Abstract
Autoantibodies (AAbs) against retinal antigens can be found in patients with cancer and unexplained vision loss unrelated to the cancer metastasis. Cancer-associated retinopathy (CAR) is a rare paraneoplastic visual syndrome mediated by AAbs. Our goal was to determine whether CAR patients with different malignancies have a specific AAb or repertoire of AAbs that could serve as biomarkers for retinal disease. We found AAbs against 12 confirmed retinal antigens, with α-enolase being the most frequently recognized. The significant finding of the study was a high incidence of anti-aldolase AAbs in colon-CAR, anti-CAII in prostate-CAR, and anti-arrestin in skin melanoma patients thus these AAbs could serve as biomarkers in the context of clinical presentation and could support the diagnosis of CAR. However, a lack of AAb restriction to any one antigenic protein or to one retinal cellular location makes screening for a CAR biomarker challenging.
1. INRODUCTION
There are autoimmune conditions of the retina that occur almost exclusively as paraneoplastic manifestations of cancer. Paraneoplastic retinopathy is rare. It is characterized by unexplained loss of vision associated with retinal dysfunction/degeneration, distant malignancy, and the presence of serum anti-retinal autoantibodies (AAbs). The most common visual paraneoplastic disorders include cancer-associated retinopathy (CAR) and melanoma–associated retinopathy (MAR)[1–3]. CAR is defined as an autoantibody-driven remote effect of systemic cancer that is associated with retinal degeneration that is not caused by tumor metastasis. Although the role of autoimmunity in retinal degeneration has not been fully explained, experimental and clinical studies corroborate that anti-retinal AAbs in high titers can penetrate into the retina, affecting function of the target antigens and, in turn, leading to retinal dysfunction and degeneration [4]. In general, about 1% of patients with cancer are estimated to be affected by various paraneoplastic syndromes and fewer related to the eye [5].
In visual paraneoplastic disorders, AAb responses are believed to be part of the anti-tumor response, triggered by tumor antigens that are released during tumor growth (neoantigens) and processed by antigen-presenting cells [6]. The persistent presence of anti-retinal AAbs is the serological hallmark of CAR and represents one of its required classification criterion [7]. Detectable levels of anti-retinal AAbs are present for many years and vision problems may manifest before detecting malignancy thus serum AAbs would have a strong predictive value in CAR/MAR [8–10]. The occurrence of an autoimmune retinal disease, including CAR might not require a new autoimmunization, but rather is a loss of control in the existing autoimmunity [11]. In addition, AAbs also occur in the presence of malignancy without paraneoplastic manifestation [12, 13]. However, sera of individuals prior to vision loss are not available, thus we cannot establish the exact timeline between the AAb generation and clinical manifestation of visual problems. It precludes us from full understanding the mechanisms that regulate such a transition.
Usually, patients with autoimmune retinopathies have a small number of anti-retinal AAbs. We are interested in a discovery of AAb profiles that are associated with unexplained vision loss that could forecast CAR or malignancy with a high probability, and in providing an immunopathogenic understanding of syndrome [14–17]. Unfortunately, CAR is rare, highly heterogeneous and complex, considering that patients differ not only in antibody profiles but also in their clinical presentation. Our approach to the analysis of AAbs in such patients was based on an unbiased western blotting methodology and immunostaining of retina with serum. This approach revealed 12 clinically relevant AAbs that were detected at high frequencies. We hypothesized that CAR patients with different malignancies have a specific AAb or specific repertoire of AAbs that could serve as biomarkers for different type of CAR. Here, we present results from retrospective study of the largest cohort of seropositive patients until today with unexplained vision loss in the presence of different malignancies.
2. METHODS
2.1. Study population
Human subjects research was approved by the by the OHSU Institutional Review Board. Our research adhered to the tenets of the Declaration of Helsinki. All patient samples were originally deposited to the Autoimmune Retinopathy Serum Research Repository OHSU under approval by the OHSU Institutional Review Board. Samples used in this study were de-identified, and stored at −80°C, and results cannot be linked to subjects. We reviewed serum samples tested for AAbs from 2014 to 2017 and selected those that met our criteria as follows: progressive nature of vision loss, visual field defects, abnormal rod and/or cone responses on the ERG, and a diagnosed cancer that often triggered the suspicion of CAR. We selected seropositive patients with cancer that included 271 females and 170 males, showing a slight female predominance (F/M ratio 1.6 to 1). The average age of a CAR patient was 66 ±13 years old. These patients had been diagnosed with 20 different kinds of cancers (CAR group). Patients with malignant melanoma were included in the “CAR group”. We present findings for 441 seropositive patients for AAbs against 12 retinal autoantigens. There were additional 54 seronegative patients with cancer that met the selection criteria. We do not know whether these patients developed AAbs in the later time (they were not re-tested). For comparison, we included 127 sera of healthy individuals that have been examined for anti-retinal AAbs.
Selection of Frequent Autoantibodies
Initially, patient’s sera were analyzed by western blotting using human retinal extract according to previously published methods [18]. A verification of specificity of anti-retinal antibodies required an additional immunoblotting with purified or recombinant proteins of the appropriate molecular mass and the use of specific antibodies against those proteins (positive controls). The antigen identity was confirmed by immunoreactivity with 12 retinal proteins that included recoverin and retinal arrestin, heat shock protein 27 (HSP27), Rab6A GTPase, carbonic anhydrase II (CAII), cellular-retinaldehyde binding protein (CRALB), α-tubulin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), aldolase C, α-enolase, pyruvate kinase M2 (PKM2) and P62 (HSP60) [19]. Details are presented in Table 1. Only patients whose sera were verified for antibody specificity have been the focus of this study.
Table 1:
List of control proteins and antibodies used in the study
| Antigen Molecular Mass | Protein Name | Source | Control Antibody | Source |
|---|---|---|---|---|
| 23-kDa | Recombinant Recoverin | Purified in the lab | Rat MAb anti-recoverin | Made in the lab |
| 23-kDa | Recombinant HSP27 | R&D | Mouse anti-human HSP27 | Thermo Fisher Scientific |
| 23-kDa | Recombinant Rab6A | NOVUS | Rabbit anti-human Rab6A | NOVUS |
| 30-kDa | Purified CAII | Sigma | Sheep anti-human CAII | Invitrogen |
| 34-kDa | Recombinant CRALBP | NOVUS | Rabbit anti-human CRALBP | Thermo Fisher |
| 36-kDa | Purified GADPH | Sigma | Rabbit anti-human GADPH | Sigma |
| 40-kDa | Aldolase C | MP Biomedical | Rabbit anti-aldolase C | Cell Signaling Technology |
| 46-kDa | Purified retinal α-Enolase | Purified in the lab | Rat MAb anti-α-enolase | Made in the lab |
| 48-kDa | Purfied retinal Arrestin | Purified in the lab | Mouse MAb anti-arrestin | Made in the lab |
| 52-kDa | Purified Tubulin-α | Sigma | Goat anti-human tubulin-α | Santa Cruz |
| 56-kDa | Recombinant PKM2 | NOVUS | Rabbit anti-human PKM2 | Invitrogen |
| 62-kDa | Recombinant HSP60 | StressMarq | Rabbit anti-human HSP60 | Cell signaling Technology |
2.2. Immunohistochemistry (IHC)
Donor human retina was fixed 1 hr in 4% paraformaldehyde and then 30% sucrose overnight. Retinal tissue was frozen in OCT as previously described [9]. Twelve microns tissue cryosections were prepared and then incubated with human serum diluted 1:30 [18]. A reference human serum, containing anti-recoverin antibodies was used as a positive control. A negative control contained secondary antibodies only. Color reaction was developed using peroxidase substrate (Pierce) for 15 min and then the tissue was counterstained with methyl green.
2.3. Statistics
The patients’ data were sorted according to their AAb positivity, cancer presence, and clinical manifestation. The Fisher’s exact test was used to compare the prevalence of positive antibodies between cases and healthy controls. ANOVA tests were performed to find statistical significant differences between CAR and control samples. Statistical significance values are as follows: *p< 0.05, **p<0.01, ***p<0.001, and ****p<0.0001. The analysis was performed using the GraphPad Prism 5.0a software.
3. RESULTS
3.1. Prevalence of Autoantibodies
A broad spectrum of antibody reactivities was observed in the series of 441 patients with cancer and unexplained vision loss. In the search for a potential target for those AAbs, we identified 12 retinal autoantigens that represented photoreceptor-specific proteins, such as recoverin, retinal arrestin, CRALBP, and the small GTPase Rab6, 2 heat shock proteins HSP27 and P62 (HSP60), CAII, α-tubulin, and 4 glycolytic enzymatic proteins that were also found in the outer segments of photoreceptor cells: GAPDH, aldolase C, α-enolase, and PKM2 [20, 21]. The occurrence of CAR AAbs was compared to those revealed in the control group and the results showed that AAbs were more frequent in the CAR group (p=0.0025), including AAbs against ubiquitous antigens, such as aldolase, enolase, GADPH, and CAII. The prevalence of specific AAbs in both groups is presented in Table 2. Reactivities against recoverin, Rab6 and CRALBP proteins were not detected in healthy individuals. The positivity rate in CAR for a single AAbs was 37% and 25% patients had double positivity, 14% had triple AAbs, 6% 4 AAbs, and 1% had 5 AAbs. This suggests that an increased number of specific AAbs was more strongly associated with CAR. Moreover, few specific AAbs occurred in same patient that created antibody profiles related to the patient.
Table 2.
Frequency of anti-retinal autoantibodies against 12 autoantigens in normal individuals and patients with cancer and vision loss
| Antigen | Normal (N=127) | CAR (N=441) |
|---|---|---|
| Aldolase | 5% | 16% |
| Arrestin | 2% | 14% |
| CAII | 15% | 28% |
| CRALBP | 0 | 3% |
| Enolase | 13% | 43% |
| GAPDH | 11% | 17% |
| HSP27 | 1% | 4% |
| p62 | 2% | 15% |
| PKM2 | 4% | 8% |
| Rab6 | 0 | 5% |
| Recoverin | 0 | 3% |
| Tubulin | 2% | 5% |
3.2. Cellular Targets of Anti-retinal Autoantibodies
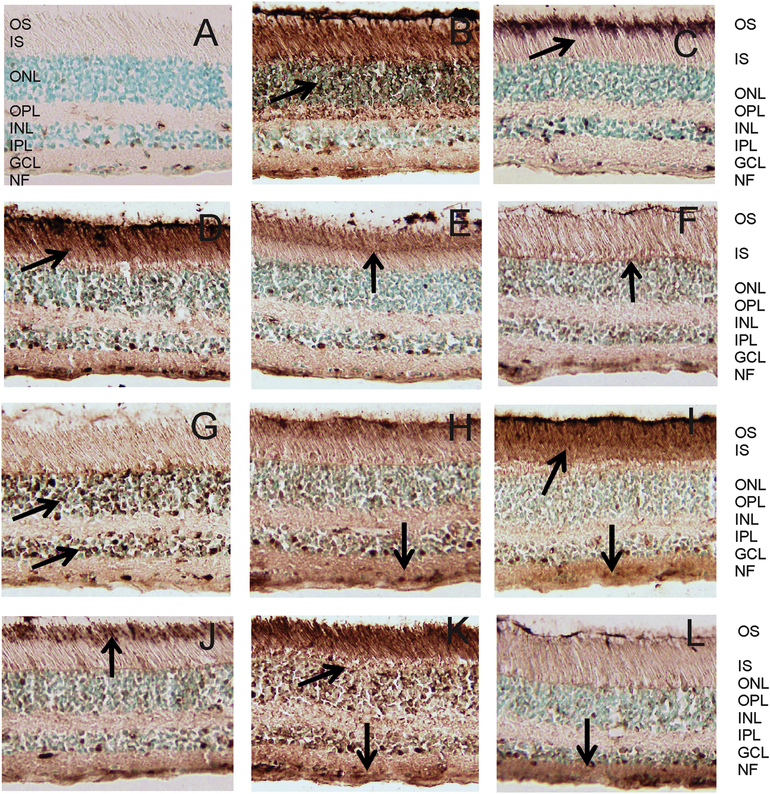
The immunostaining of human retina by different serum samples was dependent on the combination of specificity and location of target antigens in retina. Because most patients had more than 1 anti-retinal AAbs detected by western blotting it was not surprising serum AAbs shows diverse patterns of the retina immunostaining. Examples of such immunostaining are represented in Figure 1. AAbs that were against photoreceptor cell antigens immunostained outer segments (Fig. 1 CEJ), outer and inner segments (Fig 1 DIK), or the whole photoreceptor cells (Fig. 1 BK). AAbs against enolase usually stained the ganglion cell layer (Fig. 1 DFHIKL).
Figure 1.
Patterns of immunostaining of human retina cryosections with serum anti-retinal autoantibodies. Immunoperoxidase staining depended on antibody specificities and accessibility to the antigen in the tissue : (A) control staining, no serum, only secondary antibodies; (B) labeling of Ph layer; (C) labeling of OS in photoreceptor cells; (D) labeling of OS and IS in photoreceptor cells and GCL and NF; (E) labeling of OS and the junction between the IS and OS of rod and cone photoreceptors (F) labeling of OLM; (G) labeling of ONL and INL; (H) labeling of Ph cells, INL, IPL, GCL; (I) labeling of OS and IS and IPL and GCL; (J) labeling of OS of rods and cones, and outer limiting membrane; (K) Labeling of Ph cells, INL and GCL; and (L) labeling of IPL and GCL; Retinal layers are: OS, outer segments; IS, inner segments; Ph, photoreceptors; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; and GCL, ganglion cell layer; NF, nerve fiber layer. Arrows point at strongest immunostaining.
3.3. Correlation of Autoantibodies with Tumors in CAR
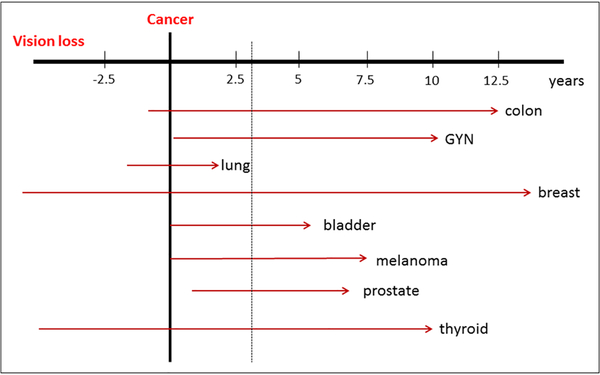
There were at least 20 different undelaying tumors reported in association of 12 specific AAbs with the most frequent being breast cancer (22%), malignant skin melanoma (19%), lung cancer (11%; SCCL, NSCCL, adenocarcinoma), and gynecologic cancers (8%; ovarian, endometrial, uterine, and cervical). Cancers like prostate, colon, thyroid cancers, and lymphomas occurred at lower incidence in association with ocular disturbances (Table 3). Figure 2 shows the occurrence of vision loss in the relation to cancer diagnosis. The majority of patients (85%) reported vision loss months to years after diagnosis of cancer and 15% developed vision problems around the time of cancer diagnosis (in particular, patients with lung cancers) or vision loss was preceding a discovery of their malignancies. The median time delay between finding cancer and ocular symptoms was ~3 years (ranged from 0 to 15 years).
Table 3:
Occurrence of cancers associated with vision loss and the presence of anti-retinal autoantibodies
| Cancer (n=441) | Total (N) | % |
|---|---|---|
| Bladder | 12 | 3% |
| Brain | 7 | 2% |
| Breast | 96 | 22% |
| Colon | 21 | 5% |
| Esophagus | 3 | >1% |
| Gynecological (ovarian, endometrial, uterine, cervical) | 32 | 7% |
| Leukemia | 9 | 2% |
| Liver | 8 | 2% |
| Lung (SCCL, NSCCL, adenocarcinoma) | 47 | 11% |
| Lymphoma | 17 | 4% |
| Melanoma | 82 | 19% |
| Multiple myeloma | 5 | 1% |
| Pancreas | 7 | 2% |
| Pituitary | 5 | 1% |
| Prostate | 30 | 7% |
| Renal | 4 | 1% |
| Sarcoma | 3 | >1% |
| Skin, not melanoma | 14 | 3% |
| Testis | 4 | 1% |
| Thyroid | 16 | 4% |
Figure 2.
A diagram showing the latency between the occurrence of vision loss and cancer diagnosis illustrated for breast bladder, colon, gynecological cancers (GYN), melanoma, lung, prostate, and thyroid cancers. The median time delay between finding cancer and ocular symptoms was ~3 years (dotted line).
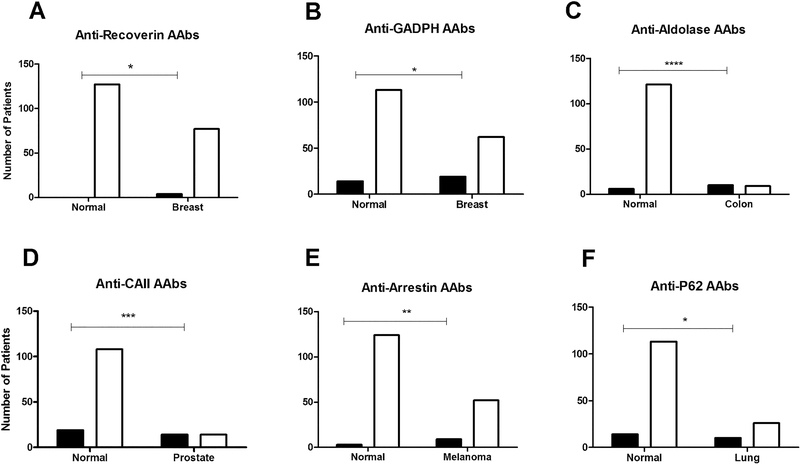
The frequency of specific AAbs differed in subgroups of cancer patients. The most frequent were anti-α-enolase AAbs (43%), followed by anti-CAII (28%), anti-GADPH (17%), anti-aldolase C (16%), anti-P62 (15%), anti-arrestin (14%), anti-PKM2 (8%), anti-tubulin-α (5%), anti-Rab6 (5%), anti-HSP27 (4%), anti-recoverin (3%), and anti-CRALBP (3%) AAbs. Some AAbs revealed strong association with particular cancers. As an example Figure 3 shows statistically relevant incidence of AAbs in breast, colon, prostate, lung cancers and melanoma. Anti-CAII AAbs were found more frequent in prostate-CAR (50%; p<0.0001), breast-CAR (32%; p=0.0003), and others, implying that AAbs against ubiquitous proteins cannot be ignored when evaluating results from the antibody screening. This includes AAbs against glycolytic enzymes (enolase, aldolase, GADPH, PKM2) that were significantly elevated in patients than those in healthy controls. A unique new finding was a high incidence of anti-aldolase C AAbs in 53% patients with colon cancer (p<0.0001), and anti-GAPDH AAbs in gynecological cancers (27%; p=0.0050) where in other cancers these AAbs were detected below 15% rate (Figure 2). Anti-PKM2 AAbs were present in 33% bladder-CAR, 19% breast-CAR, and 16% colon-CAR. Another remarkable finding was a detection of anti-arrestin AAbs in thyroid-CAR (33%), breast-CAR (23%), colon-CAR (21%), bladder-CAR (22%), and melanoma (15%, p=0.0023). Anti-Rab6 AAbs were associated with prostate-CAR and bladder-CAR at 11% frequencies. Anti-CRALBP AAbs were the most frequent in thyroid-CAR (20%). Anti-tubulin-α AAbs were strongly associated with patients with liver malignancy (60%). Anti-P62 AAbs were frequently detected in thyroid-CAR (19%) and gynecological-CAR (19%), and in 17% of lung-CAR patients (p=0.00176). Anti-recoverin AAbs were found at low frequencies and always with 3 or more of other anti-retinal AAbs. The odd ratio, sensitivity and specificity, a positive test result and a negative test result respectively, differed depending on AAbs and the overview is shown in Table 4. The results showed a very high sensitivity and moderate specificity for all autoantigens. For anti-recoverin, anti-CRAMBP, and anti-Rab6, the calculation is not included in the table because the small sample size and their absence in the control group, limited obtaining meaningful results.
Figure 3.
Prevalence of specific anti-retinal autoantibodies in normal subjects and patients with different cancers. Statistically significant increases of AAbs was determined for: (A) anti-recoverin AAbs in breast-CAR; B - anti-GADPH in breast-CAR; C – anti-aldolase AAbs in colon-CAR; D – anti-CAII AAbs in prostate-CAR; E – anti-arrestin AAbs in melanoma-CAR; and F – anti-P62 AAbs in lung-CAR. Open bars – negative, black bars – positive for antibodies
Table 4.
Analysis of Contingency Table: Overview
| Table Analyzed | P value | Odds ratio | 95% CI | Sensitivity | 95% CI | Sperifidty | 95% CI | PPV | 95% CI | NPV | 95% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldolase | 0.0009 | 3.741 | 1.584 to 8.833 | 92% | 0.8340 to 0.9701 | 25% | 0.2081 to 0.2859 | 16% | 0.1238 to 0.1938 | 95% | 0.9000 to 0.9825 |
| Arrestin | <.0001 | 6.509 | 2.005 to 21.13 | 95% | 0.8671 to 0.9901 | 25% | 0.2086 to 0.2855 | 14% | 0.1055 to 0.1716 | 98% | 0.9325 to 0.9951 |
| CAII | 0.0025 | 2.223 | 1.309 to 3.778 | 87% | 0.8003 to 0.9181 | 25% | 0.2134 to 0.2983 | 28% | 0.2397 to 0.3256 | 85% | 0.7763 to 0.9075 |
| Enolase | <0.0001 | 5.203 | 2.981 to 9.083 | 92% | 0.8763 to 0.9547 | 31% | 0.2588 to 0.3560 | 43% | 0.3819 to 0.4762 | 87% | 0.8035 to 0.9262 |
| GADPH | 0.0009 | 3.741 | 1.584 to 8.833 | 92% | 0.8340 to 0.9701 | 25% | 0.2081 to 0.2859 | 16% | 0.1238 to 0.1938 | 95% | 0.9000 to 0.9825 |
| HSP27 | 0.0906 | 5.362 | 0.7084 to 40.58 | 95% | 0.7397 to 0.9987 | 23% | 0.1950 to 0.2670 | 4% | 0.02437 to 0.06374 | 99% | 0.9569 to 0.9998 |
| p62 | <0.0001 | 10.37 | 2.503 to 42.93 | 97% | 0.8992 to 0.9965 | 24% | 0.2007 to 0.2749 | 14% | 0.1120 to 0.1771 | 98% | 0.9443 to 0.9981 |
| PKM2 | 0.122 | 2.235 | 0.8592 to 5.812 | 88% | 0.7437 to 0.9602 | 23% | 0.1965 to 0.2704 | 8% | 0.05976 to 0.1138 | 96% | 0.9105 to 0.9871 |
| Tubulin | 0.2306 | 2.274 | 0.6714 to 7.704 | 88% | 0.6985 to 0.9755 | 23% | 0.1941 to 0.2665 | 5% | 0.03334 to 0.07723 | 98% | 0.9325 to 0.9951 |
3.4. Association of Autoantibodies with Symptoms
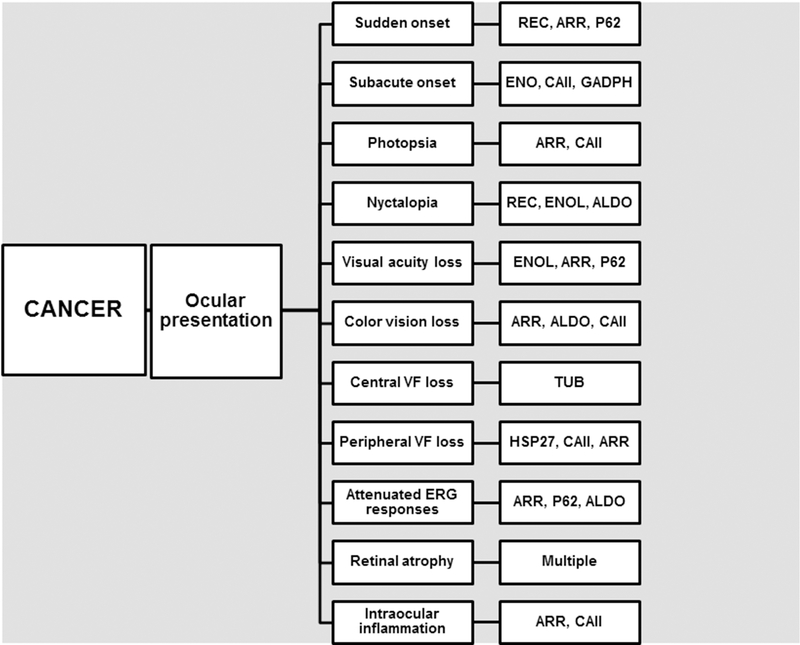
The next question was whether specific anti-retinal AAbs are associated with retinal disease symptoms. Our analysis of clinical data showed that not every patient displayed all symptoms typical of CAR, likely because their disease was at different stage when sera were collected and tested for AAbs. Figure 4 comprises the patients’ symptoms and findings with the most frequently occurring specific AAbs. Vision loss was painless and bilateral, and was sudden (7%) or subacute (10%). Sudden onset of vision loss was more often related to anti-recoverin, anti-P62, and anti-arrestin AAbs. Some patients complained of night vision problems (nyctalopia, 15%), and light sensitivity (14%). Visual testing revealed the loss of visual acuity (42%), color vision loss (15%), and visual field testing showed peripheral and ring scotomas (32%). The ERG was abnormal, presenting defects in rod and cone functions (29%). Mild intraocular inflammation (iritis, vitritis) was occasionally present (9%) in association with anti-arrestin and anti-CAII AAbs. In Table 5 summarizes the major findings related to anti-retinal AAbs and 9 most common cancers in CAR. Overall, these results suggest that an increased prevalence of at least some AAbs in the context of ocular presentation may help with diagnosis of CAR.
Figure 4.
A diagram demonstrating the correlation between ocular symptoms and findings and the most frequent anti-retinal autoantibodies in patients; VF, visual field
Table 5:
Association of anti-retinal autoantibodies with 9 most common cancers in cancer-associated retinopathy (CAR)
| Cancer | Incidence of cancer | Most frequent AAbs against retinal markers | Time from AAbs detection to cancer diagnosis | Major symptoms and findings |
|---|---|---|---|---|
| Breast | 22% (96/441) | Enolase, GAPDH, CAII, recoverin | Mostly after or at the time of diagnosis | Photopsia/photophobia, VA loss, ffERG dysfunction, pigmentary changes |
| Gynecological (ovarian, endometrial, uterine, cervical) | 7% (33/441) | GAPDH, aldolase, enolase | Can precede, mostly after | Progressive course, blurry vision, photopsia, VA loss, VF defect, retinal thinning |
| Lung (SCCL, NSCCL, adenocarcinoma) | 11% (47/441) | Enolase, p62, CAII | Can precede | Sudden onset, color vision loss, retinal thinning, vascular abnormalities |
| Colon | 5% (21/441) | Aldolase, CAII, enolase, (arrestin, recoverin) | Can precede | Sudden onset, progressive course, blurry vision, central vision loss, color vision loss, cone-ffERG dysfunction, retinal thinning |
| Prostate | 8% (35/441) | CAII, recoverin | Can precede | Progressive course, nyctalopia, VF defects, ERG dysfunction, attenuated vessels |
| Thyroid | 4% (16/441) | Enolase, arrestin | At the time diagnosis or after | Photopsia/photophobia, peripheral vision loss, VA loss, rod-ffERG dysfunction, retinal thinning |
| Melanoma | 18% (78/441) | Enolase, CAII, arrestin | After | Gradual loss, nyctalopia, VA loss, rod-ERG dysfunction |
| Lymphomas | 4% (17/441) | Arrestin, Enolase, p62 | After | VA loss, VF loss |
| Bladder | 3% (12/441) | CAII, Enolase, GADPH | At the time diagnosis or after | Progressive course, VA loss, ERG dysfunction; color vison loss |
VA = visual acuity, ERG = electroretinogram; VF = visual field
DISCUSSION
In recent years, several anti-retinal AAbs have been found in association with CAR [22, 23]. Our immunological studies identified 12 targets for AAbs that were frequently detected in the largest group of patients with unexplained vision loss and cancer so far. This study has demonstrated a striking lack of restriction of AAbs to any one antigenic protein or one retinal cellular location, thus screening for an antibody CAR marker is challenging. The complexity of cancer, degeneration of retina, and the immune response differed vastly between people and cancer type.
In the past, it has been speculated that anti-recoverin AAbs are the sole biomarker of CAR and other associated AAbs may be indirectly or synergistically involved. However, previous and current studies confirmed that anti-recoverin AAbs are rare [21]. Consequently, an absence of AAbs against recoverin does not exclude the diagnosis of paraneoplastic retinopathy in patients with the appropriate clinical profile of this disease. There are other specific AAbs present in patents at much higher frequencies in association with unexplained vision loss.
Anti-α-enolase AAbs were the most frequently found in patients with different tumors and had the highest incident in breast cancer, followed by prostate and thyroid carcinomas, and malignant melanoma. These antibodies are less predictive of associated neoplasm than are anti-recoverin AAbs [14]. The occurrence of anti-glycolytic enzymes antibodies (enolase, aldolase C, and GAPDH) was 2–3 times more frequent in CAR with gynecological cancers than normal women [17]. Glycolytic enzymes are highly expressed in the outer segment of photoreceptor cells as well as in other retinal cells, also in the majority of cancers related to paraneoplastic retinopathy [24, 25]. It is reasonable to assume that the overexpressed glycolytic antigens may elicit a humoral immunity, and in effect, attack the tumor and the retina [26]. Therefore, the generation of anti-enolase AAbs is a likely consequence of the uptake of enolase by antigen-presenting cells at the tumor site and subsequent B cell activation [26]. Healthy individuals also have some serum AAbs against retinal antigens, which makes a difficult interpretation of clinical significance of such findings. However, we have showed in the past that normal individuals had anti-enolase AAbs that bound to different epitopes [27] and were at lower incidence as compare to that of control AAbs of the same specificity. In general, the reason for the presence and abundance of AAbs in human sera, especially in younger and healthy individuals, is unknown. Some AAbs may be remnants of past immunological activities in infectious disease, but may also be present as a result of ongoing current disease [6]. Another potential source of those antigenic components is cancer (neoantigens), undergoing cell death due to anti-tumor response and necrosis, and the generation of AAbs as a direct consequence of the autoimmune process [6].
This study shows that at least some AAbs have a biomarker potential that includes the high frequency of anti-aldolase AAbs with colon-CAR. Finding anti-aldolase AAb responses in association with colon cancer may not be surprising because the aldolase protein is overexpressed in colorectal cancers and therefore, such an increased presence of this antigen could predispose the immune system to elicit anti-aldolase humoral response [28]. It is also important to mention that photoreceptor specific antigenic proteins (arrestin, recoverin, and rhodopsin) were also found to be expressed in different tumors (i.e. renal cell carcinoma, thyroid cancer, melanoma) and are considered to be a new class of cancer antigens (i.e. cancer-retina antigens) [29, 30]. The presence of anti-retinal AAbs in a patient with a history of cancer could be indicative of cancer recurrence, which is of primary concern, even in a patient who does not have typical paraneoplastic anti-retinal AAbs.
We detected anti-arrestin AAbs in thyroid-CAR, colon-CAR, bladder-CAR, and MAR, which coincides with the expression of arrestins in those tumors. Retinal arrestin (also called S-antigen) has been found to be a major pathogenic antigen in autoimmune uveitis [31]. Arrestins were initially discovered in the visual phototransduction system and include four mammalian members, two visual arrestin-1 in rod cells, arrestin-4 in cone cells, and two non-visual β-arrestin1 and β-arrestin 2 (also called arrestin-2 and arrestin-3) respectively [32]. β-Arrestins are overexpressed in late-stage cancers, such as human glioblastomas, breast cancer, and melanoma and act on the tumor cell proliferation, apoptosis, migration, and invasion [29, 33]. The expression of arrestins in different tumors is a potential source of the anti-arrestin autoimmune response. Altogether, these findings suggest that the occurrence of specific AAbs may correspond to differences in cellular location of antigens, availability of the antigen, duration of antibody exposure to its antigen, and immunopathological mechanisms CAR. It remains to be determined whether the autoantibody represents secondary autoimmunity (progression of retinal damage) or is a product of disease heterogeneity.
Due to AAbs presence in the general population as well as in multiple autoimmune diseases, showing the presence of AAbs alone does not make a diagnosis. Such results have to be interpreted in the context of clinical findings. Similarly, the absence of autoantibody does not exclude a disease. The initial AAb test result may be negative for retinal antigens but then AAbs develop over time, especially, if retinal disease progresses. Autoantibodies might not be directly responsible for the manifestation of the diseases; they could still be highly sensitive and specific marker to detect disease. Moreover, many different cell types have the same common proteins and only a very small subset of protein targets and their corresponding AAbs would be expected to be truly cell-type/disease-specific, and thus useful for disease detection and diagnosis. This process may depend on the protein accessibility in the cell and protective mechanism in the cell against adverse reaction [34, 35].
We recognize that this study has some limitations. CAR is rare and the ambiguity of its clinical picture on presentation, and limited access to clinical data in some cases makes the study difficult. To predict the development of CAR based on the AAbs presence alone or vision loss alone is problematic therefore test results must be interpreted in the context of clinical presentation. Also, our ethnic population consists of mainly of Caucasian individuals therefore the results are largely relevant for this group of patients. And finally, we limited our study to 12 autoantigens that were identified and verified but there might be others, awaiting a future discovery. Identification and verification of a discrete antigen in association with specific symptoms is very costly and therefore most antigenic proteins are known by their molecular mass. Thus, our investigation should be considered as a work-in-progress with the potential for future development of a bigger library of verified CAR autoantigens to help the medical community with better diagnosis and management strategies for their patients.
Highlights.
There is an association between the presence of anti-retinal autoantibodies, ocular symptoms and tumors in cancer-associated retinopathy (CAR)
Multiple autoantibody are detected in association with different kinds of cancer creating antibody signatures specific to patients
Some AAbs have a biomarker potential that includes the high frequency of anti-aldolase AAbs with colon-CAR
Anti-carbonic anhydrase II autoantibodies are frequently present in prostate-CAR
ACKNOWLEDGEMENT
Funding/Support: This work was supported by grant P30 EY010572 from the National Institutes of Health (Bethesda, MD) and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY)
Abbreviations used in the paper
- AAbs
autoantibodies
- AR
autoimmune retinopathy
- CAR
cancer-associated retinopathy
- MAR
melanoma-associated retinopathy
- REC
recoverin
- ARR
retinal arrestin
- HSP27
heat shock protein 27
- CAII
carbonic anhydrase II
- TUB
tubulin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- ALDO
aldolase
- ENOL
enolase
- GYN
gynecological cancers
- ERG
electroretinogram
Footnotes
Financial Disclosures: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Jacobson DM. Paraneoplastic disorders of neuro-ophthalmologic interest. Curr Opin Ophthalmol, 1996;7:30–8. [DOI] [PubMed] [Google Scholar]
- [2].Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol, 2001;21:173–87. [DOI] [PubMed] [Google Scholar]
- [3].Ohguro H, Yokoi Y, Ohguro I, Mamiya K, Ishikawa F, Yamazaki H et al. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol, 2004;137:1117–9. [DOI] [PubMed] [Google Scholar]
- [4].Lu Y, Jia L, He S, Hurley MC, Leys MJ, Jayasundera T et al. Melanoma-associated retinopathy: a paraneoplastic autoimmune complication. Arch Ophthalmol, 2009;127:1572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Honnorat J Onconeural antibodies are essential to diagnose paraneoplastic neurological syndromes. Acta Neurol Scandinavica, 2006;113:64–8. [DOI] [PubMed] [Google Scholar]
- [6].Adamus G Are Anti-Retinal Autoantibodies a Cause or a Consequence of Retinal Degeneration in Autoimmune Retinopathies? Front Immunol, 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fox AR, Gordon LK, Heckenlively JR, Davis JL, Goldstein DA, Lowder CY et al. Consensus on the Diagnosis and Management of Nonparaneoplastic Autoimmune Retinopathy Using a Modified Delphi Approach. Am J Ophthalmol, 2016;168:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Keltner JL, Roth AM, Chang S. Photoreceptor degeneration. Possible autoimmune disorder. Arch Ophthalmol, 1983;101:564–9. [DOI] [PubMed] [Google Scholar]
- [9].Polans A, Witkowska D, Haley T, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci USA, 1995;92:9176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saito W, Kase S, Ohguro H, Furudate N, Ohno S. Slowly Progressive Cancer-Associated Retinopathy. Arch Ophthalmol, 2007;125:1431–3. [DOI] [PubMed] [Google Scholar]
- [11].Cohen IR. Activation of benign autoimmunity as both tumor and autoimmune disease immunotherapy: a comprehensive review. J Autoimmun, 2014;54:112–7. [DOI] [PubMed] [Google Scholar]
- [12].Savchenko M, Bazhin A, Shifrina O, Demoura S, Kogan E, Chuchalin A et al. Antirecoverin autoantibodies in the patient with non-small cell lung cancer but without cancer-associated retinopathy. Lung Cancer, 2003;41:363–7. [DOI] [PubMed] [Google Scholar]
- [13].Bazhin AV, Savchenko MS, Shifrina ON, Demoura SA, Chikina SY, Jaques G et al. Recoverin as a paraneoplastic antigen in lung cancer: the occurrence of anti-recoverin autoantibodies in sera and recoverin in tumors. Lung Cancer, 2004;44:193–8. [DOI] [PubMed] [Google Scholar]
- [14].Adamus G Autoantibody Targets and their Cancer Relationship in the Pathogenicity of Paraneoplastic Retinopathy. Autoimmun Rev, 2009;8:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Adamus G Latest updates on antiretinal autoantibodies associated with vision loss and breast cancer. Invest Ophthalmol Vis Sci, 2015;56:1680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol, 1996;78:120–9. [DOI] [PubMed] [Google Scholar]
- [17].Adamus G, Choi D, Raghunath A, Schiffman J. Significance of Anti-retinal Autoantibodies in Cancer-associated Retinopathy with Gynecological Cancers. J Clin Exp Ophthalmol, 2013;4:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol, 2004;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Adamus G, Bonnah R, Brown L, David L. Detection of autoantibodies against heat shock proteins and collapsin response mediator proteins in autoimmune retinopathy. BMC Ophthalmol, 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hsu SC, Molday RS. Glyceraldehyde-3-phosphate dehydrogenase is a major protein associated with the plasma membrane of retinal photoreceptor outer segments. J Biol Chem, 1990;265:13308–13. [PubMed] [Google Scholar]
- [21].Yang S, Dizhoor A, Wilson DJ, Adamus G. GCAP1, Rab6, and HSP27: Novel Autoantibody Targets in Cancer-Associated Retinopathy and Autoimmune Retinopathy. Trans Vis SciTech, 2016;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Adamus G Paraneoplastic Retinal Degeneration In: Levin L, and Albert DM, editor. Ocular Disease: Mechanisms and Management: Saunders Elsevier, Inc; 2010, p. 599–608. [Google Scholar]
- [23].ten Berge JC, van Rosmalen J, Vermeer J, Hellström C, Lindskog C, Nilsson P et al. Serum Autoantibody Profiling of Patients with Paraneoplastic and Non-Paraneoplastic Autoimmune Retinopathy. PLoS One, 2016;11:e0167909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hsu S-C, Molday RS. Glycolytic enzymes and a GLUT-1 glucose transporter in the outer segments of rods and cone photoreceptor cells. J Biol Chem, 1991;266:21745–52. [PubMed] [Google Scholar]
- [25].Lincet H, Icard P. How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions? Oncogene, 2015;34:3751–9. [DOI] [PubMed] [Google Scholar]
- [26].Adamus G Impact of Autoantibodies against Glycolytic Enzymes on Pathogenicity of Autoimmune Retinopathy and Other Autoimmune Disorders. Front Immunol, 2017;8:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun, 1998;11:671–7. [DOI] [PubMed] [Google Scholar]
- [28].Yamamoto T, Kudo M, Peng W-X, Takata H, Takakura H, Teduka K et al. Identification of aldolase A as a potential diagnostic biomarker for colorectal cancer based on proteomic analysis using formalin-fixed paraffin-embedded tissue. Tumor Biology, 2016;37:13595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bazhin AV, Schadendorf D, Willner N, Smet CD, Heinzelmann A, Tikhomirova NK et al. Photoreceptor proteins as cancer-retina antigens. Inter J Cancer, 2007;120:1268–76. [DOI] [PubMed] [Google Scholar]
- [30].Michels J, Becker N, Suciu S, Kaiser I, Benner A, Kosaloglu-Yalcin Z et al. Multiplex bead-based measurement of humoral immune responses against tumor-associated antigens in stage II melanoma patients of the EORTC18961 trial. OncoImmunol, 2018;7:e1428157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Donoso LA, Yamaki K, Merryman CF, Shinohara T, Yue S, Sery TW. Human S-antigen: characterization of uveitopathogenic sites. Curr Eye Res, 1988;7:1077–85. [DOI] [PubMed] [Google Scholar]
- [32].Kang DS, Tian X, Benovic JL. Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol, 2014;0:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bonnans C, Flacelière M, Grillet F, Dantec C, Desvignes J-P, Pannequin J et al. Essential requirement for β-arrestin2 in mouse intestinal tumors with elevated Wnt signaling. PANS, 2012;109:3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mazzola JL, Sirover MA. Subcellular localization of human glyceraldehyde-3-phosphate dehydrogenase is independent of its glycolytic function. Biochim Biophys Acta, 2003;1622:50–56. [DOI] [PubMed] [Google Scholar]
- [35].Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol, 2010;6:280–289. [DOI] [PubMed] [Google Scholar]