Abstract
Objectives
To examine the prognostic significance of atrial fibrillation (AF) versus sinus rhythm (SR) on the management and outcomes of patients with severe aortic stenosis (AS).
Methods
1847 consecutive patients with severe AS (aortic valve area ≤1.0 cm2 and aortic valve systolic mean Doppler gradient ≥40 mm Hg or peak velocity ≥4 m/s) and left ventricular ejection fraction ≥50% were identified. The independent association of AF and all-cause mortality was assessed.
Results
Age was 76±11 years and 46% were female; 293 (16%) patients had AF and 1554 (84%) had SR. In AF, 72% were symptomatic versus 71% in SR. Survival rate at 5 years for AF (41%) was lower than SR (65%) (age- and sex-adjusted HR=1.66 (1.40–1.98), p<0.0001). In multivariable analysis, factors associated with mortality included age (HR per 10 years=1.55 (1.42–1.69), p<0.0001), dyspnoea (HR=1.58 (1.33–1.87), p<0.0001), ≥ moderate mitral regurgitation (HR=1.63 (1.22–2.18), p=0.001), right ventricular systolic dysfunction (HR=1.88 (1.52–2.33), p<0.0001), left atrial volume index (HR per 10 mL/m2=1.13 (1.07–1.19), p<0.0001) and aortic valve replacement (AVR) (HR=0.44 (0.38–0.52), p<0.0001). AF was not a predictor of mortality independent of variables strongly correlated HR=1.02 (0.84–1.25), p=0.81). The 1-year probability of AVR following diagnosis of severe AS was lower in AF (49.8%) than SR (62.5%) (HR=0.73 (0.62–0.86), p<0.001); among patients with AF not referred for AVR, symptoms were frequently attributed to AF instead of AS.
Conclusion
AF was associated with poor prognosis in patients with severe AS, but apparent differences in outcomes compared with SR were explained by factors other than AF including concomitant cardiac abnormalities and deferral of AVR due to attribution of cardiac symptoms to AF.
INTRODUCTION
Aortic stenosis (AS) is a common valve disorder and its prevalence steadily increases with ageing. Severe AS is associated with a poor prognosis in unoperated patients.1 Atrial fibrillation (AF) is the most common cardiac arrhythmia, frequently associated with heart failure2,3 and is present in up to 35% of patients with AS.4 However, our understanding of the natural history of severe AS and recommendations for timing of aortic valve replacement (AVR) are based on studies of patients in sinus rhythm (SR).5–7 Previous seminal studies show a negative impact of AF on outcomes in severe AS, but are few and limited by small number of patients with severe AS or include patients with mild or moderate AS.8–10 Consequently, AF is not factored into decision-making related to indications and timing of AVR for severe AS.11,12
The aim of this study was to investigate the prognostic importance of the presence of AF, as well as type and duration of AF, on management and outcomes in patients with severe AS in the context of routine clinical practice.
METHODS
Study population
From 1 January 2008 to 31 December 2012, patients with severe AS and left ventricular ejection fraction (LVEF) ≥50% were identified from the echocardiographic laboratory database at Mayo Clinic, Rochester, Minnesota. Exclusion criteria included moderate or severe coexisting aortic regurgitation, subvalvular or supravalvular AS, dynamic subaortic obstruction and active endocarditis.8 Data retrieved from the electronic medical records included medical history, ECG, echocardiography, and serum blood chemistries. Patients were grouped according to the presence or absence of AF based on clinical history and diagnosed on ECG or rhythm monitor before the index echocardiogram showing severe AS. Subgroup analyses were performed based on the type of AF (persistent vs paroxysmal), as well as on known duration of AF (<1 year or ≥1 year before diagnosis of severe AS). Vital status and cause of death were determined from the medical records, Minnesota death report, and National Death Index (to 31 December 2016). Only individuals who had not refused permission to use their medical records for research (according to Minnesota Research Authorization) were included.
Echocardiography
Assessment of the severity of AS was according to the current European Association of Echocardiography and American Society of Echocardiography13 guidelines. For patients in SR, three consecutive cardiac cycles were averaged for all measures. For patients in AF, at least five consecutive cardiac cycles were averaged. Severe AS was defined as aortic valve area ≤1.0 cm2 and aortic valve peak velocity ≥4 m/s or aortic valve mean systolic Doppler gradient ≥40 mm Hg.13 LVEF was calculated using the biplane Simpson’s method, and the left atrial volume was calculated using the method of discs14 and indexed to body surface area (left atrial volume index (LAVi)). Diastolic function and pulmonary artery systolic pressure (PASP) were assessed according to the guidelines.13,15 Quantitative and semiquantitative measures were integrated to determine the size and systolic function of the right ventricle according to the guidelines.14,16 Quantitative Doppler was preferentially used over qualitative parameters for grading severity of valvular regurgitation.17
Clinical data
Data included age, sex, symptom status, AVR and vital status at the latest follow-up visit at Mayo Clinic. Hypertension was defined as blood pressure >140/90 mm Hg or history of hypertension and current antihypertensive medications. Diabetes mellitus was defined as fasting blood sugar >126 mg/dL on two occasions or treatment with antidiabetic agents. Renal insufficiency was defined as serum creatinine ≥1.3 mg/dL. Chronic lung diseases included asthma, chronic obstructive pulmonary disease, cystic fibrosis and idiopathic pulmonary fibrosis. Charlson Comorbidity Index (CCI) was calculated. All-cause mortality was the primary outcome with data on survival ascertained as described above.
Statistical analysis
Continuous variables are expressed as mean±SD, and categorical variables as number and percentages. Continuous variables were compared across groups using two-sample t-test or Wilcoxon rank-sum test, as appropriate. Categorical variables were compared using χ2 or Fisher’s exact test. Cumulative survival curves were estimated by Kaplan-Meier methods and compared between groups using the log-rank test. Rates of AVR were estimated using the cumulative incidence function, accounting for the competing risk of death. Cox proportional hazards regression was used to examine the associations of AF with risk of mortality. Candidate variables for adjustment in the mortality multivariable analysis were those found to be significantly associated with AF. Only those variables found to be independently associated with mortality were retained. AVR was evaluated as a time-dependent covariate for overall mortality. An adjusted survival curve was created using the method of direct adjustment to illustrate outcomes between those with and without AF after taking into account variables found to be significant in the multivariable analysis. A second Kaplan-Meier analysis of mortality was performed after censoring at AVR. Propensity matching was also used to compare outcomes in AF versus SR. In this analysis, two SR subjects were propensity matched to each AF subject on clinical characteristics. Outcome analyses were then repeated within these matched groups. Two-sided tests were used and statistical significance was defined as p<0.05. SAS version 9.4 (Cary, North Carolina, USA) was used for analyses.
RESULTS
Baseline characteristics
A total of 1847 patients met the inclusion criteria and the baseline clinical characteristics are summarised in table 1. The mean age was 76±11 years and 46% were female. There were 293 (16%) patients with AF and 1554 (84%) patients with SR. The AF group was older (80±9 years vs 75±12 years, p<0.0001) with a higher CCI (2 (1.0, 4.0) vs 1 (0.0, 3.0), p<0.0001) and more frequent history of heart failure (28% vs 13%, p<0.0001). In the AF group, 72% were symptomatic and 28% asymptomatic; in the SR group, 71% were symptomatic and 29% asymptomatic (p=0.57). Regarding type of symptoms, patients with AF had less frequent angina (19% vs 27%, p=0.009), but no difference in syncope (9% vs 9%, p=0.72) or dyspnoea (69% vs 65%, p=0.17) when compared with patients with SR. Among the 293 patients with AF, 205 (70%) patients had persistent AF and 88 (30%) had paroxysmal AF. Clinical characteristics for each AF subgroup are also shown in table 1.
Table 1.
Baseline patient characteristics
| Characteristics | Sinus rhythm (n=1554) | AF (N=293) | P value | Persistent AF (n=205) | Paroxysmal AF (n=88) |
|---|---|---|---|---|---|
| Age, years | 74.9±11.5 | 80.4±8.6 | <0.0001 | 81.2±8.4 | 78.5±9.0* |
| Male | 835 (54) | 162 (55) | 0.62 | 114 (56) | 48 (55) |
| Body surface area, m2 | 1.6±0.3 | 1.6±0.3 | 0.94 | 1.6±0.3 | 1.5±0.3 |
| Charlson Comorbidity Index, median (Q1, Q3) (n=1818) | 1.0 (0, 3.0) | 2.0 (1.0, 4.0) | <0.0001 | 2.0 (1.0, 4.0) | 3.0 (1.0, 5.0)* |
| Hypertension | 846 (54) | 200 (68) | <0.0001 | 131 (64) | 69 (78)* |
| Hyperlipidemia (n=1818) | 740 (49) | 162 (55) | 0.03 | 99 (48) | 63 (72)* |
| Diabetes mellitus | 367 (24) | 80 (27) | 0.18 | 58 (28) | 22 (25) |
| Congestive heart failure | 196 (13) | 82 (28) | <0.0001 | 61 (30) | 21 (24) |
| Chronic lung diseases | 185 (12) | 42 (14) | 0.25 | 27 (13) | 15 (17) |
| Stroke | 408 (26) | 99 (34) | 0.008 | 74 (36) | 25 (28) |
| Renal failure | 112 (7) | 35 (12) | 0.006 | 23 (11) | 12 (14) |
| Angina+ (n=1844) | 414 (27) | 57 (19) | 0.009 | 36 (18) | 21 (24) |
| Syncope+ (n=1845) | 133 (9) | 27 (9) | 0.72 | 20 (10) | 7 (8) |
| Dyspnoea+ (n=1846) | 1012 (65) | 203 (69) | 0.17 | 150 (73) | 53 (60)* |
| Prior PCI (n=1818) | 85 (6) | 24 (8) | 0.08 | 18 (9) | 6 (7) |
| Prior CABG (n=1818) | 172 (11) | 33 (11) | 0.99 | 20 (10) | 13 (15) |
| NT-proBNP, median (Q1, Q3) (n=1006) | 665 (270, 1577) | 1982 (1003, 4072) | <0.0001 | 2140 (1087, 4492) | 1342 (605, 2964)* |
| Serum creatinine, median (Q1, Q3) (n=1835) | 1.0 (0.8, 1.2) | 1.1 (0.9, 1.3) | 0.002 | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.3) |
| Heart rate (n=1795) | 68.9±12.5 | 71.6±14.6 | 0.001 | 72.5±14.8 | 69.3±14.0 |
Persistent AF versus paroxysmal AF p-value<0.05.
AF, atrial fibrillation; CABG, coronary artery bypass surgery; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCI, percutaneous coronary intervention.
Comparison of echocardiographic characteristics between SR and AF groups is shown in table 2. Patients with AF had higher PASP (46±14 mm Hg vs 38±13 mm Hg, p<0.0001), LAVi (56±21 mL/m2 vs 41±13 mL/m2, p<0.0001) and more prevalent ≥ moderate mitral regurgitation (11% vs 4% p<0.0001), ≥ moderate tricuspid regurgitation (21% vs 3%, p<0.0001) as well as right ventricular systolic dysfunction (25% vs 6%, p<0.0001) compared with the SR group (table 2). Echocardiographic characteristics of patients with persistent and paroxysmal AF are also shown in table 2.
Table 2.
Baseline echocardiographic parameters
| Echocardiography parameters | Sinus rhythm (n=1554) | AF (N=293) | P value | Persistent AF (n=205) | Paroxysmal AF (n=88) |
|---|---|---|---|---|---|
| Left ventricular end-diastolic diameter, mm (n=1806) | 48.0±5.4 | 48.2±5.6 | 0.58 | 48.3±5.6 | 47.9±5.7 |
| Left ventricular end-systolic diameter, mm (n=1724) | 29.7±5.0 | 30.5±5.3 | 0.02 | 30.9±5.4 | 29.5±5.0* |
| Left ventricular stroke volume index, mL/m2 (n=1833) | 60.6±12.2 | 55.1±12.5 | <0.0001 | 52.7±11.6 | 60.9±12.8* |
| Left ventricular ejection fraction, % | 65.0±6.1 | 63.6±6.5 | 0.0003 | 62.9±6.4 | 65.3±6.3* |
| Left ventricular mass index, g/m2 (n=1768) | 117.7±29.7 | 115.8±31.6 | 0.34 | 116.0±32.7 | 115.3±29.1 |
| Aortic valve area, cm2 (n=1843) | 0.83±0.13 | 0.81±0.14 | 0.01 | 0.80±0.15 | 0.83±0.12 |
| Aortic valve area index, cm2/m2 (n=1838) | 0.55±0.11 | 0.53±0.11 | 0.04 | 0.52±0.10 | 0.56±0.11* |
| Aortic valve peak velocity, m/s | 4.62±0.54 | 4.46±0.54 | <0.0001 | 4.46±0.55 | 4.45±0.53 |
| Aortic valve mean gradient, mm Hg | 52.4±12.9 | 48.5±11.9 | <0.0001 | 48.4±11.8 | 48.7±12.0 |
| E/e’ ratio (n=1652) | 18.0±8.8 | 20.5±9.8 | <0.0001 | 20.1±9.7 | 21.2±9.9 |
| Left atrium volume index, mL/m2 (n=1417) | 41.1±13.3 | 56.1±20.8 | <0.0001 | 60.0±22.2 | 46.3±12.0* |
| MR (≥ moderate) (n=1829) | 55 (4) | 31 (11) | <0.0001 | 26 (13) | 5 (6) |
| TR (≥ moderate) (n=1829) | 43 (3) | 60 (21) | <0.0001 | 56 (28) | 4 (5)* |
| Right ventricular dysfunction (n=1827) | 98 (6) | 71 (25) | <0.0001 | 60 (31) | 11 (13)* |
| PASP, mm Hg (n=1578) | 37.9±12.7 | 46.1±14.4 | <0.0001 | 48.4±14.7 | 40.5±12.0* |
Persistent AF versus paroxysmal AF p-value <0.05.
AF, atrial fibrillation; e’, mitral annular early diastole velocity.E, transmitral inflow early diastole velocity; MR, mitral regurgitation; PASP, pulmonary artery systolic pressure; TR, tricuspid regurgitation;
Clinical outcomes
Overall survival
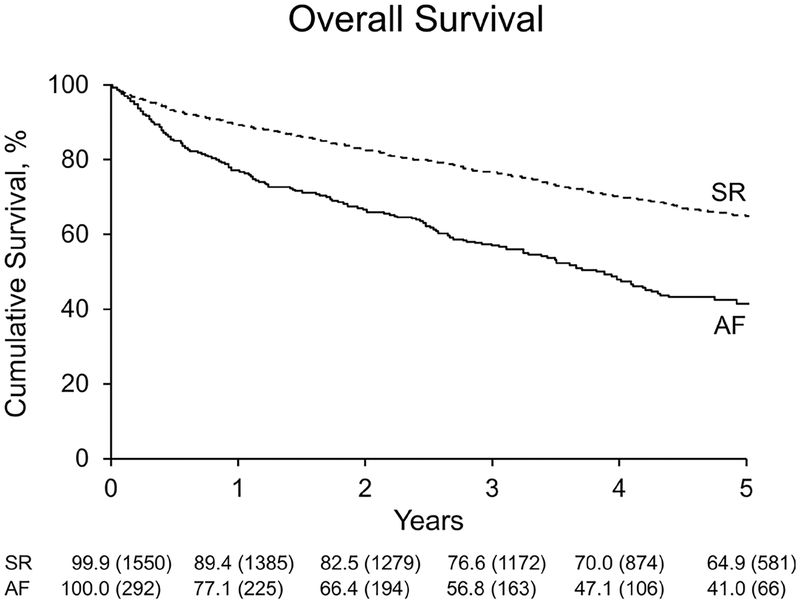
During a median follow-up of 4.2 years (IQR: 2.7–5.6), 173 patients died among the AF group and 582 patients died in the SR group (log-rank p<0.0001). Cause of death was cardiovascular in 54% of AF and in 48% of SR. Overall, the survival rate at 5 years was significantly lower in the AF group (41%) than the SR group (65%) (age- and sex-adjusted HR=1.66 (1.40–1.98), p<0.0001) (figure 1). The survival rates at 5 years for persistent AF (37.2%) and paroxysmal AF (49.2%) were significantly lower than the SR group (65%) (age- and sex-adjusted HR=1.76 (1.45–2.15), p<0.0001 and HR=1.45 (1.08–1.95), p=0.01).
Figure 1.
Kaplan-Meier cumulative survival plot of normal sinus rhythm (SR) versus atrial fibrillation (AF) in patients with severe aortic stenosis. Observed overall survival in patients with severe aortic stenosis is lower in those with AF compared with SR at the time of diagnosis of severe aortic stenosis (p<0.0001).
Factors associated with mortality
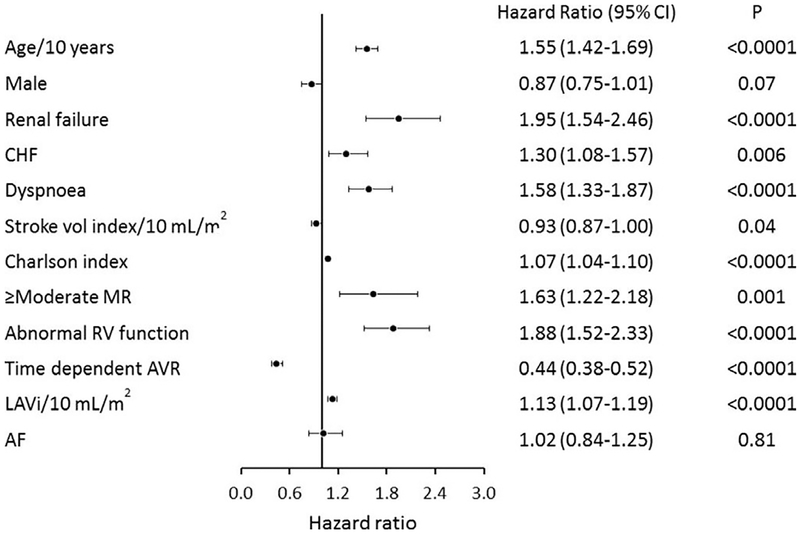
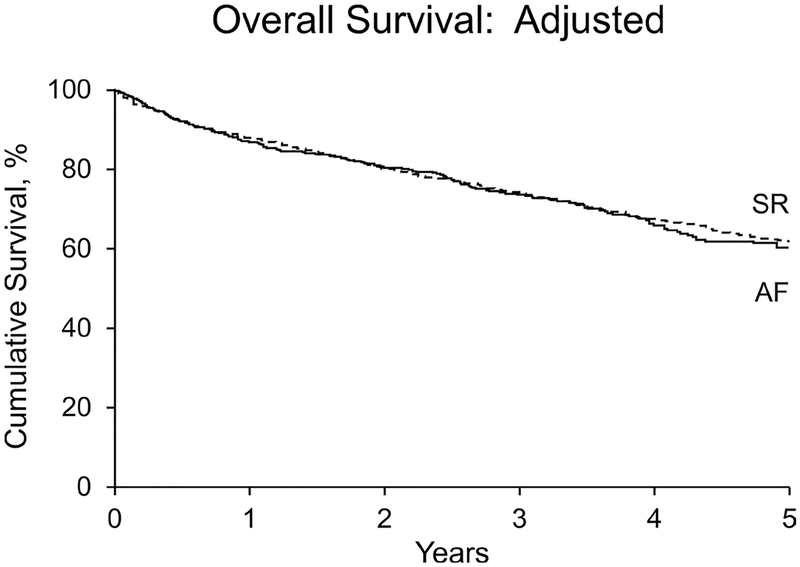
In the multivariable analysis, factors associated with mortality included age (HR per 10 years=1.55 (1.42–1.69), p<0.0001), heart failure (HR=1.30 (1.08–1.57), p=0.006), renal failure (HR=1.95 (1.54–2.46), p<0.0001), dyspnoea (HR=1.58 (1.33–1.87), p<0.0001), stroke volume index (HR per 10 mL/m2=0.93 (0.87–1.00), p=0.04), CCI (HR=1.07 (1.04–1.10), p<0.0001), ≥ moderate mitral regurgitation (HR=1.63 (1.22–2.18), p=0.001), right ventricular systolic dysfunction (HR=1.88 (1.52–2.33), p<0.0001), LAVi (HR per 10 mL/m2=1.13 (1.07–1.19), p<0.0001) and AVR (HR=0.44 (0.38–0.52), p<0.0001) (figure 2). The rhythm of AF by itself was not a predictor of overall mortality independent of variables strongly correlated with AF (figures 2 and 3) (all AF HR=1.02 (0.84–1.25), p=0.81; persistent AF HR=0.97 (0.74–1.27), p=0.84; paroxysmal AF HR=1.31 (0.94–1.83), p=0.11).
Figure 2.
Forest plot of multivariable predictors of overall survival. HRs, 95% confidence limits and p-values from the multivariable analysis are illustrated. AF, atrial fibrillation; AVR, aortic valve replacement; CHF, congestive heart failure; LAVi, left atrial volume index; MR, mitral regurgitation; RV, right ventricular.
Figure 3.
Kaplan-Meier cumulative survival plot of normal sinus rhythm (SR) versus atrial fibrillation (AF) in patients with severe aortic stenosis after adjusting for independent determinants of survival (p=0.81).
Aortic valve replacement
Overall, the probability of AVR at 1 year following the diagnosis of severe AS was significantly lower in the AF group (49.8%) than the SR group (62.5%) (HR=0.73 (0.62–0.86), p<0.001). Among those undergoing AVR, symptoms were present at baseline in 80% in the AF group versus 72% in the SR group. Type of AVR (surgical vs transcatheter) and associated procedures at the time of AVR are shown in table 3.
Table 3.
Type of AVR and associated procedures
| Sinus rhythm (n=1092) | Atrial fibrillation (n=162) | |
|---|---|---|
| Type of AVR | ||
| Surgical mechanical AVR | 142 (13%) | 17 (10.5%) |
| Surgical bioprostheticAVR | 819 (74%) | 109 (67%) |
| TAVR | 130 (11.9%) | 36 (22%) |
| Unknown | 1 (0.1%) | 0 (0%) |
| Concomitant procedures during surgical AVR | ||
| CABG | 352 (32%) | 50 (31%) |
| MVR | 30 (2.7%) | 9 (6%) |
| TVR | 23 (2%) | 18 (11%) |
| MAZE | 0 | 23 (14%) |
AVR, aortic valve replacement; CABG, coronary artery bypass surgery; MVR, mitral valve replacement or repair; TAVR, transcatheter aortic valve replacement; TVR, tricuspid valve replacement or repair.
Overall mortality under medical management
Among the 131/293 patients (45%) who did not undergo AVR in the AF group, 43/131 (33%) were referred to AVR and 88/131 (67%) were not referred to AVR; among those not referred, symptoms were present in 70/88 (80%) and attributed to AF in 21/70 (30%). Among the 462/1554 patients (30%) who did not undergo AVR in the SR group, 146/462 (32%) were referred to AVR and 316/462 (68%) were not referred to AVR. Reasons for not referring patients to AVR are summarised in table 4. Under medical therapy, the survival rate at 5 years remained significantly lower in the AF group (25.8%) than the SR group (45.9%).
Table 4.
Reasons why patients were not referred to aortic valve replacement
| Attributed Reasons | Sinus rhythm (n=316) | Atrial fibrillation (n=88) | P value |
|---|---|---|---|
| Symptoms attributed to AF | 0/316 (0%) | 21/88 (24%) | <0.0001 |
| Symptoms attributed to COPD | 27/316 (8%) | 3/88 (3%) | 0.17 |
| Symptoms attributed to other conditions | 56/316 (18%) | 3/88 (3%) | 0.0003 |
| AS not severe enough (low-gradient AS) | 12/316 (4%) | 13/88 (15%) | 0.0006 |
| Comorbidities precluded intervention | 94/316 (30%) | 30/88 (34%) | 0.44 |
| Asymptomatic | 121/316 (38%) | 18/88 (20%) | 0.002 |
| Unknown | 6/316 (2%) | 0/88 (0%) | 0.35 |
AF, atrial fibrillation; AS, aortic stenosis; COPD, chronic obstructive pulmonary disease.
Duration of AF and outcomes
Forty-seven per cent (137/293) of patients with AF had AF duration ≥1 year from diagnosis of severe AS, and of those 125 (91%) had persistent AF. Overall, there was a trend toward worse all-cause mortality in the group with AF duration ≥1 year (≥1 year vs <1 year age- and sex-adjusted HR=1.33 (0.99–1.80), p=0.06). Among those with persistent AF, there were no observed differences in all-cause mortality between the 125 patients (61%) with AF duration ≥1 year versus 80 (39%) with AF <1 year (≥1 year vs <1 year age- and sex-adjusted HR=1.15 (0.76–1.74), p=0.50).
Propensity matching
Overall 5-year mortality remained significantly higher in the AF group compared with the SR group (HR=1.68 (1.38–2.04), p<0.0001) after 2:1 propensity matching of patients with SR and AF by clinical characteristics. Differences in underlying structural cardiac abnormalities persisted between AF and SR after matching by clinical characteristics (table 5).
Table 5.
Baseline clinical and echocardiographic parameters after 2:1 propensity matching by clinical characteristics
| Clinical characteristics | Sinus rhythm (n=567) | Atrial fibrillation (n=284) | P value |
|---|---|---|---|
| Age, years | 80.6±9.0 | 80.4±8.7 | 0.80 |
| Male | 319 (56) | 157 (55) | 0.79 |
| Body surface area, m2 | 1.55±0.31 | 1.55±0.33 | 0.81 |
| Charlson Comorbidity Index, median (Q1, Q3) | 2.0 (1.0, 4.0) | 2.0 (1.0, 4.0) | 0.29 |
| Hypertension | 376 (66) | 194 (68) | 0.56 |
| Hyperlipidemia | 305 (54) | 156 (55) | 0.75 |
| Diabetes mellitus | 157 (28) | 78 (28) | 0.94 |
| Congestive heart failure | 146 (26) | 80 (28) | 0.45 |
| Chronic lung diseases | 76 (13) | 40 (14) | 0.78 |
| Stroke | 194 (34) | 98 (34) | 0.93 |
| Renal failure | 54 (10) | 34 (12) | 0.27 |
| Angina+ | 109 (19) | 56 (20) | 0.86 |
| Syncope+ | 43 (8) | 26 (9) | 0.43 |
| Dyspnoea+ | 390 (69) | 196 (69) | 0.95 |
| Prior PCI | 44 (8) | 24 (8) | 0.73 |
| Prior CABG | 67 (12) | 33 (12) | 0.93 |
| Serum creatinine, median (Q1, Q3) | 1.0 (0.9, 1.3) | 1.1 (0.9, 1.3) | 0.43 |
| Heart rate | 70.6±13.9 | 71.6±14.6 | 0.30 |
| Echocardiography parameters | |||
| Left ventricular end-diastolic diameter, mm | 47.8±5.3 | 48.2±5.6 | 0.44 |
| Left ventricular end-systolic diameter, mm | 29.8±4.9 | 30.5±5.3 | 0.05 |
| Left ventricular stroke volume index, mL/m2 | 59.2±12.0 | 55.2±12.6 | <0.001 |
| Left ventricular ejection fraction, % | 64.8±6.3 | 63.6±6.5 | 0.01 |
| Left ventricular mass index, g/m2 | 118.2±28.7 | 116.0±31.5 | 0.32 |
| Aortic valve area, cm2 | 0.81±0.31 | 0.81±0.14 | 0.64 |
| Aortic valve area index, cm2/m2 | 0.54±0.11 | 0.53±0.11 | 0.39 |
| Aortic valve peak velocity, m/s | 4.57±0.52 | 4.45±0.53 | 0.002 |
| Aortic valve mean gradient, mm Hg | 51.5±12.2 | 48.2±11.6 | <0.001 |
| E/e’ ratio | 19.0±14.9 | 20.4±9.8 | 0.06 |
| Left atrium volume index, mL/m2 | 43.5±14.9 | 56.3±21.0 | <0.001 |
| MR (≥ moderate) | 26 (5) | 31 (11) | <0.001 |
| TR (≥ moderate) | 22 (4) | 58 (21) | <0.001 |
| Right ventricular dysfunction | 39 (7) | 71 (26) | <0.001 |
| PASP, mm Hg | 39.6±13.2 | 46.2±14.5 | <0.001 |
CABG, coronary artery bypass surgery; e’, mitral annular early diastole velocity.E, transmitral inflow early diastole velocity; MR, mitral regurgitation; PASP, pulmonary artery systolic pressure; PCI, percutaneous coronary intervention; TR, tricuspid regurgitation;
DISCUSSION
The current study of a large cohort of patients with severe AS stratified by rhythm (AF vs SR) followed for a median of 4.2 years during routine clinical practice reveals the following major findings: (1) patients with severe AS and AF were older with more prevalent heart failure and echocardiographic cardiac abnormalities compared with patients in SR; (2) overall survival was lower in patients with AF compared with SR due to other factors correlated with presence of AF; (3) AVR was associated with better outcomes, but patients with AF were less likely to undergo AVR. AF signals the presence of factors that contribute to excess mortality in patients with severe AS.
In our cohort, AF was present in 16% of patients with severe AS with preserved LVEF. While AF may be a consequence of AS, a significant proportion of the patients in this study had AF diagnosed ≥1 year before the diagnosis of severe AS and the observed frequency was similar to that in the general population of the same age,18 suggesting AS was not the principal aetiology of AF. Older age is a known risk factor for the development of AF and the incidence of AF doubles with each decade of life.19 The association of advanced age and AF can be explained by older individuals having more comorbidities related to ageing and advanced underlying structural changes in the atrial myocardium, such as distension and fibrosis, which promote AF by increasing conduction heterogeneity as a result of discrete areas of slow conduction.20
History of heart failure was more common in patients with AF compared with SR. Previous studies have shown a high prevalence of heart failure with preserved ejection (HFpEF) in patients with AF and dyspnoea3 and whether a subset of patients with AS and AF represents patients with HFpEF who develop severe AS needs further investigation. Haemodynamic consequences of AF include loss of atrioventricular synchrony, loss of atrial contribution to ventricular filling, irregularity in the ventricular rhythm, increased atrial and pulmonary capillary wedge pressures, decreased forward stroke volume, and atrioventricular regurgitation.21 A number of patients with AF likely already have dyspnoea before developing severe AS, confounding the class I indication for AVR of new-onset dyspnoea.11,12 In our cohort, among symptomatic patients with AF and severe AS not referred to AVR, 30% were not referred for the expressed reason that symptoms were attributed to AF and not because of other comorbidities or patient refusal (table 4). Given the prognostic significance of dyspnoea and heart failure, the difficulty of sorting out if symptoms are due to AS or AF, and the association of better outcomes with AVR, symptoms in patients with severe AS and AF may be better attributed to severe AS.
AS associated with AF has been shown previously to have worse outcomes. Levy et al8 showed AF to be a predictor of worse outcomes irrespective of symptoms or AS severity in patients with moderate to severe AS (n=65 with severe AS) and LVEF ≥50% and reported 4-year all-cause mortality with medical and surgical therapy to be 60%±5% for the AF group compared with 24%±2% for the SR group.8 Moretti et al10 showed AF to be an independent predictor of death in low-gradient AS (mean gradient ≤30 mm Hg) and preserved LVEF ≥55%, independent of severity of symptoms, and the association with better outcomes with AVR was more pronounced in AF compared with SR.10 In a case–control study, Burup Kristensen et al9 also showed AF to be an independent risk factor for worse outcomes compared with SR irrespective of AS severity in patients with ≥mild AS and mean LVEF 42% (n approximately 50 with severe AS) and AVR was performed less frequently in AF group, but outcomes of AVR were not reported.9 Although AF has been shown in previous studies to increase mortality risk in any degree of AS, it is necessary to scrutinise the role of AF in severe AS singularly since severe AS is the principal indicator for AVR.11,12
Where our study differs from previous studies is in the finding that while AF correlated with worse outcomes in severe AS even after propensity matching, the apparent difference in outcome between patients in AF and SR was explained by other factors. Structural heart disease such as mitral22,23 and tricuspid valve regurgitation,24 left atrial enlargement25 and right ventricular dysfunction26,27 all impact prognosis and were far more common among patients with AF compared with SR, with paroxysmal AF showing intermediate degree of structural changes. This may help explain why most deaths in patients with AF are cardiac-related as previously shown by Gomez-Outes et al28 and corroborated by this current study. Thus, detection of AF, whether persistent or paroxysmal,29 in symptomatic or asymptomatic AS may be a clinical marker denoting a myriad of underlying cardiac abnormalities associated with worse outcomes30 as indicated by the significant differences in prevalent underlying structural heart disease in AF versus SR even when matched by clinical comorbidities.
In the general population, increased risk of mortality within the first 90 days of new-onset AF when associated with heart failure has previously been reported.18 Duration of AF in the current study made little difference to the poorer outcome in the AF group, suggesting even newer-onset AF is a marker of poor survivorship in patients with severe AS.
STUDY LIMITATIONS
This is a single-centre retrospective study. Continuous 24 hours of Holter monitoring was not systematically performed in all patients. Although guidelines have specific recommendations for AF, Doppler assessment during AF remains a challenge and may limit generalisability of the results of this study. Whether there are subsets of patients with severe AS and AF better suited for surgical versus transcatheter AVR is beyond the scope of this study and requires additional research.
CONCLUSIONS
AF was associated with poor prognosis in patients with severe AS, but differences in outcomes compared with SR were explained by other factors including concomitant cardiac abnormalities and deferral of AVR due to the attribution of cardiac symptoms to AF. Further studies are needed to examine the association of other structural heart disease and AF to the natural history of AS.
Key messages.
What is already known on this subject?
Atrial fibrillation is frequently associated with aortic stenosis and portends worse survival, but it is unclear how atrial fibrillation influences management of patients with aortic stenosis and if it is an independent predictor of outcomes.
What might this study add?
This study shows that atrial fibrillation was not an independent predictor of mortality in patients with severe aortic stenosis; instead other factors explain the apparent association of atrial fibrillation and mortality including associated structural heart disease and deferral of aortic valve replacement due to attribution of cardiac symptoms to atrial fibrillation.
How might this impact on clinical practice?
Symptoms in patients with severe aortic stenosis and atrial fibrillation may be better attributed to aortic stenosis. Presence of atrial fibrillation should prompt evaluation for associated structural heart diseases which, when present, further increase risk of mortality without aortic valve replacement.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was approved by the Mayo Clinic Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information.
REFERENCES
- 1.Bach DS, Cimino N, Deeb GM. Unoperated patients with severe aortic stenosis. J Am Coll Cardiol 2007;50:2018–9. [DOI] [PubMed] [Google Scholar]
- 2.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation 2009;119:2516–25. [DOI] [PubMed] [Google Scholar]
- 3.Reddy YNV, Obokata M, Gersh BJ, et al. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation 2018;137:534–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faggiano P, Frattini S, Zilioli V, et al. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. potential implications for the decision-making process. Int J Cardiol 2012;159:94–9. [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262–70. [DOI] [PubMed] [Google Scholar]
- 6.Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111:3290–5. [DOI] [PubMed] [Google Scholar]
- 7.Rosenhek R, Zilberszac R, Schemper M, et al. Natural history of very severe aortic stenosis. Circulation 2010;121:151–6. [DOI] [PubMed] [Google Scholar]
- 8.Levy F, Rusinaru D, Maréchaux S, et al. Determinants and prognosis of atrial fibrillation in patients with aortic stenosis. Am J Cardiol 2015;116:1541–6. [DOI] [PubMed] [Google Scholar]
- 9.Burup Kristensen C, Jensen JS, Sogaard P, et al. Atrial fibrillation in aortic stenosis--echocardiographic assessment and prognostic importance. Cardiovasc Ultrasound 2012;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretti M, Fabris E, Morosin M, et al. Prognostic significance of atrial fibrillation and severity of symptoms of heart failure in patients with low gradient aortic stenosis and preserved left ventricular ejection fraction. Am J Cardiol 2014;114:1722–8. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association Task force on practice guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23. quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 16.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. quiz 86–8. [DOI] [PubMed] [Google Scholar]
- 17.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain AM, Gersh BJ, Alonso A, et al. Decade-Long trends in atrial fibrillation incidence and survival: a community study. Am J Med 2015;128:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham heart study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 20.Falk RH. Atrial fibrillation. N Engl J Med 2001;344:1067–78. [DOI] [PubMed] [Google Scholar]
- 21.Clark DM, Plumb VJ, Epstein AE, et al. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol 1997;30:1039–45. [DOI] [PubMed] [Google Scholar]
- 22.Liang JJ, Silvestry FE. Mechanistic insights into mitral regurgitation due to atrial fibrillation: “Atrial functional mitral regurgitation”. Trends Cardiovasc Med 2016;26:681–9. [DOI] [PubMed] [Google Scholar]
- 23.Widgren V, Dencker M, Juhlin T, et al. Aortic stenosis and mitral regurgitation as predictors of atrial fibrillation during 11 years of follow-up. BMC Cardiovasc Disord 2012;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki N, Kondo F, Kubo T, et al. Severe tricuspid regurgitation in the aged: atrial remodeling associated with long-standing atrial fibrillation. J Cardiol 2006;48:315–23. [PubMed] [Google Scholar]
- 25.Rusinaru D, Bohbot Y, Kowalski C, et al. Left atrial volume and mortality in patients with aortic stenosis. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli E, Guirette Y, Feneon D, et al. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging 2015;16:531–8. [DOI] [PubMed] [Google Scholar]
- 27.Gorter TM, van Melle JP, Rienstra M, et al. Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail 2018;24:177–85. [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Outes A, Lagunar-Ruíz J, Terleira-Fernández A-I, et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2016;68:2508–21. [DOI] [PubMed] [Google Scholar]
- 29.Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian registry of atrial fibrillation. Am Heart J 2005;149:489–96. [DOI] [PubMed] [Google Scholar]
- 30.Généreux P, Pibarot P, Redfors B, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J 2017;38:3351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]