Abstract
Aims
The objective of this paper is to assess whether cardiac contractility modulation (via the Optimizer System) plus standard of care (SoC) is a cost‐effective treatment for people with heart failure [New York Heart Association (NYHA) III, left ventricular ejection fraction of 25–45%, and narrow QRS] compared against SoC alone from the perspective of the English National Health Service.
Methods and results
We developed a regression equation‐based cost‐effectiveness model, using individual patient data from three randomized control trials (FIX‐HF‐5 Phases 1 and 2, and FIX‐HF‐5C) to populate the majority of parameters. A series of regression equations predicted NYHA class over time, mortality, all‐cause hospitalization rates, and health‐related quality of life. We conducted the analysis in line with the National Institute for Health and Care Excellence reference case, modelling costs from an English National Health Service perspective, and considering outcomes in quality‐adjusted life years (QALYs) over a patient lifetime perspective. Our base case analysis produced an incremental cost per additional QALY of GBP22 988 (€25 750) when comparing Optimizer + SoC to SoC alone. This result was not sensitive to parameter uncertainty but was sensitive to the time horizon over which costs and QALYs were captured and the duration over which a survival benefit with Optimizer + SoC can be assumed to apply.
Conclusions
Cardiac contractility modulation is likely to be cost‐effective in people with heart failure with reduced ejection fraction, NYHA III, and narrow QRS, provided that the treatment benefit can be maintained beyond the duration of the existing clinical trial follow‐up. This analysis supports the current recommendations of the European Society of Cardiology that this therapy may be considered for such patients.
Keywords: Cost‐effectiveness analysis, Heart failure, Cardiac contractility modulation
Introduction
Despite major advances in therapies, patients with heart failure (HF) continue to experience a high degree of morbidity and mortality, and significant treatment gaps exist.1 For example, patients with HF with reduced ejection fraction (HFrEF) under 35% are eligible for cardiac resynchronization therapy (CRT) if they have a prolonged QRS duration (>150 ms),2 but only one third of HFrEF patients meet this criterion.3, 4 Furthermore, no evidence‐based device therapy currently exists for HF patients with moderately reduced ejection fractions (35–50%).
Cardiac contractility modulation (CCM) is a therapy for chronic HF that uses electrical pulses to increase the strength of contraction of cardiac muscle. The Optimizer System (Impulse Dynamics) is a device that delivers CCM. Evidence to support the efficacy of the Optimizer System comes from a series of randomized controlled trials (RCTs) and registry studies. The FIX‐HF‐5 Phase 1 (pilot) study5 enrolled people with New York Heart Association (NYHA) Class III/IV HF and left ventricular ejection fraction (LVEF) of <35%. All patients received the Optimizer System and were randomized to either have their devices programmed to deliver CCM signals (n = 25) or remain switched off (n = 24). Fewer deaths and hospitalizations occurred in the treatment group, and HF‐specific outcomes such as 6 min walk, peak VO2 (pVO2), and anaerobic threshold improved over 6 months of follow‐up in patients receiving CCM.
The FIX‐HF‐5 Phase 2 (pivotal) study6, 7 tested CCM in people with NYHA Class III/IV HF, who had narrow QRS, and LVEF of ≤45%. Patients were randomized to receive osteopathic manipulative treatment (OMT) plus CCM delivered by the Optimizer System (n = 215) or OMT alone (n = 213). After 6 months of treatment, CCM significantly improved pVO2 and quality of life indexed by Minnesota Living with Heart Failure Questionnaire (MLWHFQ) scores. The study also achieved its primary safety endpoint, demonstrating non‐inferiority of a composite of all‐cause mortality and hospitalizations at 12 months.
Most recently, the FIX‐HF‐5C (confirmatory) study8 tested CCM in people with persistent NYHA III/IV HF symptoms, QRS duration of <130 ms, and LVEF of 25–45%. Patients were randomized to receive OMT plus CCM delivered by the Optimizer System (n = 74) or OMT alone (n = 86) for 24 weeks. The analysis demonstrated improvements in pVO2, MLWHFQ scores, NYHA class, and 6 min walk with CCM versus OMT alone.
In addition to these three RCTs, a number of single and multicentre registry studies contribute additional evidence of the safety and efficacy of CCM as a treatment for HF in patients with a broad range of ejection fractions.9, 10, 11, 12, 13 In accordance with these findings, the current European Society of Cardiology guidelines for the treatment of patients with HF14 recommend that the Optimizer System may be considered in selected patients with HFrEF.
The purpose of the current study was to assess whether CCM plus standard of care (SoC) is a cost‐effective treatment for systolic HF compared with SoC alone. We developed a cost‐effectiveness model that focused on NYHA III patients with LVEF of 25–45% and used data from the three FIX‐HF‐5 RCTs to model longer term health outcomes and cost implications associated with adopting the Optimizer System from the perspective of the English National Health Service (NHS).
Methods
Study design and model structure
We developed a regression equation‐based cost‐effectiveness model, using individual patient data from three RCTs (FIX‐HF‐5 Phases 1 and 2, and FIX‐HF‐5C) to populate the majority of parameters. Figure 1 shows the model structure. Age, sex, presence of ischaemia (yes/no), diabetic status (yes/no), LVEF, and MLWHFQ score determined the patients' baseline characteristics on entering the model. We determined treatment‐specific clinical outcomes (e.g. adverse events and hospitalizations), costs, utilities, and mortality at a ‘cohort‐level’ on the basis of patients' NYHA classification. A series of regression equations predicted NYHA class as a function of time (which determined the proportion of patients in each NYHA class in each cycle), mortality, all‐cause hospitalization rates, and health‐related quality of life (HRQoL). The analysis grouped NYHA Classes I and II due to a lack of available trial data in these groups.
Figure 1.
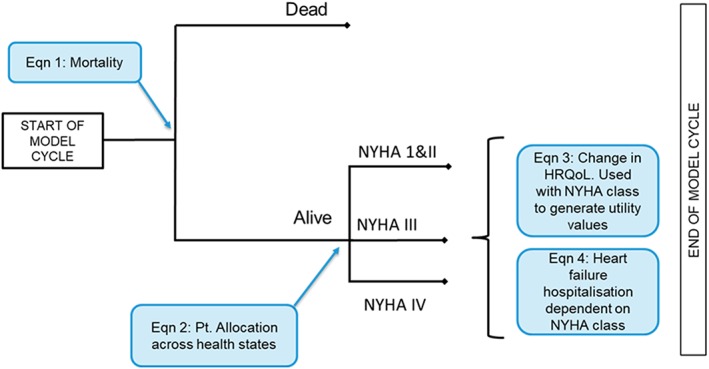
Model structure. NYHA, New York Health Association; HRQoL, health‐related quality of life.
At the beginning of the first model cycle, all patients in the Optimizer + SoC arm underwent device implantation, which could either be successful or result in procedure/device‐related adverse events. During each subsequent 30 day cycle, patients could remain stable, improve (move to NYHA I or II), deteriorate (move to NYHA IV), or die. We used a lifetime time horizon in the analysis. We conducted the analysis in line with the National Institute for Health and Care Excellence reference case, modelling costs from an English NHS perspective, considering outcomes in quality‐adjusted life years (QALYs), and discounting all costs and outcomes at 3.5% per year.15
Each arm of the model captured treatment efficacy via the distribution of patients across NYHA classes [e.g. if treatment was associated with symptomatic improvement, this would present itself as higher proportions of the cohort in NYHA I/II and lower proportions in NYHA III/IV (contingent on being alive); this had a knock‐on effect on patients' HRQoL and resource use].
Patients
Of all patients included in the three source trials, we only included data from those who were NYHA III at baseline, had LVEF of 25–45%, and had narrow QRS (<130 ms), excluding patients who did not meet all of these criteria before performing any analyses. This is aligned with the population expected to use the device in routine clinical practice. In the resulting cross‐trial cohort (N = 415), 189 received the Optimizer System + SoC and 226 received SoC alone. We used the individual patient data from the CCM registry9 to inform a scenario analysis, rather than in the model base case, because these data were non‐comparative.
Standard of care differs between patients depending on their baseline LVEF. Patients with a baseline LVEF of <35% can potentially receive an implantable cardioverter–defibrillator (ICD) as part of SoC, whereas those with a LVEF of >35% are not eligible. For this reason, we generated economic results for three patient groups: (i) all patients (LVEF of 25–45%); (ii) LVEF of 25–34%; and (iii) LVEF of 35–45%. Modelling the two LVEF‐based subgroups separately allowed us to explore any heterogeneity of clinical and cost outcomes caused by the differences in SoC therapy between the two groups. Table 1 summarizes the key characteristics of patients included in the model.
Table 1.
Key characteristics of individual patient data included in the cost‐effectiveness model (based on randomized controlled trial data)
| Characteristic | Patient population (LVEF) | ||
|---|---|---|---|
| Alla | 25–34% | 35–45% | |
| Total number of patients (n) | 415 | 320 | 95 |
| Baseline NYHA class | NYHA III | ||
| Starting age, mean (years) | 60.7 | 60.9 | 60.1 |
| Baseline LVEF, mean | 30.8% | 28.9% | 37.1% |
| Baseline MLWHFQ, mean | 55.8 | 55.4 | 57.2 |
| Males | 74.0% | 73.4% | 75.8% |
| Ischaemic | 66.7% | 68.8% | 60.0% |
| Diabetic | 46.3% | 45.3% | 49.5% |
LVEF, left ventricular ejection fraction; MLWHFQ, Minnesota Living with Heart Failure Questionnaire NYHA, New York Health association.
Refers to all patients included in the model; that is, LVEF of 25–45%.
Mortality
We used parametric time‐to‐event analysis to assess the effect of the Optimizer System on mortality, including treatment as the sole covariate.16 We fitted six standard functions (exponential, Weibull, Gompertz, log‐normal, log‐logistic, and generalized gamma) to the observable data, with the final choice for the base case analysis made on the basis of Akaike information criterion and Bayesian information criterion scores. Mortality rates in the model population never went below those in the general English population because, in these cases, the model was programmed to use general population mortality data17 adjusted for cardiovascular mortality.18
New York Heart Association class
A multinomial logit model determined the proportion of patients in each class at any given time. To account for each patient's baseline characteristics, the model considered the following as independent variables: time, treatment, sex, baseline age, baseline LVEF, ischaemic status, and diabetic status. We log transformed time and included a treatment/time interaction term to improve model fit to the RCT data.
Health‐related quality of life
The FIX‐HF‐5 trials captured patients' HRQoL via MLWHFQ. We used a generalized linear mixed model (GLMM) with a gamma error distribution and log link function to predict patients' MLWHFQ score by NYHA class. In all models using a gamma error distribution, we chose to transform values (x + 1) because the dependent variable contained 0 s. The fixed effects within the model consisted of time, treatment type, baseline age, baseline MLWHFQ score, and NYHA class, as well as the interaction between treatment type and time. Following preliminary assessments, we chose to log transform time in order to improve model fit and scale age in order for the model to converge. The random model controlled for repeated measures and nesting by incorporating a unique ID for each patient and trial.
To populate the cost‐effectiveness model, we transformed predicted MLWHFQ scores derived from the GLMM into EQ‐5D utility scores using two published algorithms: for baseline EQ‐5D19 and for change in EQ‐5D score.20
All‐cause hospitalizations
We used a GLMM with a gamma error distribution and a log link function to predict monthly all‐cause hospitalization rates by NYHA class. As well as hospitalization rates (excluding all perioperative or device‐related causes, e.g. lead problems, bleeding, erosion, or infection) and NYHA class, we included the following as independent variables: baseline age and LVEF (both scaled to improve model convergence), sex, ischaemic status, and diabetic status. The random model controlled for repeated measures and nesting by incorporating of a unique ID for each patient and trial.
Costs
The cost‐effectiveness model included costs associated with the Optimizer System, SoC (including OMT and, where applicable, ICD costs), and hospitalizations (reported in Table S1 ). The manufacturer provided the values of all costs incurred by the NHS associated with the Optimizer System and its implantation (staff time, capital costs, consumables, and hospital care) for inclusion within the analysis. The model also included functionality to apply costs associated with replacing the device at the end of its battery life. We assumed a replacement interval of 20 years in the base case (informed by data from the manufacturer), with different intervals explored in scenario analyses. We derived health care costs associated with hospitalizations (excluding hospital stay for insertion/replacement procedures and any associated adverse events) and SoC (by NYHA class), including outpatient visits, prescribed drugs, and laboratory expenses from published sources.21, 22, 23, 24
Cost‐effectiveness results
The cost‐effectiveness model generated total per patient costs and QALYs in each arm, as well as an incremental cost per QALY gained for Optimizer + SoC versus SoC alone. The analysis estimated these results for the three patient groups of interest by running individual patients relevant to the population of interest (based on their baseline characteristics) through the model and taking an average.
Sensitivity and scenario analyses
We conducted probabilistic sensitivity analysis in order to quantify the level of confidence in the model results in relation to uncertainty around the inputs. We also undertook various scenario analyses to assess the impact of key modelling assumptions on the results. These included
varying the size and duration of the survival benefit in the Optimizer + SoC arm,
use of data from CCM registry in addition to RCT data. This allowed for incorporation of follow‐up data to 36 months for some patients,
varying the length of data extrapolation for HRQoL and NYHA class over time,
varying the rate of hospitalizations applied for NYHA IV patients,
varying the assumed longevity of the Optimizer System,
considering model time horizons shorter than lifetime.
Software
We used R v.3.4.225 for all statistical analyses, with significance defined as P < 0.05. We developed the cost‐effectiveness model in Microsoft Excel (Microsoft Corporation).
Results
New York Heart Association class over time
In all three patient groups, there is a statistically significant increase in the probability of being NYHA I/II compared with NYHA III as time progresses (Figure 2; Table S3 ). However, this relationship does not hold for the probability of being NYHA Class IV versus Class I/II. The proportion of patients in NYHA I/II increases over time in the Optimizer + SoC arm. However, this effect is only statistically significant in the all patients group when the proportion in NYHA I/II is compared with the proportion NYHA IV (Table S3 ).
Figure 2.
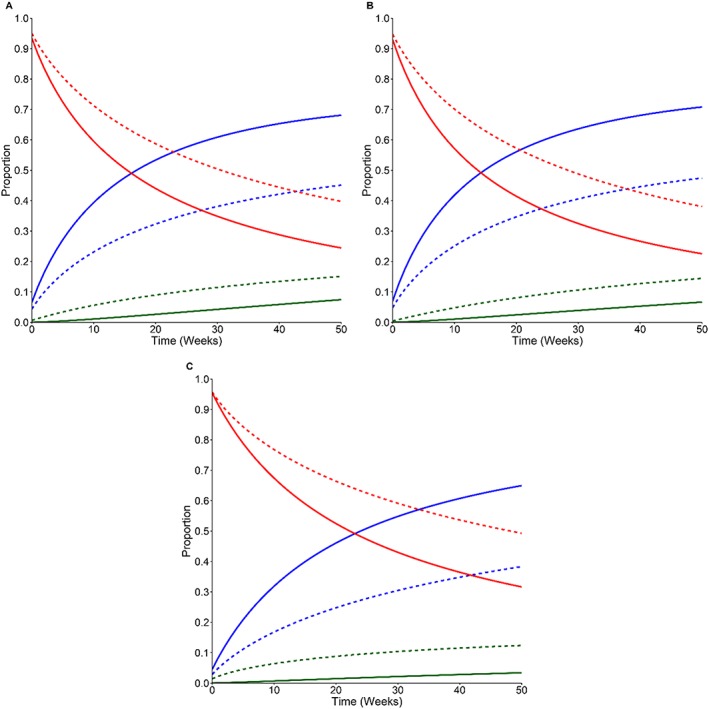
The effect of treatment on patient disease state over time. The multinomial logistic regression predicted the proportion of patients in each of the three disease states (NYHA class I/II, blue line; NYHA III, red line; NYHA IV, green line) depending on the treatment being received (Optimizer + SoC, solid line; SoC alone, dashed line). (A) All patients, (B) 25–34% LVEF, and (C) 35–45% LVEF. The model predicted disease state when baseline age and LVEF were fixed at their respective means, sex was male, and patients were ischaemic and had diabetes. LVEF, left ventricular ejection fraction; NYHA, New York Health Association; SoC, standard of care.
We judged the long‐term extrapolations of NYHA class occupancy to be clinically implausible because patients continued to improve over time despite HF being a degenerative disease. Therefore, we assumed that the proportion of patients in each NYHA class remained constant over time after a set point (12 months in the base case, aligned with the RCT follow‐up period and 36 months in the scenario analysis where registry data were included). Griffiths et al.26 previously used and clinically validated this method.
Mortality
An exponential distribution gave the best Akaike information criterion and Bayesian information criterion scores (Table S2 ), as well as the most clinically realistic long‐term extrapolation. Treatment with Optimizer + SoC non‐significantly improved all‐cause mortality in all three patient groups, with hazard ratios of 0.73 in all patients (95% CI: 0.31–1.72; P = 0.467); 0.72 in LVEF of 25–34% (95% CI: 0.27–1.91; P = 0.503); and 0.84 in LVEF of 35–45% (95% CI: 0.14–5.05; P = 0.852) when compared against SoC alone (Figure S1 ).
Health‐related quality of life
The predicted rate of improvement in HRQoL is higher in those receiving Optimizer + SoC compared against SoC alone in all patient groups. In addition, individuals receiving Optimizer + SoC show an additional decrease in MLWHFQ score per unit of time (log transformed), which is above and beyond the base effect of time.
There is an additional predicted relative decrease of 0.95 in MLWHFQ score per unit of log time in the all patients group (95% CI: 0.92–0.97; P < 0.001); 0.95 in the LVEF of 25–34% group (95% CI: 0.92–0.98; P = 0.003); and 0.93 in the LVEF of 35–45% group (95% CI: 0.88–0.99; P = 0.021) on the untransformed scale (i.e. HRQoL plus 1) (Figure 3). Individuals whose MLWHFQ scores or NYHA class were poorer at baseline are associated with a reduction in HRQoL (Table S4 ).
Figure 3.
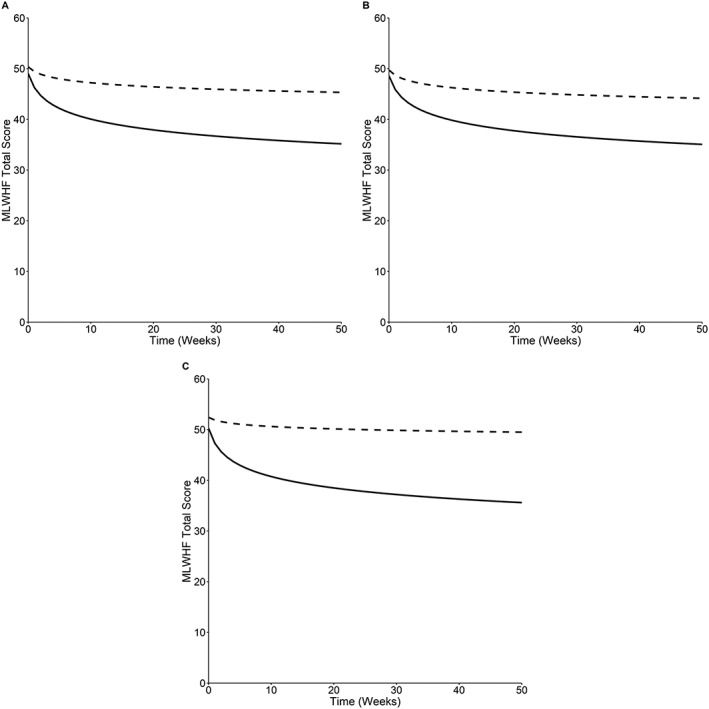
The effect of time and treatment interaction on MLWHFQ score. The generalized linear mixed model predicted MLWHFQ total scores over time for Optimizer + SoC (solid line) and SoC alone (dashed line) in the three patient groups: (A) All patients, (B) LVEF of 25–34%, and (C) LVEF of 35–45%. The model predicted MLWHFQ scores when baseline age and MLWHFQ score were fixed at their respective means, and NYHA class was set to NYHA III. Lower MLWHFQ scores indicate higher quality of life. MLWHFQ, Minnesota Living with Heart Failure Questionnaire; SoC, standard of care.
As with NYHA class, the statistical model predicted that patients' HRQoL would continue to improve over time in both treatment arms. This seems clinically implausible given the degenerative nature of HF, so in the cost‐effectiveness model, HRQoL returned to baseline at a set point in time (12 months in the base case and 36 months in the scenario using registry data). This return occurred at a constant rate in both arms.
All‐cause hospitalizations
Predicted monthly all‐cause hospitalization rates by NYHA class show that, in all three patient groups, NYHA III patients have a higher relative monthly rate of all‐cause hospitalizations compared against NYHA I/II patients by 1.02 (95% CI: 1.00–1.04; P = 0.012); 1.02 (95% CI: 1.00–1.04; P = 0.016); and 1.01 (95% CI: 0.97–1.06; P = 0.012), respectively on the untransformed scale (Table S5 ). Surprisingly, NYHA IV patients have a lower relative hospitalization rate compared against NYHA I/II by 0.96 (95% CI: 0.64–1.42; P = 0.024); 0.96 (95% CI: 0.66–1.39; P = 0.038); and 0.96 (95% CI: 0.59–1.38; P = 0.367) on the untransformed scale (i.e. monthly hospitalization rate plus 1). Table 2 shows predicted all‐cause hospitalization rates by NYHA class.
Table 2.
Hospitalization rate (per month) by NYHA class (based on randomized controlled trial data)
| NYHA class | All patients (LVEF of 25–45%) | LVEF of 25–34% | LVEF of 35–45% |
| NYHA I/II | 0.04 | 0.04 | 0.04 |
| NYHA III | 0.07 | 0.07 | 0.07 |
| NYHA IV[Link] | 0.03 | 0.03 | 0.03 |
LVEF, left ventricular ejection fraction; NYHA, New York Health association.
These rates were judged clinically implausible, and therefore, the value for NYHA III was doubled for use in the cost‐effectiveness model.
The predicted rate in NYHA IV is lower than those in NYHA I/II and III. This seems clinically implausible given the high severity of disease in these patients and is likely to be because of the limited number of hospitalizations that occurred during the trial period (20 in the whole population) and the low number of NYHA IV patients (most likely because patients with severe disease have a much higher risk of dying). Therefore, the model base case assumed the monthly rate of all‐cause hospitalizations for NYHA IV patients to be double that of NYHA III patients, as per Mealing et al.19
Cost‐effectiveness results
Base case results in each patient group are presented in Table 3. At a threshold of GBP30 000 per QALY gained, Optimizer + SoC is cost‐effective compared against SoC alone in all three groups. The estimated incremental cost‐effectiveness ratio (ICER) for the all patients group is lower than in both the LVEF of 25–34% and LVEF of 35–45% groups. However, total costs and QALYs in the all patients group fall between those in the two LVEF subgroups. This discrepancy occurs because of the ICER being a ratio and the cost‐effectiveness model being non‐linear.
Table 3.
Base case results
| Optimizer + SoC | SoC | Difference | |
|---|---|---|---|
| All patients | |||
| Cost per patient | GBP66 813 | GBP43 897 | GBP22 916 |
| QALYs per patient | 6.02 | 5.02 | 1.00 |
| ICER | GBP22 988 | ||
| Life years (undiscounted) | 10.66 | 8.91 | 1.75 |
| LVEF of 25–34% | |||
| Cost per patient | GBP72 175 | GBP48 386 | GBP23 790 |
| QALYs per patient | 6.03 | 5.00 | 1.03 |
| ICER | GBP23 118 | ||
| Life years (undiscounted) | 10.71 | 8.87 | 1.83 |
| LVEF of 35–45% | |||
| Cost per patient | GBP48 913 | GBP30 684 | GBP18 229 |
| QALYs per patient | 5.77 | 5.10 | 0.68 |
| ICER | GBP26 952 | ||
| Life years (undiscounted) | 9.97 | 9.03 | 0.93 |
ICER, incremental cost‐effectiveness ratio; LVEF, left ventricular ejection fraction; QALYs, quality‐adjusted life years; SoC, standard of care.
Optimizer + SoC has a 96% probability of being cost‐effective at the GBP30 000 threshold in the all patients group. This reduces to 35% at a GBP20 000 per QALY threshold. In the subgroups, Optimizer + SoC has 33% and 95% (LVEF of 25–34%) and 14% and 42% (LVEF of 35–45%) probability of being cost‐effective at GBP30 000 and GBP20 000 thresholds, respectively (Figure S2 ).
Scenario analyses (all patients)
Varying the assumptions around the time horizon and the survival benefit associated with Optimizer + SoC has the largest impact on cost‐effectiveness results. Removal of the survival benefit altogether increases the ICER substantially to GBP105 980 per QALY gained; and where the benefit only persists for 3 or 5 years, the model generates ICERs of GBP43 542 and GBP38 846 per QALY gained, respectively. In order for Optimizer + SoC to be cost‐effective at a GBP30 000 threshold, a hazard ratio for all‐cause mortality of 0.81 is required when comparing Optimizer + SoC with SOC alone.
Reducing the time horizon results in an increased ICER. For example, a time horizon of 10 years gives an ICER of GBP36 169 per QALY gained.
The remaining assumptions tested in scenario analyses were not key drivers of the cost‐effectiveness results:
CCM registry data: inclusion of these data in addition to RCT data results in a small reduction in the ICER to GBP21 798 per QALY.
Month at which HRQoL return to baseline: varying this assumption has only a small impact on the results of the model. A later cut‐off point results in a lower ICER (e.g. GBP18 609 per QALY for 36 months).
Month from which NYHA class remains constant: varying this assumption has only a small impact on the results of the model, for example, GBP21 335 per QALY at 36 months.
Hospitalization rate for NYHA IV patients: using the same monthly all‐cause hospitalization rate for NYHA IV patients as for NYHA III patients resulted in a slight increase in the ICER.
Longevity of Optimizer System: varying the assumption around the frequency of device replacements had only a small impact on the ICER, for example, GBP25 367 per QALY with replacement at mean of 15 years.
Discussion
The Optimizer System (as an adjunct to SoC) is likely to be a cost‐effective intervention for NYHA Class III HF patients with LVEF of 25–45% and a narrow QRS (<130 ms), with an estimated 96% likelihood of being good value for money at a reimbursement threshold of GBP30 000 per QALY gained. The ICER for the Optimizer System in this study (GBP22 988 per QALY gained) is similar to results reported for established device therapies for HF, such as CRT and ICD.19, 27 Subgroup analyses demonstrate similarly positive results in those with LVEF of 25–34% or LVEF of 35–45%, but with those for LVEF of 35–45% being more uncertain, largely because of lower number of patients in the latter group. Our results are, therefore, primarily generalizable to younger cardiac implantable electronic device eligible individuals (LVEF of <35%), who are likely to have fewer co‐morbidities than the general HF population.
It is important to consider the clinical plausibility of outcomes generated by the regression equations for NYHA class over time, mortality, HRQoL, and hospitalization rates. Where possible, we compared these outputs with estimates from the published literature. We identified some issues with statistical analyses predicting constant improvements in NYHA class over time despite the typically progressive nature of HF. We corrected this by reverting NYHA class to baseline at the point where trial follow‐up data ended. This is a conservative assumption. Real‐world data show that benefits in terms of NYHA persist for at least 2 years,28 and it is likely that such benefits would extend significantly longer.
The hazard ratio for mortality used in the cost‐effectiveness model is comparable with those reported in previous studies of cardiac implantable electronic devices,27, 29, 30 which vary from 0.73 to 0.83 (compared with 0.73 in this analysis). However, the confidence interval around this value was wide because of the small number of mortality events occurring within the follow‐up duration of the clinical studies. Utility values calculated from MLWHFQ scores for patients in NYHA I/II (0.78), III (0.65), and IV (0.52) were very similar to those reported in the literature.27
We could not identify relevant published data for all‐cause hospitalization rates by NYHA class. However, one study reported 0.36–0.43 hospitalizations per year in patients receiving medical treatment alone for HFrEF.31 This equates to a monthly rate of 0.03–0.04 hospitalizations, which is lower than the rates generated by our statistical analysis but probably represents patients with milder disease less likely to be considered for an implantable technology.
Our model predicts mean life expectancy of 10.66 years with Optimizer + SoC and 8.91 years with SoC alone based on entry to the model at the mean age of 60.7 years. This prediction is consistent with the 45% survival at 10 years observed in follow‐up of patients with LVEF of 25–45% enrolled in the original FIX‐HF‐5 Phase 2 (pivotal) study (Figure S3 ; previously unpublished data).
Study limitations
The study results indicate that the Optimizer System is cost‐effective when a lifetime horizon for costs and benefits is considered. Follow‐up in the clinical trials of the Optimizer System was relatively short, restricted to 12 months in the RCT data, and our statistical analyses extrapolated the trial data over a lifetime. However, the consistency between model predictions and observed mortality through 10 years of follow‐up does provide reassurance of model validity. Nevertheless, as we increase the time horizon, more uncertainty is introduced into the analysis. Over shorter time horizons, where this uncertainty is reduced, the ICER increases. However, these shorter time horizons are not aligned with National Institute for Health and Care Excellence recommendations for lifetime cost‐effectiveness estimation as the basis of assessment of value.15 Ongoing data collection in the Optimizer CCM registry9 will increase confidence in the durability of the treatment effect.
Scenario analyses indicate that the survival benefit associated with the Optimizer System is a key driver of the cost‐effectiveness results, and therefore, the Optimizer System is likely to be most cost‐effective in individuals at higher risk of mortality but for whom a survival benefit can be obtained. The RCT data on short‐term survival suggest a benefit, but this fails to reach statistical significance at 12 months. In order for the Optimizer System to be cost‐effective at a GBP30 000 threshold, the hazard ratio for all‐cause mortality required with the Optimizer System + SoC is 0.81 compared with SoC alone. Whilst the mortality data used in the model are uncertain, the impact of treatment on symptoms (as measured by NYHA class) is highly statistically significant and likely to be associated with better longer term survival, particularly as CCM therapy is also associated with reverse remodelling of the left ventricle32 similar to that reported for CRT, which is known to be associated with longer term survival benefit.22, 23 We therefore judge that the use of the Optimizer System + SoC will plausibly improve survival.
Conclusions
For patients with moderate‐to‐severely symptomatic HFrEF (with a narrow QRS duration who are not indicated for CRT), the addition of CCM therapy to medical therapy is likely to be cost‐effective when compared with medical therapy alone, when taking a lifetime perspective. The cost‐effectiveness model generated base case ICERs of GBP20 000–30 000 (€22 400–€33 600) across the LVEF of 25–45% range, similar to that reported for CRT or ICD therapies. Similar to CRT devices, long‐term, post‐approval studies would be necessary to confirm these findings in the real‐world setting particularly in respect to the impact of CCM therapy on mortality.
Conflict of interest
K.W. reports personal fees from York Health Economics Consortium, during the conduct of the study. G.H. reports personal fees from AstraZeneca, Berlin Chemie, Corvia, Impulse Dynamics, Novartis, Servier, Vifor Pharma, and Springer, outside the submitted work. D.B. reports personal fees from Impulse Dynamics, during the conduct of this study. M.G., J.M., A.P., and S.M. are employees of York Health Economics Consortium, which was commissioned by the device manufacturer, Impulse Dynamics, to undertake this work. I.D.Z. reports personal fees from Impulse Dynamics during the conduct of the study and personal fees from Medtronic outside the submitted work. M.R.C. reports personal fees from Impulse Dynamics outside the submitted work.
Funding
This work was supported by Impulse Dynamics.
Supporting information
Figure S1. The effect of the Optimizer system on survival in all three subgroups. An exponential survival curve was fitted to all three subgroups. The Optimizer system in combination with SoC (red line) was found to improve survival probability compared to patients receiving SoC Only (blue line). The number of patients at risk are also displayed below each graph. (A) All patients, (B) 25‐34% LVEF and (C) 35‐45% LVEF.
Figure S2. Cost‐effectiveness applicability curve for each patient group. A cost‐effectiveness acceptability curve (CEAC) was generated for each group. The Optimizer system in combination with SoC is more likely to be cost‐effective the higher the cost‐effectiveness threshold used. For all patients and LVEF 25‐34% subgroup the CEAC rises steeply between GBP15,000 and GBP30,000 per QALY. For the LVEF 35‐34% subgroup this happens at a higher threshold between GBP20,000 and GBP60,000 per QALY. (A) All patients, (B) 25‐34% LVEF and (C) 35‐45% LVEF.
Figure S3. Previously unpublished data from the FIX‐HF‐5 Phase 2 (pivotal) study showing estimated survival to 10 years post‐implantation of the Optimizer System.
Table S1. Model cost input parameters.
Table S2. AIC and BIC scores for each distribution in survival modelling.
Table S3. Disease state multinomial logistic regression results.
Table S4. HRQoL based generalised linear mixed model results.
Table S5. All‐cause hospitalisation generalised linear mixed model results.
Acknowledgements
The authors would like to thank Emily Eaton Turner for her input in the initial stages of model development and data analysis. We are also grateful to Professor Mark Sculpher and Professor Steve Palmer for their contributions at advisory boards and to the device manufacturers for making available all relevant trial data.
Witte, K. , Hasenfuss, G. , Kloppe, A. , Burkhoff, D. , Green, M. , Moss, J. , Peel, A. , Mealing, S. , Durand Zaleski, I. , and Cowie, M. R. (2019) Cost‐effectiveness of a cardiac contractility modulation device in heart failure with normal QRS duration. ESC Heart Failure, 6: 1178–1187. 10.1002/ehf2.12526.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 2. Boriani G, Nesti M, Ziacchi M, Padeletti L. Cardiac resynchronization therapy: an overview on guidelines. Heart Fail Clin 2017; 13: 117–137. [DOI] [PubMed] [Google Scholar]
- 3. Lund LH, Jurga J, Edner M, Benson L, Dahlström U, Linde C, Alehagen U. Prevalence, correlates, and prognostic significance of QRS prolongation in heart failure with reduced and preserved ejection fraction. Eur Heart J 2013; 34: 529–539. [DOI] [PubMed] [Google Scholar]
- 4. Chioncel O. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 5. Neelagaru SB, Sanchez JE, Lau SK, Greenberg SM, Raval NY, Worley S, Kalman J, Merliss AD, Krueger S, Wood M, Wish M, Burkhoff D, Nademanee K. Nonexcitatory, cardiac contractility modulation electrical impulses: feasibility study for advanced heart failure in patients with normal QRS duration. Heart Rhythm 2006; 3: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 6. Kadish A, Nademanee K, Volosin K, Krueger S, Neelagaru S, Raval N, Obel O, Weiner S, Wish M, Carson P, Ellenbogen K, Bourge R, Parides M, Chiacchierini RP, Goldsmith R, Goldstein S, Mika Y, Burkhoff D, Abraham WT. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J 2011; 161: 329–337.e2. [DOI] [PubMed] [Google Scholar]
- 7. Abraham WT, Nademanee K, Volosin K, Krueger S, Neelagaru S, Raval N, Obel O, Weiner S, Wish M, Carson P, Ellenbogen K, Bourge R, Parides M, Chiacchierini RP, Goldsmith R, Goldstein S, Mika Y, Burkhoff D, Kadish A, FIX‐HF‐5 Investigators and Coordinators . Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J Card Fail 2011; 17: 710–717. [DOI] [PubMed] [Google Scholar]
- 8. Abraham WT, Kuck K‐H, Goldsmith RL, Lindenfeld J, Reddy VY, Carson PE, Mann DL, Saville B, Parise H, Chan R, Wiegn P, Hastings JL, Kaplan AJ, Edelmann F, Luthje L, Kahwash R, Tomassoni GF, Gutterman DD, Stagg A, Burkhoff D, Hasenfuß G. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC: Heart Failure 2018; 6: 874–883. [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Borggrefe M, Neuser H, Ohlow MA, Röger S, Goette A, Remppis BA, Kuck KH, Najarian KB, Gutterman DD, Rousso B, Burkhoff D, Hasenfuss G. Cardiac contractility modulation improves long‐term survival and hospitalizations in heart failure with reduced ejection fraction. Eur J Heart Fail 2019; 21: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 10. Borggrefe MM, Lawo T, Butter C, Schmidinger H, Lunati M, Pieske B, Misier AR, Curnis A, Bocker D, Remppis A, Kautzner J, Stuhlinger M, Leclerq C, Taborsky M, Frigerio M, Parides M, Burkhoff D, Hindricks G. Randomized, double blind study of non‐excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J 2008; 29: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 11. Kuschyk J, Nagele H, Heinz‐Kuck K, Butter C, Lawo T, Wietholt D, Roeger S, Gutterman D, Burkhoff D, Rousso B, Borggrefe M. Cardiac contractility modulation treatment in patients with symptomatic heart failure despite optimal medical therapy and cardiac resynchronization therapy (CRT). Int J Cardiol 2019; 277: 173–177. [DOI] [PubMed] [Google Scholar]
- 12. Kuschyk J, Roeger S, Schneider R, Streitner F, Stach K, Rudic B, Weiß C, Schimpf R, Papavasilliu T, Rousso B, Burkhoff D, Borggrefe M. Efficacy and survival in patients with cardiac contractility modulation: long‐term single center experience in 81 patients. Int J Cardiol 2015; 183: 76–81. [DOI] [PubMed] [Google Scholar]
- 13. Lawo T, Borggrefe M, Butter C, Hindricks G, Schmidinger H, Mika Y, Burkhoff D, Pappone C, Sabbah HN. Electrical signals applied during the absolute refractory period: an investigational treatment for advanced heart failure in patients with normal QRS duration. J Am Coll Cardiol 2005; 46: 2229–2236. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence . Guide to the methods of technology appraisal 2013: the reference case. 2013. [PubMed]
- 16. Latimer N. NICE DSU Technical Support Document 14: survival analysis for economic evaluations alongside clinical trials ‐extrapolation with patient‐level data 2011. [PubMed]
- 17. Office of National Statistics . National life tables, UK: 2014 to 2016. 2017.
- 18. Kansal AR, Cowie MR, Kielhorn A, Krotneva S, Tafazzoli A, Zheng Y, Yurgin N. Cost‐effectiveness of ivabradine for heart failure in the United States. J Am Heart Assoc 2016; 5: e003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mealing S, Woods B, Hawkins N, Cowie MR, Plummer CJ, Abraham WT, Beshai JF, Klein H, Sculpher M. Cost‐effectiveness of implantable cardiac devices in patients with systolic heart failure. Heart 2016; 102: 1742–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calvert MJ, Freemantle N, Yao G, Cleland JG, Billingham L, Daubert JC, Bryan S, CARE‐HF investigators . Cost‐effectiveness of cardiac resynchronization therapy: results from the CARE‐HF trial. Eur Heart J 2005; 26: 2681–2688. [DOI] [PubMed] [Google Scholar]
- 21. Department of Health . NHS reference costs 2016‐17. London, 2017.
- 22. Joint Formulary Committee . British National Formulary (online). London: BMJ Group and Pharmaceutical Press; 2016. [Google Scholar]
- 23. Maniadakis N, Fragoulakis V, Mylonas C, Sharma R, Coats AS. Economic evaluation of cardiac contractility modulation (CCM) therapy with the optimizer IVs in the management of heart failure patients. Int Cardiov Forum J 2015; 4: 43–52. [Google Scholar]
- 24. Personal Social Services Research Unit (PSSRU) . Unit Costs of Health & Social Care 2017. Canterbury: University of Kent; 2017. [Google Scholar]
- 25. R Development Core Team . R: a language and environment for statistical computing. Vienna, Austria, 2011.
- 26. Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the U. K. National Health Service perspective. Heart 2014; 100: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Institute for Health and Care Excellence . Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure [TA314]. 2014.
- 28. Anker SD, Borggrefe M, Neuser H, Ohlow MA, Röger S, Goett A, Remppis BA, Kuck KH, Najarian KB, Gutterman DD, Rousso B, Burkhoff D, Hasenfuss G. Cardiac contractility modulation improves long‐term survival and hospitalizations in heart failure with reduced ejection fraction. Eur J Heart Fail 2019; 21: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 29. Theuns DA, Smith T, Hunink MG, Bardy GH, Jordaens L. Effectiveness of prophylactic implantation of cardioverter‐defibrillators without cardiac resynchronization therapy in patients with ischaemic or non‐ischaemic heart disease: a systematic review and meta‐analysis. Europace 2010; 12: 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xing Z, Tang L, Chen C, Huang J, Zhu Z, Hu X. Effectiveness of implantation of cardioverter‐defibrillators therapy in patients with non‐ischemic heart failure: an updated systematic review and meta‐analysis. Braz J Cardiov Surg 2017; 32: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Institute for Health and Care Excellence . Sacubitril valsartan for treating heart failure with systolic dysfunction [ID822]. 2016.
- 32. Yu CM, Chan JY, Zhang Q, Yip GW, Lam YY, Chan A, Burkhoff D, Lee PW, Fung JW. Impact of cardiac contractility modulation on left ventricular global and regional function and remodeling. J Am Coll Cardiol Img 2009; 2: 1341–1349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The effect of the Optimizer system on survival in all three subgroups. An exponential survival curve was fitted to all three subgroups. The Optimizer system in combination with SoC (red line) was found to improve survival probability compared to patients receiving SoC Only (blue line). The number of patients at risk are also displayed below each graph. (A) All patients, (B) 25‐34% LVEF and (C) 35‐45% LVEF.
Figure S2. Cost‐effectiveness applicability curve for each patient group. A cost‐effectiveness acceptability curve (CEAC) was generated for each group. The Optimizer system in combination with SoC is more likely to be cost‐effective the higher the cost‐effectiveness threshold used. For all patients and LVEF 25‐34% subgroup the CEAC rises steeply between GBP15,000 and GBP30,000 per QALY. For the LVEF 35‐34% subgroup this happens at a higher threshold between GBP20,000 and GBP60,000 per QALY. (A) All patients, (B) 25‐34% LVEF and (C) 35‐45% LVEF.
Figure S3. Previously unpublished data from the FIX‐HF‐5 Phase 2 (pivotal) study showing estimated survival to 10 years post‐implantation of the Optimizer System.
Table S1. Model cost input parameters.
Table S2. AIC and BIC scores for each distribution in survival modelling.
Table S3. Disease state multinomial logistic regression results.
Table S4. HRQoL based generalised linear mixed model results.
Table S5. All‐cause hospitalisation generalised linear mixed model results.


