Abstract
Purpose:
Significant controversy exists regarding the expression patterns of estrogen receptor beta (ERβ) in normal and diseased breast tissue. To address this issue, we have validated two ERβ antibodies, have optimized the IHC protocols for both antibodies and now report the expression patterns of ERβ in normal and malignant breast tissues.
Methods:
ERβ antibody specificity was determined using western blot and IHC analysis. ERβ protein expression patterns were assessed via IHC in normal breast tissue and invasive breast carcinoma. Further, we report the detailed protocol of the ERβ IHC assay developed in our CAP/CLIA certified laboratory to provide a standardized method for future studies.
Results:
We have confirmed the specificity of two independent ERβ monoclonal antibodies, one that detects total (i.e., full length plus splice variants 2–5, which do not include the ligand binding domain) ERβ protein (PPZ0506) and one that detects only the full-length form, which includes the ligand binding domain, of ERβ (PPG5/10). Using these two antibodies, we demonstrate that ERβ is highly expressed in normal human breast tissue as well as in 20–30% of invasive breast cancers. Further, these two antibodies exhibited similar staining patterns across multiple different tissues and were highly concordant with regard to determining ERβ positivity in breast cancers.
Conclusions:
ERβ protein was shown to be abundant in the majority of normal breast epithelial cells and is present in 20–30% of breast cancers. Use of these two antibodies, along with their standardized IHC protocols, provide a reference for future studies aimed at determining the utility of ERβ as a prognostic and/or predictive biomarker in various tissues of benign or malignant states.
Keywords: Estrogen Receptor Beta, Breast, Breast Cancer, Antibody
Introduction
Since the discovery of estrogen receptor beta (ERβ) in the mid 1990’s [1, 2], numerous studies have reported the involvement of this nuclear hormone receptor in mediating normal physiology and disease states across multiple tissues. Over the past decade, a wealth of studies have reported on the expression profiles and biological functions of ERβ in cancer, particularly in breast and prostate cancer [3, 4]. The majority of these reports demonstrated that ERβ predominantly elicits anti-proliferative and tumor suppressive effects in cancer cells, although some findings have suggested that it can function as an oncogene in certain contexts [5, 6]. Through the development of genetically manipulated cell line and animal model systems, as well as ERβ selective agonists and antagonists, it is now relatively straightforward to study the biological effects of ERβ in a given tissue, and the consequences of those effects on cellular functions, disease development and disease progression. However, translating ERβ-related laboratory findings into the clinic has been more problematic and remains controversial.
A major issue confounding a clear understanding of ERβ expression profiles in normal and diseased breast, and its association with disease related outcomes in patients, is the lack of a standardized and universally accepted methodology for accurately and reproducibly detecting ERβ protein in human specimens. Many different monoclonal and polyclonal ERβ antibodies have been developed, are widely available to the scientific community and have been indiscriminately utilized in the scientific literature. We and others have demonstrated that many of these antibodies are unfortunately non-specific and/or insensitive for detection of ERβ in multiple protein based detection methods including immunohistochemistry (IHC), western blotting and mass spectrometry [7–9]. Complicating the issue further is the fact that most ERβ antibodies also cross-react with 4 primary ERβ splice variants (ERβ2–5) that lack a portion of the ligand binding domain rendering them non-responsive to endogenous agonists and pharmacological small molecules that have been developed to target this receptor [10–12].
For these reasons, we sought to develop a reliable IHC based assay that could be used as a standard for future studies of ERβ protein expression in human tissue specimens. For this assay, we chose to employ the ERβ monoclonal antibody, PPG5/10, given that we and others have independently demonstrated that this antibody is highly specific and sensitive for ERβ detection in IHC applications [7, 13, 14] and does not cross-react with any of the ERβ splice variants [7]. It was also essential that this assay be developed in a CAP/CLIA certified environment so that it could be utilized for clinical purposes and would be available to researchers and clinicians around the world. During the development of this assay, a publication by Andersson et al. [9] described their laboratory’s experience with 13 ERβ antibodies, including the PPG5/10 antibody, and reported that 1) only the monoclonal PPZ0506 antibody specifically detects ERβ in IHC assays and 2) there is no evidence for ERβ expression in normal or malignant human breast tissue. Their findings contradict more than 20 years’ worth of research on ERβ, and questioned the relevance of this receptor in multiple tissue types and disease states. Here, we report our experience using the PPG5/10, as well as the PPZ0506 antibody recently described by Andersson et al., provide the optimized IHC protocols for both of these antibodies and detail the development of the first clinical test for ERβ protein in human tissue. Our findings demonstrate that the PPZ0506 antibody is highly specific for total ERβ protein levels. However, in contrast to Andersson et al., we provide evidence that the PPZ0506 antibody detects ERβ protein in both normal and malignant tissues under optimized IHC conditions and elicits staining patterns that are very similar to that of the PPG5/10 antibody.
Materials and Methods
Cell culture
Parental U2OS osteosarcoma cells and MDA-MB-231 breast cancer cells were originally purchased from American Type Culture Collection and grown in phenol red-free Dulbecco’s modified Eagle’s medium/F12 medium (DMEM/F12) (Corning Life Sciences, Oneonta, NY) containing 10% Fetal Bovine Serum (FBS) (Gemini Bio-Products, West Sacramento, CA) and 1% antibiotic/antimycotic (AA) (Invitrogen, Carlsbad, CA). Doxycycline (dox)-inducible Flag-tagged ERβ expressing U2OS and MDA-MB-231 cells were developed in our laboratory as previously described [15, 16]. Dox-inducible cells were routinely maintained in the same medium supplemented with 5 mg/liter blasticidin S (Roche Applied Science, Indianapolis, IN), and 500 mg/liter zeocin (Invitrogen).
Transient Transfection
Cells were plated at a density of approximately 50% in 10 cm tissue culture dishes and allowed to adhere overnight. Flag-tagged expression vectors for ERβ variants 2–5 were developed in our laboratory as previously described [7, 17]. Ten μg of ERβ variant expression vectors were transfected into cells using FuGENE 6 transfection reagent (Roche, Indianapolis, IN) following the manufacturer’s protocol. Twenty four hours following transfection, cells were washed twice with 1X PBS, protein lysates were prepared using RIPA buffer (25mM Tris pH 7.4, 150mM NaCl, 1% sodium deoxycholate, 1% NP40, 0.1% SDS) and protein concentrations were determined.
Western blotting
Western blotting was performed using 40 μg of whole cell lysates obtained from U2OS-ERβ and MDA-MB-231-ERβ cell lines cultured in the absence and presence of doxycycline as well as U2OS cells transfected with ERβ variants 2–5. Lysates were separated on 10% Criterion Tris-HCL Precast Gels (Bio-Rad, Hercules, CA) and transferred to PVDF membranes. Membranes were blocked using 5% powdered milk in tris-buffered saline with Tween 20 (TBST) for 1 hour at room temperature. Blots were incubated overnight at 4°C with primary antibodies targeting the Flag epitope (M2 Flag; MilliporeSigma, St. Louis, MO), ERβ (PPZ0506; ThermoFisher Scientific, Waltham, MA) and GAPDH (#2118S; Cell Signaling, Danvers, MA) at 1:4000, 1:500, and 1:5000 dilutions respectively. Blots were washed 5 times in TBST and incubated with an HRP-conjugated anti-mouse or anti-rabbit secondary antibody (1:2000 or 1:4000 respectively) for 1 hour at room temperature and detected via chemiluminescence on a LI-COR Odyssey® Fc Imaging System.
Preparation of cell pellets for immunohistochemistry
U2OS cells stably expressing Flag-tagged ERβ under the control of a dox-inducible promoter were expanded in a total of 10 T150 tissue culture flasks. Cells were treated with and without doxycycline to generate ERβ+ and ERβ- cultures respectively. When cells reached approximately 80% confluence, growth medium was removed and cells were washed twice with 1X PBS and fixed in 10% neutral buffered formalin for 5 minutes. Following fixation, cells were scraped and incubated in formalin at 4°C for a minimum of 12 hours. Cells were pelleted, processed, and paraffin embedded prior to use for IHC as previously described [7].
Human tissues
Formalin fixed paraffin embedded tissues used for evaluation of ERβ expression included eight independent histologically-confirmed benign breast tissues obtained from reduction mammoplasties, normal testis tissue, normal lung tissue and normal cerebral cortex tissue. ERβ protein levels were also evaluated in two independent breast cancer cohorts. The first cohort consisted of 56 invasive breast carcinomas of which 7 were ERα+, 1 was ERα+/HER2+, 15 were ERα-/HER2+ and 33 were triple negative (TN). The second cohort consisted of 643 histologically characterized TN breast carcinomas [18].
Immunohistochemistry and scoring
We developed and optimized IHC protocols for detection of ERβ using the PPG5/10 and PPZ0506 antibodies. The IHC protocol for the PPG5/10 antibody was developed in our CAP/CLIA-certified clinical Immunostains Laboratory at Mayo Clinic Rochester. The PPZ0506 IHC protocol was developed in the CAP-certified Mayo Clinic Pathology Research Core Facility.
Numerous iterations of the assays were performed and tested variables included the type of autostainer, antibody dilution, antigen retrieval method, antibody incubation time and temperature, and signal amplification steps. Details of the optimized assays are described below.
The IHC assay using the PPG5/10 antibody for detection of full-length ERβ was performed on the Ventana Discovery Ultra platform. Tissues were pretreated for 32 minutes in Discovery RiboCC followed by use of the ChromoMap peroxidase inhibitor for 12 minutes. Primary antibody (1:150 dilution in Dako Background Reducing Diluent, clone PPG5/10, Bio Rad Laboratories, Inc., Hercules, CA) was subsequently incubated for 48 minutes at room temperature followed by anti-mouse HQ for 24 minutes, anti-HQ HRP for 24 minutes, ChromoMap DAB for 8 minutes, Hematoxylin II for 8 minutes and finally Bluing Reagent for 4 minutes.
For detection of total ERβ using the PPZ0506 antibody, samples were IHC stained on-line using the Leica Bond RX stainer. Antigen retrieval was performed for 20 minutes using Leica Epitope Retrieval 1 (citrate based) and subsequently incubated in Dako Protein Block for 5 minutes. The PPZ0506 ERβ primary antibody (mouse monoclonal, Thermo #MA5–24807) was diluted 1:300 in Dako Background Reducing diluent and incubated for 15 minutes. The Polymer Refine Detection System (Leica) was used with the inclusion of DAB chromogen for stain visualization.
ERβ positivity in breast tumors was defined by moderate to strong nuclear staining intensity in ≥25% of tumor cells. Tumors with weak staining intensity and/or moderate to strong staining in <25% of tumors cells were considered to be ERβ low/negative. All IHC images were evaluated by an anatomic pathologist with expertise in breast pathology (JMC).
Results
Western blot detection of ERβ using PPZ0506
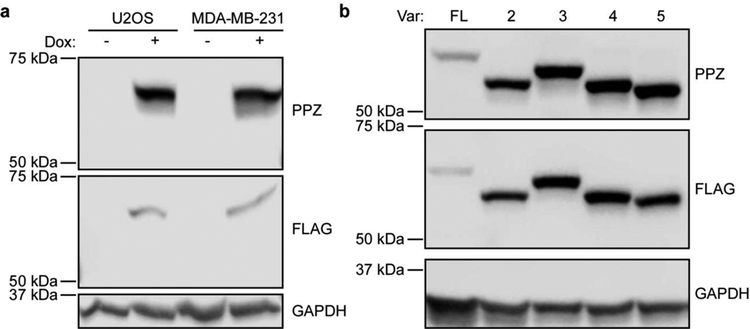
We previously demonstrated that the PPG5/10 ERβ monoclonal antibody is specific for the detection of the full-length and ligand binding form of ERβ and does not cross-react with ERβ splice variants [7]. Given that the PPZ0506 antibody targets the C-terminus of ERβ, which is conserved among all of the splice variants, we sought to confirm that this antibody is capable of detecting all forms of ERβ. Western blot analysis of dox-inducible U2OS-ERβ and MDA-MB-231-ERβ cell lines revealed detection of a highly specific band of the correct size only in dox-induced samples (Figure 1A). Western blotting with a monoclonal flag antibody also detected ERβ expression only in dox-induced samples (Figure 1A) further confirming specificity of the band detected using the PPZ0506 antibody. Western blotting of U2OS cell lysates following transient transfection of full-length ERβ, or ERβ splice variants 2, 3, 4 and 5, confirmed that the PPZ0506 antibody also detects these splice variant forms (Figure 1B). Western blotting with a Flag antibody resulted in the exact same patterns as observed with the PPZ0506 antibody (Figure 1B).
Figure 1:
Detection of ERβ using the PPZ0506 antibody via western blotting. A). Western blot analysis of whole cell lysates (40 μg) obtained from indicated cell lines grown in the absence and presence of doxycycline using the ERβ PPZ0506 and Flag antibodies. GAPDH is shown as a protein loading control. B). Western blot analysis of whole cell lysates (40 μg) obtained from U2OS cells that were transiently transfected with either a full-length (FL) ERβ expression vector, or expression vectors for ERβ variants 2, 3, 4 and 5.
Immunohistochemical detection of ERβ in cell lines
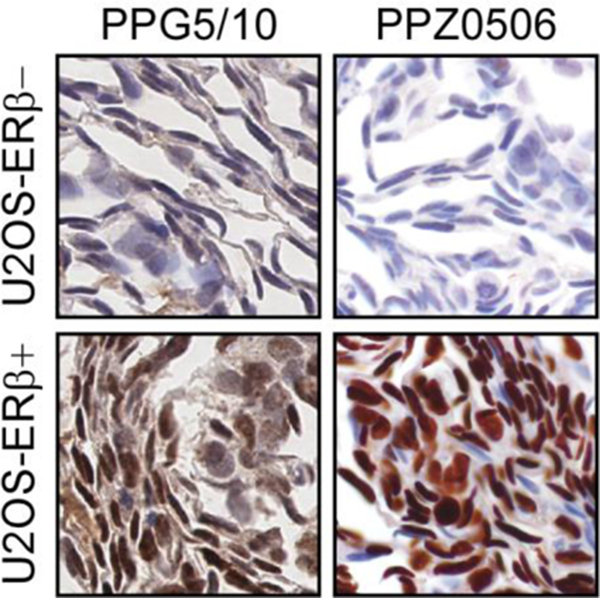
We next developed optimized immunohistochemical protocols for the PPG5/10 and the PPZ0506 antibodies. Using these protocols, we first stained sections of FFPE dox-inducible U2OS-ERβ cell line pellets. Robust nuclear staining for ERβ was observed with both antibodies only in cells that were treated with dox with no staining observed in non-dox treated cells (Figure 2). Some mild cytoplasmic staining was also observed with the PPG5/10 antibody. These results demonstrate the specificity and sensitivity of both antibodies for nuclear ERβ expression in a controlled cell line model system using IHC.
Figure 2:
Immunohistochemical detection of ERβ via IHC in cell lines. U2OS ERβ positive (+) and ERβ negative (−) cell line pellets were formalin fixed and paraffin embedded, sectioned and stained for ERβ using the PPG5/10 and PPZ0506 antibodies. Representative images using the optimized IHC conditions for each antibody are shown.
Immunohistochemical detection of ERβ in normal human tissues
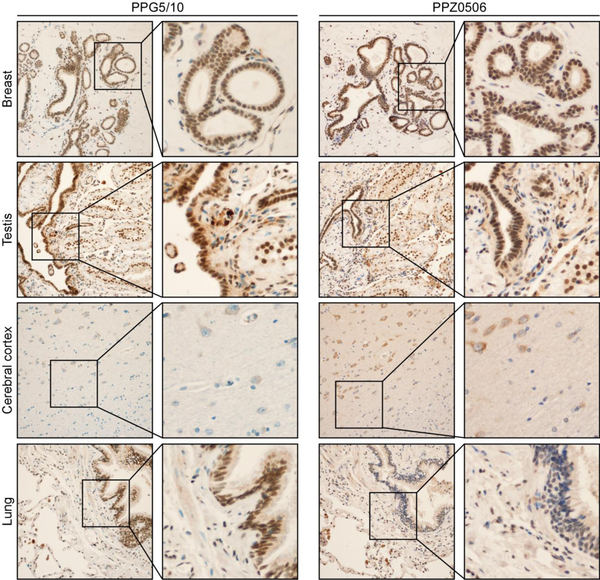
Using the same IHC conditions, we next assessed ERβ protein expression patterns as detected with the PPG5/10 and PPZ0506 antibodies in normal human tissues that are known to express ERβ including breast, testis, cerebral cortex and lung. Strong to moderate nuclear staining was observed for ERβ using both antibodies in sub-sets of normal breast epithelial and myoepithelial cells (Figure 3). Notably, subsets of fibroblasts and immune cells were negative for ERβ giving confidence to the specificity of the positive nuclear staining observed in epithelial cells (Figure 3). In normal testis, both antibodies also stained the epithelium of the seminiferous tubules and the epithelium of the rete testis, but fibroblasts and myoid cells of the tubules were largely negative (Figure 3). In the cerebral cortex, both the PPG5/10 and the PPZ0506 antibodies detected cytoplasmic ERβ staining in neurons with the PPZ0506 antibody exhibiting more robust staining (Figure 3). ERβ expression was also detected in scattered glial cells with the PPZ0506 antibody but not the PPG5/10 antibody (Figure 3). In normal lung tissue, the PPG5/10 antibody elicited strong nuclear staining of the bronchiolar epithelium and most pneumocytes as well as a subset of endothelial cells (Figure 3). The PPZ0506 antibody weakly stained the bronchiolar epithelium with strong staining of pneumocytes and endothelial cells (Figure 3).
Figure 3:
Immunohistochemical detection of ERβ via IHC in normal human tissues. Using optimized IHC protocols for the PPG5/10 and PPZ0506 antibodies, ERβ protein expression was assessed in normal human breast, testis, cerebral cortex and lung. Representative low and high magnification images of each tissue are shown.
ERβ protein expression in breast carcinomas
We also evaluated ERβ expression in a tissue microarray composed of 56 invasive breast carcinomas representative of all breast cancer sub-types. Approximately 30% of tumors exhibited nuclear ERβ positivity (moderate or high nuclear staining intensity) with the PPG5/10 assay. The PPZ0506 antibody had a slightly lower sensitivity, but a similar specificity, showing nuclear staining restricted to the same tumors deemed ERβ+ with the PPG5/10 assay, but with slightly weaker staining intensity. Both antibodies did show mild cytoplasmic immunoreactivity, both in subsets of tumors with or without nuclear ERβ expression. Representative images of two ERβ positive tumors and two ERβ negative tumors are shown in Figure 4. Given the similar performance of these two antibodies, we further evaluated full-length ERβ expression in a cohort of 643 TN breast carcinomas [18] using the PPG5/10 clinical assay. In this cohort, approximately 20% of tumors exhibited nuclear ERβ staining with, at minimum, moderate staining intensity in ≥25% of tumor cells (data not shown) further confirming ERβ expression in this sub-type of breast cancer.
Figure 4:
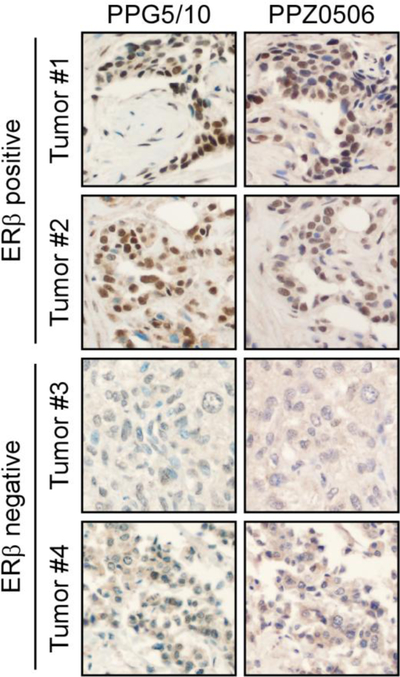
Immunohistochemical detection of ERβ via IHC in breast tumors. Using optimized IHC protocols for the PPG5/10 and PPZ0506 antibodies, ERβ protein expression was assessed in 56 invasive breast carcinomas. Two representative ERβ positive tumors and two representative ERβ negative tumors are shown.
Discussion
Here, we provide evidence that the ERβ monoclonal antibody, PPZ0506, is highly specific for the detection of ERβ protein in western blotting and IHC applications. Using ERβ positive and negative control cell lines, we have developed optimized IHC protocols for detection of total ERβ protein levels, including the splice variant forms, using the PPZ0506 antibody. We also describe the development of the first ERβ test that is conducted in a CAP/CLIA-certified laboratory specifically for detection of only the full-length and ligand binding form of ERβ using the PPG5/10 antibody. Using these two assays, we have assessed ERβ protein levels in multiple different normal tissues as well as breast cancers. Our results show that ERβ is highly expressed in normal breast tissue and in 20–30% of breast cancers. Importantly, the staining patterns for ERβ were very similar across tissues and identification of ERβ positive tumors was highly concordant between the two antibodies.
Our findings presented here largely contradict the recent report by Andersson and colleagues who concluded that the PPG5/10 antibody was not suitable for detection of ERβ via IHC and that only the PPZ0506 antibody was specific for ERβ in IHC assays. They also concluded that ERβ was not expressed in normal or malignant breast tissue based on lack of immunoreactivity when using the PPZ0506 antibody [9]. We agree that the PPZ0506 antibody is highly specific for detection of total ERβ protein, both by western blotting and by IHC. However, we strongly disagree that the PPG5/10 antibody is non-specific and that ERβ is not expressed in normal or malignant breast tissue. We believe that there are multiple reasons for these discrepancies. First, Andersson and colleagues utilized the same IHC methodology for all of the antibodies that they tested. We have clearly demonstrated that different detection methods, and even different autostainers, are required for optimized use of these two antibodies. Second, Andersson et al., used higher concentrations of the PPG5/10 antibody (1:60 dilution) in their study compared to what we identified as the ideal dilution (1:150). Indeed, use of high concentrations of any antibody will result in non-specific staining in IHC applications. Third, they employed more dilute concentrations of the PPZ0506 antibody (1:600) in their assays while we determined that a 1:300 dilution was appropriate. It is our conclusion that these factors, among others, likely explain our disparate findings and highlight the need for the implementation of standardized methodology for ERβ IHC assays across the field.
Within the context of breast cancer, the relevance of ERβ as either a prognostic or predictive biomarker has yet to be determined. A number of studies have indicated that ERβ is expressed in normal breast epithelial cells [7, 19–23]. Others have also indicated that ERβ protein expression levels decline, or are completely lost, in breast carcinomas [21, 22, 24–31]. However, there is little consensus regarding the incidence of ERβ positivity in breast cancer as published manuscripts have reported results that range from 0–100% [9, 16, 19, 20, 31–45]. Here we provide firm evidence, using two specific monoclonal ERβ antibodies, that ERβ protein is highly expressed in sub-sets of normal breast epithelial cells. Further, we have demonstrated that approximately 20–30% of breast carcinomas exhibit moderate to strong ERβ protein expression. These findings support a tumor suppressive/anti-proliferative role for ERβ in breast cancer and support a multitude of studies that have described ERβ’s anti-breast cancer effects in cell line and animal model systems [6, 16, 17, 46–57].
Although we report very similar and concordant staining patterns between the PPG5/10 and PPZ0506 antibodies, it is important to consider the form(s) of ERβ that these two antibodies detect. Specifically, we have confirmed that the PPZ0506 antibody is a pan-ERβ antibody and cross-reacts with full-length ERβ and all of its primary splice variant forms. However, the PPG5/10 antibody recognizes only the full-length and ligand binding form of ERβ. This may explain the slight differences in staining patterns that were observed with these two antibodies in normal lung tissue and in the cerebral cortex. Further, detection of total ERβ versus only full-length ERβ may contribute to the discrepancies that have been reported in the literature regarding the correlation of ERβ expression with breast cancer patient outcomes. Most studies have indicated that expression of this receptor is associated with improved rates of recurrence, disease-free survival and overall survival [16, 32, 34–37, 45, 58–62]. However, some reports indicate little to no correlation [38, 40, 59] or even worse prognosis [42, 63, 64]. Several studies have also indicated that ERβ positivity, within the context of ERα positive breast tumors, is associated with the efficacy of endocrine therapy including both tamoxifen [16, 43, 65–67] and aromatase inhibitors [68]. Finally, it is also essential to consider the hormonal status of patients within a given cohort as the functional consequences of ERβ expression are likely to differ based on menopausal status and use of hormone/endocrine therapy.
In summary, we report data on ERβ immunohistochemistry using two rigorously optimized ERβ antibodies and provide clear evidence that ERβ protein is expressed in normal breast epithelium and a sub-set of breast carcinomas, including TNBC. We provide the detailed methodology for use of these two antibodies in IHC-based applications based on optimization in our CAP/CLIA-certified laboratories. These data should provide clarity to the field and we recommend that future studies evaluating the IHC detection of ERβ protein utilize the methodologies described in this manuscript.
Acknowledgments:
We gratefully acknowledge the Mayo Clinic Immunostains Laboratory and the Mayo Clinic Pathology Research Core for their time and effort in developing the ERβ IHC assays.
Funding:
This work was supported by the National Cancer Institute of the National Institutes of Health through the Mayo Clinic Breast Cancer SPORE: P50CA116201 (MPG and JRH), as well as the Eisenberg Foundation (JRH and MS) and Mayo Clinic.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest:
M.P.G. reports personal fees from Genomic Health, consulting fees from Lilly, Biovica, Novartis, Sermonix, Context Pharm, Pfizer and Biotheranostics and grant funding from Pfizer and Lilly for efforts that are outside the context of the present study. All other authors declare that they have no conflicts of interest.
Ethics Approval:
This article does not contain any studies utilizing animals. All of the studies performed with the use of human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Collection and assessment of ERβ in human tissues was approved by the Mayo Clinic IRB under protocols 16–007352, 13–000585 and 12–004582.
Informed consent:
Informed consent for tissue collection and use for future research was obtained from all individuals included in the study.
References
- 1.Mosselman S, Polman J, Dijkema R: ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett 1996, 392(1):49–53. [DOI] [PubMed] [Google Scholar]
- 2.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA: Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A 1996, 93(12):5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haldosen LA, Zhao C, Dahlman-Wright K: Estrogen receptor beta in breast cancer. Mol Cell Endocrinol 2014, 382(1):665–672. [DOI] [PubMed] [Google Scholar]
- 4.Nelson AW, Tilley WD, Neal DE, Carroll JS: Estrogen receptor beta in prostate cancer: friend or foe? Endocr Relat Cancer 2014, 21(4):T219–234. [DOI] [PubMed] [Google Scholar]
- 5.Guillette TC, Jackson TW, Belcher SM: Duality of estrogen receptor beta action in cancer progression. Curr Opin Pharmacol 2018, 41:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner M, Huang B, Gustafsson JA: Estrogen Receptor beta as a Pharmaceutical Target. Trends Pharmacol Sci 2017, 38(1):92–99. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Subramaniam M, Negron V, Cicek M, Reynolds C, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR: Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem 2012, 113(2):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson AW, Groen AJ, Miller JL, Warren AY, Holmes KA, Tarulli GA, Tilley WD, Katzenellenbogen BS, Hawse JR, Gnanapragasam VJ et al. : Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol Cell Endocrinol 2017, 440:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson S, Sundberg M, Pristovsek N, Ibrahim A, Jonsson P, Katona B, Clausson CM, Zieba A, Ramstrom M, Soderberg O et al. : Insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun 2017, 8:15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewandowski S, Kalita K, Kaczmarek L: Estrogen receptor beta. Potential functional significance of a variety of mRNA isoforms. FEBS Lett 2002, 524(1–3):1–5. [DOI] [PubMed] [Google Scholar]
- 11.Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM: Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun 1998, 247(1):75–78. [DOI] [PubMed] [Google Scholar]
- 12.Poola I, Abraham J, Baldwin K, Saunders A, Bhatnagar R: Estrogen receptors beta4 and beta5 are full length functionally distinct ERbeta isoforms: cloning from human ovary and functional characterization. Endocrine 2005, 27(3):227–238. [DOI] [PubMed] [Google Scholar]
- 13.Weitsman GE, Skliris G, Ung K, Peng B, Younes M, Watson PH, Murphy LC: Assessment of multiple different estrogen receptor-beta antibodies for their ability to immunoprecipitate under chromatin immunoprecipitation conditions. Breast Cancer Res Treat 2006, 100(1):23–31. [DOI] [PubMed] [Google Scholar]
- 14.Wimberly H, Han G, Pinnaduwage D, Murphy LC, Yang XR, Andrulis IL, Sherman M, Figueroa J, Rimm DL: ERbeta splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Res Treat 2014, 146(3):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC: Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem 2003, 90(2):315–326. [DOI] [PubMed] [Google Scholar]
- 16.Reese JM, Suman VJ, Subramaniam M, Wu X, Negron V, Gingery A, Pitel KS, Shah SS, Cunliffe HE, McCullough AE et al. : ERbeta1: characterization, prognosis, and evaluation of treatment strategies in ERalpha-positive and -negative breast cancer. BMC Cancer 2014, 14:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Secreto FJ, Monroe DG, Dutta S, Ingle JN, Spelsberg TC: Estrogen receptor alpha/beta isoforms, but not betacx, modulate unique patterns of gene expression and cell proliferation in Hs578T cells. J Cell Biochem 2007, 101(5):1125–1147. [DOI] [PubMed] [Google Scholar]
- 18.Leon-Ferre RA, Polley MY, Liu H, Gilbert JA, Cafourek V, Hillman DW, Elkhanany A, Akinhanmi M, Lilyquist J, Thomas A et al. : Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat 2018, 167(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvinen TA, Pelto-Huikko M, Holli K, Isola J: Estrogen receptor beta is coexpressed with ERalpha and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am J Pathol 2000, 156(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skliris GP, Carder PJ, Lansdown MR, Speirs V: Immunohistochemical detection of ERbeta in breast cancer: towards more detailed receptor profiling? Br J Cancer 2001, 84(8):1095–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skliris GP, Munot K, Bell SM, Carder PJ, Lane S, Horgan K, Lansdown MR, Parkes AT, Hanby AM, Markham AF et al. : Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol 2003, 201(2):213–220. [DOI] [PubMed] [Google Scholar]
- 22.Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H: Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res 2001, 61(6):2537–2541. [PubMed] [Google Scholar]
- 23.Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, Foster CS: Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. The American journal of surgical pathology 2003, 27(12):1502–1512. [DOI] [PubMed] [Google Scholar]
- 24.Iwao K, Miyoshi Y, Egawa C, Ikeda N, Noguchi S: Quantitative analysis of estrogen receptor-beta mRNA and its variants in human breast cancers. Int J Cancer 2000, 88(5):733–736. [DOI] [PubMed] [Google Scholar]
- 25.Leygue E, Dotzlaw H, Watson PH, Murphy LC: Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res 1998, 58(15):3197–3201. [PubMed] [Google Scholar]
- 26.Leygue E, Dotzlaw H, Watson PH, Murphy LC: Expression of estrogen receptor beta1, beta2, and beta5 messenger RNAs in human breast tissue. Cancer Res 1999, 59(6):1175–1179. [PubMed] [Google Scholar]
- 27.Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K: Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene 2003, 22(48):7600–7606. [DOI] [PubMed] [Google Scholar]
- 28.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P: Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer 2004, 11(3):537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw JA, Udokang K, Mosquera JM, Chauhan H, Jones JL, Walker RA: Oestrogen receptors alpha and beta differ in normal human breast and breast carcinomas. The Journal of pathology 2002, 198(4):450–457. [DOI] [PubMed] [Google Scholar]
- 30.Park BW, Kim KS, Heo MK, Ko SS, Hong SW, Yang WI, Kim JH, Kim GE, Lee KS: Expression of estrogen receptor-beta in normal mammary and tumor tissues: is it protective in breast carcinogenesis? Breast Cancer Res Treat 2003, 80(1):79–85. [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi Y, Taguchi T, Gustafsson JA, Noguchi S: Clinicopathological characteristics of estrogen receptor-beta-positive human breast cancers. Jpn J Cancer Res 2001, 92(10):1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omoto Y, Inoue S, Ogawa S, Toyama T, Yamashita H, Muramatsu M, Kobayashi S, Iwase H: Clinical value of the wild-type estrogen receptor beta expression in breast cancer. Cancer Lett 2001, 163(2):207–212. [DOI] [PubMed] [Google Scholar]
- 33.Fuqua SA, Schiff R, Parra I, Moore JT, Mohsin SK, Osborne CK, Clark GM, Allred DC: Estrogen receptor beta protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res 2003, 63(10):2434–2439. [PMC free article] [PubMed] [Google Scholar]
- 34.Mann S, Laucirica R, Carlson N, Younes PS, Ali N, Younes A, Li Y, Younes M: Estrogen receptor beta expression in invasive breast cancer. Hum Pathol 2001, 32(1):113–118. [DOI] [PubMed] [Google Scholar]
- 35.Myers E, Fleming FJ, Crotty TB, Kelly G, McDermott EW, O’Higgins NJ, Hill AD, Young LS: Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer 2004, 91(9):1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakopoulou L, Lazaris AC, Panayotopoulou EG, Giannopoulou I, Givalos N, Markaki S, Keramopoulos A: The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol 2004, 57(5):523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiura H, Toyama T, Hara Y, Zhang Z, Kobayashi S, Fujii Y, Iwase H, Yamashita H: Expression of estrogen receptor beta wild-type and its variant ERbetacx/beta2 is correlated with better prognosis in breast cancer. Japanese journal of clinical oncology 2007, 37(11):820–828. [DOI] [PubMed] [Google Scholar]
- 38.Miller WR, Anderson TJ, Dixon JM, Saunders PT: Oestrogen receptor beta and neoadjuvant therapy with tamoxifen: prediction of response and effects of treatment. British journal of cancer 2006, 94(9):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders PT, Millar MR, Williams K, Macpherson S, Bayne C, O’Sullivan C, Anderson TJ, Groome NP, Miller WR: Expression of oestrogen receptor beta (ERbeta1) protein in human breast cancer biopsies. British journal of cancer 2002, 86(2):250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Neill PA, Davies MP, Shaaban AM, Innes H, Torevell A, Sibson DR, Foster CS: Wild-type oestrogen receptor beta (ERbeta1) mRNA and protein expression in Tamoxifen-treated post-menopausal breast cancers. Br J Cancer 2004, 91(9):1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC: Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. British journal of cancer 2006, 95(5):616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen EV, Cheng G, Palmieri C, Saji S, Makela S, Van Noorden S, Wahlstrom T, Warner M, Coombes RC, Gustafsson JA: Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci U S A 2001, 98(26):15197–15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poola I, Fuqua SA, De Witty RL, Abraham J, Marshallack JJ, Liu A: Estrogen receptor alpha-negative breast cancer tissues express significant levels of estrogen-independent transcription factors, ERbeta1 and ERbeta5: potential molecular targets for chemoprevention. Clin Cancer Res 2005, 11(20):7579–7585. [DOI] [PubMed] [Google Scholar]
- 44.Umekita Y, Souda M, Ohi Y, Sagara Y, Rai Y, Takahama T, Yoshida H: Expression of wild-type estrogen receptor beta protein in human breast cancer: specific correlation with HER2/neu overexpression. Pathology international 2006, 56(8):423–427. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Zhang C, Chen K, Tang H, Tang J, Song C, Xie X: ERbeta1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat 2015, 152(2):255–269. [DOI] [PubMed] [Google Scholar]
- 46.Shanle EK, Zhao Z, Hawse J, Wisinski K, Keles S, Yuan M, Xu W: Research resource: global identification of estrogen receptor beta target genes in triple negative breast cancer cells. Mol Endocrinol 2013, 27(10):1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F: ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology 2001, 142(9):4120–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas C, Rajapaksa G, Nikolos F, Hao R, Katchy A, McCollum CW, Bondesson M, Quinlan P, Thompson A, Krishnamurthy S et al. : ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res 2012,.14(6):R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Subramaniam M, Grygo SB, Sun Z, Negron V, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR: Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res 2011, 13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anestis A, Sarantis P, Theocharis S, Zoi I, Tryfonopoulos D, Korogiannos A, Koumarianou A, Xingi E, Thomaidou D, Kontos M et al. : Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol 2019, 145(5):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reese JM, Bruinsma ES, Monroe DG, Negron V, Suman VJ, Ingle JN, Goetz MP, Hawse JR: ERβ inhibits cyclin dependent kinases 1 and 7 in triple negative breast cancer. Oncotarget 2017, 8(57):96506–96521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reese JM, Bruinsma ES, Nelson AW, Chernukhin I, Carroll JS, Li Y, Subramaniam M, Suman VJ, Negron V, Monroe DG et al. : ERbeta-mediated induction of cystatins results in suppression of TGFbeta signaling and inhibition of triple-negative breast cancer metastasis. Proc Natl Acad Sci U S A 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bado I, Pham E, Soibam B, Nikolos F, Gustafsson JA, Thomas C: ERbeta alters the chemosensitivity of luminal breast cancer cells by regulating p53 function. Oncotarget 2018, 9(32):22509–22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao L, Huang S, Mei S, Yang Z, Xu L, Zhou N, Yang Q, Shen Q, Wang W, Le X et al. : Pharmacological activation of estrogen receptor beta augments innate immunity to suppress cancer metastasis. Proc Natl Acad Sci U S A 2018, 115(16):E3673–E3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bado I, Nikolos F, Rajapaksa G, Wu W, Castaneda J, Krishnamurthy S, Webb P, Gustafsson JA, Thomas C: Somatic loss of estrogen receptor beta and p53 synergize to induce breast tumorigenesis. Breast Cancer Res 2017, 19(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bado I, Nikolos F, Rajapaksa G, Gustafsson JA, Thomas C: ERbeta decreases the invasiveness of triple-negative breast cancer cells by regulating mutant p53 oncogenic function. Oncotarget 2016, 7(12):13599–13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang B, Warner M, Gustafsson JA: Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol 2015, 418 Pt 3:240–244. [DOI] [PubMed] [Google Scholar]
- 58.Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, Lavaill R, Mollevi C, Warner M, Gustafsson JA, Rochefort H: Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res 2004, 10(17):5769–5776. [DOI] [PubMed] [Google Scholar]
- 59.Fleming FJ, Hill AD, McDermott EW, O’Higgins NJ, Young LS: Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydroxytamoxifen in human breast cancer. J Clin Endocrinol Metab 2004, 89(1):375–383. [DOI] [PubMed] [Google Scholar]
- 60.Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA: Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res 2004, 10(22):7490–7499. [DOI] [PubMed] [Google Scholar]
- 61.Iwase H, Zhang Z, Omoto Y, Sugiura H, Yamashita H, Toyama T, Iwata H, Kobayashi S: Clinical significance of the expression of estrogen receptors alpha and beta for endocrine therapy of breast cancer. Cancer Chemother Pharmacol 2003, 52 Suppl 1:S34–38. [DOI] [PubMed] [Google Scholar]
- 62.Murphy LC, Leygue E, Niu Y, Snell L, Ho SM, Watson PH: Relationship of coregulator and oestrogen receptor isoform expression to de novo tamoxifen resistance in human breast cancer. Br J Cancer 2002, 87(12):1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Speirs V, Parkes AT, Kerin MJ, Walton DS, Carleton PJ, Fox JN, Atkin SL: Coexpression of estrogen receptor alpha and beta: poor prognostic factors in human breast cancer? Cancer Res 1999, 59(3):525–528. [PubMed] [Google Scholar]
- 64.Speirs V, Malone C, Walton DS, Kerin MJ, Atkin SL: Increased expression of estrogen receptor beta mRNA in tamoxifen-resistant breast cancer patients. Cancer Res 1999, 59(21):5421–5424. [PubMed] [Google Scholar]
- 65.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G: Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol 2008, 26(22):3727–3734. [DOI] [PubMed] [Google Scholar]
- 66.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT et al. : Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res 2008, 14(16):5228–5235. [DOI] [PubMed] [Google Scholar]
- 67.Novelli F, Milella M, Melucci E, Di Benedetto A, Sperduti I, Perrone-Donnorso R, Perracchio L, Venturo I, Nistico C, Fabi A et al. : A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res 2008, 10(5):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Motomura K, Ishitobi M, Komoike Y, Koyama H, Nagase H, Inaji H, Noguchi S: Expression of estrogen receptor beta and phosphorylation of estrogen receptor alpha serine 167 correlate with progression-free survival in patients with metastatic breast cancer treated with aromatase inhibitors. Oncology 2010, 79(1–2):55–61. [DOI] [PubMed] [Google Scholar]