Abstract
Activated PI3Kδ syndrome (APDS) Type I results from gain-of-function mutations in PIK3CD, which encodes the p110δ subunit of PI3Kδ. Abnormal actin dynamics have been hypothesized to contribute to the lymphopenia associated with this disease but have not been studied in patients with APDS. We report a patient with APDS who had widespread necrotic skin lesions that were responsive specifically to immunosuppressive therapy. EBV-transformed lymphoblastoid cells (EBV-LCLs) from patients with APDS exhibit increased polymerized actin and increased apoptosis, suggesting a contribution of impaired actin dynamics to this disease.
Keywords: PI3Kδ, APDS, actin
The importance of cytoskeletal remodeling in adaptive immunity has been underscored by mutations in genes encoding protein that regulate actin dynamics, including Coronin 1A, Wiskott-Aldrich syndrome protein, DOCK8, and WD repeat containing protein 1 (WDR1) [1,2]. The formation of immune synapses and signaling, cellular migration, phagocytosis, and survival depend on a balance between actin in its free monomeric form and polymerized filaments (F-actin) [1,3]. PI3Kδ is a member of the class I phosphoinositide 3-kinases (PI3Ks), that activates downstream effectors the regulate actin dynamics, including the kinase Akt and Rac GTPases [4,5]. Heterozygous gain-of-function mutations in PIK3CD, which encodes the catalytic p110δ subunit of PI3Kδ that is preferentially expressed in hematopoietic cells, results in activated PI3Kδ syndrome (APDS). APDS is a primary immunodeficiency characterized by recurrent infections, lymphoproliferation, autoimmunity, defective T cell function, hypogammaglobinemia, and increased risk of malignancy [6–8]. The widely expressed PI3Kα isoform has been shown to regulate actin dynamics [9]. However, the impact of mutations in PIK3CD on actin polymerization in leukocytes has not been previously investigated. Here, we report a patient with APDS who had widespread inflammatory skin lesions and lymphocytes with an accumulation of cellular F-actin and increased apoptosis.
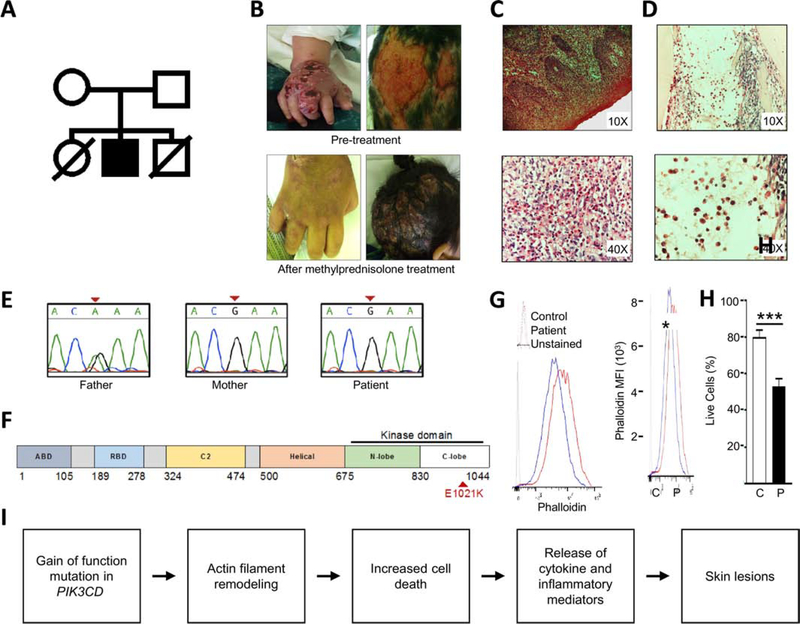
The patient is the daughter of healthy non-consanguineous Peruvian parents (Fig. 1A). She had two episodes of pneumonia within the first three months of life, followed by splenomegaly and lymphadenopathy. At four years of age, she developed inflammatory skin lesions characterized by pruritic, necrotic lesions on her scalp, extremities, torso, abdomen, and genitals (Fig. 1B, top panels). Skin biopsies revealed ulcers with an acute mixed inflammatory response and predominance of eosinophilic infiltrate in epidermis and dermis (Fig 1C), which grew P. aeruginosa, S. maltophilia, and methicillin-resistant S. aureus. The patient’s skin lesions did not improve after treatment with meropenem, vancomycin, cotrimoxazole, and fluconazole. Subsequent treatment with methylprednisolone led to rapid improvement of the lesions (Fig. 1B, bottom panels). Attempts to wean steroids led to recurrence.
Figure 1:
Characterization of patient phenotype. (A) Family pedigree. (B) Inflammatory skin lesions pre- and post-treatment with methylprednisolone. (C) Skin biopsy (hematoxylin & eosin; original magnification 10X and 40X) showing epidermis with hyperparakeratosis, acanthosis, papillomatosis, spongiosis and inflammatory infiltrate composed of neutrophils, lymphocytes and eosinophils. Dermis has mixed inflammatory infiltrate, with numerous eosinophils at magnification 40x. (D) Bone marrow biopsy (hematoxylin & eosin; original magnification 10X and 40X) showing myeloid predominance due to increased mature eosinophils. Bands, metamyelocytes and myelocytes are also observed. (E) Sanger sequencing of PIK3CD c.3061G>A, pGlu1021Lys variant. (F) Linear diagram of PIK3CD. Patient mutation in red. (G) F-actin content assessed by median fluorescent intensity of permeabilized BLCLs stained with phalloidin-FITC. N=2 pts and 2 controls in 2 independent experiments. (H) Cell death in BLCLs from 3 controls and 2 patients, pooled from 3 independent experiments. (I) Proposed mechanism by which dysregulated actin dynamics may contribute to APDS pathology. Columns and bars represent means +/− SEM. *p<0.05, *** p<0.001; Student’s t test.
The patient’s laboratory evaluation was notable for anemia, leukocytosis, eosinophilia, reduced numbers of B and NK cells, reduced numbers of naïve T cells, reduced numbers of recent thymic emigrants, and increased numbers of senescent CD57+ CD8 T cells (Table 1). Bone marrow biopsy revealed myeloid hyperplasia with predominance of eosinophils (Fig. 1D). As the patient had a brother who died from pneumonia at six weeks of age, and a sister with who died from pneumonia at two months of age (Fig 1A), a monogenic immune dysregulation syndrome was considered. Targeted next generation sequencing of 264 genes associated with immunodeficiency and immune dysregulation [10] identified the previously described pathogenic heterozygous mutation PIK3CD (c.3061G>A, pE1021K) (Fig 1E&F)[7]. The patient continues on IVIG and steroids and is undergoing evaluation for potential hematopoietic stem cell transplant. Rapamycin, an inhibitor of the PIK3/AKT/mTOR signaling pathway, reverses many of the clinical manifestations of APDS, but is not available in Peru.
Table 1.
Immunological profile of the patient at 4 years old.
| Patient (normal range) | |
|---|---|
| Hemogram | |
| Hemoglobin, g/dL | 7.8 (10.5 – 14.5) |
| WBCs, 103 cells/μL | 17.7 (5.5 – 15.5) |
| Neutrophils, 103 cells/μL | 11.0 (2.2 – 5.5) |
| Lymphocytes, 103 cells/μL | 3.4 (1.5 – 8.5) |
| Monocytes, 103 cells/μL | 0.4 (0 – 0.8) |
| Eosinophils, 103 cells/μL | 3.30 (0 – 0.65) |
| Platelets, 103 cells/μL | 112 (150 – 450) |
| Lymphocyte subsets | |
| CD3+, 103 cells/μL | 1.7 (0.7 – 4.5) |
| CD3+CD4+, 103 cells/μL | 0.92 (500 – 2400) |
| CD45RA+CD31+, % CD4+ | 11.2 (19.4 – 60.9) |
| CD45RA+CCR7+ %CD4 | 14.5 (65.2 – 85.8) |
| CD45RA+CCR7−, % CD4+ | 0.8 (0.2 – 3.0) |
| CD45RA−CCR7+, % CD4+ | 51.3 (2.9 – 9.8) |
| CD45RA−CCR7−, % CD4+ | 33.4 (10.5 – 23.2) |
| CD3+CD8+, 103 cells/μL | 0.67 (300 – 1600) |
| CD45RA+CCR7+, % CD8+ | 1.8 (39.0 – 89.0) |
| CD45RA+CCR7−, % CD8+ | 33.6 (4.8 – 30.0) |
| CD45RA−CCR7+, % CD8+ | 3.4 (3.4 – 28.2) |
| CD45RA−CCR7−, % CD8+ | 56.3 (0.9 – 5.7) |
| CD57+, %CD8+ | 58.2 (<44.3) |
| CD19+, 103 cells/μL | 0.111 (0.2 – 1.6) |
| CD27−IgD+, % CD19+ | 50.0 (76.3 – 84.9) |
| CD27+IgD+, % CD19+ | 6.2 (4.1 – 9.0) |
| CD27+IgD−, % CD19+ | 23.5 (3.3 – 7.4) |
| CD3−CD56+, 103 cells/μL | 0.037 (0.09 – 0.9) |
| Immunoglobulins | |
| IgG, mg/dL | 1602 (700 – 1600) |
| IgM, mg/dL | 200 (40 – 260) |
| IgA, mg/dL | 373 (70 – 400) |
Values in bold are outside of the reference range.
We measured the levels of F-actin in B-lymphoblastoid cell lines (B-LCLs) from the proband, as well as a second patient with the PIK3CDE102K mutation who had typical manifestations of APDS, including respiratory infections, hepatosplenomegaly, lymphopenia, and lymphadenopathy. Measuring F-actin content in B-LCLs eliminated the confounding effects of concomitant infections and medications in the patients. F-actin levels in the patients’ B-LCLs were increased compared to controls (Fig 1G). As the accumulation of F-actin is known to trigger apoptosis through a caspase-3-like protease-dependent pathway [3,11], we measured cellular viability of BLCLs. Patient-derived BLCLs exhibited increased apoptosis compared to healthy controls (Fig. 1H).
Patients with defects in WD repeat containing protein 1 (WDR1), a protein that regulates actin depolymerization, present with skins lesions similar to our patient. WDR1 deficiency overlaps with other clinical features typical of APDS, including respiratory infections, aberrant T cell activation, and B cell lymphopenia [2]. Due to defective actin depolymerization, WDR1-deficient patients have been reported to exhibit a four-fold increase in the F-actin content of leukocytes [2]. Increased F-actin content leading to cellular apoptosis can trigger an inflammatory response, leading to tissue damage, as was seen in our patient [3,12].
This report adds several notable findings to the expanding literature on APDS. Although increased susceptibility to skin infections has been described in patients with APDS [13], the rapid response of this patient to methylprednisolone demonstrates the importance of considering immunosuppressive therapies for inflammatory processes in patients with APDS, especially in low-resource settings. Prior studies have shown increased lymphocyte apoptosis in patients with APDS, but the underlying mechanisms are incompletely understood [7]. We suggest a model whereby increased F-actin contributes to lymphocyte apoptosis, leading to the release of inflammatory mediators and cytokines that culminate in inflammatory skin lesions (Fig. 1I).
Material and Methods
F-actin content and cell death
F-actin content and cell death were assessed by flow cytometry analysis. Patient B-lymphoblastoid cell lines were generated from PBMCs from patients and healthy controls. The cells were stained with fixable viability dye eFlour 506 (Invitrogen 65–0866-14), labeled with APC Annexin V (Biolegend 640919) and phalloidin-FITC (Sigma Aldrich P5282), and analyzed using a LSRFortessa (BD).
Statistical Analysis
Two-tailed Student t test was used to compare the differences between groups using the GraphPad PRISM software (GraphPad Software, La Jolla, Calif).
Highlights.
The PIK3CDE1021K mutation is associated with increased F-actin content and increased apoptosis in lymphocytes.
Patients with APDS may present with inflammatory skin lesions requiring immunosuppressive therapy.
Acknowledgments
Supported by Eleanor and Miles Shore 50th Anniversary Fellowship Award (C.D.P.), 1K08AI116979-01 (J.C.), 1R01AI139633-01(R.S.G.) and the Perkin Fund (R.S.G.).
Abbreviations
- PI3K
phosphoinositide 3-kinase
- APDS
Activated PI3Kδ syndrome
- PID
primary immunodeficiency
- BLCLs
B-lymphoblastoid cell lines
- MDM
Monocyte derived macrophages
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Moulding DA, Record J, Malinova D, Thrasher AJ, Actin cytoskeletal defects in immunodeficiency, Immunol. Rev 256 (2013) 282–299. doi: 10.1111/imr.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kuhns DB, Fink DL, Choi U, Sweeney C, Lau K, Priel DL, Riva D, Mendez L, Uzel G, Freeman AF, Olivier KN, Anderson VL, Currens R, Mackley V, Kang A, Al-Adeli M, Mace E, Orange JS, Kang E, Lockett SJ, Chen D, Steinbach PJ, Hsu AP, Zarember KA, Malech HL, Gallin JI, Holland SM, Cytoskeletal abnormalities and neutrophil dysfunction in WDR1 deficiency, Blood. 128 (2016) 2135–2143. doi: 10.1182/blood-2016-03-706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Desouza M, Gunning PW, Stehn JR, The actin cytoskeleton as a sensor and mediator of apoptosis, Bioarchitecture. 2 (2012) 75–87. doi: 10.4161/bioa.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cantley LC, The phosphoinositide 3-kinase pathway, Science. 296 (2002) 1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- [5].Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM, Cantley LC, Role of Phosphoinositide 3-Kinase Regulatory Isoforms in Development and Actin Rearrangement, Mol. Cell. Biol 25 (2005) 2593–2606. doi: 10.1128/mcb.25.7.2593-2606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nunes-Santos CJ, Uzel G, Rosenzweig SD, PI3K pathway defects leading to immunodeficiency and immune dysregulation, J. Allergy Clin. Immunol 143 (2019) 1676–1687. doi: 10.1016/j.jaci.2019.03.017. [DOI] [PubMed] [Google Scholar]
- [7].Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K, PI3Kδ and primary immunodeficiencies, Nat. Publ. Gr 16 (2016) 702–714. doi: 10.1038/nri.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wentink M, Dalm V, Lankester AC, van Schouwenburg PA, Schölvinck L, Kalina T, Zachova R, Sediva A, Lambeck A, Pico-Knijnenburg I, van Dongen JJM, Pac M, Bernatowska E, van Hagen M, Driessen G, van der Burg M, Genetic defects in PI3Kδ affect B-cell differentiation and maturation leading to hypogammaglobulineamia and recurrent infections, Clin. Immunol 176 (2017) 77–86. doi: 10.1016/j.clim.2017.01.004. [DOI] [PubMed] [Google Scholar]
- [9].Hu H, Juvekar A, Lyssiotis CA, Lien EC, Albeck JG, Oh D, Varma G, Hung YP, Ullas S, Lauring J, Seth P, Lundquist MR, Tolan DR, Grant AK, Needleman DJ, Asara JM, Cantley LC, Wulf GM, Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton, Cell. 164 (2016) 433–446. doi: 10.1016/j.cell.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abolhassani H, Chou J, Bainter W, Platt CD, Clinical, immunologic, and genetic spectrum of 696 patients with combined immunodeficiency, J. Allergy Clin. Immunol 141 (2017) 1450–1458. doi: 10.1016/j.jaci.2017.06.049. [DOI] [PubMed] [Google Scholar]
- [11].Odaka C, Sanders ML, Crews P, Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway, Clin. Diagn. Lab. Immunol 7 (2000) 947–952. doi: 10.1128/CDLI.7.6.947-952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rock KL, Kono H, The Inflammatory Response to Cell Death, Annu. Rev. Pathol. Mech. Dis 0 (2007) 70817085622001. doi: 10.1146/annurev.pathol.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Coulter TI, Chandra A, Bacon CM, Babar J, Curtis J, Screaton N, Goodlad JR, Farmer G, Steele CL, Leahy TR, Doffinger R, Baxendale H, Bernatoniene J, Edgar JDM, Longhurst HJ, Ehl S, Speckmann C, Grimbacher B, Sediva A, Milota T, Faust SN, Williams AP, Hayman G, Kucuk ZY, Hague R, French P, Brooker R, Forsyth P, Herriot R, Cancrini C, Palma P, Ariganello P, Conlon N, Feighery C, Gavin PJ, Jones A, Imai K, Ibrahim MAA, Markelj G, Abinun M, Rieux-Laucat F, Latour S, Pellier I, Fischer A, Touzot F, Casanova JL, Durandy A, Burns SO, Savic S, Kumararatne DS, Moshous D, Kracker S, Vanhaesebroeck B, Okkenhaug K, Picard C, Nejentsev S, Condliffe AM, Cant AJ, Clinical spectrum and features of activated phosphoinositide 3-kinase δ syndrome: A large patient cohort study, J. Allergy Clin. Immunol 139 (2017) 597–606.e4. doi: 10.1016/j.jaci.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]