Abstract
Studies have shown that wound pH is a potentially influential factor in the healing process. Due to the flaws of traditional pH measurement approaches, wound pH measurement has not become part of current standard of care. A near-infrared pH sensitive ratiometric film was created and characterized for measuring wound pH. This film was fabricated by physically absorbing poly (N-isopropyl Acrylamide) nanoparticles conjugated with pH sensitive (CypHer5E) and pH insensitive (Cy7) fluorescent dyes into 2-hydroxyethyl methacrylate hydrogel film. The pH pattern on wounds can be indirectly measured by pressing freshly discarded wound dressing on top of the pH sensitive film and imaging it. In vitro tests show that the film can accurately and rapidly detect a wide range of pH (from pH 4 to 8) in wound milieu. Further, patient studies showed that, by measuring pH on wound contact side of discarded wound gauze, the pH and its non-homogeneous distribution on wounds can be indirectly determined. By comparing patients with different wound conditions, we find that near-infrared pH sensing film can be used to measure wound exudate pH with high accuracy and efficiency. In addition, wound pH determination can provide an accurate assessment of wound healing activity in real time.
Keywords: Ratiometric, Wound dressing, Wound healing status, Wound imaging
Graphical Abstract

Dual dye labeled PNIPAM nanoparticles were physically embedded into PHEMA hydrogel film to form the pH sensing film. The pH sensing film can reveal wound exudate pH distribution in a two-dimensional image without directly contact to the patient’s wound.
1. BACKGROUND
Chronic wounds affect ~6.5 million patients in the United States itself. With no signs of abatement in these numbers, this highly significant burden of over 25 billion dollars on the healthcare system is only expected to increase in the years ahead [1]. Wound pH has been shown to affect many important factors in wound healing responses in many recent publications [2–7]. Thus, monitoring it can give vital information on the status of wound healing. Currently, wound surface pH can be measured in two ways in clinic: litmus paper and glass pH electrode [8]. Litmus papers lack both specificity and accuracy, while glass pH electrode is time consuming, painful and could further aggravate the wound as the electrode is rigid and contacts the wound directly. In addition, both methods acquire average pH value of one point at a time which is neither efficient nor practical [9]. It is plausible that a new imaging modality to reveal pH distribution (instead of an average single-spot measurement) in wound milieu can greatly benefit wound care.
A number of research tools are available to map the pH of the wound environment. For example, luminescent pH detection using pH sensitive dye has been widely used and referenced to measure intracellular pH [10, 11]. However, the applications of these products generally require long scanning time, rigid imaging conditions, and sophisticated equipment, which are not clinically feasible. To overcome these drawbacks, ratiometric pH probes have been created by incorporating both pH sensitive and insensitive dyes which have distinct excitation or emission wavelengths [12]. To measure tissue pH in live animals, we developed an optical ratiometric pH sensor to measure in situ pH changes using the ratiometric imaging technique [13]. Based on this early advance, this work was aimed to develop a new technology to measure the pH of wound milieu.
2. QUESTIONS ADDRESSED
To monitor the pH of wound milieu with high accuracy and without directly contacting the wound, we designed a pH sensing film using both ratiometric imaging nanoparticles and ion-exchange membrane. Firstly, both pH-sensitive and pH-insensitive dye were covalently linked to poly(N-isopropylacrylamide) (PNIPAM) nanoparticles which serve as the pH sensing part. Secondly, a2-hydroxyethyl methacrylate (HEMA) membrane was synthesized by free-radical redox polymerization employing ethylene glycol dimethacrylate (EGDMA) as a cross-linker. Thirdly, nanoparticles were physically encapsulated into the membrane which was then ready to use for the imprinting and imaging process. Finally, using the calibration curve generated by the standard simulated wound fluid on the membrane, pH on the membrane was calculated using a software that was developed in-house. A pseudo color map was produced that reflected the wound milieu pH distribution. To test its accuracy and reproducibility, wound dressings from different patients in various stages of healing were tested. Our results support that this new pH sensing film can not only measure pH of wound milieu, but also monitor the change in pH during the wound healing process.
3. EXPERIMENTAL DESIGN
See Appendix S1.
4. RESULTS
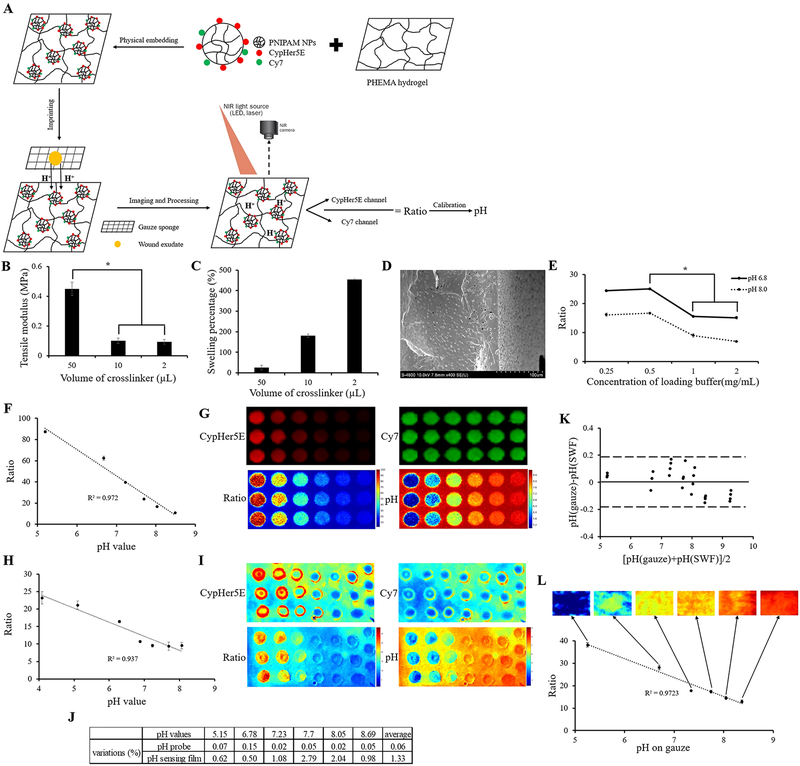
To prove that the pH of wound exudate doesn’t change through the transportation process from patients’ wound to wound dressings, pH of simulated wound fluid (SWF) before and after soaking on the dressings was measured using the skin pH meter. A Bland–Altman mean difference plot [14] was created for the data comparison (Figure 1K) and 100% of the measurements were within the 95% confidence interval which meant there was excellent concordance between two measurements. This proves that the dressing does not have any significant influence on the pH of SWF. Continuously, after imprinting the contaminated dressings on to the pH sensing film, a strong linear relationship between the fluorescence ratios on the film and the pH values measured by the skin pH meter (Ratio = −8.447 * pH + 82.667, R2 = 0.972; Figure 1L) was found within the simulated measurements. After image processing, the pseudocolor images revealed the pH of SWF on the film which equaled that on gauze sponges (Figure 1L). The pH maps here were no more just measurements of single spots but showed two-dimensional distributions on the measured surface. In conclusion, the pH sensing film can be used to map the pH distribution on simulated gauzes.
Figure 1.
Schematic diagram of pH probe embedded hydrogel film fabrication as well as application, characterization of pH sensing film, comparison of pH sensitivity between skin pH meter and pH sensing film. (A) Firstly, dual dye labeled PNIPAM nanoparticles (pH probes) were fabricated. Secondly, pH probes were physically embedded into PHEMA hydrogel film. Thirdly, discarded dressings from patients were imprinted on the pH probe embedded hydrogel film to allow ionic exchange between wound fluid and hydrogel film. Eventually, dual fluorescent images were taken and the ratio of two fluorescent intensities was determined to calculate pH value based on the calibration curve. (B) Tensile modulus of PHEMA hydrogel membrane with different crosslink densities. (C) Swelling percentage of PHEMA hydrogel membrane with different crosslink densities. (D) SEM images of lyophilized PHEMA hydrogel (10μL crosslinker), thickness~214.3μm. (E) Ratio of pH indicator film with different pH probes loading concentrations at pH 6.8 and 8.0. Student t-test was performed to compare tensile modulus of PHEMA hydrogel film with different crosslink densities and to compare ratio values of pH indicator film with different pH probes loading concentrations at pH 6.8 and 8.0. * P ≤0.05; (F) Fluorescence ratios of CypHer5E/Cy7 recorded at different known pH values with 0.5mg/mL pH probes in simulated wound fluid (SWF). (G) Pseudocolor images generated through a modified ImageJ software reflecting fluorescent intensity of CypHer5E, Cy7, ratios of CypHer5E/Cy7 and pH distribution. (H) Fluorescence ratios of CypHer5E/Cy7 recorded at different known pH values with SWF on pH probe embedded film. (I) Pseudocolor images of pH probe embedded film generated through a modified ImageJ software. (J) Comparison of measurement variations under different pH conditions using pH probe solution (0.06%) and pH indicator film (1.33%); (K) Measurement of pH values in SWF and on SWF soaked gauzes using skin pH meter. The respective Bland–Altman mean difference plot shows 100% of measurements within the 95% confidence interval. (L) Relationship of pH values measurements between skin pH meter reading and fluorescence ratios of CypHer5E/Cy7. Pseudocolor pH images of pH probe embedded film imprinted with gauzes soaked with different pH values. The images were generated through modified ImageJ software.
The pH sensing film was used to measure pH distribution of wound fluids on wound dressings recovered from different patients (Figure 2A patient #1, Figure 2B patient #2, Figure 2C patient #3, Figure 2D patient #4, Figure 2E-G patient #5 with three visits, and Figure 2H & I patient #6 with two visits). Returning patients (Figure 2E-I) came back to the clinic for dressing replacement every week and wound dressings were typically changed every 3 days. We analyzed the pH distribution on the discarded dressings isolated from several patients with chronic wounds. As shown in the images (Figure 2A-D), chronic wounds typically had uneven pH distribution with most alkaline pH (pH>7.5) and some neutral pH (6.5 <pH< 7.5). By comparing with the optical image (Figure 2B & C), we found that the acidic pH regions of wounds often coincided with the area of granulation tissue indicating localized inflammatory responses.
Figure 2.
Application of pH indicator film on measuring wound milieu of patients with different wound conditions and monitoring wound healing process of patients with repetitive visits. (A-D) Individual patients with single measurement. (E-G) One patient with three continuous visits (once a week). (H,I) One patient with two continuous visits (once a week). Wound dressing images and information of these patients. (A) Aquacel® Ag, antimicrobial dressings; (B) Aquacel® Ag, antimicrobial dressings; (C) Aquacel® Ag, antimicrobial dressings; (D) Cutimed® Siltec Sorbact®, foams; (E) Aquacel® Ag, antimicrobial dressings; (F) PolyMem®, foams; (G) Mesalt®, saline gauzes; (H) Mepilex® Border, foams; (I) Aquacel®, hydrocolloids.
We also monitored the wound pH distribution following wound debridement in two patients. Interestingly, in the first patient’s case (Figure 2E-G), imaging results clearly show that the wound pH changed from alkaline (pH 7.5–8) to acidic (pH 4.5–5.5). The changes of wound pH may reflect the dynamic changes in wound environment. Some areas of wound had a neutral pH and showed a sign of epithelialization. In the second patient’s case (Figure 2H & I), we found similar uneven wound pH distribution which may be caused by different extent of wound healing activities on wounds. The wound pH changes (from alkaline to acidic) were also observed following wound debridement.
5. CONCLUSIONS
There are many approaches for measuring the pH of wound milieu. Using indicator dyes is one of the simplest and most cost-effective methods [15]. In order to study heterogeneous tissue structures such as chronic wounds, optical imaging of pH should be observed and quantified over the whole area rather than at a single point of measurement like a pH glass electrode did [9]. Thus, many researchers incorporated their pH sensitive fluorescent probes into wound dressings [16, 17]. To eliminate errors induced by variations in concentration and distribution of fluorescent probes, a 2D ratiometric luminescence lifetime referencing pH sensor and an RGB ratiometric pH sensor film for in vivo imaging has been developed and used directly for human wound pH imaging [18, 19]. However, the most challenging part of this approach is that probes must not leach from the dressings [15]. Our pH sensing film can avoid this drawback as it measures pH of wound exudate on the wound dressing instead of measuring wound milieu directly. The equivalence relation of these two pH values has been proven through the result of the simulated measurement. Additionally, since this device can be used to detect the pH of exudate on many types of wound dressings, clinicians may utilize it to track the changes in wound pH environment.
Many studies have shown that wound milieu pH is a good indicator of wound healing status and associated activities with an alkaline pH being the hallmark of chronic wounds [20–23]. In fact, severity of chronic pressure sores has been correlated with increased wound pH (Stage I – pH 5.7, Stage II – pH 6.9, and Stage III – pH 7.6) [21]. Another study corroborated this observation and concluded that the mean wound surface pH of healing wounds is pH 6.91 compared with pH 7.42 for non-healing wounds [24]. It has also been shown that healing process is associated with the reduction of pH from a baseline of alkaline towards acidic [25]. It is also well established that wound debridement can improve healing of chronic wounds so that they granulate similar to acute wounds [26–29]. Coincidentally, inflammation and tissue granulation are typically found in acute wound with an acidic pH [21, 23, 28]. These early findings support our observation that wound debridement may reduce the pH in chronic wounds. Further study can be carried out to determine the relationship between wound pH and wound healing status which can be assessed by many factors, such as wound closure rate and granulation tissue appearance.
There is a preponderance of evidence pointing towards a strong relationship between pH of wound milieu and wound healing status. Since the change in pH is independent of the gender and ages of patients, etiology of the wound, and the types of wound dressing, it has been suggested that wound pH can be used as a universal indicator for different wounds [20]. It has also been suggested that routine monitoring and alteration of the wound pH using different wound treatments may significantly improve chronic wound therapy outcome [30]. Overall, the measurement and monitoring of wound milieu pH is critical for wound healing process and our pH sensing film can complete this mission efficiently and non-invasively.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a grant from National Institute of Health (AR064650).
Tang has a potential research conflict of interest due to a financial interest with Progenitec Inc. A management plan has been created to preserve objectivity in research in accordance with UTA policy.
Footnotes
CONFLICT OF INTEREST
No competing financial interests exist for other authors.
REFERENCES
- [1].Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT, Wound Repair and Regeneration 2009, 17, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thomas LV, Wimpenny JW, Davis JG, International journal of food microbiology 1993, 17, 289. [DOI] [PubMed] [Google Scholar]
- [3].Hunt TK, Hopf HW, Surgical Clinics of North America 1997, 77, 587. [DOI] [PubMed] [Google Scholar]
- [4].Gethin G, Cowman S, “Changes in surface pH of chronic wounds when a honey dressing was used”, presented at Wounds UK Conference Proceedings, 2006. [Google Scholar]
- [5].Rushton I, Nursing Standard 2007, 21, 68. [DOI] [PubMed] [Google Scholar]
- [6].Nagoba B, Gandhi R, Wadher B, Potekar R, Kolhe S, Journal of medical microbiology 2008, 57, 681. [DOI] [PubMed] [Google Scholar]
- [7].Percival SL, Thomas J, Linton S, Okel T, Corum L, Slone W, International wound journal 2012, 9, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gethin G, Wounds uk 2007, 3, 52. [Google Scholar]
- [9].Walpole GS, Biochemical Journal 1914, 8, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bassnett S, Reinisch L, Beebe DC, American Journal of Physiology-Cell Physiology 1990, 258, C171. [DOI] [PubMed] [Google Scholar]
- [11].Ke G, Zhu Z, Wang W, Zou Y, Guan Z, Jia S, Zhang H, Wu X, Yang CJ, ACS applied materials & interfaces 2014, 6, 15329. [DOI] [PubMed] [Google Scholar]
- [12].O’Connor N, Silver RB, Methods in cell biology 2007, 81, 415. [DOI] [PubMed] [Google Scholar]
- [13].Tsai YT, Zhou J, Weng H, Shen J, Tang L, Hu WJ, Advanced healthcare materials 2014, 3, 221. [DOI] [PubMed] [Google Scholar]
- [14].Bland JM, Altman D, The lancet 1986, 327, 307. [PubMed] [Google Scholar]
- [15].Dargaville TR, Farrugia BL, Broadbent JA, Pace S, Upton Z, Voelcker NH, Biosensors and Bioelectronics 2013, 41, 30. [DOI] [PubMed] [Google Scholar]
- [16].Trupp S, Alberti M, Carofiglio T, Lubian E, Lehmann H, Heuermann R, Yacoub-George E, Bock K, Mohr G, Sensors and Actuators B: Chemical 2010, 150, 206. [Google Scholar]
- [17].Van der Schueren L, De Clerck K, Coloration Technology 2012, 128, 82. [Google Scholar]
- [18].Meier RJ, Schreml S, Wang X. d., Landthaler M, Babilas P, Wolfbeis OS, Angewandte Chemie International Edition 2011, 50, 10893. [DOI] [PubMed] [Google Scholar]
- [19].Schreml S, Meier RJ, Wolfbeis OS, Landthaler M, Szeimies R-M, Babilas P, Proceedings of the National Academy of Sciences 2011, 108, 2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dissemond J, Witthoff M, Brauns T, Haberer D, Goos M, Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete 2003, 54, 959. [DOI] [PubMed] [Google Scholar]
- [21].Glibbery A, Int J Microcir Clin Exptl. 1992, 109. [Google Scholar]
- [22].Sayegh N, Dawson J, Bloom N, Stahl W, Current surgery 1988, 45, 23. [PubMed] [Google Scholar]
- [23].Lengheden A, Jansson L, European journal of oral sciences 1995, 103, 148. [DOI] [PubMed] [Google Scholar]
- [24].Roberts G, Chumley A, Mani R, Wound Repair and Regeneration 2006, 14, A23. [Google Scholar]
- [25].Shukla V, Shukla D, Tiwary S, Agrawal S, Rastogi A, Journal of wound care 2007, 16, 291. [DOI] [PubMed] [Google Scholar]
- [26].Steed DL, The American journal of surgery 2004, 187, S71. [DOI] [PubMed] [Google Scholar]
- [27].Brem H, Tomic-Canic M, The Journal of clinical investigation 2007, 117, 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schneider LA, Korber A, Grabbe S, Dissemond J, Archives of dermatological research 2007, 298, 413. [DOI] [PubMed] [Google Scholar]
- [29].Velnar T, Bailey T, Smrkolj V, Journal of International Medical Research 2009, 37, 1528. [DOI] [PubMed] [Google Scholar]
- [30].Schreml S, Szeimies RM, Karrer S, Heinlin J, Landthaler M, Babilas P, Journal of the European Academy of Dermatology and Venereology 2010, 24, 373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.