life; SAEs, serious adverse events.
Results from the force–frequency pacing protocol of the first 60 patients randomized in the IRON‐CRT trial at baseline before study‐specific interventions occurred. Analyses were performed using linear mixed modelling, modelling the time effect using AR(1) as times close to each other will relate more to one and another. At baseline, 12 patients had an intrinsic rhythm just above 70 b.p.m. explaining the N = 48 at DDD‐CRT 70 b.p.m. For this reason, recurrent measurements were analysed using linear mixed modelling and not repeated measures ANOVA.
Pacing protocol and protocol validation
To assess the impact of ferric carboxymaltose on cardiac contractility, a specific previously validated pacing protocol will be used to assess the force–frequency relationship. Healthy individuals normally exhibit a positive force–frequency relationship, in which contractility increases disproportionally to heart rate. Previous preclinical and clinical studies illustrate that HFrEF patients with iron deficiency have blunted or negative force–frequency relationship (decrease in contractility at higher heart rates). Briefly, patients will undergo biventricular pacing in a DDD modus (DDD‐CRT) or VVI‐CRT modus (in case of atrial fibrillation) starting at a baseline lower rate of 70 b.p.m. To avoid acute spikes in lower‐rate pacing during patients positioning, the R modus will be programmed of during the entire pacing protocol. After a 5 min adaptation period at 70 b.p.m., three blood pressure measurements will be taken, and detailed two‐dimensional (2D) echocardiography images will be collected. Subsequently, the pacing lower rate will be increased to 90 b.p.m. Again, after a 5 min adaptation period, blood pressure measurements and 2D echocardiography measurements will be collected. Finally, the lower rate of pacing will be increased to 110 b.p.m., with again collection of blood pressure measurements and 2D echocardiography data after a 5 min adaptation period. The cardiac CI will be calculated as mentioned earlier as the ratio between the averaged systolic blood pressure divided by the LVESV index.25 The LVESV for this secondary endpoint will (in contrary to the primary endpoint LVEF and secondary endpoint LVESV and LVEDV) be measured with 2D and not 3D echocardiography. This is because during higher rates of pacing (e.g. 110 b.p.m.), the electrocardiogram‐triggered collection of the 3D volumetric dataset becomes erratic, with numerous stitching artefacts and incomplete cycle lengths, rendering the 3D volume set useless. Figure 2 illustrates the downsloping relationship at baseline (before any treatment intervention) between the CI and the heart rate in the first 60 patients randomized in the IRON‐CRT trial (assessment of the entire blinded baseline population). The results are the results of a linear mixed model, modelling the time effect (DDD‐CRT 70, 90, and 110) using an AR(1) structure. This model illustrates overall a significant decrease in the slope of the cardiac CI (P < 0.001). However, post hoc least significant difference testing shows a borderline non‐significance between DDD‐CRT 70 and DDD‐CRT 90 (P = 0.051), with a significant drop in contractility in all pairwise comparisons with DDD‐CRT 110 (P < 0.001 for all).
Figure 2.
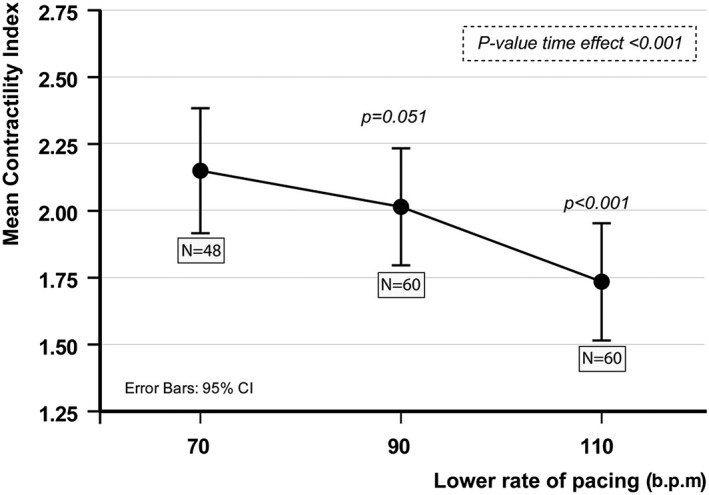
Force–frequency relationship at baseline in IRON‐CRT patients. CI, confidence interval.
Statistical considerations
Sample size calculation
The IRON‐CRT trial is powered for the primary endpoint, change in LVEF from baseline at 3 month follow‐up. The expected effect of ferric carboxymaltose on LVEF was determined based on both retrospective and prospective analyses. A previous retrospective analysis indicated that patients with iron deficiency at the time of CRT implant have 4.7% less improvement in absolute LVEF 6 months after CRT implant (after covariate adjustment).7 Additionally, a small study from Toblli et al.26 illustrated in 20 patients receiving iron sucrose that there was a mean improvement in LVEF of around 4.4%, with slight deterioration in LVEF in the control group. Considering a type I error rate α = 0.05 and a type II error rate β = 0.10 (statistical power = 90%), we calculated a total sample size of 66 patients to detect a mean 2.4% difference in LVEF, using a 3% absolute difference of standard deviation of LVEF. Additionally, to account for potential dropout and assure substantial power for the secondary objective endpoint evaluation (cardiac contractility), the sample size was rounded to 100 patients.
Methods for statistical analysis
The primary statistical analysis in the IRON‐CRT trial will be according to the intention to treat principle applied to all patients randomized to a treatment or placebo arm, who received at least one dose. Analysis will be performed using linear mixed modelling, incorporating both a fixed effect (treatment arm) and a random effect (centre effect and patient effect). Transformation will be applied when model assumptions are violated (e.g. normality). A limited amount of prespecified subgroup analysis will be performed, without correction for multiplicity. We will test heterogeneity in the treatment effect on the primary endpoint for following subgroups: (i) patients blocked randomized to high vs. low (>35%/<35%) LVEF strata, (ii) presence of baseline anaemia (haemoglobin >12 g/dL/<12 g/dL), and (iii) patients with or without isolated hypoferritinaemia (ferritin <100 μg/L with TSAT > 20%). The impact of missing data will be analysed in a sensitivity analysis using multiple imputation.
Discussion
Iron deficiency is common, affecting around 40 to 50% of HFrEF patients. In CRT patients, iron deficiency is at least equally common and has been associated with a diminished reverse remodelling and symptomatic response.1, 2 Currently, by guideline selection, patients who have an indication for CRT will also have a IIa recommendation to receive treatment with ferric carboxymaltose if they are iron deficient.22 However, a substantial proportion of patients who will have received their CRT devices will have been implanted in an iron‐deficient state. It is unclear if treatment in these patients will result in the attainment of incremental reverse remodelling. Importantly, further therapeutic options that could lead to functional improvement and reverse remodelling in CRT patients with incomplete reverse remodelling are limited, as patients by selection are on maximal tolerable doses of neurohormonal blockers.22 The IRON‐CRT trial will learn whether treatment with intravenous iron will result in incremental reverse remodelling, which is an important surrogate endpoint in heart failure. Indeed, most therapies that improve outcome such as angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, beta‐blockers, mineralocorticoid receptor antagonists, and CRT all induce reverse remodelling.27 Currently, several mortality and morbidity trials are underway to assess the impact of ferric carboxymaltose on the endpoint of heart failure hospitalization and all‐cause mortality. Currently, we only have data on functional surrogate endpoints. Additionally, data about the impact of intravenous iron on reverse remodelling in HFrEF are scarce. Toblli et al. demonstrated that repeated treatment with iron sucrose resulted in an improvement of LVEF. However, the sample size of the study was small, used an intravenous iron formulation that is currently not endorsed by European Society of Cardiology guidelines, and only included patients who had anaemia.26 One small study in eight patients indicated that treatment with ferric carboxymaltose was associated with a trend towards improvement of LVEF measured by magnetic resonance imaging.28 However, due to the limited samples size, the findings did not reach statistical significance. Perhaps more importantly, the latter study indicated that treatment with ferric carboxymaltose was capable of improving myocardial iron content measured by the T2* sequence. Changes in T2* correlated well with changes in LVEF and LVESV but not with LVEDV. In that aspect, it is important to recognize that LVEF and LVESV (even at rest) are also markers of cardiac contractility. The combination of the primary and secondary endpoints will learn if intravenous iron will have a predominant effect on true remodelling (reduction of left ventricular volumes, with LVEDV perhaps being the volumes least dependent on the contractile state) or improves mainly the contractile state of the heart, hereby furthermore underscoring the importance of the second main objective of the IRON‐CRT trial, to determine the impact of ferric carboxymaltose on cardiac contractility. Indeed, iron is an essential cofactor in the complexes of the electron transport chain.5 In both cell‐based and animal studies, iron deprivation resulted in a diminished activity of the electron transport chain, resulting in an energetic crisis (decrease in the amount of phosphocreatine and ATP), which probably contributes to the observed decreased cardiac contractility and relaxation.5, 18 Nevertheless, these artificially induced iron deficiency cell‐based and animal models exhibit extreme iron depletion, limiting the clinical relevance towards real‐world patients. In contrast, we have previously demonstrated that HFrEF patients with iron deficiency have a limited capability of increasing their cardiac output at higher heart rates.15 Indeed, the energetic demand to support contractility and relaxation increases disproportionately at higher heart rates. By inclusion of CRT patients, the IRON‐CRT trials will be capable of assessing the impact of ferric carboxymaltose on cardiac contractility improvement. Indeed, by gradually augmenting the baseline pacing rate, we can assess the force–frequency relationship in vivo, in a non‐invasive manner. In this methods paper, we report the force–frequency relationship of the first 60 patients randomized in the IRON‐CRT trial, further supporting the finding that iron deficiency is associated with a diminished contractility at higher heart rates (negative force–frequency relationship).
Conclusions
The IRON‐CRT trial will try to answer whether replenishment of iron reserve is capable of inducing reverse remodelling and restoring cardiac contractility in patients with HFrEF patients who experience incomplete reverse remodelling after CRT.
Conflict of interest
None declared.
Funding
P.M. is supported by a doctoral fellowship by the Research Foundation—Flanders (FWO, grant number: 1127917N). P.M., J.D., and W.M. are researchers for the Limburg Clinical Research Program (LCRP) UHasselt‐ZOL‐Jessa, supported by the foundation Limburg Sterk Merk (LSM), Hasselt University, Ziekenhuis Oost‐Limburg, and Jessa Hospital. P.M. has received consultancy fees from and an unrestricted research grant from Vifor Pharma.
Martens, P. , Dupont, M. , Dauw, J. , Somers, F. , Herbots, L. , Timmermans, P. , Verwerft, J. , and Mullens, W. (2019) Rationale and design of the IRON‐CRT trial: effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy. ESC Heart Failure, 6: 1208–1215. 10.1002/ehf2.12503.
References
- 1. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582. [DOI] [PubMed] [Google Scholar]
- 2. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol 2017; 73: 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Luscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 4. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, von Healing S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 5. Hoes MF, Grote BN, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018; 20: 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martens P, Dupont M, Mullens W. Cardiac iron deficiency—how to refuel the engine out of fuel. Eur J Heart Fail 2018; 20: 920–922. [DOI] [PubMed] [Google Scholar]
- 7. Martens P, Verbrugge F, Nijst P, Dupont M, Tang WH, Mullens W. Impact of iron deficiency on response to and remodeling after cardiac resynchronization therapy. Am J Cardiol 2017; 119: 65–70. [DOI] [PubMed] [Google Scholar]
- 8. Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, Samantapudi D, Paolocci N, Tomaselli GF, Kass DA, Van Eyk JE. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet 2010; 3: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aiba T, Barth AS, Hesketh GG, Hashambhoy YL, Chakir K, Tunin RS, Greenstein JL, Winslow RL, Kass DA, Tomaselli GF. Cardiac resynchronization therapy improves altered Na channel gating in canine model of dyssynchronous heart failure. Circ Arrhythm Electrophysiol 2013; 6: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chakir K, Depry C, Dimaano VL, Zhu WZ, Vanderheyden M, Bartunek J, Abraham TP, Tomaselli GF, Liu SB, Xiang YK, Zhang M, Takimoto E, Dulin N, Xiao RP, Zhang J, Kass DA. Gαs‐biased β2‐adrenergic receptor signaling from restoring synchronous contraction in the failing heart. Sci Transl Med 2011; 3 100ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeMazumder D, Kass DA, O'Rourke B, Tomaselli GF. Cardiac resynchronization therapy restores sympathovagal balance in the failing heart by differential remodeling of cholinergic signaling. Circ Res 2015; 116: 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lichter JG, Carruth E, Mitchell C, Barth AS, Aiba T, Kass DA, Tomaselli GF, Bridge JH, Sachse FB. Remodeling of the sarcomeric cytoskeleton in cardiac ventricular myocytes during heart failure and after cardiac resynchronization therapy. J Mol Cell Cardiol 2014; 72: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanderheyden M, Mullens W, Delrue L, Goethals M, de Bruyne B, Wijns W, Geelen P, Verstreken S, Wellens F, Bartunek J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol 2008; 51: 129–136. [DOI] [PubMed] [Google Scholar]
- 14. Wang SB, Foster DB, Rucker J, O'Rourke B, Kass DA, Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res 2011; 109: 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Limited contractile reserve contributes to poor peak exercise capacity in iron‐deficient heart failure. Eur J Heart Fail 2018; 20: 806–808. [DOI] [PubMed] [Google Scholar]
- 16. Mullens W, Bartunek J, Tang WH, Delrue L, Herbots L, Willems R, de Bruyne B, Goethals M, Verstreken S, Vanderheyden M. Early and late effects of cardiac resynchronization therapy on force–frequency relation and contractility regulating gene expression in heart failure patients. Heart Rhythm 2008; 5: 52–59. [DOI] [PubMed] [Google Scholar]
- 17. Neubauer S. The failing hear—an engine out of fuel. N Engl J Med 2007; 356: 1140–1151. [DOI] [PubMed] [Google Scholar]
- 18. Haddad S, Wang Y, Galy B, Korf‐Klingebiel M, Hirsch V, Baru AM, Rostami F, Reboll MR, Heineke J, Flogel U, Groos S, Renner A, Toischer K, Zimmermann F, Engeli S, Jordan J, Bauersachs J, Hentze MW, Wollert KC, Kempf T. Iron‐regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J 2017; 38: 362–372. [DOI] [PubMed] [Google Scholar]
- 19. Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, Tang WH. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol 2009; 53: 765–773. [DOI] [PubMed] [Google Scholar]
- 20. Anker SD, Comin CJ, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh TA, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Rationale and design of the CONFIRM‐HF study: a double‐blind, randomized, placebo‐controlled study to assess the effects of intravenous ferric carboxymaltose on functional capacity in patients with chronic heart failure and iron deficiency. ESC Heart Fail 2014; 1: 52–58. [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 23. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Bohm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen‐Solal A. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017; 136: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA. EAE/ASE recommendations for image acquisition and display using three‐dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012; 13: 1–46. [DOI] [PubMed] [Google Scholar]
- 25. Bombardini T, Correia MJ, Cicerone C, Agricola E, Ripoli A, Picano E. Force–frequency relationship in the echocardiography laboratory: a noninvasive assessment of Bowditch treppe? J Am Soc Echocardiogr 2003; 16: 646–655. [DOI] [PubMed] [Google Scholar]
- 26. Toblli JE, Lombrana A, Duarte P, Di GF. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 27. Nijst P, Martens P, Mullens W. Heart Failure with Myocardial Recovery ‐ The Patient Whose Heart Failure Has Improved: What Next? Prog Cardiovasc Dis 2017; 60: 226–236. [DOI] [PubMed] [Google Scholar]
- 28. Nunez J, Monmeneu JV, Mollar A, Nunez E, Bodi V, Minana G, Garcia‐Blas S, Santas E, Aguero J, Chorro FJ, Sanchis J, Lopez‐Lereu MP. Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: a pilot study. ESC Heart Fail 2016; 3: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]


