Abstract
Human amniotic mesenchymal stem cells (hAMSCs) are multiple potent progenitor cells (MPCs) that can differentiate into different lineages (osteogenic, chondrogenic, and adipogenic cells) and have a favorable capacity for angiogenesis. Schnurri-3 (Shn3) is a large zinc finger protein related to Drosophila Shn, which is a critical mediator of postnatal bone formation. Bone morphogenetic protein 9 (BMP9), one of the most potent osteogenic BMPs, can strongly upregulate various osteogenesis- and angiogenesis-related factors in MSCs. It remains unclear how Shn3 is involved in BMP9-induced osteogenic differentiation coupled with angiogenesis in hAMSCs. In this investigation, we conducted a comprehensive study to identify the effect of Shn3 on BMP9-induced osteogenic differentiation and angiogenesis in hAMSCs and analyze the responsible signaling pathway. The results from in vitro and in vivo experimentation show that Shn3 notably inhibits BMP9-induced early and late osteogenic differentiation of hAMSCs, expression of osteogenesis-related factors, and subcutaneous ectopic bone formation from hAMSCs in nude mice. Shn3 also inhibited BMP9-induced angiogenic differentiation, expression of angiogenesis-related factors, and subcutaneous vascular invasion in mice. Mechanistically, we found that Shn3 prominently inhibited the expression of BMP9 and activation of the BMP/Smad and BMP/MAPK signaling pathways. In addition, we further found activity on runt-related transcription factor 2 (Runx2), vascular endothelial growth factor (VEGF), and the target genes shared by BMP and Shn3 signaling pathways. Silencing Shn3 could dramatically enhance the expression of Runx2, which directly regulates the downstream target VEGF to couple osteogenic differentiation with angiogenesis. To summarize, our findings suggested that Shn3 significantly inhibited the BMP9-induced osteogenic differentiation and angiogenesis in hAMSCs. The effect of Shn3 was primarily seen through inhibition of the BMP/Smad signaling pathway and depressed expression of Runx2, which directly regulates VEGF, which couples BMP9-induced osteogenic differentiation with angiogenesis.
Subject terms: Mesenchymal stem cells, Stem-cell differentiation
Introduction
Bone defects occur frequently and have various causes, such as trauma, infections, and tumors1. Despite the fact that bone possesses a self-healing ability, repairing bone defects beyond a critical size still requires surgery or reconstructive surgery2. It has been reported that biological treatment modalities contribute to the repair of bone defects. Tissue-engineered bone techniques involve essential elements, such as seed cells, growth factors, and implant scaffolds, and provide new treatment options for bone defects3. Many types of mesenchymal stem cells (MSCs) have been found to originate from various types of tissue, such as bone marrow-derived MSCs (BMSCs), as well as adipose, peripheral blood, and muscle and ligament-derived MSCs4–8. Among them, BMSCs are reported to be equipped with the capacity for osteogenic, chondrogenic, and adipogenic differentiation9,10. Despite this, there are many disadvantages in the process of extracting BMSCs; invasive surgery carries a high risk of infection and bleeding and may cause immunological rejection after implantation. Recently, a new kind of MSCs, which originated from the surface membrane of human placenta, called human amniotic MSCs (hAMSCs), has been discovered11. hAMSCs possess the ability to thrive in multiple environments and are not extracted traumatically. In addition, they do not carry ethical or moral controversies with them12. hAMSCs have a multidirectional differentiation capacity as well as an advantage in therapeutic angiogenesis due to the hAMSC root in placenta, which is a vascular tissue13. Hence, hAMSCs have been extensively applied in the treatment of bone and spinal traumas and vascular reconstruction surgery.
Bone repair requires multiple stimuli, including growth factors, cytokines, differentiation factors, and extracellular matrix, which contribute to creating a conducive milieu for enhanced bone healing14,15. Bone morphogenetic proteins (BMPs), attributed to the transforming growth factor β (TGF-β) super family, comprise 14 members in humans and rodents. They are essential for the proliferation and cell differentiation that determine the fate of cells16,17. BMP9 was identified in the mouse liver and exerts some effects in maintaining the embryonic basal forebrain cholinergic neurons18. The activity of BMPs is required in cells when ligands combine with BMPRI and BMPRII receptors. The signal transduction begins and continues with phosphorylation of the R-Smads, including Smad1/5/819. After the R-Smad complex is formed, the Smad4 and complex shifts into the nucleus and regulates downstream target genes and proteins20. BMP9 can regulate a number of essential targets for osteogenic signal transduction in MSCs; however, the explicit mechanism of how Schnurri-3 (Shn3) is involved in the process of BMP9-induced osteogenic differentiation remains unclear.
Shn3, a large protein that belongs to the ZAS family of zinc finger proteins, is a critical and considerable mediator of adult skeletal formation that regulates mature osteoblast activity21,22. Shn3 is one of the three mammalian homologs of Drosophila Shn that acts as a fundamental cofactor for signaling via decapentaplegic (DPP), which is the Drosophila homolog of the BMP/TGF-β signaling pathway23,24. Recently, it was reported that mice lacking Shn3 showed increased bone mass25. This high bone mass phenotype in mice due to Shn3 indicates that Shn3 could govern the expression of Runt-related transcription factor 2 (Runx2). Runx2 is regulated by Shn3 through a complex with E3 ubiquitin ligase WWP126. Shn3 regulates the interaction by inhibiting mitogen-activated protein kinase (MAPK) activity and osteogenic differentiation, and the Shn3 expression indirectly regulates osteoclastic bone resorption27,28.
Bone formation is an intricate process that requires highly coordinated reciprocity between multiple cells, factors, and signals to form mineralized bone tissues29,30. Working as structural templates, blood vessels adjacent the region of bone formation carry key elements for bone homeostasis into the osteogenic microenvironment, as well as minerals, growth factors, and osteogenic progenitor cells31. Vascularization is necessary for striking coupling of angiogenesis and osteogenesis during skeletal development other than bone repairs32,33. Multiple factors participate in the coupling process, including vascular endothelial growth factor (VEGF), hypoxia-inducible factor 1α (HIF1α), von Willebrand factor (vWF), and CD3134,35. Owing to Shn3’s activity regulating the osteogenic differentiation through BMP/TGF-β signaling, Shn3’s capacity to regulate BMP9-induced angiogenesis and osteogenesis needs to be investigated further.
In the present study, we investigated the effect of Shn3 on BMP9-induced osteogenic differentiation both in vitro and in vivo and angiogenesis of hAMSCs. Our investigation provides another possible mechanism for the regulation of BMP9-induced differentiation in hAMSCs. These results will offer abundant benefits to BMP9-mediated bone tissue engineering.
Results
Isolation and characterization of hAMSCs
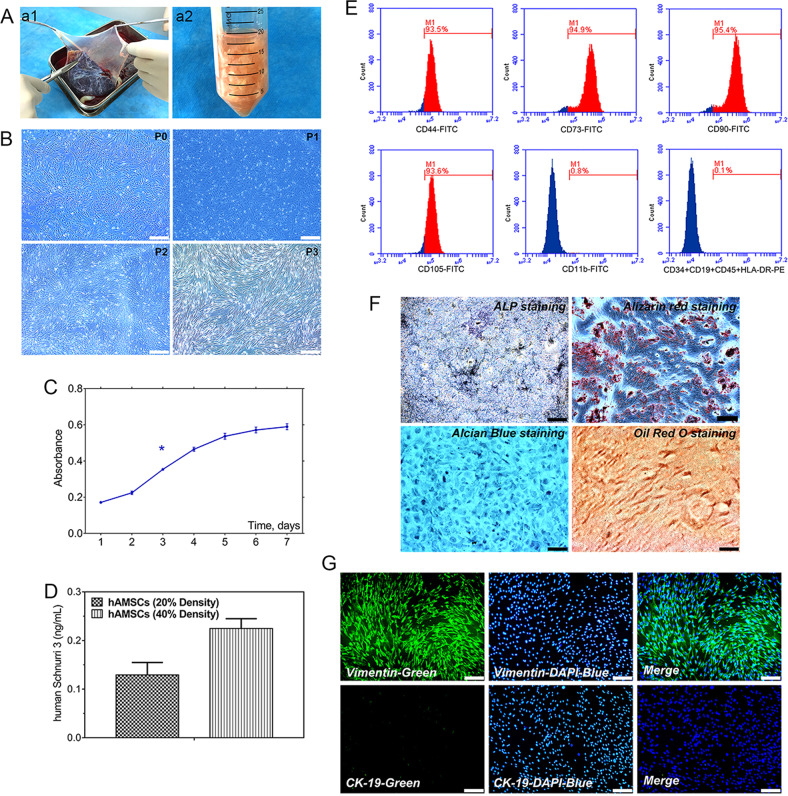
hAMSCs were isolated from superficial amnion on human placenta by using both trypsin and collagenase (Fig. 1a). Morphology of cultured primary and passaged 1, 2, and 3 (P1, P2, P3, respectively) cells showed a monolayer of adherent cells and demonstrated a spindle-shaped exterior with radial-like growth, and increasing polarization was observed with each passage. hAMSCs run up to 80% confluence over a period of approximately 5 days, whereas hAMSCs generally require 7 days to cover the culture flask (Fig. 1b). Cell Counting Kit (CCK)-8 results showed that the proliferative curve of hAMSCs exhibited an “S” pattern, and hAMSCs went through a logarithmic growth phase for 4 days after 1 day of latency. The average doubling time of hAMSCs was 72 h (Fig. 1c).
Fig. 1. Isolation and morphology of cultured hAMSCs; the proliferation potential and identification of hAMSCs; the basic expression of Shn3 in hAMSCs.
a General observation (a1), isolation, and extraction (a2) of hAMSCs. b Representative morphology of adherent hAMSCs from primary cultures (P0) were cultured through the third passage (P3) with spindle shapes on cell culture dish (original magnification ×40, scale bar = 200 μm). c Proliferation of hAMSCs as determined by the CCK-8 method showed that cells reach the doubling time at 3 days. d ELISA assay to detect the Shn3 expression in cell culture supernatants when cells reached 20% and 40% confluence, respectively. e Phenotypic properties of hAMSCs at third passage by flow cytometry and hAMSCs highly expressed CD44, CD73, CD90, and CD105 and negatively expressed CD34, CD19, CD45, and HLA-DR. f Multiple potential for osteogenic, chondrogenic, and adipogenic differentiation of hAMSCs at third passage: osteogenic (alkaline phosphatase staining and Alzarin Red S staining), chondrogenic (Alcian Blue staining), adipogenic (Oil Red O staining) (original magnification ×100, scale bar = 100 μm). g hAMSCs at third passage highly expressed vimentin and hardly expressed CK-19, and cell nuclei were stained blue by 4′,6-diamidino-2-phenylindole (DAPI) (original magnification ×100, scale bar = 100 μm). The data are shown as mean ± SD for three separate experiments. *P < 0.05.
Identification and multidirectional differentiation potential of hAMSCs
Flow cytometric results showed that P3 hAMSCs were positive for MSC markers CD44, CD73, CD90, and CD105 and weakly expressed hematopoietic markers CD34, CD19, CD45, and HLA-DR, which indicated that P3 hAMSCs were in possession of low immunogenicity (Fig. 1e). In addition, the results of alkaline phosphatase (ALP), Alizarin S Red, Alcian Blue, and Oil Red O staining showed that hAMSCs own the potential for multidirectional differentiation into osteoblasts, chondrocytes, and adipocytes (Fig. 1f). CK-19 is an exceptional marker of human amniotic epithelial cells, whereas vimentin is a specific marker of hAMSCs. During the isolation process of hAMSCs, small portions of human amniotic epithelial cells from amnion were present in hAMSCs. To reduce the rate of epithelial cells in hAMSCs, we passaged cells; epithelial cells gradually go through a conversion from epithelial to mesenchymal. The immunofluorescent staining results showed that P3 hAMSCs highly expressed vimentin and were negative for CK-19 expression (Fig. 1g). These results demonstrated that hAMSCs expressed pluripotent markers, and hAMSCs have the ability of self-renew and possess multi-linage differentiation potential.
Basic expression of Shn3 in hAMSCs
To detect the basic expression level of Shn3 in hAMSCs, we used an enzyme-linked immunosorbent assay (ELISA). Results of the ELISA assay showed that the concentration of Shn3 in the supernatant of cell cultivation was 0.1316 ng/mL when hAMSCs reached a density of approximately 20% (the number of cells reached about 1.8 × 105 cells/mL), while the concentration of Shn3 in the supernatant of cell cultivation was 0.2298 ng/mL when hAMSCs reached approximately 40% confluence (the number of cells reached about 3.7 × 105 cells/mL) (Fig. 1d). These results prompted us to determine that hAMSCs have a certain degree of expression of Shn3.
Shn3 diminishes BMP9-induced early and late osteogenic differentiation of hAMSCs in vitro
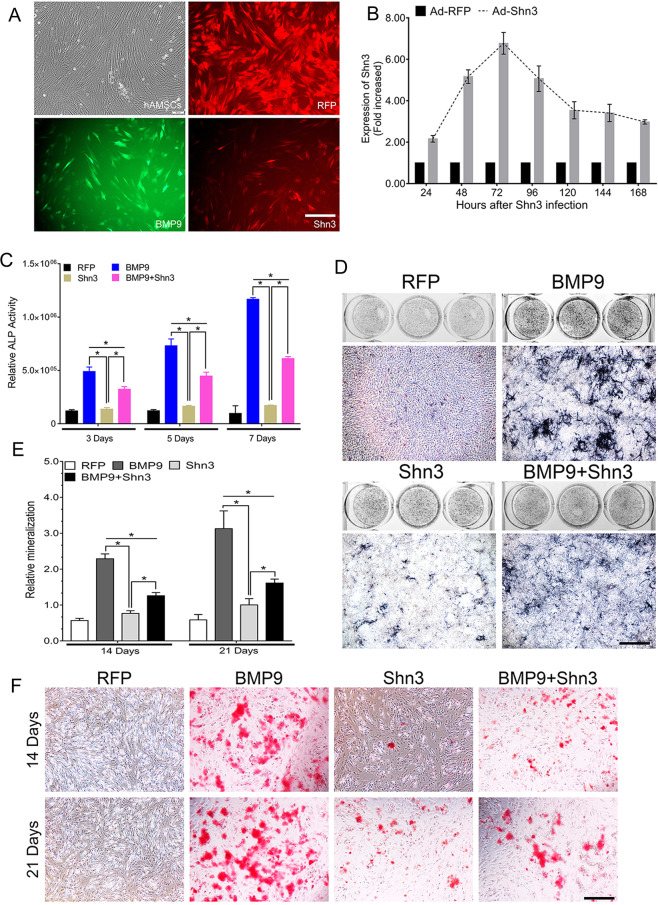
In order to assess the role of Shn3 in BMP9-induced osteogenic differentiation, we constructed an adenoviral vector system to stably overexpress Shn3 that target human Shn3. After transfection of the cells with Ad-RFP, Ad-BMP9, and Ad-Shn3 of hAMSCs for 24 h, cells were observed by fluorescence microscope (Fig. 2a). By using reverse transcription and quantitative polymerase chain reaction (RT-qPCR) assay, we found that the expression levels of Shn3 were significantly increased in hAMSCs after transfection with Ad-Shn3 for 48 and 72 h when compared with that of control hAMSCs transfected with Ad-RFP (Fig. 2b). Therefore, Ad-Shn3 was used to upregulate Shn3 levels in our study. ALP were used to determine the changes in ALP activity, which indicate early osteogenic activity. First, we examined the effect of Ad-Shn3 on early and late osteogenic differentiation on hAMSCs. Our results showed that ALP activity was dramatically decreased in the BMP9+Shn3 group compared to the BMP9 group at days 3, 5, and 7, whereas exogenous Shn3 expression alone did not exhibit any significant effect on ALP activity of hAMSCs at 7 days (Fig. 2c). Quantitatively, the Shn3-mediated synergistic effect on ALP activity in BMP9-transfected hAMSCs was decreased by 63%, 76%, and 85% on days 3, 5, and 7, respectively (Fig. 2d). Alizarin Red S staining was used to examine the calcium deposition, which is one of the late osteogenic indicators. Our results showed that the calcium deposition was redundantly decreased in the BMP9+Shn3 group compared to the BMP9 group at 14 and 21 days; quantitatively, overexpression of Shn3 reduced the matrix mineralization compared with the other groups (Fig. 2e, f). Taken together, Shn3 is shown to be able to significantly inhibit the early and late osteogenic differentiation of hAMSCs.
Fig. 2. Shn3 inhibits early and late osteogenic differentiation of hAMSCs in vitro.
a The recombinant adenovirus Ad-RFP (Red), Ad-BMP9 (green), and Ad-Shn3 (red) were shown to effectively transfect hAMSCs for 24 h (scale bar = 100 μm). b Ad-Shn3 upregulated the expression of Shn3 over 5 times at 48, 72, and 96 h. All samples were normalized with the house-keeping gene GAPDH. Each experiment was done in triplicate, Ad-Sim-Shn3 vs. Ad-RFP groups. c, d Overexpressed Shn3 inhibits BMP9-induced ALP activity in hAMSCs. ALP biochemical quantification assay (c) and ALP staining assay (d) were conducted to detect the ALP activity under the treatment as shown at 7 days after transfection (scale bar = 100 μm). e, f The quantification of mineralization (e) and calcium deposition is observed by Alizarin Red S staining assay (f) under the treatment as shown at 14 and 21 days after transfection (scale bar = 100 μm).The data are shown as mean ± SD for triplicate. *P < 0.05.
Silencing Shn3 expression potentiates BMP9-induced osteogenic differentiation of hAMSCs in vitro
To determine whether Shn3 is an essential mediator in BMP9-induced osteogenic signaling, we constructed a recombinant adenovirus that expresses a pool of three small interfering RNA (siRNA) targeting human Shn3-coding regions using the established pSOS system as recently described36, which prompted Ad-Sim-Shn3 to efficaciously knock down Shn3 expression in hAMSCs. After transfecting the cells with Ad-RFP, Ad-BMP9, and Ad-Sim-Shn3 for 24 h, cells were observed by fluorescence microscope (Fig. 3a). By using RT-qPCR, we determined the effectiveness of transfecting Ad-Sim-Shn3 on hAMSCs. We found that Ad-Sim-Shn3 could greatly inhibit mRNA expression of Shn3 from 24 to 168 h (Fig. 3b).
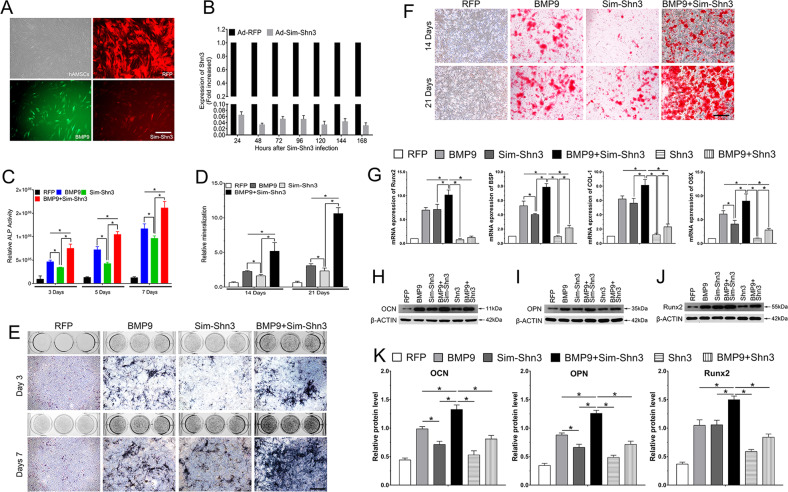
Fig. 3. Silencing Shn3 expression enhanced BMP9-induced early and late osteogenic differentiation of hAMSCs in vitro.
a The recombinant adenovirus Ad-RFP (Red), Ad-BMP9 (green), and Ad-Sim-Shn3 (red) were shown to effectively transfect hAMSCs for 24 h (scale bar = 100 μm). Effective knockdown of Shn3 expression. b The Ad-Sim-Shn3 expressing siRNA targeting Shn3 transduces hAMSCs with high efficiency after transfection. Ad-Sim-Shn3 silences the expression of Shn3 from 48 to 120 h. All samples were normalized with the house-keeping gene GAPDH. Each experiment was done in triplicate, Ad-Sim-Shn3 vs. Ad-RFP groups. c, e Silencing Shn3 promotes BMP9-induced ALP activity in hAMSCs. ALP biochemical quantification assay (c) and ALP staining assay (e) were conducted to detect the ALP activity under the treatment as shown at 3 and 7 days after transfection (Scale bar = 100 μm). d, f The quantification of mineralization (d) and calcium deposition is observed by Alizarin Red S staining assay (f) under the treatment as shown at 14 and 21 days after transfection (scale bar = 100 μm). g–k Shn3 inhibits the expression of osteogenic relative factors of hAMSCs. g RT-qPCR assay was performed to determine that Shn3 inhibits the expression of osteogenic relative factors, including Runx2, BSP, COL-1, and OSX, induced by BMP9 of hAMSCs at 7 days. h–k Western blotting assay was adopted to detect that Shn3 inhibits the expression of osteogenic relative factors OCN (h), OPN (i), and Runx2 (j) induced by BMP9 of hAMSCs. β-ACTIN served as the loading control. The quantification results of western blotting assay showed the effect of Shn3 on the expression level of OCN, OPN, and Runx2 on BMP9-induced hAMSCs (k). The data are shown as mean ± SD for triplicate. *P < 0.05.
Further, we analyzed the effect of downregulating Shn3 expression on BMP9-induced osteogenic differentiation of hAMSCs. The results showed that ALP activities were significantly increased at 3, 5, and 7 days by co-transfection with Ad-BMP9 and Ad-Sim-Shn3 compared with the control BMP9 group at each time point (Fig. 3c). However, cells transfected with Ad-Sim-Shn3 alone showed a slight increase inn ALP activity of hAMSCs compared with Ad-BMP9 at 3 and 7 days. By quantification, ALP histochemical staining returned similar results to that of the expression of Ad-Sim-Shn3; both significantly enhanced BMP9-induced ALP activity in hAMSCs at 3 and 7 days (Fig. 3e). Moreover, BMP9-induced calcium deposition in hAMSCs was notably increased at days 14 and 21 when the expression of Shn3 was silenced as illustrated by Alizarin Red S staining (Fig. 3d, f). Conjointly, these results indicate that inhibiting Shn3 expression significantly potentiates BMP9-induced early and late osteogenic differentiation in vitro, which shows that Shn3 may play a critical role in BMP9-induced osteogenesis.
Shn3 inhibits the gene and protein expression of osteogenesis-related factors
Runx2 is a crucial osteoblast-specific mediator that plays a central role in osteoblast differentiation, bone formation, and remodeling. Runx2, bone sialoprotein (BSP), collagen type I (COL-1), osterix (OSX), and osteocalcin (OCN) play an important role in regulating anabolic bone formation and calcium metabolism. Hence, we determined the effects of Shn3 on the BMP9-induced expression of Runx2, BSP, COL-1, OSX, and OCN in hAMSCs. The RT-qPCR results revealed that the mRNA levels of Runx2, BSP, COL-1, and OSX in the BMP9+Sim-Shn3 group were significantly upregulated at day 7 compared to the BMP9 group (Fig. 3g); in contrast, the gene expression levels of these factors were decreased greatly in the BMP9+Shn3 group compared with the control BMP9 group at each time point. Moreover, the expression levels of these factors were also lower in the Shn3 group when compared with the Sim-Shn3 group and the red fluorescent protein (RFP) group. Similarly, the protein expression of OCN, osteopontin (OPN), and Runx2 were clearly more upregulated in the BMP9+Sim-Shn3 group at day 7 than in the other groups; the Shn3 group showed lower protein expression level compared with the BMP9 group and the BMP9+Shn3 group and but was higher than the RFP group (Fig. 3h–k). Taken together, these results suggest that Shn3 exerts a negative effect on the mRNA and protein expression levels of osteogenesis-related factors.
Shn3 suppresses the subcutaneous ectopic bone formation in hAMSCs in nude mice
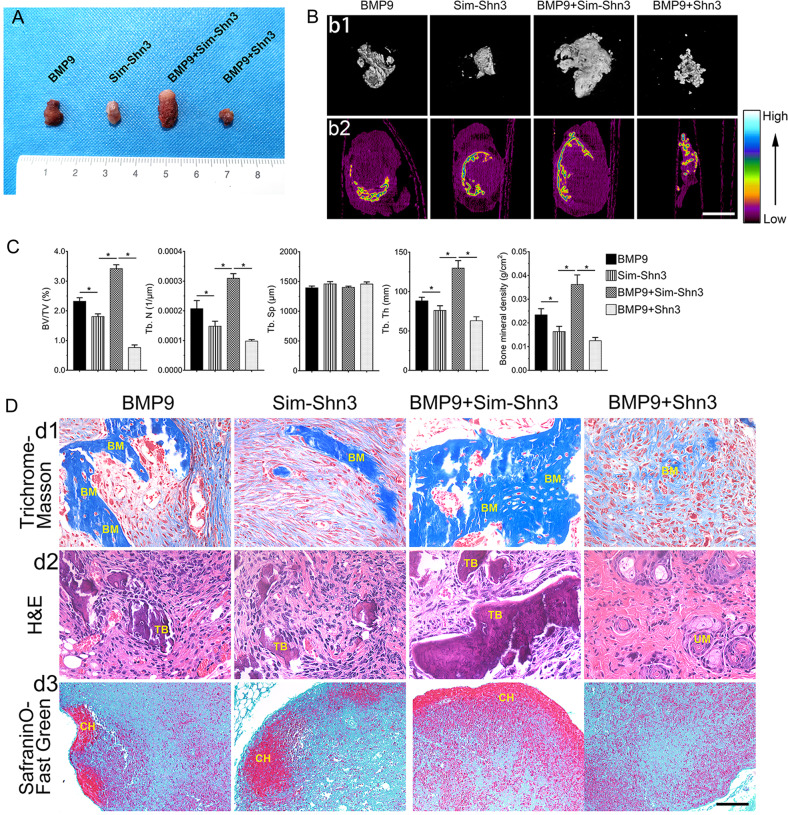
On the basis of the conclusion that Shn3 restrained BMP9-induced osteogenic differentiation of hAMSCs in vitro, we further examined the effect of Shn3 on BMP9-induced osteogenic differentiation with the well-established subcutaneous ectopic bone formation in nude mice. The general observation and the results of three-dimensional reconstruction of micro-computed tomography (micro-CT; three-dimensional (3D)) revealed that the volume of ectopic bone mass was decreased in the BMP9+Shn3 group compared to the BMP9 group. The BMP9+Sim-Shn3 group showed a strong ability to potentiate the development of osteogenic masses compared to the other groups (Fig. 4a, b1). Mineral density expressed through a heat map from a micro-CT demonstrated that silencing the expression of Shn3 increased the average mineral density of the bone masses induced by BMP9 in hAMSCs; in contrast, overexpression of Shn3 greatly decreased the average mineral density formed by BMP9-transfected cells (Fig. 4b2). Quantitative analysis of bone histomorphometry showed that the values of bone volume/total volume (BV/TV), trabecular number (Tb. N), trabecular thickness (Tb. Th), and bone mineral density (BMD) were significantly increased in the BMP9+Sim-Shn3 group compared with the BMP9 group. In the opposite set-up, these parameters were greatly decreased in the BMP9+Shn3 group compared to the control. In addition, the results showed that trabecular separation (Tb. Sp) did not create any differences in each group (Fig. 4c). Besides, histological evaluation revealed that Sim-Shn3 increases the number of trabecular bone and the formation of the ossified matrix after transfection with BMP9 compared to the BMP9 group. Shn3 inhibited the BMP9-induced formation of bone matrix (Fig. 4d2). The Masson’s trichrome staining showed that Sim-Shn3 can increase the ossified matrix induced by BMP9, and silencing Shn3 can decrease the osteoid matrix maturation after being transfected with BMP9 (Fig. 4d1). However, overexpressing or silencing Shn3 expression levels did not affect BMP9’s effect on chondrogenesis (Fig. 4d3). These in vivo data are consistent with the in vitro studies. In summary, our data strongly suggested that Shn3 is a critical mediator of BMP9-induced osteogenic signaling and that Shn3 exerts a negative regulatory effect on BMP9-induced osteogenic differentiation of hAMSCs in vitro and in vivo.
Fig. 4. Shn3 restricts the BMP9-induced subcutaneous ectopic osteogenesis of hAMSCs in nude mice.
a The general observation of the subcutaneous bone masses in nude mice. b Subcutaneous osteogenic bone formation at 4 weeks detected by micro-CT scanning to determine the 3D iso-surface (b1) and the heat map of average mineralization density (b2). In the heat map analysis, white represents the highest average mineral density and black represents the lowest (scale bar = 1 mm). c Quantification results of ectopic bone formation and the relative values of BV/TV, Tb. N, Tb. Sp, Tb. Th, and bone mineral density (BMD) were analyzed. These data are shown as mean ± SD repeat triplicate. d The samples of bone masses were subjected to Masson’s trichrome staining (d1), H&E staining (d2), and Alcian Blue staining (d3) to determine the formation of trabecular bone and bone matrix (magnification ×400, scale bar = 50 μm). Representative images are shown. BM bone matrix, UM undifferentiated MSCs, CH chondrocytes, TB trabecular bone. *P < 0.05.
Shn3 inhibits the angiogenic differentiation and vascularization in BMP9-induced osteogenic differentiation
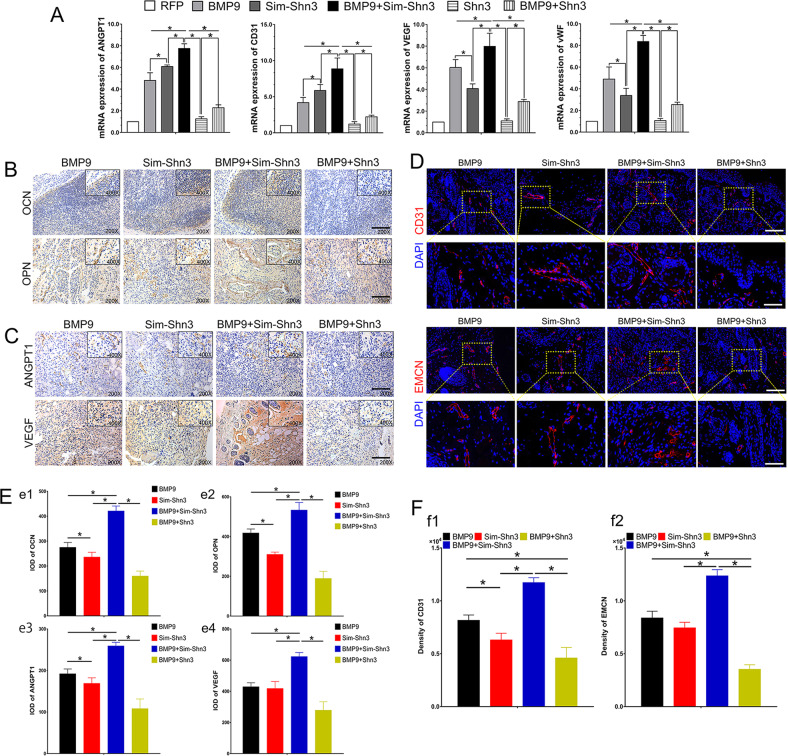
Angiogenesis and osteogenesis are closely coupled in bone development and regeneration. Thus we further determine the effect of angiogenic differentiation and vascularization regulated by Shn3. We used a RT-qPCR assay to detect the transfected hAMSCs on days 3, 5, and 7. The results showed that the mRNA level of angiopoietin 1 (ANGPT1), CD31, VEGF, and vWF were significantly upregulated in the BMP9+Sim-Shn3 group compared to the BMP9 group at day 7. Meanwhile, the gene expression levels of these factors were downregulated drastically in the Shn3 group compared to the BMP9 group at day 7 (Fig. 5a).
Fig. 5. Histological analysis of the effect of Shn3 on osteogenesis and angiogenesis.
a Shn3 downregulates the mRNA level of BMP9-induced angiogenic relative factors including ANGPT1, CD31, VEGF and vWF at day 7. b, e Immunohistochemical staining and quantification results were adopted to detect the effects of Shn3 on the expression of osteogenesis relative markers OCN (e1) and OPN (e2) in BMP9-induced ectopic bone masses of hAMSCs (magnification ×200, ×400, scale bar = 50 μm). c, e Immunohistochemical staining and quantification results was adopted to detect the effects of Shn3 on the expression of angiogenesis relative markers ANGPT1 (e3) and VEGF (e4) in BMP9-induced ectopic bone masses of hAMSCs (magnification ×200, ×400). d, f Immunofluorescence staining and quantified analysis were used to determine the effects of Shn3 on the expression of Type H vessels, including CD31 (red) (f1) and EMCN (red) (f2) and DAPI (blue) (magnification of up images = ×200, of down images ×400, scale bar = 50 μm). *P < 0.05.
Moreover, the immunohistochemical staining results of ectopic bone masses demonstrated that the expression levels of OCN and OPN were upregulated in the BMP9+Sim-Shn3 group and downregulated in the BMP9+Shn3 group compared with the BMP9 group (Fig. 5b, e1, e2). Similarly, the immunohistochemical results also revealed that the angiogenesis-related proteins of ANGPT1 and VEGF were highly expressed in the BMP9+Sim-Shn3 group and minimally expressed in the BMP9+Shn3 group compared with the BMP9 group (Fig. 5c, e3, e4). Recent studies have showed that the H-type micro-vessels highly express CD31 and endothelium (CD31hiEMCNhi), which have the function of regulating osteoblasts and serve an important role as mediators of bone regeneration37. Beyond this, we investigated the role of Shn3 in BMP9-induced CD31hiEMCNhi vascular endothelium in ectopic bone formation. The results showed that Sim-Shn3 could potentiate BMP9-induced expression of CD31hiEMCNhi endothelium as compared with the BMP9 group. However, CD31hiEMCNhi endothelium expression in the Shn3 group was significantly lower than that seen in the BMP9 group (Fig. 5d, f1, f2). In summary, these data suggest that Shn3 exhibits a negative regulatory effect on angiogenic differentiation and osteogenic differentiation of hAMSCs in vivo.
Effects of Shn3 on BMP9-induced angiogenesis in vitro
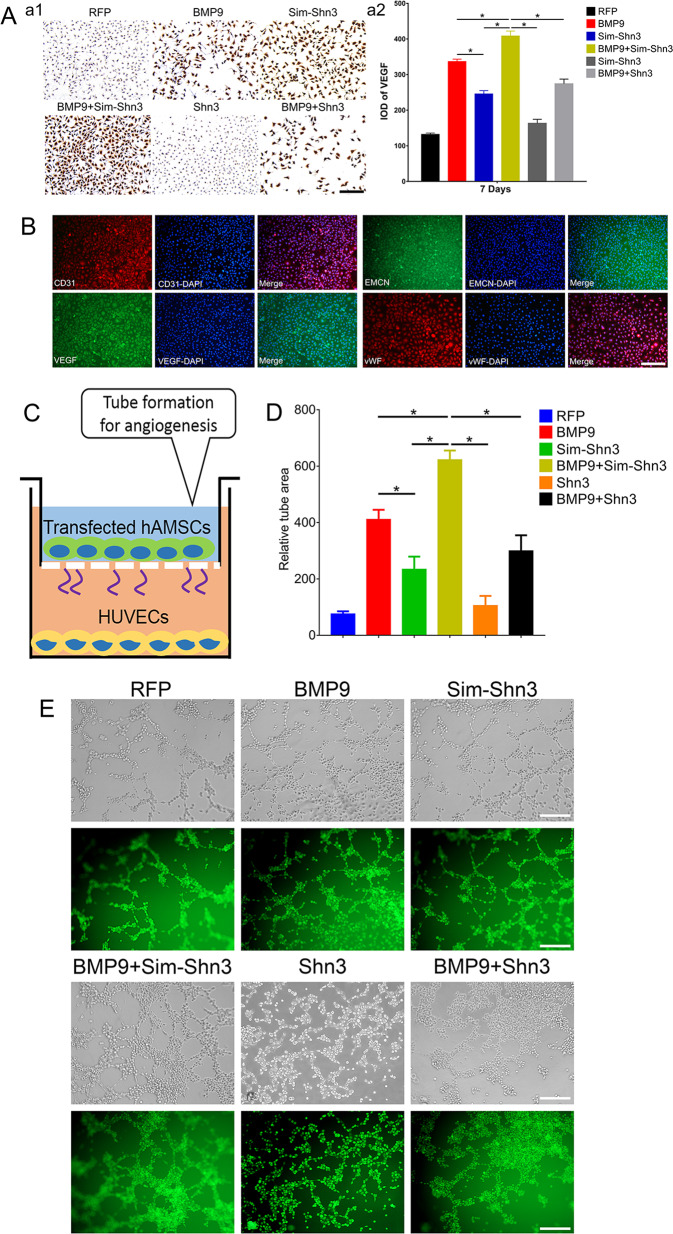
To further investigate the effects of Shn3 on BMP9-induced angiogenesis of hAMSCs, we used a VEGF assay of hAMSCs and observed the results with immunohistochemical staining. The expression of VEGF was higher in the BMP9+Sim-Shn3 group and lower in the BMP+Shn3 group compared with the control BMP9 group at day 7. In addition, the Shn3 group expressed the lowest expression of VEGF of any of the groups (Fig. 6a1, a2).
Fig. 6. Effect of Shn3 on BMP9-induced angiogenic differentiation in hAMSCs in vitro.
a Immunohistochemical staining (a1) and its quantification analysis (a2) were performed to detect the effects of Shn3 on BMP9-induced protein expression of VEGF in hAMSCs (magnification ×200, scale bar = 50 μm). b Phenotypes of HUVECs at third passage was identified by immunofluorescence staining assay that HUVECs positively expressed CD31, VEGF, EMCN, and vWF (magnification ×100, scale bar = 100 μm). c To investigate the effects of tube formation induced by HUVECs, the Matrigel was coated on Transwell culture plates and HUVECs were cultivated in the ECM medium for 12 h. The HUVECs were adjusted to 2 × 105 cells/mL/well; hAMSCs were treated as the experimental design and seeded onto the upper wells. d, e The number of tube structures was recorded beginning at 6 h. The tube formation results were observed by light and fluorescence microscope. The HUVECs treated with calcein AM (green) were observed by fluorescence microscopy. The tube-formation results (e) and the quantified number of tube area (d) are shown (magnification ×200, scale bar = 50 μm). *P < 0.05.
The phenotypes of human umbilical vein endothelial cells (HUVECs) were identified by immunofluorescence staining, and the results showed that the HUVECs highly expressed CD31, VEGF, EMCN, and vWF (Fig. 6b). The HUVEC tube-formation assay revealed that silencing Shn3 expression could markedly increase the tube area in BMP9-induced hAMSCs, which had 1.38-fold greater area of tube formation than the control (BMP group). The volume of tube area in the BMP9+Shn3 group was lower; it showed a 0.78-fold change than that from the BMP9 group (Fig. 6d, e). Taken together, these results suggest that Shn3 greatly inhibits the BMP9-induced angiogenesis of hAMSCs in vitro.
Effects of Shn3 on BMP9-induced vessel invasion in vivo
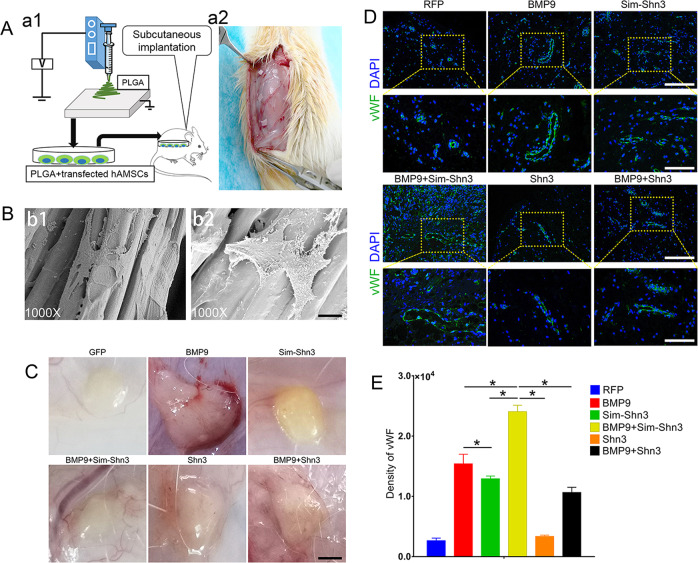
To further investigate the effect of Shn3 on angiogenesis in vivo, the cells were treated as the experimental design, seeded on poly(lactic-co-glycolic acid (PLGA) scaffolds, and implanted on the dorsal subcutaneous tissues of mice (Fig. 7a). The topological features of PLGA were demonstrated and the PLGA that had been seeded with cells for 24 h was observed by scanning electron microscope (Fig. 7b). The grafts were evaluated by general observation and by immunofluorescence staining for vWF after 4 weeks. The gross graft was observed when the vascular invasion experiment was completed. The BMP9+Sim-Shn3 group showed a strong vascularization ability as compared to the BMP9 group, while the RFP group hardly had any vessels on the surface of the graft. By contrast, the BMP9+Shn3 group showed lower angiogenesis than the BMP9 group, while the vascularization activity in the Shn3 group was also lower than the Sim-Shn3 group (Fig. 7c). The immunofluorescence results revealed that the PLGA scaffold seeded with hAMSCs that had been transfected with BMP9+Sim-Shn3 showed significantly greater expression of vWF than when transfected with BMP9 alone. However, the overexpression of Shn3 could attenuate the expression of vWF compared with the BMP9 group, and there was no significance found within the RFP group (Fig. 7d, e).
Fig. 7. Effects of Shn3 on subcutaneous vascular invasion of PLGA–hAMSCs composite in vivo.
a Illustrative diagram showing that hAMSCs treated as experimental design seeded on the electro-spun PLGA scaffolds (a1) were implanted into the dorsal subcutaneous space of the mice and harvested for analysis after 5 weeks (a2). b The scanning electron microscopy was adopted to detect the PLGA (b1) and cells cultured on PLGA scaffold (b2) (magnification ×1000, scale bar = 200 μm). c The macromorphological observation of PLGA implants located on subcutaneous tissue at 5 weeks. d, e Immunofluorescence staining (d) and its quantification analysis (e) were performed to detect the vWF expression (green) in PLGA scaffold subcutaneously (magnification of up images = ×200, of down images ×400, scale bar = 50 μm). *P < 0.05.
Effects of Runx2 and VEGF signal on the expression of BMP9 and Shn3 in hAMSCs
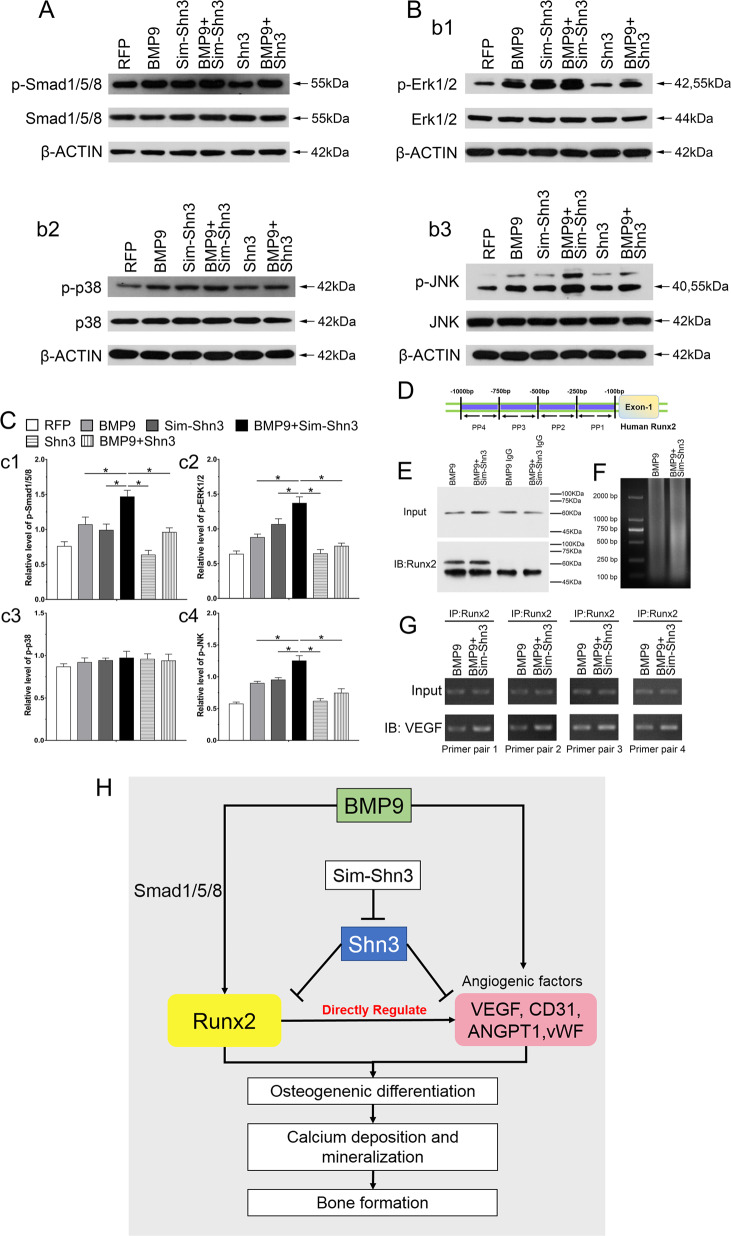
BMP9 routinely produced a marked effect on physiological function through the BMP/Smad signaling pathway or the non-canonical BMP/Smad signaling pathway, so we first examined whether Shn3 could exert any effect on the BMP/Smad signaling pathway. By analyzing western blots, the results showed that BMP9 significantly increased the phosphorylation of Smad1/5/8 (p-Smad1/5/8) and had effects on the level of total Smad1/5/8. Despite the fact that Sim-Shn3 had no explicit effects on the expression levels of p-Smad1/5/8 and Smad1/5/8, it remains relevant to the effect of BMP9 on the expression of p-Smad1/5/8 in hAMSCs. While overexpression of Shn3 did not exert any effect on the expression of p-Smad1/5/8 and Smad1/5/8, it markedly decreased the level of p-Smad1/5/8 by inducing BMP9 in hAMSCs (Fig. 8a, c1). MAPK signaling are vital part of non-Smad BMP pathway. It is reported that BMP9 was capable of activating extracellular signal–regulated kinase 1/2 (ERK1/2) and p38 MAPKs. It is unclear that Shn3 exerts any effect on MAPK signaling pathway. The western blotting results revealed that the expression levels of p-Erk1/2 and p-JNK were higher in the BMP9+Sim-Shn3 group and lower than the Shn3 group as compared with the BMP9 group. In addition, the expression level of p-p38 did not exert any differences in each group (Fig. 8b, c2–c4). These data may point to the effect of Shn3 on inhibiting BMP9-induced osteogenic differentiation, and the molecular mechanism may be mediated by diminishing the BMP/Smad and BMP/MAPK signaling pathways.
Fig. 8. Effect of Shn3 and BMP/Smad signaling on the expression of Runx2 and VEGF in hAMSCs.
a–c Western blot and quantification analysis (c) were adopted to determine the effects of Shn3 and sim-Shn3 on protein level of p-Smad1/5/8, Smad1/5/8 (a), p-Erk1/2, Erk1/2, p-p38, p38, p-JNK, and JNK (b) under the treatment as shown at 5 days after transfection. The β-ACTIN served as the loading control. The same blots of p-Smad1/5/8 and Smad1/5/8, p-Erk1/2 and Erk1/2 (b1), p-p38 and p38 (b2), and p-JNK and JNK (b3) are used for the β-ACTIN control. Total of 12 gels were ran and 12 blots were made. d–g ChIP assay analysis shows the interaction between VEGF and promoter region of human Runx2 in hAMSCs (PP1, primer pair 1; PP2, primer pair 2; PP3, primer pair 3; PP4, primer pair 4) (d). The results show that VEGF is a direct target of Runx2 regulated by Sim-Shn3 in BMP9-induced hAMSCs. The hAMSCs were transfected with Ad-BMP9 or Ad-BMP9+Ad-Sim-Shn3 for 36 h followed by formaldehyde crosslinking. The pulled down composite was detected by gel electrophoresis image and the location of primers used for ChIP assay in Runx2 promoter region (e). The crosslinked cells were lysed and subjected to enzymolysis and immunoprecipitation with Runx2 antibody or IgG antibody (f). IP assay results show the enhanced interaction between Runx2 and VEGF in the BMP9+Sim-Shn3 group of hAMSCs compared to the BMP9 group (g). *P < 0.05. h The role of Shn3 regulates Runx2 and VEGF in BMP9-induced angiogenesis–osteogenesis coupling during hAMSC-mediated bone formation. Shn3 was able to inhibit the expression of Runx2 through BMP9-mediated BMP/Smad1/5/8 signaling, thereby inhibiting osteogenic marker factors to enhance osteogenesis. Silencing Shn3 could increase the level of angiogenesis relative factor VEGF induced by BMP9. VEGF is an essential downstream target of Runx2 that could regulate the differentiation of skeletal progenitor cells into osteoblasts both in vitro and in vivo, and VEGF is directly activated by Runx2 in MSCs. Because our preliminary results and previous reports all support the point that silencing Shn3 not only positively regulates the osteogenesis of MSCs through Runx2 induced by BMP9 but also regulates the VEGF, which is directly activated by Runx2 to form the osteogenesis–angiogenesis coupling to promote osteogenic differentiation and calcium deposition, which eventually contribute to bone regeneration.
The balance of bone metabolism is dependent on the interaction between blood vessels and osteocytes. Angiogenesis and bone formation are coupled to each other through specific vascular forms and pathways. Recent studies have confirmed that VEGF participated in the initiation of angiogenesis and Runx2 as a key regulator in start-up osteoblast differentiation of MSCs induced by BMPs. Thus we further determined the relationship between Runx2 and VEGF, which is regulated by Shn3 and BMP9. Chromatin immunoprecipitation (ChIP) assay results showed that Runx2 can bind with the promoter region of VEGF in hAMSCs after being transfected with BMP9+Sim-Shn3 (Fig. 8d–g). Immunoprecipitation (IP) and western blotting results revealed that Runx2 has an interaction with VEGF in hAMSCs by transfecting with BMP9+Sim-Shn3. These results strongly indicate that silencing Shn3 may mediate the effect of BMP9 on enhancing angiogenesis and osteogenesis through the interaction between Runx2 and VEGF to some extent.
Discussion
Bone formation and angiogenesis are two closely related processes in bone development, remodeling, and repair38,39. Vascular invasion is a prerequisite for the coupling of angiogenesis and bone formation. Neovascularization is not only a bridge pathway for nutrient provisioning and bone tissue metabolism but also plays an active role in the regulation of bone formation31,40. Therefore, determining the coupling effect between bone formation and angiogenesis is of great significance for bone regeneration. In this present study, we demonstrated the effects of Shn3 on BMP9-induced osteogenic and angiogenic differentiation in hAMSCs and identified the possible mechanism underlying this process. We found that the basic expression of Shn3 is detectable in hAMSCs and BMP9 can partially downregulate the expression of Shn3. Silencing the expression of Shn3 can potentiate BMP9-induced osteogenic factors in hAMSCs, while exogenous expression of Shn3 restrains BMP9-induced ALP activities and calcium deposition in hAMSCs, as well as the ectopic bone formation. Meanwhile, we also analyzed the role of Shn3 in BMP9-induced angiogenic differentiation in hAMSCs and vascular invasion in vivo. We found that silencing the expression of Shn3 upregulates the BMP9-induced angiogenesis-related factors and enhances subcutaneous vascularization. Despite the fact that lumen size seems different among groups, the protein expression level of vWF by immunofluorescence staining is a primary indicator to evaluate the formation of blood vessels. Mechanistically, we found that inhibition of Shn3 can enhance BMP9-induced BMPs/Smad signal transduction, as well as Runx2 and VEGF expression. Shn3 may exert this function through enhancing BMP/Smad signals and we also demonstrated that restraining the expression of Shn3 can activate Runx2, which is capable of directly regulating the expression of angiogenic factor VEGF. These results strongly indicate that Shn3 may play a critical role in regulating the BMP9-induced coupling effect between osteogenesis and angiogenesis in MSCs, which may be mediated by regulating Runx2 and VEGF signaling at least.
MSCs are multi-potent, self-renewing, and undifferentiated cells. BMSCs are considered favorable sources of MSCs; however, the difficulty in obtaining BMSCs comes from their invasive extraction process41. MSCs have attracted a lot of attention in cell-based therapy to be practical in clinical settings. Recently, the placenta has gained attention because of its abundance and availability42. The placenta is a feto-maternal organ that is disposed of after delivery and can be acquired with no invasive procedures, which makes it a favorable source with no ethical limitations43. hAMSCs were derived from the amniotic membrane on the surface of placenta. Compared to other tissue-originated MSCs, hAMSCs have many advantages. Their non-invasive and convenient collection has been widely used in trauma, neurological diseases, and spinal cord injury. Notably, the hAMSCs have lower DNA methylation levels, which contribute to more congruous overlap with the human genome44,45. Owing to the placenta’s high vascularization, hAMSCs are provided with early progenitors of hemangiogenic cells. In our study, the flow cytometric results showed that the hAMSCs negatively expressed the cell surface markers CD11b and HLA-DR, which give hAMSCs low immunogenicity. Thus we chose hAMSCs as seed cells that can differentiate into osteoblasts and perform angiogenesis.
Despite of osteogenic differentiation mediated primarily by MSCs, it is likely that a bunch of factors are involved in markers, such as BMPs, Runx2, VEGF, TGF-β, and insulin-like growth factor46,47. Besides these, many tissue types exist in the bone, including vascular endothelium and connective tissues and autonomic and sensory nerves, which contribute to forming a favorable milieu for bone formation48,49. BMPs belong to the TGF-β super-family, which are recognized as playing critical roles in regulating bone formation and proliferation50,51, as well as in angiogenesis52,53. However, some members of BMPs can commit MSCs to osteoblast lineages, such BMP2, BMP4, BMP6, and BMP754,55. In a recent study, we reported that BMP9 (also termed as growth differentiation factor 2) is one of the most potent BMPs among the 14 types of BMPs in the induction of osteogenic differentiation19,56. BMP9 usually produces its marked effect through the BMP/Smad signaling pathway, which includes the canonical BMP/Smad pathway or the non-canonical BMP/Smad pathways, such as ERK and p38 MAPK pathway57. In the canonical BMP/Smad pathway, BMP9 activates the corresponding phosphorylated R-Smad (named Smad1/5/8), and the phosphorylated Smad1/5/8 (p-Smad1/5/8) recruits and phosphorylates Smad4 to form the complex. From there, the complex shifts to the nucleus where it can regulate the expression of downstream targets58. Beyond this, there are lots of factors or signals that are also implicated in regulating the BMP9-induced osteogenic and angiogenic differentiation, including VEGF, Runx2, and fibroblast growth factor (FGF)59. Our results showed that silencing the expression of Shn3 increases the phosphorylation level of Smad1/5/8, which suggested that Shn3 may participate in the BMP9-induced Smad signaling pathway. For the non-canonical BMP/Smad pathway, silencing Shn3 could significantly potentiate the level of phosphorylated ERK1/2. Taken together, we conclude that Shn3 inhibits the BMP9-induced osteogenic differentiation of hAMSCs by blocking the gene expression and downregulating the BMP/Smad and BMP/MAPK signaling pathways.
Shn3 is a large zinc finger protein that plays a vital role in the process of embryogenesis as a critical nuclear factor for DPP signaling pathway, which is the Drosophila homolog of BMP/TGF-β23. Shn3 was identified as a DNA-binding protein of the heptameric recombination signal sequence and is one of the mammalian homologs of Drosophila Shn60. Shn3 not only functions as an adaptor protein in the immune system that interacts with nuclear factor-κB to regulate tumor necrosis factor-α and interleukin-2 but also serves a function in regulating bone formation21,61. It has been reported that Shn3 mutant (Shn3−/−) mice exhibit a conspicuously high bone mass phenotype, and this phenotype was regulated by a multimerized complex containing Shn3, Runx2, and the NEDD4 (an E3 ubiquitin ligase)–WWP125. This complex was regulated by Shn3 and inhibits Runx2 function. Hence, the absence of Shn3 result in elevated levels of Runx2 protein and potentiated transcriptional activity of Runx2 that profoundly increased the degree of bone formation62. In our study, silencing the expression of Shn3 significantly increased the BMP9-induced ALP activity and late osteogenic differentiation. The RT-qPCR results showed that the osteogenic relative factors including Runx2, BSP, COL-1 and OSX were markedly upregulated by inhibiting the expression of Shn3. These results may indicate that silencing Shn3 inhibits the WWP1-complex-dependent E3 ubiquitin ligase that elevated transcription of Runx2 and then further upregulated the target genes of Runx2.
In addition, VEGF is comprehensively expressed by cranial neural crest cells and plays multiple roles in regulating cell proliferation, vascularization, and bone formation, including endochondral ossification as well as intramembranous ossification. In addition, VEGF is widely known to induce angiogenesis63,64. In the process of angiogenesis, a variety of factors are involved. The main pro-angiogenic factors are VEGF, basic FGF, TGF-β family, and HIF1α65,66. Other factors contain angiogenic components, such as angiopoietin (ANGPT1), CD31, and vWF67,68. VEGF and its receptor VEGF-R are key regulators in the cascade of molecules, which ultimately lead to the development of the vasculature, and the formation of angiogenesis is accompanied by the occurrence of vasculature. Therefore, VEGF is a key regulator of angiogenesis, and VEGF plays a significant role in bone repair and development. Our results showed that silencing Shn3 could enhance the BMP9-induced angiogenic differentiation in hAMSCs and upregulate the mRNA expression of VEGF, ANGPT1, CD31, and vWF. In addition, silencing Shn3 markedly potentiates the vascular invasion of hAMSCs in vivo. These results strongly suggest that Shn3 not only regulates osteogenic differentiation but also controls angiogenesis. Nevertheless, how can we explain the coupling effect between osteogenesis and angiogenesis being regulated by Shn3? It has been reported that the DNA sequence recognized by Runx2 is 5′-PuACCPUCA-3′ and its complementary sequence is 5′-TGPyGGTPy-3′, which can be activated with a variety of protein promoters, including COL-I, OPN, OCN, and BSP. These are used to enhance the expression of these proteins and go on to promote osteogenic differentiation and bone formation69,70. Thus we conducted the ChIP assay and the results showed that silencing Shn3 increased the transcription activity of Runx2 in BMP9-induced hAMSCs, which could directly bind with the promoter of VEGF (Fig. 8h). Taken together, these results suggest that Shn3 exerts a coupling effect between osteogenic differentiation and angiogenesis in BMP9-induced hAMSCs by reinforcing the transcription activity of Runx2 and subsequent regulation of the VEGF expression.
In summary, our findings suggested that silencing Shn3 can promote BMP9-induced early and late osteogenic differentiation as well as angiogenesis both in vitro and in vivo, which may be mediated through enhancing the activity of the BMP/Smad signaling pathway and BMP/MAPK signaling pathway. Inhibition of Shn3 plays a coupling role in regulating the key osteogenic factor Runx2 that activate its downstream target VEGF to promote osteogenesis and angiogenesis in BMP9-induced hAMSCs.
Materials and methods
Isolation and cultivation of hAMSCs
This research was approved by the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Human placentas were obtained from the Obstetrics Department of the First Affiliated Hospital of Chongqing Medical University. hAMSCs were isolated from six full-term puerperants, and informed consent was obtained from all of the patients prior to their participation. For the isolation of hAMSCs, they were first dissected bluntly from the placenta as previously described13. Following this, the amnion samples were washed three times with phosphate-buffered saline (PBS) and transferred to sterile containers at 4 °C in a laboratory facility. The amnion was washed in a sterile dish with PBS containing 1% penicillin and streptomycin three times. The amnion tissue was minced into 1–2 mm3 pieces with sterile scissors. Digestion was conducted twice and was terminated by the addition of medium with 0.05% trypsin and 0.01% ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) for 30 min each and incubated for 1–2 h with 0.75% collagenase type II in low-glucose Dulbecco’s modified Eagle’s medium (LG-DMEM) with 1% penicillin and streptomycin in water at 37 °C, until the pieces were indistinguishable. hAMSCs were collected into 50 mL centrifuge tubes by passing through a 300/mesh filter. Cells were centrifuged at 1500 rpm for 6 min and re-suspended at a density of 10 × 104 cells/ml in LG-DMEM medium with 10% fetal bovine serum (FBS), 1.176 g NaHCO3, 1% penicillin and streptomycin, 1% L-glutamine, and non-essential amino acids and placed in diameter of 10 cm dishes at 37 °C, with 5% humidified CO2. The medium was refreshed every 3 days to remove the unattached cells with PBS. In each experiment, when the cells reached 80% confluence they were digested with 0.125% trypsin/0.01% EDTA-2Na for 3 min and passaged at ratio of 1:2 or 1:3 for subculture, and only cells between passages 3 and 5 were used for subsequent experiments.
Phenotypic identification of hAMSCs
The cell markers of P3 hAMSCs were detected using flow cytometry. P3 hAMSCs were seeded at a density of 2 × 106/mL in a 6-well plate. After the cells reached 90–100% confluence, the cells were digested and obtained in a 100-µL cell suspension, which was transferred to a flow cytometric tube. Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD90, phycoerythrin (PE)-conjugated anti-CD44, peridinin chlorophyll protein-conjugated anti-CD105, and allophycocyanin-conjugated anti-CD73. The cells were incubated with the negative control antibodies, including PE-conjugated anti-CD34, anti-CD19, anti-CD45, anti-CD11b, and anti-HLA-DR for 30 min in the dark, washed by the addition of 2 mL of flow buffer, and centrifuged at 1200 × g for 5 min. The liquid supernatant was removed, and the cells were re-suspended in 250 µL of flow buffer. Flow cytometry in conjunction with C6 Plus Workstation Computer and Software (BD AccuriTM C6 Plus Corporation, USA) was used to analyze hAMSC surface marker expression.
CK-19 and vimentin expression of hAMSC was determined by immunofluorescence. Goat IgG served as the isotype control and was added to eliminate non-specific staining. P3 hAMSCs on cover slips in six-well plates were fixed with 4% paraformaldehyde, and PBS-Tween-20 (PBST) was used to wash the cover slips. Cells were blocked with Lowlenthal serum for 30 min, then were incubated with purified primary anti-CK-19 and anti-vimentin antibodies overnight or for 12 h and then with secondary FITC-labeled antibodies for 2 h. Cell nuclei were counterstained with 2-(4-amidinophenyl)-1H-indole-6-carboxamidine (DAPI) at room temperature for 5 min. The results were observed by inverted fluorescence microscopy.
Multidirectional differentiation potential of hAMSCs
P3 hAMSCs were seeded at a density of 105 cells/mL in a 6-well plate. After cells reached 50–60% confluence, for osteogenic differentiation, cells were cultured and cycled through a series of mediums with a StemPro™ Human Osteogenesis Differentiation Kit (GibicoTM, USA) according to the manufacturer’s instructions for 14 days. Afterwards, the osteogenesis was analyzed. The osteogenic differentiation results were observed by Alzarin Red S staining (0.2%, pH = 8.3) (Solarbio, Beijing, China). For chondrogenic differentiation, hAMSCs were cultured with MSC chondrogenic differentiation basal medium (Cyagen Biosciences, Shanghai, China) for 14 days and assessed by Alcian Blue staining (1%) (Solarbio, Beijing, China). For adipogenic differentiation of hAMSCs, cells were cultured with human MSC adipogenic differentiation basal medium (Cyagen Biosciences, Shanghai, China) for 21 days, and Oil Red O (0.5% in isopropanol) (Solarbio, Beijing, China) staining was conducted to determine the differentiation potential of adipogenic formation, including intracellular lipid droplets.
Determination of hAMSC proliferation by CCK-8 assays
When P3 hAMCSs reached 80% confluence, cells were collected and suspended at 105 cells/mL; 100 µL cell suspension was added to a 96-well culture plate. Cells were successively cultured for 7 days; each day included five replicate wells. Viability was evaluated in all the wells by using CCK-8 assays. The results were recorded by microplate reader (Thermo Scientific™, USA) at absorbance of 450 nm. Growth curves were drawn, and the cell proliferation activity was analyzed.
Immunofluorescence stain assay
Cells were seeded onto sterile cover slips in a Corning 12-well culture plate at density of 104 cells/mL and treated according to the experimental design. At the indicated time point, cells were washed three times with PBS for 10 min each, then fixed with 4% paraformaldehyde at 37 °C for 15 min in a thermostatic water bath, washed with PBS for 10 min each, and then permeabilized using 0.4% Triton X-100 for 30 min at 37 °C. After cells were blocked with goat serum for 30 min, cells were incubated with the primary anti-CK19 (ab52625, Abcam, Cambridge, MA, USA), anti-vimentin (ab193555, Abcam, Cambridge, MA, USA), anti-CD31 (ab134168, Abcam, Cambridge, MA, USA), VEGF (ab32152, Abcam, Cambridge, MA, USA), and anti-vWF (ab6994, Abcam, Cambridge, MA, USA) antibodies overnight, followed by incubation with the corresponding fluorophore-conjugated antibodies for 60 min, then cells were washed with PBST for 10 min each and stained with DAPI for 5 min. The cover slips were carefully removed and then mounted on slides with glycerol. The same protocol was performed in the negative control groups except that the primary antibodies were omitted. The slides were observed by confocal microscopy (DFM-80C, Nikon, Japan), and images were assessed by Nikon auxiliary systems. The results of immunofluorescence were quantified using the Image Pro Plus software.
Recombinant adenovirus construction
The recombinant adenoviruses were generated with AdEasy technology as described previously71,72. Briefly, the coding regions of RFP, BMP9, and Shn3 (HIVEP3, human immunodeficiency virus type I enhancer binding protein 3) were amplified with the RT-qPCR and cloned into adenoviral shuttle vectors and used to generate recombinant adenoviruses in HEK-293 cells subsequently. The siRNA target sites against mouse Shn3-coding region were cloned into the pSES adenoviral shuttle vector to create recombinant adenoviruses. The resulting adenoviruses were designated as Ad-BMP9, Ad-Shn3, and Ad-Sim-Shn3. The Ad-BMP9 expresses green fluorescent protein, while Ad-Shn3 and Ad-Sim-Shn3 express RFP as a visual tag for monitoring infection efficiency. The analogous adenovirus expressing only monomeric RFP (Ad-RFP) served as a control.
ALP staining and activity
Cells were seeded in 24-well plates at a density of 30–40% confluence and treated as per the experimental design. ALP activities of cells were determined by a modified Great Escape SEAP Chemi-luminescence Assay (BD Clontech) and histochemical staining assay (solution containing 0.1 mg/mL naphthol AS-MX phosphate and 0.6 mg/mL Fast Blue BB salt) as described35,73. For the chemiluminescence assay, each assay was performed in triplicate, and the results were repeated in at least three independent experiments. Normalization of ALP activities were subjected to total cellular protein concentrations of hAMSCs. ALP activities were expressed as mean ± SD.
Alizarin Red S staining and calcium quantification assay
Cells were inoculated at a density of 30–40% confluence in 24-well plates and treated as per the experimental design. Cells were cultured with the conditioned medium containing 50 mg/L Vitamin C, 0.1 μmol/L dexamethasone, and 10 mmol/L β-Glycerol phosphate disodium for 14 and 21 days. The mineralization nodules were assessed by Alizarin Red S staining as described previously74,75. In brief, cells were fixed with 0.05% (v/v) glutaraldehyde at 37 °C for 15 min and washed with PBS for three times, then the mineralization tubercle were incubated with 0.4% Alizarin Red S for 10 min, followed by careful washing with distilled water. The calcium deposits were observed under microscope. For quantification, Alizarin Red S was dissolved with 10% acetic acid and the absorbance was detected at 405 nm with a micro-plate reader as described previously76,77. The results were performed in at least three independent experiments.
Reverse transcription and quantitative polymerase chain reaction
Total RNA was extracted with RNAiso reagents (TAKARA, Japan), then the cDNA was obtained from total RNA extracted from cells through reverse transcription (RT) reaction kit (RR047a, TAKARA, Japan). The products were diluted 5–10-fold and used as templates for detection by RT-qPCR. All samples were normalized with the level of glyceraldehyde phosphate dehydrogenase. The amplification conditions included pre-denaturation at 95 °C for 30 s, denaturation for 5 s, and annealing at 60 °C for 30 s. All samples were repeated three times. The PCR primers used in this study are provided in Supplementary Table 1. The relative expression levels of mRNAs in the groups were analyzed using the 2ΔΔCT method.
Protein harvest and western blotting
Cells were seeded in six-well plates and treated as per the experimental design. Total protein were obtained after lysis, and cleared lysates were denatured by boiling for 10 min with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously78. Page Ruler Plus Pre-stained Protein Ladder (26619; Thermo Scientific, USA) was used to evaluate the bands based on molecular weights ranging from 10 to 250 kDa. Proteins were separated by electrophoresis with Tris-glycine buffer and transferred carefully onto polyvinylidene difluoride (PVDF) membranes under dark conditions, in which the PVDF membrane were blocked with 5% evaporated milk for 2 h and incubated overnight with primary antibodies against OCN (ab13421, Abcam, USA), OPN (ab8448, Abcam, USA), and Runx2 (ab192256, Abcam, USA). After being washed, the membranes were probed with a fluorescently labeled secondary antibody. Immune-reactive signals were detected captured using a Bio-Rad. In addition, the membranes were incubated with a monoclonal mouse anti-human β-ACTIN (66009-1; Proteintech) antibody used as a loading control. Relative band intensity was measured using the ImageJ analysis software.
Stem cell implantation and ectopic ossification
hAMSCs were transfected with specific adenoviruses and harvested for subcutaneous injection (5 × 106 cells per injection) into the flanks of athymic nude (nu/nu) mice (4–6-week-old males, Harlan Sprague-Dawley) until the fluorescence could be seen. At 4 weeks after injection, animals were euthanized, and the bony masses were collected for micro-CT imaging and histologic evaluation.
Micro-CT imaging analysis and hematoxylin and eosin (H&E), Masson’s trichrome, and Safranin O-fast green staining
Animals were euthanized 4 weeks after injection and the retrieved bone masses were scanned after 4 weeks with Skyscan1174 X-Ray Microtomograph (Micro-CT) (Bruker Company, Belgian) after the animals were euthanized. N-Recon software was used for 3D image reconstruction and all image data analysis was performed using the CT-AN software. Retrieved bony masses were decalcified with EDTA and then processed for paraffin embedding. BV/TV (%), Tb. N, Tb. Sp, Tb. Th, and BMD were measured.
The retrieved bone masses were decalcified, washed with PBS three times, fixed in 4% paraformaldehyde overnight at 37 °C, and embedded in paraffin. Serial sections of embedded bone masses were stained with H&E, and Masson’s trichrome or Alcian Blue staining was carried out as previously described73,79.
HUVEC cell tube-formation assay
The tube-like structures of HUVECs were developed on growth factor-reduced Matrigel (BD Bioscience, USA) in conditioned media and were assayed using Transwell plates with polycarbonate filters (pore size: 4 μm). Before the experiment, the Matrigel sterilized tips were chilled at 4 °C overnight. Twenty-four-well Transwell cultivation plates were used and daubed with the suspension of 200 μL Matrigel and 200 μL complete medium according to the manufacturer’s instructions. HUVECs were seeded in endothelial conditioned medium containing 10% FBS, 2 mM/L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/mL streptomycin, and 1% ECGS (ScienCell, CA, USA) for 12 h and plated onto the lower layer of the Transwell with diluted Matrigel at a density of 2 × 105 cells/mL/well. Then the cells treated as per the experimental design were loaded into each of the upper wells. The Matrigel in the Transwell cultivation was incubated at 37 °C and 5% CO2 for 6 h. HUVECs were stained using 2 μM calcein AM fluorescent dye (Solarbio, Beijing, China) (Fig. 6c). Tube areas were quantified by the number of tubes and relative areas of tubes. The results were recorded under the microscope at 6 h. The number of tubes and relative area of tubes were assessed from five figures of each well by Adobe Photoshop (Adobe, San Jose, CA, USA).
In vivo implantation of PLGA–hAMSC hybrids to evaluate angiogenesis
Cells were seeded at a density of 5 × 105 cells/mL on the PLGA scaffolds (diameter 3.5 mm, thickness 200 μm: bought from Foshan Lepton Precision Measurement And Control Technology Company, Guangdong, China) for 24 h. Eighteen mice (6-week-old males; BALB/cAnN, Beijing, China), weighing 18–25 g, were anesthetized with 1% pentobarbital sodium (30 mg/kg), then the cells–PLGA were implanted into the dorsal subcutaneous position. The cells–PLGA composite were collected and analyzed after 5 weeks. Procedures for the animal study were approved by the Institutional Animal Care and Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The mice were euthanized 5 weeks after implantation surgery. The cells–PLGA composite was retrieved and fixed in 4% paraformaldehyde solution, then immunohistochemical staining was performed for vWF. They were incubated with the primary antibodies against mouse vWF (ab6994, Abcam, USA), followed by incubation with the corresponding fluorophore-conjugated antibodies. The immunohistochemical staining results were observed by inverted fluorescence microscopy (Oly 3800; Olympus), and the images were analyzed using an Olympus auxiliary system.
ChIP assay
Subconfluent hAMSCs were seeded in T75 flasks and infected with Ad-RFP or Ad-Sim-Shn3. The cells were crosslinked after 48 h of infection. The cells were subjected to the ChIP analysis according to the manufacturer’s instructions. The cells were incubated with a monoclonal rabbit anti-human VEGF (Anti-VEGF Antibody, clone JH Sigma-Aldrich) antibody or IgG to pull down the DNA–protein complexes. The PCR primers and sequence of promoter used in this study are provided in Supplementary Tables 2 and 3. The presence of Runx2 promoter sequence was analyzed by three pairs of primers corresponding to the human Runx2 promoter region.
Statistical analysis
All quantitative experiments were performed in triplicate and/or repeated through three independent batches of experiments. Differences among groups were assessed using a three-way analysis, and the data are reported as the mean ± standard deviation. Statistical analyses were performed using the software package SPSS 14.0, and Fisher Exact tests and Student–Newman–Keuls q tests were used to identify significant differences among groups. Statistical significance was set at level of P < 0.05 for all post hoc comparisons.
Supplementary information
Acknowledgements
We thank Dr. Chenghao Zhang from the University of Pennsylvania for his constructive comments and valuable discussion. This work was supported by the National Natural Science Foundation of China (Nos. 81672167 and 81572142).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by Y. Shi
This article has been retracted. Please see the retraction notice for more detail: 10.1038/s41419-021-04443-8
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuwan Li, Ziming Liu
Change history
12/9/2021
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41419-021-04443-8
Contributor Information
Ning Hu, Email: 1276321387@qq.com.
Wei Huang, Email: weih68@hotmail.com.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-2279-5).
References
- 1.El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. doi: 10.1016/j.actbio.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Schemitsch EH. Size matters: defining critical in bone defect size! J. Orthop. Trauma. 2017;31(Suppl 5):S20–S22. doi: 10.1097/BOT.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 3.Blaudez F, Ivanovski S, Hamlet S, Vaquette C. An overview of decellularisation techniques of native tissues and tissue engineered products for bone, ligament and tendon regeneration. Methods. 2019 doi: 10.1016/j.ymeth.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, et al. Overexpression of MiR-335-5p promotes bone formation and regeneration in mice. J. Bone Miner. Res. 2017;32:2466–2475. doi: 10.1002/jbmr.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 6.Fu Q, et al. Engrafted peripheral blood-derived mesenchymal stem cells promote locomotive recovery in adult rats after spinal cord injury. Am. J. Transl. Res. 2017;9:3950–3966. [PMC free article] [PubMed] [Google Scholar]
- 7.Camernik K, et al. Skeletal-muscle-derived mesenchymal stem/stromal cells from patients with osteoarthritis show superior biological properties compared to bone-derived cells. Stem Cell Res. 2019;38:101465. doi: 10.1016/j.scr.2019.101465. [DOI] [PubMed] [Google Scholar]
- 8.Fu W, et al. Mesenchymal stem cells reside in anterior cruciate ligament remnants in situ. Int. Orthop. 2016;40:1523–1530. doi: 10.1007/s00264-015-2925-1. [DOI] [PubMed] [Google Scholar]
- 9.Sha Y, et al. MGF E peptide pretreatment improves the proliferation and osteogenic differentiation of BMSCs via MEK-ERK1/2 and PI3K-Akt pathway under severe hypoxia. Life Sci. 2017;189:52–62. doi: 10.1016/j.lfs.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Li B. MicroRNA regulation in osteogenic and adipogenic differentiation of bone mesenchymal stem cells and its application in bone regeneration. Curr. Stem Cell Res. Ther. 2018;13:26–30. doi: 10.2174/1574888X12666170605112727. [DOI] [PubMed] [Google Scholar]
- 11.Beeravolu N, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J. Vis. Exp. 2017 doi: 10.3791/55224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silini AR, et al. The dichotomy of placenta-derived cells in cancer growth. Placenta. 2017;59:154–162. doi: 10.1016/j.placenta.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, et al. Differentiation of human amniotic mesenchymal stem cells into human anterior cruciate ligament fibroblast cells by in vitro coculture. Biomed. Res. Int. 2017;2017:7360354. doi: 10.1155/2017/7360354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, et al. Mesenchymal stem cell sheets: a new cell-based strategy for bone repair and regeneration. Biotechnol. Lett. 2019;41:305–318. doi: 10.1007/s10529-019-02649-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Lee S, Kim K. Bone tissue engineering strategies in co-delivery of bone morphogenetic protein-2 and biochemical signaling factors. Adv. Exp. Med. Biol. 2018;1078:233–244. doi: 10.1007/978-981-13-0950-2_12. [DOI] [PubMed] [Google Scholar]
- 16.Lamplot JD, et al. BMP9 signaling in stem cell differentiation and osteogenesis. Am. J. Stem Cells. 2013;2:1–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Reddi AH, Reddi A. Bone morphogenetic proteins (BMPs): from morphogens to metabologens. Cytokine Growth Factor Rev. 2009;20:341–342. doi: 10.1016/j.cytogfr.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289:313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- 19.Kang Q, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 20.Akla N, et al. BMP9 (bone morphogenetic protein-9)/Alk1 (activin-like kinase receptor type I) signaling prevents hyperglycemia-induced vascular permeability. Arterioscler. Thromb. Vasc. Biol. 2018;38:1821–1836. doi: 10.1161/ATVBAHA.118.310733. [DOI] [PubMed] [Google Scholar]
- 21.Jones DC, et al. Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science. 2006;312:1223–1227. doi: 10.1126/science.1126313. [DOI] [PubMed] [Google Scholar]
- 22.Xu R, et al. Targeting skeletal endothelium to ameliorate bone loss. Nat. Med. 2018;24:823–833. doi: 10.1038/s41591-018-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Affolter M, Marty T, Vigano MA, Jazwinska A. Nuclear interpretation of Dpp signaling in Drosophila. EMBO J. 2001;20:3298–3305. doi: 10.1093/emboj/20.13.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducy P, et al. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim JH, et al. Schnurri-3 regulates ERK downstream of WNT signaling in osteoblasts. J. Clin. Invest. 2013;123:4010–4022. doi: 10.1172/JCI69443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 27.Wein MN, et al. Control of bone resorption in mice by Schnurri-3. Proc. Natl Acad. Sci. USA. 2012;109:8173–8178. doi: 10.1073/pnas.1205848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang YS, et al. Bone-targeting AAV-mediated silencing of Schnurri-3 prevents bone loss in osteoporosis. Nat. Commun. 2019;10:2958. doi: 10.1038/s41467-019-10809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22:1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinik Y, et al. Ablation of the mammalian lectin galectin-8 induces bone defects in mice. FASEB J. 2018;32:2366–2380. doi: 10.1096/fj.201700716R. [DOI] [PubMed] [Google Scholar]
- 31.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Miner. Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sivaraj KK, Adams RH. Blood vessel formation and function in bone. Development. 2016;143:2706–2715. doi: 10.1242/dev.136861. [DOI] [PubMed] [Google Scholar]
- 34.Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu N, et al. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J. Cell Sci. 2013;126:532–541. doi: 10.1242/jcs.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng GH, Luo Q, Jin XQ, Guo YX, Xu YH. Screening siRNAs targeting a novel gene (HA117) and the development of a derivative recombinant adenovirus delivery system. Cancer Gene Ther. 2011;18:655–662. doi: 10.1038/cgt.2011.32. [DOI] [PubMed] [Google Scholar]
- 37.Lafage-Proust MH, Prisby R, Roche B, Vico L. Bone vascularization and remodeling. Jt. Bone Spine. 2010;77:521–524. doi: 10.1016/j.jbspin.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Chim SM, et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. 2013;24:297–310. doi: 10.1016/j.cytogfr.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Xie H, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang M, et al. Kruppel-like factor 3 inhibition by mutated lncRNA Reg1cp results in human high bone mass syndrome. J. Exp. Med. 2019;216:1944–1964. doi: 10.1084/jem.20181554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 42.Alviano F, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parolini O, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first International Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder DI, et al. The human placenta methylome. Proc. Natl Acad. Sci. USA. 2013;110:6037–6042. doi: 10.1073/pnas.1215145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CY, Liu SH, Chen CY, Chen PC, Chen CP. Human placenta-derived multipotent mesenchymal stromal cells involved in placental angiogenesis via the PDGF-BB and STAT3 pathways. Biol. Reprod. 2015;93:103. doi: 10.1095/biolreprod.115.131250. [DOI] [PubMed] [Google Scholar]
- 46.Eiraku Nahoko, Chiba Norika, Nakamura Toshiaki, Amir Muhammad Subhan, Seong Chang-Hwan, Ohnishi Tomokazu, Kusuyama Joji, Noguchi Kazuyuki, Matsuguchi Tetsuya. BMP9 directly induces rapid GSK3-β phosphorylation in a Wnt-independent manner through class I PI3K-Akt axis in osteoblasts. The FASEB Journal. 2019;33(11):12124–12134. doi: 10.1096/fj.201900733RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reible Bruno, Schmidmaier Gerhard, Moghaddam Arash, Westhauser Fabian. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. International Journal of Molecular Sciences. 2018;19(6):1674. doi: 10.3390/ijms19061674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassanshahi M, Hassanshahi A, Khabbazi S, Su YW, Xian CJ. Bone marrow sinusoidal endothelium: damage and potential regeneration following cancer radiotherapy or chemotherapy. Angiogenesis. 2017;20:427–442. doi: 10.1007/s10456-017-9577-2. [DOI] [PubMed] [Google Scholar]
- 50.Zhu B, Xue F, Zhang C, Li G. LMCD1 promotes osteogenic differentiation of human bone marrow stem cells by regulating BMP signaling. Cell Death Dis. 2019;10:647. doi: 10.1038/s41419-019-1876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rezzola S, et al. VEGFR2 activation mediates the pro-angiogenic activity of BMP4. Angiogenesis. 2019 doi: 10.1007/s10456-019-09676-y. [DOI] [PubMed] [Google Scholar]
- 53.Gu Y, Becker V, Zhao Y, Menger MD, Laschke M. W. miR-370 inhibits the angiogenic activity of endothelial cells by targeting smoothened (SMO) and bone morphogenetic protein (BMP)-2. FASEB J. 2019;33:7213–7224. doi: 10.1096/fj.201802085RR. [DOI] [PubMed] [Google Scholar]
- 54.Chen S, Jia L, Zhang S, Zheng Y, Zhou Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res. Ther. 2018;9:185. doi: 10.1186/s13287-018-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KM, et al. Peroxiredoxin II negatively regulates BMP2-induced osteoblast differentiation and bone formation via PP2A Calpha-mediated Smad1/5/9 dephosphorylation. Exp. Mol. Med. 2019;51:62. doi: 10.1038/s12276-019-0263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang Q, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18:545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su X, et al. CCN3 and DLL1 co-regulate osteogenic differentiation of mouse embryonic fibroblasts in a Hey1-dependent manner. Cell Death Dis. 2018;9:1188. doi: 10.1038/s41419-018-1234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nieto L, Fuertes M, Rosmino J, Senin S, Arzt E. Crosstalk of BMP-4 and RA signaling pathways on POMC gene regulation in corticotrophs. J. Mol. Endocrinol. 2019 doi: 10.1530/JME-19-0059. [DOI] [PubMed] [Google Scholar]
- 59.Li R, et al. Influences of hucMSC-exosomes on VEGF and BMP-2 expression in SNFH rats. Eur. Rev. Med. Pharmacol. Sci. 2019;23:2935–2943. doi: 10.26355/eurrev_201904_17573. [DOI] [PubMed] [Google Scholar]
- 60.Wu LC, et al. Molecular cloning of a zinc finger protein which binds to the heptamer of the signal sequence for V(D)J recombination. Nucleic Acids Res. 1993;21:5067–5073. doi: 10.1093/nar/21.22.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oukka M, Wein MN, Glimcher LH. Schnurri-3 (KRC) interacts with c-Jun to regulate the IL-2 gene in T cells. J. Exp. Med. 2004;199:15–24. doi: 10.1084/jem.20030421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pickart CM. Back to the future with ubiquitin. Cell. 2004;116:181–190. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- 63.Schorn L, et al. Vertical bone regeneration using rhBMP-2 and VEGF. Head Face Med. 2017;13:11. doi: 10.1186/s13005-017-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 65.Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp. Physiol. 2005;90:315–326. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- 66.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 67.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 68.Liu CH, Wang Z, Sun Y, Chen J. Animal models of ocular angiogenesis: from development to pathologies. FASEB J. 2017;31:4665–4681. doi: 10.1096/fj.201700336R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu J, Hirschi KK. Endothelial cell development and its application to regenerative medicine. Circ. Res. 2019;125:489–501. doi: 10.1161/CIRCRESAHA.119.311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park MH, et al. Differential expression patterns of Runx2 isoforms in cranial suture morphogenesis. J. Bone Miner. Res. 2001;16:885–892. doi: 10.1359/jbmr.2001.16.5.885. [DOI] [PubMed] [Google Scholar]
- 71.He TC, et al. A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo J, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J. Bone Miner. Res. 2010;25:2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, et al. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J. 2019;33:8913–8924. doi: 10.1096/fj.201802711R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen H, et al. Increased glycolysis mediates Wnt7b-induced bone formation. FASEB J. 2019;33:7810–7821. doi: 10.1096/fj.201900201RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCabe KM, et al. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013;83:835–844. doi: 10.1038/ki.2012.477. [DOI] [PubMed] [Google Scholar]
- 77.He F, et al. BMP9/COX-2 axial mediates high phosphate-induced calcification in vascular smooth muscle cells via Wnt/beta-catenin pathway. J. Cell. Biochem. 2018;119:2851–2863. doi: 10.1002/jcb.26460. [DOI] [PubMed] [Google Scholar]
- 78.Yang M, et al. Role of bone morphogenetic protein-9 in the regulation of glucose and lipid metabolism. FASEB J. 2019;33:10077–10088. doi: 10.1096/fj.201802544RR. [DOI] [PubMed] [Google Scholar]
- 79.Tang N, et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J. Cell. Mol. Med. 2009;13:2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.