Abstract
Previously a calcium bentonite clay (CB) has been shown to tightly bind aflatoxins in vitro, significantly reduce mortality and morbidity in animals, and decrease molecular biomarkers of aflatoxin exposure in humans and animals. Extensive studies have shown that CB is safe for human and animal consumption. In further work, we have investigated a highly active sodium bentonite (SB) clay (SB-E) with enhanced aflatoxin sorption efficacy compared to CB and other clays. Computational models and isothermal analyses were used to characterize toxin/clay surface interactions, predict mechanisms of toxin sorption, and gain insight into: 1) surface capacities and affinities, and 2) thermodynamics and sites of toxin/surface interactions. We have also used a toxin-sensitive living organism (Hydra vulgaris) to confirm the safety and predict the efficacy of SB-E against aflatoxin toxicity. Compared to CB, SB-E had a higher capacity for aflatoxin B1 (AfB1) at pH 2 and 6.5. Results from this work suggest that high capacity clays such as SB-E can be used as effective aflatoxin enterosorbents to decrease short-term exposures in humans and animals when included in food and/or water during extended droughts and outbreaks of aflatoxicosis.
Keywords: Aflatoxin, bentonite, sorption, enterosorbent, mycotoxin, isotherms, hydra bioassay
Introduction
Aspergillus flavus and Aspergillus parasiticus are fungi that produce highly toxic and carcinogenic metabolites known as aflatoxins. The aflatoxins are widespread and can frequently cause health problems during extended periods of heat and drought. Every year a significant percentage of the world’s grain and oilseed supply is contaminated with aflatoxins, and this contamination of the diet can result in disease and death, especially in vulnerable populations of humans and animals (CAST 1989; Phillips et al. 1995). Dairy animals can also secrete carcinogenic metabolites in their milk (aflatoxin M1) following the ingestion of aflatoxin-contaminated feed, resulting in unintended aflatoxin exposures from milk and dairy products (Allcroft and Carnaghan 1963). Among the naturally occurring aflatoxins, aflatoxin B1 (AfB1) is the most toxic and is commonly detected in diverse food and feed products (Grant and Phillips 1998). Common symptoms in humans and animals from AfB1 exposure include growth stunting, weight loss, liver toxicity, immunosuppression and hepatocellular carcinoma (Murugesan et al. 2015; Wang et al. 2017).
Detection and quantification of aflatoxins in contaminated grains are difficult because of heterogeneous distribution of mold contamination. The majority of a feed may be below the action level of 20 ng g−1 (CAST 1989), but small portions can contain much higher levels (hot spots), which can affect the final concentration and toxicological outcome after processing and mixing. Because of the widespread occurrence of aflatoxins and the potent carcinogenicity of AfB1, practical and effective detoxification methods are needed.
Innovative enterosorption strategies to significantly diminish the bioavailability of aflatoxins and reduce human and animal exposures from contaminated food and feed have been developed. Smectite clays (including bentonites) consist of three-layered molecular sheets containing two layers of silica that are tetrahedrally coordinated to oxygen and one layer of alumina that is octahedrally coordinated to oxygen. The sheets are weakly bonded which allow water and chemicals to enter. They have large surface areas, which result in swelling and considerable expansion when saturated and shrinkage when drying. These clays are sticky and plastic when wet and can pose problems with shear and consolidation (USDA 1978; EC 2005). Ca2+/Na+-bentonite with 20% (or more) sodium, behaves qualitatively as a sodium bentonite clay (SB), whereas bentonite with 90% (or more) calcium in the interlayer behaves similarly to calcium bentonite (CB) (Thuresson et al. 2017). Previously, our laboratory has conducted a series of in vitro studies, which have demonstrated that a CB can tightly bind AfB1 with high capacity. Its global inclusion into feedstuffs and food has been reported to protect numerous animal species and to significantly reduce biomarkers of AfB1 exposure in humans (Phillips et al. 2019). The mechanism of protection involves sorption of AfB1 onto active interlayer surfaces of the bentonite, resulting in a reduced concentration of unbound toxin in the gastrointestinal tract and decreased bioavailability and toxicity of AfB1. Results from our earlier work with bentonites have indicated that these clays possess active sites within their interlamellar regions for aflatoxin sorption. The carbonyl moiety in the aflatoxin molecule is important for binding. Thermodynamic studies have shown that the reaction is spontaneous in the forward direction and involves chemisorption of aflatoxins to bentonite with heats of sorption, or enthapies (∆Hs) equal to > −40 kJ/mol. Stereochemistry and planar surfaces of the aflatoxin ring (minus the terminal furan), favor the interaction and result in tight binding. Other reports have suggested that aflatoxin sorption mechanisms may include electron donor-acceptor, ion-dipole interactions, coordination between exchange cations and the carbonyl oxygens, and water bridging (Phillips et al. 2019).
A variety of aflatoxin mitigants including: natural binders, such as bentonite clay and chemopreventive agents such as green tea polyphenols (Tang et al. 2008), chlorophyllin (Egner et al. 2001), oltipraz (Wang et al. 1999) and sulforaphane (Kensler et al. 2005), have been tested in human studies. The bentonite clay, unlike the chemopreventive agents, can work rapidly to bind toxins in the stomach and intestines, thus decreasing bioavailability and toxicity. Due to high affinity, high capacity and a large heat of sorption for aflatoxin onto CB surfaces, it can be used therapeutically to prevent mortality and decrease morbidity during acute outbreaks of aflatoxicosis in the vulnerable. Importantly, CB clay has been reported to be very cost-effective at $0.73 per person per year (Khlangwiset and Wu, 2010). Unlike CB, SB clay is known for its higher expansibility (swelling) in water, resulting in more prominent delamination, less selective sorption and similar, or less, binding capacity than that of the CB.
A wide range of materials used in aflatoxin sorption studies have shown limited adsorption capacities, low capacities, or no capacity for toxin binding based on isothermal data and curve fitting using Langmuir and Freundlich models. The purpose of this study was to investigate a sodium bentonite sample (SB-E) that was unique to other bentonite samples (sodium and calcium) based on a combination of expansibility, flocculation rate and aflatoxin adsorption. Computational models and isothermal analyses were used to delineate sorption mechanisms and to predict the thermodynamics of aflatoxin binding to active sites on the clays. Equilibrium isothermal analyses were conducted to derive binding parameters and to gain insight into: 1) surface capacities and affinities, and 2) thermodynamics of toxin/surface interactions. We have also used a toxin-sensitive living organism (Hydra vulgaris) to confirm the safety and predict the efficacy of these sorbents against AfB1.
Materials and Methods
Reagent
AfB1 was purchased from Sigma Aldrich (Saint Louis, MO). In this study, CB standard was obtained from BASF Chemical Company (Lampertheim, Germany) with an average total surface area as high as 850 m2 g−1, an external surface area of approximately 70 m2 g−1 and cation exchange capacity equal to 97 cmol kg−1 (Grant and Phillips 1998). SB standard was a gift from the Source Clay Mineral Repository at the University of Missouri-Columbia with an estimated cation exchange capacity equal to 75 cmol kg-1. The generic formula for these clays is (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O. Samples of both clays contain some quartz, mica, calcite, orthoclase feldspars and sanidine as impurities, and mesopores of approximately 5 nm diameter (Marroquin-Cardona et al. 2011). The novel SB sample (SB-E) listed as a hydrated sodium calcium aluminosilicate (HSCAS) from Halliburton (Houston, TX). Diverse important physico-chemical properties of CB, SB and SB-E have been summarized in Table 1. Ultrapure deionized water (18.2 MΩ) was generated in the laboratory using an Elga™ automated filtration system (Woodridge, IL) and was used in all experiments.
Table 1.
Important physico-chemical properties of CB, SB and SB-E
| CB | SB | SB-E | |
|---|---|---|---|
| Appearance | Off-white to grayish-green powder | Off-white powder | Blue, gray powder |
| Bulk density (loose) | 640.74 kg/m3 | 593 kg/m3 | 849 kg/m3 |
| Moisture | 9% | 8% | 9–12% |
| Screen analysis | 5% +100 mesh | 80% −200 mesh | 0-5% + 60 mesh |
| 18% +200 mesh | 80% −200 mesh | 15-25% +100 mesh | |
| 60% −325 mesh | 70-80% −200 mesh | ||
| Chemical analysis by X-ray fractionation (XRF) spectroscopy (weight %) | %CaO 3.2-4.8 | %CaO 1.68 | %CaO 2.64 |
| %MgO 4.0-5.4 | %MgO 0.05 | %MgO 1.83 | |
| %Fe2O3 5.4-6.5 | %Fe2O3 3.35 | %Fe2O3 3.72 | |
| %K2O 0.50-0.90 | %K2O 0.53 | %K2O 0.6 | |
| %Na2O 0.10-0.30 | %Na2O 1.53 | %Na2O 2.51 | |
| %MnO 0.01-0.03 | %MnO 0.006 | %MnO 0.08 | |
| %Al2O3 14.8-18.2 | %Al2O3 19.6 | %Al2O3 17.6 | |
| %SiO2 62.4-73.5 | %SiO2 62.9 | %SiO2 59.9 |
(Data collected from product information sheets and clays.org)
In order to confirm the importance of an intact interlayer and investigate potential aflatoxin binding sites, the interlayers of sample sorbents were collapsed by heating at 200°C for 30 min followed by 800°C for 1 hr (Wang et al. 2019).
Coefficient of Linear Expansibility in Water
Sorbent samples were added to the 2 mL mark in graduated cylinders, and then stirred with 20 mL of water. After 24 hr following hydration and swelling, the final sorbent volume was determined. The ratio that was calculated from the beginning (2 mL of clay) and the final volume was indicative of hydration and expansion of the sample. A higher ratio indicates greater hydration and expansion.
In Vitro Isothermal Sorption
The AfB1 stock solution was prepared by dissolving pure crystals of AfB1 into acetonitrile. A calculated amount of the stock solution was injected into distilled water at pH 6.5 (the average pH of human intestine) or pH 2 (the average pH of human stomach) to yield an 8 mg kg−1 (8 µg/mL) AfB1 solution that was used for each isothermal study (Fallingborg 1999). The concentration was confirmed by scanning and reading the absorbance of aflatoxin at 362 nm using the SHIMADZU UV-visible spectrophotometer (UV-1800, SHIMADZU Corporation, Japan). The maximum AfB1 concentration was set at 8 mg kg−1 (well below the solubility range of 11–33 mg kg−1 for aflatoxins) so that precipitation of AfB1 did not occur in the solutions. Then 100 μg of each sorbent was exposed to an increasing concentration gradient of AfB1 solution: 0.4, 0.8, 1.6, 2.4, 3.2, 4, 4.8, 6, 6.4, 7.2 and 8 mg kg-1. The concentration gradients were achieved by adding a calculated amount of aflatoxin solution along with a complementary volume of distilled water to sterile 17 × 100 mm polypropylene centrifuge tubes to make a total volume of 5 mL. The 100 μg sorbent inclusion was achieved by injecting 50 μL of a 2 mg mL−1 clay suspension into the reaction media. Besides testing samples, there were 3 controls consisting of 5 mL each of distilled water, 8 mg kg−1 AfB1 solution without sorbent and 100 μg sorbent in distilled water. The control and test groups were capped and agitated at 1000 rpm for 2 hr at ambient temperature (26°C) and body temperature (37°C) using the IKA® electric shaker (VIBRAX VXR basic, Werke, Germany). All samples were then centrifuged at 2000 g for 20 min to separate the clay/AfB1 complex from solution. The UV-visible spectrophotometer was used to measure the free AfB1 in the supernatant from test and control groups.
Data Calculations and Curve Fitting
The UV-visible absorbance data were used to calculate the concentration of AfB1 left in solution using Beer’s law. The amount of toxin bound by clay at each data point was derived from the concentration difference between test and control groups and expressed as mol/kg on the isotherm. Values were calculated by the difference in moles of free toxin in the test solution versus control groups and is then divided by the mass of the clays included.
These data were then plotted using Table-Curve 2D and a computer program that was developed with Microsoft Excel to derive values for the variable parameters. The best fit for the data was a Langmuir model, which was used to plot equilibrium isotherms from triplicate analysis. The isotherm equation was entered as user-defined functions.
Estimates for the Qmax and Kd were taken from the double-logarithmic plot of the isotherm. The plot displays a break in the curve. The value on the x axis where the curve breaks is an estimate of Kd-1. The value on the y axis where the curve breaks is an estimate of Qmax (Grant and Phillips 1998; Abdellaoui et al. 2019). The Qmax was taken from the fit of the Langmuir model to the adsorption data. Equations for the calculation of isothermal data are shown in Table 2.
Table 2.
Equations for the calculation of isothermal data
| Beer’s law | Absorbance = ℇ L
c ℇ is the molar extinction coefficient (ℇ for AfB1 = 21,865 cm−1mol−1), L is the path length of the cell holder = 1 cm. |
| Langmuir model |
q = AfB1 sorbed (mol/kg), Qmax = maximum capacity (mol/kg), Kd = distribution constant, Cw = equilibrium concentration of AfB1. |
| Kd |
Hydra Bioassay
Hydra vulgaris were obtained from Environment Canada (Montreal, Qc) and maintained at 18°C. The hydra classification method (Wilby et al. 1990) was used with modification to rate morphology of the adult hydra as an indicator of solution toxicity. In this assay, the scoring of hydra morphology is objective and repeatable as indicated in our previous literature. The assay included monitoring times at shorter intervals during the first two days (0, 4, 20, and 28 hr) and 24 hr intervals for the last three days (44, 68, and 92 hr). Solutions were not changed during testing. The hydra morphological response was scored and recorded after exposure to AfB1, with and without, sorbent treatment. Mature and non-budding hydra in similar sizes were chosen for testing in order to minimize differences between samples. Controls for this experiment included hydra media consisting of 18.2 MΩ water, 4 mg L−1 ethylenediaminetetraacetic acid (EDTA), 115 mg L−1 N-tris[Hydroxymethyl]methyl-2-aminoethanesulfonic acid (TES), and 147 mg L−1 calcium dichloride (CaCl2) adjusted to pH 6.9–7.0. Sorbent inclusion percentage was chosen based on previous studies (Phillips et al. 2008). Toxin treatment groups included 20 mg kg−1 AfB1 in hydra media based on the minimum effective dose (MED) that caused 100% mortality in 92 hr. All test solutions were prepared by shaking at 1000 rpm for 2 hr and centrifugation at 2000 g for 20 min prior to toxin exposure of hydra in Pyrex dishes (Brown et al 2014). For each sample, three hydra were included into 4 mL of test media and kept at 18°C. The score or average toxicity rating was determined by calculating the average score for morphological changes for a certain group at a specific time point.
Molecular Models
The molecular model for bentonite was drawn in ISIS Draw 2.0 (MDL Information Systems, Inc., Hayward, California) and then imported into HyperChem 8.0. The aflatoxin structure was energy-minimized using the semiempirical quantum mechanical AM1 method. The model was constructed using the unit cell coordinates of muscovite (Richardson and Richardson 1982). These coordinates were then converted to orthogonal coordinates in an Excel spreadsheet that was constructed from a public domain C program. The unit cells were replicated in three-dimensional space by applying the symmetry operations for a C2/c space group (Donnay 1952). The d001 spacing of the model was then set to the corresponding dimensions of the bentonite (18 Å) based on the report of Greenland and Quirk (1960). The model was constructed in a 56 Å water box and aflatoxin was added to the interlayer and on the external basal surface (Slade et al. 1978), to illustrate proposed sites of aflatoxin sorption, based on isothermal data.
Statistical Analysis
A two way t-test was used to calculate statistical significance. Each experiment was independently triplicated to derive an average and standard deviation. In the t-test, the average COLE ratio from COLE experiments, Qmax from equilibrium isothermal analyses and toxicity scores from the hydra assay were included to calculate D = control-test groups and D2. Then the t-value was calculated using the following equation (N = 3):
The t-value and degrees of freedom were compared in a p-value table to determine the statistical significance. Results were considered significant at p ≤ 0.05.
Results
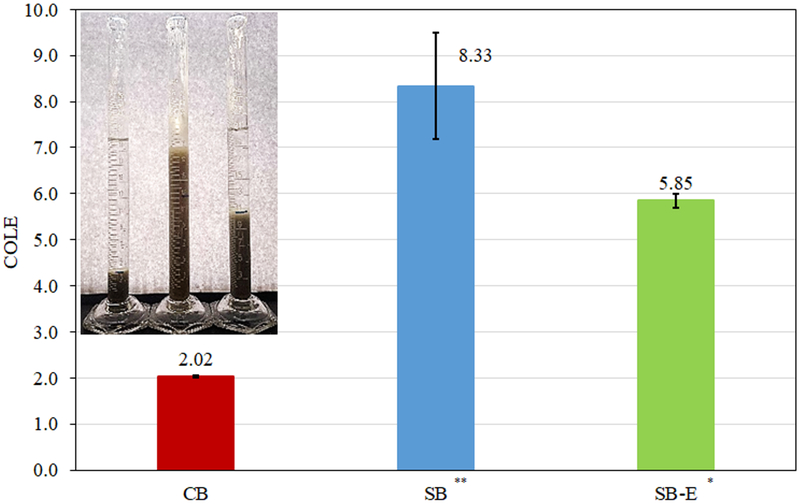
The COLE (Coefficient of Linear Expansibility) ratio indicates the expansibility of sorbents in water. COLE is equal to the expansion volume of clay divided by the original volume of clay. The higher the ratio, the more expansion and hydration of the sample. The accuracy of this method was confirmed by the COLE values for CB and SB clays, which showed limited swelling for CB compared to both SB clays (Figure 1). The COLE ratio for the test bentonite clay (SB-E) was equal to 5.85, indicating sodium predominance in its interlamellar region and the ability to swell upon hydration.
Figure 1.
Coefficient of linear expansibility for sorbents in water. The COLE value for CB confirmed its limited expansibility, whereas COLE values for SB and SB-E were significantly increased.
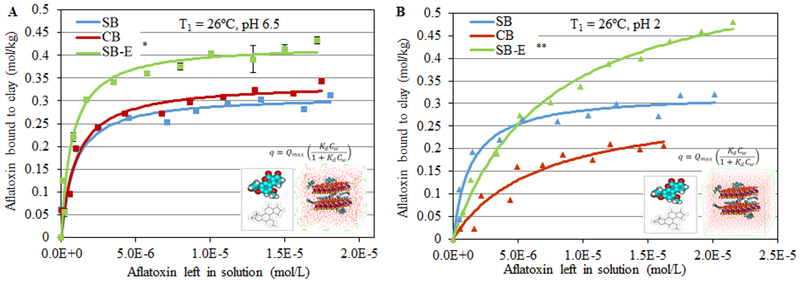
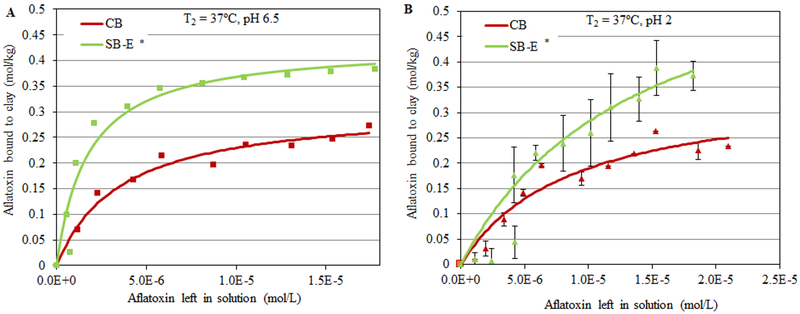
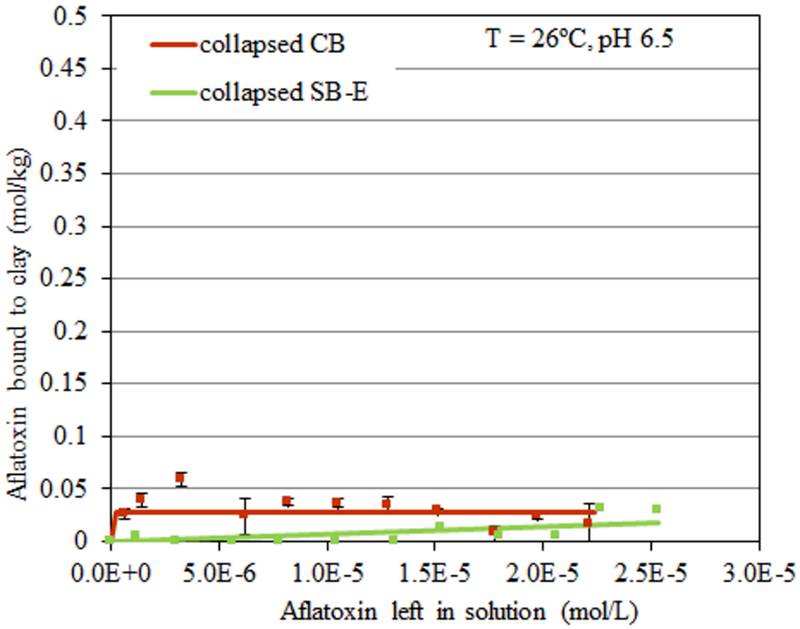
The isotherm in Figure 2A shows AfB1 sorption at pH 6.5 onto the surface of SB-E compared to standard samples of CB and SB clays. For all isotherms, the r2 values (or coefficients of determination) were above 0.8, indicating that the raw data strongly fit the Langmuir model and that AfB1 was bound tightly onto the clay surfaces and did not dissociate easily. Sample SB-E showed the highest Qmax for AfB1 with 0.42 moles of toxin bound per kg of clay. The Kd values (or binding affinities) for all sorbents were similar. In Figure 2B, isothermal plots at pH 2 indicated that the sorption of AfB1 onto the surfaces of SB-E was significantly increased (Qmax equal to 0.6 mol/kg) compared to the plots at pH 6.5. To calculate the binding enthalpy of sample SB-E for AfB1, isotherms were run at 2 different temperatures, i.e., 26°C (T1) and 37°C (T2). Calculated enthalpies (∆H) for sample SB-E were equal to −61.7 kJ mol−1 at pH 6.5 and −46.6 kJ mol−1 at pH 2, which were similar to the standard CB (−89.2 kJ mol−1 and −36.8 kJ mol−1, respectively) (Figure 3). After the interlayers of SB-E were heat-collapsed, the AfB1 sorption was dramatically decreased (Figure 4). A summary of sorption parameters for AfB1 onto active surfaces of standard CB and SB, and SB-E is shown in Table 3.
Figure 2.
Langmuir adsorption isotherm - plots of AfB1 on SB, CB and SB-E showing the observed and predicted Qmax values at 26°C (T1), pH 6.5 (A) and pH 2 (B) (* p ≤ 0.05, ** p ≤ 0.01).
Figure 3.
Langmuir adsorption isotherm - plots of AfB1 on CB and SB-E showing the observed and predicted Qmax values at 37°C (T2), pH 6.5 (A) and pH 2 (B) (* p ≤ 0.05).
Figure 4.
Langmuir adsorption isotherm - plots of AfB1 on collapsed CB and collapsed SB-E at 26°C and pH 6.5.
Table 3.
Summary table of sorption parameters for CB, SB and SB-E.
| Sorbents | pH 6.5, T1 | pH 2, T1 | pH 6.5, T2 | pH 2, T2 | Collapsed |
|---|---|---|---|---|---|
| SB | Qmax = 0.31 | Qmax = 0.32 | N/A | N/A | N/A |
| Kd = 1.2E6 | Kd = 7.5E5 | ||||
| CB | Qmax = 0.35 | Qmax = 0.3 | Qmax = 0.32 | Qmax = 0.35 | Qmax = 0.03 |
| Kd = 9.0E5 | Kd = 1.5E5 | Kd = 2.8E5 | Kd = 1.2E5 | Kd = 4.2E19 | |
| SB-E | Qmax = 0.42 | Qmax = 0.62 | Qmax = 0.43 | Qmax = 0.68 | Kd = 1.5E2 |
| Kd = 1.4E6 | Kd = 1.4E5 | Kd = 5.8E5 | Kd = 7.2E4 |
(SB, sodium montmorillonite; CB, calcium montmorillonite; SB-E, sample E; T1 = 26°C; T2 = 37°C; Qmax = maximum capacity; Kd = affinity constant; N/A, not applicable)
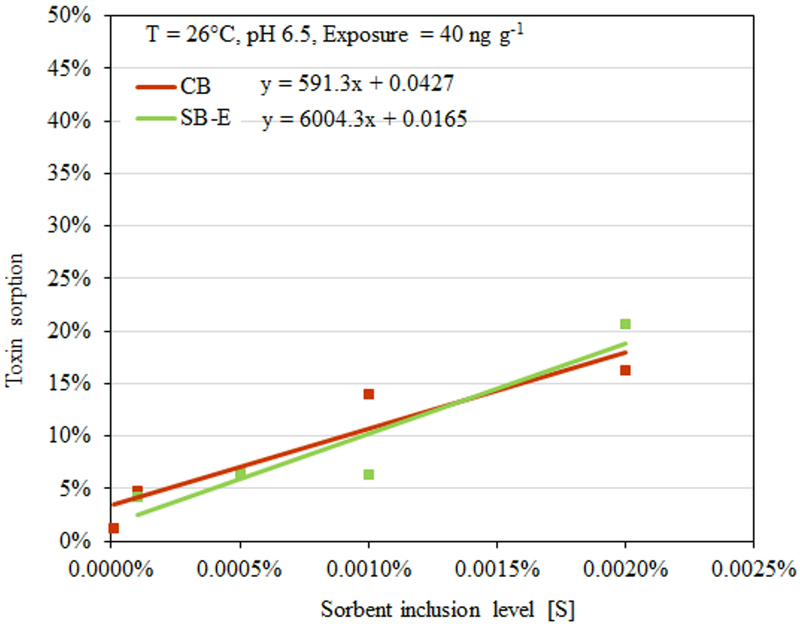
In order to establish the amount of sorbent needed to mitigate different levels of aflatoxin in a contaminated diet, % toxin sorption can be plotted against sorbent inclusion level ([S]) to derive the dose of sorbent needed to achieve the regulatory level. We exposed AfB1 at twice the 20 ng g−1 threshold level (or 40 ng g−1) to an increasing dose of sorbent ranging from 0.0001% to 0.002%. Based on our dosimetry results (Figure 5), to reduce 50% aflatoxin exposure and thus achieve below the action level, the predicted sorbent inclusion rate ([S]) for SB-E would be equal to 0.01%, which was 8 times less than the predicted dose for standard CB (i.e. 0.08%).
Figure 5.
Extrapolation of sorbent dosimetry for aflatoxin exposure at 40 ng g-1.
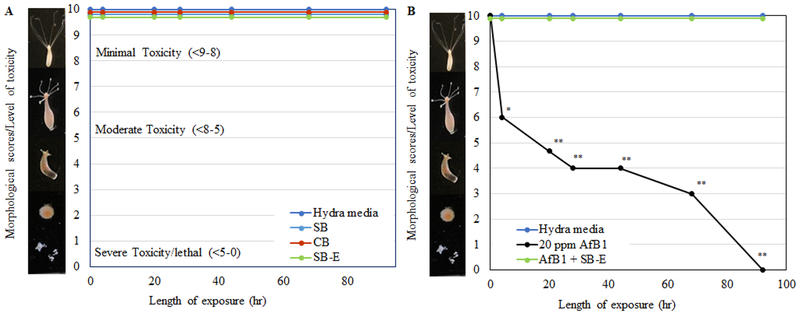
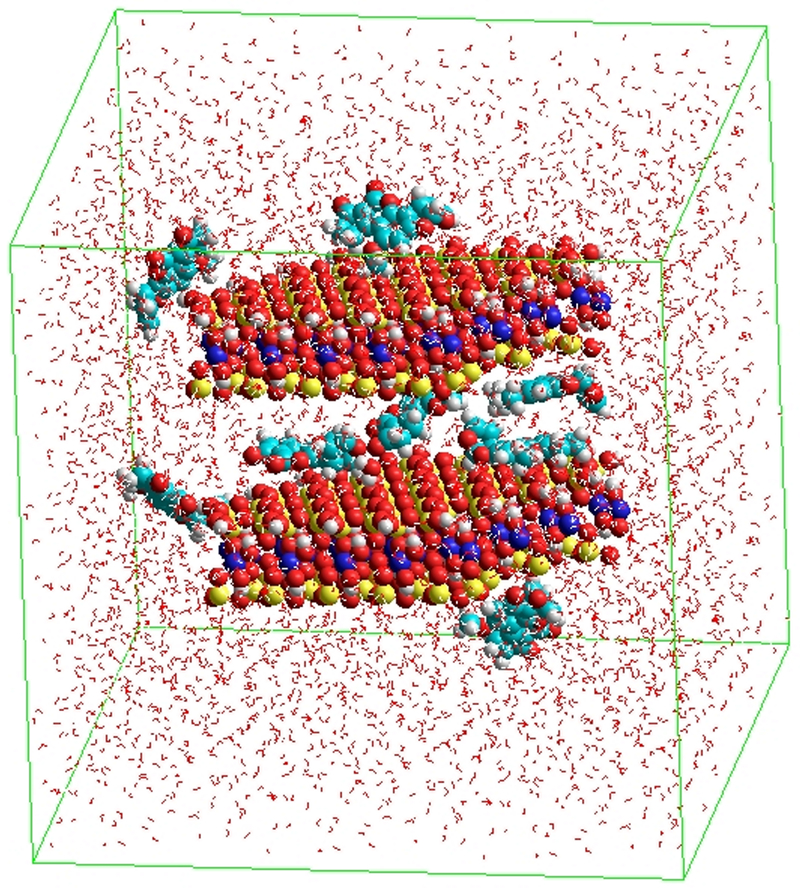
The minimum effective dose (MED) for AfB1 in the hydra bioassay has been established earlier as 20 mg kg−1, which results in 100% hydra mortality in 92 hr (Wang et al. 2019). All sorbents at the inclusion rate at 0.5% showed no adverse effects in hydra (Figure 6A). When SB-E was included at a very low clay inclusion rate of 0.02% in the presence of 20 mg kg−1 AfB1, complete protection of hydra against AfB1 was achieved compared to the hydra media control (Figure 6B). Based on the evidence from isothermal and hydra studies, a molecular model representing aflatoxin binding interactions on active surfaces within the interlamellar region of SB-E is shown in Figure 7. The molecular models of CB and SB have been previously published along with XRD analysis (Marroquin-Cardona et al. 2011a, 2011b; Zychowski 2014). There are no major differences in the unit cell formulas for SB and SB-E.
Figure 6.
Hydra bioassay of bentonite samples at a 0.5% inclusion rate (A), and protection at a 0.02% inclusion rate of SB-E are shown in the presence of 20 mg kg−1 AfB1 (B). Hydra media and toxin controls are included for comparison. No toxicity was shown by the hydra media and all clay treatment groups (* p ≤ 0.05, ** p ≤ 0.01).
Figure 7.
Energy minimized molecular model of SB-E and aflatoxin B1 in a water box (oxygen = red; silicon = yellow; aluminum = blue; carbon = cyan).
Discussion
Based on our previous in vitro work and extensive animal interventions and human clinical trials, CB clay was an effective aflatoxin enterosorbent and was safe for consumption by humans and animals (Phillips et al. 2019). The interlayers of SB are more accessible than CB, but may be less preferential than CB. The high expansibility and dispersion in water for SB was confirmed by its COLE ratio equal to 8.33, whereas CB was shown to have limited swelling as indicated by its COLE ratio of 2.02 (Figure 1). The stronger swelling tendency of SB is related to the increased hydration energy of sodium ions versus calcium ions. This facilitates the hydration and desorption of sodium from interlamellar regions following the addition of water and swelling. Sample SB-E showed a high COLE value, indicating a predominance of sodium in the structure. SB-E also showed the ability to quickly flocculate and precipitate after 24 hr of equilibrium. Thus, we suspect that the unusual combination of a high COLE value (like SB) and a high flocculation ability (like CB) could be associated with the sodium adsorption ratio and contribute to the higher aflatoxin sorption capacity of SB-E. Further work (in vitro and in vivo) will elucidate this mechanism and investigate the enhanced ability of SB-E to protect against aflatoxicosis in animals and humans.
CB and SB are commonly used as general sorbents for toxin binding (Abdellaoui et al. 2019). To investigate the binding effectiveness for AfB1, equilibrium isotherms were generated by Table-Curve 2D and a computer program was developed in our laboratory using Microsoft Excel. This program was used to derive affinities (Kd), capacities (Qmax) and the enthalpy of sorption (ΔH) for toxin-surface interactions. Based on r2 values (r2 > 0.8) and randomness of the residuals, the best fit for the data was a Langmuir model, which was used to plot equilibrium isotherms from triplicate analyses. Each point represents the values calculated for AfB1 bound to clay (mol/kg) and AfB1 left in solution (mol/L) for the corresponding dilutions. Although the expansibility of SB standard in water was larger than CB standard, its binding capacity was typically lower, as shown in Figure 2A. When isotherms were run at pH 6.5, simulating the pH of the intestine, SB-E showed the highest Qmax, which was equal to 0.42 mol/kg for AfB1 sorption. This value was higher than that of CB (i.e. 0.35 mol/kg). Further work is ongoing to delineate reasons for a uniformly higher binding capacity of SB-E vs CB. At pH 2, SB-E showed increased Qmax and decreased Kd values, compared to pH 6.5 (Figure 2B). This resulted in a significantly increased Qmax for SB-E vs CB. Since the pHPZC (pH for zero point of charge) of bentonite is 2–3, a possible mechanism for this effect in SB-E may involve acidic solution providing protons that can neutralize the overall negative charge at active surfaces in the interlamellar region and decrease binding affinity leading to increased Qmax. SB-E was a very efficient clay binder for AfB1 at pH 2, and this pH will be the first encountered by clay and the toxin in the diet of humans and monogastric animals.
As part of this study, it was important to gain insight into AfB1 binding mechanisms and the thermodynamics of toxin/surface interactions. The enthalpy (heat) of sorption (ΔH) can be calculated from Kd values of isotherms run at different temperatures. It is directly relevant to the tightness of toxin/clay interactions and can help with the derivation of mechanistic information (Fischer and Peters 1970; Stumm et al. 1992). In particular, the sorption of compounds to a surface can be categorized as either physisorption or chemisorption on the basis of ΔH (Gatta 1985). Physisorption involves weak associations, which include Van der Waals attraction, dipole−dipole interactions, induced dipole interactions, and hydrogen bonding. Chemisorption implies a chemical reaction or sharing of electrons between the adsorbent and the adsorbate. Physisorption is described as having an enthalpy of < 20 kJ mol−1, while chemisorption is generally > 20 kJ mol−1 (Gu et al. 1994). Sorption enthalpy (∆H) for clay interactions with AfB1 were calculated by the Van’t Hoff equation from isotherms at 2 different temperatures, i.e., 26°C (T1) and 37°C (T2). Derived ∆H values for sample SB-E (−61.7 kJ mol−1 and −46.6 kJ mol−1, at pH 6.5 and 2, respectively) were similar to CB (−89.2 kJ mol−1 and −36.8 kJ mol−1, respectively) (Figure 3). These high enthalpy absolute values indicate that AfB1 is chemisorbed tightly to the clay surfaces and is consistent with a Langmuir model for the isothermal interaction. Following heating, the interlayers of CB and sample SB-E were dehydroxylated and collapsed. Figure 4 showed that binding capacities of collapsed CB and collapsed SB-E were significantly reduced. This dramatic decrease of AfB1 suggests (indirectly) that a large amount of the AfB1 binds within the negatively charged interlayer of these clays and only minor amounts bind on the edges and basal surfaces. A summary of sorption parameters for AfB1 onto surfaces of CB, SB, and SB-E is shown in Table 3.
Besides isotherms, enhanced binding efficacy of SB-E was also shown in our dosimetry study that delineated the amount of clays required to maintain a regulatory threshold concentration of AfB1 (20 ng g−1). Based on our results (Figure 5), for 40 ng g−1, the predicted concentration of sorbent ([S]) for CB and was equal to 0.08% inclusion in the diet, which is equivalent to 2.4 g clay/day based on a 3 kg daily intake in the U.S. for humans. This level is in agreement with our earlier mass balance study with CB in animals, where 0.1% inclusion in the diet significantly decreased exposures from 80 and 40 ng g−1 of 14C-AfB1 labeled aflatoxin. For SB-E, [S] was equal to 0.01% or 0.3 g clay/kg of diet based on a daily intake of 3 kg for U.S. adults. This level of SB-E clay (if approved for human consumption) could be easily delivered as three 0.2 g doses before each meal.
The protective roles of CB, SB, and SB-E were confirmed in vivo using the adult hydra assay. As indicated in Figure 6A, the clays were not toxic to the hydra and were comparable to the hydra media control (morphological score of 10). In further studies, 20 mg kg−1 AfB1 resulted in 100% mortality of hydra within 92 hr. Importantly, SB-E at a 0.02% inclusion in hydra media showed complete (100%) protection against 20 mg kg−1 AfB1 (Figure 6B). Because this work was focused on characterizing a high capacity binder for aflatoxin, we included levels of clays (0.02%) in the hydra bioassay that were less than the common inclusion level in animal feed (0.2%). Our findings in vivo are consistent with our in vitro isothermal results.
Based on in vitro and in vivo results, a suggested model for AfB1 sorption to SB-E surfaces is shown in Figure 6. Sample SB-E showed the highest binding capacity for aflatoxin at pH 6.5 and pH 2 with high enthalpy (∆H) and was very effective as a binder for high doses of aflatoxins. Heat-collapsed CB and SB-E indicated that the major binding sites for AfB1 were on surfaces within the interlayer (Figure 7). The hydra bioassay also confirmed the safety of SB-E and its ability to protect against aflatoxin at low rates of clay inclusion. SB deposits are widespread, and this material, which is highly effective for aflatoxin binding, should be applicable for use in animal feed. SB binders (like SB-E) could be delivered through capsules, nutritional supplements, snacks, flavored liquids and complete feeds during emergencies and outbreaks of acute aflatoxicosis.
Acknowledgments
This work was supported by the NIEHS (Superfund hazardous Substance Research and Training Program) under Grant number P42 ES0277704; and USDA under Grant number Hatch 6215.
Footnotes
Declaration of interest statement
The authors declare no conflict of interest.
References
- Abdellaoui Y, Olguinm MT, Abatal M, Ali B, Mendez SED, Santiago AA. 2019. Comparison of the divalent heavy metals (Pb, Cu and Cd) adsorption behavior by montmorillonite-KSF and their calcium- and sodium-forms. Superlattice Microst. 127:165–175. [Google Scholar]
- Allcroft R, Carnaghan RBA. 1963. Groundnut Toxicity: An examination for toxins in human food products from animals fed toxic groundnut meal. Vet Rec. 75:259–263. [Google Scholar]
- Brown KA, Mays T, Romoser A, Marroquin-Cardona A, Mitchell NJ, Elmore SE, Phillips TD. 2014. Modified hydra bioassay to evaluate the toxicity of multiple mycotoxins and predict the detoxification efficacy of a clay-based sorbent. JAT. 34:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [CAST] Council for Agricultural Science and Technology. 1989. In mycotoxins: Economic and health risks In: Niyo K, editor. Task Force Report. Ames (USA): CAST; p. 1–91. [Google Scholar]
- Donnay JD. 1952. International tables for X-ray crystallography. Birmingham (USA): Kynoch Press; p.3. [Google Scholar]
- [EC] European Commission. 2005. Soil atlas of europe, european soil bureau network. Luxembourg: Office for Official Publications of the European Communities; p. 128. [Google Scholar]
- Egner PA, Wang JB, Zhu YR, Zhang BC, Wu Y, Zhang QN, Qian GS, Kuang SY, Gange SJJacobson LP. 2001. Chlorophyllin intervention reduces aflatoxin–DNA adducts in individuals at high risk for liver cancer. Proc Natl Acad Sci USA, 98(25): 14601–14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallingborg J 1999. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 46(3):183–196. [PubMed] [Google Scholar]
- Fischer RB, Peters DG. 1970. Chemical equilibrium. Philadelphia (USA): W. B. Saudners; p. 1. [Google Scholar]
- Gatta GD. 1985. Direct determination of adsorption heats. Thermochim. Acta. 96:349–363. [Google Scholar]
- Grant PG, Phillips TD. 1998. Isothermal adsorption of aflatoxin B(1) on HSCAS clay. J Agric Food Chem. 46:599–605. [DOI] [PubMed] [Google Scholar]
- Greenland DJ, Quirk JP. 1960. Adsorption of 1-n-alkyl pyridinium bromides by montmorillonite. Clays Clay Miner. 9:484–499. [Google Scholar]
- Gu B, Schmitt J, Chen Z, Liang L, McCarthy JF. 1994. Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ Sci Technol. 28:38–46. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y. 2005. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin–DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev, 14(11 Pt 1): 2605–2613. [DOI] [PubMed] [Google Scholar]
- Khlangwiset R, Wu F. 2010. Costs and efficacy of public health interventions to reduce aflatoxin-induced human disease. Food Addit Contam Part A, 27(7): 998–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquin-Cardona A, Deng Y, Garcia-Mazcorro J, Johnson NM, Mitchell N, Tang L, Robinson A, Taylor J, Wang JS, Phillips TD. 2011a. Characterization and safety of uniform particle size NovaSil clay as a potential aflatoxin enterosorbent. Appl Clay Sci. 54(3–4):248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquin-Cardona AG. 2011b. Characterization and safety of clays as potential dietary supplement to prevent aflatoxicosis [dissertation]. College Station (TX): Texas A&M University. [Google Scholar]
- Murugesan GR, Ledoux DR, Naehrer K, Berthiller F, Applegate TJ, Grenier B, Phillips TD, Schatzmayr G. 2015. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult Sci. 94:1298–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TD, Afriyie-Gyawu E, Williams J, Huebner H, Ankrah NA, Ofori-Adjei D, Jolly P, Johnson N, Taylor J, Marroquin-Cardona A, Xu L, Tang L, Wang JS. 2008. Reducing human exposure to aflatoxin through the use of clay: a review. Food Addit Contam Part A. 25(2):134–145. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Sarr AB, Grant PG. 1995. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. J Nat Toxins. 3:204–213; discussion 221. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Wang M, Elmore SE, Hearon S, Wang JS. 2019. NovaSil clay for the protection of humans and animals from aflatoxins and other contaminants. Clays Clay Miner. 67(1):99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SM, Richardson JM. 1982. Crystal structure of a pink muscovite from Archer’s post, Kenya: implications for reverse plechorism in dioctahedral micas. Am Miner. 67:69–75. [Google Scholar]
- Slade PG, Raupach M, Emerson WW. 1978. The Ordering of Cetylpyridinium Bromide on Vermiculite. Clays Clay Miner. 26:125–134. [Google Scholar]
- Stumm W, Sigg L, Sulzberger B. 1992. Chemistry of the solid-water interface. New York (USA): Wiley; pp. 1. [Google Scholar]
- Tang L, Tang M, Xu L, Luo H, Huang T, Yu J, Zhang L, Gao W, Cox SB, Wang JS. 2008. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis, 29(2): 411–417. [DOI] [PubMed] [Google Scholar]
- Thuresson A, Jansson M, Plivelic TS, Skepö M. 2017. Temperature response of charged colloidal particles by mixing counterions utilizing Ca2+/Na+ montmorillonite as model system. J Phys Chem C. 121(14):7951–7958. [Google Scholar]
- [USDA] United States Department of Agriculture. 1978. Engineering Classification of Earth Materials. In National Engineering Handbook, Part 631. [Google Scholar]
- Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba AEgner PA. 1999. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Cancer Inst, 91: 347–354. [DOI] [PubMed] [Google Scholar]
- Wang M, Hearon S, Phillips TD. 2019. Development of enterosorbents that can be added to food and water to reduce toxin exposures during disasters. J Environ Sci Heal B. 54(6):514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hearon SE, Johnson NM, Phillips TD. 2019. Development of broad-acting clays for the tight adsorption of benzo[a]pyrene and aldicarb. Appl Clay Sci. 168:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Maki CR, Deng Y, Tian Y, Phillips TD. 2017. Development of high capacity enterosorbents for aflatoxin B1 and other hazardous chemicals. Chem Res Toxicol. 30(9):1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Afriyie-Gyawu E, Tang Y, Johnson NM, Xu L, Tang L, Huebner HJ, Ankrah NA, Ofori-Adjei DEllis W. 2008. NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis: II. Reduction in biomarkers of aflatoxin exposure in blood and urine. Food Addit Contam, 25(5): 622–634. [DOI] [PubMed] [Google Scholar]
- Wilby OK, Tesh JM, Shore PR. 1990. Application of the hydra regeneration assay: Assessment of the potential teratogenic activity of engine exhaust emissions. Toxicology in vitro. 4:612–613. [DOI] [PubMed] [Google Scholar]
- Zychowski KE. 2014. Calcium montmorillonite for the mitigation of aflatoxicosis and gastrointestinal inflammation [Dissertation]. College Station (TX): Texas A&M University. [Google Scholar]