Abstract
While anti‐cancer therapies, including chemotherapy, immunotherapy, radiotherapy, and targeted therapy, are constantly advancing, cardiovascular toxicity has become a major challenge for cardiologists and oncologists. This has led to an increasing demand of cardio‐oncology units in Europe and a growing interest of clinicians and researchers. The Heart Failure 2019 meeting of the Heart Failure Association of the European Society of Cardiology in Athens has therefore created a scientific programme that included four dedicated sessions on the topic along with several additional lectures. The major points that were discussed at the congress included the implementation and delivery of a cardio‐oncology service, the collaboration among cardio‐oncology experts, and the risk stratification, prevention, and early recognition of cardiotoxicity. Furthermore, sessions addressed the numerous different anti‐cancer therapies associated with cardiotoxic effects and provided guidance on how to treat cancer patients who develop cardiovascular disease before, during, and after treatment.
Keywords: Cardiotoxicity, Heart failure, Cancer
1. Introduction
More than 32 million people worldwide suffer from cancer.1 In the last years, advances in anti‐cancer therapies have led to an improvement in life expectancy of different cancer types.2 These patients often suffer from multiple different co‐morbidities that may develop as consequences from anti‐cancer therapies. Frequent problems include, but are not limited to, chronic kidney disease,3, 4 liver dysfunction,5, 6 gastrointestinal disease,7, 8 anaemia,9, 10 fatigue,11, 12 infections,13, 14 anorexia15, 16, muscle wasting,17, 18 pain,19, 20 and heart failure (HF).21, 22 Depending on the cancer diagnosis and the type of anti‐cancer treatment, cardiotoxicity rates may vary from 0% to 48% of patients, with HF being a predominant presentation.23 HF is associated with a 5‐year survival rate of nearly 50%24, 25, 26 and is frequently accompanied by reduced quality of life.27 HF is characterized by multiple symptoms such as reduced physical performance,28 shortness of breath,29 fluid retention,30 general weakness 31, and prolonged hospital stays32, which ultimately also result into substantial healthcare costs.33
Besides HF, other frequent cardiovascular (CV) problems associated with anti‐cancer therapies include coronary artery disease,34 atrial fibrillation,35 arterial hypertension,36 thromboembolic disease37, valvular disease38, pulmonary hypertension,39 stroke,40 and peripheral vascular disease.41 Special populations at increased short‐term and long‐term risks for CV disease are paediatric.42 and elderly patients43 Depending on the cancer entity, up to 30% of cancer patients eventually die of CV disease.44
The ‘Heart Failure and World Congress on Acute Heart Failure 2019’ provided a great platform for experts in cardio‐oncology to meet and exchange the latest ideas and advances in cardio‐oncology. The congress was held in Athens, Greece, from 25 May 2019 to 28 May 2019, and was attended by 5431 delegates, including more than 285 faculty members in more than 120 scientific sessions. During the congress, four full‐dedicated sessions, additional lectures, various clinical cases, and posters were dedicated to cardio‐oncology. Experts in the field discussed how to identify, treat, and prevent CV problems in cancer patients and were involved in interactive discussions with the audience. A detailed list of all sessions can be found in Supporting Information, Table S1 .
2. Cardiovascular disease in cancer
Cardiovascular events in cancer patients can be caused by three main factors: (i) concomitant CV risk factors and diseases; (ii) anti‐cancer therapy (including chemotherapy, targeted therapy, immune checkpoint inhibitors, and radiation) through direct or indirect damage effects; (iii) cancer itself, through the direct invasion of CV structures or indirect release of metabolites and/or activation of adrenergic system45, 46, 47 (Figure 1 ).
Figure 1.
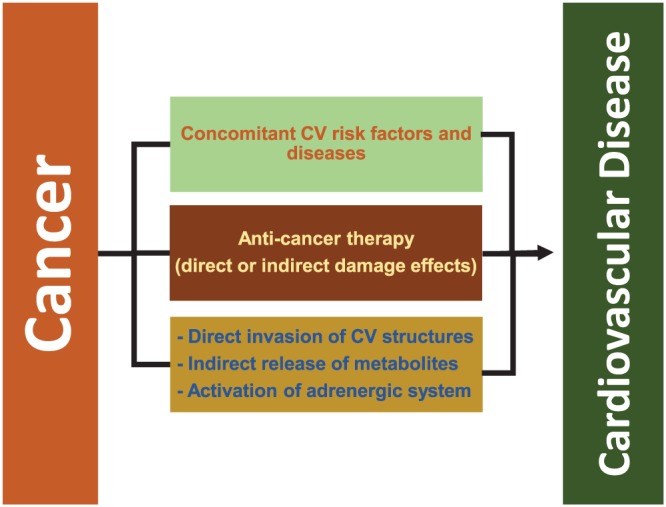
Aetiology of cardiovascular disease in cancer patients.
Dr Peter van der Meer (Groningen, the Netherlands), Dr Christian Bär (Hannover, Germany), and Associate Professor Dimitrios T. Farmakis (Athens, Greece) presented cancer therapy‐related cardiomyopathy (CTRCM).24, 48 A recent 10‐year follow‐up study with 350 breast cancer survivors previously treated with chemotherapy and/or radiation therapy compared with 350 age‐matched healthy women demonstrated an increased risk of mild left ventricular (LV) dysfunction in cancer survivors (15.3% vs. 7%).49 These data underline a long‐term risk for cardiac dysfunction in cancer patients previously treated with specific chemotherapy and/or radiotherapy.23 The intersection between HF and cancer was explored by Associate Professor Farmakis and Professor Denise Hilfiker‐Kleiner (Hannover, Germany). Both entities share several risk factors (ageing, tobacco, adiposity, physical inactivity, and infections) and an underlying systemic inflammatory status.50, 51, 52 In the CANTOS trial,53 which included 10 061 patients with myocardial infarction and increased high‐sensitive C‐reactive protein, the inhibition of the cytokine interleukin 1β with canakinumab (patients randomized to 50, 150, and 300 mg, or placebo) showed a reduction of nonfatal myocardial infarction, nonfatal stroke, or CV death with 150 mg canakinumab.54 A secondary analysis of the data by Ridker et al.55 showed a reduction of lung cancer incidence (with 150 and 300 mg canakinumab vs. placebo) and lung cancer mortality (with 300 mg of canakinumab vs. placebo). Further studies are needed to validate these results. Dr Markus Anker (Berlin, Germany) discussed CV problems of cancer patients beyond anti‐cancer therapy associated cardiotoxicity. It has previously been shown that treatment‐naïve colorectal cancer patients also demonstrated a mildly reduced left ventricular ejection fraction (LVEF) compared with healthy controls of similar age and sex56. The resting heart rate of cancer patients has also been found to be an independent predictor of all‐cause mortality in patients with pancreatic, colorectal, and non‐small cell lung cancer57. This was confirmed in a large retrospective cohort of 4786 patients with breast cancer58. A recent analysis in 305 advanced colorectal adenoma patients suggested higher adenoma recurrence rates in those patients with higher heart rates59. These effects might be related to a sympathetic activation in some patients, like it has been shown for HF60. More studies are needed to better understand the underlying mechanisms.
3. Diagnosis and prevention
Dr Teresa Lopez‐Fernandez (Madrid, Spain) addressed different cardiac imaging methods in cancer patients. Imaging is useful to stratify the risk profile at baseline, to predict recovery from cardiac injuries during cancer treatment and to detect early as well as late‐onset of cardiac dysfunction in cancer survivors61 (Figure 2). Dr Lopez‐Fernandez accentuated the fundamental role of transthoracic echocardiography in modern day cardio‐oncology, because it is ubiquitously available, involves no radiation exposure, and can also assess haemodynamics.23 Cardiotoxicity, according to the 2016 ESC Position Paper on cancer treatments and CV toxicity,23 is defined as a decrease of LVEF by >10 percentage points, below the local lower limit of normality (50–55%). According to recent opinions, 3D echocardiography may be preferable over 2D echocardiography (2DE) to assess LVEF in cancer patients, because of its low analysis time, higher reproducibility, and higher feasibility.62, 63 3D echocardiography has demonstrated to outperform 2DE in detecting LVEF changes in cancer patients.64 Global longitudinal strain has arisen as another parameter to characterize myocardial tissue in the past years. It permits the early identification of myocardial deformation abnormalities, with high reproducibility, before the detection of left ventricular systolic dysfunction (LVSD) by 2DE.65 But much more research is needed into global longitudinal strain and its implications for the treatment of cancer patients.66 In addition, Dr Lopez‐Fernandez showed new data on right ventricle strain as a novel and sensitive predictor of cardiotoxicity.67 Cardiac magnetic resonance is very accurate, has an excellent reproducibility, and allows tissue characterization of the heart.23, 68 However, Dr Lopez‐Fernandez explained that cardiac magnetic resonance is not used in daily practice due to its high costs and limited availability only in larger clinical centres.
Figure 2.
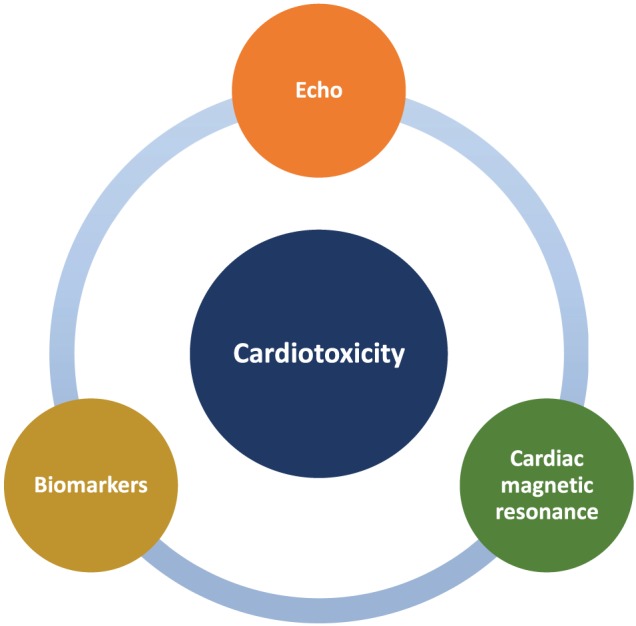
Diagnostic methods for early detection of cardiotoxicity.
Blood biomarkers as predictors of subclinical cardiotoxic injury were discussed by Dr Daniela Cardinale (Milan, Italy). Abnormal levels of troponins are considered a sensitive marker for cardiac monitoring to detect early cardiac‐specific injury and asymptomatic LVSD in cancer patients during potentially cardiotoxic anti‐cancer therapy,69 and they are also used to stratify the risk of cardiotoxicity before cancer therapy.70 Persistently increased N‐terminal pro B‐type natriuretic peptide during high‐dose chemotherapy have also shown to predict long‐term LVEF reduction in cancer patients.71 Combined measurements of N‐terminal pro B‐type natriuretic peptide as markers of CV overload and troponins for myocardial injury during cancer therapy may be useful.72 Lastly, microRNA were discussed as potential new biomarkers in cardio‐oncology.73
Professor Radek Pudil (Hradec Kralove, Czech Republic) and Dr Kalliopi Keramida, (Athens, Greece) talked about different prevention strategies in order to minimize the development of CTRCM. The proposed approach consists of three different intervention phases according to the implementation of anti‐cancer treatment. The aim of the first phase is to stratify the patients into low‐risk, medium‐risk, and high‐risk groups for cardiotoxicity during anti‐cancer treatment.74 The identification of high‐risk patients is important in order to apply adequate measures, including optimization of CV risk factors and pre‐existing CV disease, planning of active screening during therapy and interdisciplinary cardio‐oncologic decisions for the anti‐cancer treatment with the best benefit/risk ratio.75 Additionally, beneficial effects of exercise training before and during cancer therapy have been demonstrated.76, 77, 78 The second phase concentrates on identifying patients with LVSD during treatment. Frequently used and inexpensive screening tools include serial troponin measurements and regular echocardiographic monitoring of LVEF.23, 79 Both speakers agreed that in case of cardiac dysfunction, a decision for continuation of treatment of the patient can only be made by the close collaboration between cardiologists and oncologists.23 The third phase of prevention consists of long‐term surveillance of cancer survivors. Associate Professor Farmakis quoted a study by Mertens et al.80 in 20 227 childhood cancer survivors that found cardiac mortality to be 8.2 times higher compared with the general population with no previous cancer diagnosis. CV disease can develop many years after diagnosis and treatment of cancer.81 Associate Professor Farmakis recommended cardiologists to follow‐up high‐risk cancer patients after completion of anti‐cancer treatment. Surveillance tools may include electrocardiograms, echocardiography, cardiac biomarkers, carotid ultrasound, fasting lipid profile, TSH in case of neck irradiation, and regular measurement of blood pressure.23, 82
Cardio‐oncology services have become very important and continue to grow in oncological clinical care. Dr Alexander Lyon (London, UK) and Dr Lopez‐Fernandez discussed during their presentations how to establish such a service. Cardio‐oncology services can help optimizing anti‐cancer treatment, avoiding delays, and allowing adequate treatment of CV complications.83 They also play a key role in the prevention of cardiotoxicity. Cardio‐oncology units are composed of primary care departments, outpatient clinics, and educational programmes.84 Recent data from Pareek et al.84 demonstrated the effectiveness of these services. From 128 cancer patients with LVSD, 88% of patients were able to continue their cancer therapy after CV optimization, while an improvement of LVSD after 1 year of follow‐up was shown in 94% of patients. Professor Thomas Thum (Hannover, Germany) recommended the promotion of basic, translational, and clinical research, training programmes, as well as educational resources as the foundation to a successful cardio‐oncology workforce for the care of cancer patients. Lastly, the speakers also talked about the need to actively involve the patients in all decisions, so that patients can make informed decisions together with the treating physicians about possible treatment strategies.
4. Treatment of cardiotoxicity and new frontiers
Professor Alain Cohel‐Solal (Paris, France) and Professor Rudolf de Boer (Groningen, the Netherlands) talked about available CV therapies to manage CTRCM. Because of its peculiar phenotype (tachycardia, low blood pressure, LV and/or right ventricular involvement, and dilated ventricles), intervention strategies may be challenging.85 One of the largest studies regarding this question was conducted by Cardinale et al.82 The authors followed 2625 cancer patients for a median of 5 years. During the first year of follow‐up, echocardiography was conducted every 3 months, then for another 4 years every 6 months, and after that once a year. Cardiotoxicity was defined as a drop of LVEF below 50% and by >10 percentage points, which is similar to the current definition of cardiotoxicity by the ESC.23 When cardiotoxicity occurred, HF therapy with angiotensin‐conversion enzyme inhibitors (ACE‐Is) and beta‐blockers was initiated and up‐titrated to the maximum tolerated dose. In the entire study, cardiotoxicity occurred in 221 patients (9%); 98% of all cardiotoxic events occurred within the first year of follow‐up. This underlines the importance of closely monitoring cancer patients during the first year after a potentially cardiotoxic therapy. Of those patients with cardiotoxicity who initiated HF therapy with ACE‐Is and beta‐blockers, 82% had at least a partial recovery of LVEF. In patients, in whom ACE‐I or BB therapy cannot be up‐titrated due to adverse effects like hypotension, Professor Cohen‐Solal considered ivabradine as a possible additional treatment for selected patients86 and the need to be more cautious in drug titration in these patients than in other patients with cardiac dysfunction. There is also a need to investigate the effects and possible benefits of sacubitril/valsartan for the treatment of CTRCM.87
During the congress, the preliminary results of MADIT‐CHIC Study (NCT02164721) by Singh et al. 88, 89 were also discussed. Thirty patients (87% female) with CTRCM and lymphoma or breast cancer, which qualified for cardiac resynchronization–defibrillator therapy, participated in the trial. The primary endpoint, change of LVEF at 6 months, increased by 11% (95% CI 8–14; P < 0.001) and was independent of age, sex, NYHA class, and QRS duration. LV end‐diastolic volume, LV end‐systolic volume, and left atrial volume decreased. Weaknesses of the trial were the short period of follow‐up, the primarily female patient population, the lack of control group, and only 30 included patients while the trial ran for 3.5 years. Larger randomized trials will have to further investigate the potential benefits of cardiac resynchronization–defibrillator therapy in CTRCM.
Dr Javid Moslehi (Nashville, TN, USA) focused on immune checkpoints inhibitors (ICIs). Immunotherapy (e.g. anti‐CTLA‐4, anti‐PDL‐1, and anti‐PD‐1) can be used as monotherapy or in combination with other chemotherapies, for example, in advanced melanoma90 and advanced, refractory non‐small‐cell lung cancer.91 Adverse events like myositis, mucositis, colitis, and pneumonitis can accompany the administration of these anti‐cancer agents.92 ICI‐associated myocarditis is infrequently seen after the administration of 1‐2 drug doses but associated with high rates of mortality of early 50%93. According to Dr Moslehi, a deeper understanding of the underlying pathophysiological mechanisms is urgently needed.94, 95 Therefore, specific knockout models in mice have been developed to further study ICI‐related mechanisms and underlying pathways96. Professor Carlo Gabriele Tocchetti (Naples, Italy) further deepened the concept about the importance of immunology in cardio‐oncology:97 not only can immunologic pathways be exploited to fight cancer98 and predict the response to anti‐cancer therapies99 but also they are involved in the development of cardiotoxicity.100
Professor Dirk Brutsaert (Antwerp, Belgium) discussed how an impairment of the endothelium could influence the progress of HF and cancer. According to him, the endothelium has haemodynamic, mechanical, and biochemical sensors for several molecules like proteins, microvesicles, peptides, and microRNA.101 As an organ with perfusion and transport function, endothelial cells secrete growth inhibitors and permit the extravasation of cells.102 Professor Brutsaert therefore hypothesized that endothelial dysfunction might play an important role in the development of both cancer and HF.103
5. Conclusions
The ‘Heart Failure and World Congress on Acute Heart Failure 2019’ gave the participants a great overview of the current knowledge in cardio‐oncology and new directions of this research area. Some of the cornerstones of cardio‐oncology include the assessment of CV risk profiles in cancer patients before the initiation of anti‐cancer treatment, the effective and adequate monitoring techniques for cardiotoxicity, the design of patient specific management strategies, and the need to universally introduce cardio‐oncology services in hospitals working in close collaboration with oncology departments.
Conflict of interest
M.S.A. reports receiving personal fees from Servier. The UMCG, which employs R.A.dB. has received research grants and/or fees from AstraZeneca, Abbott, Bristol‐Myers Squibb, Novartis, Novo Nordisk, and Roche. R.A.dB. received speaker fees from Abbott, AstraZeneca, Novartis, and Roche. D.F. has received speaker honoraria, consultancy fees, and/or travel grants from Abbott, Boehringer‐Ingelheim, Daiichi‐Sankyo, Menarini, Novartis, Pfizer, Roche, and Servier. S.vH. has been a paid consultant to BRAHMS/Thermo Fisher, Chugai, Helsinn, Boehringer Ingelheim, Grünenthal, Novartis, Roche, and Vifor. Z.I. reports lecture fees from Novartis, Pfizer, Boehringer Ingelheim, Bayer, Novo Nordisk, Astra Zeneca, and Eli Lilly. C.M. received personal fees from Servier, Boehringer Ingelheim, AstraZeneca, Bayer, Bristol‐Myers Squibb, Novartis, Berlin Chemie, and Daiichi Sankyo. H.S. received speaker honoraria from Servier, Novartis, Boehringer, and Astra Zeneca. A.C.S. received personal fees (honoraria, grants, and travel expenses) from Novartis, Servier, Vifor, MSD, Astra Zeneca, Abbott, and Cytokinetics. C.G.T. was funded by a “Riceca di Ateneo/Federico II University” grant. A.J.S.C. has received fees from Astra Zeneca, Bayer, Menarini, Novartis, Nutricia, Servier, Vifor, Actimed, Cardiac Dimensions, CVRx, Enopace, Faraday, Gore, Respicardia, Stealth Peptides, and V‐Wave. A.R.L. has received speaker, advisory board or consultancy fees, and/or research grants from Pfizer, Novartis, Servier, Amgen, Takeda, Roche, Janssens‐Cilag Ltd, Clinigen Group, Eli Lily, Eisai, Bristol Myers Squibb, Ferring Pharmaceuticals, and Boehringer Ingelheim. All other authors report no conflict of interest.
Supporting information
Table S1. Cardio‐Oncology sessions during “Heart Failure and World Congress on Acute Heart Failure 2019”.
Anker, M. S. , Hadzibegovic, S. , Lena, A. , Belenkov, Y. , Bergler‐Klein, J. , de Boer, R. A. , Farmakis, D. , von Haehling, S. , Iakobishvili, Z. , Maack, C. , Pudil, R. , Skouri, H. , Cohen‐Solal, A. , Tocchetti, C. G. , Coats, A. J. S. , Seferović, P. M. , Lyon, A. R. , and for the Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology (2019) Recent advances in cardio‐oncology: a report from the ‘Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019’. ESC Heart Failure, 6: 1140–1148. 10.1002/ehf2.12551.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOSCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, Forsythe L, Scoppa S, Hachey M, Rowland JH. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev 2013; 22: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, Chung W, Oh KH, Jung JY. Chronic kidney disease in cancer patients: an independent predictor of cancer‐specific mortality. Am J Nephrol 2011; 33: 121–130. [DOI] [PubMed] [Google Scholar]
- 4. Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol 2010; 23: 253–262. [PMC free article] [PubMed] [Google Scholar]
- 5. Hampel PJ, Chaffee KG, King RL, Simonetto D, Larson MC, Achenbach S, Call TG, Ding W, Kenderian SS, Leis JF, Chanan‐Khan AA, Bowen DA, Conte MJ, Schwager SM, Hanson CA, Slager SL, Kay NE, Shanafelt TD, Parikh SA. Liver dysfunction in chronic lymphocytic leukemia: prevalence, outcomes, and pathological findings. Am J Hematol 2017; 92: 1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol 2013; 2013: 815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment‐induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol 2012; 25: 106–118. [PMC free article] [PubMed] [Google Scholar]
- 8. Gibson RJ, Keefe DM, Lalla RV, Bateman E, Blijlevens N, Fijlstra M, King EE, Stringer AM, van der Velden WJ, Yazbeck R, Elad S. Bowen JM; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 2013; 21: 313–326. [DOI] [PubMed] [Google Scholar]
- 9. Rodgers GM, Becker PS, Blinder M, Cella D, Chanan‐Khan A, Cleeland C, Coccia PF, Djulbegovic B, Gilreath JA, Kraut EH, Matulonis UA, Millenson MM, Reinke D, Rosenthal J, Schwartz RN, Soff G, Stein RS, Vlahovic G, Weir AB. Cancer‐ and chemotherapy‐induced anemia. J Natl Compr Canc Netw 2012; 10: 628–653. [DOI] [PubMed] [Google Scholar]
- 10. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer‐related anemia. Am J Hematol 2014; 89: 203–212. [DOI] [PubMed] [Google Scholar]
- 11. Neefjes ECW, van den Hurk RM, Blauwhoff‐Buskermolen S, van der Vorst MJDL, Becker‐Commissaris A, de van der Schueren MAE, Buffart LM, Verheul HMW. Muscle mass as a target to reduce fatigue in patients with advanced cancer. J Cachexia Sarcopenia Muscle 2017; 8: 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bohlen J, McLaughlin SL, Hazard‐Jenkins H, Infante AM, Montgomery C, Davis M, Pistilli EE. Dysregulation of metabolic‐associated pathways in muscle of breast cancer patients: preclinical evaluation of interleukin‐15 targeting fatigue. J Cachexia Sarcopenia Muscle 2018; 9: 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rolston KV. Infections in cancer patients with solid tumors: a review. Infect Dis Ther 2017; 6: 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cole CL, Kleckner IR, Jatoi A, Schwarz EM, Dunne RF. The role of systemic inflammation in cancer‐associated muscle wasting and rationale for exercise as a therapeutic intervention. JCSM Clin Rep 2018; 3. [PMC free article] [PubMed] [Google Scholar]
- 15. Molfino A, Iannace A, Colaiacomo MC, Farcomeni A, Emiliani A, Gualdi G, Laviano A, Rossi FF. Cancer anorexia: hypothalamic activity and its association with inflammation and appetite‐regulating peptides in lung cancer. J Cachexia Sarcopenia Muscle 2017; 8: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodriguez AM, Braverman J, Aggarwal D, Friend J, Duus E. The experience of weight loss and its associated burden in patients with non‐small cell lung cancer: results of an online survey. JCSM Clin Rep 2017; 2. [Google Scholar]
- 17. Lena A, Coats AJS, Anker MS. Metabolic disorders in heart failure and cancer. ESC Heart Fail 2018; 5: 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long‐term outcoes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle 2018; 9: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberto A, Deandrea S, Greco MT, Corli O, Negri E, Pizzuto M, Ruggeri F. Prevalence of neuropathic pain in cancer patients: pooled estimates from a systematic review of published literature and results from a survey conducted in 50 Italian palliative care centers. J Pain Symptom Manage 2016; 51: 1091–1102.e4. [DOI] [PubMed] [Google Scholar]
- 20. Nijs J, Leysen L, Adriaenssens N, Aguilar Ferrándiz ME, Devoogdt N, Tassenoy A, Ickmans K, Goubert D, van Wilgen CP, Wijma AJ, Kuppens K, Hoelen W, Hoelen A, Moloney N, Meeus M. Pain following cancer treatment: guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol 2016; 55: 659–663. [DOI] [PubMed] [Google Scholar]
- 21. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 1. J Am Coll Cardiol 2017; 70: 2536–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anker MS, Lena A, Hadzibegovic S, Belenkov Y, Bergler‐Klein J, Boer RA, Cohen‐Solal A, Farmakis D, Haehling S, López‐Fernández T, Pudil R, Suter T, Tocchetti CG, Lyon AR. and for the Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology. Modern‐day cardio‐oncology: a report from the ‘Heart Failure and World Congress on Acute Heart Failure 2018’. ESC Heart Fail 2018; 5: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2768–2801. [DOI] [PubMed] [Google Scholar]
- 24. Fornaro A, Olivotto I, Rigacci L, Ciaccheri M, Tomberli B, Ferrantini C, Coppini R, Girolami F, Mazzarotto F, Chiostri M, Milli M, Marchionni N, Castelli G. Comparison of long‐term outcome in anthracycline‐related versus idiopathic dilated cardiomyopathy: a single centre experience. Eur J Heart Fail 2018; 20: 898–906. [DOI] [PubMed] [Google Scholar]
- 25. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P, Buchan I, Hannaford PC, Myint PK. Do patients have worse outcomes in heart failure than in cancer? A primary care‐based cohort study with 10‐year follow‐up in Scotland. Eur J Heart Fail 2017; 19: 1095–1104. [DOI] [PubMed] [Google Scholar]
- 26. Kreusser MM, Tschierschke R, Beckendorf J, Baxmann T, Frankenstein L, Dösch AO, Schultz JH, Giannitsis E, Pleger ST, Ruhparwar A, Karck M, Katus HA, Raake PW. The need for dedicated advanced heart failure units to optimize heart failure care: impact of optimized advanced heart failure unit care on heart transplant outcome in high‐risk patients. ESC Heart Fail 2018; 5: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison JM, Davis MA, Barton DL, Janz NK, Pressler SJ, Friese CR. Functional status and quality of life among breast cancer survivors with heart failure: results of the Medicare Health Outcomes Survey. Support Care Cancer 2017; 25: 2463–2473. [DOI] [PubMed] [Google Scholar]
- 28. Saitoh M, Dos Santos MR, Emami A, Ishida J, Ebner N, Valentova M, Bekfani T, Sandek A, Lainscak M, Doehner W, Anker SD, von Haehling S. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). ESC Heart Fail 2017; 4: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riley JP, Beattie JM. Palliative care in heart failure: facts and numbers. ESC Heart Fail 2017; 4: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Costanzo MR, Ronco C, Abraham WT, Agostoni P, Barasch J, Fonarow GC, Gottlieb SS, Jaski BE, Kazory A, Levin AP, Levin HR, Marenzi G, Mullens W, Negoianu D, Redfield MM, Tang WHW, Testani JM, Voors AA. Extracorporeal ultrafiltration for fluid overload in heart failure: current status and prospects for further research. J Am Coll Cardiol 2017; 69: 2428–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Haehling S. Co‐morbidities in heart failure beginning to sprout‐and no end in sight? Eur J Heart Fail 2017; 19: 1566–1568. [DOI] [PubMed] [Google Scholar]
- 32. Platz E, Jhund PS, Claggett BL, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Solomon SD, McMurray JJ. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: recurrent hospitalizations and mortality. Eur J Heart Fail 2018; 20: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salem K, ElKhateeb O. Gender‐adjusted and age‐adjusted economic burden of congestive heart failure: cost and disability‐adjusted life‐year analysis. ESC Heart Fail 2017; 4: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hess CN, Roe MT. Treatment of coronary artery disease in cancer survivors: an emerging challenge. Coron Artery Dis 2017; 28: 1–2. [DOI] [PubMed] [Google Scholar]
- 35. Farmakis D, Parissis J, Filippatos G. Insights into onco‐cardiology: atrial fibrillation in cancer. J Am Coll Cardiol 2014; 63: 945–953. [DOI] [PubMed] [Google Scholar]
- 36. Colt JS, Schwartz K, Graubard BI, Davis D, Ruterbusch J, DiGaetano R, Purdue M, Rothman N, Wacholder S, Chow WH. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011; 134: 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Nisio M, Ferrante N, Feragalli B, De Tursi M, Iacobelli S, Cuccurullo F, Porreca E. Arterial thrombosis in ambulatory cancer patients treated with chemotherapy. Thromb Res 2011; 127: 382–383. [DOI] [PubMed] [Google Scholar]
- 38. Hering D, Faber L, Horstkotte D. Echocardiographic features of radiation‐associated valvular disease. Am J Cardiol 2003; 92: 226–230. [DOI] [PubMed] [Google Scholar]
- 39. Limsuwan A, Pakakasama S, Rochanawutanon M, Hong‐eng S. Pulmonary arterial hypertension after childhood cancer therapy and bone marrow transplantation. Cardiology 2006; 105: 188–194. [DOI] [PubMed] [Google Scholar]
- 40. Plummer C, Henderson RD. O´Sullivan JD and Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke 2011; 42: 2410–2418. [DOI] [PubMed] [Google Scholar]
- 41. Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015; 125: 901–906. [DOI] [PubMed] [Google Scholar]
- 42. Chow EJ, Leger KJ, Bhatt NS, Mulrooney DA, Ross CJ, Aggarwal S, Bansal N, Ehrhardt MJ, Armenian SH, Scott JM, Hong B. Paediatric cardio‐oncology: epidemiology, screening, prevention, and treatment. Cardiovasc Res 2019; 115: 922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Serrano C, Cortes J, De Mattos‐Arruda L, Bellet M, Gomez P, Saura C, Perez J, Vidal M, Munoz‐Couselo E, Carreras MJ, Sanchez‐Olle G, Tabernero J, Baselga J, Di Cosimo S. Trastuzumab‐related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol 2012; 23: 897–902. [DOI] [PubMed] [Google Scholar]
- 44. Anker MS, von Haehling S, Landmesser U, Coats AJS, Anker SD. Cancer and heart failure‐more than meets the eye: common risk factors and co‐morbidities. Eur J Heart Fail 2018; 20: 1382–1384. [DOI] [PubMed] [Google Scholar]
- 45. Lenneman CG, Sawyer DB. Cardio‐oncology: an update on cardiotoxicity of cancer‐related treatment. Circ Res 2016; 118: 1008–1020. [DOI] [PubMed] [Google Scholar]
- 46. Farmakis D, Keramida K, Filippatos G. How to build a cardio‐oncology service? Eur J Heart Fail 2018; 20: 1732–1734. [DOI] [PubMed] [Google Scholar]
- 47. Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH, Goodell MA, Taegtmeyer H. Oncometabolite d‐2‐hydroxyglutarate impairs α‐ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A 2016; 113: 10436–10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003; 97: 2869–2879. [DOI] [PubMed] [Google Scholar]
- 49. Boerman LM, Maass SWMC, van der Meer P, Gietema JA, Maduro JH, Hummel YM, Berger MY, de Boeck GH, Berendsen AJ. Long‐term outcome of cardiac function in a population‐based cohort of breast cancer survivors: A cross‐sectional study. Eur J Cancer 2017; 81: 56–65. [DOI] [PubMed] [Google Scholar]
- 50. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016; 133: 1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res 2019; 115: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Boer RA, Meijers WC, van der Meer P, van Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail. 2019. https://doi.org/10.1002/ejhf.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group . Antinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 54. Chabner BA, Nabel CS. Canakinumab and lung cancer: intriguing, but is it real? Oncologist 2018; 23: 637–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, CANTOS Trial Group . Effect of interleukin‐1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet 2017; 390: 1833–1842. [DOI] [PubMed] [Google Scholar]
- 56. Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, Sandek A, Valentova M, Stojakovic T, Scharnagl H, Riess H, Anker SD, von Haehling S. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol 2014; 64: 1310–1319. [DOI] [PubMed] [Google Scholar]
- 57. Anker MS, Ebner N, Hildebrandt B, Springer J, Sinn M, Riess H, Anker SD, Landmesser U, Haverkamp W, von Haehling S. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non‐small cell lung cancer: results of a prospective cardiovascular long‐term study. Eur J Heart Fail 2016; 18: 1524–1534. [DOI] [PubMed] [Google Scholar]
- 58. Lee DH, Park S, Lim SM, Lee MK, Giovannucci EL, Kim JH, Kim SI, Jeon JY. Resting heart rate as a prognostic factor for mortality in patients with breast cancer. Breast Cancer Res Treat 2016; 159: 375–384. [DOI] [PubMed] [Google Scholar]
- 59. Park J, Kim JH, Park Y, Park SJ, Cheon JH, Kim WH, Park JS, Jeon JY, Kim TI. Resting heart rate is an independent predictor of advanced colorectal adenoma recurrence. PLoS One 2018; 13: e0193753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Bilsen M, Patel HC, Bauersachs J, Böhm M, Borggrefe M, Brutsaert D, Coats AJS, de Boer RA, de Keulenaer GW, Filippatos GS, Floras J, Grassi G, Jankowska EA, Kornet L, Lunde IG, Maack C, Mahfoud F, Pollesello P, Ponikowski P, Ruschitzka F, Sabbah HN, Schultz HD, Seferovic P, Slart RHJA, Taggart P, Tocchetti CG, Van Laake LW, Zannad F, Heymans S, Lyon AR. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2017; 19: 1361–1378. [DOI] [PubMed] [Google Scholar]
- 61. López‐Fernández T, Martín García A, Santaballa Beltrán A, Montero Luis Á, García Sanz R, Mazón Ramos P, Velasco Del Castillo S, López de Sá Areses E, Barreiro‐Pérez M, Hinojar Baydes R, Pérez de Isla L, Valbuena López SC, Dalmau González‐Gallarza R, Calvo‐Iglesias F, González Ferrer JJ, Castro Fernández A, González‐Caballero E, Mitroi C, Arenas M, Virizuela Echaburu JA, Marco Vera P, Íñiguez Romo A, Zamorano JL, Plana Gómez JC, López Sendón Henchel JL. Cardio‐onco‐hematology in clinical practice. Position paper and recommendations. Rev Esp Cardiol (Engl Ed) 2017; 70: 474–486. [DOI] [PubMed] [Google Scholar]
- 62. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013; 61: 77–84. [DOI] [PubMed] [Google Scholar]
- 63. Tamborini G, Piazzese C, Lang RM, Muratori M, Chiorino E, Mapelli M, Fusini L, Ali SG, Gripari P, Pontone G, Andreini D, Pepi M. Feasibility and accuracy of automated software for transthoracic three‐dimensional left ventricular volume and function analysis: comparisons with two‐dimensional echocardiography, three‐dimensional transthoracic manual method, and cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2017; 30: 1049–1058. [DOI] [PubMed] [Google Scholar]
- 64. Zhang KW, Finkelman BS, Gulati G, Narayan HK, Upshaw J, Narayan V, Plappert T, Englefield V, Smith AM, Zhang C, Hundley WG, Ky B. Abnormalities in 3‐dimensional left ventricular mechanics with anthracycline chemotherapy are associated with systolic and diastolic dysfunction. JACC Cardiovasc Imaging 2018; 11: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Negishi T, Thavendiranathan P. Negishi K and Marwick TH; SUCCOUR investigators. Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: The SUCCOUR trial. JACC Cardiovasc Imaging 2018; 11: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 66. Lopez‐Fernandez T, Thavendiranathan P. Emerging cardiac imaging modalities for the early detection of cardiotoxicity due to anticancer therapies. Rev Esp Cardiol 2017; 70: 487–495. [DOI] [PubMed] [Google Scholar]
- 67. Keramida K, Farmakis D, Bingcang J, Sulemane S, Sutherland S, Bingcang RA, Ramachandran K, Tzavara C, Charalampopoulos G, Filippiadis D, Kouris N, Nihoyannopoulos P. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail 2019; 21: 529–535. [DOI] [PubMed] [Google Scholar]
- 68. Harries I, Biglino G, Baritussio A, De Garate E, Dastidar A, Plana J and Bucciarelli‐Ducci C4 . Long term cardiovascular magnetic resonance phenotyping of anthracycline cardiomyopathy. Int J Cardiol. 2019. pii: S0167‐5273(19)30293‐1. [DOI] [PubMed] [Google Scholar]
- 69. Cardinale D, Biasillo G, Salvatici M, Sandri MT, Cipolla CM. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert Rev Mol Diagn 2017; 17: 245–256. [DOI] [PubMed] [Google Scholar]
- 70. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, Ganame J, Sebag IA, Angler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation od adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014; 27: 911–939. [DOI] [PubMed] [Google Scholar]
- 71. Sandri MT, Salvatici M, Cardinale D, Zorzino L, Passerini R, Lentati P, Leon M, Civell M, Martinelli G, Cipolla CM. N‐terminal pro‐B‐type natriuretic peptide after high‐dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem 2005; 51: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 72. Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014; 63: 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Todorova VK, Makhoul I, Wei J, Klimberg VS. Circulating miRNA profiles of doxorubicin‐induced cardiotoxicity in breast cancer patients. Ann Clin Lab Sci 2017; 47: 116–119. [PubMed] [Google Scholar]
- 74. Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio‐oncology. Mayo Clin Proc 2014; 89: 1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lotrionte M, Biondi‐Zoccai G, Abbate A, Lanzetta G, D'Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta‐analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol 2013; 112: 1980–1984. [DOI] [PubMed] [Google Scholar]
- 76. Cavarretta E, Mastroiacovo G, Lupieri A, Frati G, Peruzzi M. The positive effects of exercise in chemotherapy‐related cardiomyopathy. Adv Exp Med Biol 2017; 1000: 103–129. [DOI] [PubMed] [Google Scholar]
- 77. Howden EJ, Bigaran A, Beaudry R, Fraser S, Selig S, Foulkes S, Antill Y, Nightingale S, Loi S, Haykowsky MJ, La Gerche A. Exercise as a diagnostic and theapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol 2019; 26: 305–315. [DOI] [PubMed] [Google Scholar]
- 78. Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Lopez G, Madan K, Oeffinger KC, Salamone J, Scott JM, Squires RW, Thomas RJ, Treat‐Jacobson DJ, Wright JS, American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease . Cardio‐oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation 2019: 139:e997–e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Asssessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes and trastuzumab. Circ Cardiovasc Imaging 2012; 5: 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME Jr, Ruccione K, Smithson WA, Robison LL. Late mortality experience in five‐year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol 2001; 19: 3163–3172. [DOI] [PubMed] [Google Scholar]
- 81. Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol 2012; 30: 3657–3664. [DOI] [PubMed] [Google Scholar]
- 82. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015; 131: 1981–1988. [DOI] [PubMed] [Google Scholar]
- 83. Lancellotti P, Suter TM, Lopez‐Fernandez T, Galderisi M, Lyon AR, Van der Meer P, Cohen Solal A, Zamorano JL, Jerusalem G, Moonen M, Aboyans V, Bax JJ, Asteggiano R. Cardio‐oncology services: rationale, organization, and implementation: a report from the ESC cardio‐oncology council. Eur Heart J 2019; 40: 1756–1763. [DOI] [PubMed] [Google Scholar]
- 84. Pareek N, Cevallos J, Moliner P, Shah M, Tan LL, Chambers V, Baksi AJ, Khattar RS, Sharma R, Rosen SD, Lyon AR. Activity and outcomes of a cardio‐oncology service in the United Kingdom‐a five‐year experience. Eur J Heart Fail 2018; 20: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 85. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio‐oncology and cardio‐oncological prevention. J Natl Cancer Inst 2010; 102: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sarocchi M, Arboscello E, Ghigliotti G, Murialdo R, Bighin C, Gualandi F, Sicbaldi V, Balbi M, Brunelli C, Spallarossa P. Ivabradine in cancer treatment‐related left ventricular dysfunction. Chemotherapy 2018; 63: 315–320. [DOI] [PubMed] [Google Scholar]
- 87. Mertens P, Belien H, Dupont M, Vandervoort P, Mullens W. The reverse remodelling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Therapy 2018; 36: e12435. [DOI] [PubMed] [Google Scholar]
- 88. Heart Rhythm Congress 2019. S‐LBCT02‐04.
- 89.https://clinicaltrials.gov/ct2/show/ NCT02164721
- 90. Hodi FS, Chiarion‐Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez‐Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4‐year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19: 1480–1492. [DOI] [PubMed] [Google Scholar]
- 91. Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez‐Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti‐PD‐1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non‐small‐cell lung cancer (CheckMate 063): a phase 2, single‐arm trial. Lancet Oncol 2015; 16: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, Lyon AR, Padera RF, Johnson DB, Moslehi J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019; 115: 854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moslehi JJ, Salem JE, Sosman JA, Lebrun‐Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor‐associated myocarditis. Lancet 2018; 391: 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 2018; 19: 447–458. [DOI] [PubMed] [Google Scholar]
- 95. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, Lyon AR, Padera RF, Johnson DB, Moslehi J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019; 115: 854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD‐1 receptor‐deficient mice. Science (New York, NY) 2001; 291: 319–322. [DOI] [PubMed] [Google Scholar]
- 97. Varricchi G, Marone G, Mercurio V, Galdiero MR, Bonaduce D, Tocchetti CG. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Curr Med Chem 2018; 25: 1327–1339. [DOI] [PubMed] [Google Scholar]
- 98. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, Magrini E, Gianni F, Kunderfranco P, Polentarutti N, Pasqualini F, Di Marco S, Supino D, Peano C, Cananzi F, Colombo P, Pilotti S, Alomar SY, Bonavita E, Galdiero MR, Garlanda C, Mantovani A, Jaillon S. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell 2019; 178: 346–360.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li M, Sala V, De Santis MC, Cimino J, Cappello P, Pianca N, Di Bona A, Margaria JP, Martini M, Lazzarini E, Pirozzi F, Rossi L, Franco I, Bornbaum J, Heger J, Rohrbach S, Perino A, Tocchetti CG, Lima BHF, Teixeira MM, Porporato PE, Schulz R, Angelini A, Sandri M, Ameri P, Sciarretta S, Lima‐Júnior RCP, Mongillo M, Zaglia T, Morello F, Novelli F, Hirsch E, Ghigo A. Phosphoinositide 3‐kinase gamma inhibition protects from anthracycline cardiotoxicity and reduces tumor growth. Circulation 2018; 138: 696–711. [DOI] [PubMed] [Google Scholar]
- 101. Segers VFM, Brutsaert DL, DeKeulenaer GW. Cardiac remodelling: endothelial cells have more to say than just no. Front Phsysiol 2018; 9: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kivelä R, Hemanthakumar KA, Vaparanta K, Robciuc M, Izumiya Y, Kidoya H, Takakura N, Peng X, Sawyer DB, Elenius K, Walsh K, Alitalo K. Endothelial cells regulate physiological cardiomyocyte growth via VEGFR2‐mediated paracrine signaling. Circulation 2019; 139: 2570–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bertero E, Canepa M, Maack C, Ameri P. Linking heart failure to cancer. Circulation 2018; 138: 735–742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cardio‐Oncology sessions during “Heart Failure and World Congress on Acute Heart Failure 2019”.


