Abstract
Objective
To examine whether initiation of interleukin (IL)-17, IL-12/23 or tumour necrosis factor (TNF) inhibitor is associated with an increased risk of serious infection among real-world psoriasis (PsO) or psoriatic arthritis (PsA) patients.
Methods
We assembled a retrospective cohort of commercially insured adults in the USA diagnosed with PsO or PsA between 2015 and 2018. Exposure was dispensation for IL-17 (ixekizumab or secukinumab), IL-12/23 (ustekinumab) or TNF (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab). The outcome was infection requiring hospitalisation after biologic initiation. Incidence rates (IRs) per 100 person-years were computed, and hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression models, adjusted for inverse probability of treatment-weighted propensity scores.
Results
A total of 11 560 new treatment episodes were included. Overall, 190 serious infections (2% of treatment episodes) were identified in 9264 person-years of follow-up. Class-specific IRs were similar among IL-17 and TNF, yet significantly lower for IL-12/23. After adjustment for propensity scores, there was no increased risk with IL-17 compared with either TNF (HR=0.89, 95% CI 0.48 to 1.66) or IL-12/23 (HR=1.12, 95% CI 0.62 to 2.03). By contrast, IL-23/23 were associated with a lower risk of infections than TNF (HR=0.59, 95% CI 0.39 to 0.90).
Conclusions
Relative to TNF and IL-17, IL-12/23 inhibitors were associated with a reduced risk of serious infection in biologic-naïve patients with PsO or PsA. In biologic-experienced individuals, there was no difference in infection risk across TNF, IL-17 or IL-12/23 inhibitors.
INTRODUCTION
Tumour necrosis factor (TNF) inhibitors have transformed the care of many rheumatologic and autoimmune conditions, including psoriasis (PsO) and psoriatic arthritis (PsA). In the past 10 years, additional biologic options approved by the US Food and Drug Administration (FDA) include the interleukin-12/23 (IL-12/23) inhibitor ustekinumab as well as the human interleukin-IL-17A (IL-17) antagonists secukinumab and ixekizumab.
Despite efficacy for the management of moderate-to-severe PsO and PsA, biologics’ immunosuppressive properties also contribute to an increased risk of serious infections in placebo-controlled randomised controlled trials (RCTs).1–4 Head-to-head RCTs between biologic agents with adequate power to inform comparative safety questions have been limited.3,5,6
It is important to understand whether these findings from RCTs persist in real-world practice, where patients are more heterogeneous and drug utilisation is far less controlled.7 Evidence from observational studies between biologic and non-biologic drugs have yielded inconsistent findings: some have shown an increased risk,8,9 while others have not found a difference.10–14 To our knowledge, no published studies have yet quantified the comparative real-world risk of serious infections among IL-17, IL-12/23 and TNF inhibitors.
We examined the absolute and relative comparative risk of serious infections in patients initiating IL-17, IL-12/23 and TNF inhibitors, among commercially insured adults in the USA diagnosed with PsO or PsA between 2015 and 2018.
METHODS
Data source
We conducted a retrospective cohort analysis using the OptumLabs Data Warehouse.15 The OptumLabs data consist of administrative claims for over 100 million individuals in all 50 states, of all ages, ethnic and racial groups. Claims include limited patient sociodemographic characteristics as well as inpatient, outpatient and pharmacy dispensation claims. Analysis of secondary, deidentified data is considered exempt by the Johns Hopkins Institutional Review Board.
Patient and public involvement
Patients were not involved in the design, recruitment or conduct of the study.
Study population
First, we identified a cohort of all prescription dispensation or medical infusion procedure claims for any of the biologics of interest between 1 January 2015 and 1 May 2018. We were not able to study brodalumab (IL-17) nor guselkumab (IL-12/23), as they were FDA approved towards the end of the study period. We then included only those with at least one diagnosis code prior to the index date for PsO (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 696.1 or ICD-10-CM code L40.9) or PsA (ICD-9-CM code 696.0; ICD-10-CM codes L40.50, L40.51, L40.52, L40.53, L40.54, L40.59) from a dermatologist or rheumatologist visit. Prior work suggests a sensitivity of 77%—91% and positive predictive value of 67%−89% for this approach.16
We defined the index date as the date of the first dispensing of any IL-17, IL-12/23 or TNF inhibitor of interest, requiring individuals to have at least 6 months of continuous enrolment with full medical and pharmacy data before the index date to establish new user status.17 Since these biologics were only approved for use in adults, we required patients to be at least 18 years old at the index date. We excluded individuals with overlapping claims for multiple biologics, due to our inability to ascertain which biologic was truly used given the contraindication of simultaneous use. We also excluded persons who had a diagnosis of rheumatoid arthritis, Crohn’s disease, ulcerative colitis, osteoarthritis, HIV, cancer, chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma at any point during 24 months prior to the index date, given the potential impact of these comorbid conditions on the incidence of serious infection.18 We further excluded persons who had a serious infection (using our outcome definition, below) in the 60 days prior to index date.
Exposures
We defined three mutually exclusive exposures (IL-17: ixekizumab and secukinumab; IL-12/23: ustekinumab; TNF: adalimumab, certolizumab pegol, etanercept, golimumab and infliximab) based on pharmacologic drug class (online supplementary table S1). We defined treatment episodes as the initiation of a new biologic agent without any claim for that specific treatment at any time previously, requiring a minimum of 6 months of medical and pharmacy coverage.18,19 We allowed for a grace period of 90 days for non-overlapping prescriptions to define periods of continuous treatment.
Each person could contribute more than one treatment episode. We defined biologic-naïve as no claims of PsO-related or PsA-related biologics prior to the index date based on all available data. Biologic-experienced was defined as having at least one other biologic, but not the index biologic, before the index date of the current treatment episode.
Self-administered biologics were identified from pharmacy claims using National Drug Codes. Prescription fill date and days of supply were used to calculate duration of treatment. Biologics that require infusions under supervision of a physician were identified through Healthcare Common Procedure Coding System procedure codes from medical claims. As these medical claims lack information on days of supply, we assigned the duration of treatment based on a typical dosage regimen. For infliximab, days of continuous drug exposure were based on a loading schedule of 0, 2, 6, 14, and then every 8 weeks. For ustekinumab, first administration was assumed to be 4-week supply, and any subsequent refill was assumed to be 12-week supply.20
Outcome
Our primary outcome was serious infection, defined as hospitalisation with the listing of infection in the inpatient claims diagnosis codes, including the primary and all non-primary positions (list available from authors on request). Subjects were followed from biologic initiation until their first hospitalisation with serious infection, or were censored if they developed a competing comorbidity that would have been exclusionary at baseline (such as Crohn’s disease), discontinued biologic therapy (defined as switching to another biologic, or a treatment gap of at least 90 days),21 lost continuous enrolment, died or 31 December 2018, whichever came first.
Covariates
We measured covariates using a 6-month lookback period from the index date. Patient covariates included demographics (age, sex, calendar year) and socioeconomic characteristics (race, education, household income level). We conducted multiple imputation by simple random sampling for missing data including race, income level and education (less than 5%).22 Other pharmacologic covariates included prior use of phototherapy, and non-biologic PsO and PsA drugs (online supplementary table S2). We also included Charlson Comorbidity Index scores using ICD-9 or ICD-10 codes from claims,23 selected comorbidities and health services utilisation (number of prior hospitalisations, emergency room, outpatient and physician specialist visits). For the number of prior biologic agents, we used all available look-back data to ascertain prior drug experience.
Propensity scores
We used inverse probability weighting for the average treatment effect of the treated weighting,24 with a propensity score to adjust for differences in baseline demographic and clinical characteristics between groups which may confound their drug treatment exposure. Propensity scores were calculated based on the probability of being exposed to either IL-17, IL-12/23 or TNF using multivariable logistic regression models.18,25 Weights were trimmed at 0.1 and 10 to minimise the influence of outliers.26 We assessed the balance of covariates after weighting by standardised mean differences (SMD). Propensity scores were recalculated for each of the stratified analyses.
Statistical analysis
We estimated the incidence rate (IR) of serious infections per 100 person-years for each drug class, with 95% confidence intervals (CIs) calculated using Poisson models. Kaplan-Meier curves were constructed to describe time from drug initiation to serious infection. We used weighted Cox proportional hazard models to estimate hazard ratios (HRs) and corresponding 95% CI for risk of serious infections, adjusted for imbalanced covariates with SMD >10% after weighting.27 We calculated corrected standard errors clustered on an individual level, to account for a patient contributing more than one treatment episode. The proportional hazards assumption was verified by Schoenfeld residuals and complementary log-log plots. For each analysis, we compared IL-17, IL-12/23 and TNF inhibitor groups to each other (ie, three pairwise comparisons).
We performed subgroup analyses stratifying by PsO or PsA, as well as by history with other biologics (naïve vs experienced). Diagnosis groups were not exclusive, as patients with PsO can also have PsA, and vice versa; stratified regression models were adjusted for the presence of the alternative disease type.
We conducted two sensitivity analyses. First, we restricted our outcome definition to the primary diagnosis code on inpatient admission diagnoses (rather than any position). We also narrowed the length of the grace period to 60 days to produce reduced estimates of treatment duration. All analyses were done in R V3.5.3.28
RESULTS
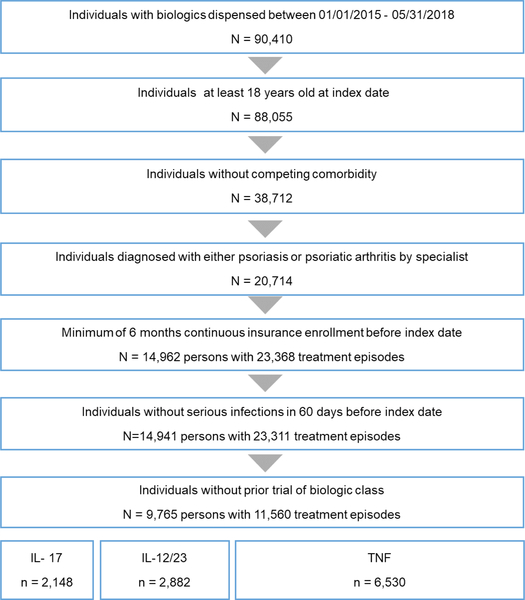
We identified a total of 11 560 treatment episodes from 9305 adults: 19% IL-17, 25% IL-12/23% and 56% in the TNF groups, respectively (figure 1). Overall, the population mean age was 46 years and 53% male (table 1).
Figure 1.
Patient selection process. IL,interleukin; TNF, tumour necrosis factor.
Table 1.
Characteristics of PsO or PsA patients at the time of index date, overall and by drug class
| All | IL-17 | IL-12/23 | TNF | |
|---|---|---|---|---|
| (n=11 560) | (n=2148) | (n=2882) | (n=6530) | |
| Age | 46 (12) | 48 (11) | 46 (12) | 46 (12) |
| Male | 6107 (53%) | 1141 (53%) | 1569 (54%) | 3397 (52%) |
| Household income | ||||
| <$40 000 | 1372 (12%) | 277 (13%) | 333 (12%) | 762 (12%) |
| $40 000—$74 999 | 2818 (24%) | 477 (22%) | 678 (24%) | 1663 (26%) |
| $75 000—$124 999 | 3694 (32%) | 682 (32%) | 922 (32%) | 2090 (32%) |
| $125 000—$199 999 | 2137 (19%) | 415 (19%) | 529 (18%) | 1193 (18%) |
| >$200 000 | 1539 (13%) | 297 (14%) | 420 (15%) | 822 (13%) |
| Diagnosis | ||||
| PsO only | 6043 (52%) | 1204 (56%) | 1994 (69%) | 2846 (44%) |
| PsA only | 1869 (16%) | 245 (11%) | 239 (8%) | 1388 (21%) |
| PsO and PsA | 3648 (32%) | 699 (33%) | 650 (23%) | 2299 (35%) |
| Charlson Comorbidity Index score | ||||
| 0 | 8248 (71%) | 1429 (67%) | 2158 (75%) | 4661 (71%) |
| 1 | 2192 (19%) | 477 (22%) | 468 (16%) | 1247 (19%) |
| 2 or more | 1120 (10%) | 242 (11%) | 256 (9%) | 622 (10%) |
| Number of previous biologics | ||||
| 0 | 5995 (52%) | 332 (16%) | 1344 (47%) | 4319 (66%) |
| 1 | 3614 (31%) | 817 (38%) | 1121 (39%) | 1676 (26%) |
| 2 or more | 1951 (17%) | 999 (46%) | 417 (14%) | 535 (8%) |
| DMARDs in past 6 months | 3115 (27%) | 519 (24%) | 619 (22%) | 1977 (30%) |
| Methotrexate | 2032 (18%) | 251 (12%) | 345 (12%) | 1436 (22%) |
| Sulfasalazine | 249 (2%) | 22 (1%) | 28 (1%) | 199 (3%) |
| Apremilast | 796 (7%) | 228 (11%) | 240 (8%) | 328 (5%) |
| Other | 369 (3%) | 77 (4%) | 81 (3%) | 211 (3%) |
| Oral steroids in past 6 months, % | 3989 (35%) | 732 (34%) | 891 (31%) | 2366 (36%) |
| NSAIDs prescribed in past 6 months | 1594 (14%) | 278 (13%) | 262 (9%) | 1054 (16%) |
| Phototherapy in past 6 months | 261 (2%) | 39 (2%) | 93 (3%) | 129 (2%) |
Continuous variables are presented as mean (SD), and categorical variables are presented as counts (percentages).
DMARD, disease-modifying antirheumatic drugs; IL, interleukin; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis; PsO, psoriasis.
Among the treatment episodes, 6044 (52%) were for persons with a recorded diagnosis of PsO only, 1872 (16%) with PsA only, and 3648 (32%) both. Using all available data, the proportion of patients with any historical exposure to previous biologics differed greatly by cohort (85% among IL-17, 53% IL-12/23% and 34% in TNF inhibitor groups, respectively), and may reflect utilisation management strategies by pharmacy benefit managers. Conventional synthetic disease-modifying antirheumatic drugs (DMARDs) were most commonly used in the TNF inhibitors group, and 35% were dispensed oral steroids during the 6-month lookback period. Additional cohort characteristics are presented in online supplementary table S3, as well as stratified for the PsO (online supplementary table S4) and PsA (online supplementary table S5) subgroups. Compared with the PsO cohort, the PsA cohort had a higher mean Charlson score, and a larger proportion of patients using other medications (disease-modifying antirheumatic drugs, steroids and non-steroidal anti-inflammatory drugs) during the lookback period. A larger proportion of biologic-naïve, compared with biologic experienced persons, used methotrexate. Steroids treatment was similar between the two subgroups.
Incidence rate of serious infections
Overall, 190 serious infections (2% of treatment episodes) occurred after initiation of study biologics. The most commonly diagnosed serious infections on hospitalisation were sepsis and pneumonia (table 2).
Table 2.
Frequency counts of ICD codes for serious infection
| Code | Description | Frequency |
|---|---|---|
| A419 | Sepsis unspecified organism | 38 |
| J189 | Pneumonia unspecified organism | 30 |
| N390 | UTI site not specified | 20 |
| L03116 | Cellulitis of left lower limb | 13 |
| L0390 | Cellulitis unspecified | 12 |
| Other types of serious infection* | 77 | |
Clinical description of cells with 10 or fewer observations suppressed per data license.
Class-specific IRs were similar among IL-17 and TNF, and significantly lower for IL-12/23 (table 3).
Table 3.
Incidence of serious infections among biologic users with PsO or PsA, overall and by drug class
| All biologic classes | IL-17 | IL-12/23 | TNF | |
|---|---|---|---|---|
| Total cohort | ||||
| Number of treatment episodes | 11 560 | 2148 | 2882 | 6530 |
| Total person-years of follow-up | 9264 | 1528 | 2461 | 5275 |
| Incident serious infections, n (%) | 190 (2) | 32 (1) | 32 (1) | 126 (2) |
| Incidence rate (95% CI), per 100 person-years | 2.1 (1.8 to 2.4) | 2.1 (1.5 to 2.9) | 1.3 (0.9 to 1.8) | 2.4 (2.0 to 2.8) |
| PsO | ||||
| Number of treatment episodes | 9691 | 1903 | 2644 | 5144 |
| Total person-years of follow-up | 8010 | 1406 | 2311 | 4293 |
| Incident serious infections, n (%) | 156 (2) | 26 (1) | 29 (1) | 101 (2) |
| Incidence rate (95% CI), per 100 person-years | 2.0 (1.7 to 2.3) | 1.9 (1.2 to2.7) | 1.3 (0.9 to 1.8) | 2.4 (1.9 to 2.8) |
| PsA | ||||
| Number of treatment episodes | 5517 | 944 | 888 | 3685 |
| Total person-years of follow-up | 4159 | 605 | 647 | 2907 |
| Incident serious infections, n (%) | 105 (2) | 14 (1) | 13 (1) | 78 (2) |
| Incidence rate (95% CI), per 100 person-years | 2.5 (2.1 to 3.1) | 2.3 (1.3 to 3.7) | 2.0 (1.1 to 3.3) | 2.7 (2.1 to 3.3) |
| Biologic-naïve | ||||
| Number of treatment episodes | 5995 | 332 | 1344 | 4319 |
| Total person-years of follow-up | 5019 | 237 | 1217 | 3565 |
| Incident serious infections, n (%) | * | * | 11 (1) | 80 (2) |
| Incidence rate (95% CI), per 100 person-years | 2.0 (1.6 to 2.4) | 3.4 (1.6 to 6.3) | 0.9 (0.5 to 1.6) | 2.2 (1.8 to 2.8) |
| Biologic experienced | ||||
| Number of treatment episodes | 5565 | 1816 | 1538 | 2211 |
| Total person-years of follow-up | 4.246 | 1292 | 1244 | 1710 |
| Incident serious infections, n (%) | 91 (2) | 24 (1) | 21 (1) | 46 (2) |
| Incidence rate (95% CI), per 100 person-years | 2.1 (1.7 to 2.6) | 1.9 (1.2 to 2.7) | 1.7 (1.1 to 2.5) | 2.7 (2.0 to 3.5) |
Clinical description of cells with 10 or fewer observations suppressed per data license. IL, interleukin; TNF, tumour necrosis factor.
While statistically significant, the absolute burden of serious infection was one or two serious infections per 100 person-years. There were no significant differences in incidence after stratification for disease type (PsO vs PsA).
Cumulative incidence of serious infections
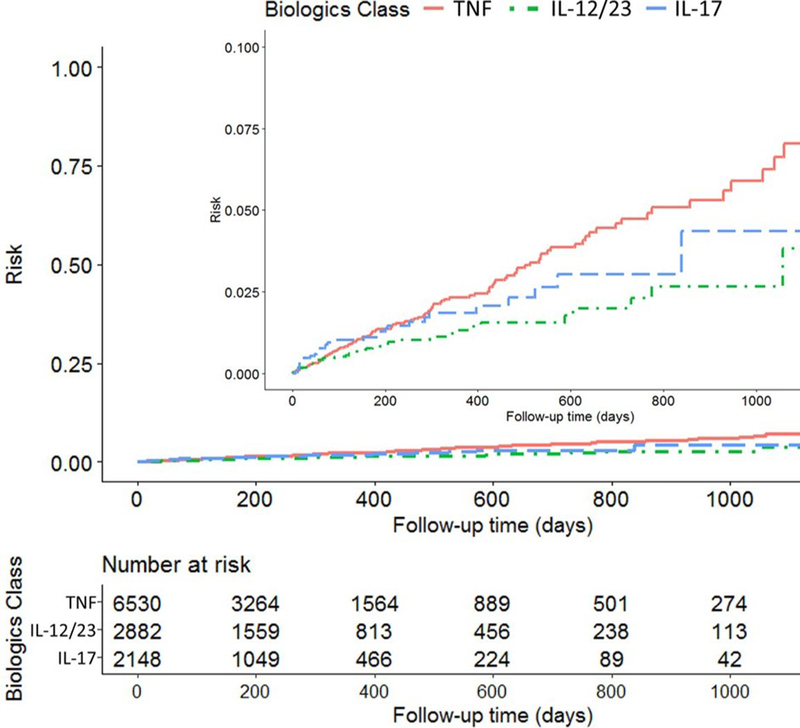
Kaplan-Meier curves were constructed to show the time from biologic drug initiation to serious infection, by drug class (figure 2). The total follow-up time was 9264 person-years, with median follow-up time of 0.6 years (IQR 0.2–1.1 years) per treatment episode. The cumulative incidence of infection was lowest for IL-12/23 over the entire follow-up period, followed by IL-17 then TNF inhibitors.
Figure 2.
Kaplan-Meier curve showing the cumulative incidence of serious infection over time, by biologic class. IL,interleukin; TNF, tumour necrosis factor.
Adjusted risk of serious infections
After propensity score weighting and adjustment for imbalanced baseline covariates, there was no increased risk of serious infections with IL-17 compared with either IL-12/23 (HR=1.12, 95% CI 0.62 to 2.03) or to TNF (HR=0.89, 95% CI 0.48 to 1.66) (table 4).
Table 4.
HRs (with 95% CIs) of risk of first serious infection among persons with psoriasis or psoriatic arthritis, by drug class
| Unadjusted | Adjusted for propensity score and imbalanced covariates |
|
|---|---|---|
| Total cohort | ||
| IL-17 vs TNF | 0.86 (0.58 to 1.27) | 0.89 (0.48 to 1.66) |
| IL-12/23 vs TNF | 0.55 (0.37 to 0.80) | 0.59 (0.39 to 0.90) |
| IL-17 vs IL-12/23 | 1.53 (0.94 to 2.51) | 1.12 (0.62 to 2.03) |
| Psoriasis | ||
| IL-17 vs TNF | 0.76 (0.50 to 1.18) | 0.73 (0.36 to 1.45) |
| IL-12/23 vs TNF | 0.53 (0.35 to 0.81) | 0.59 (0.38 to 0.92) |
| IL-17 vs IL-12/23 | 1.42 (0.83 to 2.41) | 1.01 (0.53 to 1.92) |
| Psoriatic arthritis | ||
| IL-17 vs TNF | 0.83 (0.47 to 1.47) | 0.67 (0.25 to 1.73) |
| IL-12/23 vs TNF | 0.74 (0.41 to 1.34) | 0.74 (0.40 to 1.36) |
| IL-17 vs IL-12/23 | 1.10 (0.52 to 2.35) | 1.23 (0.50 to 3.01) |
| Biologic-naive | ||
| IL-17 vs TNF | 1.45 (0.70 to 3.00) | 2.02 (0.94 to 4.33) |
| IL-12/23 vs TNF | 0.41 (0.22 to 0.76) | 0.46 (0.23 to 0.89) |
| IL-17 vs IL-12/23 | 3.63 (1.44 to 9.12) | 3.34 (1.10 to 10.12) |
| Biologic experienced | ||
| IL-17 vs TNF | 0.68 (0.42 to 1.12) | 0.72 (0.40 to 1.32) |
| IL-12/23 vs TNF | 0.62 (0.37 to 1.04) | 0.72 (0.42 to 1.26) |
| IL-17 vs IL-12/23 | 1.05 (0.59 to 1.90) | 0.92 (0.49 to 1.74) |
IL, interleukin; PsA, psoriatic arthritis; PsO, psoriasis; TNF, tumour necrosis factor.
However, we observed a 41% lower risk of serious infection in the IL-12/23 inhibitor users as compared with TNF (HR=0.59, 95% CI 0.39 to 0.90). This finding remained significant in subgroup analyses of both the PsO cohort and the biologic-naïve cohort. Additionally, within the biologic-naïve subgroup, there was a significantly increased risk of infections with IL-17 versus IL-12/23 (HR=3.34, 95% CI 1.10 to 10.12). Similar results were found in sensitivity analyses restricting to the primary admission diagnosis (online supplementary table S6), and narrowing the permissible treatment gap to 60 days (online supplementary table S7). The common imbalanced variables after weighting were cohort entry year and baseline DMARD use.
DISCUSSION
Risk of serious infections were similar among new users of IL-17 and TNF inhibitors, while persons treated with an IL-12/23 were less likely to be hospitalised with a serious infection than TNF. Risks of serious infections were not significantly different between biologics among biologic-experienced patients.
Our findings are important as IL inhibitors are relatively new products, and the comparative safety of each of the IL-17, IL-12/23 and TNF inhibitors has not yet been closely examined in a real-world cohort. Prior investigations of the safety of IL-17 inhibitors have generally been placebo-controlled or have not reported the risks for serious infection separately from overall adverse events.3,29–31 Our results build on the recent work of Kalb et al8 and Dommasch et al,14 which found reduced risk of infection with ustekinumab compared with non-biologic treatments. However, in contrast to that reports, we used more recent data, included two IL-17 inhibitors, and focused on TNF products, rather than methotrexate, as our referent group. Our findings also extend a recent report comparing the rate of serious infections of IL-17 to IL-12/23 and some, but not all, TNF inhibitors.32
Interestingly, we found that IL-12/23 inhibitors were associated with a significant reduction in risk of serious infection compared with TNF inhibitors, which held in subgroup analyses of PsO but not PsA and in biologic-naïve but not biologic-experienced patients. While we cannot exclude the possibility of residual confounding or the play of chance, PsA patients appear to have been at higher risk for infection due to other factors (older, female, more comorbidities and physician office visits, greater utilisation of DMARDs and glucocorticoids). Similarly, biologic-experienced patients are at higher risk compared with biologic naïve patients. For example, the rate in biologic naïve ustekinumab-treated patients (0.9/100py) was approximately half the rate in the biologic-experienced ustekinumab-treated patients (1.7/100py). Thus, in higher risk patients who have multiple infection-related risk factors, it may be somewhat more difficult to detect the specific contribution of biologic exposure compared with other infection-related risk factors. Additionally, the proportion of biologic-naïve episodes was larger in the PsO cohort. Nevertheless, we note that the effect estimate even in the PsA patients for IL-12/23 exposure was numerically lower (HR=0.74, 95% CI 0.40 to 1.36) and compatible with the protective association observed in the PsO patients (HR=0.59, 95% CI 0.38 to 0.92).
Among the biologic-naïve cohort, we observed a lower risk of serious infections in IL-12/23 than both IL-17 and TNF. However, given the small sample size of biologic-naïve persons using IL-17 (8 infections, 332 treatment episodes), further studies are needed to confirm these results.
Our analysis has limitations. First, the ICD codes used to define serious infections have not been fully validated in PsO and PsA patients. However, the ICD codes were derived from a combination of validation studies of patients with inflammatory arthritis (e.g., rheumatoid arthritis) and clinical expertise. Moreover, our absolute infection rates of approximately 1–2 per 100 patient-years are consistent with PsO and PsA trials and registries.3,9 Second, our study had a relatively short duration of follow-up, with a median of 6 months, and thus comparative risks between drugs should be interpreted accordingly. However, most evidence suggests that the risks of serious infections are greatest during the first months of treatment.33 Third, our data are limited to persons with commercial insurance and may not represent the Medicare, Medicaid and uninsured populations or experiences outside of the US healthcare system. Finally, as with any observational analysis, we acknowledge the potential for unmeasured or residual confounding related to confounders that were not available in these data.
While many factors inform the choice among TNF, IL-17 and IL-12/23 inhibitors, our results suggest that the risks of serious infections associated with specific biologics may differ between PsO and PsA patients, and between biologic-naïve and biologic-experienced patients. Given the relatively small magnitude of absolute effect (difference less than 1 per 100 person-years) yet strong relative reduction in risk, this potentially clinically relevant signal for reduced infections among the IL-12/23 inhibitors warrants further investigation and surveillance efforts.
Supplementary Material
Key messages.
What is already known about this subject?
-
▶
In randomised controlled trials, and some observational cohort studies, biologic therapies such as interleukin (IL) and tumour necrosis factor (TNF) inhibitors are associated with an increased risk of serious infection.
What does this study add?
-
▶
Risks of serious infections were similar among new users of IL-17 and TNF inhibitors, while persons newly treated with an IL-12/23 were less likely to be hospitalised with a serious infection than those newly treated with a TNF. Risks of serious infections were similar among the biologic experienced users who initiated a new class of biologic.
How might this impact on clinical practice or future developments?
-
▶
While many factors guide treatment choices for psoriasis and psoriatic arthritis, our results, derived from real-world cohorts, may be useful in guiding clinicians and patients regarding the selection of biologic treatments for these conditions.
Acknowledgements
Ms Andersen receives doctoral training support from the National Heart, Lung and Blood institute Pharmacoepidemiology T32 Training Program (T32HL139426–01). Dr Curtis received salary support from the Patient Centered Outcomes Research institute (PCORi).
Funding This study was funded by internal resources in the Center for Drug Safety and Effectiveness at the Johns Hopkins Bloomberg School of Public Health.
This work had been presented at the 35th international Conference on Pharmacoepidemiology & Therapeutic Risk Management in Philadelphia, PA, USA on August 2019.
Footnotes
Competing interests GCA is past Chair of FDA’s Peripheral and Central Nervous System Advisory Committee, has served as a paid advisor to iQViA, and is a consultant and holds equity in Monument Analytics, a healthcare consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement No data are available.
REFERENCES
- 1.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (Phoenix 1). The Lancet 2008;371:1665–74. [DOI] [PubMed] [Google Scholar]
- 2.Papp KA, Tyring S, Lahfa M, et al. A global phase lii randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005;152:1304–12. [DOI] [PubMed] [Google Scholar]
- 3.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CEM, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. The Lancet 2015;386:541–51. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt A, Reich K, Tsai T-F, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J Am Acad Dermatol 2017;76:60–9. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010;362:118–28. [DOI] [PubMed] [Google Scholar]
- 7.Yiu ZZN, Mason KJ, Barker JNWN, et al. A standardization approach to compare treatment safety and effectiveness outcomes between clinical trials and real-world populations in psoriasis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis. JAMA Dermatol 2015;151. [DOI] [PubMed] [Google Scholar]
- 9.Dobry AS, Quesenberiy CP, Ray GT, et al. Serious infections among a large cohort of subjects with systemically treated psoriasis. J Am Acad Dermatol 2017;77:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Doval l, Cohen AD, Cazzaniga S, et al. Risk of serious infections, cutaneous bacterial infections, and granulomatous infections in patients with psoriasis treated with anti-tumor necrosis factor agents versus classic therapies: prospective meta-analysis of Psonet registries. J Am Acad Dermatol 2017;76:299–308. [DOI] [PubMed] [Google Scholar]
- 11.Yiu ZZN, Smith CH, Ashcroft DM, et al. Risk of Serious Infection in Patients with Psoriasis Receiving Biologic Therapies: A Prospective Cohort Study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2018;138:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reich K, Mrowietz U, Radtke MA, et al. Drug safety of systemic treatments for psoriasis: results from the German psoriasis registry PsoBest. Arch Dermatol Res 2015;307:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carneiro C, Bloom R, Ibler E, et al. Rate of serious infection in patients who are prescribed systemic biologic or nonbiologic agents for psoriasis: a large, single center, retrospective, observational cohort study. Dermatol Ther 2017;30:e12529. [DOI] [PubMed] [Google Scholar]
- 14.Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace PJ, Shah ND, Dennen T, et al. Optum Labs: building a novel node in the learning health care system. Health Aff 2014;33:1187–94. [DOI] [PubMed] [Google Scholar]
- 16.Asgari MM, Wu JJ, Gelfand JM, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996–2009. Pharmacoepidemiol Drug Saf 2013;22:842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–20. [DOI] [PubMed] [Google Scholar]
- 18.Herrinton LJ, Curtis JR, Chen L, et al. Study design for a comprehensive assessment of biologic safety using multiple healthcare data systems. Pharmacoepidemiol Drug Saf 2011;20:1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun H, Xie F, Delzell E, et al. Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in Medicare. Arthritis Rheumatol 2016;68:56–66. [DOI] [PubMed] [Google Scholar]
- 20.Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol 2016;74:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grijalva CG, Chen L, Delzell E, et al. initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011;306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svan Buuren, Groothuis-Oudshoorn K. mice : Multivariate imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with iCD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh JA, Adejoro O, Chastek B, et al. Treatment patterns among patients with psoriatic arthritis treated with a biologic in the United States: descriptive analyses from an administrative claims database. J Manag Care Spec Pharm 2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010;15:234–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen T-L, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, 2018. Available: https://www.R-project.org/ [Google Scholar]
- 29.Yiu ZZN, Exton LS, Jabbar-Lopez Z, et al. Risk of serious infections in patients with psoriasis on biologic therapies: a systematic review and meta-analysis. J Invest Dermatol 2016;136:1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaç D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: clear, a randomized controlled trial. J Am Acad Dermatol 2015;73:400–9. [DOI] [PubMed] [Google Scholar]
- 31.Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol 2018;178:509–19. [DOI] [PubMed] [Google Scholar]
- 32.Ibler E, Rangel SM, Huynh TN, et al. The rate of infection in patients with psoriasis who are exposed to secukinumab compared to the rate of infection for other biologic agents: a retrospective cohort analysis from a large U.S. patient population. J Amer Acad Derm 2017;76. [Google Scholar]
- 33.Singh JA. infections with biologics in rheumatoid arthritis and related conditions: a scoping review of serious or hospitalized infections in observational studies. Curr Rheumatol Rep 2016;18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.