Abstract
Aims
Ivabradine has been approved in heart failure with reduced ejection fraction (HFrEF) and elevated heart rate despite guideline‐directed medical therapy (GDMT) to reduce cardiovascular (CV) death and hospitalization for worsening HF. The median value of 77 b.p.m. is the lower bound selected for the regulatory approval in Canada, South Africa, and Australia. Patient‐reported outcomes (PROs) including symptoms, quality of life, and global assessment are considered of major interest in the global plan of care of patients with HF. However, the specific impact of GDMT, and specifically ivabradine, on PRO remains poorly studied. In the subgroup of patients from the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) who had heart rate above the median of 77 b.p.m. (pre‐specified analysis) and for whom the potential for improvement was expected to be larger, we aimed (i) to evaluate the effects of ivabradine on PRO (symptoms, quality of life, and global assessment); (ii) to consolidate the effects of ivabradine on the primary composite endpoint of CV death and hospitalization for HF; and (iii) to reassess the effects of ivabradine on left ventricular (LV) remodelling.
Methods and results
Comparisons were made according to therapy, and proportional hazards models (adjusted for baseline beta‐blocker therapy) were used to estimate the association between ivabradine and various outcomes. In SHIFT, n = 3357 (51.6%) patients had a baseline heart rate > 77 b.p.m. After a median follow‐up of 22.9 months (inter‐quartile range 18–28 months), ivabradine on top of GDMT improved symptoms (28% vs. 23% improvement in New York Heart Association functional class, P = 0.0003), quality of life (5.3 vs. 2.2 improvement in Kansas City Cardiomyopathy Questionnaire overall summary score, P = 0.005), and global assessment [from both patient (improved in 72.3%) and physician (improved in 61.0%) perspectives] significantly more than did placebo (both P < 0.0001). Ivabradine induced a 25% reduction in the combined endpoint of CV death and hospitalization for HF (hazard ratio 0.75; P < 0.0001), which translates into a number of patients needed to be treated for 1 year of 17. Patients under ivabradine treatment demonstrated a significant reduction in LV dimensions when reassessed at 8 months (P < 0.05).
Conclusions
In patients with chronic HFrEF, sinus rhythm, and a heart rate > 77 b.p.m. while on GDMT, the present analysis brings novel insights into the role of ivabradine in improving the management of HFrEF, particularly with regard to PRO (ISRCTN70429960).
Keywords: Heart failure with reduced ejection fraction, Patient‐reported outcomes, Ivabradine, Mortality, The SHIFT trial
1. Introduction
In the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT), ivabradine, a specific If‐channel inhibitor, resulting in a pure heart rate reduction, significantly reduced the composite endpoint of cardiovascular (CV) death and heart failure (HF) hospitalization in patients with chronic HF and reduced ejection fraction [left ventricular ejection fraction (LVEF) ≤ 35%] who were in sinus rhythm and had resting heart rate ≥ 70 b.p.m. despite optimal medical therapy.1 Subgroup analyses demonstrated a constant improvement in outcomes across the whole spectrum of heart rate, with a significant relationship between baseline heart rate and treatment effect (higher being better).2 Various heart rates have been approved for this indication by the international regulatory authorities,3, 4, 5 with 77 b.p.m. being the cut‐off value in Canada, South Africa, and Australia.
Patient‐reported outcomes (PROs) such as symptoms, quality of life, and global assessment gained interest in the global plan of care of patients with HF, becoming key aspects with regard to the management of patient's experience with chronic disease and being at least as important as survival from patients' perspective.6 As such, PRO measures provide useful insights into the impacts of symptoms on patients functioning and are currently encouraged as an outcome measure in clinical trials as well as a marker of high‐quality care in clinical practice.7, 8
Although the exact interplay between medical therapy and improved PROs remains largely speculative, PROs showed to be strong predictors of mortality and recurrent admissions in HF.9 As such, baseline Kansas City Cardiomyopathy Questionnaire (KCCQ)10 was shown to be associated with prognosis in patients with HF11, 12; and even small changes in quality of life related to various pharmacological and non‐pharmacological interventions have recently been demonstrated to be associated with better outcomes,13, 14, 15, 16 especially when co‐occurring with improvements in cardiac status.17, 18 However, the specific impacts of guideline‐directed medical therapy (GDMT), and particularly ivabradine, on PRO (i.e. beyond simple assessment of quality of life) remains poorly studied in HF with reduced ejection fraction (HFrEF).
Therefore, we aimed to better characterize the potential benefit associated with ivabradine on PRO in the subgroup of patients from the SHIFT trial with heart rates at rest of at least the median value of 77 b.p.m., as the potential for improvement was expected to be larger in those patients given its mode of action.19 As such, the present analysis aimed (i) to evaluate the effects of ivabradine on PROs (symptoms, quality of life, and global assessment); (ii) to consolidate the effects of ivabradine on the primary composite endpoint of CV death and hospitalization for HF; and (iii) to reassess the effects of ivabradine on LV remodelling.
2. Methods
2.1. Study design
The overall objective of the SHIFT trial was to assess the role of ivabradine on top of current GDMT in chronic HFrEF.1 The design and primary results of the trial have extensively been described elsewhere.1, 20 Briefly, patients with HF, reduced left ventricular function (LVEF ≤ 35%), New York Heart Association (NYHA) functional class II–IV, and persistent heart rate ≥ 70 b.p.m. at rest (sinus rhythm) despite GDMT were eligible for randomization in SHIFT, with a majority of patients being already treated with the highest tolerated doses of beta‐blockers or having a contraindication or intolerance to them. A total of 6505 patients were randomized to receive either ivabradine or placebo. The primary outcome was a composite of death from CV causes or hospitalization for worsening HF. Secondary endpoints were CV hospitalization, CV death, hospitalization for HF, HF death, all‐cause hospitalization, and all‐cause death. Changes in functional capacity were assessed using the NYHA functional class and by patient‐reported and physician‐reported global assessment. Physician global assessment was based on a scoring system assessing changes in functional status (‘markedly improved’, ‘moderately improved’, ‘slightly improved’, ‘no change’, ‘slightly worsened’, ‘moderately worsened’, or ‘markedly worsened’). Patient self‐assessment was based on a separate questionnaire evaluating changes in health status (‘markedly improved’, ‘moderately improved’, ‘slightly improved’, ‘no change’, ‘slightly worsened’, ‘moderately worsened’, or ‘markedly worsened’). Patients were blinded to physician global assessments and vice versa. Quality of life was assessed using the Food and Drug Administration‐approved KCCQ. All assessments were provided under treatment at baseline, 4 months, 12 months, 24 months, or last follow‐up.1, 15
The SHIFT echocardiography sub‐study included 411 patients (ivabradine, n = 208; and placebo, n = 203), and the primary endpoint was the change in left ventricular end‐systolic volume index (LVESVi; mL/m2) over time (measures were obtained at baseline and 8 months). The ancillary study design has also been published.15, 21
2.2. Statistical considerations
We assessed outcomes in the patients with a heart rate ≥ 77 b.p.m. (i.e. median heart rate at baseline in patients included in the SHIFT trial), and comparisons were made regarding study treatment. Baseline characteristics are summarized using counts and percentages for categorical variables and mean ± standard deviation or median [inter‐quartile range (IQR)] for continuous variables. The effects of ivabradine compared with placebo on pre‐specified outcomes are provided using a Cox's proportional hazards model including treatment as a factor and adjusted for baseline beta‐blocker therapy (yes or no). Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated, and P‐values calculated from the Wald statistic. Time‐to‐event curves by treatment group were estimated using the Kaplan–Meier method. The number of patients needed to be treated (NNT) for 1 year in order to prevent one event was calculated as the inverse of the between‐treatment group difference of the estimated probability of having an event at 1 year in the Kaplan–Meier curves. All survival analyses were based on endpoints adjudicated by an independent committee blinded to treatment allocation and were conducted as time‐to‐first event using the intention‐to‐treat principle. The percentages of patients improving their NYHA class and patient‐reported and physician‐reported global assessment were compared using a χ 2 test. Changes in echocardiographic endpoints (at Week 8) and KCCQ scores (at Week 12) were compared between treatment groups on patients included in the corresponding substudies using a covariance analysis adjusted for beta‐blocker intake at randomization, country, and baseline value. The treatment effect was estimated using adjusted least square means from this model, with associated two‐sided 95% CIs and P‐values. SAS Version 9.1 was used for all analyses.
3. Results
3.1. Baseline characteristics
Baseline demographics and clinical characteristics of patients receiving either ivabradine (n = 1657) or placebo (n = 1700) in the ≥ 77 b.p.m. subgroup are demonstrated in Table 1. The median follow‐up in SHIFT was 22.9 months (IQR 18–28 months). Patient characteristics were similar in both groups. A majority of patients were in NYHA functional class II or III and were optimally treated according to current GDMT (90% having an angiotensin‐converting‐enzyme inhibitor or angiotensin II receptor blockers and up to 60% having an aldosterone antagonist). Of the patients, 86% were treated with a beta‐blocker in both groups at the time of randomization (carvedilol, bisoprolol, or metoprolol succinate), with up to 25% and 55% at target beta‐blocker dose or at least half the target dose, respectively. Overall, the proportion of patients implanted with pacemakers or implantable cardioverter defibrillators (ICDs) was low (<5% in both groups). The mean dose of ivabradine was 6.74 ± 1.19 mg bid. Median treatment duration was 20.96 months (±13.43) in the ivabradine group and 20.91 months (±13.66) in the placebo group.
Table 1.
Baseline characteristics of patients with a heart rate ≥ 77 b.p.m. at rest
| Baseline characteristics | Ivabradine group (n = 1657) | Placebo group (n = 1700) | P |
|---|---|---|---|
| Age, mean (±SD) | 59.5 (±11) | 59.2 (±12) | 0.7709 |
| Female | 23.9% (n = 396) | 23.0% (n = 392) | 0.5660 |
| Caucasian | 87.7% (n = 1454) | 87.0% (n = 1479) | 0.7745 |
| Hypertension | 65.1% (n = 1079) | 63.5% (n = 1080) | 0.3369 |
| Diabetes | 31.6% (n = 525) | 32.6% (n = 555) | 0.5503 |
| History of atrial fibrillation | 7.4% (n = 124) | 7.6% (n = 130) | 0.8577 |
| Ischaemic aetiology of HF | 65.4% (n = 1084) | 64.8% (n = 1103) | 0.7440 |
| BMI, kg/m2 mean (±SD) | 28.1 (±5.4) | 27.9 (±5.2) | 0.4930 |
| Heart rate, b.p.m. Median (IQR) | 84 (77–130) | 84 (77–142) | 0.2338 |
| Systolic blood pressure, mmHg mean (±SD) | 121.7 (±17) | 120.9 (±16) | 0.2681 |
| NYHA functional class | 0.8947 | ||
| I | 0 | 0 | |
| II | 45.7% (n = 758) | 44.9% (n = 764) | |
| III | 52.0% (n = 862) | 52.8% (n = 898) | |
| IV | 2.2% (n = 37) | 2.2% (n = 38) | |
|
LVEF, % mean (±SD) |
28.5% (±5.2) | 28.5% (±5.2) | 0.7768 |
| eGFR, mL/min/1.73 m2 mean (±SD) | 75.8 (±23.9) | 75.8 (±23.1) | 0.8961 |
| Treatments at study start or randomization | |||
| ACEi or ARBs | 89.7% (n = 1487) | 89.6% (n = 1524) | 0.9291 |
| Digitalis | 23.9% (n = 397) | 25.2% (n = 429) | 0.3907 |
| Aldosterone antagonist | 63.1% (n = 1046) | 61.1% (n = 1039) | 0.2304 |
| Beta‐blockers, at study start or randomization | 86.1% (n = 1428) | 86.8% (n = 1476) | 0.5852 |
| Beta‐blockers, target daily dose | 26.0% (n = 366) | 24.2% (n = 356) | 0.3357 |
| Beta‐blockers, at least 50% of target dose daily | 54.4% (n = 765) | 54.4% (n = 792) | 0.9900 |
| Pacemaker or ICD or ICD + CRT | 3.0% (n = 50) | 4.8% (n = 83) | 0.0056 |
ACEi, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, inter‐quartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
3.2. Effects of ivabradine on patient‐reported outcomes in the ≥77 b.p.m. subgroup
Treatment with ivabradine was associated with a significant improvement in symptoms, as over one‐quarter of patients (28.0%, n = 460) in the ivabradine group had improvement in functional status over the study period, vs. 22.7% (n = 382) in the placebo group (P = 0.0003). Global assessment also significantly improved from both patient (72.3%) and physician (61.0%) perspectives with ivabradine when compared with placebo (both P < 0.0001, Table 2). In patients completing assessment at both baseline and last post‐baseline visit (n = 510), quality of life also improved with ivabradine, as there was a significant improvement in KCCQ score over time (change in the KCCQ Clinical Summary Score 3.66 ± 18.51 vs. 1.24 ± 18.67 in the placebo group, P = 0.028; and change in the KCCQ Overall Summary Score 5.30 ± 18.54 vs. 2.19 ± 18.86 in the placebo group, P = 0.005; Table 3).
Table 2.
Change between baseline and last visit for New York Heart Association class and global assessment in patients with a heart rate ≥ 77 b.p.m. at rest
| Ivabradine group (N = 1657) | Placebo group (N = 1700) | P | |
|---|---|---|---|
| NYHA functional class, % (n) | Nobs = 1643 | Nobs = 1680 | 0.0003 |
| Improved | 28.0% (n = 460) | 22.7% (n = 382) | |
| Stable or worsening | 72.0% (n = 1183) | 77.0% (n = 1298) | |
| Change in global self‐assessment, % (n) | Nobs = 1497 | Nobs = 1515 | 0.0006 |
| Improved | 72.3% (n = 1082) | 66.6% (n = 1009) | |
| Stable or worsening | 27.7% (n = 415) | 33.4% (n = 506) | |
| Change in global assessment, physician perspective, % (n) | Nobs = 1573 | Nobs = 1596 | <0.0001 |
| Improved | 61.0% (n = 960) | 54. 5% (n = 869) | |
| Stable or worsening | 39.0% (n = 613) | 45.5% (n = 727) |
Nobs, number of observations; NYHA, New York Heart Association.
Table 3.
Quality of life, subgroup of patients with a heart rate ≥ 77 b.p.m. at rest
| KCCQ scores | Ivabradine group (N = 510) | Placebo group (N = 512) | Treatment effect (change in QoL at 1 year) | |
|---|---|---|---|---|
| Estimate (95% CI) | P | |||
| CSS, at baseline mean (±SD) | 66.58 (±20.74) | 66.38 (±20.04) | — | — |
| CSS, changes at last post‐baseline value mean (±SD) | 3.66 (±18.51) | 1.24 (±18.67) | 2.37 (0.25–4.48) | 0.028 |
| OSS, at baseline mean (±SD) | 63.27 (±20.67) | 63.13 (±19.31) | — | — |
| OSS, changes at last post‐baseline value mean (±SD) | 5.30 (±18.54) | 2.19 (±18.86) | 3.00 (0.89–5.10) | 0.005 |
CSS, clinical summary score; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, overall summary score; QoL, quality of life.
3.3. Effects of ivabradine on major outcomes in patients with heart rate ≥ 77 b.p.m.
The effects of ivabradine on major outcomes in patients with heart rate ≥ 77 b.p.m. are shown in Figures 1, 2, 3 and Table 4. In addition to GDMT for chronic HFrEF, ivabradine was associated with a 25% reduction in the primary endpoint, a composite of CV death, and hospitalization for worsening HF (HR 0.75; 95% CI 0.67–0.85; P < 0.0001). Importantly, the 25% decrease in the primary endpoint was attributable to both a decrease in CV death (HR 0.81, P = 0.0137) and a decrease in HF hospitalization (HR 0.69, P < 0.0001) (Figures 1, 2, 3). There was also a significant reduction in CV hospitalization (decreased by 21%; HR 0.79; 95% CI 0.71–0.89; P < 0.0001), in CV death (decreased by 19%; HR 0.81; 95% CI 0.69–0.96; P = 0.0137), in hospitalizations for HF (decreased by 31%; HR 0.69; 95% CI 0.59–0.80; P < 0.0001), in HF death (decreased by 39%; HR 0.61; 95% CI 0.45–0.83; P = 0.0017), in all‐cause hospitalization (decreased by 18%; HR 0.82; 95% CI 0.74–0.9; P = 0.0002), and in all‐cause mortality alone (decreased by 19%; HR 0.81; 95% CI 0.69–0.94; P = 0.0074). The estimated NNT (numbers needed to initiate treatment with ivabradine to prevent pre‐specified clinical outcomes within 1 year) in this group of patients was 17 for the primary endpoint, 18 for CV hospitalization, 64 for CV death, 18 for hospitalization for worsening of HF, 94 for HF mortality, 17 for all‐cause hospitalization, and 56 for all‐cause mortality.
Figure 1.
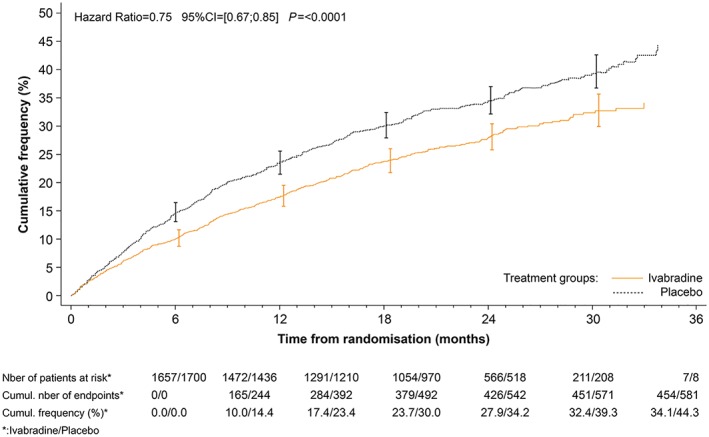
Kaplan–Meier curves for the primary endpoint (composite of cardiovascular mortality or hospitalization for worsening heart failure), patients with a heart rate ≥ 77 b.p.m. at rest.
Figure 2.
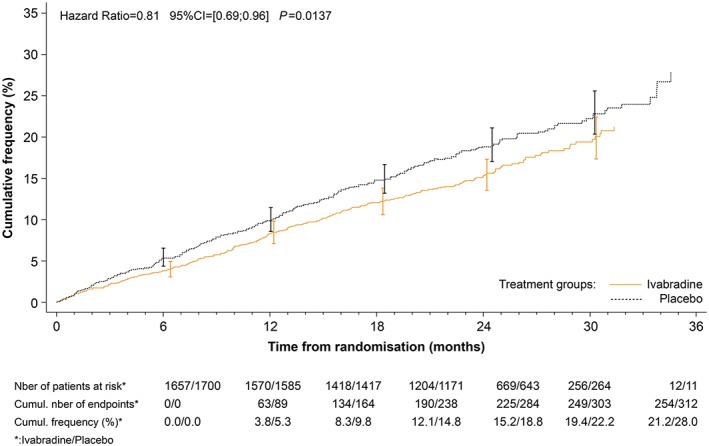
Kaplan–Meier curves for cardiovascular mortality alone in patients with a heart rate ≥ 77 b.p.m. at rest.
Figure 3.
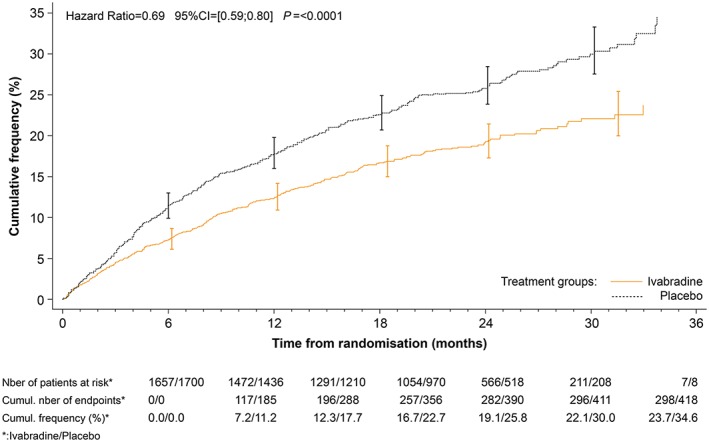
Kaplan–Meier curves for hospitalization for worsening heart failure alone in patients with a heart rate ≥ 77 b.p.m. at rest.
Table 4.
Outcomes and treatment effect in patients with a heart rate ≥ 77 b.p.m. at rest
| Outcomes, % (n) | Treatment effect of ivabradine vs. placebo | |||||
|---|---|---|---|---|---|---|
| Ivabradine (n = 1657) | Placebo (n = 1700) | NNT | HR | 95% CI | P | |
| CV mortality or hospitalization for worsening HF | 27.4% (n = 454) | 34.1% (n = 581) | 17 | 0.75 | 0.67–0.85 | <0.0001 |
| CV hospitalization | 32.2% (n = 534) | 38.0% (n = 647) | 18 | 0.79 | 0.71–0.89 | <0.0001 |
| CV mortality | 15.3% (n = 255) | 18.3% (n = 312) | 64 | 0.81 | 0.69–0.96 | 0.0137 |
| Hospitalization for worsening HF | 17.9% (n = 298) | 24.5% (n = 418) | 18 | 0.69 | 0.59–0.80 | <0.0001 |
| HF death | 4.0% (n = 67) | 6.2% (n = 107) | 94 | 0.61 | 0.45–0.83 | 0.0017 |
| All‐cause hospitalization | 40.2% (n = 667) | 45.7% (n = 778) | 17 | 0.82 | 0.74–0.91 | 0.0002 |
| All‐cause mortality | 17.2% (n = 285) | 20.5% (n = 350) | 56 | 0.81 | 0.69–0.94 | 0.0074 |
CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; NNT, number needed to be treated to prevent pre‐specified outcomes within 1 year.
3.4. Effects of ivabradine on left ventricular remodelling in the ≥77 b.p.m. subgroup
In the SHIFT echocardiography sub‐study, 95 patients in the ivabradine group and 74 in placebo group had a baseline heart rate ≥ 77 b.p.m. along with optimal image quality and are included in the present sub‐analysis. At baseline, mean LVESVi was similar in both groups (68.7 ± 29.7 and 66.0 ± 28.0 mL/m2), as was LVEF (30.3 ± 9.1% and 29.4 ± 9.4% in the ivabradine and placebo groups, respectively). At 8 months, there was a significant reduction in LVESVi in patients treated with ivabradine (−6.6 ± 17.8 vs. +2.3 ± 19.4 mL/m2, estimate standard error (SE) −8.3 (2.7), 95% CI −13.75 to −2.85; P = 0.003; Table 5), as well as in the left ventricular end‐diastolic volume index −7.5 ± 19.8 vs. +2.4 ± 21.0 ml/m2, estimate (SE) −8.9 (3.1); 95% CI −15.04 to −2.76; P = 0.0047; Table 5]. There was also an improvement in LVEF in the ivabradine group vs. placebo when reassessed at 8 months (2.7 ± 8.2 in the ivabradine group vs. ‐0.1 ± 8.9 in the placebo group, estimate (SE) 3.0 (1.3); 95% CI 0.52–5.64; P = 0.0189; Table 5).
Table 5.
Echocardiographic characteristics in patients with a heart rate ≥77 b.p.m. at rest
| Ivabradine (N = 95) | Placebo (n = 74) | Treatment effect | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 months | Change | Baseline | 8 months | Change | E (SE) | 95% CI | P | |
| LVESVi (mL/m2), mean (SD) | 68.7 (±29.7) | 62.1 (±29.8) | −6.6 (±17.8) | 66.0 (±28.0) | 68.3 (±28.3) | 2.3 (±19.4) | −8.3 (2.7) | −13.75 to −2.85 | 0.0030 |
| LVESV (mL), mean (SD) | 130.6 (±58.1) | 118.9 (±59.2) | −11.7 (±34.6) | 126.3 (±57.3) | 131.0 (±56.3) | 4.6 (±38.2) | −15.3 (5.4) | −25.98 to −4.67 | 0.0051 |
| LVEDVi (mL/m2), mean (SD) | 96.9 (±33.9) | 89.4 (±32.1) | −7.5 (±19.8) | 92.3 (±29.8) | 94.7 (±31.0) | 2.4 (±21.9) | −8.90 (3.1) | −15.04 to −2.76 | 0.0047 |
| LVEDV (mL), mean (SD) | 183.9 (±67.2) | 170.4 (±64.6) | −13.5 (±38.6) | 176.3 (±62.0) | 181.5 (±62.3) | 5.2 (±42.7) | −16.6 (6.0) | −28.91 to −5.00 | 0.0057 |
| LVEF (%), mean (SD) | 30.3 (±9.1) | 33.1 (±10.6) | 2.7 (±8.2) | 29.4 (±9.4) | 29.3 (±10.4) | −0.1 (±8.9) | 3.0 (1.3) | 0.52 to 5.64 | 0.0189 |
LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end‐systolic volume index.
4. Discussion
This study demonstrates the following: (i) patients with HFrEF and heart rate at rest > 77 b.p.m treated with ivabradine on top of GDMT had a significant improvement in symptoms, quality of life, and global assessment; (ii) adding ivabradine to GDMT significantly reduced the occurrence of the primary endpoint, a composite of CV death and hospitalization for worsening HF, by 25%, attributable to both a decrease in CV death and in HF hospitalization; and (iii) in addition, patients treated with ivabradine had a greater reduction in LV dimensions over time. These results demonstrate the effects of ivabradine on top of recommended medical therapy on PRO, and reinforce its role in improving major outcomes in patients with chronic HFrEF and baseline heart rate ≥ 77 b.p.m.. Further analyses on other subgroups of patients could consolidate these results in patients with lower heart rate values at baseline.
4.1. Ivabradine and patient‐reported outcomes in heart failure with reduced ejection fraction
Patients' health status perceptions and global functioning are increasingly considered as important outcome measures in the setting of chronic disease.7 In patients with HF, PROs, that is, symptoms, global function, and quality of life, proved to be substantially impaired and have been associated with worse prognosis.9 Moreover, PRO measures more accurately assess some of the specific aspects of the disease process than do conventional clinical tools.6, 7, 8 In this analysis, we demonstrated that treatment with ivabradine was strongly associated with improved symptoms and self‐reported global assessment. We also demonstrate a significant 5‐point improvement in KCCQ overall summary score under ivabradine treatment, which has been considered to be of significant interest in patients with HF.22 Improvement in symptoms and functional status under ivabradine treatment has also been demonstrated in elderly patients with HF, a group in which co‐morbidities and polypharmacy are frequent and may play a role in PRO impairment.23 In addition to results from SHIFT,15 the beneficial effects of ivabradine on quality of life measures have been demonstrated in various cohorts of real‐life patients,24 these effects being maintained in patients followed up for at least 12 months.25 However, whether these impacts on PRO are specifically related to ivabradine or simply related to the global improvement in cardiac status remains to be determined.
4.2. Ivabradine and clinical outcomes in patients with heart failure with reduced ejection fraction and heart rate > 77 b.p.m. at rest
Elevated heart rate at rest is associated with poor outcomes in HFrEF,1, 20, 26, 27, 28, 29, 30 and some reports plead for a significant survival benefit related to heart rate lowering per se, independently of the achievement of target doses of beta‐blockers in patients with HFrEF.31 In SHIFT, subgroup analyses showed a significant relationship between heart rate at baseline and risk reduction under therapy, the benefits from ivabradine being independent from baseline medication.2, 32 In the present analysis, we demonstrate that in patients with a baseline heart rate ≥ 77 b.p.m., the 25% decrease in the primary endpoint is attributable to a decrease both in CV death (HR 0.81, P = 0.0137) and in HF hospitalization (HR 0.69, P < 0.0001), whereas in the global SHIFT population, the effects of ivabradine were mainly driven by a reduction in the numbers of hospitalization for worsening HF (HR 0.64, P < 0.0001) and no significant effect on CV mortality (P = 0.128).20 Notably, this reduction in CV deaths has already been demonstrated in various subgroup analyses in patients with high heart rates at baseline.1 These differences in the effectiveness of ivabradine according to baseline heart rate may, at least in part, be explained by its mode of action, because If‐channel inhibition preferentially occurs when the If‐channel is in its opened state, that is, when heart rate is the highest.19, 33, 34
4.3. Ivabradine and left ventricular remodelling in patients with heart failure with reduced ejection fraction and heart rate > 77 b.p.m. at rest
In HFrEF, heart rate lowering with ivabradine has shown to be associated with significant LV structural changes that have been associated with better survival.21, 35 Furthermore, the role of ivabradine in promoting LV reverse remodelling was shown to be independent from baseline LVEF, LV volumes, and background therapy21 and to be substantially more pronounced in patients with higher heart rate at rest.2, 21 In this analysis, we demonstrate a significant decrease in LV dimensions in patients after 8 months of treatment with ivabradine, these results being consistent with those observed in the overall SHIFT trial.21
4.4. Limitations
In this analysis, patients differed from those with HFrEF in the global population, as they were younger, wre in sinus rhythm (patients with known atrial fibrillation were excluded considering the mechanism of action of the drug), a low proportion of patients had ICDs, and recommended target doses of beta‐blockers often not being reached, limiting the ability to extrapolate the results to all patients with HFrEF. Nonetheless, the aforementioned analyses provide important insights with regard to heart rate lowering in chronic HFrEF, particularly with regard to PRO in the subgroup of patients with high heart rates at baseline.
5. Conclusions
In patients with chronic HFrEF, sinus rhythm, and a baseline heart rate ≥ 77 b.p.m. included in the SHIFT trial, treatment with ivabradine on top of standard therapy for HFrEF improved major clinical outcomes, including PROs.
Conflict of interest
Dr Bouabdallaoui was consultant for AstraZeneca (2019). Dr O'Meara has received research subventions from Novartis, Bayer, AstraZeneca, and Merck; has been involved in clinical trials with Servier, Amgen, and Cardiorentis; has participated in conferences for Novartis, Pfizer, Servier, AstraZeneca, and Bayer; and is consultant for Bayer, Novartis, Servier, and Amgen. Virginie Bernier is employee of Servier Canada Inc. Dr Komajda is consultant for Servier, Novartis, and Sanofi and has speaker activity for Servier, Novartis, MSD, and Sanofi. Dr Swedberg has received honoraria from and participated in advisory boards of AstraZeneca, Amgen, Novartis, Pfizer, Servier, and Vifor Pharma. Dr Tavazzi is Trial Committee Member, Member of the speakers' bureau for the SHIFT trial, and Trial Committee Member for CVIE THERAPEUTICS. Dr Borer is a consultant to Servier, participated as a member of the Executive Committee of SHIFT, and is a consultant to AstraZeneca, Novartis, Takeda USA, General Electric, and CellAegis. Dr Bohm has received honoraria from Abbott, Amgen, Astra, Bayer, Boehringer, Medtronic, Servier, and Vifor. Dr Ford has received research grants and committee honoraria from Servier. Dr Tardif reports grands from Amarin, AstraZeneca, DalCor, Esperion, Ionis, RegenexBio, Sanofi and Servier; honoraria from Amarin, DalCor, Sanofi and Servier; minor equity interest in DalCor; patent on pharmacogenomics‐guided CETP inhibition.
Funding
The SHIFT trial was funded by Servier, France.
Bouabdallaoui, N. , O'Meara, E. , Bernier, V. , Komajda, M. , Swedberg, K. , Tavazzi, L. , Borer, J. S. , Bohm, M. , Ford, I. , and Tardif, J.‐C. (2019) Beneficial effects of ivabradine in patients with heart failure, low ejection fraction, and heart rate above 77 b.p.m. ESC Heart Failure, 6: 1199–1207. 10.1002/ehf2.12513.
References
- 1. Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, SHIFT investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet 2010; 376: 886–894. [DOI] [PubMed] [Google Scholar]
- 2. Bohm M, Borer J, Ford I, Gonzalez‐Juanatey JR, Komajda M, Lopez‐Sendon J, Reil JC, Swedberg K, Tavazzi L. Heart rate at baseline influences the effect of ivabradine on cardiovascular outcomes in chronic heart failure: analysis from the SHIFT study. Clin Res Cardiol 2013; 102: 11–22. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N, Grzeslo A, Hamilton PG, Heckman GA, Howlett JG. 2017 Comprehensive Update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol 2017; 33: 1342–1433. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 6. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation 2013; 127: 2233–2249. [DOI] [PubMed] [Google Scholar]
- 7. Stehlik J, Rodriguez‐Correa C, Spertus JA, Biber J, Nativi‐Nicolau J, Zickmund S, Steinberg BA, Peritz DC, Walker A, Hess J, Drakos SG, Kfoury AG, Fang JC, Selzman CH, Hess R. Implementation of real‐time assessment of patient‐reported outcomes in a heart failure clinic: a feasibility study. J Card Fail 2017; 23: 813–816. [DOI] [PubMed] [Google Scholar]
- 8. Kelkar AA, Spertus J, Pang P, Pierson RF, Cody RJ, Pina IL, Hernandez A, Butler J. Utility of patient‐reported outcome instruments in heart failure. JACC Heart Fail 2016; 4: 165–175. [DOI] [PubMed] [Google Scholar]
- 9. Psotka MA, von Maltzahn R, Anatchkova M, Agodoa I, Chau D, Malik FI, Patrick DL, Spertus JA, Wiklund I, Teerlink JR. Patient‐reported outcomes in chronic heart failure: applicability for regulatory approval. JACC Heart Fail 2016; 4: 791–804. [DOI] [PubMed] [Google Scholar]
- 10. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 11. Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004; 110: 546–551. [DOI] [PubMed] [Google Scholar]
- 12. Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Piña IL, Weinfurt KP. Relationships between changes in patient‐reported health status and functional capacity in outpatients with heart failure. Am Heart J 2012; 163: 88–94.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 14. Luo N, O'Connor CM, Cooper LB, Sun JL, Coles A, Reed SD, Whellan DJ, Piña IL, Kraus WE, Mentz RJ. Relationship between changing patient‐reported outcomes and subsequent clinical events in patients with chronic heart failure: insights from HF‐ACTION. Eur J Heart Fail 2019; 21: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ekman I, Chassany O, Komajda M, Böhm M, Borer JS, Ford I, Tavazzi L, Swedberg K. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J 2011; 32: 2395–2404. [DOI] [PubMed] [Google Scholar]
- 16. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 17. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 18. Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, Misier AR, Böcker D, Block M, Kirkels JH, Kramer A, Huvelle E, Pacing Therapies in Congestive Heart Failure II Study Group , Guidant Heart Failure Research Group . Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol 2003; 42: 2109–2116. [DOI] [PubMed] [Google Scholar]
- 19. DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs 2007; 67: 15–24. [DOI] [PubMed] [Google Scholar]
- 20. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 21. Tardif JC, O'Meara E, Komajda M, Böhm M, Borer JS, Ford I, Tavazzi L, Swedberg K, SHIFT Investigators . Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J 2011; 32: 2507–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough P, Pina I, Tooley J, Weintraub WS, Rumsfeld JS, Cardiovascular Outcomes Research Consortium . Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150: 707–715. [DOI] [PubMed] [Google Scholar]
- 23. Zachariah D, Stevens D, Sidorowicz G, Spooner C, Rowell N, Taylor J, Kay R, Salek MS, Kalra PR. Quality of life improvement in older patients with heart failure initiated on ivabradine: results from the UK multi‐centre LIVE:LIFE prospective cohort study. Int J Cardiol 2017; 249: 313–318. [DOI] [PubMed] [Google Scholar]
- 24. Riccioni G, Prencipe G, Benvenuto A, Masciocco L, Ventra S, Rizzo U, Russi C, Speziale G. Ivabradine improves all aspects of quality of life assessed with the 36‐item short form health survey in subjects with chronic ischemic heart disease compared with beta‐blockers. Pharmacology 2013; 91: 35–38. [DOI] [PubMed] [Google Scholar]
- 25. Zugck C, Störk S, Stöckl G. Long‐term treatment with ivabradine over 12 months in patients with chronic heart failure in clinical practice: effect on symptoms, quality of life and hospitalizations. Int J Cardiol 2017; 240: 258–264. [DOI] [PubMed] [Google Scholar]
- 26. Custodis F, Schirmer SH, Baumhäkel M, Heusch G, Böhm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol 2010; 56: 1973–1983. [DOI] [PubMed] [Google Scholar]
- 27. Metra M, Torp‐Pedersen C, Swedberg K, Cleland JGF, di Lenarda A, Komajda M, Remme WJ, Lutiger B, Scherhag A, Lukas MA, Charlesworth A, Poole‐Wilson PA. Influence of heart rate, blood pressure, and beta‐blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J 2005; 26: 2259–2268. [DOI] [PubMed] [Google Scholar]
- 28. Nikolovska Vukadinovic A, Vukadinović D, Borer J, Cowie M, Komajda M, Lainscak M, Swedberg K, Boehm M. Heart rate and its reduction in chronic heart failure and beyond. Eur J Heart Fail 2017; 19: 1230–1241. [DOI] [PubMed] [Google Scholar]
- 29. Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Sendon JLL, Steg PG, Tardif JC, Tavazzi L, Tendera M, Heart Rate Working Group . Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007; 50: 823–830. [DOI] [PubMed] [Google Scholar]
- 30. Mulder BA, Damman K, van Veldhuisen DJ, van Gelder IC, Rienstra M. Heart rate and outcome in heart failure with reduced ejection fraction: differences between atrial fibrillation and sinus rhythm‐A CIBIS II analysis. Clin Cardiol 2017; 40: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flannery G, Gehrig‐Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta‐blockers. Am J Cardiol 2008; 101: 865–869. [DOI] [PubMed] [Google Scholar]
- 32. Swedberg K, Komajda M, Böhm M, Borer J, Robertson M, Tavazzi L, Ford I, SHIFT Investigators . Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta‐blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol 2012; 59: 1938–1945. [DOI] [PubMed] [Google Scholar]
- 33. Cerbai E, Pino R, Porciatti F, Sani G, Toscano M, Maccherini M, Giunti G, Mugelli A. Characterization of the hyperpolarization‐activated current, I(f), in ventricular myocytes from human failing heart. Circulation 1997; 95: 568–571. [DOI] [PubMed] [Google Scholar]
- 34. Roubille F, Tardif JC. New therapeutic targets in cardiology: heart failure and arrhythmia: HCN channels. Circulation 2013; 127: 1986–1996. [DOI] [PubMed] [Google Scholar]
- 35. Reil JC, Tardif JC, Ford I, Lloyd SM, O'Meara E, Komajda M, Borer JS, Tavazzi L, Swedberg K, Böhm M. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. J Am Coll Cardiol 2013; 62: 1977–1985. [DOI] [PubMed] [Google Scholar]


