Abstract
Background:
Prior data evaluating risk of severe UTI infections with Sodium-Glucose Co-Transporter 2 inhibitors (SGLT2i) have reported conflicting findings.
Objective:
To assess whether patients initiating SGLT2i were at an increased risk of developing severe UTI events compared to those initiating Dipeptidyl peptidase-4 inhibitors (DPP-4i) and Glucagon-like Peptide 1 receptor agonists (GLP1a).
Design
Population-based cohort study
Setting
Two large US-based commercial claims databases (March 2013 – September 2015)
Participants
Within each database, two cohorts of 1:1 propensity score-matched patients 18 years and older with type 2 diabetes mellitus, initiating SGLT2 inhibitors vs DPP-4 inhibitors (cohort 1) or GLP1 agonists (cohort 2)
Measurements:
The primary outcome was a severe UTI event, defined as a hospitalization for either primary UTI, sepsis with UTI or pyelonephritis; secondary outcome was outpatient UTI treated with antibiotics. Hazard ratios [HRs] were estimated in each propensity-score matched cohort adjusting for more than 90 baseline characteristics.
Results
After 1:1 PS matching, we identified 123,752 patients in cohort 1 and 111,978 patients in cohort 2 in the two databases. In cohort 1, there were 61 severe UTI events among initiators of SGLT2 inhibitors (incidence rate [IR] per 1,000 person-years=1.76) v 57 in the DPP-4 inhibitor group (IR =1.77), corresponding to a HR of 0.98 (95% CI, 0.68, 1.41). In cohort 2, there were 73 events in the SGLT2 inhibitor group (IR=2.15) vs 87 in the GLP1 agonist group (IR=2.96) (HR 0.72 [ 0.53–0.99]). Findings were robust across sensitivity analyses, within several subgroups of age, sex, frailty, and for canagliflozin and dapagliflozin individually. SGLT2 inhibitors were not associated with an increased risk of outpatient UTIs (HR 0.96 [0.89–1.04] in cohort 1 and 0.91 [0.84–0.99] in cohort 2).
Limitations:
Generalizability of the study findings may be limited to patients with commercial-claims insurance.
Conclusion:
In a large cohort of patients seen in routine clinical practice, risk of severe and non-severe UTI events among those who initiated SGLT2 inhibitors was similar to the rates among those initiating other second-line anti-diabetes medications
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a newer class of anti-diabetic medications that reduce serum glucose by inhibiting its re-absorption in the proximal tubule (1). In addition to decreasing serum glucose, they exert a beneficial effect on cardiometabolic markers like blood-pressure (2) and have demonstrated reductions in cardiovascular events and mortality in both randomized and non-experimental studies (3–6).
Because SGLT2 inhibitors increase the availability of glucose in the urinary tract, they provide substrate for bacteria to grow.(7, 8) Accordingly, they have been previously linked to infections of the genitourinary tract (2, 9). Although SGLT2 inhibitors have consistently shown to increase the risk of genital infections (9, 10), their association with urinary tract infections (UTIs) is less clear, with prior meta-analyses reporting conflicting findings (2, 11, 12). While most UTIs caused by SGLT2 inhibitors were of mild to moderate severity (13), in 2015, the US Food and Drug Administration revised labels for all SGLT2 inhibitors, adding a warning for severe UTI infections. This warning was prompted by post-marketing adverse-event reports of sepsis with UTI and pyelonephritis in patients using these agents (14).
The relationship between SGLT2 inhibitor use and risk of severe UTI remains unclear, with prior evidence coming from either post-marketing reports – which have limited validity (15) or from clinical trials – which despite pooling leave substantial uncertainty for such a rare outcome. For example, a recent meta-analysis of 72 clinical trials found only 17 cases of sepsis with UTI in the SGLT2 inhibitor arm (0.67 events per 1,000 patients) (11).
Using two large commercial claims databases in the US, we aimed to assess whether the initiation of SGLT2 inhibitors was associated with an increased risk of severe UTI infections when compared to the initiation of two alternative non-gliflozin classes of anti-diabetic medications among patients with type 2 diabetes mellitus.
METHODS
The study was approved by the Brigham and Women’s Institutional Board Review Board and the appropriate data use agreements were in place for both databases.
Data Sources
The study utilized data sourced from IBM MarketScan (MarketScan) and Clinformatics Datamart (Optum), both of which are US based health-insurance databases and are comprised of patients with employer-based insurance. Optum also provides data for patients with managed-care Medicare through United Health Care plans and MarketScan for patients with supplemental Medicare coverage. Data elements of interest include patient demographics, medical and pharmacy enrollment status, inpatient and outpatient medical services (ICD-9-CM (international classification of diseases, ninth revision, clinical modification) and CPT-4 (current procedural terminology, fourth edition)) and outpatient pharmacy dispensing (including drug names, strength, units dispensed and days’ supply).
Study population and exposure definition
Within each database, a separate cohort was created for each pair-wise comparison of SGLT2 inhibitors versus an alternative non-gliflozin class, and cohort entry was restricted between March 2013 – to coincide with the approval of the first SGLT2 inhibitor in the United States – and September 2015. Cohort membership required patients to be new-users of the study medications of interest (defined as no use in the 180-day washout window), ≥ 18 years at cohort entry with a recorded diagnosis of Type II Diabetes Mellitus at any time prior to drug initiation. Patients with evidence of nursing home or hospice care, gestational diabetes, type 1 diabetes mellitus, cancer, human immunodeficiency virus, renal insufficiency (chronic kidney disease, acute renal failure or end-stage renal disease), or those with a high risk of UTI (e.g., hydronephrosis, vesicoureteral reflux, spinal cord injuries or catheter use), or a history of UTI were excluded from analysis (see appendix Table 1 for complete list of exclusion criteria).
The first cohort was comprised of patients initiating an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin; empagliflozin/linagliptin combination was not considered for Cohort 1 (see appendix Table 1 for list of included products)) or a dipeptidyl peptidase-4 (DPP4) inhibitor (sitagliptin, saxagliptin, linagliptin or alogliptin) without evidence of prior use of either SGLT2 or DPP4 inhibitors. Similarly, the second cohort included patients initiating an SGLT2 inhibitor or a glucagon-like peptide-1 receptor (GLP1) agonist (albiglutide, dulaglutide, exenatide or liraglutide) without prior use of either SGLT2 inhibitors or GLP1 agonists. Patients meeting the inclusion criteria could contribute to each cohort only once, but it was possible for the same patient to be included in both cohorts (see Appendix Figure 1 for the study design).
Propensity score matching
All analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC). To mitigate the risk for confounding, new initiators of SGLT2 inhibitors were matched to a non-gliflozin agent (DPP4 inhibitors for cohort 1; GLP1 agonists for cohort 2) on their estimated propensity score, which utilized a logistic regression to model the probability of initiating an SGLT2 inhibitor using 91 baseline covariates (PROC LOGISTIC). These baseline variables were measured in the 180-day period prior to cohort entry and included demographic variables (e.g., age, sex), microvascular and macrovascular complications of diabetes (e.g., diabetic neuropathy, myocardial infarction), insulin and other anti-diabetic therapy, risk factors for UTI (e.g., oral steroid or broad spectrum antibiotic use, history of mycotic infections, disease-modifying antirheumatic drugs), comorbid conditions (e.g., chronic obstructive pulmonary disease), claims-based frailty index – which uses claims data to create an aggregate measure of frailty, with higher scores corresponding to a higher risk of mortality and disability (16, 17) – measures of healthcare utilization (e.g., number of emergency department visits) and markers for a healthy user (e.g., immunization status). A 1:1 propensity score matched cohort was created within each cohort using a nearest neighbor matching approach within a maximum caliper width of 0.01.
Follow up and study endpoint
Patients began contributing follow-up time from the day after cohort entry up until the first occurrence of the following: end of healthcare or pharmacy eligibility, switching to a comparator class, or discontinuation of therapy (defined as a 30-day treatment gap after the last prescription), end of study-data (September 30, 2015), or occurrence of the outcome.
The primary study endpoint was the occurrence of a severe UTI event (composite of primary UTI hospitalizations, hospitalizations with sepsis and UTI, and hospitalizations with pyelonephritis). A primary UTI hospitalization required the presence of diagnosis codes related to UTI (cystitis, ureteritis or pyelonephritis: 590.xx, 595.xx, 597.xx, 599.0x) in the primary discharge diagnosis field. A hospitalization with sepsis and UTI required the co-occurrence of discharge codes related to both UTI (590.xx, 595.xx, 597.xx, 599.0x) and sepsis (bacteremia, septicemia, sepsis, and septic shock: 785.52, 790.7, 955.8x, 995.9x) at any discharge diagnosis position, while a hospitalization with pyelonephritis required codes related to pyelonephritis (590.xx) at any discharge diagnosis position.
In addition to examining the individual components of the primary composite outcome, we also examined two additional secondary outcomes, with the first being any UTI related hospitalizations (UTI related codes in any position), and second being treated outpatient UTI (required evidence of outpatient antibiotic dispensing and outpatient diagnosis codes related to UTI: see appendix Table 1 for all outcome definitions).
Statistical analysis
We assessed the performance of the propensity score by cross-tabulating the baseline covariates before- and after – propensity-score matching by exposure group, utilizing a threshold of 10% in the standardized difference as a meaningful imbalance between the two groups, (18) and examining c-statistic for the logistic regression model (with values closer to 0.50 indicating less imbalance in the covariates between the two groups) (19). Hemoglobin A1c (available for 10% of the pooled population; not included in the propensity score) was utilized to assess the presence of adequate therapeutic equipoise between the SGLT2 and their non-gliflozin counterparts prior to propensity score matching and to assess potential residual confounding after propensity score matching. There was no other missing data in our study.
In the propensity-score matched cohorts, we estimated the risk of a severe UTI event for SGLT2 inhibitors compared to non-gliflozin agents by calculating the number of events, incidence rates, and hazard ratios (HRs; PROC PHREG) with the Wald 95% confidence intervals (CIs). Analysis were performed within each database, and estimates were pooled through inverse-variance fixed-effects meta-analysis (20). Kaplan-Meier curves (PROC LIFETEST) were generated to visualize the risk of the outcome over time and log-rank tests were used to compare the survival distribution in the two groups.
Sensitivity and subgroup analyses
We conducted several sensitivity analyses to assess robustness of the study findings. First, because severe UTI events may be rare, in order to preserve power by including more patients, we altered the propensity score specification employing a variable-ratio parallel balanced matching technique (21), and also utilizing a propensity score based fine-stratification approach (50-strata) (22). Second, we varied study specifications pertaining to exposure-related censoring criteria and maximum length of follow up. Specifically, in addition to the as-treated analysis – where we censored patients at the time of treatment discontinuation or switching – we carried the index exposure forward to mimic an intention-to-treat approach. For both the as-treated and intention-to-treat analysis, we also varied the maximum follow-up duration to 3-months, 6-months, 12-months and till the end of study data.
We tested for the presence of effect modification in several relevant subgroups of patients. First, we restricted analysis to the subgroup of patients without evidence of antibiotic or disease-modifying antirheumatic drugs, or infections in the baseline period (defined as 180-days prior to the cohort entry). Second, we stratified analysis by sex to reflect the differences in incidence and severity of UTIs. (23) Third, because older patients may have a higher baseline risk of UTIs and develop a more severe UTI symptomology, (24) we also stratified analysis by age (using 60 years as the threshold to reflect the distribution of older patients in our study population). Fourth, since high frailty is likely to be associated with a higher risk of severe infections, we stratified the analysis by frailty using tertiles of a claims-based frailty index to group patients into low, moderate and high frailty.
Finally, we examined the association between individual SGLT2 inhibitors and the risk of severe UTI events, though this analysis was limited to canagliflozin and dapagliflozin because not enough patients exposed to empagliflozin accumulated during the study period. For the dapagliflozin analysis, cohort entry was restricted to after February 2014 to reflect the US market approval date.
Within each subgroup, the propensity score was re-estimated, and patients were re-matched on the newly estimated propensity score using the 1:1 nearest neighbor matching approach with a caliper width of 0.01.
RESULTS
Across the two databases, there were 270,762 patients initiating an SGLT2 inhibitor compared to 440,970 DPP4 inhibitor initiations (cohort 1; SGLT2 v DPP4), and 273,617 patients initiating an SGLT2 inhibitor compared to 211,701 initiating a GLP1 agonist (cohort 2; SGLT2 v GLP1). After the inclusion and exclusion criteria were applied, there were 86,665 SGLT2 inhibitor v 136,741 DPP4 inhibitor initiators in cohort 1 and 107,289 SGLT2 inhibitor v 67,871 GLP1 agonist initiators in cohort 2 eligible for propensity score matching, from which a 1:1 propensity score matched cohort of 123,752 patients (cohort 1) and 111,978 patients (cohort 2) was created (See Appendix Figures 2 and 3 for the CONSORT flow diagram).
Appendix Tables 3–6 show the baseline characteristics of patients prior to and after propensity score matching (stratified by database and cohort). Appendix Table 7 and Table 1 show select pooled baseline characteristics prior to and after propensity score matching respectively. For cohort 1, prior to matching, SGLT2 inhibitor initiators were more likely to be younger, to have more diabetes-related complications, and to have a history of insulin or GLP1 agonist use; for cohort 2, patients in SGLT2 inhibitor group were more likely to be male and to have a history of DPP4 inhibitor use but were less likely to have used insulin during the baseline period.
Table 1:
Select pooled baseline characteristics after propensity score matching *
| SGLT2 v DPP4 (Cohort 1) | SGLT2 v GLP1 (Cohort 2) | |||||
|---|---|---|---|---|---|---|
| SGLT2 (n=61,876) |
DPP4 (n=61,876) |
SD | SGLT2 (n=55,989) |
GLP1 (n=55,989) |
SD | |
| Male, n (%) | 33,502 (54.1) | 33,645 (54.4) | 0.5 | 27,686 (49.4) | 27,715 (49.5) | 0.1 |
| Age, mean (SD) | 54.7 (9.9) | 54.7 (10.2) | 0.2 | 54.5 (10.1) | 54.4 (10.1) | 1.4 |
| Diabetic severity, n (%) | ||||||
| Ocular complications | 2,469 (4.0) | 2,453 (4.0) | 0.1 | 2,519 (4.5) | 2,466 (4.4) | 0.5 |
| Neurological complications | 4,567 (7.4) | 4,634 (7.5) | 0.4 | 5,126 (9.2) | 5,093 (9.1) | 0.2 |
| Other or unspecified complications | 4,292 (6.9) | 4,235 (6.8) | 0.4 | 4,381 (7.8) | 4,392 (7.8) | 0.1 |
| A1c, mean (SD)† | 8.8 (1.8) | 8.8 (1.9) | 0.1 | 8.7 (1.8) | 8.8 (1.9) | 0.1 |
| Antidiabetic therapy, n (%) | ||||||
| Metformin | 48,319 (78.1) | 48,480 (78.4) | 0.6 | 42,599 (76.1) | 42,586 (76.1) | 0.1 |
| Dipeptidyl peptidase-4 inhibitors | 17,046 (30.4) | 17,113 (30.6) | 0.3 | |||
| Glucagon-like peptide agonists | 4,915 (7.9) | 4,270 (6.9) | 4.0 | |||
| Insulin | 12,067 (19.5) | 11,838 (19.1) | 0.9 | 18,237 (32.6) | 18,117 (32.4) | 0.5 |
| Sulfonylureas | 22,052 (35.6) | 22,208 (35.9) | 0.5 | 20,850 (37.2) | 20,720 (37.0) | 0.5 |
| Risk factors for UTI, n (%) | ||||||
| Broad spectrum Antibiotic | 14,487 (23.4) | 14,393 (23.3) | 0.4 | 13,648 (24.4) | 13,489 (24.1) | 0.7 |
| Oral Steroids | 6,353 (10.3) | 6,263 (10.1) | 0.5 | 5,796 (10.4) | 5,833 (10.4) | 0.2 |
| DMARDs, non-biologic | 519 (0.8) | 509 (0.8) | 0.2 | 473 (0.8) | 481 (0.9) | 0.2 |
| DMARDS, biologic | 381 (0.6) | 389 (0.6) | 0.2 | 386 (0.7) | 391 (0.7) | 0.1 |
| Mycotic infections | 3,402 (5.5) | 3,377 (5.5) | 0.2 | 3,453 (6.2) | 3,439 (6.1) | 0.1 |
| Propensity Score diagnostics | ||||||
| AUC, Clinformatics | 0.52 | 0.51 | ||||
| AUC, MarketScan | 0.52 | 0.51 | ||||
| Average Standardized difference | 0.4% | 0.3% | ||||
Abbreviations: AUC: Area Under the Curve; DPP4: Dipeptidyl peptidase-4 inhibitors; GLP1: Glucagon-like peptide 1 agonists SD: Standardized differences; SGLT2: Sodium Glucose Cotransporter-2 inhibitors
Data Select pooled variables are shown; see Appendix Tables 3-6 for baseline characteristics prior to and after propensity score matching, stratified by database and cohort. See Appendix Table 7 for the analogous table for Table 1 prior to propensity score matching.
A1c laboratory data was available for 10% of the pooled sample and not included in the propensity score model
After 1:1 propensity-score matching, the baseline characteristics were well balanced between the two groups in both cohorts, with no standardized differences exceeding 10%. After matching, the average standardized difference decreased from 6.9% to 0.5% for cohort 1 and from 5.1% to 0.3% for cohort 2; reductions were also noted for the post-matching c-statistics (the closer to 0.5 the better) compared to their pre-matching value: from 0.80 to 0.52 for cohort 1 and from 0.70 to 0.51 for cohort 2. Hemoglobin A1c values were similar between the two groups in both cohorts even prior to propensity score matching implying good therapeutic equipoise between the SGLT2 and the non-gliflozin groups and remained well-balanced after matching.
Analysis of primary and secondary outcomes
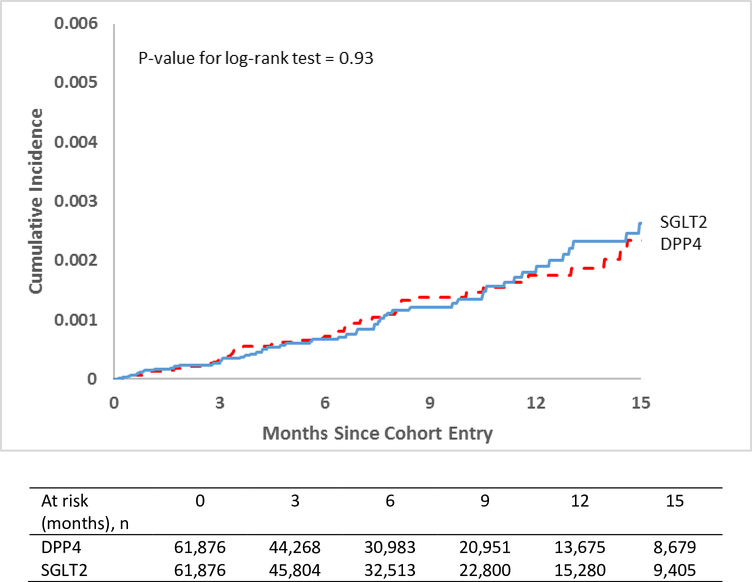
Table 2 shows the pooled and the database-specific estimates for the primary analysis (see Appendix Table 8 and 9 for further information on follow-up time and censoring reasons). In cohort 1, prior to propensity score matching, there were 89 cases of severe UTI events in the SGLT2 inhibitor group among 86,665 patients corresponding to an incidence rate [IR] of 1.86 cases per 1,000 person-years of follow-up, compared to 206 events in the DPP4 inhibitor group among 136,741 patients (IR=2.33) corresponding to an unadjusted Hazard Ratio [HR] of 0.80 (95% CI, 0.63–1.03). After propensity-score matching (n=61,876 matched pairs), there were 61 severe UTI events in the SGLT2 inhibitor group (IR=1.76) compared to 57 in the DPP4 inhibitor group (IR=1.77) corresponding to an adjusted HR of 0.98 (95% CI, 0.68–1.41). Figure 1 shows the cumulative incidence of severe UTI since start of study drugs pooled across both cohorts as Kaplan-Maier plots (see Appendix Fig. 4 and 5 for database-specific plots). The p-value for log-rank test was 0.93 for Cohort 1.
Table 2:
Risk of a severe urinary tract infection event associated with SGLT2 inhibitors prior to and after propensity score matching *
| Optum Clinformatics | Prior to propensity score matching | Propensity score matched | ||||||
|---|---|---|---|---|---|---|---|---|
| SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
|||||
| SGLT2 (n=21,976) |
DPP4 (n=38,993) |
SGLT2 (n=26,519) |
GLP1 (n=18,402) |
SGLT2 (n=16,147) |
DPP4 (n=16,147) |
SGLT2 (n=14,645) |
GLP1 (n=14,645) |
|
| Number of Events | 19 | 63 | 27 | 19 | 12 | 14 | 19 | 15 |
| Mean follow-up† | 192 | 235 | 194 | 201 | 195 | 188 | 208 | 187 |
| Incidence Rate‡ | 1.64 | 2.51 | 1.92 | 1.88 | 1.39 | 1.68 | 2.27 | 2.00 |
| HR (95% CI) | 0.65 (0.39, 1.08) | 1.01 (0.56, 1.81) | 0.82 (0.38, 1.77) | 1.14 (0.58, 2.24) | ||||
| MarketScan | Prior to propensity score matching | Propensity score matched | ||||||
|
SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
|||||
|
SGLT2 (n=64,689) |
DPP4 (n=97,748) |
SGLT2 (n=80,759) |
GLP1 (n=49,480) |
SGLT2 (n=45,729) |
DPP4 (n=45,729) |
SGLT2 (n=41,344) |
GLP1 (n=41,344) |
|
| Number of Events | 70 | 143 | 85 | 93 | 49 | 43 | 54 | 72 |
| Mean follow-up† | 205 | 237 | 205 | 207 | 208 | 191 | 225 | 193 |
| Incidence Rate‡ | 1.93 | 2.25 | 1.88 | 3.32 | 1.88 | 1.79 | 2.12 | 3.30 |
| HR (95% CI)§ | 0.86 (0.65, 1.14) | 0.56 (0.42, 0.75) | 1.03 (0.69, 1.56) | 0.64 (0.45, 0.91) | ||||
| Pooled | Prior to propensity score matching | Propensity score matched | ||||||
|
SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
|||||
|
SGLT2 (n=86,665) |
DPP4 (n=136,741) |
SGLT2 (n=107,278) |
GLP1 (n=67,882) |
SGLT2 (n=61,876) |
DPP4 (n=61,876) |
SGLT2 (n=55,989) |
GLP1 (n=55,989) |
|
| Number of Events | 89 | 206 | 112 | 112 | 61 | 57 | 73 | 87 |
| Mean follow-up† | 202 | 236 | 202 | 205 | 204 | 190 | 221 | 191 |
| Incidence Rate‡ | 1.86 | 2.33 | 1.89 | 2.93 | 1.76 | 1.77 | 2.15 | 2.96 |
| HR (95% CI)§ | 0.80 (0.63, 1.03) | 0.63 (0.48, 0.82) | 0.98 (0.68, 1.41) | 0.72 (0.53, 0.99) | ||||
Abbreviations: DPP4: Dipeptidyl peptidase-4 inhibitors; GLP1: Glucagon-like peptide 1 agonists; HR: Hazard Ratio; SGLT2: Sodium Glucose Cotransporter-2 inhibitors
Defined as composite of primary UTI hospitalizations, hospitalizations with sepsis and UTI, and hospitalizations with pyelonephritis; see text and appendix for definitions
In days
Per 1,000 person-years of follow up
Estimates were pooled across the two databases using fixed effects meta-analysis
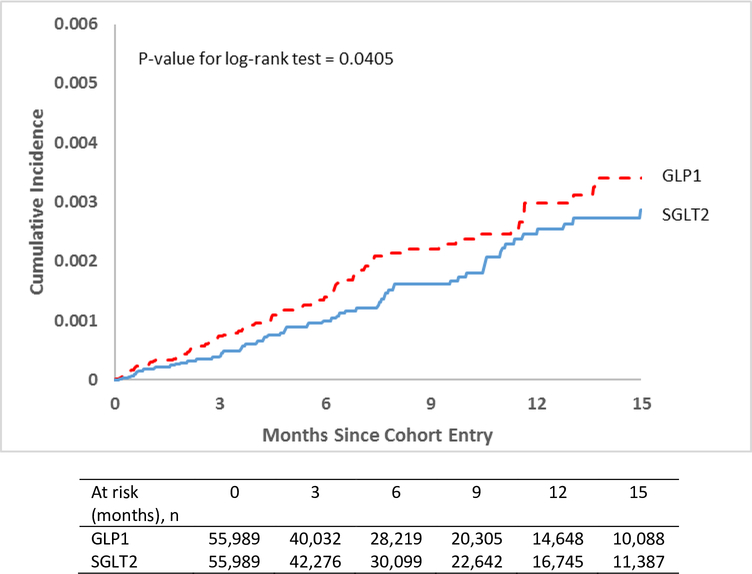
Figure 1:
Propensity-score matched Kaplan-Meier curves for cumulative incidence of severe UTI events in the pooled cohort of patients
DPP4: Dipeptidyl peptidase-4 inhibitors; GLP1: Glucagon-like peptide agonists; SGLT2: Sodium Glucose Cotransporter-2 inhibitors
In cohort 2, there were 112 events among 107,278 patients in the SGLT2 inhibitor group (IR=1.89) compared to 112 events among 67,882 patients in the GLP1 agonist group (IR=2.93), unadjusted HR 0.63 (95% CI, 0.48–0.82). After matching (n=55,989 matched pairs), there were 73 (IR=2.15) v 87 (IR=2.96) events in the SGLT2 inhibitor and GLP1 agonist group respectively [HR 0.72 (95% CI, 0.53–0.99)]. The p-value for the log-rank test was 0.04.
Findings were consistent for all secondary outcomes (Table 3 and Appendix Table 10; see appendix Table 11 for the incidence rates of the secondary outcomes). SGLT2 inhibitors were not associated with an increase in the risk of any UTI hospitalizations; HR 0.68 (95% CI, 0.54–0.87) and 0.78 (95% CI, 0.62–0.99) for cohorts 1 and 2 respectively. Likewise, SGLT2 inhibitors were not associated with an increase in the risk of treated outpatient UTIs for Cohort 1, HR 0.96 (95% CI, 0.89–1.04) or Cohort 2, 0.91 (95% CI, 0.84–0.99).
Table 3:
Risk of secondary outcomes associated with SGLT2 inhibitors in a propensity score matched analysis, HR (95% CI) *
| SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
|
|---|---|---|
| Primary Outcome † | 0.98 (0.68, 1.41) | 0.72 (0.53, 0.99) |
| Individual components of the primary outcome | ||
| Hospitalizations with sepsis and UTI | 1.11 (0.68, 1.82) | 0.54 (0.36, 0.82) |
| Hospitalizations with pyelonephritis | 0.74 (0.45, 1.21) | 0.65 (0.42, 1.00) |
| Primary UTI hospitalization | 0.81 (0.46, 1.43) | 0.86 (0.52, 1.43) |
| Other Secondary outcomes | ||
| UTI hospitalization‡ | 0.68 (0.54, 0.87) | 0.78 (0.62, 0.99) |
| Treated Outpatient UTI§ | 0.96 (0.89, 1.04) | 0.91 (0.84, 0.99) |
DPP4: Dipeptidyl peptidase-4 inhibitors; GLP1: Glucagon-like peptide 1 agonists; HR: Hazard Ratio; SGLT2: Sodium Glucose Cotransporter-2 inhibitors
See text and appendix Table 1 for outcome definitions and appendix Table 8 for database specific estimates.
Defined as the composite of hospitalizations with 1) sepsis and UTI, 2) pyelonephritis, and 3) primary UTI hospitalization. See text for details.
Defined as a hospitalization for UTI (at any diagnosis position)
Required evidence of outpatient antibiotic dispensing and outpatient diagnosis codes related to UTI
Sensitivity and subgroup analyses
Study findings were consistent across a range of sensitivity and subgroup analyses (Table 3; see Appendix Figures 6 and 7 for database-specific estimates). Changing the propensity score matching approach from a 1:1 to a variable ratio match did not appreciably change the point estimates; findings were also similar using propensity-score fine-stratification. Further, findings from the intention-to-treat analysis were similar to the as-treated analysis regardless of the maximum duration of follow-up considered in the analysis.
The risk of the outcome also did not vary meaningfully across the several subgroups of genders, age, baseline frailty, those at low risk for severe UTI events, or within the individual SGLT2 inhibitors in either cohort.
DISCUSSION
It has been previously postulated that because of their pharmacodynamic properties, SGLT2 inhibitors increase the risk of severe UTI events; however, this association has not been previously studied in a routine care setting. This study examined data from two large commercial claims databases and found that when compared to patients initiating a DPP-4 inhibitor or a GLP-1 agonist, patients initiating an SGLT2 inhibitor for the management of Type II Diabetes Mellitus had a similar rate of either severe or non-severe UTI events. Study findings were consistent across a range of pre-defined sensitivity analyses, and within several subgroups of age, sex, and frailty, and for individual SGLT2 agents.
This study has several important clinical implications. UTIs occur at a higher frequency and severity in patients with diabetes (25, 26); thus, anti-diabetic agents that increase the risk of such infections may decrease the quality of life, predisposing patients to therapy discontinuation and poor glycemic control. In addition, uroseptic and pyelonephritic infections have been found to be relevant contributors to patient mortality, making the study findings relevant in guiding the care of patients with diabetes. Further, patients who may be good candidates to receive SGLT2 inhibitors to control their diabetes but have a history of recurrent UTIs may be precluded from being prescribed these agents; given the high prevalence of UTIs in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications, which has demonstrated to decrease the risk of major cardiovascular events and mortality.
While our study did not find an increase in risk of UTI events among SGLT2 initiators, there was some evidence of a lower risk of UTI with SGLT2 (vs GLP1) in one database (MarketScan). We caution against overinterpreting these results, as these are additional sources of uncertainty including the possibility of chance finding, bias due to differential surveillance, and residual confounding caused by differences in access policies which may have impacted our final point estimates and corresponding confidence bounds.
Although, prior literature on SGLT2 inhibitors and UTIs overall has been inconsistent, with older meta-analysis with fewer clinical trials alluding to a higher UTI risk (2), our findings are consistent with a recently published meta-analysis of 72 trials that did not find an elevated risk of severe or non-severe UTI events with SGLT2 inhibitors, sepsis with UTI HR 1.41 (95% CI, 0.57–3.48) and pyelonephritis HR 0.78 (95% CI, 0.52–1.18), and overall UTI events (severe and non-severe) HR 1.03 (95% CI, 0.96–1.11) (11). A network meta-analysis by Li et al examined individual SGLT2 inhibitors and similarly did not find an elevated risk of UTI infections (with a possible exception of dapagliflozin at higher doses) (9). Similarly, a small observational study of 1,977 SGLT2 initiators in Australia also did not find a meaningful increase in the six-month risk of overall UTI infections, HR 0.90 (95% CI, 0.66–1.24) (27).
Prior data examining the risk of SGLT2 inhibitors on severe UTI events have come from randomized trials. Although randomized trials are the gold standard to assess pharmaceutical efficacy, they are often inadequately powered to detect differences in rare events such as uroseptic or pyelonephritic events. This study, which included over 55,000 propensity-score-matched SGLT2 inhibitor initiators in each pair-wise comparison, took several steps to decrease bias by characteristics associated with both treatment selection and risk of severe UTI events, i.e., confounding by indication. We assessed this risk in two large US-based commercial claims databases against two other comparable classes of anti-diabetic medications and conducted several sensitivity and subgroup analyses to test the robustness of our primary findings. The data included in the study predate the FDA drug safety communication that revised drug labels for SGLT2 products to include a warning on serious UTI infections in December 2015 (14), potentially mitigating the concern for selective physician prescribing after this time.
Our study has several limitations. As an observational study, it is susceptible to residual confounding due to the lack of randomization. For example, although we excluded patients at a higher risk of a severe UTI infection (thereby increasing the therapeutic equipoise between the groups) and adjusted for more than 90 potential confounders in the propensity score model, we could not directly account for some important variables like duration of diabetes or body mass index. Similarly, hemoglobin A1C results were available for a small proportion (10%) of our sample, which limited our ability to directly adjust for diabetes severity and glycemic control. It has been demonstrated that good balance across comparison groups for these unmeasured characteristics can be achieved by the use of claims-based proxies in studies based on healthcare utilization databases (28). Further, treatment initiation was defined using a 180-day period; thus, it is possible for patients to have been exposed to the treatment prior to this time-window. Finally, our findings are generalizable to a commercial insurance population (approximately 55% of the US) and to patients meeting the study inclusion criteria (e.g., patients without high risk of UTI or UTI history). Future studies should validate our findings in other populations, especially among older adults who may be particularly susceptible to severe UTI events.
In this large population-based cohort study of patients with type 2 diabetes mellitus, SGLT2 inhibitor use was not associated with an increase in the risk of serious – and non-serious – urinary tract infections. Based on our findings, other factors beyond the risk of UTI events should be considered in deciding whether to prescribe SGLT2 therapy in routine care patients with diabetes.
Supplementary Material
Table 4:
Sensitivity and subgroup analyses, Hazard Ratio (95% CI) *
| SGLT2 v DPP4 (Cohort 1) |
SGLT2 v GLP1 (Cohort 2) |
|
|---|---|---|
| Sensitivity analyses | ||
| Propensity Score specification † | ||
| 1:1 nearest neighbor‡ | 0.98 (0.68, 1.41) | 0.72 (0.53, 0.99) |
| 1:N variable ratio | 0.88 (0.59, 1.31) | 0.68 (0.46, 1.00) |
| Fine stratification | 0.79 (0.61, 1.02) | 0.74 (0.55, 0.98) |
| Intention to treat analysis | ||
| 3 months | 1.00 (0.50, 1.98) | 0.49 (0.28, 0.86) |
| 6 months | 0.83 (0.52, 1.34) | 0.62 (0.41, 0.92) |
| 12 months | 0.82 (0.58, 1.17) | 0.64 (0.47, 0.88) |
| Any duration | 0.85 (0.63, 1.16) | 0.67 (0.51, 0.88) |
| As treated analysis | ||
| 3 months | 0.93 (0.46, 1.89) | 0.54 (0.31, 0.95) |
| 6 months | 0.97 (0.58, 1.65) | 0.70 (0.46, 1.06) |
| 12 months | 0.99 (0.66, 1.47) | 0.76 (0.54, 1.06) |
| Any duration‡ | 0.98 (0.68, 1.41) | 0.72 (0.53, 0.99) |
| Subgroup analysis e | ||
| Exclusion criteria | ||
| No major risk factors§ | 0.92 (0.55, 1.51) | 0.64 (0.41, 1.01) |
| Active ingredient | ||
| Canagliflozin | 0.83 (0.57, 1.21) | 0.66 (0.47, 0.92) |
| Dapagliflozin | 0.57 (0.29, 1.14) | 0.52 (0.28, 0.97) |
| Gender | ||
| Male | 0.66 (0.37, 1.20) | 0.72 (0.40, 1.29) |
| Female | 0.77 (0.51, 1.17) | 0.76 (0.53, 1.10) |
| Age | ||
| Age <60 | 0.78 (0.49, 1.25) | 0.75 (0.47, 1.20) |
| Age >=60 | 1.09 (0.64, 1.84) | 0.79 (0.51, 1.23) |
| Frailty§ | ||
| Low frailty | 1.00 (0.46, 2.15) | 1.12 (0.58, 2.16) |
| Medium frailty | 0.60 (0.33, 1.09) | 0.59 (0.31, 1.10) |
| High Frailty | 0.84 (0.49, 1.43) | 0.64 (0.40, 1.02) |
DPP4: Dipeptidyl peptidase-4 inhibitors; GLP1: Glucagon-like peptide 1 agonists; HR: Hazard Ratio; SGLT2: Sodium Glucose Cotransporter-2 inhibitors
For the subgroup analysis, propensity Score was re-estimated within each subgroup and patients were re-matched on the new re-estimated score. Estimates were pooled across the two databases using fixed effects meta-analysis See Appendix Figures 6 and 7 for database-specific estimates.
1:1 NN is primary analysis, a parallel balanced variable ratio matching approach was used; fine stratification utilized 50 strata
Indicates the primary analysis
Patients without evidence of any antibiotic or DMARD use or history of any infections were included for analysis
Patients were stratified into three groups based on their estimated frailty score; see text for details.
Funding Source
This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. EP was supported by a career development grant (K08AG055670) from the National Institute on Aging.
Role of the Funding Source
This study was funded by the internal funds of Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital. The authors had complete control over design, analysis, and the decision to submit the manuscript for publication.
Footnotes
Conflict of interest: EP reports research grants from GSK and Boehringer-Ingelheim, not directly related to the topic of the submitted work. SS is consultant to WHISCON LLC and to Aetion Inc, a software manufacturer of which he also owns equity. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Genentech, Bayer, Boehringer Ingelheim, US Food and Drug Administration, and Patient-Centered Outcomes Research Institute, not directly related to the topic of the submitted work.
Protocol: Available from Dr. Patorno (epatorno@bwh.harvard.edu)
Statistical code: Available from Dr. Patorno (epatorno@bwh.harvard.edu)
Data set: Available from data vendors through a data use agreement
REFERENCES
- 1.Vallon V The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annual review of medicine. 2015;66:255–70. [DOI] [PubMed] [Google Scholar]
- 2.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium–Glucose Cotransporter 2 Inhibitors for Type 2 DiabetesA Systematic Review and Meta-analysis. Annals of internal medicine. 2013;159(4):262–74. [DOI] [PubMed] [Google Scholar]
- 3.Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine. 2017;377(7):644–57. [DOI] [PubMed] [Google Scholar]
- 4.Patorno E, Goldfine AB, Schneeweiss S, Everett BM, Glynn RJ, Liu J, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. bmj. 2018;360:k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. New England Journal of Medicine. 2016;375(4):323–34. [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, et al. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering DrugsClinical Perspective: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136(3):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GEERLINGS SE, BROUWER EC, GAASTRA W, VERHOEF J, HOEPELMAN AI. Effect of glucose and pH on uropathogenic and non-uropathogenic Escherichia coli: studies with urine from diabetic and non-diabetic individuals. Journal of medical microbiology. 1999;48(6):535–9. [DOI] [PubMed] [Google Scholar]
- 8.Geerlings SE, Meiland R, Hoepelman AI. Pathogenesis of bacteriuria in women with diabetes mellitus. International journal of antimicrobial agents. 2002;19(6):539–45. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium‐glucose co‐transporter 2 inhibitors: A meta‐analysis of randomized controlled trials. Diabetes, Obesity and Metabolism. 2017;19(3):348–55. [DOI] [PubMed] [Google Scholar]
- 10.Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium‐glucose co‐transporter‐2 inhibitors. Diabetes, Obesity and Metabolism. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puckrin R, Saltiel M-P, Reynier P, Azoulay L, Oriana H, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta diabetologica. 2018;55(5):503–14. [DOI] [PubMed] [Google Scholar]
- 12.Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. The lancet Diabetes & endocrinology. 2016;4(5):411–9. [DOI] [PubMed] [Google Scholar]
- 13.Chaplin S SGLT2 inhibitors and risk of genitourinary infections. Prescriber. 2016;27(12):26–30. [Google Scholar]
- 14.SGLT2 Inhibitors: Drug Safety Communication - Labels to Include Warnings About Too Much Acid in the Blood and Serious Urinary Tract Infections, https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm475553.htm, accessed June 1, 2018.
- 15.Goldman SA. Limitations and strengths of spontaneous reports data. Clinical therapeutics. 1998;20:C40–C4. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-based frailty index. The Journals of Gerontology: Series A. 2017;73(7):980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. The Journals of Gerontology: Series A. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate behavioral research. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Statistics in medicine. 2014;33(10):1685–99. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research synthesis methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 21.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One‐to‐many propensity score matching in cohort studies. Pharmacoepidemiology and drug safety. 2012;21(S2):69–80. [DOI] [PubMed] [Google Scholar]
- 22.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28(2):249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. The Journal of urology. 2005;173(4):1281–7. [DOI] [PubMed] [Google Scholar]
- 24.Arinzon Z, Shabat S, Peisakh A, Berner Y. Clinical presentation of urinary tract infection (UTI) differs with aging in women. Archives of gerontology and geriatrics. 2012;55(1):145–7. [DOI] [PubMed] [Google Scholar]
- 25.Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. Clinical infectious diseases. 1995;21(2):316–22. [DOI] [PubMed] [Google Scholar]
- 26.Ronald A, Ludwig E. Urinary tract infections in adults with diabetes. International Journal of Antimicrobial Agents. 2001;17(4):287–92. [DOI] [PubMed] [Google Scholar]
- 27.Gadzhanova S, Pratt N, Roughead E. Use of SGLT2 inhibitors for diabetes and risk of infection: Analysis using general practice records from the NPS MedicineWise MedicineInsight program. diabetes research and clinical practice. 2017;130:180–5. [DOI] [PubMed] [Google Scholar]
- 28.Patorno E, Gopalakrishnan C, Franklin JM, Brodovicz KG, Masso‐Gonzalez E, Bartels DB, et al. Claims‐based studies of oral glucose‐lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes, Obesity and Metabolism. 2018;20(4):974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.