Abstract
Context:
Specialized pediatric palliative care (SPPC) is increasingly involved in the care of seriously ill children, yet the evidence on its impact has not been comprehensively reviewed.
Objective:
To assess the effects of providing SPPC to seriously ill children on patient-, caregiver-, and systems-level outcomes.
Methods:
We performed a Systematic Review following Cochrane methods. Data Sources: Medline, Embase, PsycINFO, Global Health, The Cochrane Central Register of Controlled Trials, LILACS, and Web of Science were searched from January 1996 to June 2018. Study Selection/Data Extraction ; We included randomized controlled, cohort, case-control, and before-after studies in which exposure to SPPC services was the intervention of interest. All outcomes reported in these studies were included. Two investigators independently selected articles, extracted data, and assessed risk of bias of included studies using standardized criteria.
Results:
Twenty-four studies were included in qualitative synthesis: one non-randomized controlled trial, 16 cohort studies, and seven before-after studies. Evidence certainty was low. Twenty-one studies had ≥1 area with high risk of bias, most commonly selection bias, low group comparability, risk for confounding, and inadequate statistical reporting. Studies analyzed 46 domains, operationalized as 136 distinct outcomes. SPPC was associated with better child quality of life (QOL) scores in all four studies that assessed this outcome. No other outcome showed this consistency.
Conclusions:
Receiving SPPC was associated with better child QOL. However, the paucity and low certainty of the evidence precluded any firm recommendations about SPPC practice. Larger collaborative networks and greater consensus regarding SPPC research standards are needed.
PROSPERO Registration Number:
CRD42016049533
Keywords: Palliative care, pediatrics, outcome assessment, end of life, patient-reported outcomes, systematic review
Introduction
Specialized pediatric palliative care (SPPC) is an integrative model of care for children with life-threatening illnesses that aims to ease suffering, improve child and family quality of life (QOL), and support families in delineating their goals of care and making decisions accordingly.(1) For nearly two decades, the National Academy of Medicine and the American Academy of Pediatrics have recommended involvement of SPPC early in the illness course and concurrent with disease-directed therapies.(2,3) Recognized by the American Board of Medical Subspecialties since 2006 with subsequent formation of dedicated fellowship training programs,(4) SPPC has grown rapidly as a field over the last decade with increasing numbers of SPPC programs across the United States, particularly in academic hospital settings.(5) Nonetheless, significant heterogeneity exists between programs with regards to SPPC team composition, the spectrum of and availability of services provided, and funding.
Despite the recent growth of SPPC programs in hospitals nationwide, evidence-based research to guide standards of practice is lacking, deterring implementation of SPPC services.(6) Furthermore, there is no consensus as to which outcomes or outcome metrics should be used to evaluate the impact of SPPC. Two recent systematic reviews studied the instruments and indicators chosen to evaluate pediatric palliative care and identified substantial variability along with scarce use of valid child-reported measures.(7,8) Other reviews have focused on a particular SPPC model or a specific outcome domain and have revealed a small number of studies representing a scarce body of evidence.(9,10) These findings are similar to what has been reported in the adult palliative care literature, in which recent systematic reviews have also demonstrated significant methodologic limitations and outcome heterogeneity, with varying evidence of effects on patient-, family-, and systems-level outcomes.(11–17) To our knowledge, no review has reported on the full spectrum of SPPC intervention studies to date, including description of the exposure and impact and a critical assessment of the existing literature.
As part of the formative research contributing to the development of a complex SPPC intervention, we sought to systematically summarize and critically evaluate the available evidence on effects of providing SPPC to children with serious illnesses and their families on patient and health system outcomes.
Methods
The methodology for this review was informed by the Cochrane Collaboration’s Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist.(18) This review was registered with PROSPERO (http://www.crd.vork.ac.uk/PROSPERO/) under protocol registration number CRD42016049533.
Selection Criteria
We defined the following inclusion criteria: (1) study design: randomized controlled trials (RCTs), cohort studies, case-control studies, and before-after studies. Observational non-comparative studies (e.g., cross-sectional studies, case series) were excluded; (2) population: individuals eligible for SPPC care; no limitations were placed on patient age or patient diagnosis due to the heterogeneity of the population seen by SPPC teams; (3) intervention/exposure: receipt of SPPC services, defined as comprehensive care provided by individuals or teams that is distinct from and complementary to the services provided by the primary medical team. Thus, studies examining the impact of a singular component of pediatric palliative care (e.g., pain management) or primary palliative care interventions (e.g., advance care planning provided by clinicians not specialized in PPC), were excluded; also, studies that focused on a specific result (e.g., location of death), were excluded only if SPPC exposure was not the primary independent variable studied; (4) control: individuals receiving usual/standard care; (5) setting: inpatient, ambulatory, home, or inpatient hospice; (6) outcomes: although initially QOL was defined as the primary outcome, the scarcity of publications meeting this criterion led to the broadening of our search to include any type of outcome so as to better describe the range of effects represented in the literature; and (7) publication status and language: studies published in a peer-reviewed journal in English, Spanish, or French.
Search Strategy
We searched Medline, Embase, PsyclNFO, Global Health, The Cochrane Central Register of Controlled Trials, LILACS, and Web of Science (January 1996 to June 2018). In addition, we searched the following clinical trial registries through December 2018: ISCRTN (a registry of observational and interventional trials), clinicaltrials.gov, the WHO International Clinical Trials Registry Platform (IC-TRP), and the European registry. With input from the review team, a professional librarian (DC) formulated the search strategy and performed the search, including modification of the strategy based on initial results (see Appendix, Table A1 for complete search strategy). We manually reviewed reference lists of included studies and related systematic reviews to identify additional relevant studies.
Study Screening and Selection
For the screening and selection process we used the Early Review Organizing Software (EROS),(19) a web-based software program developed by the Cochrane Center at the Institute of Clinical Effectiveness and Health Policy in Buenos Aires, Argentina. EROS organizes the various phases of a systematic review and facilitates independent review of studies, discrepancy reconciliation, and risk of bias assessment.
Titles and abstracts were independently screened by pairs of co-authors. Two reviewers (KM and GS) independently screened full-text manuscripts of all potentially eligible references. If eligibility was unclear, we contacted study authors. Discrepancies were resolved at each stage through discussion and involvement of a third reviewer if necessary.
Data extraction
For each included article, we systematically collected the following variables using EROS: (1) study design; (2) setting; (3) study population (i.e., source, number of participants enrolled, diagnostic categories represented); (4) definitions of exposure and control; (5) outcomes and outcome measures; and (6) results.
Risk of Bias Assessment
Given that all but one of the studies generated by the search were observational studies, risk of bias was critically evaluated using an adapted version of the quality criteria outlined by Sanderson et al.,(20) including the following domains: 1) methods for selecting study participants; 2) methods for measuring exposure and outcome variables; 3) methods to control confounding; 4) statistical methods; 5) conflict of interest; and 6) comparability between groups, a domain added to better represent the risk of bias of included studies. For the single controlled trial identified, we additionally evaluated random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Regarding assessment of bias in the measurement of subjective outcomes (e.g., patient symptoms and quality of life), based on published recommendations(21) we developed a guide to evaluate risk of bias. We considered risk to be low when patient-reported outcomes were self-reported (or equivalent, depending on participant’s age/developmental status) through measures with proven validity, reliability and responsiveness, and we considered risk to be high when outcomes were not self-reported and instruments had not undergone any formal validation (see Appendix, Table A2 for more details). Two reviewers (KM and GS) independently assigned risk as “low,” “high,” or “unclear,” and a senior author (VD) reviewed all determinations and associated justifications. Disagreements were resolved through discussion with the full review team.
Synthesis
We planned to conduct a meta-analysis of marginal heterogeneity of study design, outcomes, and reporting of results. If not feasible, we planned to summarize results through a qualitative synthesis focused on outcomes and risk of bias. Outcomes were grouped into conceptual categories to summarize their measurement and reported effects. We decided to show the effects reported to describe potential trends, or lack thereof, that may aid future research efforts. Risk of bias was assessed by domain; we used graphs to summarize risk of bias across studies. As recommended by the Cochrane collaboration,(22) we did not estimate composite scores by paper. For domains that had more than one element (e.g., studies with more than one outcome in the same category), we assigned the highest risk level observed across elements.
Results
Description of Studies
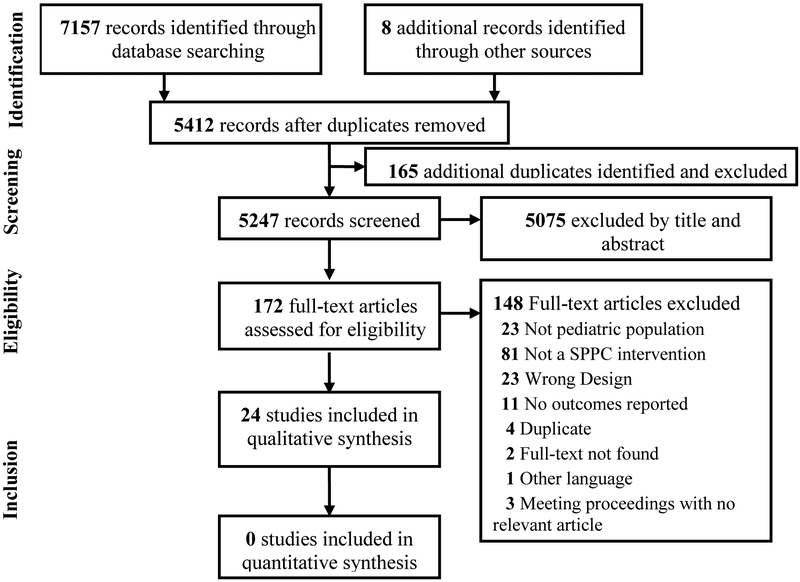
We identified 5412 individual articles by our search strategy and included twenty-four studies. The selection process and reasons for article exclusions are presented in Figure 1.
Figure 1: Flow Diagram of Study Selection Process.
Characteristics of included studies are presented in Table 1. The 24 publications spanned the years 2001–2018, with 79% (19/24) published in the last five years (2013 or later).(23–41) Sample sizes and participant characteristics varied significantly across studies. Greater than half of the studies (13/24) had a sample size of fewer than 100 subjects. (24–29,32,34,38–40,42,43) Fourteen studies exclusively represented the experience of decedents.(23,24,29–31,34,36–38,40,41,44–46) Out of the 21 studies that provided diagnostic information, nine included only patients with cancer,(23,24,29,34,37,40,41,45,46) two only patients with non-oncologic diagnoses,(39,43) and ten included patients with cancer or other diagnoses.(25–27,30,31,33,36,38,42,44) Fifteen studies conducted in the United States(24–26,30–33,35–37,39,40,42,44,45) reported the experience of ten SPPC teams and one was a large database analysis; three were based in England,(23,28,43) Germany(27,34) and Canada(29,41) had two publications each, and Israel(46) and Singapore(38) one.
Table 1:
Study Characteristics
| Author (Year) | Country | Study Design | Source Population | Participants | Diagnostic Categories | SPPC Characteristics | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Characteristics | Pt Symptoms, QOL | Caregiver | EOL Discussions | EOL Care Patterns | HC Utilization | Supportive Services | ||||||
| Chong (2018) | Singapore | RCoh | Hospital-based | N=138 (SPPC: 71; non-PPC: 67) | Pts diagnosed 1 month-19 years of age who died between 2012–2015 at home or hospital AND had received care at participating hospitals (SPPC group included 7 pts that were referred from non-participating hospitals) | Cancer (43%), Other (57%) | Home-based SPPC service consisting of 1 SPPC MD, 4 pediatric nurses, 2 social workers, and 1 administrative executive; provide home and inpatient consults; 24/7 support provided by a mixed adult-pediatric service | X | X | ||||
| Ub/a | SPPC Program-based | N=56 | Pts 0–19 years old referred to SPPC team during 2012–2015 period | Cancer (18%), Congenital defects (50%), cerebral palsy (13%), neurodegenerative disorders (14%), other (5%) | X | X | |||||||
| Fraser (2013) | England | RCoh | Population-based (county level) | N=443 (SPPC: 132; non-PPC: 311) | Pts 0–19 years old diagnosed between 1990–2009 (identified via cancer registry), who died prior to 08/2011 and who could be linked to hospital episode statistics data | Cancer (100%) | Hospice-based SPPC service; full-time SPPC consultant appointed in 2004; 24/7 on-call medical service; provide home visits and inpatient care | X | |||||
| Friedrichsdorf (2015) | US | RCoh | Hospital-based | N=60 (SPPC: 30; non-PPC: 30) | Bereaved parents of pts 0–17 years old who died between 2002–2008 | Cancer (100%) | Palliative home care and/or home hospice care; visits by SPPC nurses, social workers, child life specialists, chaplaincy, SPPC physician; providing SPPC in community, outpatient clinic, and inpatient setting; 24/7 on-call service | X | X | X | |||
| Gans (2015) | US | Ub/a | Population-based (county level) | N=93 | Primary caregivers of pts <21 years old who enrolled in SPPC program between 2010–2012, who were deemed likely to be hospitalized for >30 days in next year | Neurologic/neuromuscular (44%), respiratory failure (12%), transplant-related complications (12%), cancer (13%). other (19%) | SPPC program contracting with nurses and social workers at multiple hospice and home health agencies across 11 counties; access to 24/7 on-call nurse | X | |||||
| Golan (2008) | Israel | RCoh | Hospital-based | N=246 (historic cohort: 144; intervention cohort: 102) | Historic cohort: pts who died of cancer between 1990–1999 and received care at the participating pediatric oncology department Intervention cohort: pts who died of cancer between 1999–2005 and received care at the participating pediatric oncology department |
Cancer (100%) | Oncology-based SPPC team; full-time SPPC nurses; provide ambulatory and inpatient care (SPPC unit), home visits, and bereavement follow-up; they work with interdisciplinary oncology staff which includes oncologists, social workers, psychologists, creative art/child life specialists, nutritionists, physical therapists, and spiritual advisor. Not all pts in intervention cohort were necessarily exposed to SPPC | X | |||||
| Goldhagen (2016) | US | Ub/a | SPPC Program-based | N=40 | Pts 0–18 years old enrolled in SPPC who had documented hospital admissions during 2 years prior to and first 2 quarters after enrollment between 2002–2006 | Neurologic (27%), congenital anomalies (27%), neoplasms (13%), symptoms/signs unspecified (9%), circulatory system (6%), injury/poisoning 16%). other 112%) | Community-based SPPC program providing medical, nursing, social work, child life, spiritual, and volunteer services | X | |||||
| Groh (2013) | Germany | Ub/a | SPPC Program-based | N=40 | Primary caregivers of pts newly admitted to SPPC service between 2011–2012 | Neurologic (33%), cancer (25%), congenital anomalies (20%), metabolic (18%), cardiovascular (5%) | Home-based SPPC consisting of 3 pediatricians, 2 nurses, social worker, chaplain, all trained in SPPC; 24/7 on-call service | X | X | ||||
| Gupta (2013) | England | Ub/a | SPPC Program-based | N=23 | Parents of pts referred to SPPC program between 2005–2007 | Unknown - authors present diagnostic categories for all referrals, not subset comprising study cohort | Community-based SPPC program consisting of nurses, clinical psychology, palliative care support workers, cultural development worker, bereavement support worker | X | |||||
| Hancock(2018) | US | RCT | Hospital-based | N=40 (intervention arm: 18; control arm: 20) | Mothers of neonates with single-ventricle heart disease admitted for first-stage palliative surgery referred during pregnancy to pediatric cardiology between 04–2013 and 08–2015 | Single ventricle heart disease (100%) | Hospital-based SPPC consisting of 1 physician, nurse practitioner, nurse, and social worker all trained and certified in SPPC | X | |||||
| Hays (2006) | US | Ub/a | Hospital-based | N=41 | Parents of pts 0–21 years old with severe illness that might result in death within 24 months and who were referred to SPPC program over 2-year period | Neurologic (44%), cancer (34%), congenital anomalies (10%), metabolic (5%), cardiovascular (5%), respiratory (2%) | Hospital-based multidisciplinary team (consisting of physicians, advanced practice nurses, social worker, benefits coordinator) that implemented 3 major program components: 1) clinical decision-making; 2) provider education on pain and symptom management, EOL care, and ethical decision-making; and 3) flexible administration of benefits and co-case management | X | X | ||||
| Horrocks (2002) | England | Ub/a | SPPC Program-based | N=16 | Parents of pts who were referred to SPPC service 5 months after service was established, excluded parents of pts who were “close to death” at time of referral | Degenerative (38%), neurologic (25%), circulatory (13%) congenital anomalies (13%), metabolic (6%), other (6%) | Community-based service consisting of 3 community pediatric nurses and 2 part-time child psychologists, supported by hospital-based consultants and senior nurse managers | X | |||||
| Kassam (2015) | Canada | RCoh | Hospital-based | N=75 (SPPC: 42; non-PPC: 33) | Parents of pts who died between 2005–2011, with death occurring >4 weeks after diagnosis | Cancer (100%) | Hospital-based SPPC team, no further details provided. Exposure defined based on parent report and confirmed through medical record review | X | X | X | |||
| Keele (2013) | US | RCoh | Population-based (national level) | N=24,342 (SPPC: 919; non-PPC: 23,423) | Pts <18 years old who died >5 days after admission between 2001–2011 from 40+ children’s hospitals comprising Children’s Hospital Association (secondary analysis of Pediatric Health Information System data) | Neonatal (41%), respiratory (14%), circulatory (11%), neurologic (8%), lymphatic/hematopoietic/malignancy (6%), gastrointestinal (6%), infectious disease (5%), other (11%). 85% classified as complex chronic | Hospital-based SPPC characteristics vary by site. Exposure defined by ICD-9 code for SPPC services during final hospital admission | X | X | ||||
| Osenga (2016) | US | RCoh | Hospital-based | N=114 (SPPC: 28; non-PPC: 86) | Pts 0–18 years old who were treated for >24 hours prior to death and who died while inpatient between 2012–2013 | Neonatal (50%), trauma/other (27%), cardiovascular (15%), hematology/oncology (8%) | Hospital-based SPPC (same as Friedrichsdorf (2015) | X | X | X | |||
| Petteys (2015) | US | PCoh | Hospital-based | N=22 (SPPC: 6; non-PPC: 16) | Parents of pts in NICU who had anticipated stay of >2 weeks | Unknown | Hospital-based SPPC service consisting of advanced practice nurse and registered nurse with extensive NICU experience, both trained in SPPC; additional medical support as needed | X | |||||
| Pierucci (2001) | US | RCoh | Hospital-based | N=196 (SPPC: 25; non-PPC: 171) | Pts <1 year old at time of death and who died while inpatient between 1994–1997 | Cardiovascular (39%), trauma (11%), congenital anomalies (10%), gastrointestinal (7%), respiratory (7%), neonatal (7%), infectious disease (6%), genetic/chromosomal (5%), myopathy (3%), metabolic (2%), dehydration (2%), cancer (1%) | Hospital-based SPPC consultation service consisting of 2 clinical nurse specialists and a physician medical director | X | X | X | |||
| Postier (2014) | US | Ub/a | SPPC Program-based | N=425 | Pts 1–21 years old who initiated home- or hospice-based SPPC service between 2000–2010 | Cancer (47%), non-cancer (53%) | Hospital-based SPPC (same as Friedrichsdorf (2015) | X | |||||
| Schmidt (2013) | Germany | RCoh | Population-based (state/province level) | N=96 (historic cohort: 48; intervention cohort: 48) | Historic cohort: parents of pts who died of cancer between 1999–2000 and were seen at a participating oncology department Intervention cohort: parents of pts who died of cancer between 2005–2006 and were seen at a participating oncology department |
Cancer (100%) | No details provided on specific SPPC state teams. Authors describe a series of national initiatives (policies, laws, academic/educational, SPPC service standards) that helped increase the provision of SPPC nationwide (>pediatric oncology departments offering SPPC, >SPPC home care programs). These changes took place in the early 2000s, between historic and intervention cohorts. Not all patients in intervention cohort were necessarily exposed to SPPC | X | X | X | X | X | |
| Smith (2015) | US | RCoh | Hospital-based | N=902 (SPPC: 86; non-PPC: 816) | Pts discharged in 2010 who represent top decile of inpatient costs | Unknown | Hospital-based SPPC service consisting of medical director, advanced practice nurse, registered nurse, social worker, interfaith chaplain | X | |||||
| Snaman (2017) | US | RCoh | Hospital-based | N=69 (SPPC: 50; non-PPC: 19) | Adolescents and young adults with cancer who died at the hospital between 2008–2014 | Cancer(100%) | Hospital-based SPPC team, no further details provided. SPPC exposure defined based on medical record data | X | X | X | |||
| Ullrich (2016) | US | RCoh | Hospital-based | N=147 (SPPC: 37; non-PPC: 110) | Pts who underwent stem cell transplant for any indication and died between 2004–2012 | Hematologic malignancy (65%), solid/brain tumor (15%), non-malignancy (20%) | Hospital-based SPPC team, no further details provided (same as Wolfe, 2008) | X | X | X | |||
| Vern-Gross (2015) | US | RCoh | Hospital-based | N=191 (historic cohort: 134; intervention cohort: 57) | Historic cohort: pts <21 years old who died between 2001 2005, prior to establishment of SPPC service Intervention cohort: pts <21 years old who died between 2007–2012 and received SPPC consultation prior to death |
Cancer (100%) | Hospital-based SPPC program began as pilot program in 2007, expanded into institution-wide program available to all pts beginning in 2008; provide inpatient consultations and ongoing SPPC and hospice support for pts and families if transferred to home institutions | X | X | X | |||
| Widger (2017) | Canada | RCoh | Population-based (state/province level) | N=572 (SPPC: 166; PPC: 100; non-PPC: 306) | Nested cohort of a deceased cohort of children with cancer diagnosed at <15 years old who died between 2000 and 2012 <18 years. Patients were included if they received care at institutions with SPPC and died at a time when a clinical SPPC database was available | Cancer (100%) | Hospital-based SPPC, no further details provided about teams. Exposure levels: SPPC: patients registered in one of the SPPC databases: PPC: patients with ≥2 physician billing or inpatient diagnostic codes compatible with PC provision (no need of PC training); in both cases first PC contact had to occur > 30 days prior to death | X | X | ||||
| Wolfe (2008) | US | RCoh | Hospital-based | N=221 (historic cohort: 102; intervention cohort: 119) | Historic cohort: parents of pts who died between 1990–1997, prior to establishment of SPPC service Intervention cohort: parents of pts who died between 1997–2004, following establishment of SPPC service |
Cancer(100%) | Hospital-based SPPC team providing inpatient, outpatient, and home-based consultations and ongoing education about SPPC to pediatric oncology practitioners; team consists of physician, nurse, social worker. Not all pts in intervention cohort received SPPC consultation (approximately half) | X | X | X | X | X | |
Abbreviations: EOL - end of life; HC - healthcare; Pt(s) - patient(s); QOL - quality of life; SPPC -specialized pediatric palliative care; PPC - general pediatric palliative care provision; non-PPC - no pediatric palliative care; NICU - neonatal intensive care unit; ICD-9 - International Classification of Diseases, Ninth Revision; US - United States; PCoh - prospective cohort; RCoh - retrospective cohort; Ub/a - uncontrolled before/after; RCT - randomized control trial.
Notably, only one controlled trial (non-randomized) was identified in the search.(39) A majority of studies (16/24) used a cohort design: one prospective(32) and 15 retrospective;(23,24,29–31,34–38,40,41,44–46) the remaining were uncontrolled before/after studies.(25–28,33,38,42,43) One study used two designs: a retrospective cohort and an uncontrolled before/after.(38) Half of the studies were survey-based,(24,25,27–29,32,34,38,39,42,43,45) and only one of these used a patient-reported outcome; the other survey studies used outcomes reported by parents or primary caregivers.
Description of Exposure/Intervention groups
Regarding the SPPC exposure/intervention, 19 out of 24 studies evaluated the impact of single SPPC programs (characteristics described in Table 1), whereas five described the impact of SPPC from a population-based (i.e. county, state/province, national) perspective.
Descriptions of the intervention or exposure under study often emphasized different components and rarely included enough detail about team composition, the setting in which the team worked, or the team’s activities and frequency, rendering comparison difficult. Only three studies thoroughly described these four characteristics, either in the methods section or as part of their results.(24,25,36,39,44) Five studies did not provide any description of the team, the setting where care was provided, or their activities or frequency; three of these were population-based studies.(29,30,34,40,41) See Appendix, Table A3 for more on description of the intervention/exposure.
Most control groups consisted of “usual care,” which was operationalized as: “late or no SPPC consultation” (controlled trial), “no receipt of SPPC” (most cohort studies), and “pre-exposure” (before/after studies). Three studies used historic cohorts to examine hospital-wide(45,46) or statewide(34) systemic effects of introducing a SPPC team.
Outcomes
Across the 24 studies, 46 outcome domains and 136 outcomes were examined, representing significant heterogeneity. For ease of discussion and synthesis, we grouped outcomes into six conceptual categories. Most studies (n=14, 58%) reported on more than one outcome category and only three(23,35,39) identified a primary outcome. Therefore, we report on and evaluate risk of bias for all outcomes with equal emphasis. Outcomes are discussed in the context of their conceptual groupings. For details about individual outcomes and reported effect sizes, please refer to Table 2.
Table 2:
Outcomes Reported and Summary of Findings
| Outcome Categories | Specific Outcome | Study | Outcome Measurement | Effect Size (SPPC vs. comparison group or post-exposure to SPPC vs. pre-exposure to SPPC; arrow indicates direction of significant results) |
|---|---|---|---|---|
| Pt Symptoms/QOL | ||||
| Prevalence of symptoms at EOL | Symptom prevalence during LMOL | Friedrichsdorf (2015) | SCCC | Most symptoms NS; ↑ Constipation: 70% vs. 36%, p=0.01 |
| Schmidt (2013) | SCCC (German) | Most symptoms NS; ↑ Nausea: 65% vs. 42%, p=0.024 | ||
| Wolfe (2008) | SCCC | NS | ||
| During LMOL, number of documented: 1) Symptoms 2) Refractory symptoms 3) Physical symptoms 4) Psychosocial symptoms |
Snaman (2017) | Medical record review | 1) NS 2) NS 3) NS 4) NS |
|
| Documentation of symptom prevalence in last 3 days of life | Osenga (2016) | Medical record review | NS | |
| Prevalence of suffering at EOL | “A great deal/a lot” of suffering due to [symptoms] during LMOL | Friedrichsdorf (2015) | SCCC | Most symptoms NS; ↑ Energy loss/fatigue: 93% vs. 63%, p=0.007 |
| Schmidt (2013) | SCCC (German) | Most symptoms NS; ↑ Fatigue: 50% vs. 25%, p=0.01 | ||
| Wolfe (2008) | SCCC | Most symptoms NS; ↓ Pain: RD 19%, p=0.018; Dyspnea: RD 21%, p=0.020 | ||
| “A great deal/a lot” of overall suffering during LMOL | Friedrichsdorf (2015) | SCCC | NS | |
| Perceived success of symptom management | Perceived success of treatment of [symptoms] in LMOL | Friedrichsdorf (2015) | SCCC | NS |
| Schmidt (2013) | SCCC (German) | NS | ||
| Perceived success of symptom control, measured during first week of SPPC referral and at 6 months (presented as median score) | Groh (2013) | Ad hoc parent survey items (1–10 numeric rating scale) | ↑ 9.0 (IQR 2) vs. 5.0 (IQR 3), p<0.000056 | |
| PtQOL | 1) Report of child having had “some” to “a great deal” of fun during LMOL 2) Report of child having experienced an event that added meaning to his/her life during LMOL |
Friedrichsdorf (2015) | SCCC | 1) ↑ 70% vs. 45%, p=0.03 2) ↑ 89% vs. 63%, p-0.02 |
| Health-related QOL measured at study baseline, 3, 6, and 12 months | Chong (2018) | Health Utilities Index (self-report) | Most attributes NS; ↑ Pain-free at 3 months: OR 2.58 (95% CI: 1.12, 5.95) |
|
| HRQOL, measured at SPPC enrollment and 3 months later: 1) Total score 2) Emotional score 3) Physical score 4) Social score 5) School score 6) Psychosocial health summary score (average of emotional, social, and school functioning scores) (presented as mean scores) |
Hays (2006) | PedsQL™ 4.0, parent version (items transformed to a 0–100 scale) | 1) NS 2) ↑ 62.73 vs. 52.52 (mean difference +10.21), effect size 0.60, p=0.021 3) NS 4) NS 5) NS 6) NS |
|
| Child QOL, measured at first week of SPPC and 6 months (presented as median score) | Groh (2013) | Ad hoc parent survey items (1–10 numeric rating scale) | ↑ 4.0 (IQR 4) vs. 2.5 (IQR 2), p<0.000056 | |
| Caregiver Outcomes | ||||
| Caregiver worry | Worry about ability to manage child’s health (5-point Likert-type response option), measured at SPPC enrollment and at 6 and 12 months (presented as change [in points] from one timepoint to the next) | Gans (2015) | Ad hoc parent survey item | ↓ −0.26 (95% CI: −0.05, −0.47), p<0.05 |
| Caregiver stress | Stress (5-point Likert-type response option), measured at SPPC enrollment and at 6 and 12 months (presented as change [in points] from one timepoint to the next) | Gans (2015) | Ad hoc parent survey item | ↓ −0.26 (95% CI: −0.04, −0.48), p<0.05 |
| Stress, measured at SPPC enrollment and 12 months later | Gupta (2013) | PSI-SF | NS | |
| Stress, measured at study enrollment, 2 weeks after enrollment, at discharge | Petteys (2015) | PSS:NICU | NS | |
| Diagnosis of clinical or subclinical acute stress disorder, measured at study enrollment, 2 weeks after enrollment, at discharge | Petteys (2015) | SASRQ | NS | |
| Caregiver anxiety/depression | Anxiety and depression, measured at first week of SPPC and 6 months (presented as median score) | Groh (2013) | HADS | ↓ 19.0 (IQR 6) vs. 28.0 (IQR 8.5), p<0.001 |
| Measured at baseline and after discharge or 30 days after first stage palliative surgery: 1) Maternal depression (mean change) 2) Maternal anxiety (mean change) |
Hancock(2018) | 1) Beck Depression Inventory-ll 2) State-Trait Anxiety Index |
1) NS 2) ↓ −7.6 vs. 0.3, p=0.02 |
|
| Anxiety, measured at enrollment in SPPC and 6 months later | Horrocks (2002) | State-Trait Anxiety Index | NS | |
| Caregiver burden | Caregiver burden, measured at study baseline and at 3, 6 and 12 months (presented as mean score difference compared to baseline) | Chong (2018) | Zarit Burden Interview | 3 months: ↓ 3.4 (95% CI 1.0 – 5.9); 6 months: ↓ 4.0 (95% CI 0.5 – 7.5); 12 months: NS |
| Caregiver burden, measured at first week of SPPC and 6 months (presented as median score) | Groh (2013) | HPS | ↓ 14.5 (IQR 8.8) vs. 20.0 (IQR 10.5), p<0.001 | |
| Caregiver coping | Maternal coping, measured at baseline and after discharge or 30 days after first stage palliative surgery 1) Adaptive coping (mean scores) 2) Problematic coping (mean scores) |
Hancock(2018) | Brief Cope Inventory | 1) ↑ 6.3 (±1.4) vs. 4.9 (±2.0), p=0.03 (for positive reframing subscale) 2) NS |
| Caregiver psychological wellbeing | Psychological wellbeing, measured at SPPC enrollment and 12 months later | Gupta (2013) | GHQ12 | NS |
| Caregiver QOL | QOL, measured at first week of SPPC and 6 months later (presented as median score) | Groh (2013) | QOLLTI-F | ↑ 7.1 (IQR 1.3) vs. 5.8 (IQR 1), p<0.001 |
| Parent-reported HRQOL (measured at baseline and after discharge or 30 days after first stage palliative surgery (mean ± standard deviation): 1) Physical functioning 2) Emotional functioning 3) Social functioning 4) Cognitive functioning 5) Communication 6)Worry Parent-reported family functioning: 7) Daily activities 8) Family relationships |
Hancock(2018) | PedsQL Family Impact Module | 1) NS 2) NS 3) NS 4) NS 5) ↑ 11.3 (± 16.9) vs. 1.0 (± 23.7), effect size: 0.46 6) NS 7) NS 8) ↑ 5.0 (± 17.2) vs. −2.6 (± 17.5), effect size: 0.41 |
|
| Four domains of QOL, measured at SPPC enrollment and 6 months later: 1) Mental role limitation 2) Mental health 3) Energy/vitality 4) Health perceptions (presented as mean score, higher score=better QOL) |
Horrocks (2002) | SF-36 | 1) ↑ 51.6 vs. 31.2, mean difference 20.3 (95% CI: −4.0, −36.6), p=0.02 2) NS 3) NS 4) NS |
|
| Caregiver satisfaction with care | Satisfaction with various domains of care, measured at first week of SPPC and at 6 months | Groh (2013) | Ad hoc parent survey items (1–10 numeric rating scale) | ↑ Differences between median scores (from before to after) for items reaching significance range +1.0 to +8.0, p values range p<0.002778 to p<0.000056 |
| Satisfaction with various domains of care, measured at SPPC enrollment and 3 months later | Hays (2006) | Ad hoc parent survey items | ↑ Mean differences (from before to after) for items reaching significance range +0.43 to +1.42, p values range 0.000–0.048 | |
| Satisfaction with care, measured at discharge | Petteys (2015) | Ad hoc parent survey item | Statistical comparison not feasible due to low response rate | |
| Satisfaction with child’s EOL care | Schmidt (2013) | SCCC (German) | NS | |
| Caregiver preparedness at EOL | Whether healthcare team prepared parents for medical aspects surrounding death | Kassam (2015) | SCCC + ad hoc items | ↑ 63% vs. 36%, p=0.02 |
| 1) Parents felt “very prepared” for EOL medical problems 2) Parents felt “very prepared” for circumstances at time of death |
Wolfe (2008) | SCCC | 1) ↑ 56% (95% CI: 54, 57) vs. 27% (95% CI: 26, 28), p<0.001 2) ↑ 49% (95% CI: 49, 51) vs. 25% (95% CI: 24, 26), p=0.002 |
|
| Caregiver abilty to plan location of death | Parent able to plan child’s location of death | Friedrichsdorf (2015) | SCCC | NS |
| Parent control over location of death | Kassam (2015) | SCCC + ad hoc items | NS | |
| EOL Discussions | ||||
| Frequency/timing of discussion regarding ACP | Proportion of patients who had an ACP in place or an ACP discussion | Chong (2018) | Medical record review | ↑ OR = 5.51 (95% CI: 1.55, 19.67) |
| Frequency/timing of discussion regarding prognosis | 1) Documented discussion regarding prognosis 2) Median number of days between discussion regarding prognosis and death |
Ullrich (2016) | Medical record review | 1) ↑ 97% vs. 83%, p=0.04 2) ↑ 8 days (IQR 4–26) vs. 2 days (IQR 1–13), p<0.001 |
| 1) Median number of EOL discussions per pt 2) Median number of days between first EOL discussion and enrollment in hospice 3) Median number of days between first EOL discussion and DNR order 4) Median number of days between first EOL discussion and death 5) Median number of EOL discussions (before/after analysis among subset who received SPPC) |
Vern-Gross (2015) | Medical record review | 1) ↑ 12 (IQR 7.5–15.5) vs. 3 (IQR 2–4), p<0.001 2) ↑ 225 (IQR 36–442) vs. 18 (IQR 2–98), p<0.001 3) ↑ 195 (IQR 20–421) vs. 2 (IQR 0–35), p<0.001 4) ↑ 268 (IQR 57–474) vs. 64 (IQR 11–164), p<0.001 5) ↑ 1 (IQR 0–2) vs. 0 (IQR 0–1), p=0.026 |
|
| 1) Documented discussion about no realistic chance for cure 2) Number of days between discussion about no realistic chance for cure and death |
Wolfe (2008) | Medical record review | 1) NS 2) NS |
|
| Frequency/timing of discussion about hospice or palliative home care | 1) Whether family was informed about possibility of palliative home care 2) Whether family perceived that timing of information was appropriate |
Schmidt (2013) | SCCC (German) | 1) ↑ 79% vs. 33%, p=0.003 2) ↑ 81% vs. 42%, p=0.042 |
| Median number of discussions about transition to home/hospice (before/after analysis among subset who received SPPC) | Vern-Gross (2015) | Medical record review | ↑ 2 (IQR 1–4) vs. 1 (IQR 0–1), p=0.001 | |
| 1) Hospice discussion documented 2) Number of days between discussion about hospice and death (outcome presented as geometric mean) |
Wolfe (2008) | Medical record review | 1) ↑ 76% (95% CI: 73, 79) vs. 54% (95% CI: 51, 57), p<0.001 2) ↑ 52 days (95% CI: 41, 65) vs. 28 days (95% CI: 21, 38), p=0.002 |
|
| Frequency/timing of discussion regarding resuscitation status | Discussion of “no CPR” order with parents by healthcare team | Kassam (2015) | SCCC + ad hoc items | NS |
| 1) Discussions regarding resuscitation status 2) Median number of days between discussion regarding resuscitation status and death |
Ullrich (2016) | Medical record review | 1) ↑ 88% vs. 58%, p=0.002 2) ↑ 7 (IQR 3–18) vs. 2 (IQR 1–5), p<0.001 |
|
| DNR discussion documented | Wolfe (2008) | Medical record review | NS | |
| Frequency/timing of discussion regarding death and dying | 1) Discussion of death and dying with parents by healthcare team 2) Discussion of death and dying with child by healthcare team when appropriate 3) Provision of guidance to parents on how to talk to child about death and dying when appropriate |
Kassam (2015) | SCCC + ad hoc items | 1) ↑ 85% vs. 46%, p<0.01 2) ↑ 61% vs. 4%, p<0.01 3) ↑ 62% vs. 13%, p<0.01 |
| Median number of discussions focused on the “high likelihood of death” (before/after analysis among subset who received SPPC) | Vern-Gross (2015) | Medical record review | ↓ 1 (IQR 1–3) vs. 2.5 (IQR 1.5–4), p=0.003 | |
| Frequency/timing of discussion regarding withdrawal of life-sustaining therapies | Median number of discussions regarding transition of care/withdrawal of life support (before/after analysis among subset who received SPPC) | Vern-Gross (2015) | Medical record review | NS |
| Patterns of EOL Care | ||||
| Location of death | Home death | Friedrichsdorf (2015) | SCCC | ↑ 93% vs. 20%, p<0.001 |
| Schmidt (2013) | SCCC (German) | NS | ||
| 1) Pediatricward death | Golan (2008) | Administrative database and medical record review | ↓ 4% vs. 40%, p<0.001 | |
| 2) Oncology ward death | ↓ 8% vs. 26%, p<0.001 | |||
| 3) ICU death | NS | |||
| 4) Home death | ↓ 16%vs. 28%, p<0.003 | |||
| 5) Palliative care unit death | 66% (post PPC) | |||
| ICU death | Keele (2013) | Administrative database review | ↓ 60% vs. 88%, RR 0.67 (95% CI: 0.64, 0.72) | |
| Pierucci (2001) | Medical record review | ↓ 68%vs. 97%, p<0.001 | ||
| Snaman (2017) | Medical record review | ↓ 19 (38%) vs. 13 (68%), p=0.024 | ||
| Schmidt (2013) | SCCC(German) | NS | ||
| Ullrich (2016) | Medical record review | ↓ 58% vs. 80%, p=0.03 | ||
| ICU or outside hospital death | Wolfe (2008) | Medical record review (missing values [n=5] imputed using parental report) | ↓ 22% (95% CI: 19, 26) vs. 38% (95% CI: 35, 42), p=0.024 | |
| In-hospital death | Widger (2018) | Pediatric Oncology Group of Ontario Networked Information System (POGONIS) | ↓ OR 0.3 (0.2 to 0.4), p<0.001 | |
| Procedures/interventions at EOL (including CPR, intubation, blood draws, imaging, fluids, etc.) | Various interventions during final admission before death | Keele (2013) | Administrative database review | ↑/↓ RR ranged 0.14–2.8 (non-invasive ventilation and intracranial pressure monitoring or extraventricular device more common in intervention group; all others more common in control group) |
| 1) Documentation of ≥1 diagnostic or monitoring procedure in last 48 hours of life 2) Documentation of various interventions in last 48 hours of life |
Osenga (2016) | Medical record review | 1) ↓ 61% vs. 92%, OR 0.16 (95% CI: 0.04, 0.61) 2) ↓ OR ranged 0.07–0.18 for significant findings; several procedures/interventions were NS |
|
| Various interventions in last 48 hours of life | Pierucci (2001) | Medical record review | ↓ p<0.001 for significant findings; several procedures/interventions were NS | |
| During LMOL: 1) MV (reported as n (%)) 2) Number of medical procedures (presented as median) 3) Other interventions: CPR, dialysis |
Snaman (2017) | Medical record review | 1)↓ 17 (34%) vs. 12 (63%), p=0.028 2)↓ 1 (IQR 0–3) vs. 3 (IQR 2–4), p=0.009 3) NS |
|
| 1) Intubation in last 24 hours of life 2) CPR attempted at EOL |
Ullrich (2016) | Medical record review | 1) ↓ 42%vs. 66%, p=0.02 2) ↓ 3% vs. 20%, p=0.03 | |
| Various interventions between time of DNR order and death | Vern-Gross (2015) | Medical record review | ↑ Fluids: 75% vs. 40%, p=0.001; other interventions NS | |
| 1) MV within 14 days of death 2) High intensity EOL care | Widger (2018) | Pediatric Oncology Group of Ontario Networked Information System | 1) ↓ OR 0.2 (0.1–0.5), p<0.001 2) ↓ OR 0.2 (0.1 – 0.3), p<0.001 |
|
| Intubation in last 24 hours of life | Wolfe (2008) | Medical record review | NS | |
| Medication use at EOL | Use of various medications in last 4 days of life | Keele (2013) | Administrative database review | ↓ RR ranged 0.15–0.53 for analgesics, sedatives, paralytics, antibiotics, and inotropics |
| Orders placed for various medications in last 3 days of life | Osenga (2016) | Medical record review | NS | |
| Use of various medications in last 48 hours of life | Pierucci (2001) | Medical record review | ↓ Paralytics: 8% vs. 41%, p<0.001; other medications NS | |
| During LMOL: 1) Use of chemotherapy 2) Opioids 3) Benzodiazepines |
Snaman (2017) | Medical record review | 1) NS 2) NS 3) NS |
|
| Use of various medications between DNR order and death | Vern-Gross (2015) | Medical record review | ↑ Antibiotics: 69% vs. 43%, p=0.008; steroids: 63% vs. 22%, p=0.001; anti-convulsants: 40% vs. 6%, p=0.001; other medications NS | |
| Integrative medicine therapies at EOL | Orders placed for integrative medicine therapies in last 3 days of life | Osenga (2016) | Medical record review | NS |
| Actions to manage specific | Documentation of specific action to manage 1) pain and 2) non-pain symptoms in last 3 days of life | Osenga (2016) | Medical record review | 1) ↑ OR ranged 3.14 (95% CI: 1.08, 9.16) to 6.51 (95% CI: 1.92, 22.12) |
| symptoms at EOL | Receipt of treatment for various symptoms in LMOL | Schmidt (2013) | SCCC (German) | ↑ Anxiety: 36% vs. 9%, p=0.035; other symptoms NS |
| Pain assessments at EOL | Documentation of pain assessments in final 3 days of life (measured in 12-hour increments) | Osenga (2016) | Medical record review | ↑ RR 1.57 (95% CI: 1.16, 2.10) for last 12–24 hours of life; other 12-hour increments NS |
| Frequency/timing of documentation of resuscitation status | DNR documented prior to death | Osenga (2016) | Medical record review | ↑ 79% vs. 53%, OR 7.92 (95% CI: 2.02, 31.12) |
| Pierucci (2001) | NS | |||
| Snaman (2017) | NS | |||
| Ullrich (2016) | ↑ 97% vs. 68%, p-0.002 | |||
| Vern-Gross (2015) | ↑ 84% vs. 47%, p<0.0001 | |||
| Wolfe (2008) | NS | |||
| Days between DNR order and death | Snaman (2017) | Medical record review | ↑ 6 (IQR 2–21) vs. 2 (IQ Rl-4), p - 0.008 (median) | |
| Wolfe (2008) | ↑ 18 (95% CI: 13, 25) vs. 12 (95% CI: 9, 15), p=0.031 (geometric mean) | |||
| Withdrawal of life-sustaining | 1) Documentation of life-sustaining therapy withdrawn 2) Documentation of nutritional support withdrawn |
Osenga (2016) | Medical record review | 1) NS 2) NS |
| therapies or nutritional support | Withdrawal of vasopressors, feedings, intravenous fluids, mechanical ventilation | Pierucci (2001) | Medical record review | NS |
| Withholding of life-sustaining therapies or nutritional support | Withholding of vasopressors, feedings, intravenous fluids, mechanical ventilation | Pierucci (2001) | Medical record review | ↑ Withholding of vasopressors: 56% vs. 13%, p<0.001; withholding of mechanical ventilation: 28% vs. 4%, p<0.001; others NS |
| Timing of discontinuation of disease-directed therapy | Time before death that cancer-directed therapy was discontinued | Wolfe (2008) | Medical record review | NS |
| Documentation of pt/family emotional status prior to death | Documentation of emotional status of families by physicians and nurses | Pierucci (2001) | Medical record review | NS |
| Healthcare Utilization | ||||
| Hospital admissions | Hospital admissions over 12 months (mean difference) | Chong (2018) | Medical record review | ↓ 2.46 (95% 0:0.43, 4.48) |
| Between referral to SPPC and death (or proxy date and death for control group): 1) Total hospital admissions (“main outcome”) 2) Unplanned hospital admissions 3) Planned hospital admissions |
Fraser (2013) | Review of linked databases for hospital episode statistics | 1) NS 2) NS 3) ↓ IRR 0.60 (95% CI: 0.43, 0.85) |
|
| Hospital admissions in 12 months preceding SPPC enrollment vs. 12 months following SPPC enrollment | Postier (2014) | Administrative database review | NS | |
| Hospital admissions among: 1) Those who died within 2 years of 2010 discharge 2) Those who survived 2 years following 2010 discharge |
Smith (2015) | Administrative database review | 1) ↓ RR 0.5 (95% CI: 0.28, 0.91) 2) ↑ RR 2.1 (95% CI: 1.3, 3.4) |
|
| Hospital length of stay | 1-year cumulative length-of-stay (mean difference) | Chong (2018) | Medical record review | ↓ 52.3 days (95% CI: 25.44, 79.17) |
| Hospital length of stay: quarterly means calculated from Quarters 3 and 4 of 2005 and Quarters 1–4 of 2006 before and after enrollment in SPPC | Goldhagen (2016) | Administrative database review | ↓ 1.22 days/quarter (SE 0.39) vs. 2.92 days/quarter (SE 0.94), p<0.05 | |
| Mean length of stay (days) in 12 months preceding SPPC enrollment vs. 12 months following SPPC enrollment | Postier (2014) | Administrative database review | ↓ 19 (± 34) vs. 34 (± 60), Z = −6.175, p<0.001 | |
| Median length of stay (days) for: 1) 2010 (all pts) 2) Those who died during 2010 admission 3) Those who died within 2 years of 2010 discharge 4) Those who survived 2 years following 2010 discharge |
Smith (2015) | Administrative database review | 1) ↑ 37 vs. 26, p=0.002 2) NS 3) ↓ 0 (IQR 0–26.1) vs. 11.9 (IQR 3.6–61.2), p=0.010 4) ↑ 9.1 (IQR 0–31.4) vs. 0 (IQR 0–6.0), p<0.001 |
|
| ED Visits | Number of Emergency Department (ED) visits | Chong (2018) | Medical record review | NS |
| ICU admissions | Admission to ICU | Keele (2013) | Administrative database review | ↓ RR 0.29 (95% CI: 0.26, 0.32) |
| PICU admissions for: 1) 2010 (all pts) 2) Those who died within 2 years of 2010 discharge 3) Those who survived 2 years following 2010 discharge |
Smith (2015) | Administrative database review | 1) ↑ OR 4.8 (95% 0:1.7, 13.5) 2) NS 3) ↑ 47% vs. 17%, p<0.001 |
|
| ICU admission within 30 days of death | Widger (2018) | Pediatric Oncology Group of Ontario Networked Information System (POGONIS) | ↓OR 0.2 (0.1–0.4), p<0.001 | |
| Days spent in ICU | Days spent in ICU during final admission before death | Pierucci (2001) | Medical record review | ↓ 6 days (± 8) vs. 14 days (± 28), p=0.01 |
| Hospital costs | Difference in healthcare expenditure between patients who received PPC and those on standard care (reported as mean incremental cost) in their final year of life at: 1) 1 month prior to death 2) 3 months prior to death 3) 6 months prior to death 4) 12 months prior to death |
Chong (2018) | Medical record review | 1) ↓ -$72K (95% CI: -$76K, -$69K) 2) ↓ -$116K (95% CI: -$121K, -$111K) 3) ↓ -$151K (95% CI: -$159K, -$143K) 4) ↓ -$175K (95% CI: -$186K, -$164K) |
| 1) Total charges and 2) diagnostic charges: quarterly means calculated from Quarters 3 and 4 of 2005 and Quarters 1–4 of 2006 before and after enrollment in SPPC | Goldhagen (2016) | Administrative database review | 1) NS 2) NS |
|
| Average total charges in 12 months preceding SPPC enrollment vs. 12 months following SPPC enrollment | Postier (2014) | Administrative database review | ↓ 10.91 (± 21.3) vs. 20.97 (± 43.3), Z = −6.581, p<0.001 ($10,000 per unit) | |
| Median inpatient cost/cost-per-day for: 1) 2010 (all pts) 2) Those who died during 2010 admission 3) Those who died within 2 years of 2010 discharge 4) Those who survived 2 years following 2010 discharge 5) Subgroup who received SPPC (before/after analysis; secondary outcome) |
Smith (2015) | Administrative database review | 1) ↑ Total cost: $138, 168 vs. $90,791, p=0.000; Cost/day: $3,755 vs. $3,404, p=0.035 2) Total cost: NS; ↓ Cost/day: $4,260 (IQR 3,071–5,663) vs. $5,945 (IQR 4,735–8,940), p=0.001 3) Total cost: NS; Cost/day: NS 4) Total cost: NS; ↑ Cost/day: $2,965 (IQR 0–4,295) vs. $0 (IQR 0–2,956), p<0.001 5) ↓ Cost/day: $3,625 vs. $4,732, p<0.0001 |
|
| Involvement of Supportive Services | ||||
| Pain team consultation | Pain team involvement at EOL | Wolfe (2008) | Medical record review | NS |
| Social work | Involvement of a religious or spiritual mentor for: 1) Parent 2) Child, when appropriate |
Kassam (2015) | SCCC + ad hoc items | 1) NS 2) NS |
| Referral during EOL period | Osenga (2016) | Medical record review | ↓ 71% vs. 99%, OR 0.04 (95% CI: 0.01, 0.36} | |
| Pierucci (2001) | ↑ 80% vs. 30%, p<0.001 | |||
| Spiritual support | Involvement of a religious or spiritual mentor for: 1) Parent 2) Child, when appropriate |
Kassam (2015) | SCCC + ad hoc items | 1) NS 2) NS |
| Chaplaincy consultation during EOL period | Osenga (2016) | Medical record review | NS | |
| Pierucci (2001) | ↑ 64% vs. 23%, p<0.001 | |||
| Ethics committee | Ethics consultation during EOL period | Osenga (2016) | Medical record review | NS |
| Psychology | Psychology consultation during EOL period | Osenga (2016) | Medical record review | NS |
| Home care Hospice | Whether child received palliative home care Frequency of hospice involvement |
Schmidt (2013) | SCCC (German) Medical record review |
↑ 65% vs. 33%, p=0.007 |
| Ullrich (2016) | NS | |||
| Vern-Gross (2015) | ↑ 71%vs. 46%, p=0.002 | |||
| Time between hospice enrollment and death | Vern-Gross (2015) | Medical record review | NS | |
| Sibling support | Receipt of sibling support | Kassam (2015) | SCCC + ad hoc items | ↑ 58% vs. 26%, p=0.01 |
| Vern-Gross (2015) | Medical record review | ↑ 50% vs. 16%, p<0.0001 | ||
| Bereavement support | Receipt of bereavement support | Vern-Gross (2015) | Medical record review | ↑ 96% vs. 50%, p<0.0001 |
Abbreviations: ACP- advanced care planning; CPR - cardiopulmonary resuscitation; DNR - do-not-resuscitate order; EOL - end of life; GHQ 12 - General Health Questionnaire Short Version (parent-report); HADS - Hospital Anxiety and Depression Scale (parent-report); HPS - Hausliche Pflegeskala (home care scale) (parent-report); HUI - Health Utilities Index II and III (child/teen self-report); ICU -intensive care unit; IQR - interquartile range; IRR - incidence rate ratio; NS - non-significant; OR - odds ratio; PedsQL™ 4.0 - Pediatric Quality of Life Inventory™, Version 4.0 (parent-report); PICU - pediatric intensive care unit; PSI-SF - Parenting Stress Index, Short Form (parent-report); PSS:NICU - Parental Stressor Scale: NICU (parent-report); Pt(s) - patient(s); QOL - quality of life; QOLLTI-F - Quality of Life in Life-Threatening Illness-Family Carer Version (parent-report); RD - risk difference; RR - relative risk; SASRQ - Stanford Acute Stress Reaction Questionnaire (parent-report); SCCC - Survey About Caring for Children with Cancer (parent-report); SE - standard error; SF-36 - Short Form-36 (parent-report); SPPC - specialized pediatric palliative care; 95% Cl - 95% confidence interval.
Patient symptoms and QOL
Eight of the 24 studies reported symptom and/or QOL outcomes. Six of these studies evaluated patient symptoms using three outcome domains operationalized in seven different ways. None of the studies used validated patient-reported outcome measures (PROMs), although three used parent-report via the Survey about Caring for Children with Cancer (SCCC), which is a commonly used but not formally validated survey.(47) No clear patterns emerge with regards to effects of SPPC on patient symptoms.
Patient QOL was included as an outcome domain in four studies. Each study utilized a different outcome measure and different timeframe. One study used child-report (using the Health Utilities Index), while the rest assessed caregiver-reported QOL. All studies found that SPPC exposure was associated with better scores in at least one QOL sub-domain when compared to controls, as shown in Table 2.
Caregiver outcomes
One of the most commonly studied outcome categories, included in half of the studies, was the impact of SPPC involvement on caregivers. Studies looked at ten different domains, all measured through caregiver self-report. Only half used validated questionnaires and two studies used ad hoc survey items only. Timeframes of interest varied between studies, with some focusing on the end-of-life (EOL) period and others focusing on the time during which SPPC was involved in a child’s care.
Few studies measured the same outcome, with a total of 26 outcomes reported, rendering it difficult to identify any patterns. Most studies reported at least one nonsignificant result. Five studies reported significant results in all outcomes assessed, all in the expected direction (i.e., better caregiver outcomes in the setting of exposure to SPPC).
EOL discussions
Six studies evaluated the impact of SPPC on EOL discussions, the least frequently evaluated category. A total of 24 outcomes were used to operationalize six domains. The most frequent data source for this category was the medical record, except for two studies that used parent-report (SCCC). Each study used a different outcome measure and/or timeframe.
More consistent results were observed for discussions about hospice/palliative home care, death and dying, and prognosis, which occurred more frequently and earlier among families exposed to SPPC.
Patterns of EOL care
Eleven studies included outcomes on patterns of EOL care, with nine studies including location of death as one of the outcome domains. Over 37 different outcomes were used to operationalize 11 outcome domains, many differing in their timeframe. All studies relied on medical record or administrative database review except two, which used parent-report with SCCC.
Of the nine studies examining location of death, all but one showed that patients receiving SPPC were less likely to die in the intensive care unit and/or more likely to die at home or in the palliative care unit. In addition, SPPC exposure was more consistently associated with earlier documentation of do-not-resuscitate orders and receipt of less invasive interventions and procedures; use of pain medication was not associated with exposure to SPPC.
Healthcare utilization
Healthcare utilization was operationalized as six dimensions examined in eight studies. All studies relied on review of administrative databases or medical records. There was significant heterogeneity as to how the periods and populations of interest were defined, with 33 different outcome measures used to evaluate these domains. No substantial consistencies were observed among these results.
Utilization of supportive services
Seven studies examined whether the use of supportive services was impacted by SPPC exposure. In addition, the provision of sibling support and bereavement support were also included in this category. Five studies relied on medical record documentation and two on parent-report via SCCC. All studies focused on children who had died.
Here too, each specific outcome was examined by few studies. No consistent findings emerged with regards to the involvement of supportive services. On the other hand, the two studies that investigated sibling support reported greater support among those exposed to SPPC.
Risk of Bias Assessment
The overall quality of the studies was low. Figure 2 (a and b) graphically depicts the risk of bias assessment results for the 24 studies. Justifications for each bias domain assessment can be found in the Appendix, Table A4.
Figure 2: Risk of Bias Graphs of the 24 Included Studies.
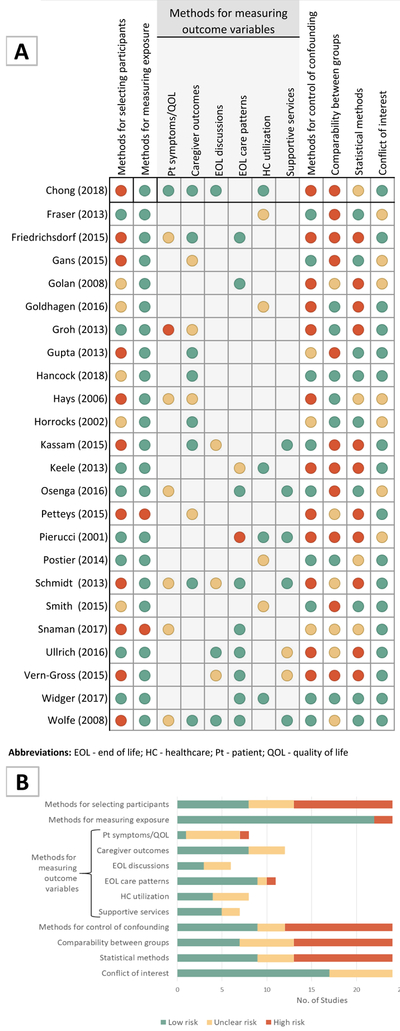
A. Risk of bias summary by study; B. Risk of bias summary by assessed item.
Abbreviations: EOL - end of life; HC - healthcare; Pt - patient; QOL - quality of life
The only controlled trial identified(39) was non-randomized and also had high risk of selection bias (allocation by date of birth/admission date) and performance bias (unblinded), but low risk of attrition and selective outcome reporting bias. Other potential sources of bias for this study are reported in the following sections and in Figure 2.
Participant selection
Almost half of the studies were considered to have high risk of participant selection bias, while only a third were categorized as low risk. Most significant limitations came from the study design. Observational, retrospective cohort, and before/after studies could not control how participants were allocated to groups. Reasons for being in the exposure group (SPPC) were often not clearly delineated and seemingly subject to provider preference. In addition, response rates for survey-based studies were variable, with several studies limited by low response rates (either initially or over time, when multiple surveys were involved), and reporting of response rates was non-standardized across studies (i.e., whether response rates were based on the number of potential participants who could be contacted or all potentially eligible participants), rendering their interpretation difficult. In several survey-based studies, there was no comparison of responders to non-responders, limiting judgment of selection bias and generalizability of the findings. Furthermore, several studies of parent-reported outcomes had cohorts that were predominantly female, educated, and/or white, limiting diversity of perspectives and experiences described.
Measurement of exposure and outcome variables
Nearly all studies had clear methods for measuring exposure to the SPPC team and thus were determined to be low risk.
With regards to measurement of outcome variables, the category that contributed the most significant risk of bias was the “patient symptoms and QOL” category: one study had high risk (caregiver-report using a non-validated tool) and six unclear risk (caregiver-report using validated measures when self-report was appropriate). Caregiver outcomes carried less bias; over two thirds of the studies used self-report and validated measures. In the case of the EOL discussions and health care utilization categories, risk of bias was related to risk for misclassification, use of partially validated tools (e.g., SCCC), use of non-validated imputation methods, and limitations with estimating costs. Finally, measurement of EOL care patterns and utilization of supportive services rated low on the risk of bias spectrum.
Confounding control
In only nine of the 24 studies, authors adequately controlled for potential confounding through multivariate analysis or regression modeling. In half of the studies, authors did not perform (or performed an incomplete) multivariate analysis, leading to a high risk for confounding bias. Three studies were assigned an unclear risk given that it was not possible to rule out confounding bias due to either small sample or a strict exploratory nature.
Comparability between groups
In eleven studies, the risk of bias due to poor comparability between SPPC and non-SPPC groups was high and related to important baseline differences, >20% attrition between baseline and follow-up, and changes in SPPC utilization over time that were not accounted for in analysis. In six studies, the risk of comparability bias was unclear due to lack of description of group differences, very small samples, and inability to account for secular trends in two of the historic cohorts.
Statistical methods
Only nine studies were considered to have low potential for bias introduced by the statistical methods used. Studies with high or unclear risk frequently had more than one limitation. Most did not report uncertainty measures (e.g., confidence intervals). Other problems identified included small samples, high attrition, multiple testing, no report on effect sizes, failure to use appropriate methods, and lack of description of statistical methods.
Discussion
The aim of this review was to summarize and critically assess the available evidence about the effects of SPPC exposure on children with serious illnesses and their families, regarding a broad spectrum of outcomes. Due to the variability of study design and study populations, the inconsistency in timing and characteristics of the SPPC exposure, and the heterogeneity of outcomes assessed, meta-analysis across studies was not feasible and any conclusions drawn from the data are limited. Nonetheless, the findings of this review provide a comprehensive assessment of the current state of the field and identify several important lessons to guide future SPPC research.
The need for high-quality intervention research in SPPC has long been identified as a national priority.(2) However, no published or completed RCTs or large prospective observational or multisite studies specifically evaluating the impact of SPPC were identified by this review. Except for two, all included studies were retrospective or uncontrolled before/after studies with strong methodological limitations; many had a poor description of the exposure and unclear or high risk for selection and confounding bias. Non-randomized designs, especially retrospective ones, are attractive because they are relatively feasible and involve lower costs. However, the ability to draw definitive conclusions from such studies is very limited. These designs are prone to different sources of bias, one of the most salient being the impossibility of balancing the distribution of known and, more importantly, unknown potential confounders among groups.(48) Only a few of the studies included in this review used bias amelioration strategies, such as prospective design, a concurrent control group, or adjustment by baseline factors and confounders during analysis. To move the field forward, we need to focus our efforts on the design and conduct of studies that provide evidence of a high level of certainty.
In recent years, palliative care studies among seriously ill adults have included a growing number of RCTs that, despite some methodological limitations, are bridging the knowledge gap.(49) Unfortunately, this experience cannot be directly applied to pediatrics. Children in need of SPPC are a small and heterogeneous population,(50,51) limiting the viability of single site RCTs. In addition, SPPC services are highly diverse,(5) research funding continues to be scarce,(52) and there seems to be a limited number of trained scientists.(53) Given this context, cluster randomized trials emerge as an ideal design to study SPPC interventions. However, because a high number of clusters are required to reach an effective sample size,(54) costs may be prohibitive. Instead, well-designed multisite parallel RCTs, Sequential Multiple Assignment Randomized Trial (SMART) trials,(55) or large multisite prospective observational studies may still help generate the evidence the field needs.(56,57) These studies will only be possible through research collaboratives and strong interdisciplinary work that incorporates statisticians and social scientists. Two such networks have been developed and have published foundational work.(58–60)
This analysis also highlights the need for authors to thoroughly describe the intervention/ exposure under scrutiny. The Equator Network CONSORT (Consolidated Standards of Reporting Trials) and STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statements(61,62) call for providing a detailed depiction of interventions/ exposures, including their main characteristics and “dose,” to allow studies to be replicable. A clear majority of the studies included in this review did not provide information pertaining to the team’s composition, let alone types of activities or frequencies. Even when there may be wide variation among teams, or perhaps because of this, it is crucial to provide a detailed account of what SPPC encompasses, as it can help identify core subspecialty components that may be worth replicating.
We also confirmed another key challenge identified by prior systematic reviews,(7,8), that is the heterogeneity and low consensus around outcome measurement. Results from this review raise several questions regarding which outcomes are being measured and how. First, the finding that caregiver factors and patterns of EOL care were the most frequent outcomes reported led us to wonder about the main drivers of outcome selection. Based on the studies included, it seems that outcome choice is influenced by feasibility and accessibility over relevance or responsiveness. In support of this hypothesis, we observed that while alleviation of suffering and improvement of QOL are considered the essence of SPPC,(1) only one third of identified studies used these as measures of effectiveness, and only one study used PROMs. Interestingly, even with all the methodological limitations described, child QOL was one of the few outcomes to show potential responsiveness to SPPC exposure. The infrequent use of symptoms, suffering, and QOL as endpoints can be explained by the challenges related to eliciting these outcomes directly from pediatric patients, including ethical concerns about burdening a vulnerable population (50) and the paucity of valid, reliable, responsive, and age-adapted outcome measures,(63) among others. An additional barrier to more generalized use of PROMs in research studies lies in the fact that these outcomes are still under scrutiny and are often considered to have a “high risk of bias,”(48) even when there has been a call to consider PROMs “reliable outcomes.”(21) Over the past 20 years, due to growing awareness of the importance of self-report and limitations of parent and clinician proxy-reports,(63) along with an increasing number of validated tools in children,(8) approaches are gradually changing, raising hope for incorporating the child’s voice as part of routine care and research.(58,64) In addition, the methods used in this paper to appraise the risk of bias of PROMs may further encourage the use of such outcomes. Notably, caregiver experience and EOL care patterns remain meaningful outcomes in SPPC research. However, the palette of outcomes should be chosen a priori based on the target of the intervention or exposure under study.
Another salient consideration pertains to the types of outcomes we are measuring. For example, is it correct to consider the outcomes from the “EOL discussions” domain as endpoints, i.e. the result of SPPC delivery? Alternatively, should they more correctly be considered processes or intermediate measures, i.e. tasks that are inherent to SPPC delivery? In this review, none of the papers provided this distinction. If used as process measures, the link to an expected endpoint should be demonstrated,(65) such as what has been reported for “planning location of death” or “early awareness about a child’s unrealistic chance of cure.” These have been associated with outcomes such as parental reports of feeling prepared for and comfortable with the place of death, lower intubation rates, and less use of cancer-directed therapy, among others.(66,67) The explicit description of the type of outcomes chosen will increase rigor and provide more ground to develop a theoretical framework of SPPC delivery.
Finally, many of the outcomes reported focused on the EOL period, which makes sense from a historical perspective given how the subspecialty developed.(47,68) However, as the scope of SPPC is changing and is being integrated earlier into care, it is important to develop outcomes that can account for the impact of early SPPC on seriously ill children and their families.
Limitations and Strengths
One limitation was that we were unable to perform a meta-analysis due the heterogeneity mentioned above. Also, our search strategy did not exhaustively look for grey literature, resulting in a risk for publication bias and the possibility that some studies may have been missed. Importantly, no abstracts were excluded because of language, suggesting that the risk for language restrictions is low. Recent systematic reviews that were similar in scope(7,8) did not capture any other papers that would have been included in this review. Moreover, given that we did not identify any completed clinical trials in existing trial databases, it seems unlikely that, even if we missed studies, the results of the review would change significantly.
On the other hand, this is one of the first systematic reviews to evaluate the existing evidence about the impact of SPPC and thoroughly assess its risk of bias using standardized criteria.(20) As such, we hope that these results will assist the design and conduct of future palliative care research in seriously ill children and their families.
Conclusions
Given the existing evidence is scarce and provides a low level of certainty, no firm recommendations can be made about the impact of SPPC in the care of seriously ill children. However, child QOL seems to emerge as a promising outcome. To overcome barriers to conducting high quality research, we need to strengthen and broaden existing collaborative research networks, conduct multicentered prospective studies, and reach greater consensus regarding meaningful and measurable outcomes.
Supplementary Material
Acknowledgments
We are grateful to Maria Victoria Riso, Kins. for her work on abstract revision and to Mg. Maria Laura Requena and Luciano Uzal, Anth. for their assistance and careful feedback with manuscript development.
Funding
This work was supported by an NIH/NINR grant (1R01 NR016720–01) and the Neil Samuel Ghiso Fellowship for Compassionate Medical Care. Sponsors had no role in the design and conduct of the study, analysis of the data, or preparation of the manuscript.
Abbreviations
- DNR
do-not-resuscitate
- EOL
end of life
- EROS
Early Review Organizing Software
- ICU
intensive care unit
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROMs
patient-reported outcome measures
- RCT
randomized controlled trial
- SCCC
The Survey about Caring for Children with Cancer
- SPPC
specialized pediatric palliative care
- QOL
quality of life
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Disclosures of Potential Conflicts of Interest:
All authors have completed and submitted the Disclosure of Potential Conflict of Interests forms. KM, GS, DC, and AC indicated no potential conflicts of interest, whereas JW and VD indicated potential academic conflict of interest as they are authors of four and one of the included articles respectively. Of note, they did not participate in data extraction of papers where authorship involved potential conflict.
References
- 1.World Health Organization: WHO Definition of Palliative Care [Internet]. [cited 2017 Jun 30]. Available from: http://www.who.int/cancer/palliative/definition/en/
- 2.Field MJ, Behrman RE, Institute of Medicine (U.S.). Committee on Palliative and End-of-Life Care for Children and Their Families When children die: improving palliative and end-of-life care for children and their families. Washington, D.C.: National Academy Press; 2003. xx, 690 p. [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics. Committee on Bioethics and Committee on Hospital Care. Palliative care for children. Pediatrics. 2000. August; 106(2 Pt 1):351–7. [PubMed] [Google Scholar]
- 4.ABMS BOARD CERTIFICATION REPORT 2015–2016. American Board of Medical Specialties. [Internet]. [cited 2018 Mar 15]. Available from: http://www.abms.org/media/131568/2015-16-abmscertreport.pdf
- 5.Feudtner C, Womer J, Augustin R, Remke S, Wolfe J, Friebert S, et al. Pediatric palliative care programs in children’s hospitals: a cross-sectional national survey. Pediatrics. 2013. December;132(6): 1063–70. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq E, Rost M, Pacurari N, Eiger BS, Wangmo T. Aligning guidelines and medical practice: Literature review on pediatric palliative care guidelines. Palliat Support Care. 2017. January 9; 1–16. [DOI] [PubMed] [Google Scholar]
- 7.Widger K, Medeiros C, Trenholm M, Zuniga-Villanueva G, Streuli JC. Indicators Used to Assess the Impact of Specialized Pediatric Palliative Care: A Scoping Review. J Palliat Med [Internet]. 2018. December 11 [cited 2019 Jan 16]; Available from: https://www.liebertpub.com/doi/10.1089/jpm.2018.0420 [DOI] [PubMed] [Google Scholar]
- 8.Friedel M, Aujoulat I, Dubois A-C, Degryse J-M. Instruments to Measure Outcomes in Pediatric Palliative Care: A Systematic Review. Pediatrics. 2019. January; 143(1):e20182379. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell S, Morris A, Bennett K, Sajid L, Dale J. Specialist paediatric palliative care services: what are the benefits? Arch Dis Child. 2017. October;102(10):923–9. [DOI] [PubMed] [Google Scholar]
- 10.Conte T, Mitton C, Trenaman LM, Chavoshi N, Siden H. Effect of pediatric palliative care programs on health care resource utilization and costs among children with life-threatening conditions: a systematic review of comparative studies. CMAJ Open. 2015. March;3(1):E68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmenlund K, Sjøgren P, Nordly M. Specialized palliative care in advanced cancer: What is the efficacy? A systematic review. Palliat Support Care. 2017. June 13; 1–17. [DOI] [PubMed] [Google Scholar]
- 12.Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ. 2017. July 4;j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of Specialized Palliative Care: A Systematic Review. JAMA. 2008. April 9;299(14):1698. [DOI] [PubMed] [Google Scholar]
- 14.Kassianos AP, loannou M, Koutsantoni M, Charalambous H. The impact of specialized palliative care on cancer patients’ health-related quality of life: a systematic review and meta-analysis. Support Care Cancer [Internet]. 2017. September 20 [cited 2017 Nov 4]; Available from: http://link.springer.eom/10.1007/s00520-017-3895-1 [DOI] [PubMed] [Google Scholar]
- 15.Haun MW, Estel S, Rücker G, Friederich H-C, Villalobos M, Thomas M, et al. Early palliative care for adults with advanced cancer. Cochrane Pain, Palliative and Supportive Care Group, editor. Cochrane Database Syst Rev [Internet]. 2017. June 12 [cited 2017 Nov 4]; Available from: http://doi.wiley.eom/10.1002/14651858.CD011129.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative Care Interventions for Patients with Heart Failure: A Systematic Review and Meta-Analysis. J Palliat Med. 2017. January;20(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brereton L, Clark J, Ingleton C, Gardiner C, Preston L, Ryan T, et al. What do we know about different models of providing palliative care? Findings from a systematic review of reviews. Palliat Med. 2017. October;31(9):781–97. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015. January 1;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glujovsky D, Bardach A, Garcia Marti S, Comande D, Ciapponi A. PRM2 EROS: A new software for early stage of systematic reviews. Value Health. 2011;14(7):A564. [Google Scholar]
- 20.Sanderson S, Tatt ID, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007. June;36(3):666–76. [DOI] [PubMed] [Google Scholar]
- 21.Johnston BC, Patrick DL, Busse JW, Schünemann HJ, Agarwal A, Guyatt GH. Patient-reported outcomes in meta-analyses--Part 1 : assessing risk of bias and combining outcomes. Health Qual Life Outcomes. 2013. July 1;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011. October 18;343(oct18 2):d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraser LK, van Laar M, Miller M, Aldridge J, McKinney PA, Parslow RC, et al. Does referral to specialist paediatric palliative care services reduce hospital admissions in oncology patients at the end of life? BrJ Cancer. 2013. April 2; 108(6): 1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrichsdorf SJ, Postier A, Dreyfus J, Osenga K, Sencer S, Wolfe J. Improved quality of life at end of life related to home-based palliative care in children with cancer. J Palliat Med. 2015. February;18(2): 143–50. [DOI] [PubMed] [Google Scholar]
- 25.Gans D, Hadler MW, Chen X, Wu S-H, Dimand R, Abramson JM, et al. Impact of a Pediatric Palliative Care Program on the Caregiver Experience: J Hosp Palliat Nurs. 2015. December;17(6):559–65. [Google Scholar]
- 26.Goldhagen J, Fafard M, Komatz K, Eason T, Livingood WC. Community-based pediatric palliative care for health related quality of life, hospital utilization and costs lessons learned from a pilot study. BMC Palliat Care. 2016. August 3;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groh G, Borasio GD, Nickolay C, Bender H-U, von Lüttichau I, Führer M. Specialized pediatric palliative home care: a prospective evaluation. J Palliat Med. 2013. December;16(12):1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta V, Prescott H. “That must be so hard” --examining the impact of children’s palliative care services on the psychological well-being of parents. Clin Child Psychol Psychiatry. 2013. January;18(1):91–9. [DOI] [PubMed] [Google Scholar]
- 29.Kassam A, Skiadaresis J, Alexander S, Wolfe J. Differences in end-of-life communication for children with advanced cancer who were referred to a palliative care team. Pediatr Blood Cancer. 2015. August;62(8):1409–13. [DOI] [PubMed] [Google Scholar]
- 30.Keele L, Keenan HT, Sheetz J, Bratton SL. Differences in characteristics of dying children who receive and do not receive palliative care. Pediatrics. 2013. July;132(1):72–8. [DOI] [PubMed] [Google Scholar]
- 31.Osenga K, Postier A, Dreyfus J, Foster L, Teeple W, Friedrichsdorf SJ. A Comparison of Circumstances at the End of Life in a Hospital Setting for Children With Palliative Care Involvement Versus Those Without. J Pain Symptom Manage. 2016. November;52(5):673–80. [DOI] [PubMed] [Google Scholar]
- 32.Petteys AR, Goebel JR, Wallace JD, Singh-Carlson S. Palliative care in neonatal intensive care, effects on parent stress and satisfaction: a feasibility study. Am J Hosp Palliat Care. 2015. December;32(8):869–75. [DOI] [PubMed] [Google Scholar]
- 33.Postier A, Chrastek J, Nugent S, Osenga K, Friedrichsdorf SJ. Exposure to home-based pediatric palliative and hospice care and its impact on hospital and emergency care charges at a single institution. J Palliat Med. 2014. February;17(2):183–8. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt P, Otto M, Hechler T, Metzing S, Wolfe J, Zernikow B. Did increased availability of pediatric palliative care lead to improved palliative care outcomes in children with cancer? J Palliat Med. 2013. September; 16(9): 1034–9. [DOI] [PubMed] [Google Scholar]
- 35.Smith AG, Andrews S, Bratton SL, Sheetz J, Feudtner C, Zhong W, et al. Pediatric palliative care and inpatient hospital costs: a longitudinal cohort study. Pediatrics. 2015. April; 135(4):694–700. [DOI] [PubMed] [Google Scholar]
- 36.Ullrich CK, Lehmann L, London WB, Guo D, Sridharan M, Koch R, et al. End-of-Life Care Patterns Associated with Pediatrie Palliative Care among Children Who Underwent Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2016. June;22(6):1049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vern-Gross TZ, Lam CG, Graff Z, Singhal S, Levine DR, Gibson D, et al. Patterns of End-of-Life Care in Children With Advanced Solid Tumor Malignancies Enrolled on a Palliative Care Service. J Pain Symptom Manage. 2015. September;50(3):305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong PH, De Castro Molina JA, Teo K, Tan WS. Paediatric palliative care improves patient outcomes and reduces healthcare costs: evaluation of a home-based program. BMC Palliat Care [Internet]. 2018. December [cited 2019 Jan 8];17(1). Available from: https://bmcpalliatcare.biomedcentral.eom/articles/10.1186/s12904-017-0267-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hancock HS, Pituch K, Uzark K, Bhat P, Fifer C, Silveira M, et al. A randomised trial of early palliative care for maternal stress in infants prenatally diagnosed with single-ventricle heart disease. Cardiol Young. 2018. April;28(04):561–70. [DOI] [PubMed] [Google Scholar]
- 40.Snaman JM, Kaye EC, Lu JJ, Sykes A, Baker JN. Palliative Care Involvement Is Associated with Less Intensive End-of-Life Care in Adolescent and Young Adult Oncology Patients. J Palliat Med. 2017. May;20(5):509–16. [DOI] [PubMed] [Google Scholar]
- 41.Widger K, Sutradhar R, Rapoport A, Vadeboncoeur C, Zelcer S, Kassam A, et al. Predictors of Specialized Pediatric Palliative Care Involvement and Impact on Patterns of End-of-Life Care in Children With Cancer. J Clin Oncol. 2018. March 10;36(8):801–7. [DOI] [PubMed] [Google Scholar]
- 42.Hays RM, Valentine J, Haynes G, Geyer JR, Villareale N, McKinstry B, et al. The Seattle Pediatric Palliative Care Project: effects on family satisfaction and health-related quality of life. J Palliat Med. 2006. June;9(3):716–28. [DOI] [PubMed] [Google Scholar]
- 43.Horrocks S, Somerset M, Salisbury C. Do children with non-malignant life-threatening conditions receive effective palliative care? A pragmatic evaluation of a local service. Palliat Med. 2002. September;16(5):410–6. [DOI] [PubMed] [Google Scholar]
- 44.Pierucci RL, Kirby RS, Leuthner SR. End-of-life care for neonates and infants: the experience and effects of a palliative care consultation service. Pediatrics. 2001. September; 108(3):653–60. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe J, Hammel JF, Edwards KE, Duncan J, Comeau M, Breyer J, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol Off J Am Soc Clin Oncol. 2008. April 1;26(10):1717–23. [DOI] [PubMed] [Google Scholar]
- 46.Golan H, Bielorai B, Grebler D, Izraeli S, Rechavi G, Toren A. Integration of a palliative and terminal care center into a comprehensive pediatric oncology department. Pediatr Blood Cancer. 2008. May;50(5):949–55. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe J, Grier HE, Klar N, Levin SB, Ellenbogen JM, Salem-Schatz S, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000. February 3;342(5):326–33. [DOI] [PubMed] [Google Scholar]
- 48.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Internet]. The Cochrane Collaboration; 2011. Available from: www.handbook.cochrane.org. [Google Scholar]
- 49.Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Metaanalysis. JAMA. 2016. November 22;316(20):2104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinds PS, Burghen EA, Pritchard M. Conducting end-of-life studies in pediatric oncology. West J Nurs Res. 2007. June;29(4):448–65. [DOI] [PubMed] [Google Scholar]
- 51.Dussel V, Orellana L, Soto N, Chen K, Ullrich C, Kang TI, et al. Feasibility of Conducting a Palliative Care Randomized Controlled Trial in Children With Advanced Cancer: Assessment of the PediQUEST Study. J Pain Symptom Manage. 2015. June;49(6):1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown E, Morrison RS, Gelfman LP. An Update: NIH Research Funding for Palliative Medicine, 2011–2015. J Palliat Med. 2018. February 1;21(2):182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feudtner Chris, Rosenberg Abby R., Boss Renee D., Wiener Lori, Lyon Maureen E., Hinds Pam, et al. Challenges and Priorities for Pediatric Palliative Care Research: Report from a Pediatric Palliative Care Research Network Workshop. Manuscript submitted to JPSM in July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am J Public Health. 2004. March;94(3):416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” Design for Building Individualized Treatment Sequences. Annu Rev Clin Psychol [Internet]. 2012. [cited 2019 Apr 10];8 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3887122/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Concato J, Shah N, Horwitz Rl. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000. June 22;342(25):1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandenbroucke JP. The HRT controversy: observational studies and RCTs fall in line. Lancet Lond Engl. 2009. April 11;373(9671): 1233–5. [DOI] [PubMed] [Google Scholar]
- 58.Wolfe J, Orellana L, Cook EF, Ullrich C, Kang T, Geyer JR, et al. Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol. 2014. April 10;32(11):1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feudtner C, Kang TI, Hexern KR, Friedrichsdorf SJ, Osenga K, Siden H, et al. Pediatric Palliative Care Patients: A Prospective Multicenter Cohort Study. Pediatrics. 2011. June 1; 127(6): 1094–101. [DOI] [PubMed] [Google Scholar]
- 60.Siden H, Steele R. Charting the Territory: Children and families living with progressive life-threatening conditions. Paediatr Child Health 2015. April;20(3): 139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010. March 24;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiol Camb Mass. 2007. November;18(6):805–35. [DOI] [PubMed] [Google Scholar]
- 63.Pinheiro LC, McFatrich M, Lucas N, Walker JS, Withycombe JS, Hinds PS, et al. Child and adolescent self-report symptom measurement in pediatric oncology research: a systematic literature review. Qual Life Res [Internet]. 2017. September 6 [cited 2017 Nov 5]; Available from: http://link.springer.eom/10.1007/s11136-017-1692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leahy AB, Feudtner C, Basch E. Symptom Monitoring in Pediatric Oncology using Patient-Reported Outcomes: Why, How, and Where Next. The patient. 2018. April; 11 (2): 147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mant J Process versus outcome indicators in the assessment of quality of health care. Int J Qual Health Care J Int Soc Qual Health Care. 2001. December;13(6):475–80. [DOI] [PubMed] [Google Scholar]
- 66.Wolfe J, Klar N, Grier HE, Duncan J, Salem-Schatz S, Emanuel EJ, et al. Understanding of prognosis among parents of children who died of cancer: impact on treatment goals and integration of palliative care. JAMA. 2000. November 15;284(19):2469–75. [DOI] [PubMed] [Google Scholar]
- 67.Dussel V, Kreicbergs U, Hilden JM, Watterson J, Moore C, Turner BG, et al. Looking beyond where children die: determinants and effects of planning a child’s location of death. J Pain Symptom Manage. 2009. January;37(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreicbergs U, Valdimarsdóttir U, Steineck G, Henter J-l. A population-based nationwide study of parents’ perceptions of a questionnaire on their child’s death due to cancer. Lancet Lond Engl. 2004. September 28;364(9436):787–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.