Forager-horticulturalist children do not spend more calories than industrialized children, but they do spend calories differently.
Abstract
Children’s metabolic energy expenditure is central to evolutionary and epidemiological frameworks for understanding variation in human phenotype and health. Nonetheless, the impact of a physically active lifestyle and heavy burden of infectious disease on child metabolism remains unclear. Using energetic, activity, and biomarker measures, we show that Shuar forager-horticulturalist children of Amazonian Ecuador are ~25% more physically active and, in association with immune activity, have ~20% greater resting energy expenditure than children from industrial populations. Despite these differences, Shuar children’s total daily energy expenditure, measured using doubly labeled water, is indistinguishable from industrialized counterparts. Trade-offs in energy allocation between competing physiological tasks, within a constrained energy budget, appear to shape childhood phenotypic variation (e.g., patterns of growth). These trade-offs may contribute to the lifetime obesity and metabolic health disparities that emerge during rapid economic development.
INTRODUCTION
Metabolic energy is needed to perform life’s essential tasks, including somatic maintenance (e.g., immune activity and cellular repair), growth, reproduction, and physical activity. Life history theory proposes that organisms have evolved to adaptively manage the allocation of energetic resources between these competing tasks, resulting in trade-offs that shape phenotypic variation across the life course (1, 2). Energetic trade-offs and evolved constraints on overall total energy expenditure (TEE; kcal/day) have been demonstrated for numerous species (3–6), including humans (7–11).
Despite wide acceptance of energetic trade-offs and constraint in evolutionary biology, prevailing models in human health and nutrition assume that energy use is additive (12). Such additive TEE models suggest that, rather than engendering trade-offs, caloric investment in any single metabolic task correspondingly increases TEE in an unbounded, dose-dependent manner (Fig. 1A). The additive TEE model implies large differences in energy budgets between populations owing to differences in lifestyle and environment. Among industrialized populations, increasingly sedentary lifestyles and minimal pathogen burdens are assumed to lower TEE, thereby promoting positive energy imbalance and accelerating the global pandemic of obesity and metabolic disease that accompanies secular dietary change (13).
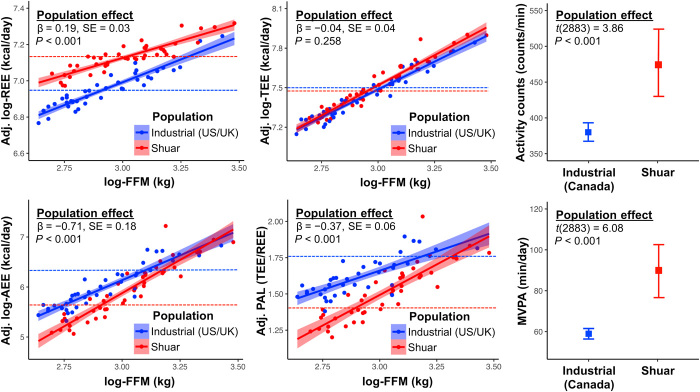
Fig. 1. Predicted, observed, and modeled patterns of children’s energy expenditure in industrial (US/UK) and subsistence-based (Shuar) populations.
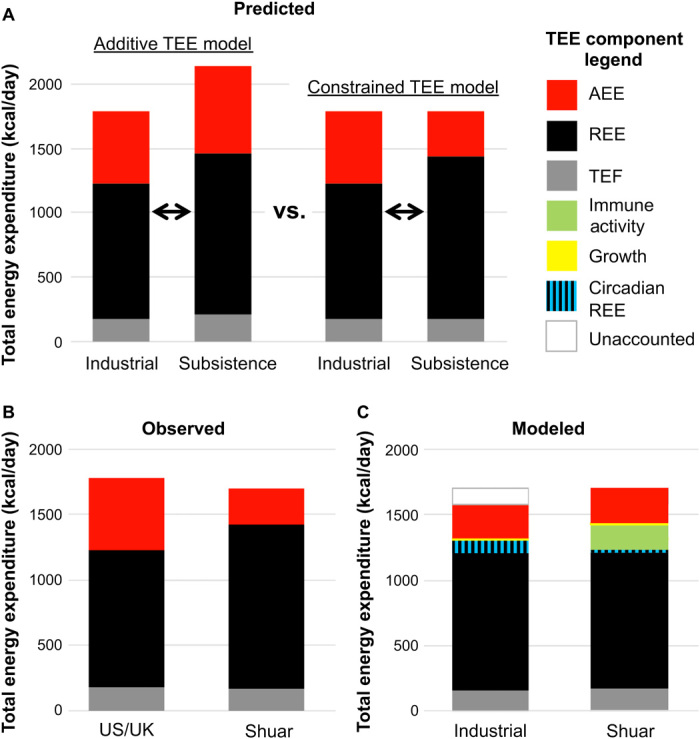
Predicted additive TEE and constrained TEE models of human energy use (A), with observed (B) and modeled (C) components of TEE among Shuar and industrialized children. (A) Children from subsistence-based populations with relatively heavy burdens of infectious disease and active lifestyles are predicted to either increase TEE as a result of increased REE and AEE (additive TEE model) or maintain TEE at industrialized levels as a result of REE and AEE trade-offs (constrained TEE model). (B) Observed energy expenditures in Shuar and U.S./U.K. cohorts follow the constrained TEE model with greater Shuar REE but lower AEE and no overall TEE difference. (C) Modeled energy expenditures accounting for immune activity and growth, as well as possible population differences in exercise efficiency and REE circadian variation, produce TEE estimates for Shuar and industrial populations that vary by only 8% (Materials and Methods). TEE, total energy expenditure; REE, resting energy expenditure; AEE, activity energy expenditure; TEF, thermic effect of food.
Determining whether human energy expenditure is constrained or additive is critical for understanding and reversing global trends in obesity and poor metabolic health. The implications of this understanding are perhaps most salient during childhood, a uniquely human life stage (14) that is exceptionally energetically demanding (9) and is critical in establishing lifetime trajectories of phenotype and metabolic health (15). Problematically, no studies of childhood TEE regulation to date have been performed among cohorts with chronically high levels of immune and physical activity. Nor have they included the objective measures of resting energy expenditure (REE; in kilocalories per day) and daily physical activity necessary to identify possible energy allocation trade-offs.
Here, we test for constraint and trade-offs in childhood energy expenditure using doubly labeled water (DLW) measures of TEE and respirometry measures of REE from forager-horticulturalist Shuar in Amazonian Ecuador. The Shuar engage in physically active, subsistence-based lifestyles and experience persistent immune activation (7). We compared Shuar TEE and REE, as well as activity energy expenditure (AEE; the portion of TEE not attributed to REE or digestion), physical activity level (PAL; TEE/REE), and measures of daily physical activity (from accelerometry) to data from children living in industrialized populations (16–18). The additive TEE model predicts that Shuar TEE should be elevated relative to industrial references, reflecting greater investment in immune activity (increased REE) and physical activity (increased AEE). In contrast, the constrained TEE model predicts that TEE should be similar between populations, the result of trade-offs between underlying energy expenditure components.
RESULTS
High Shuar REE and daily physical activity
Shuar REE and daily physical activity were elevated compared to industrialized children. In multivariable analysis controlling for age, sex, log–fat mass (FM), and log–fat free mass (FFM), Shuar REE exceeded that of U.S./U.K. children by 213 kcal/day or 20% (β = 0.19, SE = 0.03, P < 0.001; Fig. 2, Table 1, and table S1). Likewise, Shuar accelerometry averaged 96 counts/min (25%) more than Canadian references [two-sample t(2883) = 3.86, P < 0.001; Fig. 2 and Table 1], with Shuar spending 31 min/day (53%) more time than industrialized children in moderate-vigorous physical activity [MVPA; two-sample t(2883) = 6.08, P < 0.001; Fig. 2 and Table 1].
Fig. 2. Energy expenditure and daily physical activity measures for Shuar children (red) and industrialized cohorts (blue).
Scatterplot solid lines (shaded 95% confidence intervals) indicate regressions of energetic measures on log–fat-free mass (FFM) adjusting for age, sex, and log–fat mass (FM), with dotted lines denoting population estimated marginal means from final energetic models. Plots of accelerometry activity counts and MVPA display unadjusted population means (95% confidence intervals). No population difference was observed for TEE. However, Shuar children had greater REE, lower AEE and PAL, and greater activity counts and MVPA than industrialized references. MVPA, moderate-vigorous physical activity.
Table 1. Measures of interest for Shuar and industrialized cohorts.
| Population | ||
| Shuar (n = 44) | Industrial (n = 40)† | |
| Descriptive, mean (95% CI) | ||
| Sex (% male) | 50% | 56% |
| Age (years) | 8.1 (7.5 to 8.7)* | 7.1 (6.7 to 7.5) |
| Anthropometry, adjusted mean (95% CI) | ||
| Stature (cm) | 117.0 (115.2 to 118.8)*** | 124.6 (122.7 to 126.4) |
| Body mass (kg) | 22.8 (21.6 to 24.0)*** | 26.0 (24.7 to 27.3) |
| Body mass index (kg/m2) | 16.5 (16.1 to 17.0) | 16.5 (16.1 to 17.0) |
| Fat mass (kg)‡ | 2.7 (2.4 to 3.0)*** | 5.7 (5.0 to 6.4) |
| Fat-free mass (kg)‡ | 19.7 (18.9 to 20.3) | 19.5 (18.7 to 20.3) |
| Body fat percentage (%)‡ | 11.9% (11.0 to 12.9)*** | 22.4% (20.5 to 24.5) |
| Energetics, adjusted mean (95% CI) | ||
| TEE (kcal/day)‡ | 1738 (1670 to 1809) | 1811 (1733 to 1892) |
| REE (kcal/day)‡ | 1255 (1215 to 1296)*** | 1042 (1006 to 1080) |
| Activity energy expenditure (kcal/day)‡ |
276 (225 to 338)*** | 558 (447 to 698) |
| Physical activity level | 1.38 (1.31 to 1.46)*** | 1.76 (1.68 to 1.84) |
| Shuar (n = 30) | Industrial (n = 2855)§ | |
| Daily physical activity, mean (95% CI) | ||
| Activity wear time (hours/day) |
12.2 (11.6 to 12.8)*** | 13.5 (13.4 to 13.6) |
| Activity counts (CPM) | 474 (433 to 516)*** | 379 (365 to 392) |
| Sedentary activity (min/day) |
240 (228 to 252)*** | 478 (473 to 483) |
| Light activity (min/day) | 405 (382 to 428)*** | 273 (268 to 278) |
| MVPA (min/day) | 89 (76 to 102)*** | 58 (55 to 61)|| |
‡Values back-converted from analysis of log-transformed measures (table S1); *P < 0.05; ***P < 0.001; population-level differences in two-tailed t tests (daily physical activity measures) or multivariable models controlling for age and sex (anthropometry measures) or age, sex, log-FM, and log-FFM (energetics measures).
§Canadian cohort (18).
||U.K. sample reported 64 min/day of moderate-vigorous activity (17).
No difference in Shuar TEE
Despite greater REE and daily physical activity, Shuar TEE did not differ from that of children in industrialized populations. Controlling for age, sex, log-FM, and log-FFM, there was no difference between Shuar and U.S./U.K. TEE values (β = −0.04, SE = 0.04, p = 0.258; alternative models give similar results in table S2; Fig. 2, Table 1, and table S1). Results were consistent in analysis of mean TEE values from a larger and more diverse sample of children in industrialized countries (fig. S1). These results support the constrained TEE model of energy use.
Low Shuar AEE and PAL
Shuar children spent 72% of their total energy budget on REE, compared to only 58% for U.S./U.K. children. As a result, Shuar AEE was 282 kcal/day (51%) lower than for more sedentary industrialized children when adjusting for age, sex, log-FM, and log-FFM (β = −0.71, SE = 0.18; P < 0.001; Fig. 2, Table 1, and table S1). Shuar PAL was similarly 0.38 units below that of the U.S./U.K. cohort (β = −0.37, SE = 0.06, P < 0.001; Fig. 2, Table 1, and table S1). Results were consistent in models excluding FM (table S2). Low AEE and PAL among highly active Shuar children indicate that these common measures are not reliable indices of daily physical activity.
DISCUSSION
The findings of this study provide evidence for constraint and trade-offs in energy expenditure during childhood. Shuar forager-horticulturalist children of Amazonia are ~25% more physically active and, in association with elevated immune activity, have ~20% greater REE than children from industrialized populations. Despite these differences, Shuar TEE is indistinguishable from that of U.S./U.K. counterparts. These results are consistent with life history theory prediction for adaptive energy allocation and challenge the commonly used additive TEE model of human energy use.
To further investigate the nature of childhood energy constraint and trade-offs, we modeled the contributions of immune function, growth, body size/composition, and circadian rhythm to TEE and its underlying components for both Shuar and industrialized populations (Materials and Methods; Fig. 1C). Elevated Shuar REE does not result from clear differences in FFM composition (i.e., muscle-to-organ mass ratio; fig. S2) and runs counter to expectation of low REE in tropical climates entailing low thermoregulatory costs (19). As demonstrated among other Amazonian forager-horticulturalists (20), elevated Shuar REE likely results from persistent immune activation (7) in the context of high environmental pathogenicity (21). This position is supported in the present sample by a positive relationship between Shuar blood concentration of total immunoglobulin G (IgG)—the most common class of circulating antibody in humans (22)—and both log-REE (β = 0.15, SE = 0.05, P = 0.003; Fig. 3) and REE elevation above U.S./U.K. predicted values (β = 252.66, SE = 64.38, P < 0.001; Fig. 3).
Fig. 3. Shuar total immunoglobulin G (IgG) concentration predicts child REE measures.
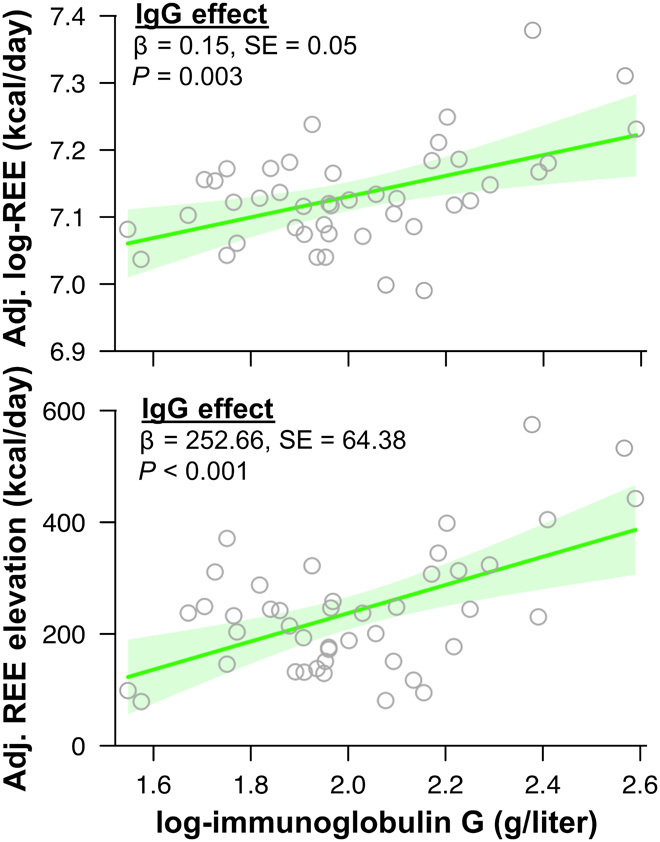
Shuar total immunoglobulin G (IgG) concentration versus measured REE (top) and REE elevation above predicted U.S./U.K. values (bottom). Solid lines (shaded 95% confidence intervals) indicate regression of log-IgG adjusting for age, sex, log-FM, log-FFM, and time of REE data collection (log-REE analysis) or time of REE data collection (REE elevation analysis). REE elevation was calculated as the difference between measured REE values and REE predicted from the best-fit model of the U.S./U.K. sample (R2 = 0.588, P < 0.001; adjusting for age, sex, log-FM, and log-FFM).
Shuar children exhibit lower AEE than expected given relatively high accelerometry–measured physical activity. This finding is not readily explained by possible minimal forager-horticulturalist differences in thermic effect of food (TEF) [owing to dietary differences (12); table S3] or by differences in sleep duration [forager-horticulturalist’s daily sleep duration is similar to that of industrialized populations (23)]. We modeled three additional factors that could contribute to this finding (Materials and Methods). First, 14% greater body mass for the U.S./U.K. population (Table 1) will increase the energy cost of movement (i.e., kcal/accelerometer count). Second, Shuar children may be somewhat more energetically efficient in movement owing to greater daily workloads (24). Third, circadian fluctuation in REE, which can approach ~10% for adults in industrialized populations (25), may be relatively blunted in energetically stressed populations such as the Shuar. Consequently, the difference between true 24-hour REE and REE extrapolated from standard early morning measurements may be greater for industrialized populations, thereby inflating their AEE calculation. Modeling the components of TEE with these considerations indicates that differences in immune activity, growth, movement costs, exercise efficiency, and circadian fluctuation in REE can largely explain the observed similarity in Shuar and U.S./U.K. TEE (8% error; Fig. 1C). Unaccounted energy expenditure in the industrial model may reflect relatively lower metabolic efficiency (e.g., greater mitochondrial proton leak) or greater 24-hour costs of thermoregulation.
Constraint in childhood TEE emphasizes the role of energetic trade-offs in shaping human developmental plasticity and life history variation. These trade-offs, while occurring throughout development, are particularly salient during childhood, when brain metabolic costs peak (9), and often involve physical growth (7, 8). Trade-offs reducing growth result in smaller body size, which decreases energy requirements. Such a response may be adaptive by reducing risk of negative energy balance and starvation in challenging environments. However, it may also have lifetime effects on metabolism (see below) and necessitate extended periods of growth to obtain target adult body size (26). Notably, constraint and trade-offs in childhood energy expenditure imply that models of human life history evolution and cooperative breeding that rely on standard additive estimates of TEE [e.g., (27)] may require restructuring to reliably calculate the energetic cost of supporting dependent offspring in subsistence-based contexts.
Exercise is essential for health in both children and adults (28), but evidence for childhood TEE constraint warrants reassessment of the mechanisms linking physical activity and lifetime well-being. Incongruity between lifestyle and TEE at the population level supports the position that secular change in diet (i.e., energy intake), not physical activity (i.e., energy expenditure), is the primary determinant of the chronic energy imbalance underlying increasing global rates of obesity (29). For populations in the developing world, TEE constraint implies that elevated physical and immune activity may reduce energy available for childhood growth, even when food is not limited and energy balance is positive. Global disparities in risk of child growth faltering and resulting lifetime metabolic dysregulation (30) may therefore be more strongly related to variation in lifestyle and infectious disease burden than variation in energy availability.
The lasting effects of childhood energetic conditions on later life health are well documented (15), and metabolic plasticity driven by TEE constraint may contribute to the rapidly emerging dual burden of childhood growth stunting and adult obesity in the developing world (31). Future research addressing these topics should build on the limitations of the present work by collecting primary data that span a range of economic development and lifestyle variation within a single transitioning population. Future studies should also investigate the nature of energy constraint and trade-offs across a wider sub-adult age range, including among infants and younger children that are more susceptible to growth faltering, typically in association with infection-induced anorexia and poor nutrient absorption/retention (32). Adopting models of childhood energy expenditure that account for constraint and trade-offs will advance strategies to promote lifetime health.
MATERIALS AND METHODS
Shuar participants and study design
The Shuar are an indigenous population of ≈50,000 individuals, many of whom continue to rely on subsistence hunting, fishing, foraging, and horticulture (33, 34). The study community of ≈300 individuals is located in the isolated cross-Cutucú geographic area. The community is not accessible by road and has no running water or health clinic. Electricity is limited and highly intermittent. Household-level economic, lifestyle, and dietary information for the study sample are provided in table S3. Data were collected over 14-day study periods during the 2016 annual dry season (October to November). All resident prepubertal children (age 5 to 12 years) were invited to participate, with a final sample of 44 children (generally healthy and nonmedicated). No participants were seriously ill at any point during the study, as determined from weekly report by children and their parents, investigator observation, and body temperature assessment (mean ± SD = 98.8° ± 0.5°C, range = 97.8° to 99.9°C). Parental informed consent with child informed assent was obtained from all participants. Study methods and procedures were approved and conducted in accordance with guidelines set by community leaders, the Federación Interprovincial de Centros Shuar, and the Committee on the Use of Human Subjects Institutional Review Boards of the University of Oregon and the City University of New York.
Anthropometry and daily physical activity
Shuar height (Seca 214 stadiometer, Hanover, MD), weight (Tanita BF-689 scale, Tokyo), triceps skinfold (Beta Technology Lange calipers, Santa Cruz, CA), and arm circumference (Seca 201 tape, Hanover, MD) were measured on day 0 using conventional methods. Body temperature was measured weekly with an aural thermometer (Welch Allyn Thermoscan Pro 6000, Skaneateles Falls, NY). Physical activity was monitored for a subsample of 30 children over the 14-day study period using Actical triaxial accelerometers (Philips Respironics, Bend, OR) worn continuously at the right hip. Retrieved data were processed using a macro program to remove non-wear time (35), with valid days defined by wear time ≥10 hours. All children had ≥5 valid days of data (mean ± SD = 13.8 ± 2.3 days). Activity levels were established using standard child-specific activity count cut points (36).
Resting energy expenditure
Shuar REE was measured in triplicate on days 0, 7, and 14 using a validated Quark RMR respirometry system (COSMED, Rome) with a canopy hood. Five children were measured only twice. Measurements were performed in a quiet room in the supine position, with parents nearby. All measures were made in the morning (mean ± SD = 06:43 ± 43 min), following overnight fast (mean ± SD = 12.4 ± 1.5 hours reported fast), and before strenuous physical activity. Device calibration was performed before each measure using a manufacturer-provided standard gas mixture and volume syringe. Child O2 consumption and CO2 production were monitored continuously for ≥30 min, with the first 10 min of data discarded and the remaining steady-state period averaged to determine REE using the modified Weir equation (37). Overall, reliability of repeated weekly REE measures was high [coefficient of variation (CV) = 6.4%], with the average of weekly values representing final REE. Model results were similar when analyzing conservative values of Shuar REE that excluded initial or single highest repeated measures (table S4).
TEE and body composition
Shuar TEE and body composition were measured using the DLW method (37). Oral doses of DLW (6% 2H2O, 10% H218O, tailored to body weight) were given to children on day 0. Urine samples were collected before dosing, ≈6 hours post-dose, and on ≈days 3, 7, and 11 (mean ± SD assessment = 11.0 ± 1.9 days). Samples were stored at −20°C until measurement of 2H and 18O isotope enrichment via cavity ring-down spectrometry (CRDS; Picarro L2120i, Santa Clara, CA). Isotope depletion rates and dilution spaces were calculated using the slope-intercept method, with rate of CO2 production subsequently calculated using a two-pool approach and converted to TEE using a food quotient of 0.93, as previously determined for Amazonian forager-horticulturalists (20). Because of common dehydration in Amazonian children, fat-free mass (FFM) was calculated using a hydration constant of 0.73. Findings were similar when using a hydration constant of 0.75 (table S5).
Reliability of TEE and body composition measures
The DLW method is the gold standard for the measurement of free-living TEE in humans (37). DLW measures of TEE and FFM are directly comparable across laboratories following standardized data collection and analytical protocols (37). To provide additional assurance that our CRDS DLW measures were reliable and directly comparable to U.S./U.K. values, duplicate measures were obtained for six Shuar participants using isotope-ratio mass spectrometry at an external laboratory (between assay CVTEE = 2.0%, CVFFM = 0.3%; table S6).
Total immunoglobulin G
Shuar circulating concentration of IgG was measured using a validated enzyme-linked immunosorbent assay protocol in finger-prick dried blood spot (DBS) samples (7). Participant DBS samples were collected following REE measurement on days 0, 7, and 14 using standard procedures (38). All DBS samples were stored at −20°C via a solar-powered freezer until shipped to the United States for storage at −30°C. Samples were measured for IgG in duplicate with two-level controls. Intra- and interassay measurement CVs for the assay are 2.0 to 2.7% and 8.0 to 10.8%, respectively (7). Final IgG concentration was obtained by averaging participant repeated weekly measures.
Comparative industrialized cohorts
Energetics data for the U.S./U.K. cohort were obtained from studies of healthy prepubertal children and were selected for their use of similar coupled DLW/respirometry protocols and complete individual-level data. Data were included for 20 children (age, 5 to 6 years) from Vermont, USA (16), and 20 children (age, 7 to 9 years) from Northern Ireland, United Kingdom (17). One child from the published U.S. sample was excluded because of negative calculated AEE. Similar to the Shuar sample, TEE was measured over a ≈2-week period, and REE was measured in the morning before any strenuous activity using a canopy hood. Measures of REE equivalent to those obtained for the Shuar were reported as “basal metabolic rate” in the U.K. study. For U.S. children only, reported nonfasted REE values were converted to fasted REE by multiplying by 0.89, a published correction factor determined and validated specifically for the U.S. study protocol (39). This had minimal effect on the analysis, as Shuar REE remained significantly greater (+169 kcal/day) than that of the U.S./U.K. cohort even when modeling uncorrected (i.e., inflated) U.S. REE values (β = 0.14, SE = 0.03, P < 0.001). U.S. and U.K. energetic measures (i.e., TEE, REE, and AEE) did not significantly differ at the group level (all P < 0.1), and TEE and REE measures were similar to values calculated by common prediction equations (table S7). This supports the treatment of the U.S. and U.K. cohorts as a single, broadly representative industrialized cohort in general linear models (see below).
Accelerometry data are not available for the U.S./U.K. cohort. Hence, physical activity data for a nationally representative Canadian cohort (Canadian Health Measures Survey 2009 to 2015; age 5 to 12 years, n = 1433 males and n = 1422 females) were used (data file S2) (18). These data were collected with Actical devices and were processed using similar methods to those for the Shuar. Canadian cohort MVPA (58 min/day) approximates that reported for the U.K. cohort (64 min/day) measured by the flex-heart rate method (17). This finding suggests that physical activity patterns are broadly similar across the two groups. Canadian physical activity measures are also comparable to those reported for the U.S. National Health and Nutrition Examination Survey references (40), suggesting general agreement in daily physical activity across the Canadian, U.K., and U.S. cohorts.
Data analysis
Participant AEE was calculated as TEE − (REE + 0.1TEE), where 0.1TEE reflects TEF (12). Population differences in daily physical activity measures were tested using two-sample t tests. Population differences in anthropometric and energetic measures were tested using generalized linear models, following conventional methods. Age, sex, log-FM, and log-FFM were included as predictors in all final energetic models. Body mass and body mass index were examined in preliminary analyses, with model fit assessed using residual sum of squares. Details for specific models are provided in figure and table captions. Post hoc diagnostic analyses revealed acceptable degrees of linearity, heteroscedasticity, and multicollinearity in final models. All analyses were performed in R (cran.us.r-project.org/), with results reported as statistically significant at P < 0.05.
Modeling TEE component energy budgets
To investigate the contribution of different metabolic tasks to child TEE in Shuar and industrialized populations, we modeled energy used in REE, diurnal REE fluctuation, physical activity, immune function, growth, and digestion.
Resting energy expenditure
Human REE is strongly related to FFM, specifically the size and tissue-specific metabolic rates of the internal organs (41). As the FFM of Shuar and U.S./U.K. children were indistinguishable (Table 1), we used the observed REE value for the U.S./U.K. sample (1042 kcal/day; Table 1) for both the Shuar and industrial TEE models.
Diurnal REE fluctuation
REE fluctuates throughout the day in a circadian rhythm, with its nadir near 06:00 and its peak near 16:00 (25). The mean diurnal difference between minimum and maximal adult REE is ~10% (25). We modeled an additional 10% increment of REE for the industrial TEE model (diurnal REE fluctuation increment = 0.1REE or 104 kcal/day). We hypothesize that diurnal fluctuation in REE is related to the diurnal production of regulatory metabolic hormones (e.g., cortisol and testosterone). In subsistence populations such as the Shuar and similarly in physically active populations, waking and diurnal salivary cortisol levels reduce as much as ~80% compared to industrialized populations (42). Thus, we modeled only a 2% increment in REE for the Shuar TEE model (diurnal REE fluctuation increment = 0.02REE or 21 kcal/day).
Immune function
Given the positive relationship between blood markers of immune activity and REE in both the present Shuar sample (Fig. 3) and in other Amazonian forager-horticulturalist populations (20), we assumed that the elevation of Shuar children’s REE relative to U.S./U.K. values was entirely due to greater infectious disease burden and resultant immune activity. This approach yields an immune function cost for Shuar children of 192 kcal/day. This estimate is similar to that reported for adult Tsimane, an Amazonian population that is ecologically and immunologically similar to the Shuar (7, 20).
Growth
Growth costs for the industrial TEE model were estimated by multiplying the mean rate of growth for U.S. children age 3 to 10 years old [7.2 g/day; (26, 43)] by the childhood-specific cost of synthesizing all new tissue [1.8 kcal/g; (12)]. This cost reflects the energy needed to synthesize new tissue and does not include the energy content of the new tissue itself, which is not captured in DLW measures of TEE. The cost of synthesizing new tissue for growth is thus 13 kcal/day for the industrial TEE model. On the basis of ~20% slower growth velocities among Shuar children (26), we estimated the growth cost for the Shuar TEE model as 10 kcal/day.
Physical activity
We estimated the energy cost of physical activity using the ratio of AEE to accelerometer-measured body movement among the Shuar sample. This approach assumes that AEE among the Shuar is entirely (or almost entirely) reflective of musculoskeletal activity, whereas AEE for the U.S./U.K. cohort is inflated by greater diurnal fluctuation in REE. For Shuar children, the ratio of AEE/mean accelerometer counts per minute (CPM; Table 1) yields 0.58 kcal/CPM. Mean body mass in the U.S./U.K. sample is 14% greater than the Shuar sample (Table 1), indicating that that the cost of movement for U.S./U.K. children should be 14% greater or 0.66 kcal/CPM. Last, we assumed that the efficiency of movement might be up to 5% greater for the Shuar due to their greater habitual physical activity (24), which yields a final U.S./U.K. cost of movement of 0.70 kcal/CPM. Multiplying this ratio by observed mean CPM for the industrial sample (379 CPM) yields a cost of physical activity of 264 kcal/day for the industrial TEE model.
Digestion
The energy cost of digestion (TEF) is closely related to TEE and was estimated as 0.1TEE (12) in both the Shuar and industrial TEE models.
Supplementary Material
Acknowledgments
We thank the Shuar for their participation and hospitality. We also thank R. Colley and Statistics Canada for providing Canadian Health Measures Survey physical activity summary data. Funding: National Science Foundation (no. SMA1606852). Author contributions: S.S.U., H.P., J.J.S., L.R.D., and L.S.S. designed the study. S.S.U. collected data. S.S.U., H.P., and C.J.J. analyzed data. All authors contributed to writing the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/12/eaax1065/DC1
Fig. S1. TEE measures for Shuar children (red) and a larger and more diverse sample of industrialized cohorts (blue).
Fig. S2. Shuar arm muscle area (AMA) measures as percentiles of US age- and sex-matched references (NHANES III).
Table S1. Parameter estimates [β (SE)] for final energetics GLM models.
Table S2. Parameter estimates [β (SE)] for energetics GLM models that do not include FM as a predictor.
Table S3. Household-level lifestyle, economic, and dietary information for the Shuar study sample (n = 18 households).
Table S4. Parameter estimates [β (SE)] for GLM models evaluating conservative values of Shuar REE that excluded initial (REEi) or single highest (REEh) repeated weekly measures.
Table S5. Parameter estimates [β (SE)] for GLM models using an alternative hydration constant of 0.75 for Shuar and US cohort FM and FFM calculation.
Table S6. Measured TEE and FFM using CRDS and duplicate measures (TEEirms; FFMirms) obtained for six participants using isotope ratio mass spectrometry.
Table S7. Measured REE and TEE for US/UK children and predicted values calculated by common prediction equations (that were developed using predominantly industrialized samples).
Data file S1. Primary study data with variable list.
Data file S2. Daily physical activity summary data for the Canadian cohort.
Data file S3. Expanded industrialized sample data.
REFERENCES AND NOTES
- 1.S. C. Stearns, The Evolution of Life Histories (Oxford Univ. Press, 1992). [Google Scholar]
- 2.E. L. Charnov, Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology (Oxford Univ. Press, 1993). [Google Scholar]
- 3.Stearns S. C., Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268 (1989). [Google Scholar]
- 4.G. Demas, R. Nelson, Ecoimmunology (Oxford Univ. Press, 2011). [Google Scholar]
- 5.Humphries M. M., Careau V., Heat for nothing or activity for free? Evidence and implications of activity-thermoregulatory heat substitution. Integr. Comp. Biol. 51, 419–431 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Speakman J. R., Król E., Maximal heat dissipation capacity and hyperthermia risk: Neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Urlacher S. S., Ellison P. T., Sugiyama L. S., Pontzer H., Eick G., Liebert M. A., Cepon-Robins T. J., Gildner T. E., Snodgrass J. J., Tradeoffs between immune function and childhood growth among Amazonian forager-horticulturalists. Proc. Natl. Acad. Sci. U.S.A. 115, E3914–E3921 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urlacher S. S., Kramer K. L., Evidence for energetic tradeoffs between physical activity and childhood growth across the nutritional transition. Sci. Rep. 8, 369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuzawa C. W., Chugani H. T., Grossman L. I., Lipovich L., Muzik O., Hof P. R., Wildman D. E., Sherwood C. C., Leonard W. R., Lange N., Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. U.S.A. 111, 13010–13015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pontzer H., The crown joules: Energetics, ecology, and evolution in humans and other primates. Evol. Anthropol. 26, 12–24 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Dugas L. R., Harders R., Merrill S., Ebersole K., Shoham D. A., Rush E. C., Assah F. K., Forrester T., Durazo-Arvizu R. A., Luke A., Energy expenditure in adults living in developing compared with industrialized countries: A meta-analysis of doubly labeled water studies. Am. J. Clin. Nutr. 93, 427–441 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FAO/WHO/UNU, Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation: Rome, 17 to 24 October 2001 (2004).
- 13.Popkin B. M., Adair L. S., Ng S. W., Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 70, 3–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogin B., Smith B., Evolution of the human life cycle. Am. J. Hum. Biol. 8, 703–716 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Dietz W. H., Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics 101, 518–525 (1998). [PubMed] [Google Scholar]
- 16.Goran M. I., Carpenter W. H., Poehlman E. T., Total energy expenditure in 4- to 6-yr-old children. Am. J. Physiol. Endocrinol. Metab. 264, E706–E711 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Livingstone M., Coward W. A., Prentice A. M., Davies P. S., Strain J. J., McKenna P. G., Mahoney C. A., White J. A., Stewart C. M., Kerr M. J., Daily energy expenditure in free-living children: Comparison of heart-rate monitoring with the doubly labeled water (2H218O) method. Am. J. Clin. Nutr. 56, 343–352 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Colley R. C., Carson V., Garriguet D., Janssen I., Roberts K. C., Tremblay M. S., Physical activity of Canadian children and youth, 2007 to 2015. Health Rep. 28, 8–16 (2017). [PubMed] [Google Scholar]
- 19.Froehle A. W., Climate variables as predictors of basal metabolic rate: New equations. Am. J. Hum. Biol. 20, 510–529 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Gurven M. D., Trumble B. C., Stieglitz J., Yetish G., Cummings D., Blackwell A. D., Beheim B., Kaplan H. S., Pontzer H., High resting metabolic rate among Amazonian forager-horticulturalists experiencing high pathogen burden. Am. J. Phys. Anthropol. 161, 414–425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cepon-Robins T. J., Liebert M. A., Gildner T. E., Urlacher S. S., Colehour A. M., Snodgrass J. J., Madimenos F. C., Sugiyama L. S., Soil-transmitted helminth prevalence and infection intensity among geographically and economically distinct shuar communities in the Ecuadorian Amazon. J. Parasitol. 100, 598–607 (2014). [DOI] [PubMed] [Google Scholar]
- 22.A. K. Abbas, A. H. Lichtman, S. Pillai, Cellular and Molecular Immunology (Elsevier Health Sciences, 2014). [Google Scholar]
- 23.Yetish G., Kaplan H., Gurven M., Wood B., Pontzer H., Manger P. R., Wilson C., McGregor R., Siegel J. M., Natural sleep and its seasonal variations in three pre-industrial societies. Curr. Biol. 25, 2862–2868 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert M. I., Burgess T. L., The effects of training, muscle damage and fatigue on running economy. Int. SportMed J. 11, 363–379 (2010). [Google Scholar]
- 25.Zitting K.-M., Vujovic N., Yuan R. K., Isherwood C. M., Medina J. E., Wang W., Buxton O. M., Williams J. S., Czeisler C. A., Duffy J. F., Human resting energy expenditure varies with circadian phase. Curr. Biol. 28, 3685–3690.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urlacher S. S., Blackwell A. D., Liebert M. A., Madimenos F. C., Cepon-Robins T. J., Gildner T. E., Snodgrass J. J., Sugiyama L. S., Physical growth of the Shuar: Height, weight, and BMI references for an indigenous Amazonian population. Am. J. Hum. Biol. 28, 16–30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurven M., Walker R., Energetic demand of multiple dependents and the evolution of slow human growth. Proc. Biol. Sci. 273, 835–841 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golubic R., Wijndaele K., Sharp S. J., Simmons R. K., Griffin S. J., Wareham N. J., Ekelund U., Brage S.; ProActive Study Group , Physical activity, sedentary time and gain in overall and central body fat: 7-year follow-up of the ProActive trial cohort. Int. J. Obes. 39, 142–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luke A., Cooper R. S., Physical activity does not influence obesity risk: Time to clarify the public health message. Int. J. Epidemiol. 42, 1831–1836 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Prendergast A. J., Humphrey J. H., The stunting syndrome in developing countries. Paediatr. Int. Child Health 34, 250–265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doak C. M., Adair L. S., Bentley M., Monteiro C., Popkin B. M., The dual burden household and the nutrition transition paradox. Int. J. Obes. 29, 129–136 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Dewey K. G., Mayers D. R., Early child growth: How do nutrition and infection interact? Matern. Child Nutr. 7, 129–142 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urlacher S. S., Liebert M. A., Josh Snodgrass J., Blackwell A. D., Cepon-Robins T. J., Gildner T. E., Madimenos F. C., Amir D., Bribiescas R. G., Sugiyama L. S., Heterogeneous effects of market integration on sub-adult body size and nutritional status among the Shuar of Amazonian Ecuador. Ann. Hum. Biol. 43, 316–329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.M. Harner, The Jivaro People of the Sacred Waterfalls (University of California Press, 1984). [Google Scholar]
- 35.Dugas L. R., Bovet P., Forrester T. E., Lambert E. V., Plange-Rhule J., Durazo-Arvizu R. A., Shoham D., Kroff J., Cao G., Cooper R. S., Brage S., Ekelund U., Luke A., Comparisons of intensity-duration patterns of physical activity in the US, Jamaica and 3 African countries. BMC Public Health 14, 882 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puyau M. R., Adolph A. L., Vohra F. A., Zakeri I., Butte N. F., Prediction of activity energy expenditure using accelerometers in children. Med. Sci. Sports Exerc. 36, 1625–1631 (2004). [PubMed] [Google Scholar]
- 37.IAEA, Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques (International Atomic Energy Agency, 2009). [Google Scholar]
- 38.McDade T. W., Williams S., Snodgrass J. J., What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 44, 899–925 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Goran M., Nagy T., Effect of the pre-testing environment on measurement of metabolic rate in children. Int. J. Obes. Relat. Metab. Disord. 20, 83–87 (1996). [PubMed] [Google Scholar]
- 40.Troiano R. P., Berrigan D., Dodd K. W., Mâsse L. C., Tilert T., McDowell M., Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 40, 181–188 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Wang Z., Bosy-Westphal A., Schautz B., Müller M., Mechanistic model of mass-specific basal metabolic rate: Evaluation in healthy young adults. Int. J. Body Compos. Res. 9, 147–152 (2011). [PMC free article] [PubMed] [Google Scholar]
- 42.Urlacher S. S., Liebert M. A., Konečná M., Global variation in diurnal cortisol rhythms: Evidence from Garisakang forager-horticulturalists of lowland Papua New Guinea. Stress 21, 101–109 (2018). [DOI] [PubMed] [Google Scholar]
- 43.R. J. Kuczmarski, Ogden C. L., Guo S. S., Grummer-Strawn L. M., Flegal K. M., Mei Z., Wei R., Curtin L. R., Roche A. F., Johnson C. L., 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat. 11 2002, 1–190 (2002). [PubMed] [Google Scholar]
- 44.Treuth M., Figueroa-Colon R., Hunter G. R., Weinsier R. L., Butte N. F., Goran M. I., Energy expenditure and physical fitness in overweight vs non-overweight prepubertal girls. Int. J. Obes. 22, 440–447 (1998). [DOI] [PubMed] [Google Scholar]
- 45.DeLany J. P., Bray G. A., Harsha D. W., Volaufova J., Energy expenditure in preadolescent African American and white boys and girls: The Baton rouge children’s study. Am. J. Clin. Nutr. 75, 705–713 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Ball E. J., O’Connor J., Abbott R., Steinbeck K. S., Davies P. S. W., Wishart C., Gaskin K. J., Baur L. A., Total energy expenditure, body fatness, and physical activity in children aged 6-9 y. Am. J. Clin. Nutr. 74, 524–528 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Abbott R., Davies P., Habitual physical activity and physical activity intensity: Their relation to body composition in 5.0–10.5-y-old children. Eur. J. Clin. Nutr. 58, 285–291 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Gurney J. M., Jelliffe D. B., Arm anthropometry in nutritional assessment: Nomogram for rapid calculation of muscle circumference and cross-sectional muscle and fat areas. Am. J. Clin. Nutr. 26, 912–915 (1973). [DOI] [PubMed] [Google Scholar]
- 49.Schofield W., Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 39, 5–41 (1985). [PubMed] [Google Scholar]
- 50.Torun B., Energy requirements of children and adolescents. Public Health Nutr. 8, 968–993 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/12/eaax1065/DC1
Fig. S1. TEE measures for Shuar children (red) and a larger and more diverse sample of industrialized cohorts (blue).
Fig. S2. Shuar arm muscle area (AMA) measures as percentiles of US age- and sex-matched references (NHANES III).
Table S1. Parameter estimates [β (SE)] for final energetics GLM models.
Table S2. Parameter estimates [β (SE)] for energetics GLM models that do not include FM as a predictor.
Table S3. Household-level lifestyle, economic, and dietary information for the Shuar study sample (n = 18 households).
Table S4. Parameter estimates [β (SE)] for GLM models evaluating conservative values of Shuar REE that excluded initial (REEi) or single highest (REEh) repeated weekly measures.
Table S5. Parameter estimates [β (SE)] for GLM models using an alternative hydration constant of 0.75 for Shuar and US cohort FM and FFM calculation.
Table S6. Measured TEE and FFM using CRDS and duplicate measures (TEEirms; FFMirms) obtained for six participants using isotope ratio mass spectrometry.
Table S7. Measured REE and TEE for US/UK children and predicted values calculated by common prediction equations (that were developed using predominantly industrialized samples).
Data file S1. Primary study data with variable list.
Data file S2. Daily physical activity summary data for the Canadian cohort.
Data file S3. Expanded industrialized sample data.