Abstract
Aims
The study aims to evaluate the prognostic significance of impaired glucose tolerance (IGT) with reference to albuminuria in patients with chronic heart failure (CHF).
Methods and results
We examined 535 CHF patients (mean 66 years, women 25%) in the control arm of our SUPPORT trial, in which we examined additive impact of olmesartan in hypertensive patients with symptomatic CHF treated with β‐blockers and/or angiotensin‐converting enzyme inhibitors. We examined the association between glycaemic abnormality (assessed by 75 g of oral glucose tolerance test) and albuminuria for a composite outcome of all‐cause death, myocardial infarction, stroke, and HF hospitalization. IGT patients (N = 113, mean 67.2 years) were older and more frequently treated with β‐blockers compared with those with normal glucose regulation (N = 142, mean 64.0 years) and those with diabetes mellitus (N = 280, mean 65.7 years). Multivariable Cox proportional hazard models revealed that, as compared with normal glucose regulation (NGR), IGT was associated with increased risk of the outcome when complicated by albuminuria [hazard ratio (HR) 2.25; 95% confidence interval (CI) 1.14–4.42; P = 0.019] but not when uncomplicated by albuminuria (HR 0.76; 95% CI 0.35–1.60, P = 0.47) (P for interaction = 0.041). This was also the case for diabetes mellitus and albuminuria (HR 2.06; 95% CI 1.17–3.61; P = 0.012). Among IGT patients without albuminuria, 21 (29%) developed albuminuria at 1‐year visit, which was again associated with poor prognosis (HR 7.36; 95% CI 1.39–38.98, P = 0.019).
Conclusions
These results indicate that IGT is associated with poor prognosis when complicated by albuminuria in CHF patients, demonstrating the importance of combined early stages of glucose intolerance and renal dysfunction in the management of CHF.
Keywords: Chronic heart failure, Impaired glucose tolerance, Diabetes mellitus, Albuminuria
1. Introduction
Glucose regulation abnormalities, including impaired glucose tolerance (IGT), are commonly noted in non‐diabetic patients with chronic heart failure (CHF).1, 2, 3, 4, 5 However, it remains unknown whether IGT itself is associated with poor prognosis, although a previous study suggested the correlation between IGT and HF severity.6 Furthermore, IGT is usually assessed by baseline serum glucose,4, 7 insulin,6 and haemoglobin A1c (HbA1c) levels8 but not necessarily by oral glucose tolerance test (OGTT), which has advantages for accurate assessment of glucose regulation.8
Accumulating evidence also indicates that albuminuria is associated with insulin resistance in patients with Types I9 and II10 diabetes mellitus (DM) as well as in non‐DM subjects,11 suggesting that it is also the case in patients with IGT. However, it is still unclear whether IGT contributes to the development for albuminuria. This issue is clinically important in the management of CHF patients with IGT, because we found that not only macroalbuminuria but also microalbuminuria is significantly associated with increased mortality in CHF patients.12 Furthermore, it has been recently shown that in Type I DM patients, myocardial deformation assessed by echocardiography was primarily associated with the presence of albuminuria,13 suggesting that combination of abnormalities of glucose regulation and renal function may impair cardiac function in CHF patients. However, the prognostic value of combined IGT and albuminuria remains to be examined. In the present study, we thus aimed to evaluate the prognostic significance of IGT in relation with albuminuria in CHF patients, as a subanalysis study of our SUPPORT (supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan) trial.15, 16
2. Methods
2.1. Study subjects
The present study is a post hoc, exploratory analysis, using the dataset of our SUPPORT trial.15, 16 The trial demonstrated that additive use of olmesartan did not improve clinical outcomes and that addition of olmesartan to the combination therapy with angiotensin‐converting enzyme inhibitors and β‐blockers was associated with increased adverse cardiac events in CHF patients.14 Thus, among 1147 patients of our SUPPORT trial, we enrolled 569 patients in the control arm in the present study. Of the 569 patients enrolled, we excluded 19 patients with impaired fasting glucose and finally enrolled 142 with normal glucose regulation (NGR), 113 with IGT, 280 with DM (277 known DM and three newly diagnosed DM) (Figure 1).
Figure 1.
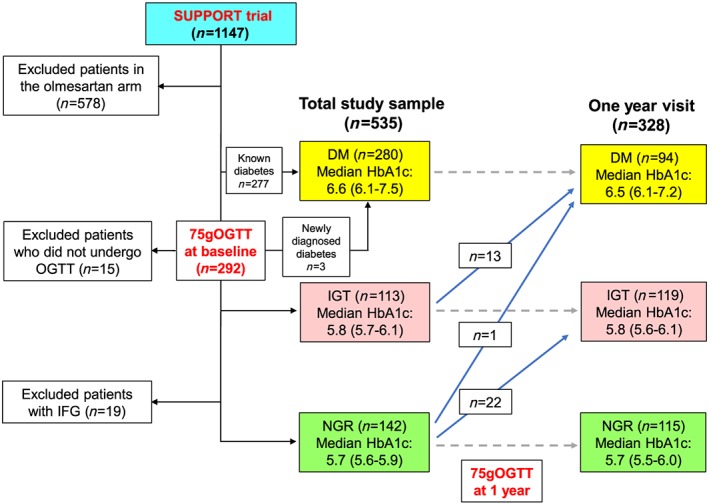
Study diagram. DM, diabetes mellitus; HbA1c, haemoglobin A1c; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NGR, normal glucose regulation; OGTT, oral glucose tolerance test.
2.2. The SUPPORT trial
The SUPPORT trial (NCT00417222) was a randomized clinical trial that evaluated the efficacy and safety of olmesartan in stable CHF patients with a history of hypertension who had been treated with angiotensin‐converting enzyme inhibitors and/or β‐blockers or both at enrolment.14, 15 In the SUPPORT trial, patients were eligible if they met all the following criteria: (i) New York Heart Association (NYHA) Classes II to IV CHF, (ii) history of hypertension or treated with anti‐hypertensive medications, (iii) aged 20 to <80 years, (iv) stable with angiotensin‐converting enzyme inhibitors and/or β‐blockers, and (v) not treated with angiotensin II receptor blockers. Exclusion criteria were (i) patients with renal dysfunction (serum creatinine ≥3.0 mg/dL) or those who were under chronic haemodialysis, (ii) drug hypersensitivity to olmesartan, (iii) severe liver dysfunction, (iv) history of angioedema, (v) history of malignant tumour or life‐threatening illness of poor prognosis, (vi) pregnant or possibly pregnant patients, (vii) cardiovascular surgery within 6 months prior to the date of the entry, (viii) acute myocardial infarction within 6 months prior to the date of the entry, and (ix) percutaneous coronary intervention with or without stent implantation within 6 months prior to the date of the entry.14, 15
The trial was approved by institutional review boards at each institution, and all participants provided written informed consent. The trial was conducted according to the Declaration of Helsinki. The primary objective of the trial was to examine whether an additive treatment with an angiotensin II receptor blocker, olmesartan, would reduce the mortality and morbidity of chronic HF patients with a history of hypertension. Between October 2006 and March 2010, a total of 1147 CHF patients with a history of hypertension were assigned to either the olmesartan or the control group (standard care), according to a 1:1 ratio of olmesartan to control and were followed up until the study ended on 31 March 2013.
2.3. Definitions of impaired glucose tolerance, diabetes mellitus, and albuminuria
In the SUPPORT trial, patients without known DM underwent a standardized procedure of 75 g of OGTT twice at enrolment [median (IQR) 5 (0–33) days from randomization] and 1‐year follow‐up visit [median (IQR) 364 (339–399) days after randomization] (Figure 1).16 The abnormalities in glucose tolerance were defined according to the 2006 WHO criteria8; newly diagnosed DM as fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or 2 h plasma glucose ≥200 mg/dL (11.1 mmol/L); IGT as fasting plasma glucose <126 mg/dL (7.0 mmol/L) and 2 h plasma glucose ≥140 mg/dL but <200 mg/dL (7.8 and 11.1 mmol/L); impaired fasting glucose as fasting plasma glucose 110–125 mg/dL (6.1–6.9 mmol/L) and 2 h plasma glucose <140 mg/dL (7.8 mmol/L). In the morning after a 9 h fast, blood and urine samples were collected at each participating site. Blood samples were centrifuged within 30 min at 4°C and were stored at −20°C until assay. Urine samples were stored at 4°C until assay. Urine and blood samples were measured in a central laboratory (SRL, Tokyo, Japan), and urinary albumin‐to‐creatinine ratio (UACR) was calculated to compensate for variations in urine concentration in spot‐check samples. Albuminuria was defined as UACR >30 mg/g to describe urine albumin levels not detected by a dipstick test.17
2.4. Outcome
The study outcome was a composite of all‐cause death, non‐fatal myocardial infraction (MI), non‐fatal stroke, and hospitalization for worsening HF, which was identical to the SUPPORT trial.15, 16 All‐cause death was adjudicated by the death certificate and/or the medical records. The definitions of MI and stroke were based on the 2001 ACC key data elements and definitions.18 A hospitalization for worsening HF was defined as an admission to a hospital necessitated by HF and primarily for its treatment or when HF became a major component of the patient's hospitalization.19 A patient hospitalized for HF had to show signs and symptoms of worsening HF and require treatment with intravenous diuretics. Evidence of worsening HF included at least one of the following items: increasing dyspnoea on exertion, orthopnoea, nocturnal dyspnoea, pulmonary oedema, increasing peripheral oedema, increasing fatigue or decreasing exercise tolerance, renal hypoperfusion (e.g. worsening renal function), raised jugular venous pressure, and radiological signs of HF.19 The independent events committee adjudicated all events for all‐cause death, non‐fatal MI, non‐fatal stroke, and hospitalization for worsening HF in a blinded fashion (SI, NI, and YM) in the SUPPORT trial.
2.5. Statistical methods
Clinical characteristics are described stratified by NGR, IGT, and DM. For comparing two groups, we used t‐test for normally distributed and Wilcoxon rank sum for non‐normally distributed data. For three or more groups, we used ANOVA for normally distributed and Kruskal–Wallis for non‐normally distributed data. We examined determinants of albuminuria or IGT by multivariable logistic regression models with comparison of backward and forward selection methods considering the following variables at baseline as candidate: IGT (for the determinant of albuminuria), diabetes, albuminuria (for the determinant of IGT), age, sex, NYHA class, systolic and diastolic blood pressure (BP), heart rate, body mass index (BMI), smoking, ischaemic aetiology, history of HF hospitalization, atrial fibrillation, history of cancer, haemoglobin, estimated glomerular filtration rate, left ventricular ejection fraction (LVEF), left ventricular mass index (LVMI), and brain natriuretic peptide (BNP). The final model was determined by the lowest value of Akaike information criterion. Event rates (per 1000 person‐years) were calculated for the composite of all‐cause death, non‐fatal MI, non‐fatal stroke, and hospitalization for worsening HF. Event rates were analysed with the exact probability test. For time to first event analysis, we plotted Kaplan–Meier survival curves and employed multivariable Cox proportional hazard models adjusted for age, sex, ischaemic aetiology, NYHA functional class, systolic BP, heart rate, BMI, LVEF, history of HF hospitalization, and BNP.20, 21 We compared the risk between NGR and DM patients, without considering the presence or absence of albuminuria. Then, we examined the risk of DM considering the presence or absence of albuminuria, where NGR without albuminuria served as a reference. We also assessed the prognostic impact of combined glycaemic status and albuminuria stratified by baseline LVEF (≥50%, preserved; ≥40 to <50%, mid‐range; and < 40%, reduced).
To estimate the survival probabilities in unbiased way, we performed landmark analysis stratified by status of albuminuria at 1‐year visit evaluated by second urinalysis, setting date at 1‐year visit as observation Time 0.22 A two‐sided P value of <0.05 was considered to be statistically significant. All analyses were performed with STATA 15 (College Station, TX).
3. Results
3.1. Baseline characteristics
Of 535 patients, the mean age was 65.6 ± 10.0 years, 25% were women, 93% had NYHA Class II, and 82% LVEF > 40% (Table 1). The median (IQR) BNP level was 78.2 (38–174) pg/mL. Median (IQR) HbA1c levels (%) were 5.7 (5.6, 5.9) in NGR, 5.8 (5.7, 6.1) in IGT, and 6.6 (6.1, 7.5) % in DM (Table 1). Patients with IGT, as compared with those with NGR and DM, were older and more likely to be treated with β‐blockers (Table 1). Median (IQR) UACR levels (mg/g) were 12.6 (6.2, 42.9) in NGR, 14.9 (8.7, 46.1) in IGT, and 26.8 (10.9, 93.0) in DM (P < 0.001). Albuminuria was noted in 47 (33%), 41 (36%), and 134 (48%) in NGR, IGT, and diabetes, respectively (P = 0.007) (Table 1 and Supporting Information, Table S1). Patients with DM and albuminuria had higher UACR [median (IQR): 103.0 (53.0, 294.0)] than those with IGT and albuminuria [77.5 (45.8, 165.0), P = 0.11] (Supporting Information, Table S1). Across the categories, patients with albuminuria had higher prevalence of atrial fibrillation, larger left atrial dimension, and higher BNP levels.
Table 1.
Baseline characteristics
| Overall (N = 535) | NGR (N = 142) | IGT (N = 113) | DM (N = 280) | P value | |
|---|---|---|---|---|---|
| Age, years | 65.6 ± 10.0 | 64.0 ± 11.8 | 67.2 ± 8.0* | 65.7 ± 9.7 | 0.035 |
| Women, n (%) | 136 (25%) | 41 (29%) | 34 (30%) | 61 (22%) | 0.13 |
| NYHA, n (%) | 0.37 | ||||
| II | 499 (93%) | 130 (92%) | 105 (93%) | 264 (95%) | |
| III | 33 (6%) | 12 (9%) | 8 (7%) | 13 (5%) | |
| Systolic BP, mmHg | 127 ± 18 | 124 ± 18 | 129 ± 19* | 128 ± 18 | 0.02 |
| Diastolic BP, mmHg | 74 ± 12 | 73 ± 11 | 75 ± 11 | 74 ± 12 | 0.32 |
| Heart rate, bpm | 71 ± 15 | 71 ± 14 | 71 ± 14 | 72 ± 15 | 0.83 |
| BMI, kg/m2 | 24.6 ± 4.1 | 23.7 ± 3.8 | 24.3 ± 3.7** | 25.3 ± 4.3 | <0.001 |
| Smoking (past or current), n (%) | 272 (48%) | 74 (52%) | 51 (45%) | 147 (53%) | 0.64 |
| Ischaemic heart disease, n (%) | 249 (47%) | 47 (33%) | 47 (42%)** | 155 (55%) | <0.001 |
| History of HF hospitalization, n (%) | 269 (50%) | 69 (49%) | 59 (52%) | 141 (50%) | 0.85 |
| Atrial fibrillation, n (%) | 226 (42%) | 64 (45%) | 52 (46%) | 110 (39%) | 0.34 |
| Cancer, n (%) | 59 (11%) | 19 (13%) | 10 (9%) | 30 (11%) | 0.50 |
| LVEF, % | 53.7 ± 14.5 | 53.9 ± 15.0 | 50.9 ± 14.8** | 54.7 ± 14.1 | 0.06 |
| LVEF categories | 0.015 | ||||
| EF ≥50%, n (%) | 310 (62%) | 85 (60%) | 60 (53%) | 184 (66%) | |
| EF 40–49%, n (%) | 110 (21%) | 35 (25%) | 21 (19%) | 54 (19%) | |
| EF < 40%, n (%) | 96 (18%) | 22 (16%) | 32 (28%) | 42 (15%) | |
| LV mass index, g/m2 | 137.0 ± 43.8 | 141.4 ± 47.0 | 143.6 ± 44.1** | 132.0 ± 41.3 | 0.024 |
| LV geometry | |||||
| Normal LV, n (%) | 35 (7%) | 9 (6%) | 8 (7%) | 18 (7%) | 0.95 |
| Eccentric LVH, n (%) | 216 (41%) | 56 (39%) | 47 (43%) | 113 (41%) | 0.84 |
| Concentric LV remodelling, n (%) | 14 (3%) | 3 (2%) | 0 | 11 (4%) | 0.08 |
| Concentric LVH, n (%) | 134 (25%) | 33 (23%) | 24 (21%) | 77 (28%) | 0.36 |
| LA dimension, mm | 43.0 ± 8.6 | 42.6 ± 9.4 | 43.6 ± 8.4 | 43.0 ± 8.1 | 0.68 |
| E/A ratio | 1.0 ± 0.7 | 1.1 ± 0.7 | 1.0 ± 0.9 | 0.9 ± 0.5 | 0.21 |
| Haemoglobin, g/dL | 13.7 ± 1.8 | 13.5 ± 1.8 | 13.7 ± 1.9 | 13.8 ± 1.9 | 0.61 |
| HbA1c, median (IQR), % | 6.1 (5.7, 6.8) | 5.7 (5.6, 5.9) | 5.8 (5.7, 6.1) | 6.6 (6.1, 7.5) | <0.001 |
| eGFR | 74.7 ± 33.5 | 80.3 ± 40.5 | 75.9 ± 33.7 | 71.4 ± 29.0 | 0.034 |
| CKD stage | 0.42 | ||||
| G1 | 115 (22%) | 39 (28%) | 26 (23%) | 50 (18%) | |
| G2 | 241 (45%) | 59 (42%) | 49 (43%) | 133 (48%) | |
| G3a | 103 (19%) | 26 (18%) | 22 (20%) | 55 (20%) | |
| G3b | 50 (9%) | 15 (11%) | 10 (9%) | 25 (9%) | |
| G4 | 25 (5%) | 3 (2%) | 6 (5%) | 16 (6%) | |
| BNP, median (IQR) | 78.2 (38.1, 174.0) | 73.3 (36.8, 198.5) | 92.7 (52.6, 237.0)** | 70.9 (32.1, 159.0) | 0.011 |
| UACR, median (IQR) | 20.9 (8.4, 65.6) | 12.6 (6.2, 42.9) | 14.9 (8.7, 46.1)** | 26.8 (10.9, 93.0) | <0.001 |
| Albuminuria, n (%) | 222 (42%) | 47 (33%) | 41 (36%)** | 134 (48%) | 0.007 |
| ACEI, n (%) | 431 (81%) | 122 (86%) | 90 (80%) | 219 (78%) | 0.16 |
| β‐blocker, n (%) | 394 (74%) | 102 (72%) | 95 (84%)* , ** | 197 (70%) | 0.017 |
| Ca channel blocker, n (%) | 201 (38%) | 46 (32%) | 42 (37%) | 113 (40%) | 0.28 |
| Loop diuretics, n (%) | 275 (51%) | 66 (47%) | 62 (55%) | 147 (53%) | 0.36 |
| Spironolactone, n (%) | 145 (27%) | 37 (26%) | 33 (29%) | 75 (27%) | 0.84 |
| Thiazide, n (%) | 22 (4%) | 4 (3%) | 4 (4%) | 14 (5%) | 0.53 |
| Statin, n (%) | 262 (49%) | 53 (37%) | 52 (46%) | 157 (56%) | 0.001 |
| Oral diabetes medication, n (%) | 132 (25%) | — | — | 132 (47%) | — |
| Insulin use, n (%) | 28 (5%) | — | — | 28 (10%) | — |
| Aspirin, n (%) | 289 (60%) | 76 (62%) | 62 (61%) | 151 (58%) | 0.73 |
| Warfarin, n (%) | 162 (33%) | 39 (32%) | 33 (32%) | 90 (35%) | 0.84 |
Two patients are missing information on NYHA functional class. Left ventricular dimensions and mass were indexed to body surface area as per European Association of Cardiovascular Imaging (EACI) guidelines.34 Left ventricular hypertrophy (LVH) was defined as LV mass index (LVMI) >115 g/m2 in men or > 95 g/m2 in women. Left ventricular geometry was categorized as normal, concentric remodelling (RWT > 0.42, normal LVMI), concentric hypertrophy (RWT > 0.42, elevated LVMI), or eccentric hypertrophy (RWT < 0.42, elevated LVMI). CKD stage was classified by the recommendation of Kidney Disease: Improving Global Outcomes (KDIGO).35 Baseline characteristics stratified by NGR, IGT, and diabetes, and albuminuria are provided in Supporting Information, Table S1.
ACEI, angiotensin‐converting enzyme inhibitor; BP, blood pressure; BMI, body mass index; BNP, brain natriuretic peptide; DM, diabetes mellitus; HbA1c, haemoglobin A1c; HF, heart failure; LA, left atrium; LVEF, left ventricular ejection fraction; LV, left ventricular; LVH, left ventricular hypertrophy; eGFR, estimated glomerular filtration rate; UACR, urinary albumin‐to‐creatinine ratio.
P < 0.05 between the NGR and IGT subgroups.
P < 0.05 between the IGT and DM subgroups.
3.2. Association between abnormality in glycaemic status, albuminuria and outcome
During the median follow‐up of 4.1 years (IQR: 3.1–5.0 years), 151 patients experienced the composite outcome. Figure 2 shows event rates (per 1000 person‐years) stratified by NGR, IGT, and DM. Patients with DM had numerically, but insignificantly, higher event rate [81.1 (65.5–100.3) per 1000 person‐years] followed by IGT [74.3 (52.8–104.5)] and NGR [71.1 (51.0–97.3)]. When stratified by the presence of albuminuria, event rates were significantly higher in patients with albuminuria compared with those without albuminuria in all the three groups. HRs (95% CI) for the outcome in patients with albuminuria over those without it were 1.00 (0.61–1.64; P = 0.99) in IGT and 1.27 (0.84–1.93; P = 0.24) in DM at baseline after adjustment for potential confounders, including age, sex, ischaemic aetiology, NYHA functional class, systolic BP, heart rate, BMI, LVEF, history of HF hospitalization, and BNP (Table 2 A). Every 1% increase in HbA1c was associated with a 18% increase in the outcome (HR 1.18; 95% CI 1.05–1.33, P = 0.007) after adjustment for age, sex, ischaemic aetiology, NYHA functional class, systolic BP, heart rate, BMI, LVEF, history of HF hospitalization, BNP, and albuminuria (Table 2 A).
Figure 2.
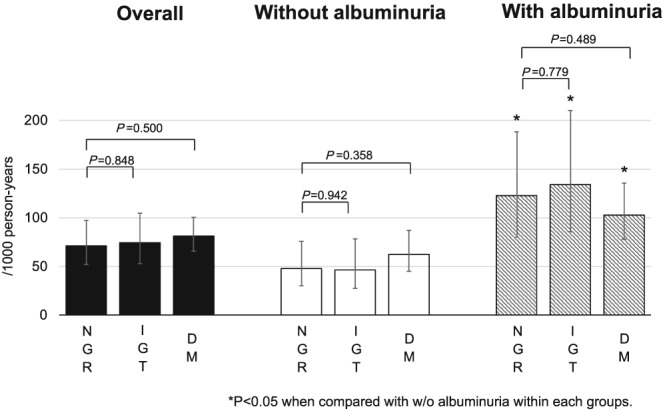
Event rates (per 1000 person‐years) for the composite of all‐cause death, non‐fatal myocardial infraction (MI), non‐fatal stroke, and hospitalization for worsening HF. Event rates were analysed with the exact probability test.
Table 2.
(A) Prognostic significance of glycaemic status, haemoglobin A1c, and albuminuria in the overall samples. (B) Prognostic significance of glycaemic status with and without albuminuria in the overall samples
| (A) Variable | HR (95% CI) | P value | Z score |
|---|---|---|---|
| NGR | Reference | ||
| IGT | 1.00 (0.61–1.64) | 0.99 | 0.00 |
| DM | 1.27 (0.84–1.93) | 0.24 | 1.14 |
| HbA1c (per 1% increase)a | 1.18 (1.05–1.33) | 0.007 | 2.70 |
| Albuminuria | 1.74 (1.22–2.47) | 0.002 | 3.09 |
| (B) Variable (overall, n = 535) | HR (95% CI) | P value | Z score |
|---|---|---|---|
| NGR without albuminuria | Reference | ||
| NGR with albuminuria | 1.77 (0.91–3.45) | 0.09 | 1.6 |
| IGT without albuminuria | 0.76 (0.36–1.60) | 0.47 | −0.72 |
| IGT with albuminuria | 2.25 (1.14–4.42) | 0.019 | 2.34 |
| DM without albuminuria | 1.45 (0.81–2.60) | 0.213 | 1.24 |
| DM with albuminuria | 2.06 (1.17–3.61) | 0.012 | 2.51 |
DM, diabetes mellitus; HbA1c, haemoglobin A1c; HR, hazard ratio; IGT, impaired glucose tolerance; NGR, normal glucose regulation.
DM, diabetes mellitus; HR, hazard ratio; IGT, impaired glucose tolerance; NGR, normal glucose regulation.
Adjusted for age, sex, ischaemic aetiology, NYHA functional class, systolic blood pressure, heart rate, body mass index, LVEF, history of HF hospitalization, BNP, and albuminuria.
Albuminuria at baseline was significantly associated with the outcome (HR 1.74; 95% CI 1.22–2.47; P = 0.002) after adjustment for age, sex, ischaemic aetiology, NYHA functional class, systolic BP, heart rate, BMI, LVEF, history of HF hospitalization, and BNP (Table 2A). In addition, as compared with NGR without albuminuria, HRs of NGR, IGT, and DM with albuminuria were 1.77 (0.91–3.45, P = 0.09), 2.25 (1.14–4.42, P = 0.019), and 2.06 (1.17–3.61, P = 0.012), respectively, whereas there was no significant association between NGR, IGT, and DM without albuminuria (Table 2 B). We also noted significant interaction for the impact on the incidence of the composite endpoint between albuminuria and IGT (P for interaction = 0.041) or DM (P for interaction = 0.001) at baseline.
When stratifying by baseline LVEF (≥50%, preserved; ≥40 to <50%, mid‐range; and < 40%, reduced), in reduced LVEF but not in mid‐range and preserved LVEF subgroups, patients with diabetes and albuminuria and those with IGT and albuminuria tended to be associated with poor prognosis (Supporting Information, Table S3 ).
Of 328 patients, 14 (4.3%, 13 in IGT and one in NGR) and 22 patients (6.7%, all in NGR) were newly diagnosed as having DM [11.2 (6.6–18.8)/1000 person‐years] and IGT [16.7 (95% CI 10.9–25.7)/1000 person‐years], respectively, at 1‐year visit [median (IQR) 364 (339–399) days from randomization] (Figure 1). At 1‐year follow‐up, 90 patients [25 (19.5%) in NGR, 19 (17.8%) in IGT, and 46 (18.0%) in DM] developed to albuminuria. In overall cohort, patients who developed albuminuria at 1 year had similar incidence of the composite outcome compared with those with known albuminuria at baseline (Supporting Information, Figure S1A). When stratified by the presence of albuminuria at 1 year, NGR, IGT, and DM were associated with poor prognosis in patients with NGR (2.97; 95% CI 1.02–8.64; P = 0.045) and IGT (3.59; 95% CI 1.04–12.46; P = 0.044), but not in DM (2.13; 95% CI 0.62–7.34; P = 0.230) (Supporting Information, Figure S1B–D).
3.3. Determinant factors of impaired glucose tolerance or albuminuria in chronic heart failure
Determinants of IGT were identified as systolic BP (per 5 mmHg increase, 1.09; 1.02–1.17; P = 0.016), age (per 5 years increase, 1.16; 1.02–1.32; P = 0.025), and LVEF (per 5% decrease, 1.10; 1.01–1.20; P = 0.037) (Supporting Information, Table S2 ). Table 3 shows the determinant factors of albuminuria by multivariable logistic models, including systolic BP, BNP, AF, and BMI. Of note, DM was also associated with albuminuria (odds ratio, 1.75; 95% CI 1.11–2.75; P = 0.016) but IGT was not (0.94; 0.54–1.63; P = 0.816). When we evaluated the association between abnormalities of glycaemic status and development of albuminuria, IGT and DM had numerically, but not statistically, higher odds ratios, while advanced NYHA class and history of HF hospitalization were associated with development of albuminuria (Table 3).
Table 3.
Relationships between abnormalities in glucose regulation and albuminuria at baseline and 1‐year follow‐up by multivariable logistic regression models
| Variables | Odds ratio (95% CI) | P value | Z score |
|---|---|---|---|
| Albuminuria at baseline | |||
| IGT | 0.94 (0.54–1.63) | 0.816 | −0.23 |
| Diabetes | 1.75 (1.11–2.75) | 0.016 | 2.41 |
| Systolic BP (per 5 mmHg increase) | 1.12 (1.07–1.19) | <0.001 | 4.27 |
| BNP (log) | 1.40 (1.18–1.66) | <0.001 | 3.82 |
| Atrial fibrillation | 1.66 (1.13–2.44) | 0.010 | 2.41 |
| BMI | 1.06 (1.01–1.11) | 0.017 | 2.38 |
| Albuminuria at 1 year | |||
| IGT | 0.96 (0.47–1.96) | 0.916 | 2.38 |
| Diabetes | 1.33 (0.74–2.40) | 0.338 | 2.57 |
| History of HF hospitalization | 1.94 (1.17–3.22) | 0.010 | 2.57 |
| NYHA class | 3.28 (1.23–8.75) | 0.017 | 2.38 |
We used backward and forward selection methods. The final model was determined as the one using backward selection by the lowest value of Akaike information criterion (AIC).
BP, blood pressure; BMI, body mass index; BNP, brain natriuretic peptide; HF, hear failure; LVEF, left ventricular ejection fraction.
4. Discussion
The novel findings of the present study are as follows: (i) although IGT itself was not associated with poor prognosis, it was a significant prognostic factor in CHF patients when complicated with albuminuria. (ii) Newly developed albuminuria was associated with poor prognosis in CHF patients with NGR or IGT. To the best of our knowledge, this is the first study that demonstrates the clinical significance of combined early stages of IGT and renal dysfunction in CHF patients. The present study also indicates the clinical importance of combined tests of OGTT and urinalysis to more accurately assess long‐term prognosis of CHF patients.
4.1. Prevalence and incidence of impaired glucose tolerance and diabetes mellitus in chronic heart failure patients
By repeating OGTT and urinalysis, we confirmed higher prevalence of IGT in CHF patients.1, 2, 3, 4, 5 Indeed, 23% of our CHF patients had IGT, a higher prevalence than in Japanese general population (14.6%).3 A previous study with OGTT showed that in outpatients with HF and LVEF ≤ 45%, the prevalence of IGT was 23%,5 a consistent finding with our present finding. In the present study, IGT was related with age, systolic BP, and LVEF in the logistic regression analysis. These results suggest that insulin resistance is associated with severity of HF, a consistent finding with the previous study that insulin resistance was associated with exercise intolerance partly through reduced coronary flow reserve.23 In the present study, we have extended the clinical relevance of IGT and DM by repeated evaluation of glucose regulation; in CHF, the incidence rate of newly diagnosed IGT and DM was 16.7 (95% CI 10.9–25.7)/1000 person‐years and 11.2 (6.6–18.8)/1000 person‐years, respectively. This incident rate of newly diagnosed DM was higher than in Japanese general population (age 19–82 years) with 8.8/1000 person‐years,24 although no report is yet available for the incidence of IGT. Thus, it is important to continuously monitor glucose tolerance in CHF patients.
4.2. Prognostic implications of impaired glucose tolerance and diabetes mellitus in chronic heart failure
In terms of the prognostic importance of IGT and DM in CHF patients, our findings extend on the previous studies assessing the prognostic significance of combined glucose intolerance and albuminuria. In the previous studies, IGT was assessed in relation to baseline serum glucose,4, 7 insulin,6 and HbA1c25 levels. In the present study, we employed OGTT as a gold standard to evaluate IGT and DM. A recent study with OGTT at baseline showed an association of known or newly diagnosed DM and prognosis in CHF patients.5 In this study with 413 CHF patients, the mortality rate was almost two‐fold higher in DM patients than in those without it,5 although prognostic impact of IGT was not examined. In the present study, for the first time, we evaluated the prognostic significance of IGT in CHF patients by OGTT. In the present study, IGT was associated with systolic BP and LVEF, whereas no association was noted between IGT and long‐term prognosis, suggesting that IGT is related to the severity of HF, but a longer period would be needed for future adverse effects.
In terms of the prognostic impact of DM in CHF, the adjusted HR of DM was numerically higher but statistically insignificant. In contrast, elevated HbA1c was significantly associated with higher event rate, as in the previous report.25 In the present study, HbA1c levels were lower (mean 6.6%) compared with the previous study (7.7%),8 suggesting that DM was well controlled and thus adverse prognostic impacts of DM were not so evident.
4.3. Prognostic significance of albuminuria in chronic heart failure with references to normal glucose regulation, impaired glucose tolerance, and diabetes mellitus
In the present study, we first demonstrated the prognostic significance of the combined early stages of glycaemic abnormality (IGT) and renal dysfunction (albuminuria) in CHF patients by repeating OGTT and urinalysis. In fact, patients with NGR and albuminuria had higher event rate than in those with IGT or DM without albuminuria, suggesting that albuminuria has more prognostic impact than glycaemic abnormalities in CHF patients.
We and others have previously shown the prognostic impacts of DM and albuminuria in CHF patients12, 26, 27, 28, 29 (Table 4). A previous study showed that albuminuria was associated with poor prognosis in CHF patients.28 The present findings have extended this observation by demonstrating the prognostic interaction between glycaemic abnormalities and albuminuria in CHF patients. In the present study, newly developed albuminuria had similar prognostic significance to known albuminuria in patients with NGR and IGT, suggesting usefulness of repeating urinalysis to assess the prognostic risk of renal dysfunction in this population. Furthermore, after adjusting confounders, we noted a 125% increase in the prognostic risk of albuminuria in HF patients with IGT. A recent study by echocardiography has shown that in patients with Type I DM, myocardial deformation was associated primarily with the presence of microalbuminuria.17 We also previously demonstrated that combined abnormalities of glycaemic regulation and albuminuria impair cardiac function, especially diastolic function, and influence the prognosis in CHF patients.13 Indeed, in the present study, NGR and IGT patients with newly developed albuminuria had similar prognosis compared with those with known albuminuria, and severity of CHF was associated with progression of albuminuria, suggesting that reduction in albuminuria may be beneficial for CHF patients with NGR and IGT. However, it is still controversial whether albuminuria is a therapeutic target. This question may be answered by the ongoing clinical trials in HF patients with any glycaemic status to evaluate the effects of sodium‐glucose co‐transporter 2 inhibitors (empagliflozin/dapagliflozin), which are known to reduce UACR and renal events in Type II DM.30, 31
Table 4.
Comparison of patients, measurements, and outcomes with previous heart failure studies
| Reference | Number of patients | Study patients | Measurements | Outcomes |
|---|---|---|---|---|
| Prevalence of IGT or diabetes | ||||
| Suskin et al.1 | 663 | NYHA II–IV, EF < 40% | Fasting plasma glucose and insulin levels | 27%, diabetes; 8%, newly diagnosed diabetes; 9%, elevated glucose levels |
| Witteles et al.2 | 43 | Idiopathic dilated cardiomyopathy | OGTT | 49%, IGT |
| Kim et al.3 | 56 | Dilated cardiomyopathy | OGTT | 50%, IGT; 26.8%, newly diagnosed diabetes |
| Berry et al.4 | 454 | Acute HF | Plasma glucose level | 13%, IGT |
| Egstrup et al.5 | 413 | Outpatients with HF and LVEF ≤ 45% | OGTT | 23%, IGT; 18%, newly diagnosed diabetes |
| Present study | 535 | Chronic HF with a history of hypertension (82%; LVEF > 40%) | OGTT |
<At enrolment>23%, IGT; 0.5%, newly diagnosed diabetes <At 1 year>5%, newly diagnosed IGT; 2.5%, newly diagnosed diabetes |
| Prognostic significance of abnormalities in glucose regulation | ||||
| Doehner et al.6 | 105 (male) | Chronic HF | ivGTT | HR 0.28 (0.14–0.55, P = 0.0003) of insulin sensitivitya for all‐cause death |
| Gerstein et al.25 | 2412 | Chronic HF | HbA1c | HR 1.22 (1.16–1.29, P < 0.001) per 1% HbA1c increase for all‐cause death |
| Berry et al.4 | 454 | Acute HF | Plasma glucose level | HR 1.41 (0.92–2.16, P = 0.12) of IGT for all‐cause death |
| Kosiborod et al.7 | 50 532 | Post discharge after acute HF, Age > 65 years (retrospective) | Plasma glucose level | HR 1.00 (0.99–1.01, P = 0.75) for all‐cause mortality (per 10 mg/dL admission serum glucose increase) |
| Present study | 535 | Chronic HF with a history of hypertension (82%; LVEF > 40%) | OGTT |
HR 1.18 (1.05–1.33, P = 0.007) per 1% HbA1c increase for composite outcome HR 1.00 (0.61–1.64, P = 0.99) for IGT HR 2.25 (1.14–4.42, P = 0.019) for IGT complicated with albuminuria |
EF, ejection fraction; HbA1c, haemoglobin A1c; HF, heart failure; HR, hazard ratio; IGT, impaired glucose tolerance; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OGTT, oral glucose tolerance test.
Insulin sensitivity—the inverse of insulin resistance—is defined as the fraction of the glucose distribution space cleared per minute by insulin‐dependent glucose disposal relative to the concentration of insulin and is expressed in min/μU/mL.
4.4. Study limitations
Several limitations should be mentioned for the present study. First, as our patient population is relatively homogeneous with Japanese patients, external validation studies should be conducted. Second, in the present study, we enrolled CHF patients with hypertension based on the study design, majority of them had preserved EF, which limits the data on clinical significance of IGT and albuminuria in patients with reduced EF. Third, timing of follow‐up OGTT (approximately 1 year) may be relatively short to fully evaluate the impact of IGT or DM. Finally, because we excluded 19 patients with IGF, we were unable to evaluate the association between IGF and prognosis. However, IGF has been shown to be less significantly associated with cardiovascular events and mortality.32, 33
5. Conclusions
In the present study, we were able to demonstrate that IGT is associated with poor prognosis when complicated by albuminuria in CHF patients, demonstrating the importance of combined early stages of glucose intolerance and renal dysfunction in the management of HF.
Conflict of interest
The Department of Evidence‐based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, is supported in part by the unrestricted research grants from Daiichi Sankyo Co., Ltd. (Tokyo, Japan), Bayer Yakuhin, Ltd. (Osaka, Japan), Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan), Kowa Pharmaceutical Co., Ltd. (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), Dainippon Sumitomo Pharma, Co., Ltd. (Osaka, Japan), and Nippon Boehringer Ingelheim Co., Ltd. (Tokyo, Japan). H.S. has received lecture fees from Bayer Yakuhin, Ltd. (Osaka, Japan), Daiichi Sankyo Co., Ltd. (Tokyo, Japan), and Novartis Pharma K.K. (Tokyo, Japan).
Funding
The SUPPORT trial was carried out, supported by grants‐in‐aid from the Ministry of Health, Labour and Welfare and from the Ministry of Education, Culture, Sports, Science and Technology, Japan (KAKENHI‐PROJECT‐17K15821).
Supporting information
Table S1. Baseline characteristics stratified by glycemic control condition and albuminuria.
Table S2. Determinants of IGT in HF patients by multivariable logistic regression model.
Table S3. Prognostic significance of glycemic status and albuminuria after adjusting for age, sex, and BNP levels stratified by preserved, mid‐range, and reduced LVEF subgroups.
Figure S1. Kaplan–Meier survival curves in (A) CHF patients in overall, (B) those with NGR, (C) those with IGT, and (D) those with DM stratified by the status of albuminuria at 1‐year visit; without albuminuria or developed albuminuria at 1 year, and known albuminuria., setting date at 1‐year visit as observation time 0. HRs are adjusted for age, sex and BNP at 1 year. Abbreviations; w/o, without.
Acknowledgements
The authors wish to thank the patients and staff of the SUPPORT trial for their important contributions.
Nochioka, K. , Sakata, Y. , Miura, M. , Shiroto, T. , Takahashi, J. , Saga, C. , Ikeno, Y. , Shiba, N. , Shinozaki, T. , Sugi, M. , Nakagawa, M. , Komaru, T. , Kato, A. , Nozaki, E. , Iwabuchi, K. , Hiramoto, T. , Inoue, K. , Ohe, M. , Tamaki, K. , Tsuji, I. , and Shimokawa, H. (2019) Impaired glucose tolerance and albuminuria in patients with chronic heart failure: a subanalysis of the SUPPORT trial. ESC Heart Failure, 6: 1252–1261. 10.1002/ehf2.12516.
References
- 1. Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, Rouleau JL, Sigouin C, Solymoss CB, Tsuyuki R, White M, Yusuf S. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J 2000; 21: 1368–1375. [DOI] [PubMed] [Google Scholar]
- 2. Witteles RM, Tang WH, Jamali AH, Chu JW, Reaven GM, Fowler MB. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol 2004; 44: 78–81. [DOI] [PubMed] [Google Scholar]
- 3. Kim J, Nakatani S, Hashimura K, Komamura K, Kanzaki H, Asakura M, Asanuma H, Kokubo Y, Tomoike H, Kitakaze M. Abnormal glucose tolerance contributes to the progression of chronic heart failure in patients with dilated cardiomyopathy. Hypertens Res 2006; 29: 775–782. [DOI] [PubMed] [Google Scholar]
- 4. Berry C, Brett M, Stevenson K, McMurray JJ, Norrie J. Nature and prognostic importance of abnormal glucose tolerance and diabetes in acute heart failure. Heart 2008; 94: 296–304. [DOI] [PubMed] [Google Scholar]
- 5. Egstrup M, Schou M, Gustafsson I, Kistorp CN, Hildebrandt PR, Tuxen CD. Oral glucose tolerance testing in an outpatient heart failure clinic reveals a high proportion of undiagnosed diabetic patients with an adverse prognosis. Eur J Heart Fail 2011; 13: 319–326. [DOI] [PubMed] [Google Scholar]
- 6. Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJ, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol 2005; 46: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 7. Kosiborod M, Inzucchi SE, Spertus JA, Wang Y, Masoudi FA, Havranek EP, Krumholz HM. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation 2009; 119: 1899–1907. [DOI] [PubMed] [Google Scholar]
- 8. Authors/Task Force M , Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, ESCCfP G, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck‐Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG. ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013; 34: 3035–3087. [DOI] [PubMed] [Google Scholar]
- 9. Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh epidemiology of diabetes complication study. Kidney Int 2002; 62: 963–970. [DOI] [PubMed] [Google Scholar]
- 10. Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, Remuzzi G, Ruggenenti P. Insulin resistance and microalbuminuria: a cross‐sectional, case‐control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 2006; 55: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 11. Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes 1998; 47: 793–800. [DOI] [PubMed] [Google Scholar]
- 12. Miura M, Sakata Y, Miyata S, Nochioka K, Takada T, Tadaki S, Ushigome R, Yamauchi T, Takahashi J, Shimokawa H. Investigators C. Prognostic impact of subclinical microalbuminuria in patients with chronic heart failure. Circ J 2014; 78: 2890–2898. [PubMed] [Google Scholar]
- 13. Jensen MT, Sogaard P, Andersen HU, Bech J, Fritz Hansen T, Biering‐Sorensen T, Jorgensen PG, Galatius S, Madsen JK, Rossing P, Jensen JS. Global longitudinal strain is not impaired in type 1 diabetes patients without albuminuria: the Thousand & 1 study. JACC Cardiovasc Imaging 2015; 8: 400–410. [DOI] [PubMed] [Google Scholar]
- 14. Sakata Y, Shiba N, Takahashi J, Miyata S, Nochioka K, Miura M, Takada T, Saga C, Shinozaki T, Sugi M, Nakagawa M, Sekiguchi N, Komaru T, Kato A, Fukuchi M, Nozaki E, Hiramoto T, Inoue K, Goto T, Ohe M, Tamaki K, Ibayashi S, Ishide N, Maruyama Y, Tsuji I, Shimokawa H, Investigators ST, Investigators ST. Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: the supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial. Eur Heart J 2015; 36: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakata Y, Nochioka K, Miura M, Takada T, Tadaki S, Miyata S, Shiba N, Shimokawa H. Supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial—rationale and design. J Cardiol 2013; 62: 31–36. [DOI] [PubMed] [Google Scholar]
- 16. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Expert Committee on the D, Classification of Diabetes M. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160–3167. [DOI] [PubMed] [Google Scholar]
- 17. Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline development work group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 18. Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Brindis RG, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins‐Nakai RL, Ellis SG, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, McKay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol 2001; 38: 2114–2130. [DOI] [PubMed] [Google Scholar]
- 19. Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S, Investigators C, Committees . Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet 2003; 362: 759–766. [DOI] [PubMed] [Google Scholar]
- 20. Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014; 2: 440–446. [DOI] [PubMed] [Google Scholar]
- 21. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H, Investigators C. Characterization of heart failure patients with mid‐range left ventricular ejection fraction—a report from the CHART‐2 study. Eur J Heart Fail 2017; 19: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 22. Dafni U. Landmark analysis at the 25‐year landmark point. Circ Cardiovasc Qual Outcomes 2011; 4: 363–371. [DOI] [PubMed] [Google Scholar]
- 23. Snoer M, Monk‐Hansen T, Olsen RH, Pedersen LR, Simonsen L, Rasmusen H, Dela F, Prescott E. Insulin resistance and exercise tolerance in heart failure patients: linkage to coronary flow reserve and peripheral vascular function. Cardiovasc Diabetol 2012; 11: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goto A, Goto M, Noda M, Tsugane S. Incidence of type 2 diabetes in Japan: a systematic review and meta‐analysis. PLoS ONE 2013; 8: e74699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, Olofsson B, Pfeffer MA, Yusuf S, Investigators CP. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Arch Intern Med 2008; 168: 1699–1704. [DOI] [PubMed] [Google Scholar]
- 26. Miura M, Sakata Y, Miyata S, Nochioka K, Takada T, Tadaki S, Ushigome R, Yamauchi T, Sato K, Onose T, Tsuji K, Abe R, Takahashi J, Shimokawa H, Investigators C. Prognostic impact of diabetes mellitus in chronic heart failure according to presence of ischemic heart disease—with special reference to nephropathy. Circ J 2015; 79: 1764–1772. [DOI] [PubMed] [Google Scholar]
- 27. Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol 2001; 38: 421–428. [DOI] [PubMed] [Google Scholar]
- 28. Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ, Investigators C, Committees . Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374: 543–550. [DOI] [PubMed] [Google Scholar]
- 29. Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, Shimokawa H, Investigators C. Urinary albumin excretion in heart failure with preserved ejection fraction: an interim analysis of the CHART 2 study. Eur J Heart Fail 2012; 14: 367–376. [DOI] [PubMed] [Google Scholar]
- 30. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 31. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, Investigators E‐RO . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 32. Decode Study Group tEDEG . Glucose tolerance and cardiovascular mortality: comparison of fasting and 2 h diagnostic criteria. Arch Intern Med 2001; 161: 397–405. [DOI] [PubMed] [Google Scholar]
- 33. Decode Study Group EDEG . Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003; 26: 688–696. [DOI] [PubMed] [Google Scholar]
- 34. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 35. Summary of Recommendation Statements . Kidney Int Suppl (2011) . 2013;3:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics stratified by glycemic control condition and albuminuria.
Table S2. Determinants of IGT in HF patients by multivariable logistic regression model.
Table S3. Prognostic significance of glycemic status and albuminuria after adjusting for age, sex, and BNP levels stratified by preserved, mid‐range, and reduced LVEF subgroups.
Figure S1. Kaplan–Meier survival curves in (A) CHF patients in overall, (B) those with NGR, (C) those with IGT, and (D) those with DM stratified by the status of albuminuria at 1‐year visit; without albuminuria or developed albuminuria at 1 year, and known albuminuria., setting date at 1‐year visit as observation time 0. HRs are adjusted for age, sex and BNP at 1 year. Abbreviations; w/o, without.


