Abstract
Introduction
Trauma-induced coagulopathy (TIC) and the tissue injury-provoked procoagulant profile are prevalent in severely injured patients, but their mechanisms remain unclear. Myosin, exposed by or released from tissue injury, may play a role in promoting thrombin generation and attenuating fibrinolysis. The objective of the study is to examine the effects of cardiac and skeletal muscle myosins on coagulation in whole blood using thrombelastography (TEG).
Materials and Methods
Whole blood was collected from healthy adult volunteers (n=8) and native TEGs were performed to evaluate the global coagulation response in the presence of cardiac or skeletal muscle myosin by measuring reaction (R) time (minutes), clot angle (°), and maximum amplitude (MA, mm). TEG measurements were compared using paired t-tests.
Results
Cardiac and skeletal muscle myosins decreased R, from 10.8 min to 8.0 min (p<0.0001) and 6.9 min (p=0.0007), respectively. There were no effects observed on clot propagation (angle) or clot strength (MA) with myosin addition. In the presence of tPA, both cardiac and skeletal muscle myosins shortened R from 11.1 min to 8.62 min (p=0.0245) and 7.75 min (p=0.0027), respectively), with no changes on angle or MA.
Conclusions
Cardiac and skeletal muscle myosins exhibit procoagulant effects in TEG assays. These whole blood TEG results support the hypothesis that cardiac and skeletal muscle myosins may be either pro-hemostatic or prothrombotic depending on context.
Introduction
Trauma induced coagulopathy (TIC) is common in severely injured patients and is the leading cause of early death in trauma patients(1, 2). Despite its prevalence, the mechanisms behind TIC remain elusive. Skeletal muscle myosin promotes thrombosis ex vivo when exposed to flowing blood, and in mechanistic studies, myosin enhances thrombin generation (3). Thus, myosin may elucidate why tissue injury is procoagulant and serve as a danger signal, promoting coagulation and inflammation. In vivo, myosin is known to be elevated after tissue injury in animal models(4, 5). While inhibition of skeletal muscle myosin reduces thrombin generation in vitro in plasma from trauma patients(3), detecting circulating elevated myosin proteins and linking these with viscoelastic measurements remains to be described. Collectively, the influences of cardiac or skeletal muscle myosin in whole blood on hemostasis are unknown and merit further investigation, as elevations of myosin in severely injured patients with TIC may serve as a therapeutic target for myosin-targeted inhibitors. The aim of this investigation was to examine the effects of skeletal and cardiac muscle myosins on coagulation in vitro using TEG.
Materials and Methods
Blood was collected from healthy volunteers after informed consent (COMIRB 18-0625) and citrated native TEGs (TEG) were performed. To evaluate the global coagulation response in the presence of skeletal muscle or cardiac myosin, myosin (Cytoskeleton Inc, Denver, CO) was solubilized in Tris buffer (0.6M NaCl), diluted in Hepes Buffered Saline (HBS) carrier, and then added to whole blood before initiation of TEG at 100 nM final concentration based on previous descriptions of peak myosin levels in the blood of acutely injured patients(3). TEG measurements with and without myosin were compared with paired t-test in R.
Results
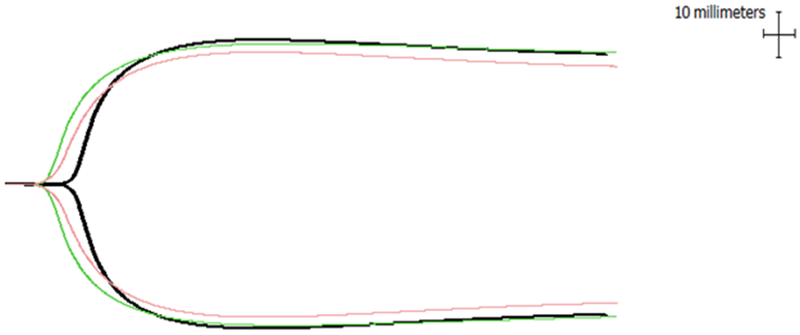
Eight healthy volunteers were included (50% male; average age 30.7 years). Cardiac muscle myosin shortened time to clot formation (R of 10.8 to 6.9 minutes, p<0.01) (Table 1, Figure 1). While cardiac muscle myosin was associated with an increase in clot propagation (angle) and clot strength (MA), we failed to detect statistically significant differences. Similarly, skeletal muscle myosin shortened R time (10.8 to 8.0 minutes, p<0.01), but it had no effect on angle or MA (Table 1, Figure 1). There were no differences in response to myosin by sex.
Table 1. Changes in coagulation in the presence of cardiac or skeletal myosin (n=8).
Expressed as mean values with standard deviation.
| Whole blood | + Cardiac myosin | p value | + Skeletal myosin | p value | |
|---|---|---|---|---|---|
| Reaction time (min) | 10.8 (1.9) | 6.9 (1.5) | <0.01 | 8.0 (2.6) | <0.01 |
| Angle (°) | 47.5 (11.8) | 48.9 (11.2) | 0.79 | 45.6 (12.5) | 0.69 |
| Max amplitude (mm) | 58.3 (6.6) | 59.9 (6.6) | 0.49 | 60.4 (9.6) | 0.39 |
Figure 1.
Thrombelastographic changes in the presence of cardiac (red) and skeletal muscle myosin (green) compared to control (black).
Discussion
These findings indicate that both cardiac and skeletal muscle myosins have procoagulant effects, shortening the clotting time in healthy adults. TIC includes a spectrum of phenotypes, ranging from the hypocoagulopathic profile of hemorrhagic shock to pathologic hypercoagulability associated with tissue injury. It has been suggested that myosins may play a role in hypercoagulability after tissue injury based on skeletal muscle myosin release into circulation in small(6) and large(5) animal and human(7) models after severe injury. However, a dearth of data exists regarding the effects of skeletal muscle or cardiac myosin on coagulation in humans. Deguchi et. al. showed that rabbit skeletal muscle myosin is prothrombotic in flowing whole human blood and that it enhances plasma thrombin generation, likely due to myosin’s binding of factor Xa and enhancement of prothrombin activation(3). Our TEG data confirm that, in human whole blood, both cardiac and skeletal muscle myosins accelerate the early amplification phase of clotting in blood, likely due to myosin’s ability to enhance thrombin generation by factors Xa and Va. This shortening of R time has important clinical translational significance, as a growing body of literature has detailed that a shortening of reaction time (by even smaller degrees than revealed in our data) can predict thrombotic complications in trauma patients(8).
Anti-myosin antibodies reduce thrombin generation in plasma from trauma patients, suggesting myosin may play a role in the hypercoagulable phenotype of trauma(3). While ex vivo work suggests a procoagulant role for myosin in normal whole blood, there is a lack of knowledge regarding the relationship between elevated circulating myosin levels in trauma and hemostatic capacity as assessed by TEG. Thus, this report establishes an important foundation for studies of the effects of endogenous cardiac and skeletal muscle myosins on coagulation in blood from trauma patients. The data here also suggest that myosin-targeted anticoagulants may exert useful effects when myosins provoke excessive thrombin generation. Future work will entail mechanistic investigations behind myosin’s procoagulant role, as well myosin quantification and inhibition in a large animal model of tissue injury and TIC.
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (T32 GM008315 and P50 GM049222). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors of the project.
Footnotes
Conflict of Interest Statement
The authors Julia R Coleman, Jevgenia Zilberman-Rudenko, Jason M Samuels, Mitchell J Cohen, Christopher C Silliman MD PhD, Anirban Banerjee PhD, Angela Sauaia MD PhD, John H Griffin PhD, Hiroshi Deguchi MD PhD have no conflicts of interest related to this work to report. Ernest E Moore receives consumable support from Haemonetics and Instrumentation Laboratories, as well as holds a patent for the tPA-challenge TEG.
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma 38(2):185–93, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma 54(6):1127–30, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Deguchi H, Sinha RK, Marchese P, Ruggeri ZM, Zilberman-Rudenko J, McCarty OJT, Cohen MJ, Griffin JH. Prothrombotic skeletal muscle myosin directly enhances prothrombin activation by binding factors Xa and Va. Blood 128(14):1870–8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contreras-Munoz P, Fernandez-Martin A, Torrella R, Serres X, De la Varga M, Viscor G, Jarvinen TA, Martinez-Ibánez V, Peiró JL, Rodas G, et al. A New Surgical Model of Skeletal Muscle Injuries in Rats Reproduces Human Sports Lesions. Int J Sports Med 37(3):183–90, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Vlasakova K, Lane P, Michna L, Muniappa N, Sistare FD, Glaab WE. Response of Novel Skeletal Muscle Biomarkers in Dogs to Drug-Induced Skeletal Muscle Injury or Sustained Endurance Exercise. Toxicological Sciences 156(2):422–7, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Burch PM, Greg Hall D, Walker EG, Bracken W, Giovanelli R, Goldstein R, Higgs RE, King NM, Lane P, Sauer JM, et al. Evaluation of the Relative Performance of Drug-Induced Skeletal Muscle Injury Biomarkers in Rats. Toxicological Sciences 150(1):247–56, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero M, Guiu-Comadevall M, Cadefau JA, Parra J, Balius R, Estruch A, Rodas G, Bedini JL, Cussó R. Fast and slow myosins as markers of muscle injury. Br J Sports Med 42(7):581–4, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V, Shackford SR. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J Trauma Acute Care Surg 83(3):413–9, 2017. [DOI] [PubMed] [Google Scholar]